Abstract
Left ventricular structural and functional changes in patients with arterial hypertension are well established. However, the influence of arterial hypertension on right ventricular (RV) remodeling is still being investigated. The introduction of strain analysis provided an insight into RV function and mechanics. Previous research has demonstrated the predictive value of RV longitudinal strain in patients with various cardiovascular conditions, such as pulmonary hypertension, heart failure, congenital heart diseases, and valvular disease. Nowadays, we are aware of the fact that conventional echocardiographic methods usually do not provide necessary information about RV dysfunction in patients with arterial hypertension, which is why the evaluation of new parameters that could detect RV subtle changes in hypertension is essential. The present review article is an overview of the main principles of RV deformation and a summary of the current knowledge and clinical significance of RV strain in patients with arterial hypertension.
Keywords: arterial hypertension, function, right ventricle, strain
1. INTRODUCTION
For a long time, the right ventricle (RV) was considered as a dispensable cardiac chamber, which was not indispensible for overall heart function. The development of cardiovascular medicine, particularly the improvement of cardiac imaging techniques, has revealed that the RV has a vital role in global cardiac function, cardiovascular and total morbidity, and mortality. Not only in diseases that predominantly involve pulmonary circulation, such as pulmonary hypertension and congenital heart diseases, but also in conditions that primarily affect the left ventricle (LV), such as heart failure and mitral and aortic valve disease.1
Anatomically the RV could be separated into 3 segments: inflow, apex, and outflow tract. However, the position of the RV in the chest makes the RV assessment challenging for a conventional imaging technique—echocardiography. Cardiac magnetic resonance and cardiac computer tomography are more accurate and provide better insight, but their availability and cost still represent the major limitation to their usage in everyday clinical practice. The introduction of three‐dimensional echocardiography (3DE) and speckle tracking imaging has significantly changed the approach to the RV evaluation providing the information regarding RV size, shape, function, and mechanics.1
Previous investigations showed that arterial hypertension impacted pulmonary circulation and provoked RV remodeling.2 However, a majority of these studies was focused on RV hypertrophy or perhaps RV diastolic function.3, 4, 5, 6, 7 Only recently have the studies that investigate RV mechanics in hypertensive patients appeared. They have shown significant deterioration of RV longitudinal deformation8, 9, 10, 11 and revealed the association between RV longitudinal strain and functional capacity in hypertensive patients.12, 13 Furthermore, the studies have detected that RV longitudinal strain represents the strongest independent predictor of RV function,14 as well as the strong relationship between RV longitudinal strain and cardiovascular mortality and morbidity in a wide range of cardiovascular conditions.15, 16
The purpose of this review article is to provide the overview of the current knowledge regarding the role of RV structure, function, and particularly mechanics in hypertensive population.
2. RV STRUCTURE IN ARTERIAL HYPERTENSION
RV structure is specific because the RV free wall predominantly consists of transverse fibers that surround the septum, whereas the septum contains oblique helical fibers without a transverse component.17 This is the reason why the RV, unlike the LV, does not have a helicoid shape, but it includes an outflow tract with oblique ascending fibers. The interventricular septum represents a dominant biventricular helical structure that determines systolic functions of both the ventricles and it is considered as “the lion of the RV function.” However, the RV function represents the result of the interaction of transverse fibers in RV free wall and oblique fibers of the septum.
The evaluation of the RV structure in the clinical practice considers the assessment of RV wall thickness, which was already confirmed as an important predictor of morbidity and mortality in the global population free of cardiovascular disease.18
Previous investigations have shown that RV wall thickness is increased in patients with arterial hypertension.3, 4, 5, 6, 7, 10, 11, 12, 19
There are several possible mechanisms that could explain RV hypertrophy in systemic hypertension. First, overstimulation of the sympathetic system and the renin—angiotensin—aldosterone system that is typical for hypertensive patients could be responsible for increased pulmonary arteriolar resistance and, furthermore, for RV hypertrophy.20 Second, the mechanical interaction between the 2 ventricles through interventricular septum could also be a reasonable explanation for RV hypertrophy. Third, oxidative stress and endothelial dysfunction could induce changes in pulmonary circulation and consecutive RV hypertrophy.
3. RV FUNCTION IN ARTERIAL HYPERTENSION
RV contraction is developed as a result of the constriction of the circumferential basal loop during isovolumetric contraction, followed by the septal shortening of the ascending and descending helical fibres.17 This shortening motion represents tricuspidannular plane systolic excursion (TAPSE) towards the apex, and its excursion velocity. Table 1 summarizes the results of the studies that used conventional echocardiographic parameters in patients with arterial hypertension.
Table 1.
The conventional RV echocardiographic parameters in patients with arterial hypertension
| Reference | Sample size and subjects included in the study | Methods | Main findings |
|---|---|---|---|
| Cuspidi et al4 | 330 untreated and treated uncomplicated essential hypertensives | Echo | RV hypertrophy was observed in 33.6%; normal cardiac morphology was observed in 49.6%, isolated RV hypertrophy in 15.7%, isolated LV hypertrophy in 16.6% and bi‐ventricular hypertrophy in 17.8% |
| Todiere et al5 | 25 hypertensive patients and 24 controls | CMR | RV mass index and RV wall thickness were higher in hypertensive subjects, whereas RV peak filling rate was reduced. Significant correlation between RV indexed mass, RV early peak filling rate and RV ejection fraction in the hypertensive group. |
| Pedrinelli et al6 | 98 never‐treated, nonobese patients with blood pressure varying from the optimal to the mild hypertensive range | Echo (PWD+TDI) | Tricuspid early diastolic flow velocity and systolic velocity assessed by tissue Doppler gradually decreased with elevation of blood pressure. Both parameters correlated negatively with septal thickness. |
| Cicala et al7 | 30 normotensive controls and 30 untreated hypertensive patients | Echo (PWD + TDI) | Tricuspid e′/a′ ratio was reduced and relaxation time was prolonged in hypertensive patients. RV relaxation time positively correlated with RV wall thickness while tricuspid E/A ratio correlated with mitral E/A ratio. |
| Tumuklu et al9 | 35 patients with arterial hypertension and 30 age‐ and sex‐adjusted control subjects | Echo (PWD + TDI) | Diastolic measurements were altered at the level of RV lateral tricuspid annulus and RV free wall mid region in hypertensive patients. |
| Hanboly NH21 | 80 patients with untreated mild to moderate systemic hypertension and 40 healthy controls | Echo (PWD + TDI) | Increased RV wall thickness in hypertensive group. The RV diastolic dysfunction defined as tricuspid E/A ratio <0.8 was found in 60% hypertensive patients. Pulmonary acceleration time was significantly reduced in hypertensive patients. Decreased TAPSE and tricuspid s′ in hypertensive patients. |
| Karaye et al22 | 128 hypertensive patients | Echo (PWD + TDI) |
A, late flow velocity measured by pulsed Doppler; a′, late flow velocity measured by tissue Doppler; CMR, cardiac magnetic resonance; E, early flow velocity measured by pulsed Doppler; e′, early flow velocity measured by tissue Doppler; LV, left ventricle; LVEF, left ventricular ejection fraction; PWD, pulsed wave Doppler; RV, right ventricle; s′, systolic flow velocity measured at the level of lateral segment of tricuspid annulus by tissue Doppler; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging.
Using different techniques and parameters of RV systolic function, the studies obtained various results. Todiere et al5 did not find any significant difference in RV volumes and RV ejection fraction (EF) between the hypertensives and the controls. However, the limited number of participants (25 hypertensive patients and 24 controls) was most likely the main reason why the statistical significance was not achieved for RV EF (64 ± 7% vs 69 ± 8%).5 The usage of tissue Doppler parameters (isovolumetric contraction time, systolic peak velocity of the RV free wall at the level of tricuspid valve), fractional area shortening (FAC), or TAPSE resulted in controversial results. Some authors found the deterioration of RV systolic function,6, 21, 22 some disagreed,7, 9 and the others partly agreed.23 Interestingly, if myocardial performance index (Tei index) is used as a parameter of RV dysfunction, most investigations concur about the negative influence of arterial hypertension on RV systolic‐global function.22
Unlike the uncertainty that exists in the relationship between arterial hypertension and RV systolic function, almost all investigators agree that arterial hypertension impacts RV diastolic function.5, 6, 7, 8, 9, 10, 11, 12 The evaluation of RV diastolic function was mainly performed by pulsed and/or tissue Doppler indices.
The impairment of RV systolic function in arterial hypertension could be related with increased RV filling pressures, RV hypertrophy, and ventricular interaction.24 The deterioration of RV diastolic function might possibly be explained by increased stiffness of the RV caused by hypertrophy, retrograde transmission of increased LV filling pressure to the pulmonary circulation and ultimately to the RV, negative influence of biohumoral systems (renin‐angiotensin‐aldosterone and sympathetic nervous system) on pulmonary circulation and the RV, and inevitable ventricular interaction.
4. RV MECHANICS—BASICS AND APPLICATION
Strain represents a percentage change of myocardial length, circumference, or thickness. It can be derived from the tissue Doppler or the speckle tracking imaging. Tissue Doppler‐derived strain is faced with many limitations of the tissue Doppler technique: angle‐, age‐, and load‐dependency. Besides these obvious restrictions, this kind of strain is also a time‐consuming analysis. On the other hand, the speckle tracking‐derived strain is angle‐independent and significantly less age‐ and load‐dependent in comparison with the tissue Doppler‐derived strain. Additionally, it represents a rapid evaluation of cardiac mechanics in the whole myocardium thickness rather than in only one small myocardial segment within the sample volume.
Unlike the LV multidirectional strain that can be longitudinal, circumferential, radial, and area, the RV strain mostly refers to the assessment of the longitudinal strain. RV fibers in the subendocardial layer are predominately oriented in the longitudinal direction and their shortening is mainly responsible for the RV EF. At an early stage, RV remodeling is characterized by progressive reduction of longitudinal function with preserved and even increased transversal function due to circumferential fibers of the subepicardial layer. Therefore, RV longitudinal strain can be a sensitive parameter of RV function that could detect subtle changes at subclinical levels. This also means that fractional area change (FAC) remains within the normal range for a long time and deteriorates last in the cascade.
Nowadays, RV global longitudinal strain (GLS) is known as an accurate parameter of RV systolic function that correlates well with CMR RV EF (r = −.69, P < .001).14 Additionally, the authors have shown that RV GLS < −20% is the most sensitive and specific predictor of RV dysfunction.14 Investigations have revealed RV GLS as one of the most important predictors of cardiovascular and total morbidity and mortality in a large number of cardiovascular disorders.1, 15, 16 Figure 1 represents the evaluation of RV longitudinal strain.
Figure 1.
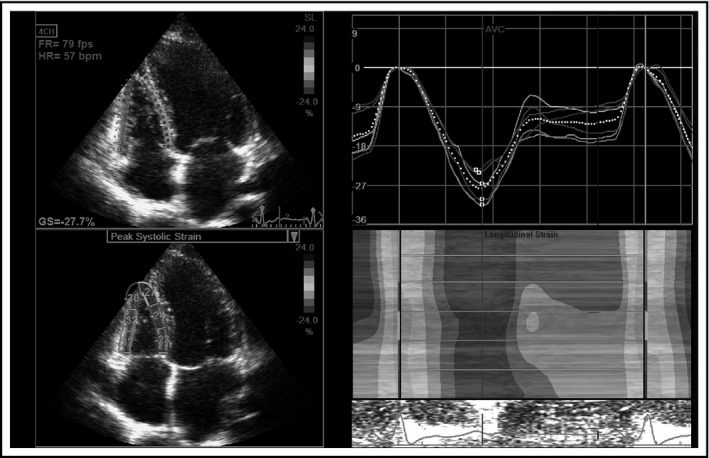
Right ventricular global longitudinal strain
5. RV MECHANICS IN ARTERIAL HYPERTENSION
The number of studies that investigated RV strain in the patients with arterial hypertension is limited. Table 2 reviews the results of the studies that used strain in patients with arterial hypertension.
Table 2.
RV strain in patients with arterial hypertension
| Reference | Sample size and subjects included in the study | Main findings |
|---|---|---|
| Pedrinelli et al8 | 89 never‐treated, non‐obese subjects with office blood pressure varying from the optimal to mildly hypertensive range | RV peak systolic strain and early diastolic strain rate reduced in the mid‐tertile of blood pressure distribution without further changes in the upper tertile. RV systolic and diastolic strain indices correlated inversely with increasing septal thickness. |
| Hanboly NH21 | 80 patients with untreated mild to moderate systemic hypertension and 40 healthy controls | RV GLS was reduced in hypertensive patients. Apical and mid segments of RV free wall were more deteriorated than basal RV segment in the hypertensive subjects |
| Tumuklu et al9 | 35 patients with arterial hypertension and 30 age‐ and sex‐adjusted control subjects | RV peak systolic strain was significantly lower in hypertensive patients with and without LV hypertrophy in comparison with normotensive controls. |
| Tadic et al25 | 174 recently diagnosed hypertensive patients | RV GLS was significantly lower in the non‐dippers. 3D RV end‐diastolic and end‐systolic volumes were increased, whereas 3D RV ejection fraction was reduced in non‐dipper hypertensive patients. |
| Tadic et al26 | 256 untreated patients (57 normotensives and 199 hypertensives) | RV mechanics is worse in night‐time and daytime‐night‐time hypertensive patients than in normotensive controls and isolated daytime hypertensive patients. 24‐h systolic blood pressure is independently associated with RV and right atrial global strain. |
| Tadic et al11 | 55 untreated hypertensive patients and 40 patients with no risk factors, similar by sex and age. | RV wall thickness, tricuspid E/e′ ratio, RV GLS, and 3D RVEF correlated with heart rate variability parameters. RV diastolic function, RV GLS, and 3D RVEF were independently associated with cardiac autonomic nervous function. |
| Tadic et al12 | 59 recently diagnosed, untreated, hypertensive patients, 62 treated subjects with well‐controlled arterial hypertension, 58 treated participants with treated but uncontrolled hypertension, and 55 controls adjusted by gender and age. | RV GLS was significantly decreased in untreated and uncontrolled hypertensive patients comparing with controls and well controlled participants. RV GLS and 3D RV stroke volume were independently associated with peak VO2. |
| Tadic et al34 | 232 hypertensive subjects | RV wall thickness was higher in concentric and concentric‐dilated LVH patients than in patients with normal LV geometry and LV concentric remodeling. RV GLS was reduced in concentric and concentric‐dilated patients compared with other hypertensive groups. 3D RV volumes were significantly higher in eccentric, dilated, and concentric‐dilated LVH hypertensive subjects. 3D RVEF was lower in these groups. |
3D, three‐dimensional; E, early flow velocity across tricuspid annulus measured by pulsed Doppler; e′, early flow velocity across lateral segment of tricuspid annulus measured by tissue Doppler; GLS, global longitudinal strain; LV, left ventricle; LVH, left ventricular hypertrophy; RV, right ventricle; RVEF, right ventricular ejection fraction; VO2, peak oxygen consumption.
The first study that used speckle‐tracking imaging in young, never‐treated hypertensive patients with mild hypertension showed that RV strain and strain rates were deteriorated.8 The RV changes completely followed LV remodeling. Figure 2 represents the calculation of RV longitudinal strain rates.
Figure 2.
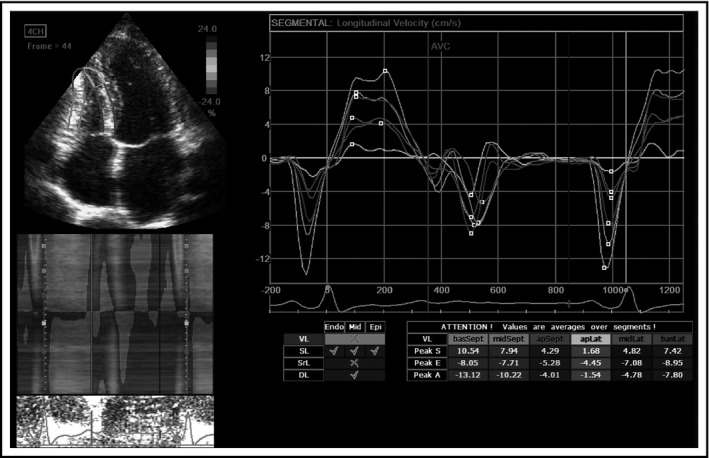
Right ventricular longitudinal strain rates
Hanboly showed not only that RV GLS was reduced in hypertensive patients, but also that apical and mid segments of RV free wall were more deteriorated than the basal RV segment in the hypertensive subjects.21 The author also found a significant correlation between systolic pulmonary pressure and RV GLS in hypertensive patients.
A small investigation that involved 20 hypertensive patients with LV hypertrophy and 15 hypertensive patients without LV hypertrophy and compared them with 30 normotensive subjects reported that free wall RV peak systolic strain was significantly lower in both groups of hypertensive patients (with and without LV hypertrophy) than in the normotensive controls.9
Our study group contributed significantly to the current knowledge regarding the influence of arterial hypertension on RV mechanics. In the investigation that studied the influence of a 24‐hour blood pressure pattern on RV remodeling, we showed that non‐dippers had significantly lower RV GLS and deteriorated strain rates than dippers.25 This could partly be explained by the increased 3D RV volumes and reduced 3D RV EF in the non‐dippers compared to the dippers.25 However, the reduced RV GLS strain could also be related with deteriorated overall right atrial and impaired RV diastolic function in non‐dippers. Although the mean arterial blood pressure correlated with RV GLS, the overall blood pressure was not independently associated with RV GLS.25 On the other hand, LV mass index, RV thickness, and 3D RV EF were independently associated with RV GLS.
A more recently published study revealed that RV mechanics was significantly worse in the nighttime and day–nighttime hypertensive patients than in the normotensive controls and isolated daytime hypertensive patients.26 This emphasized the importance of 24‐hour blood pressure monitoring in hypertensive patients because of the evident influence of blood pressure patterns on cardiac remodeling.
The investigation that involved patients with prehypertension showed that RV GLS was gradually decreased from the subjects with optimal blood pressure, across the prehypertensive subjects to the hypertensive individuals.10 Furthermore, we showed that untreated hypertensive patients have similar RV GLS to the patients with inadequately regulated hypertension, which is also significantly lower when compared to the patients with well‐regulated hypertension and the controls.12 More importantly, our study, for the first time showed that functional capacity estimated by peak oxygen consumption (peak VO2) correlated with RV free wall longitudinal strain, 3D RV end‐diastolic volume, 3D RV stroke volume, 3D RV EF, and right atrial longitudinal strain.12 However, only RV free wall strain and 3DRV stroke volume were independently associated with peak oxygen uptake in the study population.
Reduced functional capacity in the hypertensive patients was previously reported.27 This could be explained by 2 large groups of mechanisms: (1) biohumoral and (2) mechanical.13 Biohumoral mechanisms, such as catecholamines, the renin–angiotensin–aldosterone system, and brain natriuretic peptide induce oxidative stress and consequent endothelial dysfunction that might lead to increased pressure in pulmonary vascular bed, and ultimately to reduced functional capacity. The group of mechanical reasons for impaired VO2 includes LV and RV hypertrophy, increased LV and RV filling pressure (LV and RV diastolic dysfunction), as well as LV and RV systolic dysfunction.
Leong et al28 reported the significant correlation between peak VO2 and RV systolic function parameters, such as magnetic resonance‐derived RV ejection fraction, tricuspid annular plane systolic excursion, tissue Doppler tricuspid velocity, and tissue Doppler‐derived RV systolic strain rate in patients with systolic heart failure. Nevertheless, the investigators did not find any statistically significant correlation between peak VO2 and speckle tracking‐derived RV longitudinal strain (r = .39, P = .1).28
Previous studies established the relation between LV remodeling and functional capacity,29 which is why the ventricular interaction could be one of the major determinants of the relationship between RV mechanics and oxygen consumption.
The impact of the autonomic nervous system on RV deformation was shown in the previous study, in which we demonstrated the negative correlation between heart rate indexes and RV remodeling, including RV longitudinal strain.11 Our findings showed that both sympathetic and parasympathetic heart rate variability parameters correlated with RV thickness, tricuspid E/e′ ratio, 3D RV EF and RV GLS.11 Interestingly, RV GLS remained independently associated with heart rate variability indexes even after adjustment for blood pressure values, LV structure, and diastolic function parameters.
6. THE IMPACT OF LV GEOMETRY ON RV MECHANICS
The importance of LV geometry on cardiovascular morbidity and mortality was previously confirmed in the large number of observational and longitudinal studies and meta‐analyses.30 The inclusion of LV volume/diameter as a new criterion for determination of LV geometry patterns enabled reclassification of concentric and eccentric LV hypertrophy in dilated and non‐dilated forms.31 This updated classification stratified LV hypertrophy into subgroups with differential risk of adverse cardiovascular outcomes.32 Our study group demonstrated the importance of this updated classification in the hypertensive patients by suggesting that new classification could improve mortality risk stratification in a general population.33 There are many hypotheses that try to find the connection between LV geometry and cardiovascular risk, but most of them are based only on the influence of increased LV mass on morbidity and mortality.
The influence of LV geometry on RV remodeling is intriguing. Karaye et al23 reported that hypertensive patients with eccentric LV hypertrophy had the most dilated RV and lowest RV systolic function assessed by tissue Doppler (s’) and TAPSE. The authors did not find any significant difference between the hypertensive patients with normal geometry, concentric remodeling, and concentric hypertrophy.
The investigation that used an updated classification for LV geometry that involved the parameter of LV dilatation and not only LV hypertrophy showed that the hypertensive patients with concentric and concentric‐dilated LV hypertrophy had significantly lower RV strain than patients with other types of LV geometry.34 Although hypertensive patients with eccentric hypertrophy had lower RV strain and higher 3D RV volumes than patients with normal geometry and concentric remodeling, it did not reach statistical significance.
The relationship between LV geometry and RV remodeling can be explained by interplay among the sympathetic nervous system, the renin‐angiotensin‐aldosterone system, and insulin sensitivity that induce LV and RV hypertrophy, diastolic dysfunction, and deformation impairments. The ventricular interdependence is particularly important in the explanation of this association.
7. RV STRAIN IN WHITE COAT AND MASKED HYPERTENSION
There are conflicting data about the predictive value of white coat hypertension (WCH) and masked hypertension (MH) on cardiovascular morbidity and mortality. The evidence is more consistent regarding the target organ damage in WCH and MH. In the large meta‐analyses, our study group showed the influence of WCH and MH on LV hypertrophy and diastolic dysfunction.35, 36
The impact of prehypertension, WCH and MH on RV remodeling has been recently studied10, 37, 38 and the main results are briefly summarized in Table 3. We showed that even prehypertension could deteriorate RV mechanics10 and subsequently revealed that RV thickness and RV diastolic function in the WCH subjects were between the normal subjects and the hypertensive patients.37 RV GLS in WCH was also lower than in the controls, but still significantly higher than in the hypertensive patients. Multilayer RV strain assessment showed that both endocardial and mid‐myocardial longitudinal strains in the WCH individuals were worse than in the normotensive controls, but still better than in the persistently hypertensive patients. The other investigation that involved the MH subjects showed similar results as for the WCH patients, but with more deteriorated RV mechanics.38 Therefore, RV GLS, endocardial, and mid‐myocardial longitudinal strains in untreated MH individuals were not significantly different from sustained hypertensive patients, but they were significantly better than in normotensive controls.38 Figure 3 represents the estimation of RV multilayer longitudinal strain.
Table 3.
RV mechanics in patients with prehypertension, white coat and masked hypertension
| Reference | Sample size and subjects included in the study | Main findings |
|---|---|---|
| Tadic et al10 | 58 individuals with optimal blood pressure, 57 individuals with high‐normal blood pressure, and 59 recently diagnosed untreated hypertensive patients of similar age and sex distribution. | RV GLS gradually reduced from controls, across prehypertensive individuals, to hypertensive patients. RV wall thickness simultaneously associated with global RV and right atrial longitudinal strain, and RV diastolic function. |
| Tadic et al37 | 153 untreated patients (50 normal blood pressure, 48 WCH, 55 hypertensive subjects) | Results showed that LV and RV GLS gradually deteriorated from controls to patients with sustained hypertension. Endocardial RV longitudinal strain was lower in WCH and hypertensive patients than in controls. Mid‐myocardial RV longitudinal strain was decreased in hypertensive patients compared with the other 2 groups, whereas subepicardial RV longitudinal strain was similar between the observed groups. 24‐h systolic blood pressure was associated with subendocardial RV strain. |
| Tadic et al38 | 186 untreated subjects (56 normal blood pressure, 60 MH, 70 hypertensive subjects) | Global and free‐wall RV longitudinal strains were significantly lower in MH and sustained hypertensive patients comparing with controls. Endocardial and mid‐myocardial RV longitudinal and circumferential strains were significantly reduced in MH and hypertensive patients. There was no difference between MH and subjects with sustained hypertension. |
GLS, global longitudinal strain; LV, left ventricle; MH, masked hypertension; RV, right ventricle; WCH, white coat hypertension.
Figure 3.
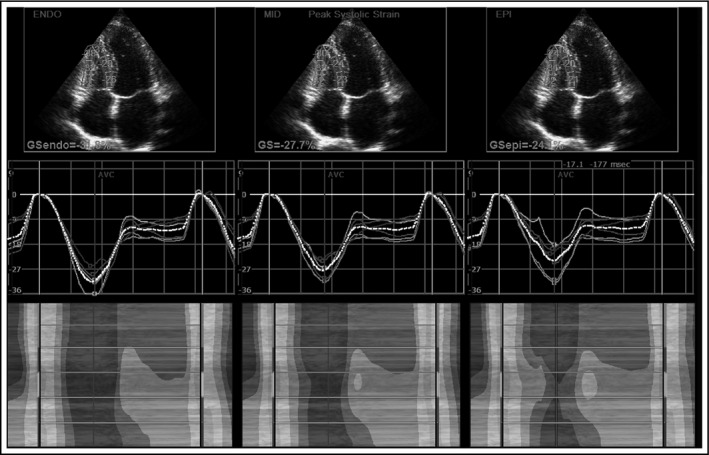
Right ventricular multilayer strain
These findings are probably the consequence of myocardial hypertrophy and interstitial fibrosis. Our results suggest that hypertensive heart disease develops in the endocardium—epicardium direction, as it was previously described in animal studies.39 In conclusion, longitudinal strain could be a surrogate marker of subendocardial fibrosis and a useful parameter for risk stratification in hypertensive patients.
8. CONCLUSION
The number of studies that showed RV remodeling in arterial hypertension is increasing. Novel imaging methods are providing insight into RV structure, function, and mechanics. RV strain is a particularly important parameter that has already been used for initial and follow‐up evaluation in many cardiovascular conditions. Strain analysis is providing important information about RV function and mechanics—it is widely available, easy to perform, and not time‐consuming with a favorable cost‐effectiveness ratio. All these advantages suggest that RV strain assessment should be present in everyday clinical practice in hypertensive patients. Further longitudinal studies are necessary to estimate the prognostic value of RV strain in the hypertensive population.
CONFLICT OF INTEREST
None.
Tadic M, Cuspidi C, Bombelli M, Grassi G . Right heart remodeling induced by arterial hypertension: Could strain assessment be helpful?. J Clin Hypertens. 2018;20:400–407. 10.1111/jch.13186
REFERENCES
- 1. Tadic M. Multimodality evaluation of the right ventricle: an updated review. Clin Cardiol. 2015;38:770‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fiorentini C, Barbier P, Galli C, et al. Pulmonary vascular over‐reactivity in systemic hypertension. A pathophysiological link between the greater and the lesser circulation. Hypertension. 1985;7:995‐1002. [DOI] [PubMed] [Google Scholar]
- 3. Cuspidi C, Sala C, Muiesan ML,, et al. Right ventricular hypertrophy in systemic hypertension: an updated review of clinical studies. J Hypertens. 2013;31:858‐865. [DOI] [PubMed] [Google Scholar]
- 4. Cuspidi C, Negri F, Giudici V, et al. Prevalence and clinical correlates of right ventricular hypertrophy in essential hypertension. J Hypertens. 2009;27:854‐860. [DOI] [PubMed] [Google Scholar]
- 5. Todiere G, Neglia D, Ghione S, et al. Right ventricular remodelling in systemic hypertension: a cardiac MRI study. Heart. 2011;97:1257‐1261. [DOI] [PubMed] [Google Scholar]
- 6. Pedrinelli R, Canale ML, Giannini C, et al. Right ventricular dysfunction in early systemic hypertension: a tissue Doppler imaging study in patients with high‐normal and mildly increased arterial blood pressure. J Hypertens. 2010;28:615‐621. [DOI] [PubMed] [Google Scholar]
- 7. Cicala S, Galderisi M, Caso P, et al. Right ventricular diastolic dysfunction in arterial systemic hypertension: analysis by pulsed tissue Doppler. Eur J Echocardiogr. 2002;3:135‐142. [DOI] [PubMed] [Google Scholar]
- 8. Pedrinelli R, Canale ML, Giannini C, Talini E, Dell'Omo G, Di Bello V. Abnormal right ventricular mechanics in early systemic hypertension: a two‐dimensional strain imaging study. Eur J Echocardiogr. 2010;11:738‐742. [DOI] [PubMed] [Google Scholar]
- 9. Tumuklu MM, Erkorkmaz U, Ocal A. The impact of hypertension and hypertension‐related left ventricle hypertrophy on right ventricle function. Echocardiography. 2007;24:374‐384. [DOI] [PubMed] [Google Scholar]
- 10. Tadic M, Cuspidi C, Pencic B, et al. High‐normal blood pressure impacts the right heart mechanics: a three‐dimensional echocardiography and two‐dimensional speckle tracking imaging study. Blood Press Monit. 2014;19:145‐152. [DOI] [PubMed] [Google Scholar]
- 11. Tadic M, Cuspidi C, Pencic B, Jozika L, Celic V. Relationship between right ventricular remodeling and heart rate variability in arterial hypertension. J Hypertens. 2015;33:1090‐1097. [DOI] [PubMed] [Google Scholar]
- 12. Tadic M, Cuspidi C, Suzic‐Lazic J, et al. Is there a relationship between right‐ventricular and right atrial mechanics and functional capacity in hypertensive patients? J Hypertens. 2014;32:929‐937. [DOI] [PubMed] [Google Scholar]
- 13. Tadic M, Ivanovic B. Why is functional capacity decreased in hypertensive patients? From mechanisms to clinical studies. J Cardiovasc Med (Hagerstown). 2014;15:447‐455. [DOI] [PubMed] [Google Scholar]
- 14. Lu KJ, Chen JX, Profitis K, et al. Right ventricular global longitudinal strain is an independent predictor of right ventricular function: a multimodality study of cardiac magnetic resonance imaging, real time three‐dimensional echocardiography and speckle tracking echocardiography. Echocardiography. 2015;32:966‐974. [DOI] [PubMed] [Google Scholar]
- 15. Peyrou J, Chauvel C, Pathak A, Simon M, Dehant P, Abergel E. Preoperative right ventricular dysfunction is a strong predictor of 3 years survival after cardiac surgery. Clin Res Cardiol. 2017;106:734‐742. [DOI] [PubMed] [Google Scholar]
- 16. D'Andrea A, Stanziola A, D'Alto M, et al. Right ventricular strain: an independent predictor of survival in idiopathic pulmonary fibrosis. Int J Cardiol. 2016;222:908‐910. [DOI] [PubMed] [Google Scholar]
- 17. Buckberg GD, Hoffman JI, Coghlan HC, Nanda NC. Ventricular structure‐function relations in health and disease: Part I. The normal heart. Eur J Cardiothorac Surg. 2015;47:587‐601. [DOI] [PubMed] [Google Scholar]
- 18. Kawut SM, Barr RG, Lima JA, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi‐Ethnic Study of Atherosclerosis (MESA) – right ventricle study. Circulation. 2012;126:1681‐1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivanovic BA, Tadic MV, Celic VP. To dip or not to dip? The unique relationship between different blood pressure patterns and cardiac function and structure. J Hum Hypertens. 2013;27:62‐70. [DOI] [PubMed] [Google Scholar]
- 20. Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanboly NH. Right ventricle morphology and function in systemic hypertension. Nig J Cardiol. 2016;13:11‐17. [Google Scholar]
- 22. Gregori M, Tocci G, Giammarioli B, et al.. Abnormal regulation of renin angiotensin aldosterone system is associated with right ventriculardysfunction in hypertension. Can J Cardiol. 2014;30:188‐194. [DOI] [PubMed] [Google Scholar]
- 23. Karaye KM, Sai'du H, Shehu MN. Right ventricular dysfunction in a hypertensive population stratified by patterns of left ventricular geometry. Cardiovasc J Afr. 2012;23:478‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferlintz J. Right ventricular performance in essential hypertension. Circulation. 1980;61:156‐162. [DOI] [PubMed] [Google Scholar]
- 25. Tadic M, Cuspidi C, Pencic B, et al. Circadian blood pressure pattern and right ventricular and right atrial mechanics: a two‐ and three‐dimensional echocardiographic study. J Am Soc Hypertens. 2014;8:45‐53. [DOI] [PubMed] [Google Scholar]
- 26. Tadic M, Cuspidi C, Celic V, Pencic‐Popovic B, Mancia G. Nocturnal hypertension and right heart remodeling. J Hypertens. 2017;36:136‐142. [DOI] [PubMed] [Google Scholar]
- 27. Kokkinos P, Pittaras A, Manolis A, et al. Exercise capacity and 24‐h blood pressure in prehypertensive men and women. Am J Hypertens. 2006;19:251‐258. [DOI] [PubMed] [Google Scholar]
- 28. Leong DP, Grover S, Molaee P, et al. Nonvolumetric echocardiographic indices of right ventricular systolic function: validation with cardiovascular magnetic resonance and relationship with functional capacity. Echocardiography. 2012;29:455‐463. [DOI] [PubMed] [Google Scholar]
- 29. Celic V, Tadic M, Suzic‐Lazic J, et al. Two‐ and three‐dimensional speckle tracking analysis of the relation between myocardial deformation and functional capacity in patients with systemic hypertension. Am J Cardiol. 2014;113:832‐839. [DOI] [PubMed] [Google Scholar]
- 30. Nakanishi K, Jin Z, Homma S, et al. Left ventricular mass‐geometry and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Am Heart J. 2017;185:85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4‐tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging. 2010;3:164‐171. [DOI] [PubMed] [Google Scholar]
- 32. Garg S, de Lemos JA, Ayers C, et al. Association of a 4‐tiered classification of LV hypertrophy with adverse CV outcomes in the general population.JACCCardiovasc. Imaging. 2015;8:1034‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cuspidi C, Facchetti R, Bombelli M, et al. Risk of mortality in relation to an updated classification of left ventricular geometric abnormalities in a general population: the Pamela study. J Hypertens. 2015;33:2133‐2140. [DOI] [PubMed] [Google Scholar]
- 34. Tadic M, Cuspidi C, Vukomanovic V, Kocijancic V, Celic V. Right ventricular remodeling and updated left ventricular geometry classification: is there any relationship? Blood Press. 2016;25:292‐297. [DOI] [PubMed] [Google Scholar]
- 35. Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G, Mancia G. White‐coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: a meta‐analysis. J Hypertens. 2015;33:24‐32. [DOI] [PubMed] [Google Scholar]
- 36. Cuspidi C, Sala C, Tadic M, Rescaldani M, Grassi G, Mancia G. Untreated masked hypertension and subclinical cardiac damage: a systematic review and meta‐analysis. Am J Hypertens. 2015;28:806‐813. [DOI] [PubMed] [Google Scholar]
- 37. Tadic M, Cuspidi C, Ivanovic B, et al. The impact of white‐coat hypertension on cardiac mechanics. J Clin Hypertens (Greenwich). 2016;18:617‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tadic M, Cuspidi C, Vukomanovic V, Celic V, Pavlovic T, Kocijancic V. The influence of masked hypertension on the right ventricle: is everything really masked? J Am Soc Hypertens. 2016;10:318‐324. [DOI] [PubMed] [Google Scholar]
- 39. Ishizu T, Seo Y, Kameda Y, et al. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heartdisease. Hypertension. 2014;63:500‐506. [DOI] [PubMed] [Google Scholar]


