Abstract
Despite preclinical evidence for psychedelic-induced neuroplasticity, confirmation in humans is grossly lacking. Given the increased interest in using low doses of psychedelics for psychiatric indications and the importance of neuroplasticity in the therapeutic response, this placebo-controlled within-subject study investigated the effect of single low doses of LSD (5, 10, and 20 μg) on circulating BDNF levels in healthy volunteers. Blood samples were collected every 2 h over 6 h, and BDNF levels were determined afterward in blood plasma using ELISA. The findings demonstrated an increase in BDNF blood plasma levels at 4 h (5 μg) and 6 h (5 and 20 μg) compared to that for the placebo. The finding that LSD acutely increases BDNF levels warrants studies in patient populations.
Keywords: psychedelics, LSD, neuroplasticity, BDNF, microdosing, placebo-controlled clinical trial
Preclinical research has demonstrated that psychedelic substances, including 2,5-dimethoxy-4-iodoamphetamine (DOI), lysergic acid diethylamide (LSD), N,N-dimethyltryptamine (DMT), and psilocybin, as well as alkaloids present in ayahuasca (harmine, tetrahydroharmine, and harmaline) affect neuroplasticity after acute and chronic administration.1−5 Catlow and colleagues, for example, demonstrated the increased formation of neurons (neurogenesis) in mice’ dentate gyrus after an average psilocybin dose of 3.5 μg/35 g bodyweight (intraperitoneal (i.p.)), while this was slightly decreased after a dose of 35 μg/35 g (psilocybin/bodyweight).6 Interestingly, when repeatedly given i.p. psilocybin four times interspersed with 1 week, a higher dose of 52 μg/35 g (psilocybin/bodyweight) increased neuroplasticity.2 Chronic administration in rats of twice the ritualistic dose of ayahuasca (150 mL/70 kg bodyweight containing 0.26 mg/kg DMT) for 28 days resulted in increased in brain-derived neurotrophic factor (BDNF) levels in the hippocampus of the female rats, compared to that in control animals.7
A recent in vitro study in cultured cortical neurons of animals showed increased formation of new neurites, as expressed by the number of dendritic branches, the total length of the arbors, and formation of synapses, after extended (24 h) treatment with a range of psychedelics like DOI, LSD, and DMT.1 While these effects were similar across psychedelic classes and the dissociative ketamine, LSD was the most potent, as shown via neuritogenesis assay.1 Also in cultured human cortical neurons, the neuro-regenerative effects of DMT8 and modulation of proteins involved in dendritic spine formation by 5-MeO-DMT have been shown.9
In light of the increased scientific interest in using low psychedelic doses,10 also known as “microdosing”,11 critical preclinical work with DMT has also shown that neuroplastic changes even take place after administration of low DMT doses that are considered to be subhallucinogenic.1 Examples are morphological changes in the prefrontal cortex of adult rats and functional changes ex vivo.1 The practice of microdosing entails repeatedly taking low doses, which are usually one-tenth of a recreational dose that causes a psychedelic experience. For LSD, that would, for example, be between 10 and 20 μg.12
User claims suggest the effectivity of self-medication with low doses of psychedelics in the treatment of disorders related to neuroplasticity, including depression.10 Interestingly, depression has been linked with impairments in neuroplasticity, and pharmacologically induced symptom improvement is linked with increases in BDNF levels.13,14 BDNF is highly expressed in limbic brain regions, which are involved in emotional processes, memory, and mood. Notably, Bershad et al. recently demonstrated connectivity changes in the limbic areas after a low dose of LSD (13 μg, tartrate).15 These biological changes correlated positively with the enhanced mood in healthy volunteers.15
Together, these findings add scientific evidence to the idea that LSD in low doses could have therapeutic potential in mood-related disorders.10 Given the interest in BDNF as a key player in several neurodegenerative and neuropsychiatric disorders13,16,17 and preclinical data showing psychedelics-induced neuroplasticity even at low doses of psychedelics,1 the present double-blind, placebo-controlled, within-subject (WS) study aimed to investigate whether LSD base in low doses (0, 5, 10, and 20 μg) affects BDNF plasma levels in healthy volunteers. Blood samples were collected every 2 h over 6 h, and BDNF levels were determined afterward in blood plasma using ELISA.18 Previously, it has been demonstrated that blood plasma BDNF concentrations reflect mammalian brain-tissue BDNF levels.19
Results
Difficulties with the peripheral venous catheter during blood sample collection resulted in missing data. For one participant, no blood samples were collected; for the remaining 23 participants, the percentage of samples over all time points ranged from 6 to 100%. Only five (21.7%) of the participants had a complete data set; therefore, we opted to run the analyses per dose for complete cases (placebo–LSD dose) to be able to perform statistical analyses. In Table 1, the demographic details of participants included in the statistical analyses are presented. Four trapezoidal areas under the curves (AUC) for BDNF were calculated for the three LSD doses and placebo; the same procedure was used for LSD concentrations.
Table 1. Participant Age and Sex per Complete Within-Placebo-LSD Dose Case.
| LSD dose (μg) | participants (number) | mean age (SD) | sex (male/female) |
|---|---|---|---|
| 5 | 10 | 21.5 (3.06) | 4:6 |
| 10 | 9 | 22.89 (2.80) | 5:4 |
| 20 | 8 | 23.75 (2.66) | 6:2 |
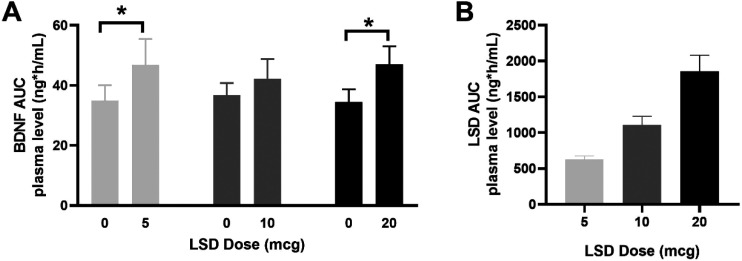
Wilcoxon signed-rank (S-R) tests revealed a statistically significant difference between AUC BDNF levels following 5 ug of LSD (Z = −2.60, p = 0.009, r = 0.58, 95% CI [0.11;1.06]) and 20 μg of LSD (Z = −2.52, p = 0.01, r = 0.63, 95% CI [0.09;1.17]) compared to placebo; the difference between AUC BDNF levels after 10 μg of LSD and placebo was not significant (Z = −1.01, p = 0.31) (Figure 1A). AUC LSD plasma levels for the selection of complete WS cases per dose are shown in Figure 1B for illustrative purposes to show that LSD plasma levels increased with increasing LSD doses.
Figure 1.
Total mean AUC (SEM) of BDNF (A) and of LSD (B) plasma levels for complete WS LSD dose-placebo cases. N(5 μg LSD) = 10; N(10 μg LSD) = 9; N(20 μg LSD) = 8. *, statistical significance at p ≤ 0.05.
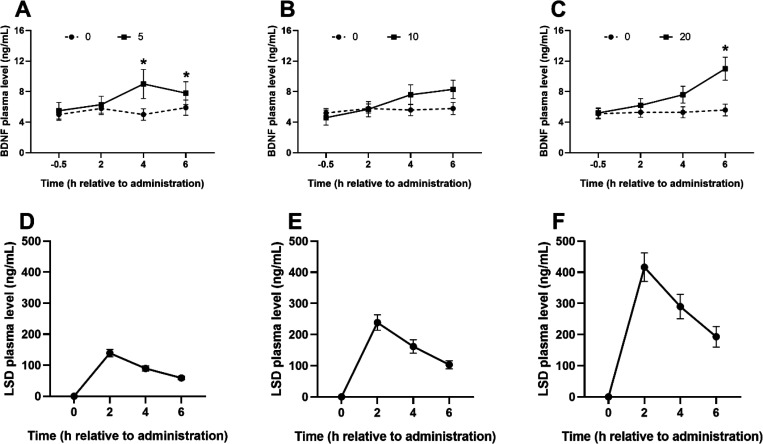
Wilcoxon S-R tests revealed higher BDNF levels at +4 h after administration of 5 μg of LSD (Z = −2.80, p < 0.01, r = 0.63, 95% CI [0.15; 1.11]) and 10 μg LSD (Z = −1.95, p = 0.05, r = 0.46, 95% CI [−0.04; 0.97]) compared to placebo. Although analysis including the 10 μg dose revealed a statistically significant p value (0.05), the CI included zero, indicating nonsignificance. Tests at +6 h after LSD administration revealed significant effects of 5 μg of LSD (Z = −2.29, p = 0.02, r = 0.51, 95% CI [0.03; 0.99]) and 20 μg of LSD (Z = −2.52, p = 0.01, r = 0.63, 95% CI [0.09; 1.17]) on BDNF levels compared to placebo (Figure 2A–C). Corresponding LSD plasma levels are presented in Figure 2D,E.
Figure 2.
Mean (SEM) BDNF plasma levels for each LSD dose with the corresponding WS placebo condition per time of testing (A–C) and corresponding mean (SEM) LSD plasma levels (D–F). *, statistical significance at p ≤ 0.05 in A–C; no statistical tests were performed over data in D–F.
Friedman tests investigating BDNF changes in the function of time demonstrated that BDNF plasma levels remained stable in the placebo conditions throughout the test frame. BDNF plasma levels under the LSD conditions showed their highest levels at 4 h after administration of 5 μg of LSD (8.95 ng/mL), and at 6 h after administration of 10 μg of LSD (8.28 ng/mL) and 20 μg of LSD (11 49 ng/mL) (Table 2).
Table 2. Friedman Test of Time on BDNF Plasma Levels in the LSD Conditions and Dunn’s Pairwise Comparisons.
| Friedman test main time effect | Dunn’s pairwise comparisons (Z(1))a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LSD dose (μg) | participants number | (x2(3)) | p | effect size Cramer’s V | 0 h–2 h | 0 h–4 h | 0 h–6h | 2 h–4 h | 4 h–6 h |
| 5 | 10 | 15.72 | <0.01 | 0.51 | 1.21 | 3.64* | 2.77* | 2.42* | 0.87 |
| 10 | 9 | 13.00 | <0.01 | 0.49 | 1.09 | 2.56* | 3.29* | 1.46 | 0.73 |
| 20 | 8 | 19.05 | <0.01 | 0.63 | 0.77 | 2.13 | 4.07* | 1.36 | 1.94 |
*, statistical significance at a sequential Bonferroni corrected p value < 0.05.
Discussion
This study provides preliminary evidence that low doses of LSD increase BDNF plasma levels in healthy volunteers up to 6 h after administration, suggesting a window of opportunity for a therapeutic response20,21 and cognitive enhancement22,23 that might be of use in patient populations. This line of thinking is supported by recent findings with ketamine and ayahuasca (containing the psychedelic DMT) demonstrating increased serum BDNF levels 24 and 48 h after a single (high) dose, respectively, compared to placebo, which was related to fast antidepressant actions.21,24,25
Of interest is the different time-course of BDNF levels for the 5 μ and 20 μg doses. While BDNF levels peaked at 4 h for the 5 μg dose, these levels significantly increased 2 h later for the 20 μg dose. Recently, Zhang et al. showed that a subanesthetic dose of IV ketamine (10 mg/kg/2h) increased BDNF in the amygdala at 2 h after administration, while 40 mg/kg/2 h did not affect BDNF levels, instead elevating levels of other proteins involved in plasticity (cFos, pERK) in the mPFC and hippocampus.26 Earlier, they showed that IV ketamine (20 mg/kg/2h) induced a decrease in BDNF plasma in rats at 2 h after ketamine administration, while 5 mg/kg/2h did not affect the levels.27 Their findings emphasize that BDNF levels undergo time-dependent changes following ketamine administration that can be influenced by the dose and timing of assay,26 something that might also explain the absence of effects in our study after dosing with 10 μg of LSD. Looking at the BDNF levels under the 20 μg dose condition suggests that the peak had yet to come. While the multiple assessment points in the present study were a strength, future studies might want to include extra assessment points beyond the 6 h postdrug period, even assessing the next day to understand the time-course of the effect.
While microdosing implies taking repeated doses of a psychedelic for a prolonged time, the present study only assessed the acute effects of a single administration on BDNF levels. Future studies will have to assess the effects of repeated dosing on neuroplasticity to understand whether or not this practice is beneficial to neuroplasticity. Previous studies investigating the repeated administration of ketamine and classical psychedelics have provided mixed results. Preclinical studies, and studies in ketamine abusers, for example, have shown that long term administration decreases the BDNF production in animals and humans.28,29 However, preclinical studies with repeated administration of high doses of serotonergic psychedelics demonstrated increased neuroplasticity.27
Concerning the underlying pathway, previously it was shown that the structural changes induced by psychedelics appear to result from stimulation of the TrkB, mTOR, and 5-HT2A signaling pathways.30 Ketamine is known to set off a signaling cascade by antagonizing NDMA receptors on presynaptic GABA neurons, resulting in an increased postsynaptic production of BDNF. The intermediate steps are increased presynaptic glutamate release and activation of mTOR pathways.31−33 Ketamine and LSD might share a final common pathway when it comes to stimulation of BDNF. Future studies might include other proteins as well, to understand the neurobiological pathways underlying neuroplasticity and the potential (therapeutic) implications of these induced changes. Potential foci might be proteins such as cFos and Perk, implicated in synaptic plasticity and memory formation,26 as ketamine is known to impact these.
While the present study had a small final sample size due to the difficulty in collecting blood over the 6 h course the participants were in the lab, the strength was the within-subject set up which neutralized the variation in the placebo–LSD comparisons and the multiple measurements after administration. Besides emphasizing the need to sample BDNF beyond the LSD elimination stage, in addition to including behavioral and imaging measures, future studies could focus on similarities between underlying biological pathways of the well-studied ketamine and LSD, as these will contribute to understanding the scope of effects LSD might have, based on ketamine findings. This first evidence of neuroplasticity in humans after low doses of a psychedelic provides a foundation to explore and replicate this finding in patient populations, to understand the therapeutic value of it, if any exists.
Materials and Methods
Participants were 24 recreational psychedelic users who provided informed consent, fell within the inclusion criteria,34 and passed medical screening including standard blood chemistry, hematology, and urinalysis before inclusion.
Test days were scheduled with minimally 5 days in between. A test day started at 9:00 AM with a screen for the presence of drugs of abuse in urine and alcohol in the breath, as well as a urine pregnancy test for female participants. When tests were negative, a venous catheter was placed to draw blood. LSD (5, 10, and 20 μg LSD base) was dissolved in 96% ethanol; the placebo consisted of 1 mL of ethanol (96%) without LSD. LSD and placebo were administered orally at 10:00 AM. A dose of 5, 10, and 20 μg LSD base would be equivalent to respectively 6.2, 12.3, and 24.6 μg pure LSD tartrate (1:0.5 without any crystal water). Participants were allocated to unique treatment orders. Blood samples were taken at −0.5 h, +2 h, +4 h, and +6 h relative to drug administration using BD vacutainer heparin tubes spray-coated with lithium heparin. Samples were centrifuged, and plasma was transferred into a clean tube and frozen subsequently at −20 °C until analysis. BDNF determination was assessed using an ELISA kit (Biosensis Mature BDNF Rapid ELISA kit: human, mouse, rat; Thebarton, Australia).18 Plasma samples were appropriately diluted (1:20), and detection of BDNF was carried out on a precoated mouse monoclonal anti-mature BDNF 96-well plate as described in the manufacturer’s protocol. The intra- and interassay coefficients of variation of this assay are below 10% (intra-assay CV 4.29%, interassay CV 7.14%). Samples were analyzed in duplicate, and mean values of respective measurements were calculated and used in statistical analyses. All measurements were done in a blinded fashion. LSD concentrations were determined using ultra-high-performance liquid chromatography/tandem mass spectrometry (UHPLC–MS/MS) as previously described.35 A different extraction procedure reanalyzed samples with an LSD concentration below 5 pg/mL. In brief, aliquots of 150 μL of plasma were extracted with 450 μL of methanol. The samples were rigorously mixed and subsequently centrifuged. The supernatant was evaporated under a constant stream of nitrogen and resuspended in 200 μL of mobile phases A and B (10:90 v/v). An LLOQ of 2.5 pg/mL was reached by this extraction.
This study is part of a more extensive study, including cognitive, psychological, and physiological parameters which are reported elsewhere.34 The study adhered to the code of ethics on human experimentation,36 was approved by the Medical Ethics Committee of the Academic Hospital of Maastricht and Maastricht University, and was registered in the Dutch Clinical Trial register (number: NTR7102 https://www.trialregister.nl/). A permit for obtaining, storing, and administering LSD was obtained from the Dutch Drug Enforcement Administration.
Statistical Analysis
Complete WS cases entered statistical analyses performed by the statistical program SPSS (version 25.0). Nonparametric Wilcoxon S-R tests for related samples (placebo–LSD dose) were conducted on BDNF AUC’s and BDNF plasma levels at −0.5 h, +2 h, +4 h, and +6 h after dose administration of 5, 10, and 20 μg of LSD. In order to understand at which time points BDNF levels were statistically different, separate Friedman tests per treatment condition were performed, and in the case of a main effect, followed by Dunn’s tests for pairwise comparisons including baseline (0 h) versus 2 h, 4 h, and 6 h, as well as 2 h–4 h and 4 h–6 h.
In the case of statistically significant effects at alpha = 0.05, effect sizes and their 95% confidence intervals (95% CI) are given; to that end, (point-biserial) correlations are calculated for Wilcoxon tests where 0.10, 0.24, and 0.37 signify small, moderate, and large effect sizes;37,38 in case of Friedman tests, Cramer’s V was calculated where 0.06, 0.17, and 0.29 signify small, moderate, and large effect sizes.39 The alpha level was corrected for multiple comparisons with sequential Bonferroni in case of Dunn’s tests.
Acknowledgments
The authors thank the medical supervisor, Cees van Leeuwen, and the interns who worked on this project. A financial contribution was received from the Beckley Foundation.
Glossary
Abbreviations
- 5-HT2A
Serotonin 2A receptor
- BDNF
Brain-derived neurotrophic factor
- CI
Confidence interval
- ELISA
Enzyme-linked immunosorbent assay
- i.p.
Intraperitoneal
- IV
Intraveneous
- LSD
d-Lysergic acid diethylamide
- mPFC
Medial prefrontal cortex
- mTOR
Mammalian target of rapamycin
- NMDA
N-Methyl-d-aspartate
- pERK
Phosphorylation of extracellular signal-regulated kinase
- S-R
Signed-rank
- WS
Within subject
Author Contributions
N.R.P.W.N., N.L.M., P.C.D., A.F., J.G.R., K.P.C.K. conceptualized the study and wrote the protocol. N.R.P.W.N., F.H., M.E.L., N.V., A.E., K.P.C.K. collected and/or analyzed the data. N.R.P.W.N. and K.P.C.K. wrote the manuscript. All authors contributed to versions leading up to the final manuscript.
The authors declare no competing financial interest.
References
- Ly C.; Greb A. C.; Cameron L. P.; Wong J. M.; Barragan E. V.; Wilson P. C.; Burbach K. F.; Soltanzadeh Zarandi S.; Sood A.; Paddy M. R.; Duim W. C.; Dennis M. Y.; McAllister A. K.; Ori-McKenney K. M.; Gray J. A.; Olson D. E. (2018) Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 23 (11), 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlow B. J., Jalloh A., and Sanchez-Ramos J. (2016) Chapter 77 - Hippocampal Neurogenesis: Effects of Psychedelic Drugs, in Neuropathology of Drug Addictions and Substance Misuse (Preedy V. R., Ed.), Academic Press, San Diego, CA, pp 821–831. [Google Scholar]
- Morales-García J. A.; de la Fuente Revenga M.; Alonso-Gil S.; Rodríguez-Franco M. I.; Feilding A.; Perez-Castillo A.; Riba J. (2017) The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci. Rep. 7 (1), 5309. 10.1038/s41598-017-05407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin D.; Mansouri N. (2006) Antidepressant-like effect of harmane and other β-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 16 (5), 324–328. 10.1016/j.euroneuro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fortunato J. J.; Réus G. Z.; Kirsch T. R.; Stringari R. B.; Fries G. R.; Kapczinski F.; Hallak J. E.; Zuardi A. W.; Crippa J. A.; Quevedo J. (2010) Chronic administration of harmine elicits antidepressant-like effects and increases BDNF levels in rat hippocampus. Journal of neural transmission 117 (10), 1131–1137. 10.1007/s00702-010-0451-2. [DOI] [PubMed] [Google Scholar]
- Catlow B. J.; Song S.; Paredes D. A.; Kirstein C. L.; Sanchez-Ramos J. (2013) Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res. 228 (4), 481–491. 10.1007/s00221-013-3579-0. [DOI] [PubMed] [Google Scholar]
- Colaço C. S.; Alves S. S.; Nolli L. M.; Pinheiro W. O.; de Oliveira D. G. R.; Santos B. W. L.; Pic-Taylor A.; Mortari M. R.; Caldas E. D. (2020) Toxicity of ayahuasca after 28 days daily exposure and effects on monoamines and brain-derived neurotrophic factor (BDNF) in brain of Wistar rats. Metab. Brain Dis. 35 (5), 739–751. 10.1007/s11011-020-00547-w. [DOI] [PubMed] [Google Scholar]
- Szabo A.; Kovacs A.; Riba J.; Djurovic S.; Rajnavolgyi E.; Frecska E. (2016) The endogenous hallucinogen and trace amine N,N-dimethyltryptamine (DMT) displays potent protective effects against hypoxia via sigma-1 receptor activation in human primary iPSC-derived cortical neurons and microglia-like immune cells. Front. Neurosci. 10, 423. 10.3389/fnins.2016.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakic V.; Minardi Nascimento J.; Costa Sartore R.; Maciel R. d. M.; de Araujo D. B.; Ribeiro S.; Martins-de-Souza D.; Rehen S. K. (2017) Short term changes in the proteome of human cerebral organoids induced by 5-MeO-DMT. Sci. Rep. 7 (1), 12863. 10.1038/s41598-017-12779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten N. R. P. W.; Mason N. L.; Dolder P. C.; Kuypers K. P. C. (2019) Self-Rated Effectiveness of Microdosing With Psychedelics for Mental and Physical Health Problems Among Microdosers. Front. Psychiatry 10, 672. 10.3389/fpsyt.2019.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiman J. (2011) The Psychedelic Explorer’s Guide: Safe, Therapeutic, and Sacred Journeys, Simon and Schuster. [DOI] [PubMed] [Google Scholar]
- Kuypers K. P.; Ng L.; Erritzoe D.; Knudsen G. M; Nichols C. D; Nichols D. E; Pani L.; Soula A.; Nutt D. (2019) Microdosing Psychedelics: more questions than answers? An overview and suggestions for future research. J. Psychopharmacol. 33, 1039. 10.1177/0269881119857204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A. R.; Lopes M.; Fregni F. (2008) A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 11 (8), 1169–80. 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Price R. B.; Duman R. (2020) Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol. Psychiatry 25 (3), 530–543. 10.1038/s41380-019-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershad A. K.; Preller K. H.; Lee R.; Keedy S.; Wren-Jarvis J.; Bremmer M. P.; de Wit H. (2020) Preliminary Report on the Effects of a Low Dose of LSD on Resting-State Amygdala Functional Connectivity. Biological psychiatry. Cognitive neuroscience and neuroimaging 5 (4), 461–467. 10.1016/j.bpsc.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S. J.; Dawbarn D. (2006) Clinical relevance of the neurotrophins and their receptors. Clin. Sci. 110 (2), 175–91. 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- Nagahara A. H.; Merrill D. A.; Coppola G.; Tsukada S.; Schroeder B. E.; Shaked G. M.; Wang L.; Blesch A.; Kim A.; Conner J. M.; Rockenstein E.; Chao M. V.; Koo E. H.; Geschwind D.; Masliah E.; Chiba A. A.; Tuszynski M. H. (2009) Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 15 (3), 331–7. 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto H.; Oshima S.; Sugiyama T.; Negishi A.; Nemoto T.; Kobayashi D. (2019) Changes in brain metabolites related to stress resilience: Metabolomic analysis of the hippocampus in a rat model of depression. Behav. Brain Res. 359, 342–352. 10.1016/j.bbr.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Klein A. B.; Williamson R.; Santini M. A.; Clemmensen C.; Ettrup A.; Rios M.; Knudsen G. M.; Aznar S. (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 14 (3), 347–353. 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- Lepack A. E.; Fuchikami M.; Dwyer J. M.; Banasr M.; Duman R. S. (2015) BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 18 (1), pyu033. 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida R. N.; Galvão A. C. M.; da Silva F. S.; Silva E.; Palhano-Fontes F.; Maia-de-Oliveira J. P.; de Araújo L. B.; Lobão-Soares B.; Galvão-Coelho N. L. (2019) Modulation of Serum Brain-Derived Neurotrophic Factor by a Single Dose of Ayahuasca: Observation From a Randomized Controlled Trial. Front. Psychol. 10, 1234. 10.3389/fpsyg.2019.01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin É. W.; Bechara R. G.; Birch A. M.; Kelly Á. M. (2009) Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus 19 (10), 973–980. 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Hwang J.; Brothers R. M.; Castelli D. M.; Glowacki E. M.; Chen Y. T.; Salinas M. M.; Kim J.; Jung Y.; Calvert H. G. (2016) Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci. Lett. 630, 247–253. 10.1016/j.neulet.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Duman R. S.; Monteggia L. M. (2006) A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59 (12), 1116–1127. 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Woelfer M.; Li M.; Colic L.; Liebe T.; Di X.; Biswal B.; Murrough J.; Lessmann V.; Brigadski T.; Walter M. (2019) Ketamine-induced changes in plasma brain-derived neurotrophic factor (BDNF) levels are associated with the resting-state functional connectivity of the prefrontal cortex. world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry 1–15. 10.1080/15622975.2019.1679391. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Radford K. D.; Driscoll M.; Purnomo S.; Kim J.; Choi K. H. (2019) Effects of subanesthetic intravenous ketamine infusion on neuroplasticity-related proteins in the prefrontal cortex, amygdala, and hippocampus of Sprague-Dawley rats. IBRO Reports 6, 87–94. 10.1016/j.ibror.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford C. K. D.; Park T. Y.; Osborne-Smith L.; Choi K. H. (2018) Effects of Subanesthetic Intravenous Ketamine Infusion on Corticosterone and Brain-Derived Neurotrophic Factor in the Plasma of Male Sprague-Dawley Rats. AANA J. 86 (5), 393–400. [PubMed] [Google Scholar]
- Fraga D. B.; Réus G. Z.; Abelaira H. M.; De Luca R. D.; Canever L.; Pfaffenseller B.; Colpo G. D.; Kapczinski F.; Quevedo J.; Zugno A. I. (2013) Ketamine alters behavior and decreases BDNF levels in the rat brain as a function of time after drug administration. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999) 35 (3), 262–6. 10.1590/1516-4446-2012-0858. [DOI] [PubMed] [Google Scholar]
- Ke X.; Ding Y.; Xu K.; He H.; Zhang M.; Wang D.; Deng X.; Zhang X.; Zhou C.; Liu Y.; Ning Y.; Fan N. (2014) Serum brain-derived neurotrophic factor and nerve growth factor decreased in chronic ketamine abusers. Drug Alcohol Depend. 142, 290–4. 10.1016/j.drugalcdep.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C.; Greb A. C.; Cameron L. P.; Wong J. M.; Barragan E. V.; Wilson P. C.; Burbach K. F.; Soltanzadeh Zarandi S.; Sood A.; Paddy M. R.; et al. (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep. 23 (11), 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B.; Adams B.; Verma A.; Daly D. (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17 (8), 2921–7. 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C. A.; Lucki I. (2013) Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front. Pharmacol. 4, 161–161. 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R. S.; Li N.; Liu R.-J.; Duric V.; Aghajanian G. (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62 (1), 35–41. 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers J. G., Hutten N. R. P. W., Mason N. L., Dolder P. C., Theunissen E. L., Holze F., Liechti M. E., Feilding A., and Kuypers K. P. (2020) A low dose of lysergic acid diethylamide (LSD) decreases pain perception in healthy volunteers. J. Psychopharmacol. 10.1177/0269881120940937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holze F.; Duthaler U.; Vizeli P.; Müller F.; Borgwardt S.; Liechti M. E. (2019) Pharmacokinetics and subjective effects of a novel oral LSD formulation in healthy subjects. Br. J. Clin. Pharmacol. 85, 1474. 10.1111/bcp.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310 (20), 2191–2194. 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Ivarsson A.; Andersen M. B.; Johnson U.; Lindwall M. (2013) To adjust or not adjust: Nonparametric effect sizes, confidence intervals, and real-world meaning. Psychology of Sport and Exercise 14 (1), 97–102. 10.1016/j.psychsport.2012.07.007. [DOI] [Google Scholar]
- Kornbrot D. (2014) Point Biserial Correlation, in Wiley StatsRef: Statistics Reference Online, Wiley. https://onlinelibrary.wiley.com/doi/10.1002/9781118445112.stat06227.
- Kim H.-Y. (2017) Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor Dent Endod 42 (2), 152–155. 10.5395/rde.2017.42.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]