Abstract
The authors evaluated the differences between evening home blood pressure (HBP) readings taken before dinner and those taken at bedtime, which were documented in a European and a Japanese guideline, respectively. Forty‐eight patients (mean age, 76.4 years) measured their evening HBP twice each day (two measurements both before dinner and at bedtime) for 14 days. The authors defined the at‐bedtime (B) minus the before‐dinner (D) systolic HBP as the B‐D difference. The mean B‐D difference was −8.7 mm Hg (P<.001). The depressor effect of bathing was significantly prolonged for 120 minutes. The B‐D difference with alcohol consumption was significantly greater than that without alcohol. In the linear mixed model analysis, time after bathing ≤120 minutes and alcohol consumption were significantly associated with the B‐D difference after adjustment with covariates. There was a marked difference between evening HBP values. When patients' evening HBP is measured according to the guidelines, their daily activities should be considered.
Keywords: alcohol consumption, bathing, evening home blood pressure, hypertension guideline
1. Introduction
Home blood pressure (HBP) had been reported to be strongly associated with target organ damage1 and to have stronger predictive power for cardiovascular mortality and mortality than office blood pressure (BP).2, 3 The Japanese Society of Hypertension (JSH) emphasizes the importance of HBP measurement in its JSH 2014 guideline,4 and HBP measurement is widespread in Japan. Most of the guidelines for the management of hypertension recommend that HBP should be measured both in the morning and in the evening,4, 5, 6, 7, 8 whereas the Eighth Joint National Committee (JNC 8) guideline does not comment on HBP.10 The relevant guidelines are as follows: the European Society of Hypertension (ESH)/European Society of Cardiology (ESC) 2013 guidelines,6 the ESH Practice Guideline for Home BP Monitoring,5 the UK's National Institute for Health and Care Excellence (NICE)/British Hypertension Society (BHS) 2011 guidelines,7 Call to Action on Use and Reimbursement for Home Blood Pressure Monitoring: A Joint Scientific Statement From the American Heart Association (AHA), American Society of Hypertension (ASH), and Preventive Cardiovascular Nurses Association (PCNA),8 the ASH/International Society of Hypertension (ISH) 2014 guidelines,9 the JNC 8 guidelines,10 and the 2014 JSH guidelines for the management of hypertension.4
A summary of these guidelines for HBP measurement, with a focus on the monitoring schedules, is given in Table 1. The guidelines that emphasize the indications for HBP measurement provided the consistent recommendation of morning HBP measurement, but there are inconsistent recommendations regarding the timing of evening HBP measurement.4, 5, 6, 7, 8 For example, the JSH 2014 guidelines and the AHA/ASH/PCNA statement recommends that evening BP should be measured just before bedtime, whereas the ESH practice guideline for HBP monitoring,5 which is a specific guideline for HBP monitoring that is different from the ESH/ESC 2013 guidelines for the management of arterial hypertension6 recommended that evening BP should be obtained before dinner. Although evening BP measurements are recommended in the ESH/ESC 2013 and NICE/BHS 20117 guidelines, neither of these guidelines specify the timing of evening BP measurement. The ASH/ISH 2014 guidelines9 did not comment on whether HBP should be measured in the morning and/or in the evening.
Table 1.
Comparison of Major Guidelines: Home BP Monitoring Schedule
| ESH/ESC 20136 | ESH Practice Guideline on Home BP Monitoring5 | NICE/BHS 20117 | AHA/ASH/PCNA Statement on Home BP Monitoring8 | ASH/ISH 20149 | JNC 810 | JSH 20144 | |
|---|---|---|---|---|---|---|---|
| Timing |
|
|
|
|
No definition | None |
|
| Frequency | Two measurements per occasion (1–2 min apart) | Two measurements per occasion (1–2 min apart) | Two measurements per occasion (at least 1 min apart) | Two to three measurements per occasion (1 min apart) | Two measurements per occasion | None | Two measurements per occasion |
| Duration |
|
|
|
|
5–7 d | None | 5 d or more |
Abbreviations: AHA, American Heart Association; ASH, American Society of Hypertension; BHS, British Hypertension Society; BP, blood pressure; ESC, European Society of Cardiology; ESH, European Society of Hypertension; ISH, International Society of Hypertension; JNC 8, Eighth Joint National Committee; JSH, Japanese Society of Hypertension; NICE, National Institute for Health and Care Excellence; PCNA, Preventive Cardiovascular Nurses Association.
In healthy humans, BP fluctuates throughout the 24‐hour day, and it also depends on individual lifestyles. The day‐by‐day variability of lifestyles that can affect evening BP levels is likely to be markedly different among individuals. In the evening, taking a warm or hot bath and consuming alcohol in particular will affect the ambulatory BP readings taken as HBP measurements, as it was reported that hot‐water bathing and alcohol consumption had depressor effects on BP.11, 12, 13 However, to the best of our knowledge, no study has compared HBP readings taken before dinner with those taken at bedtime, and the day‐by‐day effects of hot‐water bathing and alcohol consumption on HBP have not been studied. Therefore, we hypothesized that the BP measurements taken after bathing and alcohol consumption may underestimate evening HBP levels compared with those taken before these activities. Moreover, the additive or synergistic effects of bathing and alcohol consumption have not been analyzed thus far.
The objectives of the present study were to evaluate the differences in evening HBP levels by using different timing of evening HBP monitoring, ie, BP measurements taken before dinner and those taken at bedtime, as this issue is ambiguous or inconsistent in the present guidelines for the management of hypertension. We evaluated the evening HBP differences from the point of depressor effects of bathing and alcohol consumption, taking into account the day‐by‐day variability of lifestyles among elderly Japanese hypertensive patients.
2. Methods
2.1. Patients
Forty‐eight outpatients with essential hypertension recruited from two clinics were asked to measure their HBP. Twenty‐four of the patients were being treated at the Higashiagatsuma‐machi National Health Insurance Clinic, Gunma, Japan, and the other 24 patients were being treated at the Minamisanriku Public Medical Clinic, Miyagi, Japan. All patients were treated for hypertension.
2.2. HBP measurements
The patients measured their own BP at home using an automatic information and communication technology (ICT)–based device (HEM‐7252G‐HP, Omron Healthcare, Kyoto, Japan) based on the cuff‐oscillometric principle. This device also recorded the time that the wearer's BP was measured. All data obtained by the device were transmitted automatically to a cloud‐based remote monitoring system, the Medical LINK software program provided by Omron Healthcare,14 and the data were managed in an independent facility, the Jichi Medical University Center of Global Home and Ambulatory BP Analysis at the Jichi Medical University Center of Excellence Community Medicine Cardiovascular Research and Development, Shimotsuke, Japan.
The patients were instructed to measure their HBP in a sitting position after resting for 1 to 2 minutes with their legs uncrossed in an appropriate environment. The cuff used was 14.5‐cm wide and 46.6‐cm long (target arm girth: 22–32 cm). The arm cuff position was maintained at the heart level. Patients measured their HBP for 14 consecutive days, and the HBP monitoring protocol was as follows: two measurements in the morning, two measurements before dinner, two measurements at bedtime, and three measurements during sleep, for a total of nine measurements per day. The patients measured their morning BP within 1 hour after waking, after urination, before breakfast, and before ingesting medications. The before‐dinner BP was measured within 60 minutes before dinner. The at‐bedtime BP was measured just before the patient went to bed.
The patients' nighttime HBP was measured during sleep periods. A nighttime BP ICT‐based device was preset to take three BP measurements at fixed times: 2 am, 3 am, and 4 am during the “fixed time phase” of the experiment or at regular times (2, 3, and 4 hours after their chosen bedtime) during the “specific length of time phase.” All patients were randomized to either phase for 7 nights and followed the other phase for 7 days. Once the first phase was finished, the patients were immediately switched to the other phase by the data center via a Web‐based network system. The patients were instructed to wear the BP cuff and press the button to start the timer when they went to bed and to measure their nighttime HBP on as many nights as possible for 14 nights.
2.3. Daily information
The patients were asked to record their daily information every day in the same notebook that we provided. The information consisted of the times of waking, bathing, and going to bed; whether they had consumed any alcoholic beverage during their dinner times; and whether they had smoked a tobacco product (eg, cigarettes or cigars). The information about the amount of alcohol the patient had consumed on a given day was classified in terms of ethanol 20 to 30 mL in men and 10 to 20 mL in women (equivalent to 180 mL of sake, 500 mL of beer, <70 mL of shochu, a double whisky or brandy, or two glasses of wine per day) as described by the JSH 2014 guidelines.4 The details of this information were provided in the notebook, and the patients were instructed to check one of the following regarding alcohol consumption: none, appropriate, or overdose.
We designed the notebook to make it easy for the patients to provide detailed information, and the patients brought their notebooks to each visit to the clinic. We evaluated the time interval between the patients' completion of bathing and the time that they went to bed (ie, retired) from the patients' records and defined the “B‐D difference” as the at‐bedtime (B) systolic HBP value minus the before‐dinner (D) systolic HBP value.
2.4. Statistical analysis
The data are presented as the mean±standard deviation. We evaluated the differences in BP values and heart rate using paired Student t test and nonpaired t test. We calculated the standardized regression coefficients by performing univariate regression analyses. Taking into account the fact that the within‐subject variance (ie, the day‐by‐day variability of lifestyle among the patients) was different from the between‐subject variance, we conducted a linear mixed model analysis to detect the differences among groups after adjustment for age, sex, body mass index (BMI), dyslipidemia, diabetes mellitus, chronic kidney disease, history of cardiovascular disease, number of antihypertensive drugs, and medication after breakfast/dinner. In order to detect the existence and severity of multicollinearity among independent factors, the variance inflation factor was used to assess the extent to which the variances of the estimated coefficients were inflated. Considered a factor with variance inflation factor >10 as an indicative of serious multicocollinearity with other factors, we excluded antihypertensive drugs (ie, angiotensin‐converting‐enzyme inhibitors, angiotensin receptor blockers, β‐blockers, and diuretics) from adjusting factors. We then used the least‐squares method for multiple pairwise comparisons. All statistical analyses were performed using SPSS version 22.0 (SPSS Inc, Chicago, IL) at the Jichi Medical University Center of Global Home and Ambulatory BP Analysis, Tochigi, Japan. A P value <.05 was considered significant. All patients provided written informed consent to participate and to have their data published, and the study was approved by the ethics committee of Jichi Medical University, Shimotsuke, Japan.
3. Results
3.1. Patient characteristics
The ages of the 48 patients (20 men and 28 women) ranged from 50 to 89 years (mean±standard deviation: 76.4±7.8 years). The patients' characteristics and their daily schedules are shown in Table 2. The percentage of regular drinkers (alcohol consumption ≥ 3×/wk) was 18.8%. The mean interval from bathing to retiring was 104 minutes.
Table 2.
Characteristics of Hypertensive Patients
| Characteristic | Total (N=48) |
|---|---|
| Age, y | 76.4±7.8 |
| Male:female ratio | 20:28 |
| Body mass index, kg/m2 | 24.7±4.2 |
| Regular drinkers, % | 18.8 |
| Current smokers, % | 2.1 |
| Dyslipidemia, % | 55.7 |
| Diabetes mellitus, % | 22.2 |
| eGFR, mL/min/1.73 m2 | 66.4±13.2 |
| Hyperuricemia, % | 6.2 |
| History of cardiovascular disease, % | 6.8 |
| Antihypertensive medications | |
| No. of antihypertensive drugs | 2.0±1.0 |
| CCB, % | 78.2 |
| ACE inhibitor, % | 2.6 |
| ARB, % | 73.9 |
| β blocker, % | 6.6 |
| α blocker, % | 8.6 |
| Diuretic, % | 19.6 |
| Timing of antihypertensive medications | |
| After breakfast, % | 79.2 |
| After dinner, % | 29.2 |
| Time of daily schedules | |
| Wake‐up, h:min | 6:14±00:39 |
| BP measurement in the morning, h:min | 6:40±01:00 |
| BP measurement before dinner, h:min | 17:34±01:07 |
| Bathing, h:min | 19:56±01:43 |
| BP measurement at bedtime, h:min | 21:06±01:32 |
| Bedtime, h:min | 21:40±01:29 |
Values are the expressed as mean±standard deviation or number (percentage). Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BP, blood pressure; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate.
3.2. HBP measurements
The expected total numbers of BP measurements for the morning, before‐dinner, and at‐bedtime measurements were each 1344 (2 measurements×14 days×48 patients). The rates of completed BP measurements by the patients were as follows: 99.2% (1333 of 1344) in the morning, 95.7% (1286 of 1344) before dinner, and 94.6% (1272 of 1344) at bedtime (Figure 1).
Figure 1.
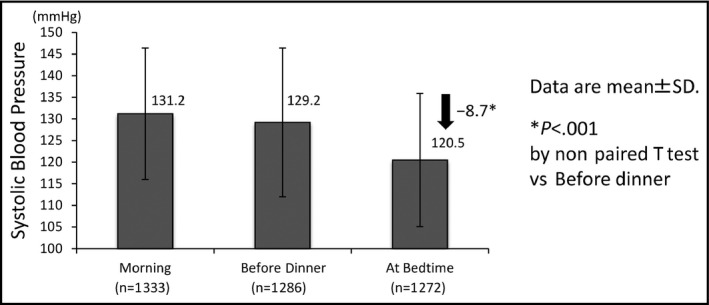
The differences in systolic blood pressure values in the morning, before dinner, and at bedtime. SD indicates standard deviation
3.3. Differences in the evening BP values and heart rate between before‐dinner and at‐bedtime levels
The SBP values for each measurement point are illustrated in Figure 1. The mean B‐D difference was −8.7 mm Hg for the total group of 48 patients (P<.001).
When we divided the day‐by‐day evening HBP readings into four groups according to the time interval from bathing to retiring (≤60 minutes [n=267], 61 to 120 minutes [n=276], 121 to 180 minutes [n=140], and ≥181 minutes [n=128]) and compared the SBP values between before‐dinner and at‐bedtime levels according to the four groups, the data revealed that the depressor effect of bathing was significantly prolonged for 120 minutes (Figure 2A). The B‐D difference for within 60 minutes after bathing was −12.8 mm Hg (P<.001) and that for 61 to 120 minutes was −8.7 mm Hg (P<.001). There was also a significant difference in the before‐dinner BP levels among the four groups (P trend=.031).
Figure 2.
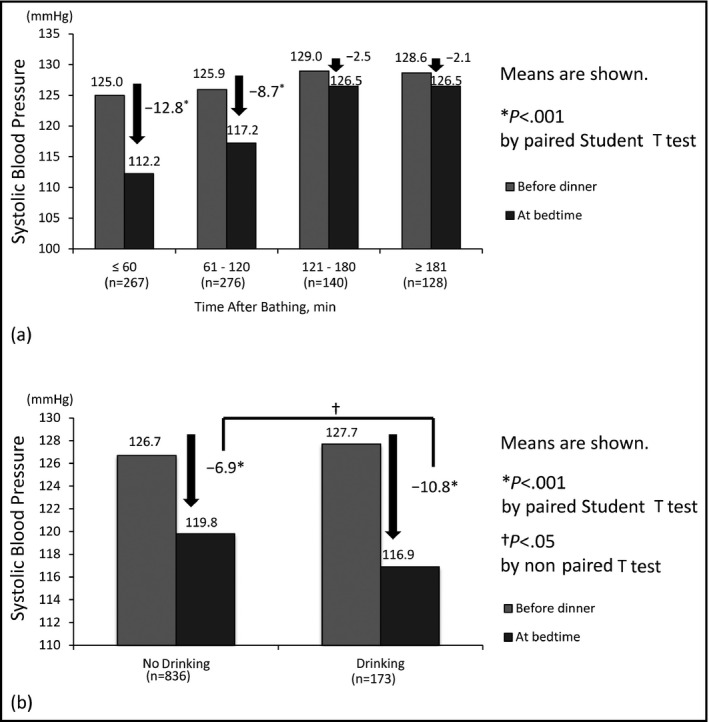
Systolic blood pressure (SBP) difference on each measurement time. (a) Differences in SBP between before dinner and at bedtime according to the interval between bathing and going to bed. (b) Differences in SBP between before dinner and at bedtime according to no drinking vs drinking alcohol
Next, to evaluate the influence of alcohol consumption on the HBP readings, we divided the evening HBP readings into two groups composed of occasions of drinking (n=173) and no drinking (n=836) before the HBP measurement in the evening (Figure 2B). Although there was a significant difference in the SBP values between the values obtained before dinner and those obtained at bedtime in both the drinking and no drinking groups, the B‐D difference with alcohol consumption was significantly greater than the corresponding difference without alcohol consumption (−10.8 vs −6.9 mm Hg, P=.007; Figure 2B).
Regarding diastolic BP (DBP), the mean B‐D difference was −5.5 mm Hg for the total group of patients (P<.001; Figure S1). The depressor effect of bathing was significantly prolonged for more than 181 minutes (P<.05; Figure S2a), and that of alcohol was significant in the patients who did consume alcohol (P<.001; Figure S2b). Regarding the heart rate results, only slight B‐D differences were observed, even in the conditions that included bathing and alcohol consumption (Figures S3 and S4).
3.4. The univariate and multivariate analysis results for the B‐D difference in BP
In the univariate analysis, the B‐D difference in SBP values was significantly associated with the time after bathing ≤120 minutes, alcohol consumption, and other factors (Table 3). Even in the multivariate analysis with the linear mixed model after the covariates were adjusted, both the time after bathing ≤120 minutes (−6.3 mm Hg, 95% confidence interval [CI], −8.3 and −4.4; P<.001) and alcohol consumption (−4.7 mm Hg, 95% CI, −7.5 to −1.9; P=.001) were significantly associated with the B‐D difference in SBP (Table 4, model 1). However, the time after bathing ≤120 minutes×alcohol consumption was not significantly associated with the B‐D difference in SBP, which meant that there was no synergistic effect between the time after bathing ≤120 minutes and alcohol consumption for the B‐D difference in SBP (Table 4, model 2).
Table 3.
Univariate Regression Analysis for B‐D Difference of Systolic Blood Pressure
| Characteristic | β | P Value |
|---|---|---|
| Age | 0.03 | .306 |
| Men | −0.06 | .053 |
| Body mass index | −0.02 | .572 |
| Current smokers | 0.03 | .321 |
| Dyslipidemia | 0.10 | .001 |
| Diabetes mellitus | 0.04 | .163 |
| eGFR | −0.01 | .720 |
| Hyperuricemia | −0.02 | .526 |
| History of cardiovascular disease | 0.10 | .002 |
| No. of antihypertensive drugs | 0.13 | <.001 |
| Antihypertensive medications | ||
| CCB | <0.001 | .994 |
| ACE inhibitor | −0.20 | <.001 |
| ARB | 0.13 | <.001 |
| β‐Blocker | 0.12 | <.001 |
| α‐Blocker | <0.001 | .992 |
| Diuretic | 0.15 | <.001 |
| Timing of antihypertensive medications | ||
| After breakfast | 0.21 | <.001 |
| After dinner | −0.13 | <.001 |
| Time after bathing ≤120 min | −0.25 | <.001 |
| Drinking | −0.10 | .002 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; B‐D, at‐bedtime systolic home blood pressure value minus the before‐dinner systolic home blood pressure value; CCB, calcium channel blocker; eGFR, estimated glomerular filtration rate.
Table 4.
Linear Mixed Model Estimates for B‐D Difference of Systolic Blood Pressure
| Variable | Adjusted Estimates, mm Hg | 95% CI | P Value |
|---|---|---|---|
| Model 1 | |||
| Time after bathing ≤120 min | −6.34 | −8.30 to −4.38 | <.001 |
| Drinking | −4.66 | −7.47 to −1.86 | .001 |
| Model 2 | |||
| Time after bathing ≤120 min | −5.94 | −8.15 to −3.74 | <.001 |
| Drinking | −3.46 | −7.63 to 0.71 | .104 |
| Time after bathing ≤120 min x Drinking | −2.14 | −7.61 to 3.33 | .443 |
Abbreviations: B‐D, at‐bedtime systolic home blood pressure value minus the before‐dinner systolic home blood pressure value; CI, confidence interval. Adjusted for age, sex, body mass index, dyslipidemia, diabetes mellitus, chronic kidney disease, history of cardiovascular disease, number of antihypertensive drugs, medication after breakfast, and medication after dinner.
Similar results were observed for the B‐D difference in DBP. Both the time after bathing ≤120 minutes (−2.5 mm Hg, 95% CI, −3.7 to −1.3; P<.001) and alcohol consumption (−4.6 mm Hg, 95% CI, −6.3 to −2.9; P=.001) were significantly associated with the B‐D difference in DBP (Table S1).
We next evaluated the B‐D difference in the patient subgroups classified by time after bathing ≤120 minutes and/or alcohol consumption. The group with both time after bathing ≤120 minutes and alcohol consumption had significantly greater B‐D differences in SBP (−15.0 mm Hg, 95% CI, −18.3 to −11.8; P<.05) compared with the other groups, which meant that time after bathing ≤120 minutes and alcohol consumption had additive effects for the B‐D difference in SBP, as illustrated in Figure 3. Regarding the B‐D difference in DBP, the patient subgroup with both time after bathing ≤120 minutes and alcohol consumption had a greater B‐D difference in DBP (−9.7 mm Hg) than the other groups, but a significant additive effect was not observed (Figure S5).
Figure 3.
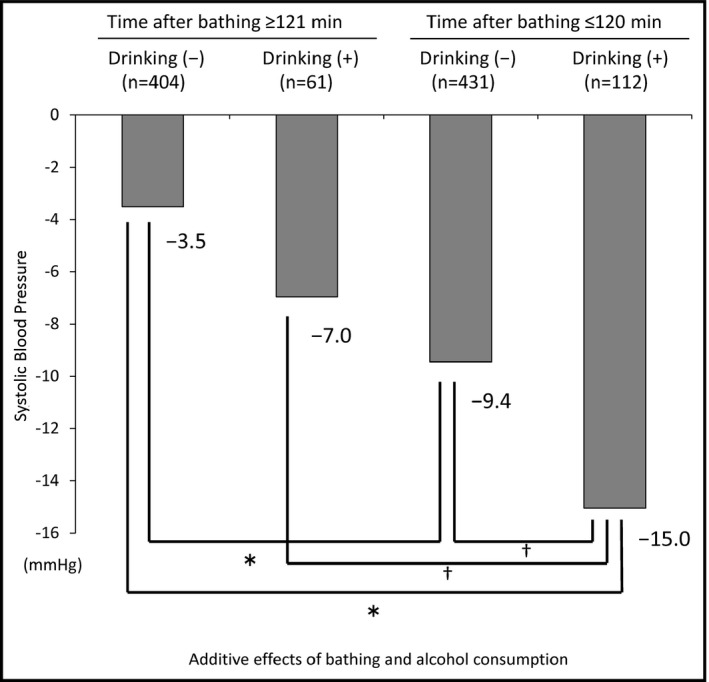
Additive effects of time after bathing ≤120 minutes and alcohol consumption for the at‐bedtime systolic home blood pressure value minus the before‐dinner systolic home blood pressure value differences in systolic blood pressurevalues. Adjusted for age, sex, body mass index, dyslipidemia, diabetes mellitus, chronic kidney disease, history of cardiovascular disease, number of antihypertensive drugs, medication after breakfast, and medication after dinner. Means are shown. *P<.001; † P<.05 by linear mixed model analysis
4. Discussion
The main findings of this study are as follows. First, there were marked differences in the evening HBP levels between the levels measured before dinner and those measured at bedtime. Second, the BP‐lowering effect of bathing was prolonged for 120 minutes, and consuming alcohol affected the evening HBP measured at bedtime. Third, these evening HBP‐lowering effects of bathing and alcohol consumption were additive but not synergistic. These findings indicate that the different conditions in which evening HBP is measured—in accord with the various guidelines for hypertension—lead to different HBP values. The evening HBP measured at bedtime according to the Japanese guidelines may underestimate the evening HBP levels due to the effects of bathing and alcohol consumption.
There was an 8.7‐mm Hg difference in the SBP values measured before dinner and those measured at bedtime in our patients, which indicates that evening HBP readings differ markedly due to the timing of HBP measurement. Despite the differences in measurement conditions for evening HBP among the Japanese, European, and American guidelines, few studies have compared the evening HBP readings between before dinner and at bedtime. In our results, the SBP values measured before dinner according to the evening HBP measurement timing recommended by the Western guidelines were close to those in the morning (129.2 vs 131.2 mm Hg). In contrast, the SBP values measured at bedtime according to the Japanese guidelines' recommendation for evening HBP measurement were lower than those measured in the morning (120.5 vs 131.2 mm Hg).
These results are consistent with those of previous studies that measured evening BP at different times. In the Finn‐Home study (treated only; n=464, mean age 56.6±8.6 years; 46.9% men)15 conducted in Finland with the evening HBP measured in accord with Western guidelines (which recommend evening BP measurement before dinner according to the ESH guideline), the evening systolic HBP levels were almost the same as those of the morning systolic HBP (139.7 vs 139.2 mm Hg, respectively). Conversely, in the Ohasama study (treated only; n=326, mean age 66.0±9.2 years; 36.5% males)16 and the Japan Morning Surge Home Blood Pressure (J‐HOP) study (treated majority; n=4278, mean age 64.9±10.9 years; 46.9% men)17, 18 conducted in Japan, the evening systolic HBP values measured at bedtime according to the Japanese guidelines were lower than the morning systolic HBP values (the Ohasama study: 129.5 vs 132.8 mm Hg, the J‐HOP study: 130.1 vs 138.4 mm Hg, respectively). Thus, the ethnic difference may be due in part to the different timing of the evening BP measurement.
In the present study, the significant depressor effects of bathing on SBP levels were prolonged for 120 minutes. Evening bathing in a bathtub full of hot water is a widespread traditional custom in Japan and most Japanese individuals take a bath every day at this time, but there is a paucity of information on the effects of bathing on BP values in Japan as well as in Western countries. Kawabe and colleagues11 reported a significant depressor effect of bathing on BP in Japanese patients aged 41.6±11.7 years. The depressor effect remained for 60 minutes after bathing, and disappeared in the persons in whom evening HBP was measured at ≥61 to 120 minutes after bathing. The difference in the ages of the patients in the present study and Kawabe's study might explain the difference in results. The depressor effects of bathing are mainly attributed to vasodilator action. In general, aging reduces the function of the autonomic nervous system. Vasoconstriction would be needed to maintain BP with sympathetic activity during and after bathing. However, the autonomic nervous system is dysregulated in the elderly and thus would not compensate for vasodilation, and it is thus difficult to maintain homeostasis after bathing. This may be why the depressor effect of bathing in the present study lasted longer in the present study compared with Kawabe's study.11 In addition, in this study, the mean interval from bathing to retiring was 104 minutes, which meant that many of the evening HBP values measured at bedtime according to the Japanese guidelines were underestimated by the depressor effects of bathing. Our findings indicate that physicians should know the timing of their patients' evening HBP measurements in detail as it is related to bathing timing.
Alcohol consumption has been reported to have biphasic effects on BP, ie, an early acute depressor effect and a pressor effect the morning after its consumption.12 In studies of Japanese alcohol drinkers, the BP values were significantly low, and the depressor effects lasted for up to 8 hours after alcohol consumption.12, 19 In an investigation of Western alcohol drinkers, the mean decreased SBP level throughout 6 hours of alcohol consumption was 4 mm Hg.20 Here, we observed that the B‐D difference in SBP in our patients who drank alcohol was significantly greater than the difference in the patients who did not drink alcohol. Our data thus support the thesis that alcohol consumption has a depressor effect on BP.
This is the first study to reveal that both taking a warm or hot bath and alcohol consumption additively increased the B‐D difference in SBP. Both hot baths and alcohol consumption have the effect of vasodilation. When they take place at the same time, the autonomic nervous system might not be able to sufficiently compensate for the excessive vasodilatation, especially in the elderly. Most Japanese bathe every night, and habitual drinkers usually drink an alcoholic beverage during their dinner times. The combination of bathing and alcohol consumption should thus be taken into consideration when evening HBP is measured. Our present findings also suggest the need for careful attention to bathing after alcohol consumption because there are many accidents during and after bathing in Japan,21 which may be triggered in part by the additive hypotensive effects.
JSH recommends that evening BP be measured at bedtime in order to improve the patients' compliance,4 but this timing might underestimate high BP levels. The results of previous studies might confirm this speculation. The Ohasama study reported that evening hypertension defined by morning HBP <135/85 mm Hg and evening HBP ≥135/85 mm Hg did not present a risk of stroke incidence compared with normotension defined by morning HBP <135/85 mm Hg and evening HBP <135/85 mm Hg, especially in antihypertension‐medicated patients.22 The J‐HOP study also reported that morning home SBP provided superior discrimination and risk reclassification for incident stroke compared with evening SBP. The predictive ability of morning SBP was attenuated by the simultaneous assessments of morning and evening SBP.18 However, the Finn‐Home study demonstrated that morning HBP and evening HBP had equally good predictive ability for cardiovascular events.23 To avoid the underestimation of cardiovascular risks, physicians should know their patients' lifestyles to interpret their evening HBP.
4.1. Study strengths and limitations
The strengths of this study are that: (1) the BP levels and the times that the BP values were measured were quite accurate and without selection bias, because we used an ICT‐based HBP monitoring device; (2) we were able to collect and analyze the detailed daily information of the patients from the same notebook format; and (3) we conducted a linear mixed model analysis taking into account the day‐by‐day variability of lifestyles among patients. In light of these strengths, our results appear to be reliable.
Our study also has some limitations. First, the number of patients (n=48) was relatively small. However, the patients measured their HBP on each occasion almost perfectly, and the numbers of BP measurements were sufficient: before dinner (n=1286) and at bedtime (n=1272), respectively. We conducted a linear mixed model analysis taking into account the within‐subject variance. Second, it is possible that the B‐D difference would be affected by a postprandial BP decrease or physiological circadian BP variation. We did not check the times and contents of the patients' dinners in this study. Ambulatory BP monitoring is needed to evaluate the effects of postprandial and circadian BP changes on the B‐D difference. Thus, the results of this study cannot be extrapolated to younger patients who might have a smaller B‐D difference. Third, we did not analyze the precise amounts of day‐by‐day alcohol consumption because few patients had consumed an “overdose” amount. We also did not take into account the opportunity for alcohol consumption after dinner and before bedtime because alcohol consumption at dinner is common in Japan. Fourth, we did not define the bath temperature, the method of bathing (whole‐body or partial‐body or shower only), or the temperature of the bathroom and dressing room. These factors may differ between Western and Japanese populations. Finally, there may be racial and genetic variations in aldehyde dehydrogenase 2,24, 25 which could affect the depressor effect of alcohol. The B‐D difference could also have been affected by factors such as the genetic difference responsible for salt‐sensitive hypertension26; differences in sodium consumption27; differences in lifestyles, eg, evening activities28; and environmental factors caused by geographical differences.29 Our results should be tested in other countries and populations.
5. Conclusions
The evening HBP values measured by our elderly Japanese patients just before they went to bed were 8.7 mm Hg lower than those measured before dinner, demonstrating a marked difference in evening HBP levels assessed at the different times recommended by the European and Japanese guidelines for hypertension. Physicians should take into account the influence of bathing (especially the first 120 minutes after bathing) and alcohol consumption on the measurement of evening HBP in order to avoid the underestimation of cardiovascular risks.
Conflicts of interest
The authors state that they have no potential conflicts of interest.
Supporting information
Acknowledgments
We gratefully acknowledge Ms Maiko Kondo and Ms Yukie Okawara for their coordination and data management of this study, and Ms Ayako Okura for her editorial assistance. The authors alone are responsible for the content and writing of the paper. The first (T.F.) and second author (S.H.) equally contributed to the writing process.
Fujiwara T, Hoshide S, Nishizawa M, Matsuo T, Kario K. Difference in evening home blood pressure between before dinner and at bedtime in Japanese elderly hypertensive patients. J Clin Hypertens. 2017; 19: 731–739. 10.1111/jch.12985
References
- 1. Jula A, Puukka P, Karanko H. Multiple clinic and home blood pressure measurements versus ambulatory blood pressure monitoring. Hypertension. 1999;34:261–266. [DOI] [PubMed] [Google Scholar]
- 2. Ohkubo T, Imai T, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population‐based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. [DOI] [PubMed] [Google Scholar]
- 3. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 4. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–392. [DOI] [PubMed] [Google Scholar]
- 5. Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779–785. [DOI] [PubMed] [Google Scholar]
- 6. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension. J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Williams H, Northedge J, et al. Hypertension: the clinical management of primary hypertension in adults. Update of clinical guidelines 18 and 34. NICE clinical guideline No. 127 2011. http://guidance.nice.org.uk/CG127. Accessed August 2011.
- 8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. [DOI] [PMC free article] [PubMed]
- 9. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 11. Kawabe H, Saito I. Influence of night‐time bathing on evening home blood pressure measurements: how long should the interval be after bathing? Hypertens Res. 2006;29:129–133. [DOI] [PubMed] [Google Scholar]
- 12. Kawano Y, Abe H, Kojima S, et al. Acute depressor effect of alcohol in patients with essential hypertension. Hypertension. 1992;20:219–226. [DOI] [PubMed] [Google Scholar]
- 13. Kawabe H, Saito I, Saruta T. Effects of night‐time alcohol intake on evening and next morning home blood pressure in Japanese normotensives. Clin Exp Hypertens. 2007;29:43–49. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi H, Yoshika M, Yokoi T. Validation of two automatic devices: Omron HEM‐7252G‐HP and Omron HEM‐7251G for self‐measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20:286–290. [DOI] [PubMed] [Google Scholar]
- 15. Niiranen TJ, Jula AM, Kantola IM, Reunanen A. Comparison of agreement between clinic and home‐measured blood pressure in the Finnish population: the Finn‐HOME Study. J Hypertens. 2006;24:1549–1555. [DOI] [PubMed] [Google Scholar]
- 16. Imai Y, Nishiyama A, Sekino M, et al. Characteristics of blood pressure measured at home in the morning and in the evening: the Ohasama study. J Hypertens. 1999;17:889–898. [DOI] [PubMed] [Google Scholar]
- 17. Hoshide S, Kario K, Yano Y, et al. on behalf of the J‐HOP study group: association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP study. Am J Hypertens. 2014;27:939–947. [DOI] [PubMed] [Google Scholar]
- 18. Hoshide S, Yano Y, Haimoto H, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: the Japan morning surge‐home blood pressure study. Hypertension. 2016;68:54–61. [DOI] [PubMed] [Google Scholar]
- 19. Abe H, Kawano Y, Kojima S, et al. Biphasic effects of repeated alcohol intake on 24‐hour blood pressure in hypertensive patients. Circulation. 1994;89:2626–2633. [DOI] [PubMed] [Google Scholar]
- 20. Seppa K, Sillanaukee P. Binge drinking and ambulatory blood pressure. Hypertension. 1999;33:79–82. [DOI] [PubMed] [Google Scholar]
- 21. Hayasaka S, Shibata Y, Noda T, Goto Y, Ojima T. Incidence of symptoms and accdidents during baths and showers among the Japanese general public. J Epidemiol. 2011;21:305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asayama K, Ohkubo T, Kikuya M, et al. Prediction of stroke by home “morning” versus “evening” blood pressure value: the Ohasama study. Hypertension. 2006;48:737–743. [DOI] [PubMed] [Google Scholar]
- 23. Niiranen TJ, Johansson JK, Reunanen A, Jula AM. Optimal schedule for blood pressure measurement based on prognostic data: the Finn‐Home study. Hypertension. 2011;57:1081–1086. [DOI] [PubMed] [Google Scholar]
- 24. Minami J, Todoroki M, Ishimitsu T, et al. Effects of alcohol intake on ambulatory blood pressure, heart rate, and heart rate variability in Japanese men with different ALDH2 genotype. J Hum Hypertens. 2002;16:345–351. [DOI] [PubMed] [Google Scholar]
- 25. Kawano Y. Physio‐pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens Res. 2010;33:181–191. [DOI] [PubMed] [Google Scholar]
- 26. Katsuya T, Ishikawa K, Sugimoto K, Rakugi K, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–525. [DOI] [PubMed] [Google Scholar]
- 27. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. [DOI] [PubMed] [Google Scholar]
- 28. Imai Y, Obara T, Asayama K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36:661–672. [DOI] [PubMed] [Google Scholar]
- 29. Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


