Abstract
The objective of this study was to test our hypothesis that nocturnal home blood pressure (BP) measurement adapted to the chosen bedtime of participants (measurement at 2, 3, and 4 hour after the chosen bedtime) would be more reliable than measurement at fixed time points (2:00, 3:00, and 4:00 am). Forty‐eight hypertensives were randomized to two groups undergoing two seven‐night measurement phases in a crossover manner and were asked to measure nocturnal home BP for 14 consecutive nights using a validated automatic information/communication technology‐based device. The intraclass correlation coefficients (ICCs) of systolic BP (SBP) obtained by a single measurement per night over two nights showed lower agreement than those of systolic BP obtained by multiple measurements based on a participant‐specified bedtime (0.539‐0.625 vs 0.675‐0.768) and multiple measurements at fixed times (0.468‐0.505 vs 0.661‐0.790). The ICCs obtained using specific bedtime‐based time points and those obtained using fixed time points showed major agreement when SBP was obtained by multiple measurements. The standard errors of measurement for SBP were similar between the bedtime‐based measurement phase (1.4‐1.7 mm Hg) and the fixed‐time measurement phase (1.2‐1.6 mm Hg). Neither a fixed bias nor a proportional bias was observed between the SBP values measured by the specific bedtime‐based time points and those measured by the fixed‐time measurement phase. In conclusion, the reliability of nocturnal home BP measurement appeared to be similar between nocturnal home BP adapted to the chosen bedtime of participants and that measured at fixed time points.
Keywords: blood pressure, communication technology‐based device, information, nocturnal home blood pressure, reliability
1. INTRODUCTION
Nocturnal blood pressure (BP) measured by ambulatory BP monitoring (ABPM) is a better predictor of future cardiovascular events than daytime BP in hypertensive patients.1, 2, 3 In recent years, nocturnal BP measured by home BP monitoring (HBPM) has become available for clinical practice, and a recent meta‐analysis showed that the clinical significance of nocturnal BP measured by HBPM is comparable to that of nocturnal BP measured by ABPM.4
Owing to its simplicity, convenience, and tolerability, HBPM has been rapidly adopted by many clinics and is now recommended by numerous hypertension guidelines.5, 6, 7, 8, 9, 10 The potential advantages of nocturnal HBPM as a substitute for nocturnal ABPM are that it permits the collection of nocturnal home BP values over multiple nights, in the manner of conventional home BP measurement, which can be measured in the morning and evening. On the other hand, participants are likely to measure nocturnal BP levels depending on their own lifestyle. Recently developed HBPM devices permit participants to set specific lengths of time after their chosen bedtime for the measurement of nocturnal BPs.11 Although there may be differences between the nocturnal BP values measured at intervals after a participant‐specified bedtime and those measured at fixed time points, the previous studies on nocturnal HBPM have not taken such measurement conditions into consideration.12, 13, 14 Nonetheless, such considerations are likely to be important, since a previous study showed that nocturnal BP measured by ABPM with fixed time points was less reliable than nocturnal BP evaluated by self‐report or using actigraphy approaches, which could reflect the lifestyles of patients directly.15 There is thus need of a study comparing nocturnal BPs measured based on the participant‐specified bedtime and those measured at fixed time points by using validated HBPM.
Recently developed HBPM devices can store home BP readings in a cloud database and can permit not only participants but also experimenters to set the timing of nocturnal BP measurement via automatic information/communication technology (ICT), resulting in a precise schedule for the measurement of nocturnal home BP.16 In the present study, we compared different schedules of nocturnal home BP measurement using an ICT‐based device in light of their reliability in multiple nocturnal home BP readings measured based on the participant‐specified bedtime and those measured at fixed time points.
2. METHODS
2.1. Participants
This Condition study is a substudy of the Prediction of ICT‐Home blood Pressure Variability (PREDICT) study. The participants of this Condition study16, 17 were selected from among the participants of the PREDICT study. The protocol of the PREDICT study was registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR) Web site (trial no. UMIN000019871). Briefly, the PREDICT study is a prospective observational study that aims to evaluate the use of home BP based on ICT for predicting cardiovascular events. Enrolled participants were asked to continuously measure their home BP over 2 years.
Fifty outpatients recruited from two clinics were asked to measure their nocturnal home BP. Twenty‐five of the participants were being treated at the Higashiagatsuma‐machi National Health Insurance Clinic, Gunma, Japan, and the other 25 participants were being treated at the Minamisanriku Public Medical Clinic, Miyagi, Japan. All participants were treated for hypertension, and their medications were not changed during the study period. All participants provided written informed consent to participate and to have their data published, and the study was approved by the Ethics Committee of Jichi Medical University, Shimotsuke, Japan.
We defined a regular drinker as a person who drank over the appropriate dose three times a week or more. The appropriate dose of alcohol consumed on a given day was classified in terms of ethanol 20‐30 mL in men and 10‐20 mL in women (equivalent to 180 mL of sake, 500 mL of beer, <70 mL of shochu, a double whiskey or brandy, or two glasses of wine/d) as described in the JSH2014 guidelines.9
2.2. Home BP measurements
The participants measured their own BP at home using an automatic ICT‐based device (HEM‐7252G‐HP; Omron Healthcare, Kyoto, Japan) based on the cuff‐oscillometric principle. All data obtained by the device were transmitted automatically to a cloud‐based remote monitoring system, the “Medical LINK” software program provided by Omron Healthcare,18 and the data were managed in an independent facility, the Jichi Medical University Center of Global Home and Ambulatory BP Analysis (GAP) at the Jichi Medical University Center of Excellence Community Medicine Cardiovascular Research and Development (JCARD), Shimotsuke, Japan.
The participants were instructed to measure their daytime home BP (morning, before dinner, and at bedtime) in a sitting position after resting for 1‐2 minutes with their legs not crossed in a quiet room that was not too cold. The cuff used was 14.5 cm wide and 46.6 cm long (target arm girth: 22‐32 cm). This cuff size could cover a width of ≥40% of the brachial girth and a length of ≥80% of the brachial girth in all study participants. The arm cuff position was maintained at the heart level. Participants measured their home BP for 14 consecutive days, and the home BP monitoring protocol was as follows: two measurements in the morning, two measurements before dinner, two measurements at bedtime, and three measurements during sleep, for a total of nine measurements per day. The participants measured their morning BP within 1 hour after waking, after urination, before breakfast, and before ingesting medications. The before‐dinner BP was measured within 60 minutes before dinner. The at‐bedtime BP was measured just before the participant went to bed.
2.2.1. Nocturnal home BP measurements
The participants were asked to measure nocturnal home BP for 14 consecutive nights using the same device as used to measure the daytime home BP. Figure 1 summarizes the study protocol. The nocturnal home BP ICT‐based device was preset to take three BP measurements at regular intervals of 2, 3, and 4 hour after the chosen bedtime of participants in the “bedtime‐based measurement phase” or at fixed times of 2:00, 3:00, and 4:00 am during the “fixed‐time measurement phase” of the experiment. Our hypothesis was that nocturnal home BP measurement adapted to the bedtime‐based measurement phase would be more reliable than measurement in the fixed‐time measurement phase.
Figure 1.
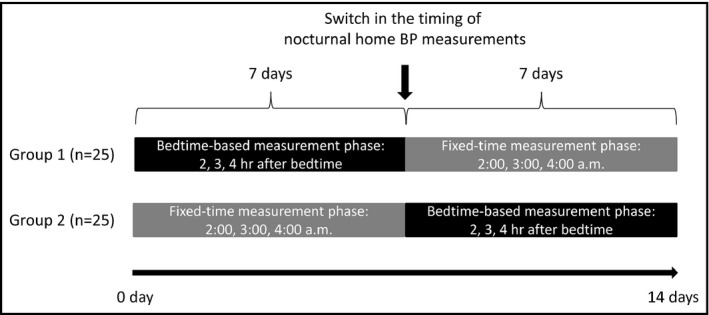
The study protocol
For the randomization, the physicians who enrolled the study participants entered the participant characteristics into the “Medical LINK” software program. Then, a computer‐generated, pseudo‐random sequence was used to randomize each participant to either phase for seven nights, after which he or she was immediately switched to the other phase for seven nights. All these procedures were performed by the GAP center, an independent facility, via a web‐based network system.
The participants were instructed to wear the BP cuff and press the button to start the timer when they went to bed and to measure their nocturnal home BP on as many nights as possible for 14 nights.
2.3. Sleep quality
The participants were asked to make a daily record of their sleep quality in a notebook that we provided. We defined sleep quality using the following four categories based on the degree of sleep disturbance and awareness of nocturnal BP measurement: satisfied, slightly dissatisfied, very dissatisfied, or no sleep at all. The participants were instructed to check one of the four sleep‐quality categories based on their subjective evaluation on the morning after their measurement of nocturnal home BP. They brought their notebooks to each clinic visit.
2.4. Sample size
We conducted an a priori power analysis using the sample size calculation software package G*Power version 3.1.9.2 (a program conceived, designed, and written by Franz, Universitat Kiel, Germany; the program is freely available as Windows application software).19, 20 Since there are no previous studies comparing BP levels between the bedtime‐based measurement phase and the fixed‐time measurement phase, the sample size required for each phase was calculated as 34 in order to realize a power of 80%, statistical significance level of 0.05, and effect size of 0.5.21
2.5. Statistical analysis
The data are presented as the mean ± standard deviation (SD) unless stated otherwise. The percentages of nocturnal home BP measurements on each night of the experiment were compared using chi‐squared statistics. That is, in cases of <45 participants, there was a significant difference in the number of participants who were able to measure the number of nights within each seven‐night phase on which the nocturnal home BP at all three of the time points in each phase. We calculated the average nocturnal home BP level for all time combinations within bedtime‐based measurement phase and the fixed‐time measurement phase. We evaluated the differences in nocturnal home BP values and pulse rate using the paired t test. The reliability of each method of nocturnal home BP measurement was separated into relative and absolute reliabilities.
To investigate relative reliability, we calculated the intraclass correlation coefficients (ICCs) for agreement by using the two‐way random model of absolute agreement, that is, the ICC (2,1),22 to assess the relationship between nocturnal home BP values and nights. The ICCs were scored as follows: 0 = poor agreement; 0‐0.20 = minor agreement; 0.21‐0.40 = fair agreement; 0.41‐0.60 = moderate agreement; 0.61‐0.80 = major agreement; and 0.81‐1.00 = almost perfect agreement.23
To investigate absolute reliability, we conducted a Bland‐Altman analysis.24 The standard error of measurement (SEM) represents the variation among individuals.25 The SEM was calculated as SDd/√n,26, 27 where SDd is the SD of the (the mean difference between the nocturnal home BP values of Night‐1 and those of Night‐2). The smaller the SEM, the greater the reliability of the measurement.25
In the Bland‐Altman analysis, we investigated whether there was a systemic bias, including either a fixed bias or a proportional bias. We assessed the fixed bias by calculating the 95% confidence interval (CI) of the . The 95% CI of the was calculated as , where t n−1 corresponds to the value of t distribution with n−1 degrees of freedom, and n corresponds to the number of participants.28 If the 95% CI of the did not include zero, the presence of fixed bias would be confirmed. We assessed the proportional bias by determining the linear regression between the averages and the differences of nocturnal home BP values between Night‐1 and Night‐2. If the slope of the linear regression was statistically significant, the presence of proportional bias would be confirmed.
If a systemic bias was denied by Bland‐Altman analysis, then what was initially considered an error that degrades the reliability of nocturnal home BP measurement would in fact be only a random error. The minimal detectable change (MDC) at the 95% confidence level was calculated only when random errors were observed. The MDC was calculated as SEM × 1.96 × √2 and represents the smallest change in BP level29; this could be interpreted as indicating that the changes in values within MDC were due to measurement errors, while changes larger than MDC were judged as “true changes” with a risk rate of 5%.30
All statistical analyses were performed using SPSS ver. 24.0 software (SPSS, IBM, Armonk, NY, USA). A P‐value <0.05 was considered significant.
3. RESULTS
We confirmed that the sample size of this study was sufficient before starting the study. After the study was completed, we learned that the participants did not always measure their nocturnal home BP on consecutive nights or on all seven nights of either phase. In Figure 2, the horizontal line indicates the number of nights within each seven‐night phase for which the nocturnal home BP was measured at all three of the time points. In the bedtime‐based measurement phase, the number of participants who measured their nocturnal home BP fell significantly on the fourth night. In the fixed‐time measurement phase, the number of participants who measured their nocturnal home BP dropped significantly on the third night. The study participants could measure nocturnal home BP only two nights at the measurement achievement rate over 90% in 1 week. Therefore, the following analysis was performed using the data of participants who had measured their nocturnal home BP three times perfectly during a night on both the first and the second nights (ie, Night‐1 and Night‐2) in both the bedtime‐based measurement phase and the fixed‐time measurement phase. Namely, among the 50 participants, the nocturnal home BP values of 4:00 am in Night‐2 in the fixed‐time measurement phase were missing for two participants. Therefore, we excluded the data of these two participants from subsequent analysis.
Figure 2.
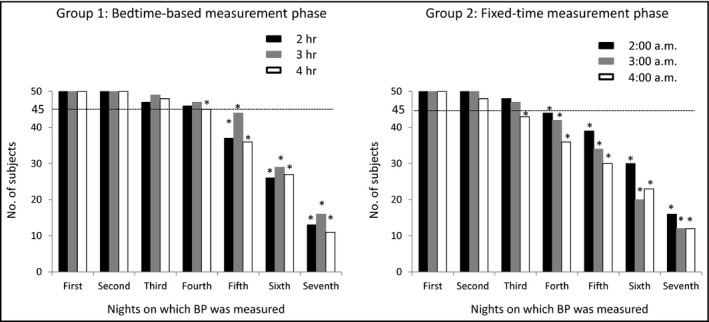
The numbers of participants who measured their nocturnal home blood pressure. In cases of less than 45 participants, there was a significant difference in the number of participants who were able to measure the number of nights within each seven‐night phase on which the nocturnal home BP at all three of the time points in each phase by chi‐squared test (*P < 0.05)
3.1. The participants’ characteristics
Table 1 provides the demographic and clinical characteristics of the present study participants. The ages of the 48 patients (21 males and 27 females) ranged from 50 to 89 years (mean ± SD: 76.5 ± 8.0 years). The percentage of regular consumers of alcohol was 18.8%. Self‐reported sleep quality during the study period was as follows: satisfied 55.2%, slightly dissatisfied 35.5%, very dissatisfied 7.0%, and no sleep at all 2.3%.
Table 1.
Characteristics of study participants (n = 48)
| Characteristics | Total |
|---|---|
| Age, y | 76.5 ± 8.0 |
| Male, n (%) | 21 (43.8) |
| Body mass index, kg/m2 | 24.4 ± 3.2 |
| Regular drinker, n (%) | 9 (18.8) |
| Current smoker, n (%) | 1 (2.1) |
| Diabetes mellitus, n (%) | 12 (25.0) |
| Pre‐existing CVD, n (%) | 3 (6.3) |
| Fasting glucose, mg/dL | 99.8 ± 23.9 |
| Total cholesterol, mg/dL | 184.0 ± 33.4 |
| High‐density lipoprotein cholesterol, mg/dL | 56.7 ± 13.2 |
| Antihypertensive medications | |
| No. of antihypertension medication drugs, n | 2.0 ± 1.0 |
| Timing of antihypertensive medications | |
| After breakfast, n (%) | 38 (79.2) |
| After dinner, n (%) | 14 (29.2) |
| Before bedtime, n (%) | 2 (4.2) |
| BP measures | |
| Morning SBP, mm Hg | 131.2 ± 15.3 |
| Morning DBP, mm Hg | 76.5 ± 10.1 |
| Morning PR, bpm | 64.3 ± 11.2 |
| Before‐dinner SBP, mm Hg | 129.2 ± 17.3 |
| Before‐dinner DBP, mm Hg | 75.6 ± 11.3 |
| Before‐dinner PR, bpm | 69.0 ± 11.3 |
| At‐bedtime SBP, mm Hg | 120.7 ± 15.4 |
| At‐bedtime DBP, mm Hg | 70.3 ± 10.0 |
| At‐bedtime PR, bpm | 67.8 ± 10.4 |
CVD, coronary vascular disease; DBP, diastolic blood pressure; PR, pulse rate; SBP, systolic blood pressure; SD, standard deviation.
Data are expressed as the mean ± SD or number (%).
3.2. Nocturnal home BP measurements
Table 2 shows the mean nocturnal home BP parameters for Night‐1 and Night‐2. Both in the bedtime‐based measurement phase and in the fixed‐time measurement phase, there were no significant differences in the systolic BP (SBP), diastolic BP (DBP), or pulse rate (PR) between the two nights.
Table 2.
Mean of nocturnal home BP parameters between Night‐1 and Night‐2 (n = 48)
| Timing of measurement | SBP | DBP | PR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Night‐1 | Night‐2 | Difference (95% CI) | P value | Night‐1 | Night‐2 | Difference (95% CI) | P value | Night‐1 | Night‐2 | Difference (95% CI) | P value | |
| Bedtime‐based measurement phase | ||||||||||||
| At 2 h after bedtime | 109.1 ± 16.6 | 113.0 ± 16.3 | −3.9 ± 14.2 (−8.0, 0.2) | 0.064 | 64.7 ± 11.8 | 66.5 ± 11.5 | −1.8 ± 10.6 (−4.9, 1.3) | 0.248 | 60.5 ± 7.7 | 60.7 ± 10.0 | −0.1 ± 8.3 (−2.6, 2.3) | 0.903 |
| At 3 h after bedtime | 108.5 ± 16.5 | 110.4 ± 16.8 | −1.8 ± 16.0 (−6.5, 2.8) | 0.432 | 65.3 ± 11.1 | 64.3 ± 12.5 | 1.0 ± 12.4 (−2.6, 4.6) | 0.579 | 59.6 ± 7.7 | 59.2 ± 9.5 | 0.5 ± 7.2 (−1.6, 2.5) | 0.659 |
| At 4 h after bedtime | 109.0 ± 17.4 | 107.1 ± 17.6 | 1.9 ± 15.2 (−2.5, 6.3) | 0.381 | 64.8 ± 11.9 | 63.5 ± 11.3 | 1.3 ± 9.6 (−1.6, 4.1) | 0.374 | 59.2 ± 8.9 | 59.4 ± 8.6 | −0.2 ± 6.6 (−2.1, 1.7) | 0.811 |
| Average at 2 and 3 h after bedtime | 108.8 ± 14.8 | 111.7 ± 15.1 | −2.9 ± 11.9 (−6.3, 0.6) | 0.102 | 65.0 ± 9.8 | 65.4 ± 10.6 | −0.4 ± 8.3 (−2.8, 2.0) | 0.742 | 60.1 ± 7.4 | 59.9 ± 9.4 | 0.2 ± 6.7 (−1.8, 2.1) | 0.872 |
| Average at 2 and 4 h after bedtime | 109.0 ± 15.7 | 110.0 ± 14.6 | −1.0 ± 11.2 (−4.2, 2.3) | 0.546 | 64.7 ± 10.9 | 65.0 ± 9.8 | −0.3 ± 7.9 (−2.6, 2.0) | 0.814 | 59.9 ± 7.8 | 60.0 ± 8.7 | −0.2 ± 5.6 (−1.8, 1.4) | 0.818 |
| Average at 3 and 4 h after bedtime | 108.8 ± 15.7 | 108.7 ± 15.6 | 0.1 ± 11.5 (−3.3, 3.4) | 0.975 | 65.0 ± 10.5 | 63.9 ± 10.9 | 1.1 ± 8.5 (−1.3, 3.6) | 0.364 | 59.4 ± 7.7 | 59.3 ± 8.7 | 0.1 ± 5.0 (−1.3, 1.6) | 0.874 |
| Average at 2, 3, and 4 h after bedtime | 108.9 ± 14.9 | 110.1 ± 14.4 | −1.3 ± 10.0 (−4.2, 1.6) | 0.386 | 64.9 ± 10.0 | 64.8 ± 10.0 | 0.2 ± 7.1 (−1.9, 2.2) | 0.882 | 59.8 ± 7.5 | 59.8 ± 8.8 | 0.0 ± 5.2 (−1.5, 1.5) | 0.970 |
| Fixed‐time measurement phase | ||||||||||||
| At 2:00 am | 108.5 ± 14.9 | 111.0 ± 14.7 | −2.4 ± 14.8 (−6.7, 1.9) | 0.263 | 64.3 ± 11.3 | 66.2 ± 10.2 | −1.9 ± 11.1 (−5.2, 1.3) | 0.232 | 58.5 ± 9.3 | 58.5 ± 7.6 | 0.0 ± 6.1 (−1.7, 1.8) | 0.962 |
| At 3:00 am | 109.6 ± 14.1 | 107.6 ± 17.5 | 2.0 ± 16.4 (−2.7, 6.8) | 0.394 | 66.0 ± 11.8 | 63.6 ± 12.2 | 2.4 ± 13.1 (−1.4, 6.2) | 0.207 | 58.4 ± 8.4 | 58.5 ± 8.1 | −0.1 ± 5.6 (−1.7, 1.6) | 0.938 |
| At 4:00 am | 112.3 ± 13.5 | 112.4 ± 15.5 | −0.1 ± 14.6 (−4.3, 4.2) | 0.976 | 65.8 ± 10.5 | 65.3 ± 9.7 | 0.5 ± 10.5 (−2.5, 3.6) | 0.721 | 57.9 ± 8.1 | 58.0 ± 7.7 | −0.1 ± 5.1 (−1.6, 1.4) | 0.888 |
| Average at 2:00 and 3:00 am | 109.1 ± 12.6 | 109.3 ± 13.8 | −0.2 ± 10.0 (−3.1, 2.7) | 0.897 | 65.1 ± 9.9 | 64.9 ± 9.2 | 0.2 ± 8.1 (−2.1, 2.6) | 0.838 | 58.5 ± 8.6 | 58.5 ± 7.4 | −0.0 ± 4.6 (−1.3, 1.2) | 0.988 |
| Average at 2:00 and 4:00 am | 110.4 ± 12.2 | 111.7 ± 12.5 | −1.2 ± 9.8 (−4.1, 1.6) | 0.383 | 65.1 ± 9.6 | 65.8 ± 8.1 | −0.7 ± 8.1 (−3.0, 1.7) | 0.553 | 58.2 ± 8.3 | 58.2 ± 8.0 | −0.0 ± 4.2 (−1.2, 1.2) | 0.959 |
| Average at 3:00 and 4:00 am | 110.9 ± 12.2 | 110.0 ± 14.5 | 1.0 ± 11.1 (−2.2, 4.2) | 0.539 | 65.9 ± 9.6 | 64.4 ± 9.5 | 1.5 ± 8.4 (−0.9, 3.9) | 0.227 | 58.1 ± 8.0 | 58.2 ± 7.5 | −0.1 ± 4.0 (−1.2, 1.1) | 0.886 |
| Average at 2:00, 3:00, and 4:00 am | 110.1 ± 11.6 | 110.3 ± 12.7 | −0.2 ± 8.0 (−2.5, 2.2) | 0.900 | 65.4 ± 9.1 | 65.0 ± 8.3 | 0.3 ± 6.7 (−1.6, 2.3) | 0.725 | 58.3 ± 8.2 | 58.3 ± 7.2 | −0.0 ± 3.7 (−1.1, 1.0) | 0.939 |
CI, confidence interval; DBP, diastolic blood pressure; PR, pulse rate; SBP, systolic blood pressure; SD, standard deviation.
Data are expressed as the mean ± SD.
3.2.1. Relative reliability of nocturnal home BP measurement
As shown in Table 3, the ICCs for the single measurement per night of SBP ranged from 0.468 to 0.625 and those of DBP ranged from 0.403 to 655, whereas the ICCs for the average of multiple measurements per night of SBP ranged from 0.661 to 0.790 and those of DBP ranged from 0.590 to 0.751. The results were similar between the bedtime‐based measurement phase and the fixed‐time measurement phase. That is, the ICCs for the single measurement per night of SBP and DBP showed moderate agreement and the ICCs for the average of multiple measurements per night of SBP and DBP showed major agreement, regardless of whether the bedtime‐based measurement phase or fixed‐time measurement phase was in effect.
Table 3.
Reliability of nocturnal home BP between Night‐1 and Night‐2 (n = 48)
| Timing of measurement | SBP | DBP | ||||||
|---|---|---|---|---|---|---|---|---|
| ICC (2, 1) (95% CI) | Bland‐Altman analysis | ICC (2, 1) (95% CI) | Bland‐Altman analysis | |||||
| Slope (P value) | SEM (mm Hg) | MDC (mm Hg) | Slope (P value) | SEM (mm Hg) | MDC (mm Hg) | |||
| Bedtime‐based measurement phase | ||||||||
| At 2 h after bedtime | 0.612 (0.401‐0.762)** | 0.021 (0.881) | 2.1 | 5.8 | 0.581 (0.361‐0.741)** | 0.032 (0.832) | 1.5 | 4.2 |
| At 3 h after bedtime | 0.539 (0.304‐0.713)** | −0.022 (0.892) | 2.3 | 6.4 | 0.455 (0.197‐0.653)* | −0.169 (0.352) | 1.8 | 5.0 |
| At 4 h after bedtime | 0.625 (0.418‐0.771)** | −0.012 (0.935) | 2.2 | 6.1 | 0.655 (0.459‐0.791)** | 0.067 (0.622) | 1.4 | 3.9 |
| Average at 2 and 3 h after bedtime | 0.675 (0.487‐0.803)** | −0.023 (0.856) | 1.7 | 4.7 | 0.675 (0.485‐0.804)** | −0.093 (0.480) | 1.2 | 3.3 |
| Average at 2 and 4 h after bedtime | 0.731 (0.567‐0.840)** | 0.089 (0.446) | 1.6 | 4.4 | 0.712 (0.538‐0.828)** | 0.126 (0.299) | 1.1 | 3.2 |
| Average at 3 and 4 h after bedtime | 0.735 (0.571‐0.842)** | 0.006 (0.957) | 1.7 | 4.7 | 0.687 (0.503‐0.811)** | −0.046 (0.720) | 1.2 | 3.4 |
| Average at 2, 3, and 4 h after bedtime | 0.768 (0.622‐0.863)** | 0.036 (0.738) | 1.4 | 3.9 | 0.751 (0.594‐0.852)** | 0.003 (0.976) | 1.0 | 2.8 |
| Fixed‐time measurement phase | ||||||||
| At 2:00 am | 0.502 (0.259‐0.686)** | 0.021 (0.904) | 2.1 | 5.8 | 0.469 (0.219‐0.662)** | 0.135 (0.445) | 1.6 | 4.4 |
| At 3:00 am | 0.468 (0.215‐0.662)** | −0.290 (0.100) | 2.4 | 6.7 | 0.403 (0.141‐0.614)* | −0.037 (0.847) | 1.9 | 5.2 |
| At 4:00 am | 0.505 (0.258‐0.689)** | −0.180 (0.290) | 2.1 | 5.8 | 0.467 (0.211‐0.662)** | 0.102 (0.569) | 1.5 | 4.2 |
| Average at 2:00 and 3:00 am | 0.717 (0.544‐0.831)** | −0.096 (0.420) | 1.4 | 3.9 | 0.650 (0.449‐0.788)** | 0.086 (0.533) | 1.2 | 3.2 |
| Average at 2:00 and 4:00 am | 0.689 (0.507‐0.813)** | −0.037 (0.771) | 1.4 | 3.9 | 0.590 (0.369‐0.747)** | 0.207 (0.167) | 1.2 | 3.2 |
| Average at 3:00 and 4:00 am | 0.661 (0.467‐0.795)** | −0.207 (0.118) | 1.6 | 4.4 | 0.615 (0.406‐0.764)** | 0.010 (0.942) | 1.2 | 3.2 |
| Average at 2:00, 3:00, and 4:00 am | 0.790 (0.654‐0.877)** | −0.102 (0.318) | 1.2 | 3.3 | 0.714 (0.540‐0.829)** | 0.112 (0.359) | 1.0 | 2.7 |
CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure.
ICC (2, 1): two‐way random model of absolute agreement, single measure.
P < 0.01.
P < 0.001.
3.2.2. Absolute reliability of nocturnal home BP measurement
The SEMs for the single measurement of Night‐1 and Night‐2 in SBP ranged from 2.1 to 2.4 mm Hg and those in DBP ranged from 1.4 to 1.9 mm Hg, whereas the SEMs for the average of multiple measurements of Night‐1 and Night‐2 in SBP ranged from 1.2 to 1.7 mm Hg and those in DBP ranged from 1.0 to 1.2 mm Hg (Table 3). The results were similar between the bedtime‐based measurement phase and the fixed‐time measurement phase.
After confirming the normal distribution of SBP, DBP, or PR, we confirmed that neither a fixed bias nor a proportional bias was observed between the measurement values of Night‐1 and Night‐2 for SBP, DBP, or PR (Tables 2 and 3). The MDCs for the single measurement of Night‐1 and Night‐2 in SBP ranged from 5.8 to 6.7 mm Hg and those in DBP ranged from 3.9 to 5.2 mm Hg, whereas the MDCs for the average of multiple measurements of Night‐1 and Night‐2 in SBP ranged from 3.3 to 4.7 mm Hg and those in DBP ranged from 2.7 to 3.4 mm Hg (Table 3).
When the average of multiple measurements of nocturnal BP parameters was calculated using the mixed times in the bedtime‐based measurement phase and the fixed‐time measurement phase, the results were similar: The ICCs of SBP ranged from 0.647 to 0.749 and those of DBP ranged from 0.591 to 0.704. Neither a fixed bias nor a proportional bias was observed between the measurement values of Night‐1 and Night‐2 for SBP, DBP, or PR (Tables S1 and S2).
4. DISCUSSION
This is the first study to compare different schedules of nocturnal home BP measurement, that is, bedtime‐based measurement vs fixed‐time measurement, from the viewpoint of their reliability, using an ICT‐based home BP monitoring device. The two main findings of this study are as follows. First, the average of multiple measurements of nocturnal home BP taken over a single night is a more reliable value than a single measurement. Second, the reliabilities of the nocturnal home BP values measured using bedtime‐based measurement and those measured using fixed‐time measurement were similar. These results indicate that we could obtain reliable nocturnal BP values regardless of the timing of the measurements, provided that participants measured their nocturnal BP multiple times per night.
From the viewpoint of the reliability of nocturnal home BP values, we propose that the average of multiple measurements of nocturnal home BP taken over a single night would be a more reliable value than the single measurement. In previous studies, Hosohata et al31 reported that the reproducibility of nocturnal BP taken on a single occasion per night (automatically measured at the programmed clock time, 0200 hour) was not good in a comparison of two sessions with an average interval of 5.9 days, although the reproducibility of multiple measurements per night was not considered. Kollias et al32 showed that a schedule of three automated measurements at intervals of 1 hour on each of two nights was the minimum requirement for the reliable assessment of nocturnal home BP values. The present study adds further evidence to support the thesis that the reliability of single nocturnal home BP measurement is poor. Since the higher reliability of HBPM is generally the reason it is considered superior to ABPM,33 it would be preferable to measure nocturnal home BP more than two times in a single night rather than once in a single night, regardless of whether a bedtime‐based measurement or fixed‐time measurement protocol is used.
In the present study, there were no differences in the reliability of multiple nocturnal home BP measurements between the bedtime‐based measurement and the fixed‐time measurement. Indeed, the nocturnal home BP values showed moderate‐to‐major agreement between Night‐1 and Night‐2 irrespective of the timing of the measurement. These results ran counter to our expectations. They showed that the nocturnal home BP level was not greatly affected by the lifestyle of participants and that it might be possible to perform highly reliable measurements using methods such as ABPM, which automatically measures nocturnal BP without consideration of the lifestyle. ABPM can evaluate various types of BP parameters, such as mean BP levels (including 24‐hours, daytime, and nocturnal BPs), BP circadian rhythm (dipping status), and BP variabilities, including morning BP surge. For nocturnal BP measurement only, HBPM has the potential to replace ABPM, which has previously been the gold standard of nocturnal BP measurement. However, our findings should be confirmed in large‐scale and long‐term studies, and the association between nocturnal BP measured by HBPM and cardiovascular outcomes of the study participants should also be evaluated.
The present study also suggested that the measurement of nocturnal home BP on two nights per week is a feasible schedule in clinical practice. The main rationale for replacing ABPM with HBPM for the measurement of nocturnal BP levels is that HBPM can provide nocturnal BP values over multiple nights. However, nocturnal home BP measurement using HBPM has not been widely adapted yet. In addition, taking nocturnal BP measurements over multiple nights might create a burden for participants, such as a sleep disturbance, which would lead to poor compliance of nocturnal home BP measurement. Even in the present clinical trial, although all participants were available to measure their nocturnal home BP on the first night, they were not able to continue the measurements for the seven consecutive nights of either phase of the protocol. In a previous study, one of the main purposes of which was to assess the changes in nocturnal home BP over three nights following an antihypertensive medication, only 26% of the 206 participants successfully measured their nocturnal home BP on all three nights.11 On the other hand, since there has been much evidence that nocturnal BP is a better predictor of future cardiovascular events than daytime BP,1, 2, 3 we concluded that nocturnal BP should be measured in clinical practices, and thus it is important to measure nocturnal home BP values over the long term under standard conditions. Therefore, from the viewpoints of feasibility and acceptability, the measurement of nocturnal home BP on two nights in 1 week should be recommended in clinical practice.
Interestingly, there was no difference in the nocturnal home BP levels between Night‐1 and Night‐2 in our study. The guidelines for conventional home BP measurement recommended that the optimal schedule was 7 days worth of BP values, excluding the first day’s data.6 Based on the results of the present study, we suggested that it is not necessary to discard the nocturnal home BP values obtained on the first night. Our findings show that the nocturnal home BP values obtained on the first night should not be discarded.
Our results confirmed that the ICT‐based approach was successful at providing reliable nocturnal home BP data, which were transmitted automatically to the data server. The nocturnal home BP values directly transmitted from the participants’ homes were quite accurate and were not participant to any selection or reporting bias. In addition, the ICT‐based HBPM device could be successfully used even by elderly hypertensive participants who had no prior experience with it, suggesting that the device had high practicability. Thus, the ICT‐based HBPM has the potential to be used widely in clinical practice.
A limitation of this study is that the number of participants (n = 48) was small, and most of the participants were elderly (age range, 50‐89 years). It is unclear whether the results in this study could be extrapolated to younger hypertensive participants.
5. CONCLUSIONS
In regard to the schedule of nocturnal home BP monitoring using an ICT‐based device, it appears that multiple measurements in a single night could provide reliable information of the nocturnal home BP values regardless of whether a bedtime‐based measurement or fixed‐time measurement protocol is used. In addition, two nights of nocturnal home BP measurement per week should be recommended in consideration of its feasibility. If a physician can obtain reliable nocturnal home BP values by ensuring that the participant will take multiple measurements over a limited number of nights, this schedule would be reasonable in clinical practice. Further large‐scale and long‐term studies are needed to investigate the reliability of nocturnal home BP monitoring.
CONFLICT OF INTEREST
None.
Supporting information
Fujiwara T, Nishizawa M, Hoshide S, Kanegae H, Kario K. Comparison of different schedules of nocturnal home blood pressure measurement using an information/communication technology‐based device in hypertensive patients. J Clin Hypertens. 2018;20:1633–1641. 10.1111/jch.13407
Takeshi Fujiwara and Masafumi Nishizawa contributed equally to the present study.
REFERENCES
- 1. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause‐specific cardiovascular events in hypertension. Hypertension. 2008;51:55‐61. [DOI] [PubMed] [Google Scholar]
- 2. Hermida RC, Ayala DE, Mojón A, Fernández JR. Decreasing sleep‐time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58:1165‐1173. [DOI] [PubMed] [Google Scholar]
- 3. Salles GF, Reboldi G, Fagard RH, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the ambulatory blood pressure collaboration in patients with hypertension (ABC‐H) meta‐analysis. Hypertension. 2016;67:693‐700. [DOI] [PubMed] [Google Scholar]
- 4. Kollias A, Ntineri A, Stergiou GS. Association of night‐time home blood pressure with night‐time ambulatory blood pressure and target‐organ damage: a systematic review and meta‐analysis. J hypertens. 2017;35:442‐452. [DOI] [PubMed] [Google Scholar]
- 5. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779‐785. [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Williams H, Northedge J, et al. Hypertension: the clinical management of primary hypertension in adults. Update of clinical guidelines 18 and 34. NICE clinical guideline No. 121. https://guidance.nice.org.uk/CG127. Accessed 24 March 2018.
- 8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 9. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253‐390. [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Soc Hypertension. 2018;12:579.e1‐579.e73. [DOI] [PubMed] [Google Scholar]
- 11. Hosaka M, Metoki H, Satoh M, et al. Randomized trial comparing the velocities of the antihypertensive effects on home blood pressure of candesartan and candesartan with hydrochlorothiazide. Hypertens Res. 2015;38(10):701‐707. [DOI] [PubMed] [Google Scholar]
- 12. Ishikawa J, Hoshide S, Eguchi K, et al. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921‐928. [DOI] [PubMed] [Google Scholar]
- 13. Kario K, Hoshide S, Haimoto H, et al. Sleep blood pressure self‐measured at home as a novel determinant of organ damage: Japan morning surge home blood pressure (J‐HOP) study. J Clin Hypertens (Greenwich). 2015;17:340‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujiwara T, Tomitani N, Kanegae H, Kario K. Comparative effects of valsartan plus either cilnidipine or hydrochlorothiazide on home morning blood pressure surge evaluated by information and communication technology‐based nocturnal home blood pressure monitoring. J Clin Hypertens (Greenwich). 2018;20:159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Booth JN 3rd, Muntner P, Abdalla M, et al. Differences in night‐time and daytime ambulatory blood pressure when diurnal periods are defined by self‐report, fixed‐times, and actigraphy: improving the detection of hypertension study. J Hypertens. 2016;34:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujiwara T, Hoshide S, Nishizawa M, Matsuo T, Kario K. Difference in evening home blood pressure between before dinner and at bedtime in Japanese elderly hypertensive patients. J Clin Hypertens (Greenwich). 2017;19:731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fujiwara T, Hoshide S, Kanegae H, Nishizawa M, Kario K. Reliability of morning, before‐dinner, and at‐bedtime home blood pressure measurements in patients with hypertension. J Clin Hypertens (Greenwich). 2018;20:315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices: Omron HEM‐7252G‐HP and Omron HEM‐7251G for self‐measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20:286‐290. [DOI] [PubMed] [Google Scholar]
- 19. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175‐191. [DOI] [PubMed] [Google Scholar]
- 20. Faul F, Erdfelder E, Lang AG, Buchner A. Statistical power analyses using G*Power 3.1: test for correlation and regression analyses. Behav Res Methods. 2009;41:1149‐1160. [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. Statistical power analysis for the behavioral sciences, (2nd edn). Hillsdale, NJ: Lawrence Erlbaum Associates; 1998:1‐567. [Google Scholar]
- 22. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420‐428. [DOI] [PubMed] [Google Scholar]
- 23. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 24. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135‐160. [DOI] [PubMed] [Google Scholar]
- 25. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217‐238. [DOI] [PubMed] [Google Scholar]
- 26. de Vet HC, Terwee CB, Knol DL, Bouter LM. When to use agreement versus reliability measures. J Clin Epidemiol. 2006;59:1033‐1039. [DOI] [PubMed] [Google Scholar]
- 27. Haley SM, Fragala‐Pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Phys Ther. 2006;86:735‐743. [PubMed] [Google Scholar]
- 28. Balaguier R, Madeleine P, Vuillerme N. Intra‐session absolute and relative reliability of pressure pain thresholds in the low back region of vine‐workers: effect of the number of trials. BMC Musculoskelet Disord. 2016;17:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2006;60:34‐42. [DOI] [PubMed] [Google Scholar]
- 30. Faber MJ, Bosscher RJ, van Wieringen PC. Clinimetric properties of the performance‐oriented mobility assessment. Phys Ther. 2006;86:944‐954. [PubMed] [Google Scholar]
- 31. Hosohata K, Kikuya M, Ohkubo T, et al. Reproducibility of nocturnal blood pressure assessed by self‐measurement of blood pressure at home. Hypertens Res. 2007;30:707‐712. [DOI] [PubMed] [Google Scholar]
- 32. Kollias A, Andreadis E, Agaliotis G, Kolyvas GN, Achimastos A, Stergiou GS. The optimal night‐time home blood pressure monitoring schedule: agreement with ambulatory blood pressure and association with organ damage. J Hypertens. 2018;36:243‐249. [DOI] [PubMed] [Google Scholar]
- 33. Imai Y, Obara T, Asamaya K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36:661‐672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


