Abstract
The 5-HT2A receptor is thought to be the primary target for psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) and other serotonergic hallucinogens (psychedelic drugs). Although a large amount of experimental work has been conducted to characterize the pharmacology of psilocybin and its dephosphorylated metabolite psilocin (4-hydroxy-N,N-dimethyltryptamine), there has been little systematic investigation of the structure–activity relationships (SAR) of 4-substituted tryptamine derivatives. In addition, structural analogs of psilocybin containing a 4-acetoxy group, such as 4-acetoxy-N,N-dimethyltryptamine (4-AcO-DMT), have appeared as new designer drugs, but almost nothing is known about their pharmacological effects. To address the gap of information, studies were conducted with 17 tryptamines containing a variety of symmetrical and asymmetrical N,N-dialkyl substituents and either a 4-hydroxy or 4-acetoxy group. Calcium mobilization assays were conducted to assess functional activity at human and mouse 5-HT2 subtypes. Head-twitch response (HTR) studies were conducted in C57BL/6J mice to assess 5-HT2A activation in vivo. All of the compounds acted as full or partial agonists at 5-HT2 subtypes, displaying similar potencies at 5-HT2A and 5-HT2B receptors, but some tryptamines with bulkier N-alkyl groups had lower potency at 5-HT2C receptors and higher 5-HT2B receptor efficacy. In addition, O-acetylation reduced the in vitro 5-HT2A potency of 4-hydroxy-N,N-dialkyltryptamines by about 10- to 20-fold but did not alter agonist efficacy. All of the compounds induce head twitches in mice, consistent with an LSD-like behavioral profile. In contrast to the functional data, acetylation of the 4-hydroxy group had little effect on HTR potency, suggesting that O-acetylated tryptamines may be deacetylated in vivo, acting as prodrugs. In summary, the tryptamine derivatives have psilocybin-like pharmacological properties, supporting their classification as psychedelic drugs.
Keywords: hallucinogen, psychedelic drug, behavior, functional assay, serotonin, 5-HT2A
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), a prototypical serotonergic hallucinogen that produces effects similar to those of lysergic acid diethylamide (LSD) and mescaline, is the major active constituent of Psilocybe mexicana and other species of hallucinogenic mushrooms (“magic mushrooms”). Psilocybin is rapidly dephosphorylated to psilocin (4-hydroxy-N,N-dimethyltryptamine, 4-HO-DMT) by alkaline phosphatase in vitro(15−38) and in vivo.32,39 Although psilocybin and psilocin have equivalent molar potencies in vivo,77 psilocybin has considerably lower potency at the receptor level,69 indicating that it may serve as a prodrug for psilocin. Over the past decade there has been a renewed interest in the pharmacology and effects of psilocybin due to accumulating evidence that it possesses therapeutic efficacy against disorders such as anxiety, depression, obsessive-compulsive disorder, and substance abuse.6 In addition, psilocybin continues to be a popular recreational drug.49−59
Following the isolation of psilocybin and psilocin by Hofmann and colleagues in 1957,34 various 4-substituted tryptamines were reported in the literature. For example, Hofmann synthesized 4-hydroxy-N,N-diethyltryptamine (CZ-74, 4-HO-DET) and 4-phosphoryloxy-N,N-diethyltryptamine (CEY-19).75 Similar to psilocybin and psilocin, CEY-19 and CZ-74 have equivalent molar potencies in humans.3,50 Repke synthesized several other psilocin homologues, including 4-hydroxy-N-methyl-N-ethyltryptamine (4-HO-MET), 4-hydroxy-N-methyl-N-isopropyltryptamine (4-HO-MIPT), 4-hydroxy-N,N-dipropyltryptamine (4-HO-DPT), and 4-hydroxy-N,N-diisopropyltryptamine (4-HO-DIPT).63,64 Tryptamines containing 4-acetoxy groups have also been synthesized. 4-Acetoxy-N,N-dimethyltryptamine (O-acetylpsilocin, 4-AcO-DMT) was patented by Hofmann in 1963.33 Shulgin and Shulgin71 experimented with 4-AcO-DMT and its N,N-diethyl (4-AcO-DET) and N-methyl-N-isopropyl (4-AcO-MIPT) homologues and found them to be potent psychedelic drugs. Subsequently, Nichols published an improved synthesis for 4-AcO-DMT and proposed that it could serve as an alternative prodrug for psilocin in scientific studies.56
Although psilocin and psilocybin have been available on the illicit market since the 1960s, the recreational use of other 4-substituted tryptamines is a more recent development, fueled by marketing and distribution via the internet. 4-Acetoxy-N,N-diisopropyltryptamine (4-AcO-DIPT) was first detected in Europe in 2005,16 followed by 4-AcO-DMT,17 4-AcO-MET,17 and 4-AcO-DPT.18 4-HO-MET and 4-HO-DPT have also been detected.17,18 In 2011, Kjellgran and Soussan published a detailed description of the phenomenological effects of 4-HO-MET in Swedish users.43 Overall, reports indicate that most 4-substituted tryptamines produce psilocybin-like psychedelic effects.26−74
Despite the increasing popularity and availability of 4-acetoxy-N,N-dialkyltryptamines, there is a lack of information about their pharmacological and behavioral properties. Furthermore, although numerous N,N-dialkyltryptamines have been explored,5−54 there has been little systematic investigation of the effect of N-alkyl substitution on their activity. The goal of the present investigation was to address the gap of knowledge regarding the structure–activity relationships (SARs) of tryptamine hallucinogens containing an oxygenated substituent at the 4-position of the indole ring. We focused on activity at the 5-HT2A receptor, which is thought to be the primary target for psilocybin and other psychedelic drugs in humans and rodents.27,55 Calcium mobilization assays were conducted to assess functional activation at human and mouse 5-HT2A, as well as human 5-HT2B and 5-HT2C receptors. Behavioral data from the mouse head-twitch response (HTR) assay were used as a measure of 5-HT2A receptor activation in vivo.9,29 The HTR assay is widely used as a behavioral proxy in rodents for human hallucinogenic effects because it is one of only a few behaviors that can reliably distinguish hallucinogenic and nonhallucinogenic 5-HT2A receptor agonists.25 The studies were conducted with 16 tryptamine derivatives containing a variety of symmetrical and asymmetrical N,N-dialkyl substituents and either a 4-hydroxy or a 4-acetoxy group. Activity was assessed in vitro and in vivo to generate converging evidence and to evaluate the likelihood that the 4-acetoxy-N,N-dialkyltryptamines are serving as prodrugs for their 4-hydroxy counterparts.
Methods
Animals
Male C57BL/6J mice (6–8 weeks old) obtained from Jackson Laboratories (Bar Harbor, ME) were housed in a vivarium at the University of California San Diego, an AAALAC-approved animal facility that meets all federal and state requirements for care and treatment of laboratory animals. Mice were housed up to four per cage in a climate-controlled room on a reverse-light cycle (lights on at 1900 h, off at 0700 h) and were provided with ad libitum access to food and water, except during behavioral testing. Testing was conducted between 1000 and 1800 h. All animal experiments were carried out in accordance with NIH guidelines and were approved by the UCSD Institutional Animal Care and Use Committee.
Drugs
4-Acetoxy-N,N-dimethyltryptamine (4-AcO-DMT) fumarate, 4-hydroxy-N,N-diethyltryptamine (4-HO-DET) hydrochloride, 4-acetoxy-N,N-diethyltryptamine (4-AcO-DET) fumarate, 4-hydroxy-N-methyl-N-ethyltryptamine (4-HO-MET) hemifumarate, 4-acetoxy-N-methyl-N-ethyltryptamine (4-AcO-MET) fumarate, 4-hydroxy-N-methyl-N-propyltryptamine (4-HO-MPT) fumarate, 4-acetoxy-N-methyl-N-propyltryptamine (4-AcO-MPT) fumarate, 4-hydroxy-N-ethyl-N-propyltryptamine (4-HO-EPT) 3:2 fumarate, 4-acetoxy-N-ethyl-N-propyltryptamine (4-AcO-EPT) fumarate, 4-hydroxy-N,N-dipropyltryptamine (4-HO-DPT) hemifumarate, 4-acetoxy-N,N-dipropyltryptamine (4-AcO-DPT) fumarate, 4-hydroxy-N-methyl-N-isopropyltryptamine (4-HO-MIPT) hemifumarate, 4-acetoxy-N-methyl-N-isopropyltryptamine (4-AcO-MIPT) fumarate, 4-hydroxy-N,N-diisopropyltryptamine (4-HO-DIPT) hydrochloride, and 4-hydroxy-N-methyl-N-allyltryptamine (4-HO-MALT) 3:2 fumarate were available from previous studies performed in our laboratories. 4-Acetoxy-N,N-diisopropyltryptamine (4-AcO-DIPT) acetate was obtained from Cayman Chemical (Ann Arbor, MI). Psilocin was obtained from the National Institute on Drug Abuse (Rockville, MD). The identity and analytical purity of the test substances were confirmed using mass spectrometry and nuclear magnetic resonance spectroscopy. All test substances had a minimum purity of >95%. For behavioral studies, psilocin was dissolved in water containing 5 mM tartaric acid (pH ∼5.0); all other compounds were dissolved in isotonic saline. Test substances were administered intraperitoneally (IP) at a volume of 5 mL/kg. For in vitro studies, all compounds were dissolved in DMSO at 10 mM concentration before serial dilution.
Head-Twitch Response Studies
The HTR was assessed using a head-mounted neodymium magnet and a magnetometer coil.29,30 Briefly, mice were anesthetized, a small incision was made in the scalp, and a neodymium magnet was attached to the dorsal surface of the cranium using dental cement. Following a 1 week recovery period, HTR experiments were carried out in a well-lit room with at least 7 days between sessions to avoid carryover effects. After magnet implantation, mice were tested in multiple HTR experiments for up to 4–5 months. Test compounds were injected immediately prior to testing, and then HTR activity was recorded for 30 min in a 12.5 cm glass cylinder surrounded by a magnetometer coil. Coil voltage was low-pass-filtered (2–10 kHz cutoff frequency), amplified, and digitized (20 kHz sampling rate) using a Powerlab/8SP data acquisition system with LabChart v 7.3.2 (ADInstruments, Colorado Springs, CO), then filtered off-line (40–200 Hz band-pass). Head twitches were identified based on the following criteria: (1) sinusoidal wavelets; (2) evidence of at least three sequential head movements (usually exhibited as bipolar peaks) with a frequency of ≥40 Hz; (3) amplitude exceeding the level of background noise; (4) duration < 0.15 s; and (5) stable coil voltage immediately preceding and following each response.
The entire 30 min recordings were examined for head twitches, but in some instances, a shorter block of time was used for analysis to accommodate compounds with a relatively brief duration of action. HTR counts were analyzed using one-way analyses of variance (ANOVA). Post hoc pairwise comparisons between selected groups were performed using Tukey’s studentized range method. Significance was demonstrated when an α-level of 0.05 was surpassed. Median effective doses (ED50 values) and 95% confidence intervals (95% CI) for HTR dose–response experiments were calculated by nonlinear regression (Prism 7.00, GraphPad Software, San Diego, CA).
5-HT2 Receptor Functional Assays
5-HT2 functional experiments (measuring Gq-mediated calcium flux) were performed with Flp-In T-REx 293 cells (Invitrogen, Carlsbad, CA) expressing either human 5-HT2A (h5-HT2A), mouse 5-HT2A (m5-HT2A), human 5-HT2B (h5-HT2B), or human 5-HT2C INI (h5-HT2C) receptor cDNA under the tetracycline repressor protein. Cells were plated into black 384-well clear-bottomed tissue culture plates in 40 μL of DMEM containing 1% dialyzed fetal bovine serum (FBS) at a density of approximately 10 000 cells per well, and receptor expression was induced with 2 μg/mL tetracycline. After approximately 20–24 h, the medium was decanted and replaced with 20 μL per well of drug buffer (HBSS, 20 mM HEPES, pH 7.4) containing Fluo-4 Direct dye (Invitrogen) and incubated for between 1 and 2 h at 37 °C. Test substances were diluted in drug buffer (HBSS, 20 mM HEPES, 0.1% bovine serum albumin, 0.01% ascorbic acid, pH 7.4). Before the experiment, plates were allowed to equilibrate to room temperature, and calcium flux was measured using a FLIPRTETRA cellular screening system (Molecular Devices, Sunnyvale, CA). Plates were read for fluorescence initially for 10 s (1 read per second) to establish a baseline and then stimulated with drug dilutions or buffer and read for an additional 120 s. Peak fluorescence in each well was normalized to the maximum fold increase over baseline. Data were normalized to the maximum peak fold over basal fluorescence produced by 5-HT (100%) and baseline fluorescence (0%). Data were analyzed using the sigmoidal dose–response function of Prism 5.0 or 8.0 (GraphPad Software, San Diego, CA). Relative activity (RA) was expressed as the logarithm of the ratio of Emax over EC50 parameter estimates.
Results
4-Hydroxy-N,N-dialkyltryptamines Induce the Head-Twitch Response
Previous studies, conducted using traditional experimental methods, have shown that psilocin is active in the HTR paradigm.25,11,31 Using a magnetometer assessment method,29 we confirmed that psilocin induces the HTR in C57BL/6J mice with ED50 = 0.17 mg/kg, which is equivalent to 0.81 μmol/kg. As shown in Table 1, all of the 4-hydroxy-N,N-dialkyltryptamines induced the HTR (full experimental details are provided in Table S1). Similar to other tryptamine hallucinogens,19−44 the HTR followed an inverted-U-shaped dose–response function. The dose–response curves for psilocin and 4-HO-MET are shown in Figure 1A as representative examples. Overall, some variation in potency was noted, ranging from 4-HO-MET (ED50 = 0.65 μmol/kg) to 4-HO-DIPT (ED50 = 3.46 μmol/kg). Potency in the HTR assay appears to be related to the steric properties of the alkyl groups on the amine nitrogen. For example, the rank order of potency for 4-hydroxytryptamines with symmetrical alkyl chains was as follows: psilocin (ED50 = 0.81 μmol/kg) > 4-HO-DET (ED50 = 1.56 μmol/kg) > 4-HO-DPT (ED50 = 2.47 μmol/kg) > 4-HO-DIPT (ED50 = 3.46 μmol/kg). A similar relationship exists for the psilocin analogs with asymmetrical alkyl substituents: 4-HO-MET (ED50 = 0.65 μmol/kg) > 4-HO-MPT (ED50 = 1.92 μmol/kg) > 4-HO-MIPT (ED50 = 2.97 μmol/kg). We examined the relationship between HTR potency and the steric properties of the amine substituents using Charton’s upsilon parameter υ (which is based on van der Waals radii) as a steric descriptor.10 For the eight N,N-dialkyl-4-hydroxytryptamines, −log ED50 values in the HTR assay were negatively correlated (R = −0.8283, p = 0.011) with the sum of the values of υ for the two amine substituents.
Table 1. Potency of 4-Hydroxy-N,N-dialkyltryptamines in HTR Experiments Conducted in C57BL/6J Mice.
| ED50 (95% CI) |
||
|---|---|---|
| drug | mg/kg | μmol/kg |
| psilocin (4-HO-DMT) | 0.17 (0.12–0.23) | 0.81 (0.57–1.15) |
| 4-HO-MET | 0.18 (0.12–0.27) | 0.65 (0.44–0.97) |
| 4-HO-MPT | 0.67 (0.46–0.97) | 1.92 (1.33–2.78) |
| 4-HO-MIPT | 0.86 (0.72–1.21) | 2.97 (2.49–3.54) |
| 4-HO-DET | 0.42 (0.26–0.67) | 1.56 (0.98–2.51) |
| 4-HO-EPT | 0.42 (0.26–0.68) | 1.01 (0.63–1.62) |
| 4-HO-DPT | 0.79 (0.46–1.35) | 2.47 (1.43–4.25) |
| 4-HO-DIPT | 1.03 (0.67–1.57) | 3.46 (2.27–5.28) |
| 4-HO-MALT | 0.91 (0.59–1.40) | 2.24 (1.45–3.46) |
Figure 1.
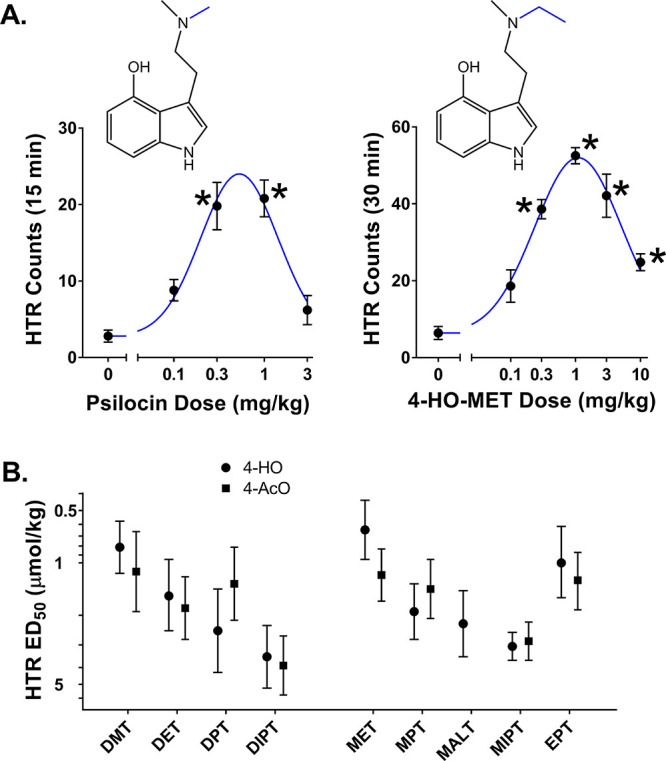
4-Substituted tryptamines induce the HTR in C57BL/6J mice. (A) Effect of psilocin (left) and 4-HO-MET (right) on the HTR. Data are presented as group means ± SEM for the entire test session. *, p < 0.05, significant difference from the vehicle control group (Tukey’s test). (B) Comparison of the potencies (ED50 values, in μmol/kg) of 4-hydroxytryptamines (●) and 4-acetoxytryptamines (■) in HTR experiments.
Effect of O-Acetylation on Activity in the HTR Paradigm
Similarly to the 4-hydroxy-N,N-dialkyltryptamines, the 4-acetoxy-N,N-dialkyltryptamines were also active in the HTR assay (see Table 2). O-Acetylation did not reliably alter the potency of psilocin or its homologues; potency was increased in some cases and reduced in others (Figure 1B). In general, however, there was little difference in potency between the 4-hydroxy-N,N-dialkyltryptamines and their acetate esters. For the eight 4-acetoxy-N,N-dialkyltryptamines, −log ED50 values in the HTR assay were also negatively correlated (R = −0.7208, p = 0.043) with the sum of the values of υ for the two amine substituents, although the relationship was not as robust as was found for the 4-hydroxy-N,N-dialkyltryptamines.
Table 2. Potency of 4-Acetoxy-N,N-dialkyltryptamines in HTR Experiments Conducted in C57BL/6J Mice.
| ED50 (95% CI) |
||
|---|---|---|
| drug | mg/kg | μmol/kg |
| 4-AcO-DMT | 0.41 (0.24–0.69) | 1.12 (0.66–1.90) |
| 4-AcO-MET | 0.44 (0.31–0.63) | 1.17 (0.82–1.67) |
| 4-AcO-MPT | 0.55 (0.37–0.81) | 1.41 (0.96–2.08) |
| 4-AcO-MIPT | 1.11 (0.87–1.41) | 2.84 (2.22–3.62) |
| 4-AcO-DET | 0.71 (0.47–1.07) | 1.81 (1.20–2.74) |
| 4-AcO-EPT | 0.51 (0.35–0.74) | 1.26 (0.86–1.84) |
| 4-AcO-DPT | 0.55 (0.35–0.89) | 1.32 (0.82–2.12) |
| 4-AcO-DIPT | 1.41 (0.95–2.08) | 3.88 (2.63–5.72) |
Comparison of the Time Course and Magnitude of the In Vivo Responses
Figure 2 depicts the time course of effects on the HTR for a subset of the compounds. After binning all the experiments in 2-min time blocks, a few of the acetate esters appeared to produce a maximal response greater than that of the corresponding 4-hydroxytryptamines. Nevertheless, as shown in Figure 2, O-acetylation did not alter the time course of the response, with the maximal response typically occurring during the first 10 min after drug administration. There was some variation in the duration of action of the tryptamines. For example, the response to 4-HO-DPT and 4-AcO-DPT began to decline within the first 10 min, whereas 4-HO-DIPT and 4-AcO-DIPT produced longer-lasting effects.
Figure 2.
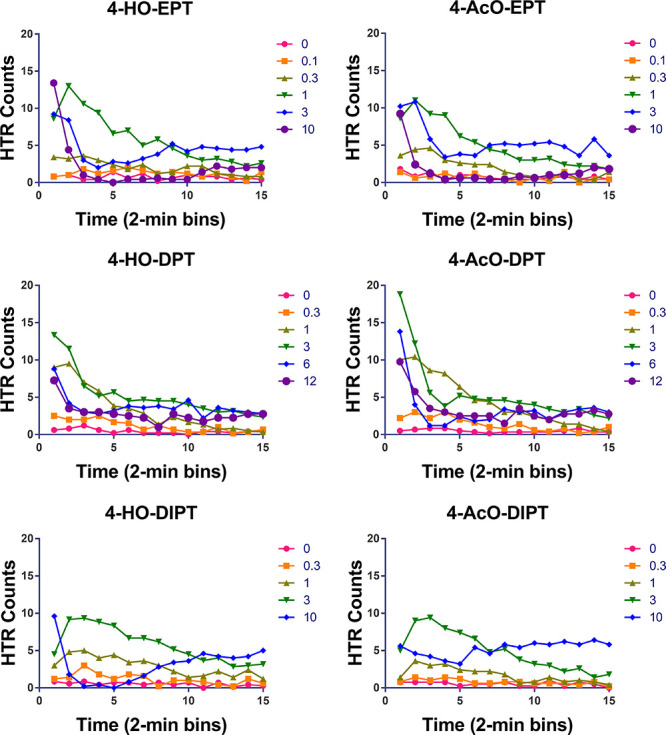
Time-course of the HTR induced by 4-substituted tryptamines. Data are presented as group means during consecutive 2-min time bins. Drug doses are shown in mg/kg.
4-Substituted N,N-Dialkyltryptamines Act as 5-HT2 Receptor Agonists
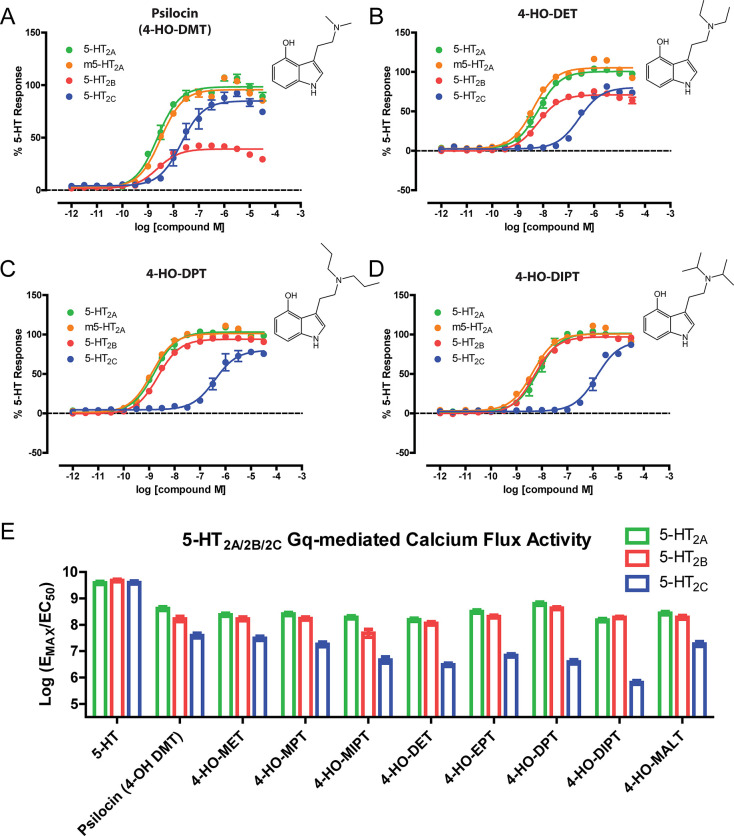
In vitro functional activity at 5-HT2A, 5-HT2B, and 5-HT2C receptors was assessed using calcium flux assays (see Figure 3 and Table 3). All of the 4-substituted tryptamines stimulated calcium mobilization via activation of human and mouse 5-HT2A receptors. In fact, most of the 4-hydroxy tryptamines had similar potency and efficacy at mouse and human 5-HT2A receptors. Almost all of the compounds behaved as highly efficacious 5-HT2A agonists (Emax range of 90–100% relative to 5-HT), with the exception of the O-acetylated tryptamines 4-AcO-DMT (Emax = 79.2%) and 4-AcO-DIPT (Emax = 74.6%). Notably, the 4-hydroxy tryptamines had high potency at 5-HT2A (EC50 values ranging from about 1–10 nM), while the potency of the O-acetylated tryptamines was about an order of magnitude weaker (ranging from 10- to 40-fold) compared to their 4-hydroxy counterparts.
Figure 3.
In vitro activity of 4-substituted tryptamines at 5-HT2 receptors. All compounds were assayed in parallel measuring Gq-mediated calcium flux using the same drug dilutions for psilocin (A), 4-HO-DET (B), 4-HO-DPT (C), and 4-HO-DIPT (D) at h5-HT2A (green), m5-HT2A (orange), h5-HT2B (red), and h5-HT2C (blue) receptors expressing Flp-In T-REx 293 stable cell lines. Data represent concentration–response curves plotting mean and SEM of data points performed in triplicate from at least three independent experiments. (E) Relative activities of the compounds (log(EMAX/EC50) are plotted for h5-HT2A (green), h5-HT2B (red), and h5-HT2C (blue) and represent data from at least three independent experiments.
Table 3. Gq-Mediated Calcium Flux of 4-Substituted Tryptamines at 5-HT2 Receptor Subtypesa.
| 5-HT2A |
m5-HT2A |
5-HT2B |
5-HT2C |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compound | EC50 (nM) | pEC50 (± SEM) | Emax% 5-HT (± SEM) | EC50 (nM) | pEC50 (± SEM) | Emax% 5-HT (± SEM) | EC50 (nM) | pEC50 (± SEM) | emax % 5-HT (± SEM) | EC50 (nM) | pEC50 (± SEM) | Emax% 5-HT (± SEM) |
| 5-HT | 0.26 | 9.58 ± 0.04 | 100 | 0.16 | 9.81 ± 0.06 | 100 | 0.21 | 9.67 ± 0.03 | 100 | 0.25 | 9.60 ± 0.04 | 100 |
| psilocin | 2.40 | 8.62 ± 0.05 | 98.4 ± 1.3 | 3.21 | 8.49 ± 0.04 | 95.7 ± 1.0 | 2.37 | 8.63 ± 0.08 | 39.2 ± 0.8 | 21.8 | 7.66 ± 0.07 | 85.1 ± 1.9 |
| 4-HO-MET | 4.04 | 8.39 ± 0.04 | 96.8 ± 1.1 | 2.49 | 8.60 ± 0.04 | 98.2 ± 1.2 | 2.64 | 8.58 ± 0.05 | 43.8 ± 0.6 | 29.7 | 7.53 ± 0.06 | 90.8 ± 1.7 |
| 4-HO-MPT | 3.82 | 8.42 ± 0.04 | 98.1 ± 1.1 | 2.85 | 8.55 ± 0.05 | 99.9 ± 1.3 | 3.40 | 8.47 ± 0.04 | 58.4 ± 0.7 | 45.8 | 7.34 ± 0.07 | 82.9 ± 2.1 |
| 4-HO-MIPT | 5.20 | 8.28 ± 0.04 | 99.6 ± 1.2 | 6.51 | 8.21 ± 0.03 | 103 ± 1.0 | 10.3 | 7.99 ± 0.12 | 49.1 ± 1.8 | 166 | 6.78 ± 0.10 | 76.2 ± 2.8 |
| 4-HO-DET | 6.47 | 8.19 ± 0.04 | 100 ± 1.2 | 4.19 | 8.38 ± 0.04 | 105 ± 1.1 | 6.27 | 8.20 ± 0.04 | 71.1 ± 0.8 | 264 | 6.58 ± 0.04 | 80.4 ± 1.3 |
| 4-HO-EPT | 3.15 | 8.50 ± 0.04 | 99.5 ± 1.2 | 1.88 | 8.73 ± 0.04 | 101 ± 1.1 | 4.34 | 8.36 ± 0.03 | 89.2 ± 0.7 | 129 | 6.89 ± 0.06 | 89.0 ± 2.1 |
| 4-HO-DPT | 1.64 | 8.79 ± 0.04 | 103 ± 1.0 | 1.28 | 8.89 ± 0.04 | 101 ± 0.9 | 2.23 | 8.65 ± 0.03 | 94.1 ± 0.7 | 212 | 6.67 ± 0.06 | 83.3 ± 2.0 |
| 4-HO-DIPT | 6.82 | 8.17 ± 0.04 | 102 ± 1.3 | 4.37 | 8.36 ± 0.04 | 101 ± 1.1 | 5.12 | 8.29 ± 0.02 | 97.0 ± 0.6 | 1408 | 5.85 ± 0.05 | 91.4 ± 2.6 |
| 4-HO-MALT | 3.67 | 8.43 ± 0.04 | 101 ± 1.2 | 3.81 | 8.42 ± 0.04 | 102 ± 1.2 | 3.16 | 8.50 ± 0.05 | 60.4 ± 0.8 | 44.2 | 7.36 ± 0.07 | 82.7 ± 2.0 |
| 4-AcO-DMT | 103 | 6.99 ± 0.02 | 79.2 ± 0.7 | N.T. | 100 | 7.00 ± 0.13 | 22.1 ± 1.0 | 268 | 6.57 ± 0.08 | 22.8 ± 0.6 | ||
| 4-AcO-MET | 92.3 | 7.04 ± 0.03 | 94.3 ± 1.1 | N.T. | 45.7 | 7.34 ± 0.06 | 48.9 ± 1.0 | 575 | 6.24 ± 0.06 | 41.2 ± 0.9 | ||
| 4-AcO-MPT | 42.4 | 7.37 ± 0.03 | 96.0 ± 1.1 | N.T. | 28.8 | 7.54 ± 0.06 | 62.7 ± 1.2 | 401 | 6.40 ± 0.05 | 32.7 ± 0.6 | ||
| 4-AcO-MIPT | 43.9 | 7.36 ± 0.04 | 93.2 ± 1.4 | N.T. | 44.7 | 7.35 ± 0.08 | 49.1 ± 1.3 | 542 | 6.27 ± 0.16 | 33.7 ± 2.1 | ||
| 4-AcO-DET | 184 | 6.74 ± 0.02 | 90.7 ± 0.8 | N.T. | 79.4 | 7.10 ± 0.07 | 64.2 ± 1.6 | 851 | 6.07 ± 0.04 | 53.5 ± 1.0 | ||
| 4-AcO-EPT | 66.4 | 7.18 ± 0.03 | 98.1 ± 0.9 | N.T. | 24.0 | 7.62 ± 0.04 | 95.8 ± 1.2 | 315 | 6.50 ± 0.04 | 87.6 ± 1.4 | ||
| 4-AcO-DPT | 23.7 | 7.63 ± 0.04 | 100 ± 1.0 | N.T. | 5.50 | 8.26 ± 0.06 | 97.4 ± 1.8 | 991 | 6.00 ± 0.05 | 68.2 ± 1.5 | ||
| 4-AcO-DIPT | 70.7 | 7.15 ± 0.16 | 74.6 ± 4.3 | N.T. | 70.8 | 7.15 ± 0.04 | 93.0 ± 1.2 | 1303 | 5.89 ± 0.06 | 80.8 ± 2.4 | ||
All data were acquired with at least three independent experiments performed in triplicate and in parallel. N.T., not tested.
Results at the 5-HT2B receptor were similar to those observed at 5-HT2A, although efficacy at 5-HT2B was more variable, ranging from 22.1 to 97.4%. There was also a relationship between 5-HT2B efficacy and the N,N-dialkyl substitution pattern: tryptamines containing N-ethyl-N-propyl, N,N-dipropyl, or N,N-diisopropyl groups had high efficacy (Emax of about 90–100%), whereas psilocin and other compounds containing shorter alkyl chains had lower efficacy at the 5-HT2B receptor.
The tryptamines had relatively lower potency at 5-HT2C receptors compared to 5-HT2A receptors. O-Acetylation tended to reduce potency and efficacy at 5-HT2C, whereas N-alkyl chain length had striking effects on 5-HT2C activity. For example, N,N-diisopropyl substitution not only was optimal for efficacy at 5-HT2C (e.g., 4-HO-DIPT Emax = 92.1%) but also caused a marked reduction of potency at that site (e.g., 4-HO-DIPT EC50 = 1408 nM). These results indicate that the 5-HT2C receptor does not tolerate longer and bulkier N-substitutions to the same degree as the 5-HT2A receptor (Figure 3). Notably, because N,N-dipropyl and N,N-diisopropyl substitution is detrimental for activity at 5-HT2C but has little effect on activity at 5-HT2A, 4-HO-DPT and 4-HO-DIPT show considerable selectivity for 5-HT2A over 5-HT2C (4-HO-DPT is 129-fold selective for 5-HT2A; 4-HO-DIPT is 206-fold selective for 5-HT2A).
Discussion and Conclusions
The present investigation examined the pharmacology and behavioral effects of N,N-dialkyltryptamines containing either a hydroxy or acetoxy group at the 4-position. One of the main findings of these studies is that psilocin and its homologues activate calcium mobilization via 5-HT2A with high efficacy and nanomolar potency, whereas the acetate esters have about 10-fold lower potency. All of the compounds induced head twitches in mice, a behavior known to be mediated by the 5-HT2A receptor. In contrast to the in vitro functional assays, however, O-acetylation of the 4-hydroxy group had little effect on potency in the HTR assay. In summary, 4-acetoxy-N,N-dialkyltryptamines have LSD-like pharmacological activity, supporting their classification as psychedelic drugs. Similar to the present results, N,N-dialkyltryptamines containing 4-acetoxy and 4-hydroxy groups reportedly have identical potencies in humans.71 Hence, there appears to be a discrepancy between the activity of 4-acetoxy-N,N-dialkyltryptamines at the receptor level and after in vivo administration to mice and humans.
It has been known for several decades that psilocybin acts as a prodrug for psilocin. According to Sard et al.,69 psilocin (EC50 = 24 nM) has a potency more than 100-fold higher than that of psilocybin (EC50 = 3475 nM) at h5-HT2A receptors. By contrast, psilocin and psilocybin have equivalent molar potencies in humans.77 Because psilocybin is rapidly metabolized to psilocin in human and animal tissues,15−39 the simplest explanation for the discrepancy between the activity of psilocybin in vivo and in vitro is that psilocin is the active species in the CNS. Indeed, blocking the enzyme alkaline phosphatase using a competitive substrate (β-glycerophosphate) attenuates the behavioral response to psilocybin.39 Similar to psilocybin, there has been speculation that 4-AcO-DMT and its homologues may also act as prodrugs.71,56 In the case of the 4-acetoxy-N,N-dialkyltryptamines, however, definite conclusions regarding their mechanism of action have not been possible because little was known about their pharmacological properties. On the basis of the present results, the 4-acetoxy-N,N-dialkyltryptamines have higher behavioral potency than would be anticipated based on their activity at the receptor level, which is consistent with the expectation that these compounds serve as prodrugs for their 4-hydroxy counterparts. Nevertheless, controlled biotransformation studies are necessary to definitively show that the 4-acetoxy-N,N-dialkyltryptamines are acting as prodrugs.
4-Hydroxy- and 4-acetoxy-N,N-dialkyltryptamines reportedly produce very similar effects in humans.71 These two sets of compounds also produced very similar effects on the HTR. Nevertheless, a few of the 4-acetoxy-N,N-dialkyltryptamines (e.g., 4-AcO-DMT and 4-AcO-DET) produced peak responses in the HTR assay larger than those of their O-desacetyl counterparts. One possible explanation for these differences is that the acetoxy group may be facilitating brain uptake. Transport of drugs across the blood–brain barrier is largely dependent on their lipophilicity.57 In tryptamines, esterification of a free phenolic group can markedly enhance lipid solubility.22 Alternatively, the 4-acetoxy group may enhance absorption from the injection site. The same phenomenon is believed to explain why the concentration of 6-monoacetylmorphine (6-MAM) in the brain is higher after heroin (3,6-diacetylmorphine) administration than after administration of an equimolar dose of 6-MAM.1 Similar to heroin, hydrolysis of 4-AcO-DMT and other homologues may occur rapidly in peripheral tissues and blood prior to brain uptake.
As far as we are aware, these are the first studies conducted with the N-allyl-N-methyl-substituted tryptamine 4-HO-MALT. Structurally, this compound is closely related to 4-HO-MPT, with the N-propyl group in the latter compound replaced with an N-allyl group. Interestingly, 4-HO-MALT had about the same potency and efficacy as 4-HO-MPT at 5-HT2 subtypes. These two compounds also had fairly similar potencies in the HTR assay (4-HO-MPT: ED50 = 1.92 μmol/kg; 4-HO-MALT: ED50 = 2.24 μmol/kg). Thus, in N,N-disubstituted tryptamines, the presence of a single allyl substituent on the terminal amine does not have a detrimental effect on activity at the 5-HT2A receptor. 4-HO-MALT likely acts as a serotonergic hallucinogen, with a potency similar to that of 4-HO-MPT. Consistent with these predictions, both 4-HO-MALT and its acetate ester are currently available online as new psychoactive substances.
The interaction of tryptamine hallucinogens with the 5-HT2B receptor is noteworthy. Psilocin and its homologues have similar nanomolar potency at 5-HT2B and act as less efficacious partial agonists compared to their activities at 5-HT2A. Similar findings have been reported previously with psilocin,69 although conflicting data have also appeared.65 These interactions are potentially significant because 5-HT2B activation is responsible for valvular heart disease in patients treated chronically with ergot alkaloids such as methysergide, pergolide, cabergoline, ergonovine, and ergotamine.21−68 Several other 5-HT2B agonists have been linked to cardiac-valve disorders, including fenfluramine, dexfenfluramine, and 3,4-methylenedioxymethamphetamine (MDMA).14,42 The primary pulmonary hypertension observed in patients treated chronically with fenfluramine and aminorex may also be mediated by 5-HT2B.48,67 Notably, some of the medications linked to these effects have about the same 5-HT2B efficacy as psilocin. Ergonovine, for example, activates calcium flux via h5-HT2B with an Emax of 39.7%.40 Methylergonovine, the primary metabolite of methysergide,7−73 also acts as a partial agonist at h5-HT2B (Emax = 49.5%).40 Recreational use of hallucinogens probably poses little risk of valvular heart disease because hallucinogen intake for recreational purposes is usually limited and occurs sporadically. However, repeated ingestion of low doses of hallucinogens (a practice known as microdosing) is becoming more common.2,41,60 Given the high potency of psilocin and its homologues at 5-HT2B, it should not be assumed that repeated, daily use of low doses poses no risk of valvular heart disease, especially considering our results with 4-HO-DPT and 4-HO-DIPT which show greater 5-HT2B agonist efficacy compared to psilocin.
One of the goals of these studies was to examine how the N,N-dialkyl substitution pattern influences the interaction of tryptamine hallucinogens with 5-HT2 subtypes. Previous SAR studies with tryptamine hallucinogens have focused on the influence of ring-substituents.24,51,44,4,13 By contrast, there has been relatively little systematic investigation of the effect of the substituents on the side-chain nitrogen. To determine how the N,N-dialkyl substitution pattern influences potency at 5-HT2 sites, we examined the effect of progressively lengthening one or both of the N-methyl groups in psilocin. Our studies showed that the size of the N-alkyl group has little effect on agonist potency at 5-HT2A or 5-HT2B, whereas potency at 5-HT2C declined when there was a relatively bulky substituent on the terminal amine. Similar to our results, N,N-dimethyltryptamine (EC50 = 38.3 nM), N,N-diethyltryptamine (EC50 = 67.8 nM), N,N-dipropyltryptamine (EC50 = 26.1 nM), N,N-diisopropyltryptamine (EC50 = 33.5 nM), and N-methyl-N-isopropyltryptamine (EC50 = 44.9 nM) all have about the same potency in h5-HT2A calcium flux assays.5 Likewise, McKenna et al.54 found that the N-alkyl group in had little effect on the 5-HT2A affinity of N,N-dialkyltryptamines unless the groups were larger than isopropyl. Although the N,N-dialkyl substitution pattern does not appear to be an important determinant of 5-HT2A agonist potency, it does seem to have an effect on potency in the HTR assay. Increasing the size or bulk of one or both of the alkyl chains tends to reduce HTR potency. It is not clear why the steric properties of the alkyl chains would affect activity in vivo but not in vitro. DMT seems to be actively transported into brain tissue,78 so steric factors could potentially influence central transport mechanisms.
In summary, 4-substituted N,N-dialkyltryptamines activate 5-HT2A receptors in vitro and in vivo. These findings support the classification of these compounds as psychedelic drugs. Indeed, the psychedelic effects produced by psilocybin and other hallucinogens are largely attributable to 5-HT2A activation because ketanserin (a 5-HT2A antagonist) blocks the response.35−76 Additionally, the intensity of the subjective response to psilocybin is correlated with the level of central 5-HT2A occupation, measured using the PET tracer [11C]Cimbi-36 ([11C]25B-NBOMe).52 We also found that 4-acetoxytryptamines are likely serving as prodrugs for the corresponding 4-hydroxytryptamines. In addition to activating 5-HT2A, psilocin and its homologues also act as 5-HT2B agonists, which is a potentially worrisome property. The findings in this report will facilitate predictions regarding the psychoactive potential of new tryptamine derivatives based on the substitution pattern on the terminal amine and the indole ring.
Acknowledgments
These studies were supported by an award from NIDA (R01 DA041336, A.L.H.), a Medical College of Wisconsin Research Affairs Counsel Pilot Grant (J.D.M.), the UCSD T32 Fellowship in Biological Psychiatry & Neuroscience (NIMH T32 MH018399, A.K.K.), and by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center (A.L.H.). Psilocin was donated by the NIDA Drug Supply Program (Rockville, MD). The authors thank Mark Geyer, Ph.D., for donating some of the compounds used for behavioral testing, as well as Paul Stamets for providing the image of Psilocybe azurescens used in the graphical abstract. This investigation is dedicated to the memory of Jochen Gartz, Ph.D., a pioneering scientist and mycologist.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.0c00176.
Results of the behavioral experiments, including HTR counts, statistical analyses, and ED50 values and 95% CI (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Eivindvik K.; Rasmussen K. E.; Sund R. B. (1989) Handling of psilocybin and psilocin by everted sacs of rat jejunum and colon. Acta. Pharm. Nord. 1, 295–302. [PubMed] [Google Scholar]
- Horita A.; Weber L. J. (1961) Dephosphorylation of psilocybin to psilocin by alkaline phosphatase. Exp. Biol. Med. 106, 32–34. 10.3181/00379727-106-26228. [DOI] [PubMed] [Google Scholar]
- Horita A.; Weber L. J. (1961) The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochem. Pharmacol. 7, 47–54. 10.1016/0006-2952(61)90124-1. [DOI] [PubMed] [Google Scholar]
- Hasler F.; Bourquin D.; Brenneisen R.; Bar T.; Vollenweider F. X. (1997) Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm. Acta Helv. 72, 175–184. 10.1016/S0031-6865(97)00014-9. [DOI] [PubMed] [Google Scholar]
- Horita A.; Weber L. J. (1962) Dephosphorylation of psilocybin in the intact mouse. Toxicol. Appl. Pharmacol. 4, 730–737. 10.1016/0041-008X(62)90102-3. [DOI] [PubMed] [Google Scholar]
- Wolbach A. B. Jr.; Miner E. J.; Isbell H. (1962) Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia 3, 219–223. 10.1007/BF00412109. [DOI] [PubMed] [Google Scholar]
- Sard H.; Kumaran G.; Morency C.; Roth B. L.; Toth B. A.; He P.; Shuster L. (2005) SAR of psilocybin analogs: discovery of a selective 5-HT 2C agonist. Bioorg. Med. Chem. Lett. 15, 4555–4559. 10.1016/j.bmcl.2005.06.104. [DOI] [PubMed] [Google Scholar]
- Bogenschutz M. P.; Ross S. (2016) Therapeutic Applications of Classic Hallucinogens. Curr. Top. Behav. Neurosci. 36, 361–391. 10.1007/7854_2016_464. [DOI] [PubMed] [Google Scholar]
- Leonard J. B.; Anderson B.; Klein-Schwartz W. (2018) Does getting high hurt? Characterization of cases of LSD and psilocybin-containing mushroom exposures to national poison centers between 2000 and 2016. J. Psychopharmacol. 32, 1286–1294. 10.1177/0269881118793086. [DOI] [PubMed] [Google Scholar]
- McCambridge J.; Winstock A.; Hunt N.; Mitcheson L. (2006) 5-Year trends in use of hallucinogens and other adjunct drugs among UK dance drug users. Eur. Addict. Res. 13, 57–64. 10.1159/000095816. [DOI] [PubMed] [Google Scholar]
- Peden N. R.; Pringle S. D.; Crooks J. (1982) The problem of psilocybin mushroom abuse. Hum. Toxicol. 1, 417–424. 10.1177/096032718200100408. [DOI] [PubMed] [Google Scholar]
- Hofmann A.; Heim R.; Brack A.; Kobel H. (1958) Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen Rauschpilz Psilocybe mexicana Heim. Experientia 14, 107–109. 10.1007/BF02159243. [DOI] [PubMed] [Google Scholar]
- Troxler F.; Seemann F.; Hofmann A. (1959) Abwandlungsprodukte von psilocybin und psilocin. 2. Mitteilung über synthetische indolverbindungen. Helv. Chim. Acta 42, 2073–2103. 10.1002/hlca.19590420638. [DOI] [Google Scholar]
- Baer G. (1967) Statistical results on reactions of normal subjects to the psilocybin derivatives CEY 19 and CZ 74. In Neuropsychopharmacology; Brill H., Ed.; Excerpta Medica Foundation, Amsterdam, pp 400–404. [Google Scholar]
- Leuner H., and Baer G. (1965) Two new short-acting hallucinogens of the psilocybin group. In Neuropsychopharmacology, Vol. 4; Bente D., and Bradley P. B., Eds.; Elsevier, Amsterdam, pp 471−474. [Google Scholar]
- Repke D. B.; Ferguson W. J.; Bates D. K. (1977) Psilocin analogs. 1. Synthesis of 3-[2-(dialkylamino)ethyl]- and 3-[2-(cycloalkylamino)ethyl]indol-4-ols. J. Heterocycl. Chem. 14, 71–74. 10.1002/jhet.5570140113. [DOI] [Google Scholar]
- Repke D. B.; Ferguson W. J.; Bates D. K. (1981) Psilocin analogs II. Synthesis of 3-[2-(dialkylamino)ethyl]-, 3-[2-(N-methyl-N-alkylamino)ethyl]- and 3-[2-(cycloalkylamino)ethyl]indol-4-ols. J. Heterocycl. Chem. 18, 175–179. 10.1002/jhet.5570180131. [DOI] [Google Scholar]
- Hofmann A. (1963) Esters of indoles, US3075992A.
- Shulgin A. T., and Shulgin A. (1997) TIHKAL: The Continuation; Transform Press, Berkeley. [Google Scholar]
- Nichols D. E.; Frescas S. (1999) Improvements to the synthesis of psilocybin and a facile method for preparing the O-acetyl prodrug of psilocin. Synthesis 1999, 935–938. 10.1055/s-1999-3490. [DOI] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction . (2006) EMCDDA-Europol 2005 Annual Report on the Implementation of Council Decision 2005/387/JHA, Publications Office of the European Union, Lisbon.
- European Monitoring Centre for Drugs and Drug Addiction . (2010) New Drugs in Europe, 2009. EMCDDA-Europol 2009 Annual Report on the implementation of Council Decision 2005/387/JHA, Publications Office of the European Union, Luxembourg.
- European Monitoring Centre for Drugs and Drug Addiction . (2013) EMCDDA-Europol 2012 Annual Report on the Implementation of Council Decision 2005/387/JHA, Publications Office of the European Union, Lisbon.
- Kjellgren A.; Soussan C. (2011) Heaven and hell—A phenomenological study of recreational use of 4-HO-MET in Sweden. J. Psychoact. Drugs 43, 211–219. 10.1080/02791072.2011.605699. [DOI] [PubMed] [Google Scholar]
- Greene S. L. (2013) Tryptamines. In Novel Psychoactive Substances: Classification, Pharmacology and Toxicology; Dargen P, and Wood D, Eds., Academic Press, London, pp 363–381. [Google Scholar]
- Palamar J. J.; Acosta P. (2020) A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines. Hum. Psychopharmacol. 35, e2719. 10.1002/hup.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittarelli R.; Mannocchi G.; Pantano F.; Romolo F. (2015) Recreational use, analysis and toxicity of tryptamines. Curr. Neuropharmacol. 13, 26–46. 10.2174/1570159X13666141210222409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough B. E.; Landavazo A.; Decker A. M.; Partilla J. S.; Baumann M. H.; Rothman R. B. (2014) Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology (Berl.) 231, 4135–4144. 10.1007/s00213-014-3557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon R. A.; Young R.; Jacyno J. M. (1983) Indolealkylamine and phenalkylamine hallucinogens. Effect of alpha-methyl and N-methyl substituents on behavioral activity. Biochem. Pharmacol. 32, 1267–73. 10.1016/0006-2952(83)90281-2. [DOI] [PubMed] [Google Scholar]
- Lyon R. A.; Titeler M.; Seggel M. R.; Glennon R. A. (1988) Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens. Eur. J. Pharmacol. 145, 291–297. 10.1016/0014-2999(88)90432-3. [DOI] [PubMed] [Google Scholar]
- McKenna D. J.; Repke D. B.; Lo L.; Peroutka S. J. (1990) Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 29, 193–198. 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L. (2015) Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 277, 99–120. 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E. (2016) Psychedelics. Pharmacol. Rev. 68, 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C. E.; Morgan D. (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test. Anal. 4, 556–576. 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. (2013) Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl.) 227, 727–39. 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J.; Weisstaub N. V.; Zhou M.; Chan P.; Ivic L.; Ang R.; Lira A.; Bradley-Moore M.; Ge Y.; Zhou Q.; Sealfon S. C.; Gingrich J. A. (2007) Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452. 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77, 200–207. 10.1016/j.neuropharm.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne S. J.; Pickering R. W. (1967) A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 11, 65–78. 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Koedood L.; Powell S. B.; Geyer M. A. (2011) Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 25, 1548–1561. 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Harrington A. W.; Kiessel C. L.; Eckler J. R.; Rabin R. A.; Winter J. C.; Coop A.; Rice K. C.; Woods J. H. (2006) Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol., Biochem. Behav. 83, 122–129. 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Reissig C. J.; Katz E. B.; Yarosh H. L.; Rice K. C.; Winter J. C. (2008) Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol., Biochem. Behav. 88, 358–365. 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Chatha M.; Klein A. K.; Wallach J.; Brandt S. D. (2020) Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 167, 107933. 10.1016/j.neuropharm.2019.107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L. M.; Cozzi N. V.; Daley P. F.; Brandt S. D.; Halberstadt A. L. (2018) Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology 142, 231–239. 10.1016/j.neuropharm.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charton M. (1983) The upsilon steric parameter — definition and determination. In Steric Effects in Drug Design; Springer, Berlin; pp 57–91. [Google Scholar]
- Oldendorf W. H. (1974) Lipid solubility and drug penetration of the blood brain barrier. Exp. Biol. Med. 147, 813–815. 10.3181/00379727-147-38444. [DOI] [PubMed] [Google Scholar]
- Gessner P. K.; Godse D. D.; Krull A. H.; McMullan J. M. (1968) Structure-activity relationships among 5-methoxy-N:N-dimethyltryptamine, 4-hydroxy-N:N-dimethyltryptamine (psilocin) and other substituted tryptamines. Life Sci. 7, 267–77. 10.1016/0024-3205(68)90200-2. [DOI] [PubMed] [Google Scholar]
- Andersen J. M.; Ripel A.; Boix F.; Normann P. T.; Morland J. (2009) Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. J. Pharmacol. Exp. Ther. 331, 153–161. 10.1124/jpet.109.152462. [DOI] [PubMed] [Google Scholar]
- Anderson T.; Petranker R.; Rosenbaum D.; Weissman C. R.; Dinh-Williams L. A.; Hui K.; Hapke E.; Farb N. A. S. (2019) Microdosing psychedelics: personality, mental health, and creativity differences in microdosers. Psychopharmacology (Berl.) 236, 731–740. 10.1007/s00213-018-5106-2. [DOI] [PubMed] [Google Scholar]
- Rickli A.; Moning O. D.; Hoener M. C.; Liechti M. E. (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 26, 1327–37. 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. W.; Burn T. C.; Brown B. S.; Patterson J. P.; Corjay M. H.; Valentine P. A.; Sun J. H.; Link J. R.; Abbaszade I.; Hollis J. M.; et al. (2000) Possible role of valvular serotonin 5-HT2B receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol. 57, 75–81. [PubMed] [Google Scholar]
- Huang X. P.; Setola V.; Yadav P. N.; Allen J. A.; Rogan S. C.; Hanson B. J.; Revankar C.; Robers M.; Doucette C.; Roth B. L. (2009) Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine2B receptor agonists: implications for drug safety assessment. Mol. Pharmacol. 76, 710–722. 10.1124/mol.109.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B. L. (2007) Drugs and valvular heart disease. N. Engl. J. Med. 356, 6–9. 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Rothman R. B.; Baumann M. H.; Savage J. E.; Rauser L.; McBride A.; Hufeisen S. J.; Roth B. L. (2000) Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102, 2836–2841. 10.1161/01.CIR.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Droogmans S.; Cosyns B.; D’haenen H.; Creeten E.; Weytjens C.; Franken P. R.; Scott B.; Schoors D.; Kemdem A.; Close L.; Vandenbossche J. L.; Bechet S.; Van Camp G. (2007) Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am. J. Cardiol. 100, 1442–1445. 10.1016/j.amjcard.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Jick H.; Vasilakis C.; Weinrauch L. A.; Meier C. R.; Jick S. S.; Derby L. E. (1998) A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N. Engl. J. Med. 339, 719–724. 10.1056/NEJM199809103391102. [DOI] [PubMed] [Google Scholar]
- Launay J. M.; Hervé P.; Peoc’h K.; Tournois C.; Callebert J.; Nebigil C. G.; Etienne N.; Drouet L.; Humbert M.; Simonneau G.; Maroteaux L. (2002) Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat. Med. 8, 1129–1135. 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- Rothman R. B.; Ayestas M. A.; Dersch C. M.; Baumann M. H. (1999) Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation 100, 869–875. 10.1161/01.CIR.100.8.869. [DOI] [PubMed] [Google Scholar]
- Bredberg U.; Eyjolfsdottir G. S.; Paalzow L.; Tfelt-Hansen P.; Tfelt-Hansen V. (1986) Pharmacokinetics of methysergide and its metabolite methylergometrine in man. Eur. J. Clin. Pharmacol. 30, 75–77. 10.1007/BF00614199. [DOI] [PubMed] [Google Scholar]
- Bredberg U.; Paalzow L. (1990) Pharmacokinetics of methysergide and its metabolite methylergometrine in the rat. Drug Metab. Dispos. 18, 338–343. [PubMed] [Google Scholar]
- Tfelt-Hansen P.; Bredberg U.; Eyjolfsdottir G. S.; Paalzow L.; Tfelt-Hansen V. (1985) Kinetics of methysergide and its main metabolite, methylergometrine, in man. Cephalalgia 5 (Suppl), 54–55. 10.1177/03331024850050S314. [DOI] [PubMed] [Google Scholar]
- Hutten N.; Mason N. L.; Dolder P. C.; Kuypers K. P. C. (2019) Motives and side-effects of microdosing with psychedelics among users. Int. J. Neuropsychopharmacol. 22, 426–434. 10.1093/ijnp/pyz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito V.; Stevenson R. J. (2019) A systematic study of microdosing psychedelics. PLoS One 14, e0211023. 10.1371/journal.pone.0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J. B.; Kurrasch-Orbaugh D.; Marona-Lewicka D.; Cumbay M. G.; Watts V. J.; Barker E. L.; Nichols D. E. (2000) Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J. Med. Chem. 43, 4701–4710. 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- Cozzi N. V.; Daley P. F. (2016) Receptor binding profiles and quantitative structure-affinity relationships of some 5-substituted-N,N-diallyltryptamines. Bioorg. Med. Chem. Lett. 26, 959–964. 10.1016/j.bmcl.2015.12.053. [DOI] [PubMed] [Google Scholar]
- Yanai K.; Ido T.; Ishiwata K.; Hatazawa J.; Takahashi T.; Iwata R.; Matsuzawa T. (1986) In vivo kinetics and displacement study of a carbon-11-labeled hallucinogen, N,N-[11C]dimethyltryptamine. Eur. J. Nucl. Med. 12, 141–146. 10.1007/BF00276707. [DOI] [PubMed] [Google Scholar]
- Holze F., Vizeli P., Ley L., Müller F., Dolder P., Stocker M., Duthaler U., Varghese N., Eckert A., Borgwardt S., and Liechti M. E. (2020) Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology, 10.1038/s41386-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometer M.; Schmidt A.; Jancke L.; Vollenweider F. X. (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 33, 10544–10551. 10.1523/JNEUROSCI.3007-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R.; Pokorny D.; Vollenweider L.; Preller K. H.; Pokorny T.; Seifritz E.; Vollenweider F. X. (2017) Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl) 234, 2031–2046. 10.1007/s00213-017-4610-0. [DOI] [PubMed] [Google Scholar]
- Preller K. H.; Herdener M.; Pokorny T.; Planzer A.; Kraehenmann R.; Stämpfli P.; Liechti M. E.; Seifritz E.; Vollenweider F. X. (2017) The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr. Biol. 27, 451–457. 10.1016/j.cub.2016.12.030. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Bäbler A.; Vogel H.; Hell D. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport 9, 3897–3902. 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Madsen M. K.; Fisher P. M.; Burmester D.; Dyssegaard A.; Stenbæk D. S.; Kristiansen S.; Johansen S. S.; Lehel S.; Linnet K.; Svarer C.; et al. (2019) Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44, 1328–1334. 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.