Abstract
A successful Staphylococcus aureus vaccine remains elusive, and one controversy in the field is whether humans generate a protective adaptive immune response to infection. We developed a bacterial challenge murine assay that directly assesses the protective capacity of adoptively transferred human serum samples. We first validated the model by showing that postpneumococcal vaccine serum samples from humans induced effective clearance of Streptococcus pneumoniae in mice. We then found that human serum samples adoptively transferred from children with invasive S. aureus infections exhibited protection from disease in a murine model, with some samples conferring near complete protection. These findings demonstrate that human serum samples are capable of conferring a protective adaptive response generated by humans during invasive staphylococcal disease, allowing for the study of protective factors in a murine model. Identification of the protective factors present in the most efficacious serum samples would be of high interest as potential staphylococcal vaccine candidates or passive therapeutics.
Keywords: Staphylococcus aureus, pediatric infection, adaptive host response, antibodies
Adoptive transfer of human serum samples from children invasively infected with Staphylococcus aureus ameliorated disease in a murine sepsis model, with protective effects peaking in serum samples obtained during disease convalescence, although the specific serologic components conferring protection remain to be determined.
Staphylococcus aureus is now the most common invasive bacterial pathogen infecting children in the United States [1–3], owing in part to successful vaccines against Hemophilus influenzae and Streptococcus pneumoniae [4]. Despite these successes, an effective human vaccine against S. aureus remains elusive. Although many vaccines have shown success in laboratory animals, all vaccines that have advanced to clinical trials have failed [5]. Speculation for the failures abound and include improper antigen selection or inadequately characterized host responses to S. aureus during human infection [6, 7]. S. aureus is well known to express numerous factors to combat the humoral immune response which have also been proposed to interfere with vaccination [8, 9]. The differential expression or variation in species tropism of critical S. aureus virulence factors are additional speculated factors [10–12]. In total, the poor predictive value of animal models threatens to derail new efforts to bring investigative vaccines to clinical trials.
A reliable approach to assess direct human immune responses to S. aureus or staphylococcal vaccines is crucially needed to advance vaccine development. Various in vitro correlates of human responses to S. aureus have been reported. We and others have observed that serum samples obtained from human subjects with invasive S. aureus disease are capable of facilitating neutrophil-mediated killing in vitro [13]. These effects may occur via a number of potential mechanisms, including antibody-dependent facilitation of neutrophil uptake (ie, opsonophagocytosis) [14, 15]; antibody-mediated neutralization of crucial secreted S. aureus virulence factors (eg, the leukocidins) [16–18]; Fc fragment–mediated effects (eg, antibody-dependent complement deposition) [14]; or nonantibody components of serum (eg, proteases, cathelicidins, etc) [19, 20]. To date, however, the critical factors that confer protection by human serum samples are inadequately defined, and a serologic correlate of protection for S. aureus remains elusive.
In the current study, we report the development of a bacterial challenge mouse assay that directly assesses the protective capacity of adoptively transferred human serum samples. Using the model, we showed that postpneumococcal vaccine serum samples induced effective clearance of S. pneumoniae in mice. Furthermore, we described application of the platform to human serum samples collected from children invasively infected with S. aureus at multiple time points. We sought to (1) determine whether adoptively transferred human serum was capable of protecting mice from S. aureus infection, (2) determine whether protective efficacy varies by postinfection kinetics, and (3) compare in vivo protection with hypothesized in vitro correlates of protection.
METHODS
Donor Subjects
We enrolled a series of 14 children over a 12-month period admitted to the Monroe Carell, Jr, Children’s Hospital at Vanderbilt, a large tertiary care children’s hospital, with culture-proven invasive S. aureus infection (bacteremia, endocarditis, or musculoskeletal infection). Serum samples were obtained on enrollment in the study (V1), 4–6 weeks after enrollment (V2), and, when possible, 6–12 months after enrollment (V3). Serum samples were obtained by centrifugation of unheparinized whole-blood samples and stored at –20°C. (See Supplementary Methods for study approvals and details for patients with invasive disease and healthy control subjects).
For proof-of-principle studies involving pneumococcal serum samples, leftover samples were obtained from a vaccine clinical trial (see Supplementary Methods for details). For the neutrophil phagocytosis assay, human whole-blood samples collected at Massachusetts General Hospital from healthy donors and used as sources of uninfected primary neutrophils.
Murine Model of Disseminated S. aureus Infection
Overnight culture of S. aureus strain of USA300 lineage LAC, a methicillin-resistant S. aureus strain, was diluted 1:00 in Todd Hewitt broth and grown to an optical density of 0.6. Female C57BL/6 mice, 6–8 weeks old, were injected intraperitoneally with 100 μL of human serum. After 24 hours, all mice were challenged intraperitoneally with 107 colony-forming units (CFUs) of S. aureus. Spleen and kidneys were harvested after 24 hours, homogenized in 1 mL of phosphate-buffered saline (PBS). and plated on agar plates, and CFUs were enumerated the next day. Kidneys and spleens were of equivalent size across animals within the same experiment. For samples that demonstrated efficacy and for which sufficient residual sample remained, these experiments were repeated with heat inactivated serum samples at 55°C and 60°C for complement and antibody inactivation, respectively.
Enzyme-Linked Immunosorbent Assay and Toxin Neutralization Assays
Binding of serum samples to LukAB or α-hemolysin (Hla) was detected by indirect enzyme-linked immunosorbent assay. The antigens were immobilized (62.5 µg per well) on microtiter plates. Serum samples in serial PBS dilutions from 1:10 to 1:1000 were added, and bound antibodies were detected using anti-human immunoglobulin (Ig) G antibodies conjugated to peroxidase. The data were plotted using Prism software (GraphPad), and nonlinear regression analysis was performed to calculate the half-maximal binding concentrations.
For toxin neutralization, polymorphonuclear leukocyte (PMN)–like HL-60 cells were used as described elsewhere [21]. Serial dilutions of each serum sample were mixed with 1.25 µg/mL of purified LukAB. Samples were preincubated for 30 minutes at room temperature (RT) before adding 1.26 × 105 PMNs in a final reaction volume of 100 µL. Cells were incubated for 1 hour at 37°C and 5% carbon dioxide before addition of CellTiter CellTiter metabolic dye, as described elsewhere [13].
Neutrophil-Mediated Killing of S. aureus
Overnight cultures of a WT S. aureus strain of USA300 lineage LAC [3, 15], grown in Roswell Park Memorial Institute 1640 medium (RPMI; Invitrogen) supplemented with 0.05 mol/L sodium bioate and 1% casamino acids (RPMI + CAS), were subcultured 1:100 in RPMI + CAS and incubated for 5 hours with shaking at 180 rpm. Cell pellets were washed and normalized to equal density before infection [4]. Normalized S. aureus cultures were used to infect PMN-like HL-60 cells, seeded at 2 × 105 cells per well, at a multiplicity of infection of 2.5, in the presence of 5% guinea pig complement and serial dilutions of human serum samples in a final volume of 190 µL, and incubated at 37°C and 5% carbon dioxide. Next, 5 µL samples were removed and plated for CFU counts at 0, 30, 60, 120, 180, and 240 minutes after infection. CFU counts were obtained after overnight incubation at 37°C.
Antigen Coupling to Fluorescent Beads
Supernatant from overnight culture of S. aureus strain Newman, selected for its known ability to abundantly produce a variety of virulence factors in vitro [22], was prepared by subculture 1:100 in RPMI + CAS, incubated for 5 hours with shaking at 180 rpm, followed by centrifugation at 4°C and4000 rpm for 15 minutes and filter sterilization (0.22 µm). Supernatant proteins were covalently coupled to 5 × 106 Magplex-C microspheres (Luminex; MC100XX) or 9 × 108 carboxylate-modified, 1-μm fluorescent microspheres (Thermo Fisher; F8823), using a 2-step carbodiimide reaction. The beads were first washed and resuspended in 100 mmol/L monosodium phosphate, pH 6.2, and then activated by incubation with 500 μg of sulfo-NHS (Pierce; A39269) and 500 μg of 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) (Pierce; A35391) at RT for 30 minutes. The beads were washed 3 times with coupling buffer (50 mmol/L 2-(N-morpholino)ethanesulfonic acid (MES), pH 5.0), then incubated with 25 μg of S. aureus supernatant protein in 100 μL of coupling buffer for 2 hours at RT. The beads were washed 3 times with PBS-TBN (1× PBS, 0.1% bovine serum albumin, 0.02% Tween-20, and 0.05% sodium azide, pH 7.4) and then blocked with PBS-TBN for 30 minutes at RT. The beads were then washed 3 times with PBS, 0.05% Tween 20, and resuspended in storage buffer (1× PBS, 0.05% sodium azide).
S. aureus–Specific IgG Quantification
Antigen-specific IgG levels were quantified using a method described elsewhere [23]. Detailed methods are provided in the Supplementary Methods.
Antibody-Dependent Phagocytosis Assays
Assays for measuring antibody-dependent cellular phagocytosis and neutrophil phagocytosis were used as described elsewhere [24, 25]. Detailed methods are provided in the Supplementary Methods.
Statistical Analysis
For murine infections, differences in CFU burdens in each organ were compared using the Wilcoxon rank sum test, assuming nonparametric distribution. Differences were considered statistically significant at P < .05. For in vitro characterizations, bivariate correlations were measured by calculation of the Pearson correlation coefficient, with Bonferroni correction for multiple comparisons. Statistical analyses were performed using Prism 8.3.0 software (GraphPad).
RESULTS
Proof of Principle: Pneumococcal Model
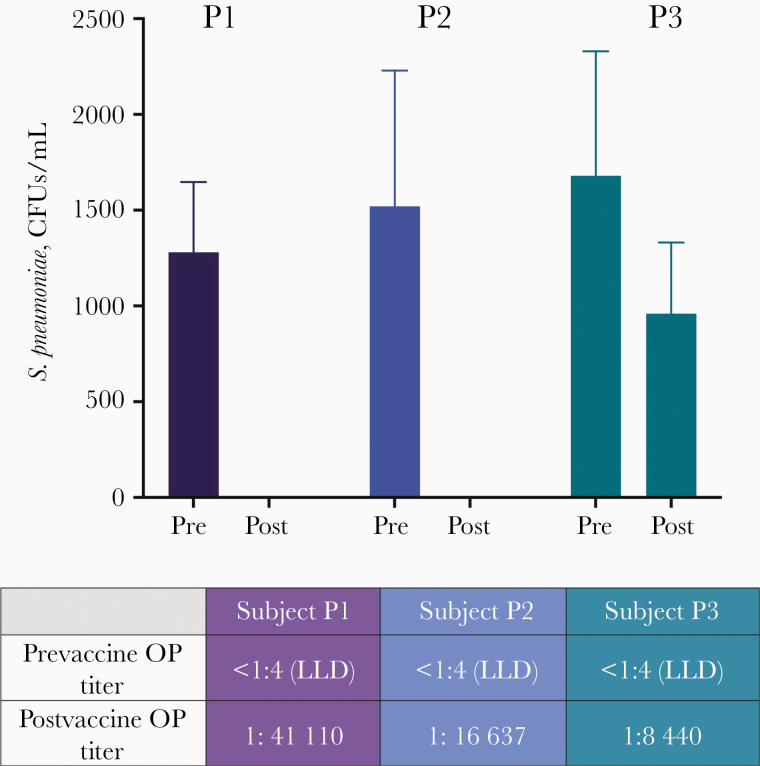
We first validated the utility of the human serum adoptive transfer model using prevaccine and postvaccine serum samples from individuals who received the 13-valent pneumococcal conjugate vaccine; this vaccine has proved to be highly efficacious in generating a capsular type-specific functional antibody response in humans, with reliable correlates of protection including opsonophagocytic titers [26]. On transfer of human serum samples into the murine model, samples from high- and moderate-titer responders, but not from low-titer responders, completely protected against pneumococcal disease in vivo, confirming the retention of antibody function and capacity of transferred human serum samples to protect against murine disease with efficacy equivalent to successful primary vaccination (Figure 1).
Figure 1.
Adoptive transfer proof of principle with postvaccine pneumococcal serum samples. Prevaccine (Pre) and postvaccine (Post) serum samples were adoptively transferred to mice from human subjects who received the conjugate pneumococcal vaccine and had a high (P1), moderate (P2), or low (P3) vaccine response by opsonophagocytic (OP) titer. Colony-forming units (CFUs) per milliliter were enumerated from whole homogenized kidney (n = 5 mice per group). Mice receiving serum samples from high- and medium-titer vaccine respondents exhibited no detectable Streptococcus pneumoniae 24 hours after infection. Abbreviation: LLD, lower limit of detection.
Human Samples After Invasive S. aureus Infection
The human antibody response after S. aureus infections has been abundantly studied, but it is unknown whether these antibodies confer short- or long-term protection against S. aureus in the host [27–29]. Therefore, after proof-of-principle studies involving pneumococcal serum samples, we sought next to apply the adoptive transfer platform to study protective antibody generation in children with invasive S. aureus infections.
Fourteen children were enrolled during the study period, all with culture-proven invasive S. aureus disease. The mean age of children enrolled in the study was 11.0 years (standard deviation, 3.4 years), and 50% were female. All children had culture-proven invasive S. aureus infection, defined as S. aureus in an otherwise sterile site, such as the bloodstream, bone, or joint. The majority of children (9 of 14) had acute hematogenous osteomyelitis and/or bacteremic septic arthritis (Table 1).
Table 1.
Clinical and Molecular Epidemiologic Characteristics of Enrolled Subjects and the Infecting Staphylococcus aureus Isolates
| Subject ID | Age, y | Sex | Diagnosis | Isolate | USA Type | MLST (ST) | ST Complex |
|---|---|---|---|---|---|---|---|
| Patients | |||||||
| A | 5 | F | Endocarditis with septic emboli to brain | MSSA | 100 | 5 | CC5 |
| B | 10 | M | Femoral osteomyelitis with subperiosteal abscess | MRSA | 300 | 8 | CC8 |
| C | 7 | M | Pyomyositis (thigh) | MSSA | 400 | 88 | NA |
| D | 11 | M | Septic arthritis and osteomyelitis | MRSA | 300 | 8 | CC8 |
| E | 14 | F | Bacteremia | MSSA | 300 | 8 | CC8 |
| F | 8 | M | Tibial osteomyelitis with subperiosteal abscess | MRSA | 300 | 8 | CC8 |
| G | 13 | F | Pyomyositis of iliopsoas and sacroiliitis | MSSA | NT | 106 | NA |
| H | 11 | F | Septic arthritis (hip) | MRSA | 300 | 8 | CC8 |
| I | 16 | M | Pelvic osteomyelitis | MSSA | NT | 87 | NA |
| J | 9 | F | Pyomyositis and pelvic osteomyelitis | MSSA | 600 | 45 | CC45 |
| K | 14 | M | Sepsis with multifocal osteomyelitis | MSSA | 300 | 8 | CC8 |
| L | 10 | M | Pneumonia | MRSA | NT | 72 | CC8 |
| M | 9 | F | Osteomyelitis (hip) with pulmonary septic emboli | MRSA | 300 | 8 | CC8 |
| N | 17 | F | Clavicular osteomyelitis | MSSA | 100 | 5 | CC5 |
| Health controls | |||||||
| H1 | 10 | M | … | … | … | … | … |
| H2 | 8 | F | … | … | … | … | … |
| H3 | 13 | F | … | … | … | … | … |
Abbreviations: ID, identifier; MLST, multilocus sequence typing; MRSA, methicillin-resistant Staphylococcus aureus strain; MSSA, methicillin-susceptible S. aureus; NA, could not be assigned; ST, sequence type.
Protection Against S. aureus Sepsis
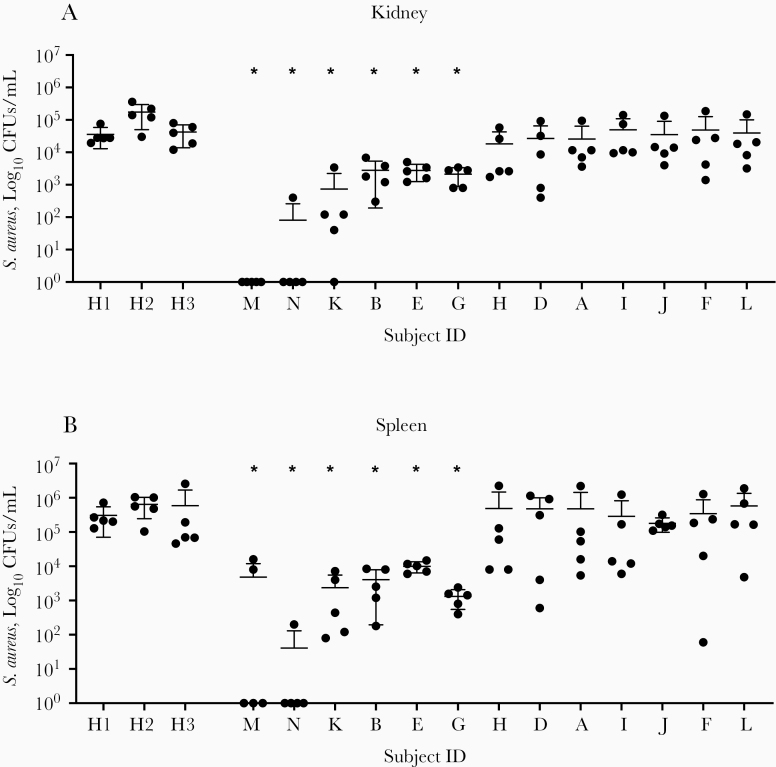
In total, 35 serum samples, obtained at multiple time points from 14 distinct patients, were adoptively transferred into C57BL/6 mice before systemic challenge of the mice with S. aureus LAC (USA300) by the intraperitoneal route. Of these, samples from 6 patients exhibited significant protection against end-organ S. aureus disease compared to healthy control serum samples, with samples from 2 of the subjects demonstrating nearly complete protection against S. aureus infection by end-organ CFU count (Figure 2). The serum samples with near-complete protection against disease (0 CFUs in kidney in spleen in most replicates) were obtained approximately 6 weeks after infection from a 9-year-old girl with severe USA300 methicillin-resistant S. aureus sepsis, and a 17-year-old girl with USA100 methicillin-susceptible S. aureus osteomyelitis. Broadly, samples could be classified as highly protective (n = 2), moderately protective (n = 4), or nonprotective (n = 7).
Figure 2.
Adoptive transfer of human sera after invasive Staphylococcus aureus infection. Serum samples obtained from pediatric subjects at disease convalescence (4–6 weeks after infection) were adoptively transferred to C57BL/6 mice. In the 13 subjects in whom convalescent serum samples could be obtained, samples from 6 exhibited significant protection from disease, by S. aureus colony-forming units (CFUs) per milliliter in kidney (A) and spleen (B) 24 hours after infection, with serum samples from 2 children (subjects M and N) exhibiting near-complete protection from disease. H1, H2, and H3 represent healthy control subjects with no known history of S. aureus infection. CFUs were enumerated from whole homogenized organ in 1 mL of phosphate-buffered saline.*P < .01 (Wilcoxon rank sum test; n = 5 animals per group). Abbreviation: ID, identifier.
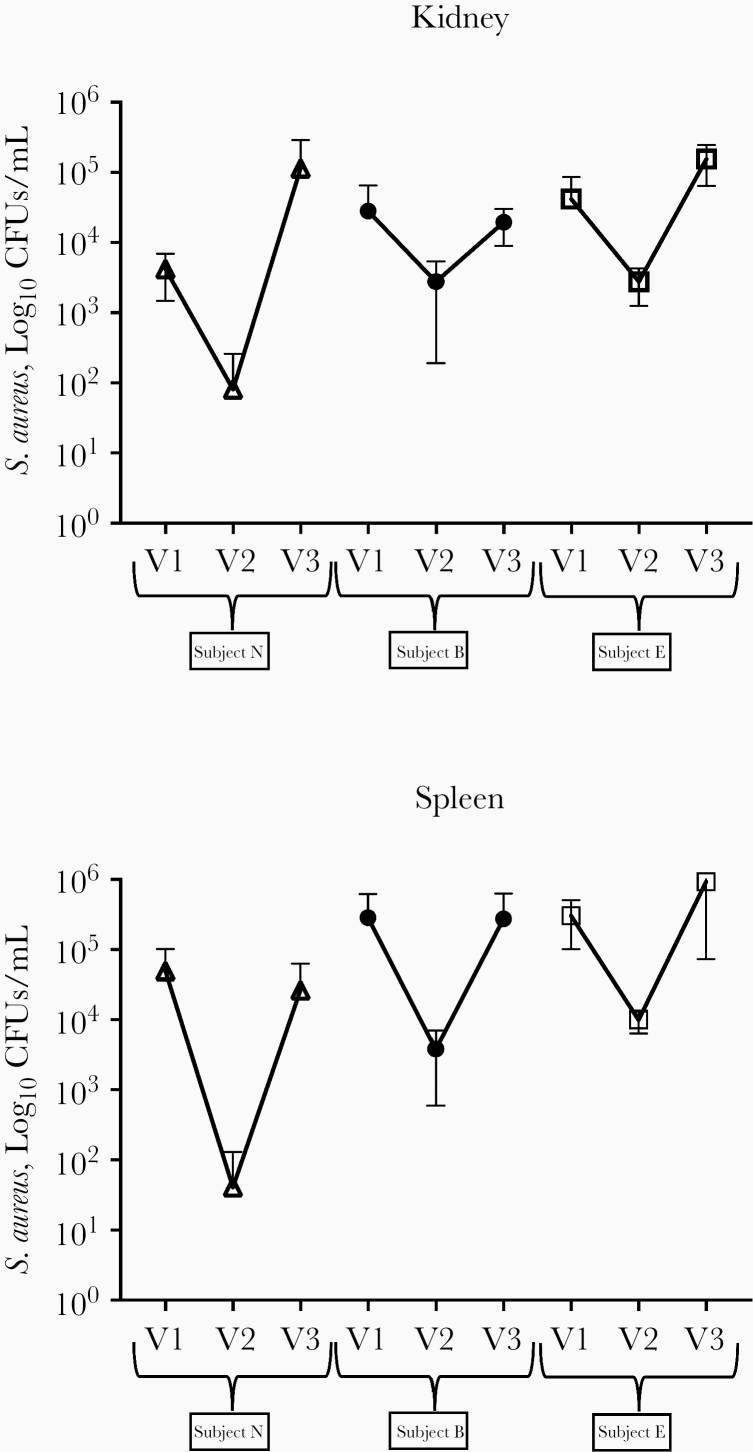
Of note, all of the highly or moderately protective samples resulted from the convalescent time point (V2, obtained 4–6 weeks after infection), and all paired serum samples exhibited greater protective function at the V2 compared to the V1 (immediately after infection) time point. Three of the children with highly or moderately protective serum samples at V2 were willing and able to return to provide a sample for a late (V3) time point (6 months after infection). In all 3 cases, protective capacity of the serum had waned and was significantly reduced compared with the V2 time point (Figure 3). Four samples that demonstrated efficacy in the primary experiments had sufficient residual sample to be tested by heat inactivation at 55°C and 60°C (for complement and antibody inactivation, respectively). Of those, at least partial loss of protective efficacy after heat inactivation occurred for 3 samples by kidney CFUs and 2 samples by spleen CFUs (Supplementary Figure 1).
Figure 3.
Protective efficacy of human sera peaks in convalescence. End-organ Staphylococcus aureus colony-forming units (CFUs) after adoptive transfer of serum samples obtained at 3 distinct time points (V1, within 72 hours of hospitalization for invasive S. aureus infection; V2, 4–6 weeks after infection; and V3, 6 months after infection). Protective efficacy is present from samples obtained at V2, but protection has waned by V3 (n = 5 animals per group). Error bars represent 95% confidence intervals around the mean. CFUs were enumerated from whole homogenized organ in 1 mL of phosphate-buffered saline.
Clinical and In Vitro Correlations With Protective Function
We hypothesized that specific clinical or in vitro factors might predict which human serum samples would be most efficacious in the prevention of S. aureus sepsis in vivo. Although this study was not powered to detect subtle clinical differences that might be associated with the generation of a protective host response, there were no clear distinctions between infection types or patient characteristics that resulted in a protective serologic response. Of the subjects with highly or moderately protective serum samples, there was no predominant infection type, host age/sex, or infecting isolate strain type compared with those samples with minimal protection in vivo.
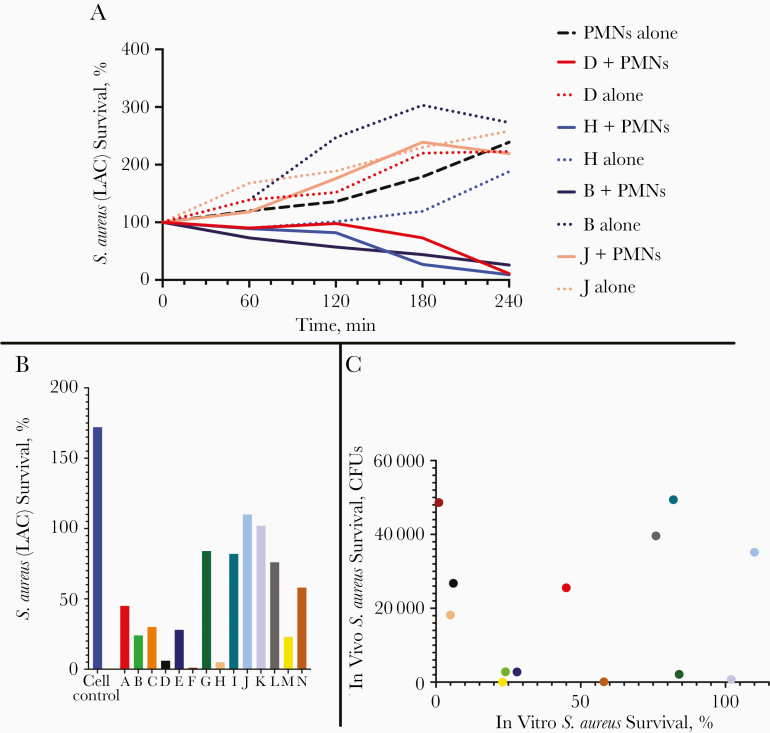
Given the role of the neutrophil as the primary innate mediator of antistaphylococcal host defense, we hypothesized that facilitation of neutrophil-mediated killing in vitro may represent a surrogate marker for evaluating the protective capacity of serum samples against S. aureus disease. We did observe marked differences in the ability of specific human serum samples to facilitate phagocyte-mediated S. aureus killing in vitro (Figure 4A–4B), and efficacy was not due to direct serum toxicity effects, because S. aureus killing only took place when cells were present. There was no correlation, however, between the samples most active in vitro and those that functionally protected in the mouse model (Figure 4C).
Figure 4.
Human serum samples facilitate neutrophil-mediated killing of Staphylococcus aureus in vitro. A, Representative human serum samples demonstrating that serum samples are capable of facilitating neutrophil-mediated S. aureus killing (dotted lines represent serum and bacteria alone, demonstrating lack of direct serum toxicity; serum samples plus cells are required for killing). B, Human serum samples vary widely with regard to facilitation of neutrophil-mediated killing of S. aureus in vitro. C, No significant correlation exists between the ability of serum samples to facilitate S. aureus killing in vitro and serum activity after adoptive transfer and in vivo protection (colors indicate specific subjects as shown in B. Abbreviations: CFUs, colony-forming units; PMNs, polymorphonuclear leukocytes.
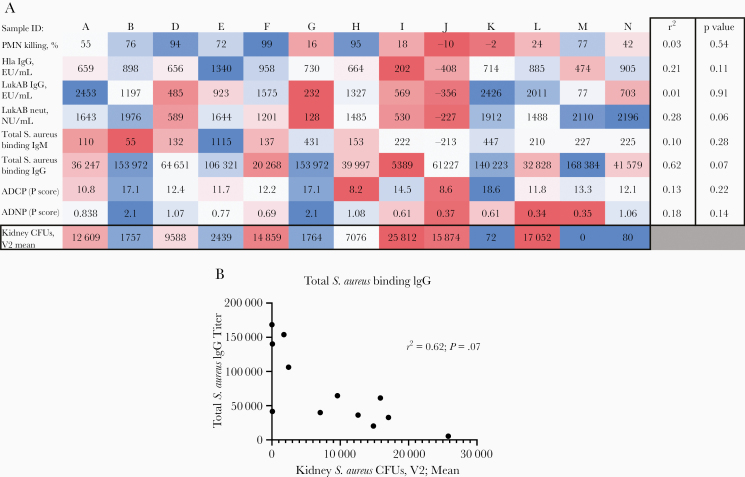
Serologic factors that were considered and tested included IgG levels against the pore-forming toxins Hla and LukAB, along with total binding IgM and IgG against S. aureus culture supernatants, and the titer of neutralization against LukAB-mediated cytotoxicity. Similar to what was observed with facilitation of phagocyte-mediated killing in vitro, no evidence of significant correlation was found between these factors and functional activity of the serum samples when adoptively transferred to mice (Figure 5A). Total binding IgG against S. aureus was moderately correlated with function in vivo, though not statistically significant after correction for multiple comparisons (r2 0.62; P = .07) (Figure 5B). Finally, we assessed functional serologic markers, such as antibody-dependent neutrophil phagocytosis and antibody-dependent cellular phagocytosis. While we again observed substantial differences between serum samples in this cohort, these were not correlated to protective capacity against murine sepsis (Figure 5A).
Figure 5.
Correlation of in vitro characteristics of human serum samples compared with efficacy of serum samples to protect against Staphylococcus aureus sepsis in mice after adoptive transfer. A, Correlation matrix with Pearson correlation coefficient of specific in vitro characteristics: facilitation of neutrophil-mediated killing; antiβα-hemolysin (Hla) immunoglobulin (Ig) G; anti-LukAB IgG; neutralization of LukAB-mediated phagocyte killing (neut); total S. aureus binding IgM and IgG by normalized enzyme-linked immunosorbent assay units (EU) per milliliter, antibody-dependent cellular phagocytosis (ADCP), and neutrophil phagocytosis (ADNP) (by phagocyte [P] score) compared with S. aureus colony-forming units (CFUs) in mice after adoptive transfer. Blue color on heat map indicates higher titer/improved function. After correction for multiple comparisons, the closest correlation was seen with total binding IgG against S. aureus (r2 = 0.62, suggesting strong correlation; P = .07). B, Correlation of total binding S. aureus IgG and mean CFU counts (n = 5 mice per group) for individual serum samples. CFUs were enumerated from whole homogenized organ in 1 mL of phosphate-buffered saline. Abbreviations: NU, neutralization units; PMNs, polymorphonuclear leukocytes; V2, 4–6 weeks after enrollment.
DISCUSSION
It has been long debated whether humans develop protective immunity after S. aureus infection, a question of fundamental importance for development of an effective S. aureus vaccine. Our study provides important insight toward this question and shows that, selectively, mice adoptively transferred serum samples from 6 of 13 children with invasive staphylococcal disease were protected from S. aureus sepsis in a murine model. Furthermore, the samples differed in their protective capacity, with some samples leading to undetectable CFU counts after transfer, and protective efficacy was maximal during disease convalescence (approximately 6 weeks after infection). This report of protective function of human serum samples in a murine model of disease has particularly important implications for the development of an effective S. aureus vaccine, which is currently at a crossroad owing to the failure to date of all staphylococcal vaccines tested in humans.
Our study proposes 2 new approaches to address this problem. First, the adoptive transfer technique creates a platform in which natural immunity to human disease can be explored for the identification of critical mediators of host defense and, therefore, potential target antigens for vaccines or therapeutics. Moreover, as demonstrated by the pneumococcal proof-of-concept model, we suggest that a novel vaccine could theoretically be examined for efficacy in a small group of individuals before expanding to large-scale clinical trials, a concept that could reinvigorate vaccine development at a time when there is little confidence in vaccines derived purely from murine models.
Prior studies have used adoptive transfer of cells or serum between mice to demonstrate key features of the host response in ameliorating certain S. aureus infections. For example, the adoptive transfer of T cells expressing the interleukin 36 receptor protected recipient mice against cutaneous inflammation due to S. aureus [30], and the roles of primed macrophages or human neutrophils in S. aureus–mediated dermonecrosis have also been evaluated via adoptive transfer [31, 32]. Furthermore, transfer of human monoclonal antibodies against S. aureus antigens such as IsdB and Hla have also demonstrated protection against subsequent challenge [33–35]. Notably for our study, a serologic adoptive transfer model was used to demonstrate the critical role of antibody-mediated protection against S. aureus skin infection when serum samples were transferred between mice [36].
The current study significantly extends this concept to provide evidence of a functional serologic response that occurs in humans after invasive disease. This is compatible with clinical observations that invasive disease may potentially be an “immunizing event” [37–40]; while recurrent noninvasive infections (eg, cutaneous abscesses) are common, S. aureus infection after invasive disease is exceedingly rare in the absence of immune compromise, indwelling hardware, or other factors that perturb the host response.
The lack of a correlate of durable protection for invasive S. aureus disease in humans poses a substantial challenge to the development of vaccines and novel therapeutics against this major human pathogen. S. aureus is a highly human-evolved pathogen, and one potential explanation for the failure of prior S. aureus vaccine constructs is that antigens that are important targets of the host response in murine models (which may vary based on the genetic background of the mice [36]) have less relevance in human infection. Furthermore, S. aureus clearly regulates and expresses virulence factors differently in various conditions [41, 42], and it remains unclear which in vitro conditions most closely recapitulate the human host environment. Serum samples from humans infected with S. aureus therefore represent an opportunity to assess the host response directly from the site of optimal relevance for evaluating which virulence factors are actively expressed and recognized by the host in the setting of invasive disease.
The serum samples obtained in this study differed substantially in their ability to protect against sepsis via adoptive transfer, but there was no clear in vitro correlate for this protection. Total antibody binding to S. aureus was most closely correlated of the factors tested, though the lack of strong correlation likely suggests that it is a functional antibody response against a specific combination of targets that confers the potent protection observed in vivo, as the total binding IgG pool includes nonfunctional antibody response to factors such as teichoic acid [27].
A prior study determined that IgG levels against Hla were correlated with protection against recurrent staphylococcal cutaneous infections in humans [43], but functional, protective serologic responses that are generated after invasive human disease remain undefined. Although none of the factors assessed were found to predict which serum samples would be protective, there are several caveats to this. For example, the bicomponent leukocidins are known to be highly specific to human (rather than murine) neutrophils [10], particularly LukAB [11], Panton-Valentine leukocidin (PVL) [44], and LukED. It may be, therefore, that some serologic factors of critical importance in the setting of human disease would appear less important in a nonhumanized mouse model. Identifying crucial factors conferring protective properties to serum samples, as well as determining whether serum samples gain or lose protective function when humanized mouse models are used, will be important future directions for this work.
Several caveats exist for the interpretation of these data. First, the model is not expected to represent a complete transfer of the adaptive host response, because T-cell responses are not accounted for. In addition, there are likely differences in Fc-mediated functions when human antibodies interact with murine effector cells, and these differences will need to be defined in future work. Furthermore, the age range of the infected subjects represents a period during which immune maturation is occurring, and this may partially explain differences in the efficacy of the host response in some samples. Finally, the function of human antibody responses to bacterial virulence factors with high tropism for human cells (eg, LukAB and other leukocidins) may be masked or underrepresented after transfer to the murine model. Nevertheless, a highly protective serologic response (as seen in several samples in this study) certainly holds important information regarding which factors are important for the inhibition of bacterial pathogenesis.
It remains unclear which specific factor(s) confer the highly protective effect to certain serum samples after invasive human infection, and this is the target of a larger-scale future work. It should be noted that the samples with the greatest protective function were obtained from follow-up visits in convalescence from disease, the approximate time at which the adaptive response is expected to be at its peak. Most samples tested lost protective efficacy after heat inactivation, further suggesting an antibody-mediated mechanism, though these experiments were conducted asynchronously and residual sample volume was not sufficient to conclude this definitively.
It is likely that a complex interaction of the humoral response (both Fab- and Fc-mediated functions) with certain key antigens and other components of the host response is required. It is also notable that serologic protection had waned by 6 months after infection. This may be owing, in part, to the known ability of S. aureus to perturb the development of an effective memory response via SpA-mediated B-cell apoptosis [45, 46] a phenomenon that could be subverted by vaccination if effective mediators of protective factors in serum samples can be identified. Importantly, protective responses were generated by patients infected with diverse strain types and methicillin resistance patterns, a crucial observation because an effective intervention or prevention target will need to be broadly applicable rather than restricted to specific strains.
In conclusion, the current study describes novel approaches to assessing the host response after invasive S. aureus disease in humans, a concept with important implications for staphylococcal vaccine development. The adoptive transfer of human serum after infection allows for a unique assessment of the functional adaptive response and may provide a means to elucidate critical protective factors that result from natural human disease. Application of the model to acute and convalescent serum samples provided important insight into the longstanding question as to whether humans develop protective immunity after infection, strongly suggesting that protective immunity is generated under certain conditions and peaks during disease convalescence. This platform provides the opportunity to identify human-disease-relevant staphylococcal vaccine antigens and represents a potential novel mechanism to directly test the protective efficacy of serum samples after vaccination, a modality that could broadly inform not only a S. aureus vaccine program, but also other challenging vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Buddy Creech, MD, MPH, and the National Institute for Allergy and Infectious Diseases, National Institutes of Health for assistance with obtaining leftover serum samples from pneumococcal vaccine trials for proof-of-principle studies.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01 AI139172 to I. T. and R01 AI127406 and R01 144694 to G. Y. L.), the Ragon Institute (G. A.)., and the SAMANA Kay Massachusetts General Hospital Scholarship (G. A.).
Potential conflicts of interest. G. A. is a founder of SeromYx Systems, which is unrelated to this study. I. T. has received research support from GlaxoSmithKline and served as a consultant for Horizon Therapeutics, both unrelated to this study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Gordon Research Conference on Staphylococcal Diseases, Barcelona, Spain, 4–9 August 2019.
References
- 1. Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics 2016; 137:e20153099. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan SL. Staphylococcus aureus infections in children: the implications of changing trends. Pediatrics 2016; 137:e20160101. [DOI] [PubMed] [Google Scholar]
- 3. Klevens RM, Morrison MA, Nadle J, et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators . Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–71. [DOI] [PubMed] [Google Scholar]
- 4. Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects.” Vaccine 2013; 31:2723–30. [DOI] [PubMed] [Google Scholar]
- 5. Thomsen I, Dudney H, Creech CB. Searching for the holy grail of a staphylococcal vaccine. Hum Vaccin 2010; 6:1068–70. [DOI] [PubMed] [Google Scholar]
- 6. Fowler VG Jr, Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 2014; 20(suppl 5):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2012; 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 2015; 13:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 2014; 12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diep BA, Le VT, Visram ZC, et al. . Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob Agents Chemother 2016; 60:6333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DuMont AL, Yoong P, Day CJ, et al. . Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 2013; 110:10794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarthy AJ, Lindsay JA. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol 2010; 10:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomsen IP, Dumont AL, James DB, et al. . Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infect Immun 2014; 82:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Kessel KP, Bestebroer J, van Strijp JA. Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front Immunol 2014; 5:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu T, Porter AR, Kennedy AD, Kobayashi SD, DeLeo FR. Phagocytosis and killing of Staphylococcus aureus by human neutrophils. J Innate Immun 2014; 6:639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomsen IP, Sapparapu G, James DBA, et al. . Monoclonal antibodies against the Staphylococcus aureus bicomponent leukotoxin AB isolated following invasive human infection reveal diverse binding and modes of action. J Infect Dis 2017; 215:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chadha AD, Thomsen IP, Jimenez-Truque N, et al. . Host response to Staphylococcus aureus cytotoxins in children with cystic fibrosis. J Cyst Fibros 2016; 15:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wood JB, Jones LS, Soper NR, et al. . Serologic detection of antibodies targeting the leukocidin LukAB strongly predicts Staphylococcus aureus in children with invasive infection. J Pediatric Infect Dis Soc 2019; 8:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolar SL, Ibarra JA, Rivera FE, et al. . Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiologyopen 2013; 2:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pietrocola G, Nobile G, Rindi S, Speziale P. Staphylococcus aureus manipulates innate immunity through own and host-expressed proteases. Front Cell Infect Microbiol 2017; 7:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reyes-Robles T, Lubkin A, Alonzo F 3rd, Lacy DB, Torres VJ. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep 2016; 17:428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenul C, Horswill AR. Regulation of Staphylococcus aureus virulence. Microbiol Spectr 2018; 6:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown EP, Licht AF, Dugast AS, et al. . High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J Immunol Methods 2012; 386:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ackerman ME, Moldt B, Wyatt RT, et al. . A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 2011; 366:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karsten CB, Mehta N, Shin SA, et al. . A versatile high-throughput assay to characterize antibody-mediated neutrophil phagocytosis. J Immunol Methods 2019; 471:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berical AC, Harris D, Dela Cruz CS, Possick JD. Pneumococcal vaccination strategies. An update and perspective. Ann Am Thorac Soc 2016; 13:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colque-Navarro P, Jacobsson G, Andersson R, Flock JI, Möllby R. Levels of antibody against 11 Staphylococcus aureus antigens in a healthy population. Clin Vaccine Immunol 2010; 17:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adhikari RP, Ajao AO, Aman MJ, et al. . Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 2012; 206:915–23. [DOI] [PubMed] [Google Scholar]
- 29. Clarke SR, Brummell KJ, Horsburgh MJ, et al. . Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis 2006; 193:1098–108. [DOI] [PubMed] [Google Scholar]
- 30. Liu H, Archer NK, Dillen CA, et al. . Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL-36-mediated T cell responses. Cell Host Microbe 2017; 22:653–66e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan LC, Rossetti M, Miller LS, et al. ; MRSA Systems Immunobiology Group . Protective immunity in recurrent Staphylococcus aureus infection reflects localized immune signatures and macrophage-conferred memory. Proc Natl Acad Sci U S A 2018; 115:E11111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tseng CW, Biancotti JC, Berg BL, et al. . Increased susceptibility of humanized NSG mice to Panton-Valentine leukocidin and Staphylococcus aureus skin infection. PLoS Pathog 2015; 11:e1005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennett MR, Bombardi RG, Kose N, et al. . Human mAbs to Staphylococcus aureus IsdA provide protection through both heme-blocking and Fc-mediated mechanisms. J Infect Dis 2019. ; 219:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bennett MR, Dong J, Bombardi RG, et al. . Human VH1-69 gene-encoded human monoclonal antibodies against Staphylococcus aureus IsdB use at least three distinct modes of binding to inhibit bacterial growth and pathogenesis. mBio 2019; 10:e02473-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tkaczyk C, Hamilton MM, Sadowska A, et al. . Targeting alpha toxin and ClfA with a multimechanistic monoclonal-antibody-based approach for prophylaxis of serious Staphylococcus aureus disease. mBio 2016; 7: e00528-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montgomery CP, David MZ, Daum RS. Host factors that contribute to recurrent staphylococcal skin infection. Curr Opin Infect Dis 2015; 28:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Espersen F, Hedström SA. Recurrent staphylococcal furunculosis: antibody response against Staphylococcus aureus. Scand J Infect Dis 1984; 16:413–4. [DOI] [PubMed] [Google Scholar]
- 38. Monteil MA, Kaniuk AS, Hobbs JR. Staphylococcal opsonization and anti-Staphylococcus aureus IgG subclass antibodies in patients with severe or recurrent S. aureus infections. FEMS Microbiol Immunol 1990; 2:259–62. [DOI] [PubMed] [Google Scholar]
- 39. Verkaik NJ, Boelens HA, de Vogel CP, et al. . Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2010; 29:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dryla A, Prustomersky S, Gelbmann D, et al. . Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immunol 2005; 12:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malachowa N, Whitney AR, Kobayashi SD, et al. . Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 2011; 6:e18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H. Expression of virulence factors by Staphylococcus aureus grown in serum. Appl Environ Microbiol 2011; 77:8097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fritz SA, Tiemann KM, Hogan PG, et al. . A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013; 56:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Löffler B, Hussain M, Grundmeier M, et al. . Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 2010; 6:e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim HK, Falugi F, Thomer L, Missiakas DM, Schneewind O. Protein A suppresses immune responses during Staphylococcus aureus bloodstream infection in guinea pigs. mBio 2015; 6:e02369-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pauli NT, Kim HK, Falugi F, et al. . Staphylococcus aureus infection induces protein A-mediated immune evasion in humans. J Exp Med 2014; 211:2331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.