Abstract
Brain-derived neurotrophic factor (BDNF) is involved in the regulation of neuronal cell growth, differentiation, neuroprotection and synaptic plasticity. Although aberrant BDNF/TrkB signaling is implicated in several neurological, neurodegenerative and psychiatric disorders, neurotrophin-based therapy is challenging and is limited by improper pharmacokinetic properties of BDNF. Dimeric dipeptide compound GSB-106 (bis-(N-monosuccinyl-l-seryl-l-lysine) hexamethylenediamide) has earlier been designed to mimic the TrkB-interaction 4 loop of BDNF. It displayed protective effect in various cell-damaging models in vitro. Animal studies uncovered antidepressive and neuroprotective properties upon GSB-106 per os administration. Current study shows that GSB-106 acts similarly to BDNF, promoting survival of serum-deprived neuronal-like SH-SY5Y cells. 100 nmol concentration of GSB-106 provided maximum neurotrophic effect, which corresponds to about 37% of the maximum effect provided by BDNF. Protective properties of GSB-106 arise from its ability to counteract cell apoptosis via activation of TrkB-dependent pro-survival mechanisms, including inactivation of pro-apoptotic BAD protein and suppression of caspases 9 and 3/7. Thus, our study has characterized neurotrophic activity of small dimeric compound GSB-106, which mimics certain biological functions of BDNF and neurotrophin-specific protective mechanisms. GSB-106 also displays similarities to some known low weight peptide and non-peptide TrkB ligands.
Subject terms: Biochemistry, Drug discovery, Neuroscience
Introduction
Brain-derived neurotrophic factor (BDNF) is a secreted protein that serves neuronal survival, cell differentiation and synaptic plasticity through interactions with tropomyosin-related kinase-B receptor (TrkB) and tumor necrosis factor receptor superfamily member 16 (p75NTR)1. BDNF binding to TrkB evokes receptor dimerization and initial phosphorylation of tyrosine residues within the autoregulatory loop of the kinase domain (human TrkB Tyr706/707) followed by autophosphorylation of cytoplasmic conserved tyrosine residues (human TrkB Tyr515, Tyr816)2. Phosphorylated TrkB Tyr516 through the interaction with scaffold protein Shc (Src homologous and collagen-like) mediates the activation of PI3K (Phosphatidylinositol 3-kinase) via Grb2 (Growth factor receptor-bound protein 2) and Gab1 (Grb2-associated binder-1) proteins, which in turn leads to Akt (Rac-alpha serine/threonine-protein kinase) activation, promotion of Akt-dependent neurotrophin survival effects and increased protein translation (regulated by mTOR-p70S6 kinase). Additionally, a Shc binding site at Tyr515 initiates Ras/Raf/MEK/Erk cascade responsible for the neuronal differentiation, neuronal outgrowth and synaptic plasticity via recruitment of Grb2 and SOS (Son of sevenless) proteins1. Phosphorylation of Tyr816 leads to PLC-γ1 (phospholipase Cγ) activation, which through DAG (diacylglycerol) and IP3 (inositol tris–phosphate) results in activation of Ca2+—and protein kinase C-regulated pathways that facilitate synaptic plasticity1.
In addition to binding with TrkB BDNF also engages p75NTR and this interaction mediate distinct outcomes, depending on the cellular milieu. In brief, when TrkB and p75NTR co-express in the same cells, p75NTR enhances neurotrophin binding to TrkB, augmenting both ligand affinity and selectivity, hence, reinforcing the TrkB-mediated trophic response via up-regulation of PI3K/Akt and/or IRAK/NF-κB signaling3. Moreover, in specific context, p75NTR can activate TrkB-independent pro-survival signals, which involve activation of transcription factor NF-κB and PI3K/Akt pathway4,5. Degenerative pathways would dominate, promoting cell death upon neurotrophin binding to p75NTR through activation of pro-apoptotic JNK-p53-Bax pathway or suppressing Ras/PI3K/Akt activation when conditions of either lacking or deficient Trk signaling or increased p75NTR expression occur6. Implication of p75NTR in modulation of myelin formation, neurite outgrowth and pro-neurotrophin-mediated signaling also widely discussed7.
Deficiency of neurotrophin and/or disturbances in BDNF/TrkB/p75NTR—dependent signaling contributes to pathogenesis and progression of numerous neurological and psychiatric disorders, denoting protective and regenerative properties of BDNF8. Given the important role that BDNF plays in human pathology, intensive efforts have been made to develop strategies for therapeutic applications of neurotrophins or their functional analogs. Clinical trials showed that exogenous BDNF had limited success as therapeutic, mainly due to suboptimal pharmacokinetic properties and side effects. Nevertheless, encouraging pre-clinical data have emerged for certain substances with favorable pharmacokinetic profiles, targeting the BDNF/TrkB-associated pathway to restore or balance the altered neurotrophin signaling9. A vast number of agents targeting neurotrophin receptors with different action mechanisms have been developed and studied in details in vitro and in various animal disorders models recently10–14.
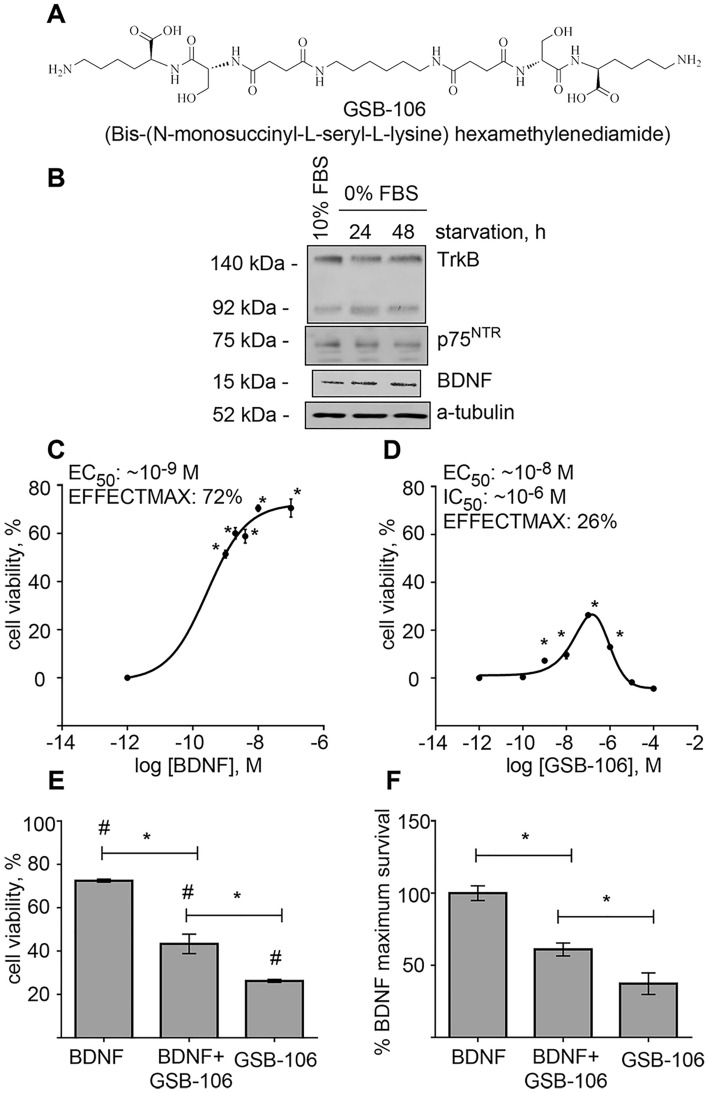
Orally available dimeric dipeptide GSB-106 (Fig. 1A; bis-(N-monosuccinyl-l-seryl-l-lysine) hexamethylenediamide) has earlier been designed based on BDNF loop 4 β-turn amino acid sequence -Asp93-Ser94-Lys95-Lys96-, where -Ser94-Lys95-residues were retained, the upstream residue -Asp93- was replaced by its bioisostere, a succinic acid residue, and -Lys96- residue was substituted by the amide group15,16. Previous studies demonstrated that GSB-106 exerted a trophic effect, up-regulated the TrkB phosphorylation as well as the downstream PI3K/Akt, MAPK/Erk and PLCγ signaling pathways in vitro17–19. It has been shown earlier that GSB-106 over concentration range of 10 nmol -1 µmol rescues HT-22 cells from oxidative stress and glutamate-induced cell death and protects SH-SY5Y cells against 6-hydroxydopamine damage20. The substance was found to possess the favorable pharmacokinetic properties, such as proteolytic stability, blood–brain barrier penetration, retention in rat blood plasma for 4 h upon single per os administration; T1/2 for GSB-106 is 0.7 h21. GSB-106 has been shown to counteract depression-like behavior in a number of rodent tests and in animal models of depression15,22–24 and exhibited pronounced neuroprotective activity in animal ischemic stroke model upon per os administration25,26.
Figure 1.
GSB-106 promotes survival of serum-deprived SH-SY5Y cells. (A) GSB-106 chemical structure. (B) BDNF, TrkB and p75NRT proteins expression in serum starved SH-SY5Y cells. Cells were incubated in serum-free culture medium (“0% FBS”) for 24 and 48 h. After incubation, cells were collected, and protein extracts were subjected to polyacrylamide gel electrophoresis and transferred for Western blotting. Blots were probed with anti-BDNF, anti-TrkB, anti-p75NTR antibodies and anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (C,D) Dose–response survival curves of SH-SY5Y cells (2 × 105/well) treated with BDNF (C) or GSB-106 (D) for 48 h in serum-free condition. Cell viability was measured by MTT metabolism. Cell viability was normalized to viability in control group (“0% FBS”) shown as “10–12 M” (*p < 0.05; n = 7; one-way ANOVA with Newman-Keul’s post-hoc test). (E) Survival of cells (2 × 105/well) incubated with BDNF (100 nmol), GSB-106 alone (100 nmol) and GSB-106 + BDNF (100 nmol for BDNF and GSB-106) in serum-free DMEM for 48 h. Cell viability was normalized to viability in control group (“0% FBS”) (*p < 0.05; #p < 0.05 in relation to control cells (“0% FBS”); n = 7; Wilcoxon t-test). (F) Survival of cells (2 × 105/well) incubated with BDNF (100 nM), GSB-106 alone (100 nmol) and GSB-106 + BDNF (100 nmol for BDNF and GSB-106) in serum-free DMEM for 48 h. Cell viability was normalized to viability in group with BDNF alone (shown as 100% on Y-axis) (*p < 0.05; n = 7; Wilcoxon t-test). Hereinafter (overall) the data is expressed as means ± S.E.M.
Our research aimed to characterize the activity profile of GSB-106 as a small molecule TrkB ligand and to find out whether GSB-106 could alleviate the cell death triggered by serum-deprivation via mechanisms similar to those of BDNF.
Results
GSB-106 provides neuroprotection from serum withdrawal-induced cell apoptosis
Neurotrophic activity of GSB-106 was studied in undifferentiated SH-SY5Y neuroblastoma cells, cultured in the serum free conditions to exclude the presence of exogenous trophic factors. Although controversial reports on the expression of TrkB in undifferentiated SH-SY5Y cells in culture exist27, we used immunoblotting to show that these cells indeed express TrkB receptor proteins during cultivation in complete serum (Fig. 1B). Expression of TrkB, p75NTR receptors and small amount of BDNF protein has also been confirmed in serum-deprived cells (Fig. 1B).
Based on the observation that SH-SY5Y cells express TrkB and p75NTR receptors, we investigated whether BDNF and its low molecular weight mimetic protect SH-SY5Y cells from serum withdrawal. Recombinant human BDNF was used as internal standard. 48 h treatment with GSB-106 alone (0.1 nmol–100 µmol) produced a bell-shaped concentration–response curve with a maximum survival effect (Emax) of 26.25 ± 0.67% at 100 nmol compared to control group (0% FBS; p < 0.05; one-way ANOVA with Newman–Keul’s post-hoc test); EC50 value is 10 nmol. Higher concentrations of the compound tended to inhibit the cell viability (IC50 of 1 µmol) (Fig. 1D). Neurotrophic activity studies with BDNF (1 nmol–1 µmol) demonstrated a sigmoidal curve with a maximum survival BDNF effect (Emax) of 72.5 ± 0.7% at 100 nmol compared to control group (0% FBS; p < 0.01; one-way ANOVA with Newman–Keul’s post-hoc test); EC50 value is 1 nmol (Fig. 1C). Statistically significant differences were established when comparing the values of the maximum effects of BDNF and GSB-106 (p < 0.05; Wilcoxon t-test). Thus, GSB-106 promoted cell survival with lower potency compared to BDNF, as evident from the half-maximum concentrations of BDNF and GSB-106, which differ by 1 order: EC50 of 1 nmol for BDNF versus EC50 10 nmol for GSB-106. To be noted, maximum neurotrophic effect was observed at 100 nmol concentrations for both BDNF and GSB-106, despite the clear differences in their efficiency (Emax of 72.5 ± 0.7% for BDNF versus Emax of 26.25 ± 0.67% for GSB-106). Obtained data indicate that survival of serum-deprived cells is dependent on the intrinsic trophic activity of GSB-106 solely, although this effect was less pronounced compared to the native neurotrophin. 100 nmol concentration of GSB-106 provided maximum neurotrophic effect, which corresponds to about 37% of the maximum effect provided by BDNF (p < 0.05; Wilcoxon t-test; Fig. 1F).
An increase of serum-depleted SH-SY5Y cells survival by 26.25 ± 0.67%, comparing with control group (0% FBS; p < 0.05; Wilcoxon t-test; Fig. 1E) was observed when GSB-106 (100 nmol) was added alone. Co-addition of GSB-106 with BDNF at concentrations, inducing maximum survival effect (100 nmol for each compound), resulted in 43.33 ± 4.48% enhancement of trophic response compared with control group (0% FBS; p < 0.05; Wilcoxon t-test; Fig. 1E), that is 60.98 ± 4.5% of BDNF maximum effect (p < 0.05; Wilcoxon t-test; Fig. 1F). It is worth noting that co-incubation with GSB-106 and BDNF raised pro-survival activity of dimeric dipeptide by 17.08 ± 1.6% (p < 0.05; Wilcoxon t-test; Fig. 1E), supposing that GSB-106 might act additively (or cooperatively) with BDNF. Competition analysis revealed that GSB-106 in the presence of saturated concentration of BDNF inhibited the maximum effect of BDNF by 39.02 ± 1.8% (p < 0.05; Wilcoxon t-test; Fig. 1F), suggesting that GSB-106 could function as partial agonist at concentration evoking maximum response.
Serum withdrawal commonly triggers cells apoptosis and represents a relevant model for studying the underlying mechanisms of neuroprotection arised from neurotrophic support28. We further examined in more depth, whether apoptosis could be downregulated by GSB-106 to elucidate the survival-promoting activity of compound in serum-deprived SH-SY5Y cells. Annexin V/SYTOX staining followed by flow cytometry has been used to detect apoptotic events. This approach allows distinguishing early apoptotic cells and late apoptotic/necrotic cells.
Upon 72-h serum starvation (0% FBS) percentage of early and late apoptotic cells has remarkably increased compared to that of cells cultured in complete serum media (10% FBS) as represented in Table 1. Treatment of serum-deprived SH-SY5Y cells with GSB-106 at concentrations of 10 nmol to 1 µmol alleviated the levels of early and late apoptosis to the nearly same extent as that of BDNF at concentration of ~ 1 nmol. Notably, an increase in both early and late apoptotic cells was shown for GSB-106 at concentrations of 10 µmol to 100 µmol, which is consistent with the data on cell viability (Fig. 1D). Taken together, these data indicates, that GSB-106 protects SH-SY5Y cells from serum withdrawal initiated apoptosis in a concentration-dependent manner.
Table 1.
GSB-106 attenuates the apoptosis of serum withdrawn SH-SY5Y cells.
| Groups | Apoptosis, % of cells | Necrosis | |
|---|---|---|---|
| Early | Late | ||
| 10% FBS | 0.5 ± 0.01 | 1.8 ± 0.1 | 2.75 ± 0.5 |
| 0% FBS | 4.32 ± 0.2 | 7.08 ± 0.09 | 2.92 ± 0.4 |
| BDNF, ~ 1 nmol | 3.85 ± 0.1* | 5.9 ± 0.1* | 2.74 ± 0.5 |
| GSB-106 | |||
| 10 nmol | 3.68 ± 0.1* | 5.85 ± 0.3* | 3.15 ± 0.2 |
| 100 nmol | 3.65 ± 0.09* | 6.2 ± 0.3* | 2.42 ± 0.6 |
| 1 µmol | 3.9 ± 0.3 | 5.1 ± 0.2* | 2.82 ± 0.4 |
| 10 µmol | 5.2 ± 0.2 | 8.91 ± 0.1 | 2.81 ± 0.5 |
| 100 µmol | 5.67 ± 0.1 | 8.18 ± 0.3 | 2.44 ± 0.3 |
SH-SY5Y cells were cultured in serum-free culture medium (0% FBS) and treated with BDNF.
(~ 1 nmol) or GSB-106 (10 nmol to 100 µmol) for 72 h. After incubation cells were collected and stained with Metabolic Activity Dead Cell Apoptosis Kit with C12 Resazurin, Annexin V APC, and SYTOX Green according to the manufacturer's recommendations. The stages of apoptosis were determined by staining: early—Annexin V+/SYTO Green−; late—Annexin V+/SYTO Green+; necrosis—Annexin V−/SYTO Green+. The data is presented as the mean ± S.E.M. of percentage of total cell population (n = 3; *p < 0.05 compared with the “0% FBS” group; Wilcoxon t-test).
GSB-106 mediates neuroprotection through Trk signaling
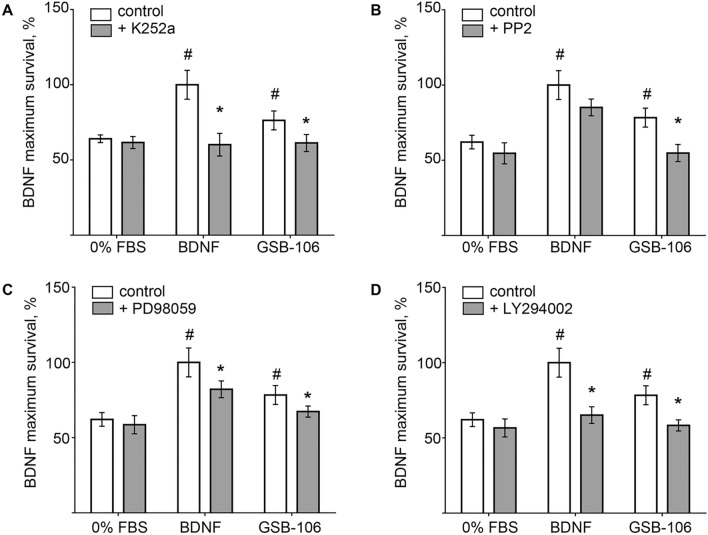
Survival responses in the presence of the kinase inhibitor K252a or the MEK1 (PD98059) and the PI3K (LY294002) inhibitors were evaluated to determine whether GSB-106 ability to support viability of serum-deprived cells is dependent on TrkB receptors activation and MAPK/Erk and PI3K/Akt pathways. K252a decreased BDNF neurotrophic activity by 40 ± 2.5% (p < 0.05; Wilcoxon t-test) and decreased GSB-106 activity by 15 ± 4.9% (p < 0.05; Wilcoxon t-test), as shown in Fig. 2A. These findings demonstrate that compound’s activity partially depends on TrkB kinase activation and could suggest also non-TrkB mediated effects of GSB-106. Indeed, Src kinases inhibitor PP2 (Fig. 2B) reduced the BDNF-stimulated cell viability by 15 ± 5.5% (p < 0.05; Wilcoxon t-test), whereas GSB-106-stimulated cell survival was inhibited by 23.5 ± 4.9% (p < 0.05; Wilcoxon t-test), intimating the plausible contribution of Src kinase-dependent cell survival mechanisms. Applying PD98059 (Fig. 2C) to serum-free cells blocked the survival promoted by BDNF or GSB-106 by 18 ± 5.5% (p < 0.05; Wilcoxon t-test) and by 14 ± 4.9% (p < 0.05; Wilcoxon t-test) respectively. LY294002 (Fig. 2D) inhibited BDNF-stimulated cell viability by 35 ± 6.8% (p < 0.05; Wilcoxon t-test) and GSB-106-supported survival by 20 ± 7.9% (p < 0.05; Wilcoxon t-test). Notably, PD98059 affected BDNF- or GSB-106-promoted survival to a lesser extent compared to LY294002, which is consistent with previous observations29. Although these inhibitors are not completely specific, obtained data suggests dependence of GSB-106-promoted survival on TrkB activation/Src-regulated transactivation and downstream MAPK/Erk, PI3K/Akt signaling.
Figure 2.
GSB-106 promotes survival of serum-deprived SH-SY5Y cells through TrkB and downstream signaling pathways. (A) Survival analysis of serum-deprived SH-SY5Y cells (2 × 105/well) incubated with BDNF (1 nmol), GSB-106 (100 nmol) in the presence of K252a (500 nmol) for 48 h. Cell viability was normalized to viability in BDNF group (n = 5; #p < 0.05 in relation to 0% FBS-control; *p < 0.05; in relation to agonist without inhibitor; Wilcoxon t-test). (B) Survival analysis of serum-deprived SH-SY5Y cells (2 × 105/well) incubated with BDNF (1 nmol), GSB-106 (100 nmol) in the presence of PP2 (100 μmol) for 48 h. Cell viability was normalized to viability in BDNF group (n = 5; #p < 0.05 in relation to 0% FBS-control; *p < 0.05; in relation to agonist without inhibitor; Wilcoxon t-test). (C) Survival analysis of serum-deprived SH-SY5Y cells (2 × 105/well) incubated with BDNF (1 nmol), GSB-106 (100 nmol) in the presence of PD98059 (50 μmol) for 48 h. Cell viability was normalized to viability in BDNF group (n = 5; #p < 0.05 in relation to 0% FBS-control; *p < 0.05; in relation to agonist without inhibitor; Wilcoxon t-test). (D) Survival analysis of serum-deprived SH-SY5Y cells (2 × 105/well) incubated with BDNF (1 nmol), GSB-106 (100 nmol) in the presence of LY294002 (1 μmol) for 48 h. Cell viability was normalized to viability in BDNF group (n = 5; #p < 0.05 in relation to 0% FBS-control; *p < 0.05; in relation to agonist without inhibitor; Wilcoxon t-test).
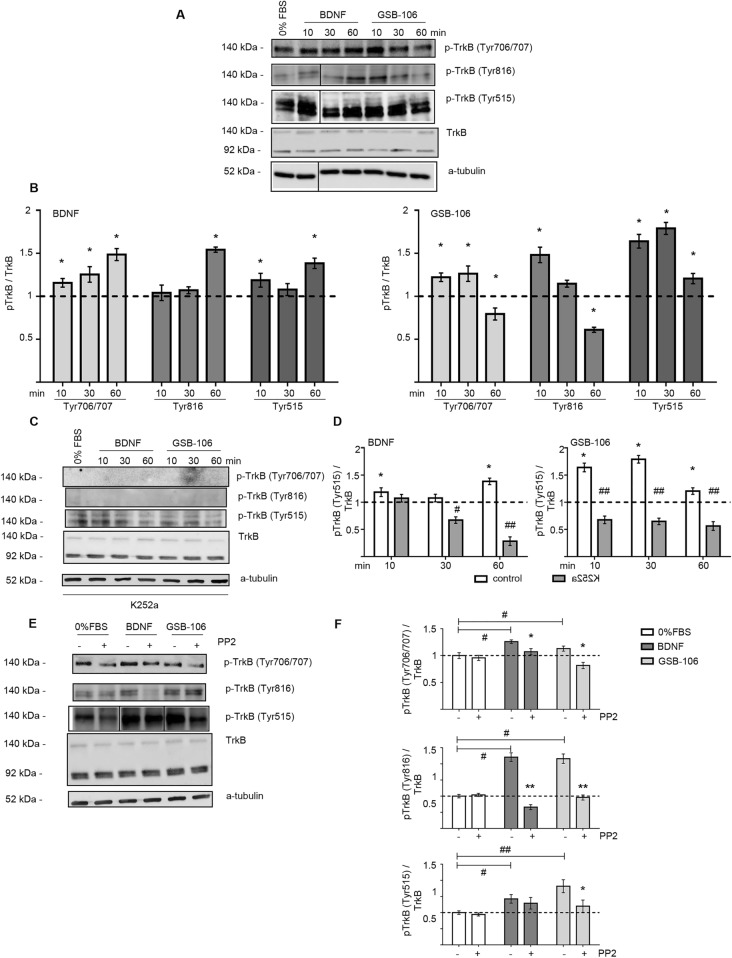
Immunoblotting of TrkB with antibodies to specific phospho tyrosines has been performed to ascertain whether TrkB phosphorylation is induced by GSB-106. Figures 3A,B show that GSB-106 induced the transient and similar to BDNF moderate TrkB Tyr706/707 phosphorylation within 10 min, followed by a decrease at 60 min, whilst BDNF caused substantial Tyr706/707 phosphorylation at 60 min. The opposite time-dependent activation of Tyr816 was also observed. Maximum of Tyr816 phosphorylation (48.1 ± 9%; p < 0.05; Wilcoxon t-test) by GSB-106 has been detected at 10 min, and pick of BDNF-triggered activation (54.2 ± 2%; p < 0.05; Wilcoxon t-test) was observed at 60 min. Tyr515 phosphorylation was affected significantly more by GSB-106, than by BDNF. Particularly, the enhanced level of Tyr515 activation appeared as early as 10 min (64.0 ± 8%; p < 0.05; Wilcoxon t-test) and maintained for up to 60 min, although BDNF caused an increase in phosphorylation at this site only after 60 min (38.4 ± 6%; p < 0.05; Wilcoxon t-test).
Figure 3.
The neuroprotective effect of GSB-106 is mediated through the activation of TrkB. (A) SH-SY5Y cells were cultured in serum-free culture medium (“0% FBS” for 24 h) with or without BDNF (28.6 ng/ml, ~ 1 nmol) or GSB-106 (100 nmol). After incubation with BDNF or GSB-106 for 10, 30, 180 min cells were collected, and protein extracts were subjected to polyacrylamide gel electrophoresis and transferred for Western blotting. Blots were probed with anti-phosphorylated pTrkB (Tyr706/707), anti-phosphorylated pTrkB (Tyr816), anti-phosphorylated pTrkB (Tyr515), anti-TrkB antibody and anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; separation bars indicate non-contiguous lanes on the same image acquisition (the original blots are shown in the Supplementary Information file). (B) The ratio of signal derived from phosphorylated TrkB over TrkB bands was calculated (n = 3; *p < 0.05 in relation to corresponding “0% FBS”, taken for 1 unit; Wilcoxon t-test). (C) SH-SY5Y cells in serum-free medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) were also pre incubated in the presence or absence of K252a (500 nmol, 60 min). Blots were first probed with anti-phosphorylated antibodies and then reprobed with anti-TrkB antibody, anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (D) The ratio of signal derived from phosphorylated TrkB (Tyr515) over TrkB bands was calculated (n = 3; *p < 0.05 in relation to corresponding “0% FBS” (dashed line); #p < 0.05, ##p < 0.01 in relation to corresponding groups without K252a; Wilcoxon t-test). (E) SH-SY5Y cells in serum-free culture medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) were also pre incubated with or without PP2 (100 µmol, 30 min). Blots were probed with anti-phosphorylated pTrkB (Tyr706/707), anti-phosphorylated pTrkB (Tyr816) or anti-phosphorylated pTrkB (Tyr515) antibodies and anti-TrkB antibody, anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (F) The ratio of signal derived from phosphorylated TrkB over TrkB bands was calculated (n = 3; #p < 0.05, ##p < 0.01 in relation to corresponding “0% FBS”; *p < 0.05, **p < 0.01 in relation to corresponding “- PP2”; Wilcoxon t-test).
BDNF and GSB-106 effects on TrkB Tyr706/707 and Tyr816 phosphorylation were nearly completely suppressed by K252a (Fig. 3C). However, Tyr515 phosphorylation, induced by BDNF was inhibited by 32.8 ± 6% at 30 min and by 71.7 ± 8% at 60 min (p < 0.05; Wilcoxon t-test), whereas cells incubation with GSB-106 resulted in 43.7 ± 8% (p < 0.01; Wilcoxon t-test) sustained inhibition at 60 min upon K252a treatment (Fig. 3C,D). Together, these findings suggested, that effects of GSB-106 and, at lesser extent, BDNF on phosphorylation of Tyr515 could be mediated not only by TrkB, but also by mechanisms, which act downstream of TrkB. K252a is a potent Trk inhibitor, capable to activate Akt and Erk kinases via Src-dependent pathway, thus supporting cell survival in serum withdrawn cells30. Currently, several TrkB-independent mechanisms described insensitive to K252a inhibition, including Src-dependent transactivation of neurotrophin receptor and maintaining of calcium signaling by truncated TrkB receptor isoforms31.
TrkB receptor is capable of autophosphorylation and activation of downstream cascades independent on BDNF through the transactivation mechanisms, which may involve GPCRs and are mediated by Src family kinases (SFK)32. To find out whether GSB-106 might affect TrkB signaling via Src-dependent transactivation mechanisms, the effect of Src kinases inhibitor PP2 on site-specific TrkB phosphorylation (Tyr706/707, Tyr515, and Tyr816) was determined. PP2 alone (100 µmol) did not affect the phosphorylation of studied tyrosines in control serum-deprived cells (Fig. 3E). Addition of PP2 resulted in a slightly reduced BDNF-induced phosphorylation of Tyr706/707 and Tyr515, while a more significant inhibition of Tyr816 phosphorylation was found (Fig. 3E,F). These data are consistent with observations, that chemically diverse inhibitors of Src family kinases suppressed the BDNF-evoked Tyr706/707, Tyr515, and Tyr816 TrkB phosphorylation, implicating SFKs as regulators of TrkB activation by BDNF33. Pre-incubation of cells with PP2 led to a modest reduction of GSB-106-induced Tyr706/707 phosphorylation compared to that of Tyr515, and more apparent decline of Tyr816 phosphorylation (p < 0.05, p < 0.01; Wilcoxon t-test; Fig. 3E,F). Remarkably, PP2 altered the GSB-106-elicited Tyr706/707 and Tyr515 phosphorylation more profoundly than that induced by BDNF, whereas the decrease of Tyr816 phosphorylation was less striking in case of GSB-106. Altogether, our data shows that SFK inhibitor PP2 preferentially reduced both BDNF- and GSB-106-mediated phosphorylation of Tyr816 compared to Tyr706/707 and Tyr515 under given experimental conditions. Collectively, our findings suggest that TrkB activation by GSB-106 was, at least, partially dependent on Src kinases-implicated TrkB transactivation mechanisms.
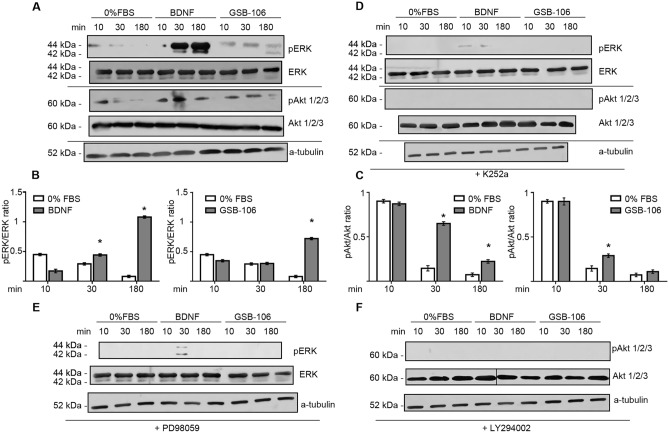
The pattern of induction of downstream effector protein kinases (Erk1/2 and Akt) was examined to further characterize the activity profile of small BDNF mimetic GSB-106 in serum starved cells. As shown in Fig. 4A,B, Erk1/2 activation occurred at 180 min in the presence of GSB-106, whereas Erk1/2 phosphorylation, induced by BDNF, registered in 30 min, reaching a maximum increase at 180 min. Notably, a greater (1.5 fold; p < 0.05; Wilcoxon t-test) Erk1/2 activation at 180 min was produced by BDNF compared to GSB-106. Figures 4A,C demonstrate that treatment of serum free cells with BDNF led to robust raise in Akt phosphorylation at 30 min, followed by a decrease at 180 min (p < 0.05; Wilcoxon t-test). Similar, although less pronounced profile of Akt activation has been established upon GSB-106 exposure: a moderate stimulation of Akt phosphorylation at 30 min with a consequent kinase activation decline at a later time point.
Figure 4.
The neuroprotective effect of GSB-106 is mediated through the activation of MAPK/Erk and PI3K/Akt pathways. (A) SH-SY5Y cells were incubated in serum-free culture medium (“0% FBS”) with or without BDNF (28.6 ng/ml, ~ 1 nmol) or GSB-106 (100 nmol) for 10, 30 or 180 min. After incubation, cells were collected, and protein extracts were subjected to polyacrylamide gel electrophoresis and transferred for Western blotting. Blots were first probed with anti-phosphorylated antibodies and then reprobed with anti-Erk or anti-pan-Akt antibodies, anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (B) The ratio of signal derived from phosphorylated Erk over Erk bands was calculated (n = 3; *p < 0.05 in relation to corresponding “0% FBS”; Wilcoxon t-test). (C) The ratio of signal derived from phosphorylated Akt over Akt bands was calculated (n = 3; *p < 0.05 in relation to corresponding “0% FBS”; Wilcoxon t-test). (D) SH-SY5Y cells in serum-free culture medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) were also pre incubated in the presence or absence of K252a (500 nmol, 60 min). Blots were first probed with anti-phosphorylated antibodies and then reprobed with anti-Erk or anti-pan-Akt antibodies, anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (E) SH-SY5Y cells in serum-free culture medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) were also pre incubated with or without PD98059 (50 µmol, 60 min). Blots were first probed with anti-phosphorylated Erk (pErk) antibody and then reprobed with anti-Erk antibody, anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (F) SH-SY5Y cells in serum-free culture medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) were also pre incubated with or without LY290042 (50 µmol, 60 min). Blots were first probed with anti-phosphorylated pan-Akt (pAkt1/2/3) antibody and then reprobed with anti-pan-Akt antibody, anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; separation bars indicate non-contiguous lanes on the same image acquisition (the original blots are shown in the Supplementary Information file).
Pretreatment of cells with a pharmacological Trk inhibitor K252a led to suppression of BDNF- or GSB-106-induced activation of Erk1/2 and Akt (Fig. 4D), thus suggesting that the pro-survival signaling evoked by GSB-106 in serum withdrawn SH-SY5Y cells requires TrkB activation. To further confirm the engagement of MAPK/Erk1/2 and PI3K/Akt pathways in GSB-106-mediated cell’s protection, cells were pretreated with PD98059 and LY294002. As shown in Fig. 4E PD98059 prevented both GSB-106 and BDNF-evoked activation of Erk1/2 phosphorylation and had no effect on the activity of Akt kinase (Supplementary Fig. S1A). Similarly, LY294002 abrogated the ability of GSB-106 and BDNF to stimulate the phosphorylation of Akt (Fig. 4F) and did not affect the phosphorylation of Erk1/2 (Supplementary Fig. S1B). Together, these findings indicate that GSB-106 promotes serum deprived cells survival through activation of TrkB and downstream MAPK/Erk1/2 and PI3K/Akt pathways.
Activation of PI3K/Akt pathway is essential for GSB-106-mediated neuroprotection
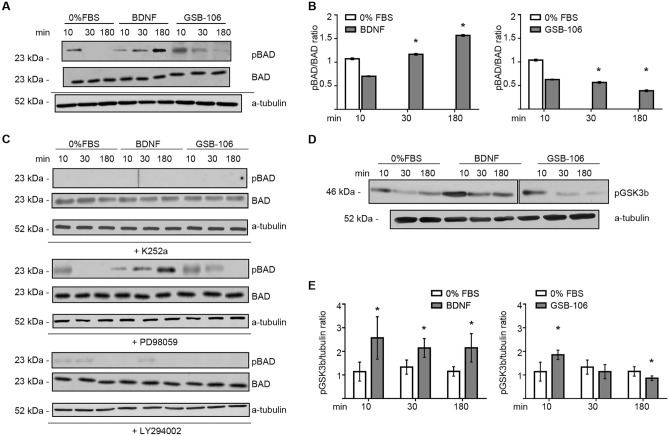
TrkB-dependent activation of the PI3K/Akt cascade regulates number of proteins essential for neuronal survival, growth and differentiation such as GSK-3β (glycogen synthase kinase 3β) and certain substrates that directly regulate the caspase cascade, in particular, the proapoptotic factor—BAD (Bcl-2 antagonist of cell death)34. The proapoptotic activity of BAD is regulated by several protein kinases (e.g. Akt, Rsk) through phosphorylation at sites known to inhibit its pro-apoptosis function and thus suppress BAD-mediated apoptosis in neurons35. We evaluated the effects of GSB-106 on activity of BAD, GSK-3β proteins and caspases-9, 3/7 in serum starved cells to characterize the survival-promoting and anti-apoptotic mechanisms of the BDNF mimetic in more detail. Figures 5A,B show that serum starvation results in a loss of phosphorylated BAD protein at 30 min, whereas incubation of cells with BDNF was accompanied with an incremental rise of BAD phosphorylation (Ser136) lasting for up to 180 min (p < 0.05; Wilcoxon t-test), which is consistent with conventional knowledge of BDNF ability to inhibit apoptosis through the BAD phosphorylation, along with other mechanisms28. GSB-106 has eliminated the serum withdrawal-induced decrease of BAD phosphorylation, maintaining the moderate level of pBAD within 180 min (p < 0.05; Wilcoxon t-test) as shown in Fig. 5A,B. Although the extent of BAD activation caused by GSB-106 was less prominent compared to BDNF, these findings still point out GSB-106 ability to mimic the neurotrophin-specific anti-apoptotic mechanism mediated by BAD protein.
Figure 5.
GSB-106-induced neuroprotection involves the BAD and GSK3β proteins. (A) GSB-106 activates Bad phosphorylation. SH-SY5Y cells were incubated in serum-free culture medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) for 10 min, 30 min, or 180 min. Blots were first probed with anti-phosphorylated BAD (pBAD) antibody and then reprobed with anti-BAD antibody, anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (B) The ratio of signal derived from phosphorylated BAD over BAD bands was calculated (n = 3; *p < 0.05 in relation to “0% FBS”; Wilcoxon t-test). (C) SH-SY5Y cells in serum-free culture medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) were also pre incubated with or without K252a (500 nmol, 60 min), LY294002 (50 µmol, 60 min) or PD98059 (50 µmol, 60 min). The figure shows data from one independent experiment (n = 2; the original blots are shown in the Supplementary Information file). (D) SH-SY5Y cells were incubated in serum-free culture medium (“0% FBS”) with or without BDNF (~ 1 nmol) or GSB-106 (100 nmol) for 10, 30, 180 min. Blots were first probed with anti-pGSK-3β antibody and then were reprobed with anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (E) The ratio of signal derived from p-GSK-3β over a-tubulin bands was calculated (n = 3; *p < 0.05 in relation to corresponding “0% FBS”; Wilcoxon t-test).
Next, we determined the role of upstream components of TrkB/PI3K/Akt cascade in BAD phosphorylation, induced by BDNF and GSB-106. BDNF and GSB-106 both failed to phosphorylate BAD protein in the presence of K252a and LY294002 (Fig. 5C). Thus, TrkB and Akt are likely required for BDNF- and GSB-106-stimulated BAD activation in serum withdrawn SH-SY5Y cells. In contrast, BAD Ser136 phosphorylation was not altered by PD98059 treatment, corroborating the insensitivity of this site to activation by Ras/MAPK pathway (Fig. 5C). As it is known, MAPK-activated kinase Rsk (non-specific serine/threonine protein kinase) catalyzes the BAD phosphorylation at Ser112 followed by suppression of BAD-mediated apoptosis36.
We have evaluated phosphorylation of GSK-3β, a downstream target of PI3K/Akt aiming to learn about additional mechanisms of PI3K/Akt-dependent protection of SH-SY5Y cells from apoptotic cell death induced by serum deprivation under BDNF and GSB-106 treatment. Western blot analysis showed the decreased level of p-GSK-3β in serum-free SH-SY5Y cells which was reversed and remained elevated for 3 h upon BDNF treatment. Addition of GSB-106 to serum-deprived cells caused a transient increase of GSK-3β Ser9 phosphorylation peaking at 10 min followed by a gradual decline over the next 3 h (p < 0.05; Wilcoxon t-test; Fig. 5D,E).
It is well known that intrinsic apoptotic pathway triggered in response to withdrawal of growth factors, results in the mitochondrial release of cytochrome c, which activates initiator caspase-9. Once activated, caspase-9 cleaves and induces executioner caspase-337. Figures 6A,B show that serum deprivation increased cleaved caspase-9 (detected by appearance of processed caspase-9 which yielded p35 and p37 subunits) within short time period, beginning at 30 min and maintaining for up to 180 min, and for a prolonged period lasting up to 72 h, thus implying permanent activation of caspase-9 (Fig. 6E). The decrease in p35 and p37 of caspase-9 subunits levels has been observed in the presence of both BDNF and GSB-106 at an early time point (p < 0.05, p < 0.01; Wilcoxon t-test; Fig. 6A,B). This reduction continued for 72 h, reflecting the sustained supression of caspase-9 activity (Fig. 6E). It is remarkable that time course of caspase-9 inhibition is consistent with the dynamic of Akt and BAD activation elicited by both GSB-106 and BDNF, which suggests that GSB-106 promotes suppression of apoptosis exerted by serum withdrawal through Akt-dependent protection mechanisms, which are attributive to BDNF. Moreover, these data further corroborated the neurotrophin-like mode of neuroprotection, displayed by GSB-106.
Figure 6.
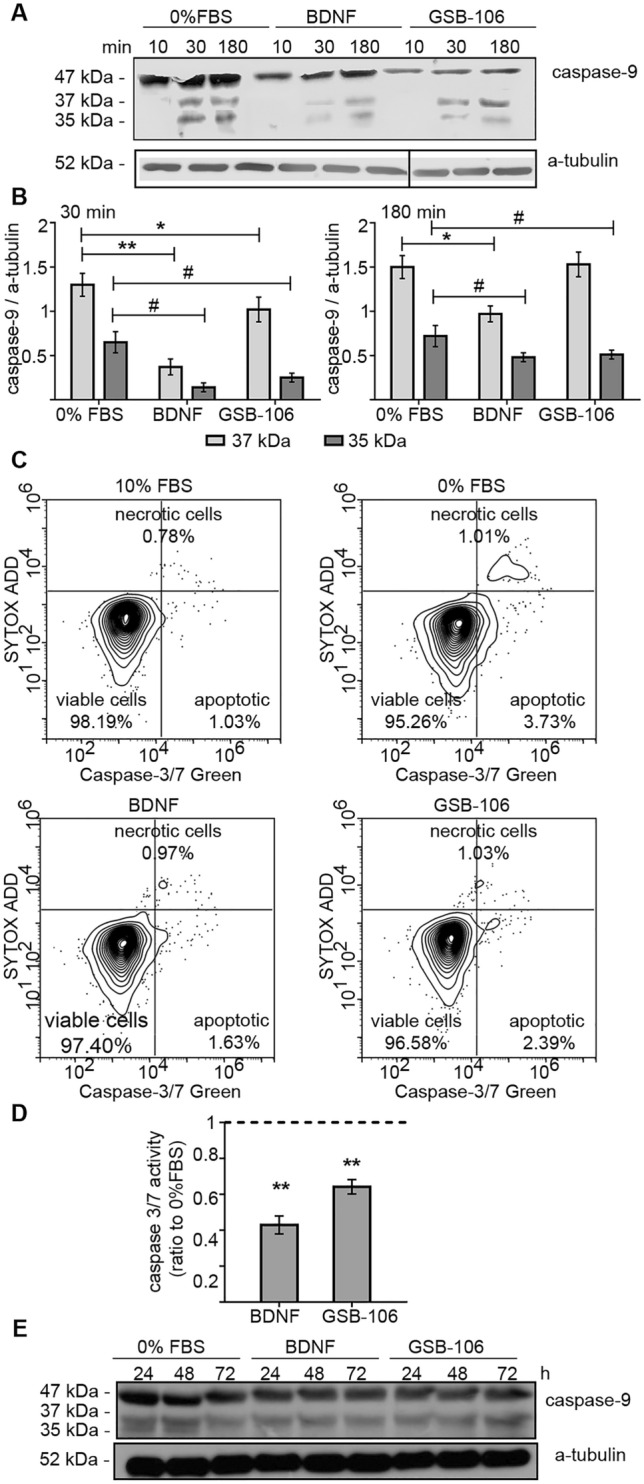
GSB-106 inhibits caspase-9 and caspase-3/7 activities induced by in serum deprivation. (A) SH-SY5Y cells were incubated in serum-free culture medium (“0% FBS”) with or without BDNF (28.6 ng/ml, ~ 1 nmol) or GSB-106 (100 nmol) for 10, 30, 180 min. After incubation, cells were collected and protein extracts were subjected to polyacrylamide gel electrophoresis and transferred for Western blotting. Blots were first probed with anti-caspase-9 antibody and then reprobed with anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file). (B) The ratio of signal derived from caspase-9 over a-tubulin bands was calculated (n = 3; *p < 0.05, **p < 0.01 in relation to 37 kDa caspase-9 form in “0% FBS” group; #p < 0.05 in relation to 35 kDa caspase-9 form in “0% FBS” group; Wilcoxon t-test). (C) SH-SY5Y cells were incubated in serum-free culture medium (“0% FBS”) with or without BDNF (28.6 ng/ml, ~ 1 nmol) or GSB-106 (100 nmol) for 6 h. After incubation, cells were collected and stained with or CellEvent Caspase-3/7 Green Flow Cytometry Assay Kit (C10427; Thermo Fisher Scientific) according manufacture’s recommendations. The figure shows contour plots from one independent experiment (n = 3). (D) The ratio of CellEvent Caspase-3/7 Green fluorescence signals derived from caspase3/7+/SYTOX− cell populations was calculated. Data is expressed as means ± S.E.M. Three independent experiments were carried out in triplicate (**p < 0.01 vs “0% FBS” (dashed line); Wilcoxon t-test). (E) GSB-106 induced a long-term decrease of caspase-9 activity. SH-SY5Y cells were incubated in serum-free culture medium (“0% FBS”) with or without BDNF (28.6 ng/ml, ~ 1 nmol) or GSB-106 (100 nmol) for 24, 48, 72 h. After incubation, cells were collected and protein extracts were subjected to polyacrylamide gel electrophoresis and transferred for Western blotting. Blots were first probed with anti-caspase-9 antibody and then reprobed with anti-a-tubulin antibody. The figure shows data from one independent experiment (n = 3; the original blots are shown in the Supplementary Information file).
Caspase-3/7 activity was directly assessed by CellEvent Caspase-3/7 Green Flow Cytometry Assay Kit in order to further evaluate the effect of GSB-106 and BDNF on downstream apoptotic pathway. This detection reagent consists of a nucleic acid-binding dye that harbors the caspase-3/7 cleavage sequence DEVD and is fluorescent after being cleaved and bound to DNA. As observed in Fig. 6C,D, activated (cleaved) caspases-3/7 signals decreased after BDNF and GSB-106 treatment by 57.0 ± 5% and 35.8 ± 4% respectively in comparison with signals in control serum deprived cells (p < 0.01; Wilcoxon t-test). These findings indicate that GSB-106 and BDNF attenuate activity of caspases-9 and 3/7, thus supporting antiapoptotic signaling.
Discussion
This study reports on BDNF small dipeptide mimetic GSB-106 being able to protect trophic-deprived cells from apoptosis and promote their survival by triggering the TrkB-dependent activation of downstream pro-survival pathways. Current study also suggests that GSB-106 behaves as a partial BDNF-like agonist, since GSB-106 was found to intrinsically provide the cell survival (~ 26% increase over control) and is able to inhibit BDNF-mediated cell viability (by ~ 37%), when added competitively, thus exhibiting the profile of partial agonist. Remarkably, an increase in GSB-106-promoted survival effect in the presence of BDNF, compared to cells, treated by GSB-106 alone, has been observed, suggesting the additional (or parallel) mechanisms of cell viability promotion upon serum withdrawal conditions.
Studied compound reproduces some functions inherent for BDNF. Particularly, neuroprotection of serum-deprived cells, provided by GSB-106, involved inhibition of apoptosis and was shown to be dependent on a transient TrkB receptor activation, which was assayed by phosphorylation at Tyr706/707. Furthermore, GSB-106 increased TrkB phosphorylation at Tyr515 and Tyr816 and promoted activation of Ras/MAPK and PI3K/Akt pathways. Patterns for Erk1/2 and Akt activation induced by GSB-106 were weaker, than those triggered by BDNF. However, it had similar temporal pattern, and this activation was prevented by K252a kinase inhibitor and corresponding MEK1 and PI3 kinase inhibitors (PD98059 and LY294002 respectively). Intriguingly, Tyr515 phosphorylation was affected by GSB-106 more significantly, than that induced by BDNF; particularly, the enhanced level of Tyr515 activation has already appeared at 10 min (~ 64%) and maintained up to 60 min, although BDNF elicited an increase of phosphorylation at this site (~ 38%) only after 60 min. While TrkB Tyr706/707 and Tyr816 phosphorylation were almost completely eliminated by K252a, a residual Tyr515 phosphorylation has retained upon both BDNF and GSB-106 treatment, hence, suggesting the contribution of non-TrkB-dependent mechanisms. Experiments with Src kinases inhibitor PP2 showed that Src kinases, apparently, implicated in GSB-106-induced TrkB activation and, potentially, transactivation mechanisms could account for elevated pro-survival response seen during cell treatment by GSB-106 in the presence of BDNF.
Most importantly, protection of serum-deprived cells by GSB-106 also engages TrkB/Akt-dependent inactivation of pro-apoptotic BAD protein and suppression of caspases-9 and 3/7. Once activated by trophic factors, e.g. NGF (nerve growth factor) and BDNF, Akt phosphorylates BAD at Ser136, which in turn, sequestered in the cytosol in the complex with chaperone 14-3-4 protein and allows anti-apoptotic Bcl-2/Bcl-xL proteins inhibit the activity of pro-apoptotic Bax protein, thereby preventing release of mitochondrial cytochrome c, activation of caspase cascade, thus promoting cell survival34. Moreover, GSB-106 increases the phosphorylation of GSK-3β at Ser9 transiently, to a substantially lesser extent than BDNF, thus inactivating the kinase; this mechanism shown to be involved in protective function of PI3K/Akt pathway38. Collectively, it appears that survival-promoting effect of GSB-106 as well as of BDNF in serum-deprived cell is related to TrkB-dependent activation of MAPK/Erk1/2 and, mainly, PI3K/Akt pathways. Furthermore, PI3K/Akt—dependent inactivation of pro-apoptotic BAD protein followed by subsequent downregulation of appropriate caspases could account for the protective mechanisms governed by the compound. Presumably, inactivation of GSK-3β is also relevant to protective mechanisms provided by GSB-106. There is no current evidence that existing small peptide or non-peptide BDNF mimetics promote survival through inactivation of BAD proteins in various damaging cell context considering the available data. Hereby, this is the first time when BDNF peptide mimetic effect has been identified to require the TrkB/Akt-dependent inactivation of BAD protein in serum-deprived cells to support the survival.
GSB-106 produced a bell-shaped concentration–response survival curve in serum-deprived SH-SY5Y cells, which is generally a characteristic of compounds that mimic receptors agonist ligands, capable of homodimerization, mainly of receptors of growth factors, some hormones and cytokines39,40. Bell-shaped concentration–response curves and partial agonist profiles have been also reported for a number of domain-specific small molecule mimetics of BDNF, which are capable of engaging with and induce the dimerization and/or activation of TrkB receptor13,41–43. Several mechanistic models have been rendered to explain how the small neurotrophin peptidomimetics and non-peptide ligands can elicit the TrkB-dependent cellular response, although the exact structural basis for peptidomimetics/Trks interaction and subsequent activation remain obscure44. Under conventional interpretation, small monomeric or dimeric molecules activate Trks upon binding by inducing receptor dimerization. There is also a possibility that small neurotrophin-like ligands might interact with existing pre-formed, yet inactive Trk dimers or Trk/p75NTR heterodimers. It was suggested that neurotrophin mimetics could stabilize preformed dimers, hence, encourage the receptor activation, considering such a mode of natural ligands action. Furthermore, additional option is associated with probable allosteric regulation of Trk receptors conferred by low molecular mass neurotrophin mimetics44.
Taking into account structural features of homodimeric dipeptide GSB-106, such as resemblance of one of the TrkB-interacting motif (amino acids 4 loop of BDNF -Asp93-Ser94-Lys95-Lys96-), presence of dimerizing linkage (oligomethylenediamine spacer) and functional in vitro and in vivo data, it is feasible that GSB-106 capable of binding to functional sites of TrkB and promoting or facilitating dimerization of the receptor, which provides the conformational changes, sufficient to trigger at a certain extent receptor autophosphorylation followed by activation of downstream signaling pathways. Interestingly, -Ser-Lys- motif occurred in both sites of neurotrophin (within the 2 loop: -Ser45-Lys46-Gly47-Gln48-Lys49- and within the 4 loop: -Asp93-Ser94-Lys95-Lys96-), which are critical for BDNF/TrkB binding and activity and possess functional importance. As noted above, this motif represents core dipeptide fragment of GSB-106 that, probably, affords BDNF-like trophic activity of the compound. Conceivably, bell-shaped concentration–response curve could reflect the ability of GSB-106 to bind and stimulate/support receptor dimerization. Nevertheless, given that GSB-106 was designed to mimic only one of multiple BDNF/TrkB-interacting domains, incomplete receptor binding could arise, thus contributing to conformational changes distinct from those inherent for BDNF. That may result in a fewer degree of receptor and effector kinases activations and temporal discrepancy of receptor phosphorylation patterns, compared to that of BDNF. Presumably, partial agonist profile of the GSB-106 could represent different from BDNF temporal and activation patterns of TrkB phosphorylation, reduced activation of downstream effectors and less pronounced inactivation of BAD and GSK-3β. However, mechanisms, underlying the partial agonistic effect and the bell-shaped curve evoked by GSB-106 require further investigations.
Considering that residues located in the loop 4 BDNF (Lys95, Lys96, Arg97) appeared to contribute both to TrkB and p75NTR binding45, we do not rule out the possible interaction of GSB-106 with p75NTR, although it is unlikely that compound could interact with both receptors simultaneously46,47, whilst this issue has not been addressed in the current study. Insofar we did not evaluate the direct interaction of GSB-106 with TrkB, as well as with p75NTR, the role that distinct receptor plays in pro-survival properties of GSB-106 couldn't be precisely resolved and requires further detailed investigations. Nevertheless, several lines of indirect evidence allow us to speculate that GSB-106 could function preferentially through the TrkB -, rather than, p75NTR—dependent pathways. First, serum-starved SH-SY5Y cells co-express TrkB and p75NTR receptors (Fig. 1B), therefore, TrkB-dependent survival signaling would be prevalent following neurotrophin addition3. Indeed, GSB-106 alone acts in a manner similar to that of BDNF in a such cellular context, rendering cell survival, which is partially TrkB-dependent and accompanied by TrkB Tyr707/707, Tyr516, Tyr817 phosphorylation and TrkB-regulated activation of MAPK/Erk and PI3K/Akt signaling. Second, in instances when TrkB and p75NTR expressed concomitantly, Trks suppress the p75NTR-mediated apoptotic JNK-p53-Bax pathway through Ras/PI3K/Akt and Ras/MAPK/Erk pathways or inhibit cell death proteins activities, such as Forkhead and members of the Bcl-2 family (Bcl-2, Bcl-xL, Bim and BAD)6. As it was mentioned above, growth factors-promoted inhibition of apoptosis, mediated by Akt activation, results in phosphorylation of BAD at Ser 112, 136 and 155, thus decreasing BAD association with Bcl-xL followed by inhibition of pro-apoptotic Bax protein and caspases-9, 3/7, thereby promoting cell survival34. Our data showed that GSB-106, as well as BDNF, prevented apoptosis, induced by serum-withdrawal, mainly via TrkB/Akt-mediated phosphorylation (Figs. 4D, 5C) and inactivation of the proapoptotic BAD protein (Fig. 5A) with subsequent suppression of downstream initiator (caspase-9) and effector (caspases-3/7) caspases (Fig. 6). Obtained results suggest, that pro-survival activity of GSB-106 could also relay on the counteraction of deleterious pathways arising from p75NTR, although, this assumption requires faithful delineation.
Based on our findings we also presumed indirect TrkB activation by GSB-106 in serum-deprived SH-SY5Y cells, which could explain the gain (by ~ 17%) of GSB-106 survival activity in the presence of BDNF. TrkB signaling undergoes transactivation, which involves several GPCRs (G-protein-coupled receptors), such as A2A adenosine receptors32, PACAP (pituitary adenylate cyclase-activating polypeptide) receptors PACR148, dopamine D1 receptors49, cannabinoid receptors CB1R50. BDNF-independent TrkB-activation also occurs via epidermal growth factor (EGF)51, glucocorticoids52, zinc53, H2O254. The finding by Lee and Chao provided evidences of Src kinases implication in phosphorylation of intracellular TrkB tyrosines and receptor activation32. Huang and McNamara carefully elucidated the role of SFKs in activation of TrkB by zinc and BDNF. It has been reported that BDNF activation of TrkB resulted in increased SFKs activity, promotion of protein complex formation consisting of TrkB and SFKs—Fyn and Src in vitro. Additional experimental data supported the hypothesis that SFKs can provide the positive feedback regulation of TrkB signaling provoked by BDNF33. According to our data, SFKs inhibitor PP2 suppressed the GSB-106-evoked Tyr706/707, Tyr515 and Tyr816 phosphorylation, thus supporting the assumption that recruitment of SFKs required for GSB-106-regulated TrkB activation, yet not excluding other non-TrkB targets or mechanisms which could be responsible for improving of cell survival, stimulated by GSB-106 in the presence of BDNF. Remarkably, SFKs inhibition led to a smaller reduction of BDNF-induced Tyr706/707 and Tyr515 phosphorylation than that produced by GSB-106, although decrease in Tyr816 phosphorylation was more profound. Distinct patterns of different TrkB tyrosines phosphorylation inhibition imply that BDNF and GSB-106 could function non-identically on certain sites via involvement of divergent mechanisms. To insight into mechanisms of GSB-106 action further analysis of GSB-106/TrkB and GSB-106/p75NTR interactions, possible non-TrkB targets and associated signaling pathways will be needed.
In summary, this report shows that GSB-106, similarly to BDNF, promotes survival of serum-deprived neuronal-like cells. This protective effect is governed by the ability of compound to counteract cell apoptosis through activation of TrkB-dependent pro-survival mechanisms, including inactivation of pro-apoptotic BAD protein and suppression of caspases-9 and 3/7. We demonstrated that GSB-106 acted as a partial TrkB receptor agonist and transactivation mechanisms also could contribute to pro-survival properties of the compound. Taken together, multiple lines of evidence indicate that GSB-106 functions as BDNF-like TrkB ligand mimicked the pro-survival activity and the principal protective mechanisms inherent for the native neurotrophin.
Materials and methods
Substance
GSB-106 (bis-(N-monosuccinyl-l-seryl-l-lysine) hexamethylenediamide; MW 746.85) was synthesized as described previously [Fig. 1A; 15] at Medicinal Chemistry Department of “Zakusov Institute of Pharmacology”. GSB-106 and BDNF were dissolved in Dulbecco’s modified Eagle’s medium (DMEM) without serum for all experiments.
Cells and reagents
Neuroblastoma cell line SH-SY5Y was kindly provided by Dr. Galina Pavlova (Institute of Gene Biology Russian Academy of Sciences, Moscow). Cells (at passages 2–10) were maintained in DMEM, supplemented with 10% fetal bovine serum (FBS), 10–3 M l-glutamine in a 5% CO2 incubator at 37 °C. In cell-based assays, SH-SY5Y cells were seeded on 48-, 12- or 6-well plates (Corning). After reaching 85–95% confluency, cells were gently washed with PBS (2–3 times) and starved in 0% FBS DMEM for at least 24 h (unless otherwise specified). Cells were incubated with BDNF or GSB-106 (in one or various concentrations, depending on the type of experiment) for different time intervals after starvation. Chemicals and cell culture reagents were obtained from Sigma-Aldrich, Thermo Fisher Scientific, Tocris.
Antibodies
Following primary antibodies were used in this study: rabbit polyclonal anti-BDNF (1:1000, #ab226843, Abcam), rabbit monoclonal anti-TrkB (1:1000, #4603, Cell Signaling Technology), anti-p75NTR (1:1000, #4201, Cell Signaling Technology), anti-phospho-TrkA (Tyr674/675)/TrkB (Tyr706/707) (1:1000, #4621, Cell Signaling Technology), anti-p44/42 MAPK (Erk1/2) (1:1000, #4695, Cell Signaling Technology), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (1:1000, #4377, Cell Signaling Technology), anti-Akt (pan) (1:1000, #4691, Cell Signaling Technology), anti-phospho-Akt (Ser473) (1:1000, #4058 Cell Signaling Technology), anti-BAD (1:1000, #9239, Cell Signaling Technology), anti-phospho-BAD (Ser136) (1:1000, #4366, Cell Signaling Technology), anti-phospho-GSK-3β (Ser9) (1:1000, #9323, Cell Signaling Technology), rabbit polyclonal anti-phospho-TrkB (Tyr816) (1:1000, #NBP1-03499SS, Novus Biologicals), anti-phospho-TrkB (Tyr515) (1:1000, # PA5-36695, Thermo Fisher Scientific), anti-Caspase-9 Antibody (1:1000, #9502, Cell Signaling Technology), mouse monoclonal anti-α-Tubulin (1:1000, #2125, Cell Signaling Technology). Secondary antibodies conjugated to HRP (used at 1:12,000 dilution) were from Cell Signaling Technology (anti-mouse IgG, #7076; anti-rabbit IgG, #7074).
MTT assay (viability assay)
SH-SY5Y cells were seeded in 48-well plates (Corning) (2 × 105 cells/200 µl/well) in DMEM containing 10% FBS, 10–3 M l-glutamine in a 5% CO2 incubator at 37 °C. Cells were gently washed with PBS (2–3 times) upon reaching 85–95% confluency. Media was changed to DMEM (0% FBS), containing exogenous BDNF (1–2–4–10–100 nmol; #B3795, Sigma-Aldrich) or/and GSB-106 (0.1 nmol to 100 µmol) after washing. For TrkB—and downstream signaling pathways—mediated survival assays cells were incubated with BDNF (1 nmol) or GSB-106 (100 nmol) in the presence either of K252a (500 nmol), or PP2 (100 μmol), or PD98059 (50 μmol), or LY294002 (1 μmol). Cells were cultured for 48 h. 20 μl of the MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; final concentration 0.5 mg/ml; #M6494, Thermo Fisher Scientific) were added to each well after incubation period was completed. Cells were incubated for 4 h in a humidified atmosphere. Cultural media was completely removed, and the pellets were solubilized in 100% DMSO (Sigma-Aldrich) for 10 min in room temperature. Absorbance was assessed using Varioskan LUX microplate reader (Thermo Fisher Scientific) after purple formazan crystals fully dissolved. Absorbance of the formazan product was measured at 590 nm wavelength. 750 nm reference wavelength was used. 590/750 nm ratio of absorbance was used (A590/750) for further analysis. After BDNF or GSB-106 treatment cell viability rates were calculated as follows:
The comparative analysis of cell survival upon BDNF, GSB-106 and BDNF + GSB-106 incubation, as well as analysis of GSB-106-dependent viability upon K252a, PP2, PD98059, LY294002 treatments was performed as follows:
Protein preparation and Western blot analysis
SH-SY5Y cells were cultured in 6-well plates for 24 h (Corning) (3 × 106 cells/well) in DMEM, containing 10% FBS and after washing cells were maintained in serum-free DMEM. Neurotrophin or compound was added at different time points, depending on the experiment. At the definite time points, cells were lysed with RIPA buffer (10–2 M Tris–Cl (pH 8.0), 10–3 M EDTA, 5 × 10–4 M EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 0.14 × 10–3 M NaCl, 1 × protease inhibitor cocktail (#P8340), 1 × phosphatase inhibitor cocktail 2 and 3 (#P5726, P0044), 10–3 M PMSF (all obtained from Sigma-Aldrich). Protein concentration was determined using BCA Protein Assay Kit (#23225, Thermo Fisher Scientific). Lysates were loaded on SDS-PAGE, and separated proteins were transferred onto the nitrocellulose membranes (#GE10600002, Sigma-Aldrich). The membranes were blocked in 5% not-fat milk or 5% BSA in TBS-T for 60 min at room temperature, incubated with the appropriate primary antibody (+ 4 °C, overnight) and then with a secondary antibody conjugated with HRP (60 min at room temperature). Bands were visualized using SignalFire Plus ECL Reagent (#12630, Cell Signaling Technology). Membranes were scanned using Amersham Imager 680 (GE Healthcare) and quantified in Image Quant TL v.8.1 (GE Healthcare). Measurement of each protein was then normalized on the related α-tubulin loading control.
Caspase 3/7 activity assay
SH-SY5Y were cultured in 12-well plates (Corning) (5 × 105 cells/well) in DMEM, containing 10% FBS for 24 h for flow cytometry detection of activated caspase-3 and caspase-7 in apoptotic cells. Cells were washed in PBS and maintained in serum-free DMEM for various time intervals upon BDNF or GSB-106 treatment. After incubation cells were harvested and stained with CellEvent Caspase-3/7 Green Flow Cytometry Assay Kit (#C10427, Thermo Fisher Scientific) according to the manufacturer’s recommendations. The samples were analyzed by NovoCyte 2060 flow cytometer (Agilent Technologies), using 488 nm excitation and collecting fluorescence emission; a 530/30 bandpass filter for CellEvent Caspase-3/7 Green Detection Reagent and a 690/50 BP filter for SYTOX AADvanced dead cell staining. After a standard fluorescence compensation technique, percent of caspase-3/7+/ SYTOX−cell populations on a dual parameter dot plot was used for statistical analysis (30,000 events were collected in each probes gated as “live cells”).
Statistical analysis
Western blots data were statistically analyzed using Wilcoxon t-test (Statistica 12.5 (StatSoft. Inc.). Data is expressed as means ± S.E.M. Graphs plotting and IC50, EC50, EFFECTMAX calculations were carried out in the “bell-shape module” (nonlinear regression) of GraphPad Prism v.7.0 (GraphPad Software Inc.) for cell viability assays data. Comparison of survival curves was analyzed by one-way ANOVA with Newman-Keul’s post-hoc. Normal distribution of data was evaluated by the Shapiro–Wilk’s test. Levene’s test was used to check equality of dispersion.
Supplementary Information
Acknowledgements
We thank PhD student Tatyana Ivanova (Institute of Biochemistry and Genetics of Ufa Scientific Centre RAS) for assistance with some experiments. This work was conducted under the government contracts of the Ministry of Science and Higher Education of the Russian Federation (Project 0521-2019-0002).
Author contributions
L.F.Z. designed and performed the experiments, analyzed the data, prepared the figures, wrote the paper (methods, figure legends). Yu.V.V. conceptualized the experiments, interpreted the data, wrote and compiled the paper. A.Yu.L. performed the experiments. T.A.G. provided GSB-106 and revised the manuscript. S.B.S. conceived and conceptualized the study, wrote the paper. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: L. F. Zainullina and Yu. V. Vakhitova.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87435-0.
References
- 1.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 3.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 4.Carter BD, et al. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 5.Roux PP, Bhakar AL, Kennedy TE, Barker PA. The p75 neurotrophin receptor activates Akt (protein kinase B) through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 2001;276:23097–23104. doi: 10.1074/jbc.m011520200. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 7.Gentry JJ, Barker PA, Carter BD. The p75 neurotrophin receptor: Multiple interactors and numerous functions. Prog. Brain Res. 2004;146:25–39. doi: 10.1016/s0079-6123(03)46002-0. [DOI] [PubMed] [Google Scholar]
- 8.Tejeda GS, Diaz-Guerra M. Integral characterization of defective BDNF/TrkB signalling in neurological and psychiatric disorders leads the way to new therapies. Int. J. Mol. Sci. 2017;18:268. doi: 10.3390/ijms18020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoenen H, Sendtner M. Neurotrophins: From enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 10.Josephy-Hernandez S, Jmaeff S, Pirvulescu I, Aboulkassim T, Saragovi HU. Neurotrophin receptor agonists and antagonists as therapeutic agents: An evolving paradigm. Neurobiol. Dis. 2017;97(Pt B):139–155. doi: 10.1016/j.nbd.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhao H, et al. Molecular mechanisms of brain-derived neurotrophic factor in neuro-protection: Recent developments. Brain Res. 2017;1665:1–21. doi: 10.1016/j.brainres.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary PD, Hughes RA. Structure–activity relationships of conformationally constrained peptide analogues of loop 2 of brain-derived neurotrophic factor. J. Neurochem. 1998;70:1712–1721. doi: 10.1046/j.1471-4159.1998.70041712.x. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary PD, Hughes RA. Design of potent peptide mimetics of brain-derived neurotrophic factor. J. Biol. Chem. 2003;278:25738–25744. doi: 10.1074/jbc.m303209200. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher JM, et al. Design of a conformationally defined and proteolytically stable circular mimetic of brain-derived neurotrophic factor. J. Biol. Chem. 2008;283:33375–33383. doi: 10.1074/jbc.m802789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudasheva TA, et al. Design and synthesis of dipeptide mimetics of brain-derived neurotrophic factor. Russ. J. Bioorg. Chem. 2012;38:243–252. doi: 10.1134/S1068162012030053. [DOI] [PubMed] [Google Scholar]
- 16.RU Patent No 2410392, 2010; CN Patent CN 102365294 B, 2016; US Patent US 9, 683, 014 B2, 2017.
- 17.Gudasheva TA, Logvinov IO, Antipova TA, Seredenin SB. Brain-derived neurotrophic factor loop 4 dipeptide mimetic GSB-106 activates TrkB, Erk, and Akt and promotes neuronal survival in vitro. Dokl. Biochem. Biophys. 2013;451:212–214. doi: 10.1134/s1607672913040121. [DOI] [PubMed] [Google Scholar]
- 18.Zainullina LF, Gudasheva TA, Vakhitova YV, Seredenin SB. Low-molecular-weight compound GSB-106 mimics the cellular effects of BDNF after serum deprivation. Dokl. Biochem. Biophys. 2019;489:396–398. doi: 10.1134/s1607672919060139. [DOI] [PubMed] [Google Scholar]
- 19.Gudasheva TA, et al. Dipeptide mimetics of different NGF and BDNF loops activate PLC-γ1. Dokl. Biochem. Biophys. 2020;494:244–247. doi: 10.1134/s1607672920050075. [DOI] [PubMed] [Google Scholar]
- 20.Logvinov IO, et al. Neuroprotective effects of dipeptide analogue of brain-derived neurotrophic factor GSB-106 in vitro experiments. Bull. Exp. Biol. Med. 2013;155:343–345. doi: 10.1007/s10517-013-2149-6. [DOI] [PubMed] [Google Scholar]
- 21.Kolyvanov GB, et al. Comparative preclinical pharmacokinetics and bioavailability of antidepressant GSB-106 tablet form. Bull. Exp. Biol. Med. 2019;167:637–640. doi: 10.1007/s10517-019-04587-w. [DOI] [PubMed] [Google Scholar]
- 22.Gudasheva TA, Povarnina PY, Tallerova AV, Seredenin SB. Antidepressant-like activity of dimeric dipeptide mimetics of different BDNF hairpin loops is determined by the activation pattern of TrkB receptor signaling pathways. Int. J. Pharm. Sci. Sci. Res. 2018;4:74–79. [Google Scholar]
- 23.Seredenin SB, et al. Antidepressant effect of dimeric dipeptide GSB-106, an original low-molecular-weight mimetic of BDNF. Acta Nat. 2013;5:105–109. doi: 10.32607/20758251-2013-5-4-105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Povarnina PY, Garibova TL, Gudasheva TA, Seredenin SB. Antidepressant effect of an orally administered dipeptide mimetic of the brain-derived neurotrophic factor. Acta Nat. 2018;10:81–84. doi: 10.32607/20758251-2018-10-3-81-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudasheva TA, Povarnina PYu, Logvinov IO, Antipova TA, Seredenin SB. Mimetics of brain-derived neurotrophic factor loops 1 and 4 are active in a model of ischemic stroke in rats. Drug. Des. Dev. Ther. 2016;10:3545–3553. doi: 10.2147/dddt.s118768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Povarnina P, Gudasheva T, Seredenin S. Dimeric dipeptide mimetics of NGF and BDNF are promising agents for post-stroke therapy. J. Biomed. Sci. Eng. 2018;11:100–107. doi: 10.4236/jbise.2018.115009. [DOI] [Google Scholar]
- 27.Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ. Induction of Trkb by retinoic acid mediates biologic responsiveness to Bdnf and differentiation of human neuroblastoma cells. Neuron. 1993;11:321–331. doi: 10.1016/0896-6273(93)90187-v. [DOI] [PubMed] [Google Scholar]
- 28.Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J. Biol. Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- 29.Encinas M, Iglesias M, Llecha N, Comella JX. Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J. Neurochem. 1999;73:1409–1421. doi: 10.1046/j.1471-4159.1999.0731409.x. [DOI] [PubMed] [Google Scholar]
- 30.Roux PP, et al. K252a and CEP1347 are neuroprotective compounds that inhibit mixed-lineage kinase-3 and induce activation of Akt and ERK. J. Biol. Chem. 2002;277:49473–49480. doi: 10.1074/jbc.m203428200. [DOI] [PubMed] [Google Scholar]
- 31.Rose CR, et al. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 32.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YZ, McNamara JO. Mutual regulation of Src family kinases and the neurotrophin receptor TrkB. J. Biol. Chem. 2010;285:8207–8217. doi: 10.1074/jbc.m109.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 35.Datta SR, et al. Akt phosphorylation of BAD couple survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 36.Bonni A, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 37.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 38.Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 2000;20:2567–2574. doi: 10.1523/jneurosci.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilondo MM, et al. Receptor dimerization determines the effects of growth hormone in primary rat adipocytes and cultured human IM-9 lymphocytes. Endocrinology. 1994;134:2397–2403. doi: 10.1210/endo.134.6.8194466. [DOI] [PubMed] [Google Scholar]
- 40.Wells JA. Binding in the growth hormone receptor complex. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1–6. doi: 10.1073/pnas.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher JM, Hughes RA. Novel monocyclic and bicyclic loop mimetics of brain-derived neurotrophic factor. J. Pept. Sci. 2006;12:515–524. doi: 10.1002/psc.760. [DOI] [PubMed] [Google Scholar]
- 42.Massa SM, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Investig. 2010;120:1774–1785. doi: 10.1172/jci41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fobian K, et al. Peptides derived from the solvent-exposed loops 3 and 4 of BDNF bind TrkB and p75(NTR) receptors and stimulate neurite outgrowth and survival. J. Neurosci. Res. 2010;88:1170–1181. doi: 10.1002/jnr.22285. [DOI] [PubMed] [Google Scholar]
- 44.Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 2013;12:507–525. doi: 10.1038/nrd4024. [DOI] [PubMed] [Google Scholar]
- 45.Rydén M, et al. Functional analysis of mutant neurotrophins deficient in low-affinity binding reveals a role for p75LNGFR in NT-4 signalling. EMBO J. 1995;14:1979–1990. doi: 10.1002/j.1460-2075.1995.tb07190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibanez CF, Ilag LL, Murray-Rust J, Persson H. An extended surface of binding to Trk tyrosine kinase receptors in NGF and BDNF allows the engineering of a multifunctional pan-neurotrophin. EMBO J. 1993;12:2281–2293. doi: 10.1002/j.1460-2075.1993.tb05882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson RC, et al. The structures of the neurotrophin 4 homodimer and the brain-derived neurotrophic factor/neurotrophin 4 heterodimer reveal a common Trk-binding site. Protein Sci. 1999;8:2589–2597. doi: 10.1110/ps.8.12.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J. Biol. Chem. 2002;277:9096–9102. doi: 10.1074/jbc.m107421200. [DOI] [PubMed] [Google Scholar]
- 49.Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J. Biol. Chem. 2008;283:15799–15806. doi: 10.1074/jbc.m801553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berghuis P, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puehringer D, et al. EGF transactivation of Trk receptors regulates the migration of newborn cortical neurons. Nat. Neurosci. 2013;16:407–415. doi: 10.1038/nn.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4862–4867. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–558. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 54.Huang YZ, McNamara JO. Neuroprotective effects of reactive oxygen species mediated by BDNF-independent activation of TrkB. J. Neurosci. 2012;32:15521–15532. doi: 10.1523/jneurosci.0755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.