Abstract
Background
Some concomitant medications including antibiotics (ATB) have been reproducibly associated with worse survival following immune checkpoint inhibitors (ICIs) in unselected patients with non-small cell lung cancer (NSCLC) (according to programmed death-ligand 1 (PD-L1) expression and treatment line). Whether such relationship is causative or associative is matter of debate.
Methods
We present the outcomes analysis according to concomitant baseline medications (prior to ICI initiation) with putative immune-modulatory effects in a large cohort of patients with metastatic NSCLC with a PD-L1 expression ≥50%, receiving first-line pembrolizumab monotherapy. We also evaluated a control cohort of patients with metastatic NSCLC treated with first-line chemotherapy. The interaction between key medications and therapeutic modality (pembrolizumab vs chemotherapy) was validated in pooled multivariable analyses.
Results
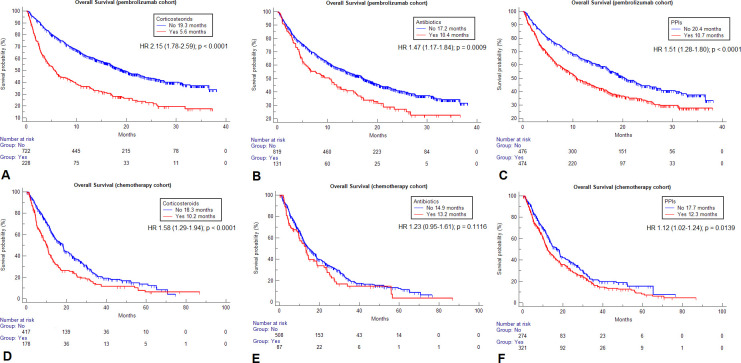
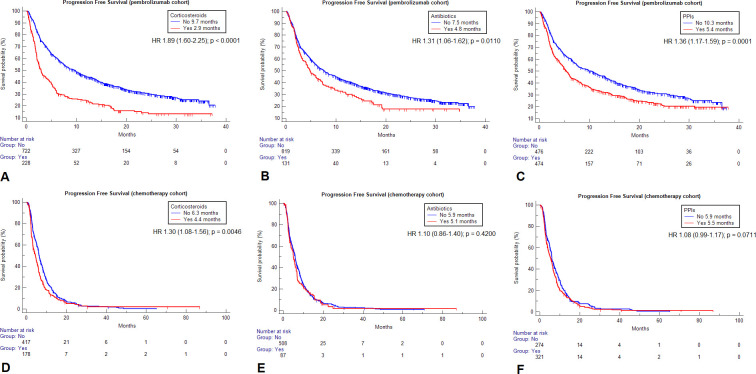
950 and 595 patients were included in the pembrolizumab and chemotherapy cohorts, respectively. Corticosteroid and proton pump inhibitor (PPI) therapy but not ATB therapy was associated with poorer performance status at baseline in both the cohorts. No association with clinical outcomes was found according to baseline statin, aspirin, β-blocker and metformin within the pembrolizumab cohort. On the multivariable analysis, ATB emerged as a strong predictor of worse overall survival (OS) (HR=1.42 (95% CI 1.13 to 1.79); p=0.0024), and progression free survival (PFS) (HR=1.29 (95% CI 1.04 to 1.59); p=0.0192) in the pembrolizumab but not in the chemotherapy cohort. Corticosteroids were associated with shorter PFS (HR=1.69 (95% CI 1.42 to 2.03); p<0.0001), and OS (HR=1.93 (95% CI 1.59 to 2.35); p<0.0001) following pembrolizumab, and shorter PFS (HR=1.30 (95% CI 1.08 to 1.56), p=0.0046) and OS (HR=1.58 (95% CI 1.29 to 1.94), p<0.0001), following chemotherapy. PPIs were associated with worse OS (HR=1.49 (95% CI 1.26 to 1.77); p<0.0001) with pembrolizumab and shorter OS (HR=1.12 (95% CI 1.02 to 1.24), p=0.0139), with chemotherapy. At the pooled analysis, there was a statistically significant interaction with treatment (pembrolizumab vs chemotherapy) for corticosteroids (p=0.0020) and PPIs (p=0.0460) with respect to OS, for corticosteroids (p<0.0001), ATB (p=0.0290), and PPIs (p=0.0487) with respect to PFS, and only corticosteroids (p=0.0033) with respect to objective response rate.
Conclusion
In this study, we validate the significant negative impact of ATB on pembrolizumab monotherapy but not chemotherapy outcomes in NSCLC, producing further evidence about their underlying immune-modulatory effect. Even though the magnitude of the impact of corticosteroids and PPIs is significantly different across the cohorts, their effects might be driven by adverse disease features.
Keywords: immunotherapy, lung neoplasms
Introduction
Several drugs have been investigated for their possible detrimental effects on immune checkpoint inhibitors (ICIs) clinical outcomes in patients with cancer.1 Beyond mere pharmacodynamic and pharmacokinetic interactions, the putative immune-disrupting effect also relieson the unbalancing of the gut microbiome2 and on drug-induced immune suppression.3 While a number of studies have now reproducibly shown that some concomitant baseline medications such as corticosteroids, systemic antibiotics (ATB) and proton pump inhibitors (PPIs), are consistently linked with poor radiological response and survival following ICIs across a number of oncological indications,4–9 it is still unclear whether the mechanisms underlying these associations are to be found in the connection with adverse prognostic factors (ie, symptomatic malignancy, disease burden, poorer performance status or exacerbation of underlying chronic airways disease) as opposed to a true immune-modulatory effect.
In a recent observational study of over 1000 ICI recipients, ATB emerged as strong predictor of outcome, irrespective of the indication for their administration (prophylaxis vs treatment of active infections), whereas corticosteroids were only associated with worse outcomes if administered for palliative indications.10 Moreover, in the same study population, both corticosteroids and PPIs were significantly associated with a higher baseline burden of disease,10 suggesting that the indication for prescription of concomitant medications and their association with negative prognostic features could be important confounding factors assessing their supposed immune-modulatory profile. ATB are usually indicated to treat infections rather than cancer-related symptoms, therefore the ATB-ICI paradigm might represent the proper model to verify whether the effect on clinical outcomes truly depends on an immune-modulatory effect.
In patients with non-small cell lung cancer (NSCLC), alongside growing evidence from retrospective observational studies,4 5 11 a pooled analysis from the OAK and POPLAR trials lent prospective confirmation that baseline PPIs and ATB were associated with decreased progression-free survival (PFS) and overall survival (OS) in patients receiving atezolizumab, but not in patients receiving docetaxel chemotherapy.8 Patients with NSCLC with high PD-L1 (programmed death-ligand 1) tumor expression, who are candidate to frontline ICI monotherapy, might represent an intrinsically different population with respect to clinicopathological characteristics, including prevalence of ATB use (eg, no previous risk of chemotherapy-induced neutropenia). To our knowledge, there is no evidence to suggest whether the same association has been confirmed in this setting.
To address these questions, we performed a retrospective clinical outcomes analysis according to some key baseline medications among a large real-world multicenter cohort of patients with metastatic NSCLC with a PD-L1 expression ≥50%, who received first-line single agent pembrolizumab at 34 European institutions.12–16 As a comparator arm, we performed the same analysis among a second cohort of patients with NSCLC treated with first-line chemotherapy, in order to estimate their potential different impact on clinical outcomes, depending on the anti-cancer treatment received.
Materials and methods
Study design
The aim of this study was to evaluate the impact of concomitant baseline medications postulated to affect responsiveness to pembrolizumab monotherapy in a cohort of patients with metastatic NSCLC with a PD-L1 expression ≥50% treated with first-line pembrolizumab monotherapy outside clinical trials.12–16 In total, 31 institutions participated to the study (online supplemental table S1) and retrospectively included patients treated from January 2017 to May 2020. We accrued a second cohort of metastatic epidermal growth factor receptor wild type patients with NSCLC treated with first-line chemotherapy as part of routine clinical practice from January 2013 to May 2020, across 13 out of the 31 above-mentioned institutions.15 16
jitc-2021-002421supp001.pdf (112.7KB, pdf)
Study endpoint included objective response rate (ORR), PFS and OS. Detailed methodology regarding clinical outcomes estimation can be found elsewhere.12–16 Data cut-off period was September 2020.
First, we evaluated the impact of each class of concomitant baseline medications (corticosteroids, systemic ATB, PPIs, statins, aspirin, β-blockers and metformin) on ORR, PFS and OS within the pembrolizumab cohort. Those medication categories which proved to be significantly correlated with outcomes on the univariate analysis (with an entry significance level of 0.05) were subsequently evaluated in multivariable models. A fixed regression model was used, including those covariates which already proved to be major determinants of clinical outcomes within the population on the basis of prior analyses.12–16 The key covariates were age (<70 vs ≥70 years old), gender (male vs female), Eastern Cooperative Oncology Group-Performance Status (ECOG-PS) (0–1 vs ≥2), smoking status (current/former vs never smokers) presence of central nervous system metastases (yes vs no), bone metastases (yes vs no) and liver metastases (yes vs no). In order to further evaluate the role of concomitant baseline medication, we estimate the association with baseline ECOG-PS for those who showed to be significantly related with clinical outcomes.17
In parallel, we explored the impact of selected concomitant baseline therapies on clinical outcomes of patients receiving first-line chemotherapy on univariate analysis. Finally, to take into account the differential impact of each medication within the two cohorts, we performed a pooled analysis, using a multivariable regression model (inclusive of the previously selected covariates) for each drug category, including the interaction term between each drug class and the therapeutic modality (pembrolizumab vs chemotherapy), used as covariate.
Concomitant baseline medications
Concomitant medication at the initiation of first-line treatment was determined from patients’ clinical records retrospectively. For the purpose of this study, we focused on the following drug categories, in view of their postulated effect on ICIs outcomes in cancer patient:
Corticosteroids (dose ≥10 mg prednisone equivalent per day, with a minimum 24 hours of dosing) within the 30 days before first-line treatment initiation (excluding chemotherapy pre-medications).5 6 11
Systemic ATB within the 30 days before first-line treatment initiation.7 10
Baseline PPIs.8–10
Baseline statins (yes vs no).10 18
Baseline aspirin (considered as low-dose daily intake for cardiovascular prevention) (yes vs no).10 19
Baseline β-blockers (yes vs no).10 20 21
Baseline metformin (yes vs no) and other oral antidiabetics (yes vs no).10 22
Statistical analysis
The sample size was estimated only for the pembrolizumab cohort, on the basis of the expected number of patients on baseline ATB. We hypothesized a 10% prevalence of ATB therapy and assumed a possible survival benefit for the non-ATB group with a reduction of the risk of death by 55%. With a probability of type I error of 0.05 and of type II error of 0.20, 255 total events were necessary and at least 615 patients had to be recruited overall from the original cohort.
Baseline patient characteristics were reported with descriptive statistics (means, medians and proportions) as appropriate. The χ2 test was used to compare categorical variables and ORRs between the two cohorts, and to evaluate the associations between concomitant baseline medication and ECOG-PS. PFS and OS were evaluated using the Kaplan-Meier method, with differences being estimated using the log-rank test. Duration of follow-up was calculated according to the reverse Kaplan-Meier method. Logistic regression was used for the univariable and multivariable analysis of ORR and to compute ORs with 95% CIs. Cox proportional hazards regression was used for the univariable and multivariable analysis of PFS and OS and to compute the HRs with 95% CIs. The alpha level for all analyses was set to p<0.05, without correction for multiplicity. All statistical analyses were performed using MedCalc Statistical Software V.19.3.1 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2020).
Results
Patient characteristics
In total, 950 patients were included in the pembrolizumab cohort, and 595 patients were included in the chemotherapy cohort. Within the chemotherapy cohort, 545 patients (91.6%) received platinum-based doublets, while 50 patients received single-agent chemotherapy (8.4%). Table 1 summarizes patient characteristics and concomitant baseline medications of both cohorts. There was a significantly higher proportion of elderly patients (≥70 years old) in the pembrolizumab cohort, compared with the chemotherapy cohort (50.8% vs 40.2%, p<0.0001), as well as of patients with an ECOG-PS ≥2 (17.4% vs 13.3%, p<0.0001). There was a lower proportion of patients receiving baseline corticosteroids (24.0% vs 29.9%, p=0.0102) and a higher proportion of patients receiving beta-blockers (27.2% vs 19.2%, p=0.0003) within the pembrolizumab cohort, compared with the chemotherapy cohort. Overall, 307 patients (51.6%) in the chemotherapy cohort received programmed death-1 (PD-1)/PD-L1 checkpoint inhibitors as later line of treatment. The median follow-up was 21.8 months (95% CI 20.5 to 37.3) for the pembrolizumab cohort and 39.3 months (95% CI 33.1 to 86.7) for the chemotherapy cohort. In both the pembrolizumab and chemotherapy cohorts, a higher baseline ECOG-PS was significantly associated with corticosteroids (p<0.0001 and p=0.0001, respectively) and PPIs (p=0.0192 and p=0.0059, respectively), but not with ATB (p=0.1209 and p=0.1285, respectively) (online supplemental table S2).
Table 1.
Patient characteristics
| Pembrolizumab cohort 950 N° (%) |
Chemotherapy cohort 595 N° (%) |
||
| Age (years) | χ2 test | ||
| Median | 70.1 | 67 | p<0.0001 |
| Range | 28–92 | 31–91 | |
| Elderly (≥70) | 483 (50.8) | 239 (40.2) | |
| Gender | p=0.4314 | ||
| Male | 625 (65.8) | 403 (67.7) | |
| Female | 325 (34.2) | 192 (32.3) | |
| ECOG-PS | p=0.0319 | ||
| 0–1 | 785 (82.6) | 516 (86.7) | |
| ≥2 | 165 (17.4) | 79 (13.3) | |
| Histology | p=0.4097 | ||
| Squamous | 210 (22.1) | 121 (20.3) | |
| Non-squamous | 740 (77.9) | 474 (79.7) | |
| Smoking status | p=0.5094 | ||
| Never smokers | 103 (10.8) | 71 (11.9) | |
| Current/former smokers | 847 (89.2) | 524 (88.1) | |
| CNS metastases | p=0.9766 | ||
| Yes | 173 (18.2) | 108 (18.2) | |
| No | 777 (81.8) | 487 (81.8) | |
| Bone metastases | p=0.0753 | ||
| Yes | 319 (33.6) | 174 (29.2) | |
| No | 631 (66.4) | 421 (70.8) | |
| Liver metastases | p=0.5615 | ||
| Yes | 146 (15.4) | 85 (14.3) | |
| No | 804 (84.6) | 510 (85.7) | |
| Corticosteroids | p=0.0102 | ||
| No | 722 (76.1) | 417 (70.1) | |
| Yes | 228 (24.0) | 178 (29.9) | |
| Antibiotics | p=0.6475 | ||
| No | 819 (86.2) | 508 (85.4) | |
| Yes | 131 (13.8) | 87 (14.6) | |
| PPIs | p=0.1208 | ||
| No | 476 (50.1) | 274 (46.1) | |
| Yes | 474 (49.9) | 321 (53.69) | |
| Statins | p=0.0701 | ||
| No | 698 (73.5) | 462 (77.6) | |
| Yes | 252 (26.5) | 133 (22.4) | |
| Aspirin | p=0.0746 | ||
| No | 696 (73.3) | 460 (77.3) | |
| Yes | 254 (26.7) | 135 (22.7) | |
| β-blockers | p=0.0003 | ||
| No | 692 (72.8) | 481 (80.8) | |
| Yes | 258 (27.2) | 114 (19.2) | |
| Metformin | p=0.0556 | ||
| No | 825 (86.8) | 536 (90.1) | |
| Yes | 125 (13.2) | 59 (9.9) |
CNS, central nervous system; ECOG-PS, Eastern Cooperative Oncology Group-Performance Status; PPIs, proton pump inhibitors.
jitc-2021-002421supp002.pdf (33.5KB, pdf)
Impact of baseline medications within the pembrolizumab cohort
Table 2 summarizes the univariable and multivariable analyses for ORR, PFS and OS according to each medication category within the pembrolizumab cohort. Multivariable analyses revealed baseline corticosteroids (OR=0.42 (95% CI 0.28 to 0.62); p<0.0001), ATB (OR=0.57 (95% CI 0.37 to 0.87); p=0.0093) and PPIs (OR=0.63 (95% CI 0.48 to 0.84); p=0.0014) to significantly correlate with a reduced probability of radiological response. Baseline corticosteroids (HR=1.69 (95% CI 1.42 to 2.03); p<0.0001), ATB (HR=1.29 (95% CI 1.04 to 1.59); p=0.0192) and PPIs (HR=1.32 (95% CI 1.13 to 1.54); p=0.0003) were also significantly associated to a higher risk of disease progression. Concordantly, corticosteroids (HR=1.93 (95% CI 1.59 to 2.35); p<0.0001), ATB (HR=1.42 (95% CI 1.13 to 1.79); p=0.0024) and PPIs (HR=1.49 (95% CI 1.26 to 1.77); p<0.0001) were significantly related to a higher risk of death. No association with clinical outcomes was found according to baseline administration of statins, aspirin, β-blockers, and metformin.
Table 2.
Univariate and multivariate analyses of objective response rate, progression free survival and overall survival within the pembrolizumab cohort according to each baseline medication
| Variable (comparator) |
Objective response rate | Progression free survival | Overall survival | |||
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|
| OR (95% CI); p value |
aOR (95% CI); p value |
HR (95% CI); p value |
aHR (95% CI); p value |
HR (95% CI); p value |
aHR (95% CI); p value |
|
| Corticosteroids Yes vs No |
0.42 (0.29 to 0.59); p<0.0001 |
0.42 (0.28 to 0.62); p<0.0001 |
1.89 (1.60 to 2.25); p<0.0001 |
1.69 (1.42 to 2.03); p<0.0001 |
2.15 (1.78 to 2.59); p<0.0001 |
1.93 (1.59 to 2.35); p<0.0001 |
| Antibiotics Yes vs No |
0.53 (0.35 to 0.81); p=0.0032 |
0.57 (0.37 to 0.87); p=0.0093 |
1.31 (1.06 to 1.62); p=0.0110 |
1.29 (1.04 to 1.59); p=0.0192 |
1.47 (1.17 to 1.84); p=0.0009 |
1.42 (1.13 to 1.79); p=0.0024 |
| PPIs Yes vs No |
0.63 (0.47 to 0.82); p=0.0008 |
0.63 (0.48 to 0.84); p=0.0014 |
1.36 (1.17 to 1.59); p=0.0001 |
1.32 (1.13 to 1.54); p=0.0003 |
1.51 (1.28 to 1.80); p<0.0001 |
1.49 (1.26 to 1.77); p<0.0001 |
| Statins Yes vs No |
1.15 (0.85 to 1.56); p=0.3407 |
– | 0.99 (0.83 to 1.17); p=0.9250 |
– | 1.06 (0.88 to 1.29); p=0.4908 |
– |
| Aspirin Yes vs No |
1.21 (0.89 to 1.63); p=0.2240 |
– | 1.01 (0.85 to 1.20); p=0.8858 |
– | 1.06 (0.87 to 1.28); p=0.5264 |
– |
| β-blockers Yes vs No |
1.17 (0.86 to 1.58); p=0.3000 |
– | 1.03 (0.86 to 1.22); p=0.7260 |
– | 1.03 (0.85 to 1.25); p=0.7085 |
– |
| Metformin Yes vs No |
0.83 (0.55 to 1.24); p=0.3626 |
– | 1.03 (0.82 to 1.29); p=0.7545 |
– | 1.14 (0.89 to 1.46); p=0.2902 |
– |
At the multivariate analysis, each drug category was adjusted for the pre-planned covariates separately. The pre-planned covariates were age (<70 vs ≥70 years old), gender (male vs female), Eastern Cooperative Oncology Group-Performance Status (0–1 vs ≥2), smoking status (current/former vs never smokers), central nervous system, metastases (yes vs no), bone metastases (yes vs no) and liver metastases (yes vs no).
aHR, adjusted hazard ratio; aOR, adjusted odd ratio; PPIs, proton pump inhibitors.
Different impact of baseline medications between the two cohorts
Table 3 summarizes the univariable analyses of ORR, PFS and OS according to baseline corticosteroids, ATB and PPIs for both cohorts. Within the chemotherapy cohort, neither corticosteroids (p=0.7618), ATB (p=0.5018), nor PPIs (p=0.7292) were significantly associated with ORR. Patients on corticosteroids had a significantly higher risk of disease progression (HR=1.30 (95% CI 1.08 to 1.56), p=0.0046) and death (HR=1.58 (95% CI 1.29 to 1.94), p<0.0001), patients on PPIs had a significantly higher risk of death (HR=1.12 (95% CI 1.02 to 1.24), p=0.0139), but not of disease progression (HR=1.08 (95% CI 0.99 to 1.17), p=0.0711), while no significant association with PFS (p=0.4200), nor with OS (p=0.1116) was found for baseline ATB within the chemotherapy cohort. Table 3 also reports all the median PFS and OS values according to baseline medications for both the cohorts, while figures 1 and 2 report the survival curves for OS and PFS, respectively.
Table 3.
Summary of the univariate analyses of objective response rate, progression free survival and overall survival according to the selected baseline medications within the pembrolizumab and the chemotherapy cohorts
| Pembrolizumab cohort | Chemoterapy cohort | |||||
| CORTICOSTEROIDS | Response/ratio | ORR (95% CI) | χ2 test | Response/ratio | ORR (95% CI) | χ2 test |
| NO | 317/678 | 46.8% (41.7 to 52.2) | p<0.0001 | 123/329 | 37.4% (31.7 to 44.6) | p=0.7618 |
| YES | 52/193 | 26.9% (20.1 to 35.3) | 51/142 | 35.9% (26.7 to 47.2) | ||
| PFS (95% CI) [events] | HR (95% CI); p value | PFS (months) (95% CI) [events] | HR (95% CI); p value | |||
| NO | 9.7 months (7.8 to 11.1) [479] | 1.89 (1.60 to 2.25); p<0.0001 | 6.3 months (5.9 to 6.9) [380] | 1.30 (1.08 to 1.56); p=0.0046 | ||
| YES | 2.9 months (2.4 to 3.9) [183] | 4.4 months (3.2 to 5.3) [164] | ||||
| OS (95% CI) [events] | OS (months) (95% CI) [events] | |||||
| NO | 19.3 months (17.2 to 22.7) [370] | 2.15 (1.78 to 2.59); p<0.0001 | 18.3 months (14.9 to 18.9) [283] | 1.58 (1.29 to 1.94); p<0.0001 | ||
| YES | 5.6 months (4.1 to 8.1) [161] | 10.2 months (8.2 to 11.1) [142] | ||||
| ANTIBIOTICS | Response/ratio | ORR (95% CI) | χ2 test | Response/ratio | ORR (95% CI) | χ2 test |
| NO | 332/748 | 44.4% (39.7 to 49.4) | p=0.0029 | 151/402 | 37.6% (31.8 to 44.0) | p=0.5018 |
| YES | 37/123 | 30.1% (21.1 to 41.4) | 23/69 | 33.3% (21.1 to 50.0) | ||
| PFS (95% CI) [events] | HR (95% CI); p value | PFS (months) (95% CI) [events] | HR (95% CI); p value | |||
| NO | 7.5 months (6.3 to 9.1) [560] | 1.31 (1.06 to 1.62); p=0.0110 | 5.9 months (5.4 to 6.4) [465] | 1.10 (0.86 to 1.40); p=0.4200 | ||
| YES | 4.8 months (3.3 to 6.9) [102] | 5.1 months (3.8 to 6.4) [79] | ||||
| OS (95% CI) [events] | OS (months) (95% CI) [events] | |||||
| NO | 17.2 months (14.8 to 19.5) [442] | 1.47 (1.17 to 1.84); p=0.0009 | 14.9 months (12.7 to 17.2) [359] | 1.23 (0.95 to 1.61); p=0.1116 | ||
| YES | 10.4 months (6.1 to 13.7) [89] | 13.2 months (9.7 to 17.3) [66] | ||||
| PPIs | Response/ratio | ORR (95% CI) | χ2 test | Response/ratio | ORR (95% CI) | χ2 test |
| NO | 213/445 | 47.9% (41.6 to 54.7) | p=0.0008 | 89/236 | 37.7% (30.3 to 46.4) | p=0.7292 |
| YES | 156/426 | 36.6% (31.1 to 42.8) | 85/235 | 36.2% (28.9 to 44.7) | ||
| PFS (95% CI) [events] | HR (95% CI); p value | PFS (months) (95% CI) [events] | HR (95% CI); p value | |||
| NO | 10.3 months (7.5 to 13.1) [314] | 1.36 (1.17 to 1.59); p=0.0001 | 5.9 months (5.7 to 6.9) [245] | 1.08 (0.99 to 1.17); p=0.0711 | ||
| YES | 5.4 months (4.6 to 6.1) [348] | 5.5 months (4.4 to 6.2) [299] | ||||
| OS (95% CI) [events] | OS (months) (95% CI) [events] | |||||
| NO | 20.4 months (18.1 to 23.7) [370] | 1.51 (1.28 to 1.80); p<0.0001 | 17.7 months (13.9 to 18.7) [119] | 1.12 (1.02 to 1.24); p=0.0139 | ||
| YES | 10.7 months (9.2 to 13.4) [161] | 12.3 months (11.0 to 15.9) [17] | ||||
PPIs, proton pump inhibitors.
Figure 1.
Kaplan-Meier survival estimates for overall survival according to the selected baseline medications within the two cohorts. Pembrolizumab cohort: corticosteroids (A), antibiotics (B), proton pump inhibitors (PPIs) (C). Chemotherapy cohort: corticosteroids (D), antibiotics (E), PPIs (F).
Figure 2.
Kaplan-Meier survival estimates for progression free survival according to the selected baseline medications within the two cohorts. Pembrolizumab cohort: corticosteroids (A), antibiotics (B), proton pump inhibitors (PPIs) (C). Chemotherapy cohort: corticosteroids (D), antibiotics (E), PPIs (F).
Online supplemental tables S3–S5 summarize the multivariable regression analyses from the pooled population for OS, PFS and ORR, respectively. At the pooled analysis, the interaction term with the therapeutic modality was statistically significant for corticosteroids (p=0.0020) and PPIs (p=0.0460) with respect to OS (online supplemental table S3), for corticosteroids (p<0.0001), ATB (p=0.0290) and PPIs (p=0.0487) with respect to PFS (online supplemental table S4), and only corticosteroids (p=0.0033) with respect to ORR (online supplemental table S5).
jitc-2021-002421supp003.pdf (33KB, pdf)
jitc-2021-002421supp004.pdf (33KB, pdf)
jitc-2021-002421supp005.pdf (32.8KB, pdf)
Discussion
PD-1 inhibitors have reshaped the landscape of NSCLC treatment as a monotherapy and in combination with chemotherapy. While PD-L1 is an imperfect biomarker, patients with PD-L1 expression ≥50% are characterized by a favorable response to pembrolizumab monotherapy in first-line. However, PD-L1 expression status is not the sole determinant of response, and concomitant baseline medications may impair the effectiveness of ICI in this exquisitely ICI-sensitive patient subpopulation.
This study is the first to offer a comprehensive analysis of the role of concomitant baseline medications in a large, real-world cohort of patients with NSCLC with a PD-L1 expression ≥50% treated with pembrolizumab monotherapy. The most striking and practice-informing finding is that antibiotic therapy exerts a detrimental effect on ORR, PFS and OS exclusively in patients treated with pembrolizumab monotherapy but not with chemotherapy. This is an important step forward in understanding the mechanistic basis of such relationship, adding further evidence to the interpretation that ATB might act as true immune-modulators rather than by masking an unrecognized association with underlying adverse prognostic features. Prescription of ATB was in fact independent from patients’ performance status in the study population. Additionally, two recent study have independently confirmed that ATB therapy concordantly affect the gut microbiome composition, impairing clinical outcomes with ICI in renal cell carcinoma and patients with NSCLC.23 24
Unlike ATB, corticosteroids and PPIs were associated with worse outcome across therapeutic modality. Within the chemotherapy cohort PPIs significantly affected OS, and corticosteroids retain their negative effect on PFS and OS. An important study published by Ricciuti et al11 has highlighted the relevance of indication for corticosteroid therapy in dictating their relationship with prognosis, a finding that was replicated across different malignancies.10 25 While PPIs have been proposed to modify the gut microbiome,26 it should be noted that this class of drugs is often coadministered with corticosteroids, making it difficult to fully appreciate whether the detrimental role is linked to the effect of steroids. However, for both corticosteroids and PPIs, we found a statistically significant interaction with the type of anticancer treatment. Our pooled analysis, confirmed that even though concomitant baseline medications exert a similar role within the two cohorts, the magnitude of the effect was different between pembrolizumab and chemotherapy treated patients.
Interestingly, we did not report a significant interaction for ATB on OS, when therapeutic modality (chemotherapy vs pembrolizumab) was tested as an interaction term. This finding does not detract from the analyses of ATB in the two independent cohorts, where the effect of ATB on OS was restricted to ICI-recipients. Patient heterogeneity and the relative small proportion of patients on ATB in both cohorts might explain the results. In addition, over 50% of chemotherapy recipients were subsequently treated with ICIs in second line, highlighting that post-progression treatments might have mitigated the differences across subgroups, while PFS analysis provide more reliable evidence on the putative role of ATB.
Unlike previous studies, where baseline use of β-blockers, aspirin and statins were somehow associated with improved outcomes,10 18–21 our analysis has not reproduced these findings in NSCLC. Likewise, our study further confirmed that metformin does not seem affect response and survival of patients with NSCLC receiving ICI.10 Even though the alleged immune-modulating effect of these medications have not been reproducibly confirmed, these differences could be also related to the differences between the study populations (patients with NSCLC receiving first-line pembrolizumab only).
Our study acknowledges several limitations beyond the retrospective design and the consequent selection bias, which could have impaired also the retrieving process about baseline medications. The chemotherapy and pembrolizumab cohorts were biologically and clinically heterogeneous, and their sample size was also significantly different. As likely result of the clinicians’ attitude to reserve chemotherapy for fitter patients, there was a higher proportion of elderly patients and with an ECOG-PS ≥2 within the chemotherapy cohort. While we purposely included among it, patients receiving single agent chemotherapy, in order to achieve a better balance regarding baseline functional status, the majority of them was treated with platinum-based doublets, which has historically been the standard approach for first-line treatment of NSCLC. Our choice might have affected the results too, as patients treated with single agent chemotherapy might be characterized by unique features of frailty, eventually affecting concomitant medications.
As other possible source of bias, we have to state that even though we aimed at being comprehensive with respect of baseline medications, some of them, including PPIs, statins, aspirin, β-blockers and metformin, are generally indefinite prescriptions, and their exact time frame might be unretrievable also in prospective clinical trials. Additionally, we were not able to discriminate the potential different impact of timing and duration (within the 30 days before pembrolizumab initiation) for neither corticosteroids nor ATB. As specified within the methods, we excluded chemotherapy premedication with corticosteroids on purpose, as it was administered to all the chemotherapy recipients and would thus have unabled any form of comparative analysis. However, in the way we analyzed them, corticosteroids retained their negative impact in both the cohorts, preserving the interpretation of the results. Moreover, immune-suppressive effects of corticosteroids are known to be dose and time-dependent.27 With respect to ABT, even though some evidence suggested that spectrum, duration and route have their own role affecting the gut microbiome,28 and also the administration within broader time ranges could affect the outcome of patients with cancer treated with ICIs,23 we chose to collect them as previously done in similar studies.7 10 Additionally, we specifically chose to collect ATB up until first-line therapy initiation, excluding concurrent ATB (within the first 30 days of treatment) as done elsewhere,4 8 in order to avoid the possible lead-in time bias caused by the time-dependent nature of any concomitant medication. Regretfully, we did not have detailed data about specific antibiotic class within out cohort.
We have to consider also the different time period of data collection and the different median follow-up of the two cohorts. Furthermore, even though it is reasonable to think that PD-L1 expression does not affect the impact of concomitant baseline medications on clinical outcomes, we lack PD-L1 expression data for the chemotherapy cohort, and it is plausible that a greater sample size is needed to obtain a significant effect according to a baseline characteristic (such as concomitant medications) on clinical outcomes among a biomarker selected population. Nevertheless, considering the real-world prevalence of PD-L1 expression in NSCLC, we can presume that 30% of them had a high PD-L1 tumor expression.29
Conclusion
In spite of the acknowledged limitations, our study provides novel clinical evidence to support the detrimental effect of ATB in patients with NSCLC treated with pembrolizumab monotherapy. Restriction of this relationship to the pembrolizumab cohort adds indirect but important confirmatory evidence as to their potential immune-modulatory effect. While a direct relationship between ATB and disruption of the gut microbiome cannot be proven in our study, the differential effect seen for ATB, corticosteroids and PPIs points towards different levels of biological plausibility for their association with adverse outcomes. Although a significant interaction was shown for corticosteroids and PPI, outcomes of patients assuming these drugs was worse even in patients receiving chemotherapy, and this might suggest an associative link more than (or in addition to) a causative link. As mechanistic evidence around the relationship between ATB and the gut microbiome evolves, clinicians should continue to exert judicious use of ATB in the context of ICI treatments.
Acknowledgments
AC and DJP would like to acknowledge the infrastructure support provided by Imperial Experimental Cancer Medicine Centre, Cancer Research UK Imperial Centre and the Imperial College Healthcare NHS Trust Tissue Bank.
Footnotes
Twitter: @emilio.bria, @Al3ssandroRusso, @gbanna74
Contributors: All authors contributed to the publication according to the ICMJE guidelines for the authorship (study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision). All authors read and approved the submitted version of the manuscript (and any substantially modified version that involves the author’s contribution to the study). Each author have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved and the resolution documented in the literature.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AC received speaker fees and grant consultancies by Astrazeneca, MSD, BMS, Roche, Novartis, Istituto Gentili and Astellas. RG received speaker fees and grant consultancies by Astrazeneca and Roche. JA reports receiving commercial research grants from Amphera and Roche, holds ownership interest (including patents) in Amphera BV, and is a consultant/advisory board member for Amphera, Boehringer Ingelheim, Bristol-Myers Squibb, Eli-Lilly, MSD and Roche. AF received grant consultancies by Roche, Pfizer, Astellas and BMS. FM received grant consultancies by MSD and Takeda. RC received speaker fees by BMS, MSD, Takeda, Pfizer, Roche and Astrazeneca. CG received speaker fees/grant consultancies by Astrazeneca, BMS, Boehringer-Ingelheim, Roche and MSD. MR received honoraria for scientific events by Roche, Astrazeneca, BMS, MSD and Boehringer Ingelheim. EB received speaker and travel fees from MSD, Astra-Zeneca, Pfizer, Helsinn, Eli-Lilly, BMS, Novartis and Roche; grant consultancies by Roche and Pfizer. MCG received grants from MSD, Astrazeneca, Novartis, Roche, Pfizer, Celgene, Tiziana Sciences, Clovis, Merck, Bayer, GSK, Spectrum, Blueprint, personal fees from Eli Lilly, Boheringer, Otsuka Pharma, Astrazeneca, Novartis, BMS, Roche, Pfizer, Celgene, Incyte, Inivata, Takeda, Bayer, MSD, Sanofi, Seattle Genetics, Daichii Sankyo, other financial supports from Eli Lilly, Astrazeneca, Novartis, BMS, Roche, Pfizer, Celgene, Tiziana Sciences, Clovis, Merck Serono, MSD, GSK, Spectrum and Blueprint. AA received grant consultancies by Takeda, MSD, BMJ, Astrazeneca, Roche and Pfizer. MDM received research funding from Tesaro-GlaxoSmithKline; acted in a consulting/advisory role for Novartis, Pfizer, Eisai, Takeda, Janssen, Astellas, Roche, AstraZeneca. FP received grant consultancies by MSD and Astrazeneca. PB received grant consultancies by Astrazeneca and Boehringer-Ingelheim. DJP received lecture fees from ViiV Healthcare, Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, Astra Zeneca; received research funding (to institution) from MSD, BMS. All other authors declare no competing interests. DJP is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and has received direct project funding by the NIHR Imperial Biomedical Research Centre (BRC), ITMAT Push for Impact Grant Scheme 2019. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The datasets used during the present study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All patients provided written, informed consent to treatment with immunotherapy. The procedures followed were in accordance with the precepts of Good Clinical Practice and the declaration of Helsinki. The study was approved by the respective local ethical committees on human experimentation of each institution, after previous approval by the coordinating center (Comitato Etico per le province di L’Aquila e Teramo, verbale N.15 del Novembre 28, 2019).
References
- 1.Hussain N, Naeem M, Pinato DJ. Concomitant medications and immune checkpoint inhibitor therapy for cancer: causation or association? Hum Vaccin Immunother 2021;17:55–61. 10.1080/21645515.2020.1769398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopalakrishnan V, Helmink BA, Spencer CN, et al. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018;33:570–80. 10.1016/j.ccell.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malmberg K-J. Effective immunotherapy against cancer: a question of overcoming immune suppression and immune escape? Cancer Immunol Immunother 2004;53:879–92. 10.1007/s00262-004-0577-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018;29:1437–44. 10.1093/annonc/mdy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed Death-Ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 6.Petrelli F, Signorelli D, Ghidini M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers 2020;12:546. 10.3390/cancers12030546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol 2019;5:1774–8. 10.1001/jamaoncol.2019.2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the oak and poplar trials. Ann Oncol 2020;31:525–31. 10.1016/j.annonc.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Homicsko K, Richtig G, Tuchmann F, et al. Proton pump inhibitors negatively impact survival of PD-1 inhibitor based therapies in metastatic melanoma patients. Annals of Oncology 2018;29:x40. 10.1093/annonc/mdy511.001 [DOI] [Google Scholar]
- 10.Cortellini A, Tucci M, Adamo V, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer 2020;8:e001361. 10.1136/jitc-2020-001361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricciuti B, Dahlberg SE, Adeni A, et al. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus Nonpalliative indications. J Clin Oncol 2019;37:1927–34. 10.1200/JCO.19.00189 [DOI] [PubMed] [Google Scholar]
- 12.Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother 2020;69:2209–21. 10.1007/s00262-020-02613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortellini A, Friedlaender A, Banna GL, et al. Immune-related Adverse Events of Pembrolizumab in a Large Real-world Cohort of Patients With NSCLC With a PD-L1 Expression ≥ 50% and Their Relationship With Clinical Outcomes. Clin Lung Cancer 2020;21:498–508. 10.1016/j.cllc.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 14.Banna GL, Cortellini A, Cortinovis DL. The Lung Immuno-oncology Prognostic Score (LIPS-3): a prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small cell lung cancer. ESMO Open 2021;15;6(2):100078. 10.1016/j.esmoop.2021.100078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortellini A, Ricciuti B, Tiseo M, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer 2020;8:e001403. 10.1136/jitc-2020-001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortellini A, De Giglio A, Cannita K, et al. Smoking status during first-line immunotherapy and chemotherapy in NSCLC patients: a case-control matched analysis from a large multicenter study. Thorac Cancer 2021;12:880–9. 10.1111/1759-7714.13852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das V. Interpreting the coefficients of a regression model with an interaction term: a detailed explanation. Available: https://levelup.gitconnected.com/interpreting-the-coefficients-of-a-regression-model-with-an-interaction-term-a-detailed-748a5e031724 [Accessed 12 Feb 2021].
- 18.Cantini L, Pecci F, Hurkmans DP, et al. High-Intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur J Cancer 2021;144:41–8. 10.1016/j.ejca.2020.10.031 [DOI] [PubMed] [Google Scholar]
- 19.Botti G, Fratangelo F, Cerrone M, et al. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J Transl Med 2017;15:46. 10.1186/s12967-017-1150-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol 2018;4:e172908. 10.1001/jamaoncol.2017.2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokolus KM, Zhang Y, Sivik JM, et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2017;7:e1405205. 10.1080/2162402X.2017.1405205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer 2018;6:64. 10.1186/s40425-018-0375-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derosa L, Routy B, Fidelle M, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol 2020;78:195–206. 10.1016/j.eururo.2020.04.044 [DOI] [PubMed] [Google Scholar]
- 24.Hakozaki T, Richard C, Elkrief A, et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res 2020;8:1243–50. 10.1158/2326-6066.CIR-20-0196 [DOI] [PubMed] [Google Scholar]
- 25.Pinato DJ, Kaseb A, Wang Y, et al. Impact of corticosteroid therapy on the outcomes of hepatocellular carcinoma treated with immune checkpoint inhibitor therapy. J Immunother Cancer 2020;8:e000726. 10.1136/jitc-2020-000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson MA, Goodrich JK, Maxan M-E, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749–56. 10.1136/gutjnl-2015-310861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasir M, Goyal A, Bansal P. Corticosteroid Adverse Effects. : StatPearls. Treasure Island (FL): StatPearls Publishing, 2021. https://www.ncbi.nlm.nih.gov/books/NBK531462/ [PubMed] [Google Scholar]
- 28.Schwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med 2020;12:82. 10.1186/s13073-020-00782-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietel M, Savelov N, Salanova R, et al. Real-World prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter express study. Lung Cancer 2019;134:174–9. 10.1016/j.lungcan.2019.06.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002421supp001.pdf (112.7KB, pdf)
jitc-2021-002421supp002.pdf (33.5KB, pdf)
jitc-2021-002421supp003.pdf (33KB, pdf)
jitc-2021-002421supp004.pdf (33KB, pdf)
jitc-2021-002421supp005.pdf (32.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets used during the present study are available from the corresponding author upon reasonable request.