Abstract
This study aimed to examine whether the diagnostic accuracy of four noninvasive tests (NITs) for detecting advanced fibrosis in nonalcoholic fatty liver disease (NAFLD) is maintained or is inferior to with or without the presence of type 2 diabetes. Overall, 874 patients with biopsy‐proven NAFLD were enrolled. After propensity‐score matching by age, sex, and the prevalence of dyslipidemia, 311 patients were enrolled in each group of with or without diabetes. To evaluate the effect of diabetes, we compared the diagnostic accuracy of the fibrosis‐4 (FIB‐4) index, the NAFLD fibrosis score (NFS), the aspartate aminotransferase to platelet ratio index (APRI), and type IV collagen 7S (COL4‐7S) in patients with NAFLD with and without diabetes. The areas under the receiver operating characteristic curve (AUROC) for identifying advanced fibrosis in patients without diabetes were 0.879 for the FIB‐4 index, 0.851 for the NFS, 0.862 for the APRI, and 0.883 for COL4‐7S. The AUROCs in patients with diabetes were 0.790 for the FIB‐4 index, 0.784 for the NFS, 0.771 for the APRI, and 0.872 for COL4‐7S. The AUROC of COL4‐7S was significantly larger than that of the other NITs in patients with NAFLD with diabetes than in those without diabetes. The optimal high and low cutoff points of COL4‐7S were 5.9 ng/mL and 4.8 ng/mL, respectively. At the low cutoff point, the accuracy of COL4‐7S was better than that of the other NITs, especially in patients with diabetes. Conclusion: COL4‐7S measurement might be the best NIT for identifying advanced fibrosis in NAFLD, especially in NAFLD with diabetes.
Abbreviations
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic curve
- BMI
body mass index
- COL4‐7S
type IV collagen 7S
- DM
diabetes mellitus
- FIB‐4
fibrosis‐4
- JSG‐NAFLD
Japan Study Group of NAFLD
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- NIT
noninvasive test
- PS
propensity score
- ROC
receiver operating characteristic
- T2DM
type 2 diabetes mellitus
Nonalcoholic fatty liver disease (NAFLD) has a higher rate in developed countries than in nondeveloped countries. NAFLD has a wide spectrum of conditions, including simple steatosis, nonalcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma.( 1 ) Mortality of patients with NAFLD is determined by progression of liver fibrosis rather than by the grade of liver steatosis. Because the main causes of death are cardiovascular disease, cancers and liver‐related disease,( 2 ) early detection and estimation of liver fibrosis are important.( 3 , 4 )
Type 2 diabetes mellitus (T2DM), which is similar to NAFLD, is associated with metabolic syndrome. The Japan Diabetes Society reported that the leading cause of death in patients with diabetes mellitus (DM) from 2001 to 2010 in Japan was malignant neoplasm (40.7%), followed by infectious disease (17.8%), vascular disorders (13.8%), including cardiovascular disease, cerebrovascular disease and chronic kidney disease, and liver cirrhosis (3.3%).( 5 ) In this report, mortality related to liver disease, such as liver cirrhosis and hepatocellular carcinoma, was estimated to be approximately 10%, and the importance of identifying advanced fibrosis in patients with T2DM was emphasized. In patients with T2DM, the prevalence of advanced fibrosis in those with NAFLD is 7%‐12%.( 6 , 7 , 8 )
NAFLD and DM affect each other. As these conditions progress, managing treatment becomes difficult. NAFLD should be recognized as one of the final organ disorders of DM. The prevalence of NAFLD is often affected by an increase in obesity, and this is thought to be one phenotype of metabolic syndrome, although there are patients with NAFLD who are not obese and taken to metabolic syndrome. NAFLD is a complicated disease, and the relation of NAFLD and DM is still unclear. Therefore, making a diagnosis and treatment for liver disease and DM are difficult for clinicians.( 9 , 10 )
In a previous study, diabetes was significantly associated with incident NAFLD and hepatocellular carcinoma. Interventions that improve metabolic abnormalities in patients with diabetes (weight loss, glycemic control, and treatment with specific drugs for hyperglycemia or dyslipidemia) are also beneficial for fatty liver disease.( 11 ) An effective treatment has not been established for NAFLD, and kinesitherapy and improvement of lifestyle are currently the only treatments. However, examination of an effective treatment on the basis of the pathophysiological relationship of DM and NAFLD is necessary in the future.( 12 )
To diagnose the stage of fibrosis in NAFLD, percutaneous liver biopsy is still the gold standard. However, disadvantages of this technique include the risk of complications because of invasiveness of the procedure, sampling errors, and high cost.( 13 ) Therefore, there is considerable interest in using validated noninvasive tests (NITs) of fibrosis in clinical practice. Of these scoring systems, the fibrosis‐4 (FIB‐4) index and NAFLD fibrosis score (NFS) are the most useful for excluding advanced fibrosis (brunt stage; F = 3, 4) in patients with NAFLD.( 14 ) The area under the receiver operating characteristic curve (AUROC) for predicting advanced fibrosis in NAFLD using the FIB‐4 index and NFS is 0.86 and 0.88, respectively. The American Association for the Study of Liver Diseases (AASLD) created a practice guidance for NAFLD in 2018.( 15 ) In this guideline, the NFS and FIB‐4 index are clinically more useful NITs for identifying patients with NAFLD with a higher likelihood of having bridging fibrosis (stage 3) or cirrhosis (stage 4) than other scores, such as the body mass index (BMI), aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, and diabetes mellitus (BARD) score, the AST to platelet ratio index (APRI), and the AST/ALT ratio. As previously reported, the utility of some noninvasive scoring systems (BAAT [BMI, age, alt, and triglycerides] score, BARD score, and NFS) was limited in a population with T2DM by inclusion of age, BMI, and high levels of indeterminate results.( 15 )
In this study, we compared four NITs for detecting advanced fibrosis of NAFLD in a clinical population with biopsy‐proven NAFLD. We evaluated the diagnostic accuracy of these NITs in patients with NAFLD and T2DM.
Patients and Methods
We performed a cross‐sectional study to estimate the accuracy of NITs in patients with NAFLD and T2DM. In this study, 874 patients were enrolled in the Japan Study Group of NAFLD (JSG‐NAFLD), and all patients underwent a liver biopsy from 2002 to 2015. The JSG‐NAFLD included the following nine Japanese hepatology centers: Kyoto Prefectural University of Medicine, Nara City Hospital, Yokohama City University Graduate School of Medicine, Hiroshima University, Kochi Medical School, Saga University, Osaka City University, Asahikawa Medical College, and Saiseikai Suita Hospital.
All patients were diagnosed with the presence or absence of T2DM in accordance with the guideline of the Japan Society of Diabetes as follows: patients who had a diagnosis of diabetes; patients with documented use of oral hypoglycemic medication, whose random glucose levels exceeded 200 mg/dL; or patients with fasting plasma glucose levels >126 mg/dL and hemoglobin A1c values ≥ 6.6%.( 16 )
The diagnosis of NAFLD was based on previously reported criteria( 17 ) and appropriately excluded liver disease with clear etiologies, such as viral and autoimmune hepatitis, and alcoholic liver disease. Written, informed consent was obtained from all patients at the time of their liver biopsy. This study was approved by the institutional review boards of all participating institutions, and it conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Covariates
Physical characteristics and clinical laboratory data were examined for each patient. Physical factors included age, sex, and BMI, and clinical characteristics included a confirmed diagnosis of hypertension, diabetes, and dyslipidemia. Blood samples were taken in the morning after a 12‐hour overnight fast, and clinical laboratory data were collected. These parameters were measured using standard techniques in clinical laboratories. Type IV collagen 7S (COL4‐7S) was measured by double antibody radioimmunoassay. The cutoff point was set two cutoff points, like other NITs, so that sensitivity and specificity for advanced fibrosis were at least 90% with using the overall population. BMI was calculated as weight in kilograms divided by height in square meters. Obesity was defined as BMI > 25.0 kg/m2 according to the criteria of the Japan Society for the Study of Obesity.( 18 ) Dyslipidemia was diagnosed if cholesterol levels were >220 mg/dL, low‐density lipoprotein cholesterol levels were >140 mg/dL, high‐density lipoprotein cholesterol levels were <40 mg/dL, and/or triglyceride levels were >160 mg/dL. Hypertension was diagnosed if the patient was on antihypertensive medication and/or had a resting recumbent blood pressure ≥140/90 mm Hg on at least two occasions.
Liver Histology and Diagnosis of NAFLD and NASH
All patients underwent percutaneous liver biopsy under ultrasonic guidance. Liver specimens were embedded in paraffin and stained with hematoxylin and eosin, Masson’s trichrome, and reticulin silver stain. Two pathologists and one hepatologist (S.I., K.S, and Y.S.), who were blinded to all clinical and identifying data, reviewed the liver biopsy specimens. An adequate liver biopsy sample was defined as a biopsy specimen with a length greater than 1.5 cm and/or more than six portal tracts. NASH was defined as steatosis with lobular inflammation and ballooning degeneration with or without Mallory‐Denk bodies or fibrosis. Patients whose liver biopsy specimens showed steatosis or steatosis with nonspecific inflammation were identified as the NAFL cohort. The severity of hepatic fibrosis (stage) was defined as follows: stage 1, zone 3 perisinusoidal fibrosis; stage 2, zone 3 perisinusoidal fibrosis with portal fibrosis; stage 3, zone 3 perisinusoidal fibrosis and portal fibrosis with bridging fibrosis; and stage 4, cirrhosis.( 17 ) Advanced fibrosis was defined as having a severity greater than stage 3. Scoring of steatosis included microvesicular and macrovesicular steatosis, and was based on the percentage area of the parenchyma that was fatty as follows: <33% was considered as mild, 33%–65% as moderate, and >66% as advanced. The activity of NAFLD was estimated by the NAFLD Activity Score (NAS), as proposed by Kleiner et al.,( 19 ) which was the unweighted sum of the scores for steatosis (0‐3), lobular inflammation (0‐3), and ballooning degeneration (0‐2).
NITs for Predicting Hepatic Fibrosis
The FIB‐4 index, the NFS, the APRI, and COL4‐7S were assessed in each patient. The FIB‐4 index was calculated using the following formula: (age [years] × AST/platelet count [×109/L] × ALT1/2).( 20 ) The NFS was calculated using the following formula: −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired glucose tolerance/DM (yes = 1, no = 0) + 0.99 × AST/ALT ratio × 0.013 × platelet count (×109/L) − 0.66 × albumin (g/dL).( 21 ) The APRI was calculated using the following formula: ([AST/upper limit of normal]/platelet count [×109/L] × 100 [× upper limit of normal, AST upper level of normal, or 56 IU/L]).( 22 ) According to accumulating evidence, the high cutoff points were 2.67 for the FIB‐4 index, 0.675 for the NFS, and 2.0 for the APRI, and the low cutoff points were 1.30 for the FIB‐4 index, −1.45 for the NFS, and 0.5 for the APRI.( 21 , 22 , 23 )
Statistical Analysis
Data are expressed as the median and range for quantitative data or as number and percentage for qualitative data. Statistical differences between two or three groups were analyzed using the Mann‐Whitney U test and Kruskal‐Wallis analysis for quantitative data, and using Fisher’s test or the chi‐square test for qualitative data. Normality was confirmed using Shapiro‐Wilk analysis. The propensity score (PS) model was estimated using a logistic regression model that was adjusted for the patients’ characteristics listed in Table 1, as well as for age, sex, and the prevalence of dyslipidemia, because these variables were prognostically significant in other studies.( 24 ) Possible confounders were chosen for their potential association with the outcome of interest on the basis of clinical knowledge. One patient with NAFLD and T2DM was matched to many patients with NAFLD without T2DM using nearest‐neighbor matching without replacement. The PSs were matched using a caliper width of 1/5 of the logit of the SD. After PS matching, receiver operating characteristic (ROC) curve analysis was performed for each NIT to identify advanced fibrosis in NAFLD. Furthermore, each AUROC was compared with the DeLong test for the presence or absence of DM.
TABLE 1.
Characteristics of Patients With NAFLD With or Without T2DM
| Variables | Diabetes Absence (n = 497) | Diabetes Presence (n = 377) | P Value |
|---|---|---|---|
| Age (years) | 51 (14‐84) | 60 (17‐92) | <0.001 |
| Gender (female) | 207 of 497 (41.6%) | 214 of 377 (56.8%) | <0.001 |
| BMI (kg/m2) | 27.0 (10.3‐49.5) | 27.3 (16.5‐44) | 0.752 |
| AST (IU/L) | 44 (16‐331) | 43 (15‐202) | 0.993 |
| ALT (IU/L) | 73 (16‐547) | 60 (11‐358) | 0.003 |
| Albumin (mg/dL) | 4.5 (3.1‐5.8) | 4.3 (2.8‐5.4) | <0.001 |
| Platelets (×108/uL) | 22.8 (6.8‐75.1) | 21.1 (4.6‐78.5) | <0.001 |
| T‐Bil (mg/dL) | 0.66 (0.30‐3.59) | 0.74 (0.25‐2.31) | 0.396 |
| TG (mg/dL) | 152 (43‐659) | 147 (49‐920) | 0.489 |
| Total cholesterol (mg/dL) | 206 (77‐436) | 207 (77‐372) | 0.726 |
| HDL‐C (mg/dL) | 48 (23‐290) | 49 (22‐99) | 0.115 |
| LDL‐C (mg/dL) | 129 (44‐246) | 126 (8‐294) | 0.668 |
| NASH (yes) | 320 of 497 (64.4%) | 279 of 378 (73.8%) | 0.003 |
| Dyslipidemia (yes) | 345 of 490 (70.4%) | 296 of 374 (79.1%) | 0.004 |
| Hypertension (yes) | 106 of 413 (25.7%) | 152 of 318 (47.8%) | <0.001 |
| COL4‐7S (ng/mL) | 4.1 (0.2‐13) | 4.5 (1.0‐55.5) | <0.001 |
| FIB‐4 index | 1.09 (0.17‐7.51) | 1.60 (0.24‐10.74) | <0.001 |
| NFS | 2.07 (9.08 to 2.15) | 0.56 (8.77 to 2.83) | <0.001 |
| APRI | 0.67 (0.13‐4.78) | 0.72 (0.15‐4.42) | 0.046 |
| Brunt grade (1/2/3) | 197/211/82 (40.2%/43.1%/16.7%) | 139/145/58 (40.6%/42.4%/17.0%) | — |
| Brunt stage (0/1/2/3/4) | 222/170/66/25/16 (44.5%/34.1%/13.2%/5.0%/3.2%) | 129/112/67/47/23 (34.1%/29.6%/17.7%/12.4%/6.1%) | — |
| Advanced fibrosis (F3 and F4) | 41 of 499 (8.2%) | 70 of 378 (18.5%) | <0.001 |
|
NAS total (1/2/3/4/5/6/7/8) |
23/39/57/113/91/61/28/1 (5.6%/9.4%/13.8%/27.4%/22.0%/14.8%/6.8%/0.2%) |
3/13/40/37/60/52/18/1 (1.3%/5.4%/17.9%/18.3%/25.4%/21.9%/9.4%/0.4%) |
— |
| Steatosis (0/1/2/3) | 2/193/201/88 (0.4%/39.9%/41.5%/18.2%) | 0/114/115/49 (40.7%/42.5%/16.8%) | — |
| Inflammation (0/1/2/3) | 76/222/97/18 (18.4%/53.8%/23.5%/4.4%) | 26/111/69/18 (11.6%/49.6%/30.8%/8.0%) | — |
| Ballooning (0/1/2) | 78/146/189 (18.9%/35.3%/45.8%) | 23/72/129 (10.3%/32.%1/57.6%) | — |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; T‐Bil, total bilirubin; TG, triglycerides.
All analyses were performed using JMP 12 (SAS Institute Inc., Cary, NC) and R statistics version 3.4.0. Nominal, two‐sided P‐values were used and were considered statistically significant for values <0.05 a priori.
Results
Characteristics of Patients With NAFLD and T2DM After PS Matching With Age, Sex, and Prevalence of Dyslipidemia
A total of 874 patients with NAFLD were included in this study. Of them, 497 (43.1%) patients had T2DM (Table 1). Age, the rate of female sex, and the prevalence of dyslipidemia, hypertension, NASH, and advanced liver fibrosis were significantly higher in patients with NAFLD with T2DM than in those without T2DM. Furthermore, NIT results, including COL4‐7S, the FIB‐4 index, the NFS, and the APRI, were significantly higher in patients with NAFLD with T2DM compared to those without T2DM.
PS matching was performed to adjust for each group with NAFLD with or without T2DM by age, sex, and the prevalence of dyslipidemia. Finally, 311 patients were enrolled in each group of patients with NAFLD with and without T2DM after PS matching. The characteristics of patients with NAFLD and T2DM were a higher rate of obesity, lower albumin levels, higher prevalence of hypertension, and higher rate of advanced liver fibrosis than those without T2DM. There was no significant difference in the prevalence of liver cirrhosis between the groups. COL4‐7S levels and the NFS were significantly higher in patients with NAFLD with T2DM than the levels in those without T2DM, whereas the FIB‐4 index and APRI were not different between the two groups (Table 2). The distribution of these NITs in the presence or absence of T2DM after PS matching is shown in Fig. 1. In NAFLD with DM and NAFLD without DM, there were significantly equilateral correlations between these NITs and NASs histologically in the total NAS, inflammation, and ballooning (Supporting Table S1).
TABLE 2.
Characteristics of Patients With NAFLD After PS Matching by Age, Sex, and Presence of Dyslipidemia
| Clinical Parameters | Diabetes Absence (n = 311) | Diabetes Presence (n = 311) | P Value |
|---|---|---|---|
| Age (years) | 58 (16‐84) | 58 (17‐83) | 0.9704 |
| Gender (female) | 168 of 311 (54.0%) | 164 of 311 (52.7%) | 0.8095 |
| BMI (kg/m2) | 26.7 (17.7‐43.5) | 27.4 (17.3‐44.0) | 0.0369 |
| AST (IU/L) | 44 (16‐208) | 43 (15‐202) | 0.7037 |
| ALT (IU/L) | 66 (16‐298) | 64 (12‐358) | 0.7683 |
| Albumin (mg/dl) | 4.4 (3.1‐5.7) | 4.3 (2.8‐5.4) | 0.0219 |
| Platelets (×108/uL) | 22 (6.8‐75.1) | 21.4 (4.6‐78.5) | 0.2182 |
| T‐Bil (mg/dL) | 0.66 (0.36‐3.59) | 0.79 (0.37‐2.31) | 0.2893 |
| GGT (IU/L) | 60 (14‐534) | 63 (12‐1464) | 0.065 |
| Total cholesterol (mg/dL) | 208 (77‐335) | 207 (77‐372) | 0.351 |
| TG (mg/dL) | 149 (44‐642) | 150 (49‐920) | 0.9632 |
| HDL‐C (mg/dL) | 51 (23‐290) | 49 (22‐99) | 0.2185 |
| LDL‐C (mg/dL) | 131 (44‐246) | 125 (8‐294) | 0.3082 |
| HbA1c (%) | 5.8 (4.5‐6.5) | 6.7 (5.1‐12.8) | <0.001 |
| Dyslipidemia (yes) | 240 of 311 (77.2%) | 242 of 311 (77.8%) | 0.9235 |
| Hypertension (yes) | 86 of 266 (32.3%) | 126 of 271 (46.5%) | <0.001 |
| COL4‐7S (ng/mL) | 4.2 (2.5‐13) | 4.4 (1‐55.5) | 0.0032 |
| FIB‐4 index | 1.33 (0.17‐7.51) | 1.53 (0.24‐9.32) | 0.1638 |
| NFS | 1.847 (9.081 to 2.147) | 0.743 (8.764 to 3.250) | <0.001 |
| APRI | 0.694 (0.126‐4.277) | 0.725 (0.149‐4.420) | 0.3891 |
| Brunt grade (1/2/3) | 137/118/51 (44.8%/38.6%/16.6%) | 120/115/48 (42.4%/40.6%/17.0%) | — |
| Brunt stage (0/1/2/3/4) | 128/104/44/23/12 (41.1%/33.4%/14.1%/7.4%/3.9%) | 106/96/55/39/15 (34.1%/30.9%/17.7%/12.5%/4.8%) | — |
| Advanced fibrosis (F3 and F4) | 35 of 311 (11.3%) | 54 of 311 (17.4%) | 0.0388 |
| Liver cirrhosis (F4) | 12 of 311 (3.9%) | 15 of 311 (4.8%) | 0.695 |
|
NAS total (1/2/3/4/5/6/7/8) |
12/19/34/66/60/42/18/1 (4.8%/7.5%/13.5%/26.2%/23.8%/16.7%/7.1%/0.4%) |
3/11/33/31/49/43/12/1 (1.6%/6.0%/18.0%/16.9%/26.8%/23.5%/6.6%/0.5%) |
— |
| Steatosis (0/1/2/3) | 135/117/52 (44.4%/38.5%/17.1%) | 120/115/48 (42.4%/40.6%/17.0%) | — |
| Inflammation (0/1/2/3) | 43/132/62/15 (17.0%/52.4%/24.6%/6.0%) | 24/91/56/12 (13.1%/49.7%/30.6%/6.6%) | — |
| Ballooning (0/1/2) | 34/87/131 (13.5%/34.5%/52.0%) | 21/61/101 (11.5%/33.3%/55.2%) | — |
Caliper: 0.20.
Abbreviations: HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; T‐Bil, total bilirubin; TG: triglycerides.
FIG. 1.
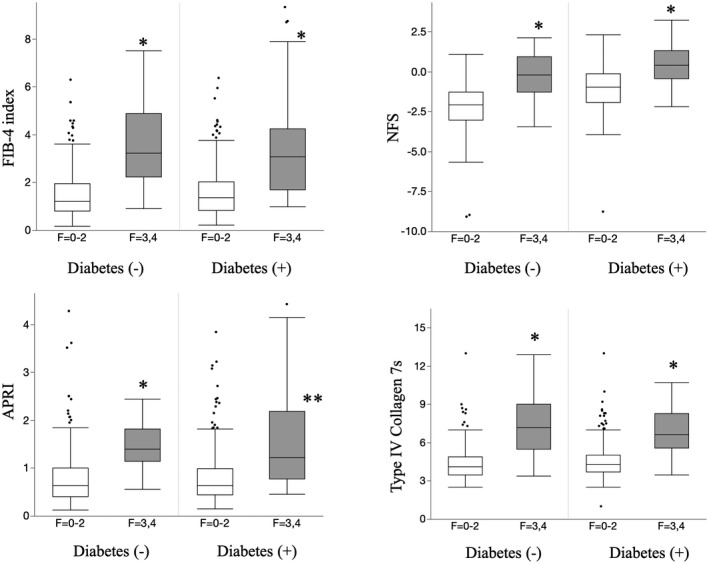
Box plot of NITs for advanced fibrosis of NAFLD with or without T2DM. *P < 0.01 versus F = 0‐2.
ROC Curve Analysis
ROC curves were developed to estimate the utility of each NIT in predicting advanced fibrosis (stages 3 and 4) with or without T2DM (Fig. 2A). The AUROCs in the group of patients without T2DM were 0.879 for the FIB‐4 index, 0.851 for the NFS, 0.862 for the APRI, and 0.883 for COL4‐7S. The AUROCs in the group of patients with T2DM were 0.790 for the FIB‐4 index, 0.784 for the NFS, 0.771 for the APRI, and 0.872 for COL4‐7S. The AUROCs of the FIB‐4 index (P = 0.038) and APRI (P = 0.026) in patients with T2DM were significantly smaller than those in patients without T2DM. Additionally, the AUROC of the NFS in patients with T2DM tended to be smaller than that in patients without T2DM (P = 0.159). There was no significant difference in the AUROC of COL4‐7S between patients with and without T2DM.
FIG. 2.
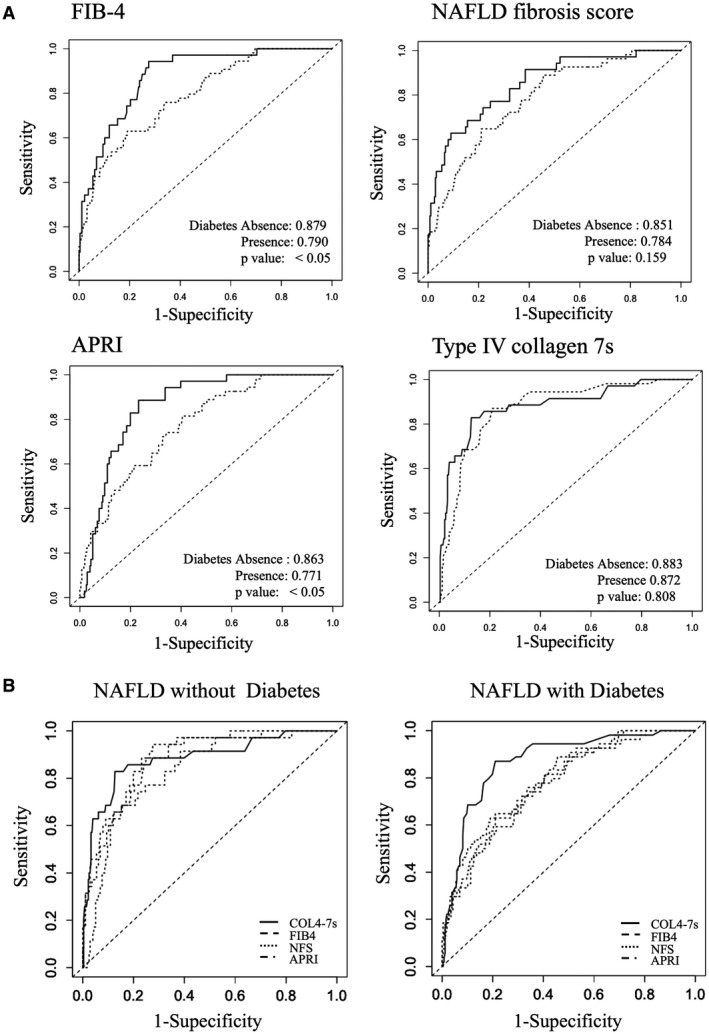
(A) ROC curve analysis of the FIB‐4 index, NFS, APRI, and COL4‐7S. The solid line indicates the absence of T2DM, and the broken line indicates the presence of T2DM. (B) DeLong test of each NIT of NAFLD with or without T2DM.
To compare the AUROC of COL4‐7S with that of other fibrosis scores in patients with or without T2DM (Fig. 2B), the change in the AUROC was tested using the DeLong test. In NAFLD with T2DM, the AUROC of COL4‐7S was significantly higher than those of other NITs. However, there were no significant differences between AUROCs of COL4‐7S and the other NITs in NAFLD without T2DM.
To estimate the difference in the AUROC between patients with NAFLD with T2DM and those without T2DM, we examined the clinical background of these patients with advanced fibrosis or without fibrosis (Table 3). In patients without T2DM, age, AST levels, and COL4‐7S levels were significantly higher and the platelet count was significantly lower in patients with stages 3 and 4 than in those with stages 0‐2. In patients with T2DM, similarly, age, BMI, AST levels, and COL4‐7S levels were significantly higher and the platelet count was significantly lower in stages 3 and 4 than in stages 0‐2. Results of NITs, such as the FIB‐4 index, NFS, and APRI, were significantly higher in stages 3 and 4 than in stages 0‐2 in patients with and those without T2DM. In patients with stages 3 and 4, the platelet count tended to be higher and age tended to be lower in the advanced fibrosis group with T2DM (n = 54) than in the advanced fibrosis group without T2DM (n = 35).
TABLE 3.
Comparison Between Factors of Noninvasive Fibrosis Markers for Diagnosing Advanced Fibrosis in Patients With NAFLD, With or Without T2DM
| Clinical Parameters | Diabetes Absence | P Value | Diabetes Presence | P Value | ||
|---|---|---|---|---|---|---|
| F0‐F2 (n = 276) | F3 and F4 (n = 35) | F0‐F2 (n = 257) | F3 and F4 (n = 54) | |||
| Age (years) | 56 (16‐84) | 65 (42‐79) | <0.01 | 57 (17‐82) | 60 (45‐83) | 0.035 |
| BMI (kg/m2) | 26.7 (17.7‐43.5) | 26.9 (20.4‐39.0) | 0.621 | 27.2 (17.3‐44.0) | 28.6 (19.6‐40.1) | 0.018 |
| Albumin (mg/dL) | 4.5 (3.2‐5.7) | 4.1 (3.1‐4.8) | <0.01 | 4.4 (2.8‐5.4) | 4.2 (3.1‐4.9) | <0.01 |
| AST (IU/L) | 42 (16‐208) | 55 (36‐119) | <0.01 | 41 (15‐202) | 58 (27‐186) | <0.01 |
| ALT (IU/L) | 67 (16‐298) | 62 (25‐227) | 0.826 | 64 (12‐358) | 62 (17‐218) | 0.520 |
| Platelets (×108/uL) | 22.7 (10.5‐75.1) | 14.6 (6.8‐28.7) | <0.01 | 22.2 (8.2‐78.5) | 17.6 (4.6‐31.1) | <0.01 |
| FIB‐4 index | 1.23 (0.17‐6.29) | 3.24 (0.91‐7.51) | <0.01 | 1.37 (0.24‐6.36) | 3.08 (1.00‐ 9.32) | <0.01 |
| NFS | 2.08 (9.08 to 1.10) | 0.21 (3.45 to 2.15) | <0.01 | 0.97 (8.76 to 2.34) | 0.43 (2.19 to 3.25) | <0.01 |
| APRI | 0.64 (0.13‐4.28) | 1.41 (0.56‐2.45) | <0.01 | 0.64 (0.15‐3.84) | 1.23 (0.46‐4.42) | <0.01 |
| COL4‐7S (ng/mL) | 4.1 (2.5‐13) | 7.2 (3.4‐12.9) | <0.01 | 4.3 (1‐55.5) | 6.7 (3.5‐23) | <0.01 |
Clinical Utility of NITs for Predicting Advanced Fibrosis
Using the overall population, two cutoff points of COL4‐7S were selected, so that sensitivity and specificity for advanced fibrosis were at least 90%. The high cutoff point was 5.9 ng/mL and the low cutoff point was 4.8 ng/mL for COL4‐7S. Low and high cutoff points of each NIT are shown in Fig. 3. We compared the clinical utility of these NITs for identifying advanced fibrosis in patients with T2DM when the proposed cutoff point of each NIT was applied (Table 4). Using the high cutoff points, sensitivity, the false negative rate, and the negative predictive value were best for COL4‐7S among the NITs in both groups, regardless of the presence of T2DM. In NAFLD with T2DM, sensitivity and the negative predictive value for advanced fibrosis of COL4‐7S were better than those of the other NITs. The accuracy of COL4‐7S did not differ from that of other NITs. In the low cutoff points, specificity, the false positive rate, and the positive predictive value of COL4‐7S were better than those of the other NITs. The accuracy of COL4‐7S was superior to that of the other NITs, especially in patients with NAFLD with T2D.
FIG. 3.
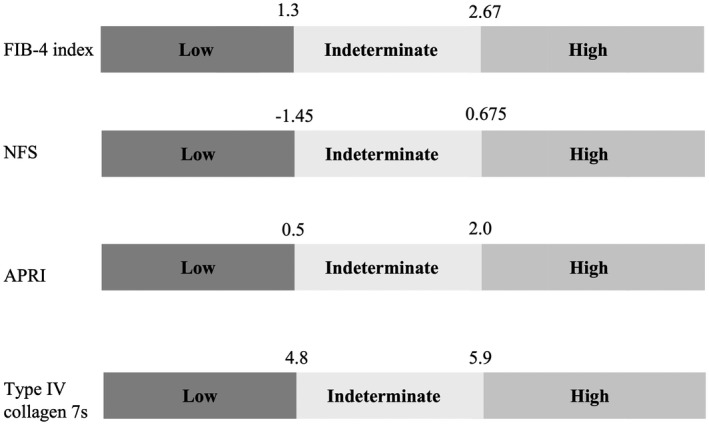
Low and high cutoff points of each NIT applied in this study.
TABLE 4.
Comparison of the Clinical Utility of Each NIT at High and Low Cutoff Points for Advanced Fibrosis in NAFLD
| High cutoff point | NIT | Sensitivity | Specificity | FP | FN | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Diabetes absence | FIB‐4 index > 2.67 | 0.657 | 0.877 | 0.123 | 0.343 | 0.404 | 0.953 | 0.852 |
| NFS > 0.675 | 0.286 | 0.989 | 0.011 | 0.714 | 0.769 | 0.916 | 0.913 | |
| APRI > 2.0 | 0.114 | 0.964 | 0.036 | 0.886 | 0.286 | 0.896 | 0.865 | |
| COL4‐7S > 5.9 | 0.686 | 0.899 | 0.101 | 0.314 | 0.462 | 0.958 | 0.875 | |
| Diabetes presence | FIB‐4 index > 2.67 | 0.537 | 0.856 | 0.144 | 0.463 | 0.439 | 0.898 | 0.797 |
| NFS > 0.675 | 0.426 | 0.891 | 0.109 | 0.574 | 0.451 | 0.881 | 0.813 | |
| APRI > 2.0 | 0.278 | 0.957 | 0.043 | 0.722 | 0.577 | 0.863 | 0.842 | |
| COL4‐7S > 5.9 | 0.685 | 0.872 | 0.128 | 0.315 | 0.529 | 0.929 | 0.839 | |
| Low cutoff point | ||||||||
| Diabetes absence | FIB‐4 index > 1.30 | 0.971 | 0.547 | 0.453 | 0.029 | 0.214 | 0.993 | 0.595 |
| NFS > 1.45 | 0.771 | 0.685 | 0.315 | 0.229 | 0.237 | 0.959 | 0.695 | |
| APRI > 0.5 | 1.00 | 0.362 | 0.638 | 0 | 0.166 | 1.000 | 0.434 | |
| COL4‐7S > 4.8 | 0.886 | 0.725 | 0.275 | 0.114 | 0.290 | 0.980 | 0.743 | |
| Diabetes presence | FIB‐4 index > 1.30 | 0.889 | 0.475 | 0.525 | 0.111 | 0.262 | 0.953 | 0.547 |
| NFS > 1.45 | 0.926 | 0.358 | 0.642 | 0.074 | 0.233 | 0.958 | 0.457 | |
| APRI > 0.5 | 0.944 | 0.327 | 0.673 | 0.056 | 0.228 | 0.966 | 0.434 | |
| COL4‐7S > 4.8 | 0.889 | 0.689 | 0.311 | 0.111 | 0.375 | 0.967 | 0.723 |
Abbreviations: FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value.
Correlations Between Age and Platelet Count or COL4‐7S
We investigated the relation between age and platelet count or COL4‐7S (Fig. 4). There was a significant negative correlation between age and platelet count in patients with NAFLD with T2DM (correlation coefficient: −0.4305, P < 0.001) and in those without T2DM (correlation coefficient: −0.4069, P < 0.001). However, there was a significant positive correlation between age and COL4‐7S in patients with NAFLD with T2DM (correlation coefficient: 0.1977, P < 0.001) and in those without T2DM (correlation coefficient: 0.3055, P < 0.001).
FIG. 4.
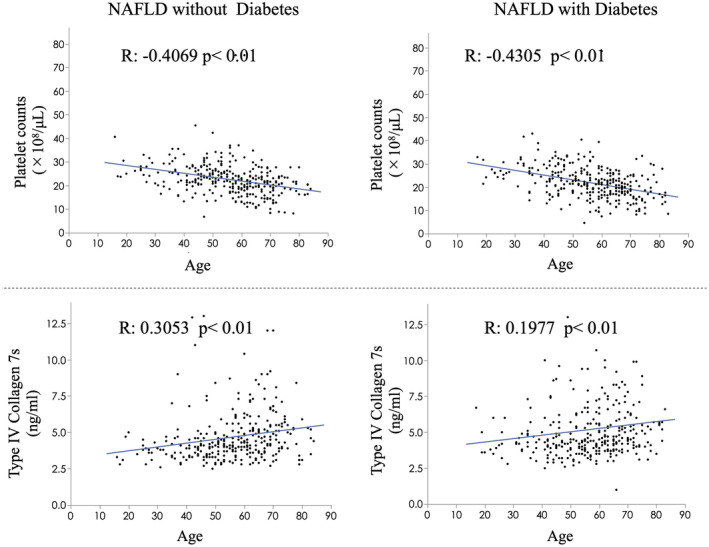
Scatter plot of the correlation between age and platelet count or COL4‐7S, with or without T2DM.
Discussion
In this study, we showed that COL4‐7S measurement was significantly better than conventional NITs for excluding advanced fibrosis of NAFLD with T2DM. Furthermore, COL4‐7S was similar to other NITs in NAFLD without T2DM. In this study, we compared the diagnostic accuracy of NITs, including the FIB‐4 index, the NFS, the APRI, and COL4‐7S, for detecting advanced fibrosis between patients with NAFLD and T2DM and those without T2DM. In patients without T2DM, each test was similar for diagnosing advanced fibrosis in NAFLD. However, in patients with T2DM, the AUROCs of the NITs of the FIB‐4 index, NFS, and APRI were smaller than 0.80 (Fig. 2A). The AUROC of COL4‐7S was 0.876 in patients with NAFLD and T2DM. The diagnostic accuracy of NITs other than COL4‐7S in patients with NAFLD with T2DM was lower than that in those without T2DM. However, the diagnostic accuracy of COL4‐7S in patients with NAFLD with T2DM was similar to that in those without T2DM. COL4‐7S is considered useful for excluding advanced fibrosis of NAFLD, especially with the presence of T2DM. The diagnostic accuracy of the FIB‐4 index and NFS for advanced fibrosis fluctuates by age, and resetting the cutoff point by each age group is necessary.( 25 , 26 ) In our study, we matched NAFLD with and without T2DM by age, sex, and the prevalence of dyslipidemia, and found that these variables were significantly different between the two groups.
COL4‐7S is a fragment of collagen type IV and is part of the extracellular matrix, which forms the basement membrane. COL4‐7S is an established biochemical marker of liver fibrosis.( 27 , 28 ) An increase in serum COL4‐7S levels is accompanied by progression of liver fibrosis in various chronic types of hepatitis, including NASH.( 28 ) Additionally, the efficacy for diagnosis of advanced fibrosis in NASH has been established.( 29 , 30 ) A previous study showed the efficacy of COL4‐7S for diagnosing NASH or advanced fibrosis of NAFLD.( 30 ) Sumida et al. reported that the NAFIC score, which consists of ferritin, insulin, and COL4‐7S, predicted NASH.( 29 ) These authors found that the AUROC for predicting NASH was 0.851 in the estimation group and 0.782 in the validation group. Okanoue et al. reported that the CA index, which consists of COL4‐7S and AST, predicted NASH or NASH‐related fibrosis.( 31 ) The AUROC of the CA‐fibrosis index for predicting NASH‐related fibrosis was 0.918. Therefore, COL4‐7S might be an important parameter for diagnosing NASH or advanced fibrosis in NAFLD.
The FIB‐4 index and NFS are recommended for diagnosing advanced liver fibrosis of NAFLD primarily according to the guideline of the AASLD and the European Association for the Study of the Liver.( 12 , 32 ) However, our study showed that the diagnostic accuracy of the FIB‐4 index and NFS was inferior to that of COL4‐7S in NAFLD with T2DM. The reason why these conventional NITs are inferior, especially in patients with T2DM, remains to be resolved. One reason for our finding is that the FIB‐4 index and NFS include age. In the advanced stage (stages 3 and 4) in our study, patients with T2DM tended to younger (60 [45‐83] years) compared to those without T2DM (65 [42‐79] years) (Table 3). Cross‐sectional data from a multicenter study by the JSG‐NAFLD showed that the presence of T2DM was strongly associated with the severity of liver fibrosis in Japanese patients with NASH.( 33 ) Adams et al. reported that T2DM and a low initial stage of fibrosis were associated with a higher rate of progression of fibrosis in 103 patients with NAFLD who underwent serial liver biopsies.( 34 ) They showed a mean interval of 3.2 ± 3.0 years between biopsies. In another cohort of Japanese patients with NAFLD, the presence of T2DM was an independent risk factor for progression to advanced liver fibrosis (hazard ratio, 1.879), as evaluated by the FIB‐4 index in addition to advanced age and low albumin concentrations. Furthermore, a longer duration of T2DM is an independent risk factor for progressive fibrosis in patients with NAFLD.( 35 ) The reason why patients with T2DM are younger in the advanced stage may be explained by T2DM being a significant risk factor for rapid progression of hepatic fibrosis in NAFLD.( 36 ) Another reason for our finding is that conventional NITs, such as the FIB‐4 index, NFS and APRI, are affected by the platelet count, because their formulas include the platelet count. In the advanced stage (stages 3 and 4) in our study, the platelet count appeared to be higher in patients with T2DM compared to those without T2DM, but this was not significant (Table 3). The reason for this lack of finding remains unknown. We found that the platelet count was negatively correlated with age in patients with T2DM and in those without T2DM (Fig. 4). The platelet count is decreased in parallel with aging. In the advanced stage, patients with T2DM might have shown a higher platelet count than those without T2DM, because patients with T2DM were younger. Another explanation for our finding is that the platelet count is elevated in patients with metabolic syndrome.( 37 ) Jesri et al. reported that the platelet count increased in parallel with obesity, insulin resistance, and physical inactivity. Obesity, insulin resistance, and diabetes are associated with higher levels of several inflammatory markers, including interleukin‐6. Elevated interleukin‐6 levels may be responsible for increased platelets in NAFLD with T2DM.( 38 , 39 , 40 , 41 )
The FIB‐4 index and NFS are useful NITs for diagnosing advanced fibrosis in NAFLD without T2DM. However, there must be caution in diagnosing advanced fibrosis of NAFLD. This is because some studies have noted the problem that diagnostic accuracy of these tests decreases with increasing age of patients. Therefore, our research group and others have proposed adaptive cutoff points for the age of patients.( 25 , 26 ) Researchers must be aware that these NITs are affected by age. Similar to our study, previous studies have reported that the AUROC tends to decrease in NAFLD with T2DM compared to NAFLD without T2DM for diagnosis of advanced fibrosis ≥ stage 3 and significantly decreases for diagnosis of cirrhosis.( 42 ) The FIB‐4 index, NFS, and APRI are useful diagnostic methods, but in the diagnosis of advanced fibrosis of NAFLD, age and the presence of T2DM should be taken into consideration.
Our study showed a significant correlation between age and COL4‐7S in both NAFLD groups. The correlation coefficient, however, was weak in NAFLD without T2DM and there was a poor correlation in NAFLD with T2DM. The correlation between age and COL4‐7S was weaker than that between age and the platelet count. COL4‐7S reflects the fibrosis process of synthesis and degradation in the liver.( 43 , 44 ) Therefore, COL4‐7S may be expected to not be greatly affected by aging. In our study, the platelet count and COL4‐7S were only a little affected by aging in practice. However, there are normally no changes in the platelet count or COL4‐7S with older age in healthy people,( 44 , 45 ) The reason why COL4‐7S was affected by age, although a little, was that subjects in this study were patients with NAFLD and liver fibrosis, and these parameters were affected by progression of liver fibrosis with aging. The present NITs are diagnostic methods for advanced fibrosis ≥ stage 3, but they may be necessary to identify it and screen from stage F2 for intervention and a cure. A model study reported that the cost‐effectiveness was better at identifying the early stage of F2 and intervening in patients’ lifestyles with vibration‐controlled transient elastography than with the FIB‐4 index identifying advanced fibrosis and starting medications.( 46 ) Identifying fibrosis stage F2 in the future may be important.
This study has several limitations that need to be acknowledged. First, this was a cross‐sectional study; therefore, estimating the diagnostic accuracy of COL4‐7S was necessary. Second, the efficacy of COL4‐7S has been shown in previous studies, but its efficacy was not validated in our study because of the limitation of a daily blood test as the hepatic fibrosis marker in our daily clinical practice. An international, multicenter, validation study is required to confirm our conclusions. Third, a recent report showed that the amount of domain of collagen in liver fibrosis is related to patients’ convalescence( 47 ); however, our study could not consider this because it was a multicenter joint research study, and the specimens were not able to be stained again. The collagen proportionate area of biopsy specimens and its relation to COL4‐7S need to be examined in the future. Fourth, the usefulness of diagnosing fibrosis with imaging methods, such as ultrasound elastography and magnetic resonance imaging, instead of liver biopsy has been reported; however, in our study, the availability of these equipment varied between facilities, so we were not able to compare the imaging methods with NITs. Examination comparing these methods with NITs will be necessary for the future. Finally, because this is a cross‐sectional study that estimates the relations between NITs and liver fibrosis at the time of liver biopsy, we cannot evaluate an effect of the diabetic medicine for liver fibrosis and the levels of NITs. It will be necessary to examine the influence of the DM medicine on liver fibrosis by a longitudinal study in future.
In conclusion, COL4‐7S measurement might be the best NIT to predict advanced fibrosis of NAFLD, especially in NAFLD with T2DM. The diagnostic accuracy of conventional NITs, such as the FIB‐4 index, NFS and APRI, is inferior to that of COL4‐7S in NAFLD with T2DM.
Supporting information
Table S1
Acknowledgment
This research was supported by AMED under Grant Number 20fk0210040h0002. [Corrections added on March 10, 2021, after first online publication: Acknowledgment section has been added.]
Potential conflict of interest: Dr. Itoh received grants from Fujirebio. Dr. Kawaguchi is on the speakers’ bureau for MSD KK, Mitsubishi Tanabe Pharma Corporation, and Otsuka Pharmaceutical. Dr. Nakajima is on the speakers’ bureau for and received grants from EA Pharma and Myland EPD. He is on the speakers’ bureau for Astellas. Dr. Yoneda received grants from AbbVie and Bayel. He is on the speakers’ bureau for and received grants from DaiNippon‐Sumitomo Pharmaceuticals.
SEE EDITORIAL ON PAGE 553
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sumida Y, Yoneda M, Seko Y, Ishiba H, Hara T, Toyoda H, et al. Surveillance of hepatocellular carcinoma in nonalcoholic fatty liver disease. Diagnostics 2020:10:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sumida Y, Shima T, Mitsumoto Y, Katayama T, Umemura A, Yamaguchi K, et al. Epidemiology: pathogenesis, and diagnostic strategy of diabetic liver disease in Japan. Int J Mol Sci 2020;21:4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Investig 2017;8:397‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India 2009;57:205‐210. [PubMed] [Google Scholar]
- 7. Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Non‐alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol 2004;19:854‐858. [DOI] [PubMed] [Google Scholar]
- 8. Leite NC, Villela‐Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int 2011;31:700‐706. [DOI] [PubMed] [Google Scholar]
- 9. Arrese M, Barrera F, Triantafilo N, Arab JP. Concurrent nonalcoholic fatty liver disease and type 2 diabetes: diagnostic and therapeutic considerations. Expert Rev Gastroenterol Hepatol 2019;13:849‐866. [DOI] [PubMed] [Google Scholar]
- 10. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 2017;40:419‐430. [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association . Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes 2019;37:11‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do diabetologists stand? Clin Diabetes Endocrinol 2020;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population‐based study including 4275 biopsies. Liver Int 2008;28:705‐712. [DOI] [PubMed] [Google Scholar]
- 14. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 15. Williamson RM, Price JF, Hayes PC, Glancy S, Frier BM, Johnston GI, et al. Prevalence and markers of advanced liver disease in type 2 diabetes. QJM 2012;105:425‐432. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183‐1197. [DOI] [PubMed] [Google Scholar]
- 17. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467‐2474. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Society for the Study of Obesity . New criteria of obesity in Japanese. J Jpn Soc Study Obes 2000;6:18‐28. [Google Scholar]
- 19. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 20. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 21. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 22. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 23. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol 2018;68:335‐352. [DOI] [PubMed] [Google Scholar]
- 25. Ishiba H, Sumida Y, Tanaka S, Yoneda M, Hyogo H, Ono M, et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi‐center study. J Gastroenterol 2018;53:1216‐1224. [DOI] [PubMed] [Google Scholar]
- 26. McPherson S, Hardy T, Dufour JF, Petta S, Romero‐Gomez M, Allison M, et al. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leeming DJ, Nielsen MJ, Dai Y, Veidal SS, Vassiliadis E, Zhang C, et al. Enzyme‐linked immunosorbent serum assay specific for the 7S domain of collagen type IV (P4NP 7S): a marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol Res 2012;42:482‐493. [DOI] [PubMed] [Google Scholar]
- 28. Sakugawa H, Nakayoshi T, Kobashigawa K, Yamashiro T, Maeshiro T, Miyagi S, et al. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol 2005;11:255‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol 2011;46:257‐268. [DOI] [PubMed] [Google Scholar]
- 30. Yoneda M, Mawatari H, Fujita K, Yonemitsu K, Kato S, Takahashi H, et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J Gastroenterol 2007;42:375‐381. [DOI] [PubMed] [Google Scholar]
- 31. Okanoue T, Ebise H, Kai T, Mizuno M, Shima T, Ichihara J, et al. A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J Gastroenterol 2018;53:129‐139. [DOI] [PubMed] [Google Scholar]
- 32. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 33. Nakahara T, Hyogo H, Yoneda M, Sumida Y, Eguchi Y, Fujii H, et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol 2014;49:1477‐1484. [DOI] [PubMed] [Google Scholar]
- 34. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132‐138. [DOI] [PubMed] [Google Scholar]
- 35. Tada T, Toyoda H, Sone Y, Yasuda S, Miyake N, Kumada T, et al. Type 2 diabetes mellitus: a risk factor for progression of liver fibrosis in middle‐aged patients with non‐alcoholic fatty liver disease. J Gastroenterol Hepatol 2019;34:2011‐2018. [DOI] [PubMed] [Google Scholar]
- 36. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019;71:793‐801. [DOI] [PubMed] [Google Scholar]
- 37. Jesri A, Okonofua EC, Egan BM. Platelet and white blood cell counts are elevated in patients with the metabolic syndrome. J Clin Hypertens (Greenwich) 2005;7:705‐711; quiz 712‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taniguchi A, Fukushima M, Seino Y, Sakai M, Yoshii S, Nagasaka S, et al. Platelet count is independently associated with insulin resistance in non‐obese Japanese type 2 diabetic patients. Metabolism 2003;52:1246‐1249. [DOI] [PubMed] [Google Scholar]
- 39. Cho NH, Becker DJ, Ellis D, Kuller LH, Drash AL, Orchard TJ. Spontaneous whole blood platelet aggregation, hematological variables and complications in insulin‐dependent diabetes mellitus: the Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications 1992;6:12‐18. [DOI] [PubMed] [Google Scholar]
- 40. Brown AS, Hong Y, de Belder A, Beacon H, Beeso J, Sherwood R, et al. Megakaryocyte ploidy and platelet changes in human diabetes and atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:802‐807. [DOI] [PubMed] [Google Scholar]
- 41. Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin‐6 causes hepatic insulin resistance in mice. Diabetes 2003;52:2784‐2789. [DOI] [PubMed] [Google Scholar]
- 42. Bertot LC, Jeffrey GP, de Boer B, MacQuillan G, Garas G, Chin J, et al. Diabetes impacts prediction of cirrhosis and prognosis by non‐invasive fibrosis models in non‐alcoholic fatty liver disease. Liver Int. 2018;38:1793‐1802. [DOI] [PubMed] [Google Scholar]
- 43. Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon‐Jensen T, et al. The good and the bad collagens of fibrosis—their role in signaling and organ function. Adv Drug Deliv Rev 2017;1:43‐56. [DOI] [PubMed] [Google Scholar]
- 44. Karsdal MA, Genovese F, Madsen EA, Manon‐Jensen T, Schuppan D. Collagen and tissue turnover as a function of age: implications for fibrosis. J Hepatol 2016;64:103‐109. [DOI] [PubMed] [Google Scholar]
- 45. Fang KC, Cheng YL, Su CW, Wang YJ, Lan KH, Huo TI, et al. Higher platelet counts are associated with metabolic syndrome independent of fatty liver diagnosis. J Chin Med Assoc 2017;80:125‐132. [DOI] [PubMed] [Google Scholar]
- 46. Noureddin M, Jones C, Alkhouri N, Gomez EV, Dieterich DT, Rinella ME; NASHNET . Screening for non‐alcoholic fatty liver disease in persons with type 2 diabetes in the u.s. is cost effective: a comprehensive cost‐utility analysis. Gastroenterology 2020. Aug 5. 10.1053/j.gastro.2020.07.050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47. Calvaruso V, Burroughs AK, Standish R, Manousou P, Grillo F, Leandro G, et al. Computer‐assisted image analysis of liver collagen: relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology 2009;49:1236‐1244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


