Abstract
Small-for-gestational age (SGA) human newborns have an increased risk of hyperphagia and obesity, as well as a spectrum of neurologic and neurobehavioral abnormalities. We have shown that the SGA hypothalamic (appetite regulatory site) neuroprogenitor cells (NPCs) exhibit reduced proliferation and neuronal differentiation. DNA methylation (DNA methyltransferase; DNMT1) regulates neurogenesis by maintaining NPC proliferation and suppressing premature differentiation. Once differentiation ensues, DNMT1 preferentially promotes neuronal and inhibits astroglial fate. We hypothesized that the programmed dysfunction of NPC proliferation and differentiation in SGA offspring is epigenetically mediated via DNMT1. Pregnant rats received either ad libitum food (Control) or were 50% food-restricted to create SGA offspring. Primary hypothalamic NPCs from 1 day old SGA and Controls newborns were cultured and transfected with nonspecific or DNMT1-specific siRNA. NPC proliferation and protein expression of specific markers of NPC (nestin), neuroproliferative transcription factor (Hes1), neurons (Tuj1) and astrocytes (GFAP) were determined. Under basal conditions, SGA NPCs exhibited decreased DNMT1 and reduced proliferation and differentiation, as compared to Controls. In both SGA and Controls, DNMT1 siRNA in complete media inhibited NPC proliferation, consistent with reduced expression of nestin and Hes1. In differentiation media, DNMT1 siRNA decreased expression of Tuj1 but increased GFAP. In vivo data replicated these findings. In SGA offspring, impaired neurogenesis is epigenetically mediated, in part, via reduction in DNMT1 expression and suppression of Hes1 resulting in NPC differentiation. It is likely that the maturation of regions beyond the hypothalamus (e.g., cerebral cortex, hippocampus) may be impacted, contributing to poor cognitive and neurobehavioral competency in SGA offspring.
Keywords: DNMT1, hypothalamus, neurogenesis, neurons, astrocytes, developmental programming
INTRODUCTION
The fetal nutritional environment has been demonstrated to have a remarkable effect on potential neural programming, including effects on offspring appetite, reward function, addiction potential, neurobehavior, cognitive function, and a predisposition to Alzheimer's disease (Kyle & Pichard, 2006; Ross et al., 2007; Roseboom et al., 2011; Arcangeli et al., 2012; O'Neill et al., 2017). Among the multiple associations, the small for gestational age (SGA) human newborn has clearly been demonstrated to have an increased risk of hyperphagia and obesity (Jaquet et al., 2005; Painter et al., 2005; Meas et al., 2010; Ross & Desai, 2014) as well as the aforementioned spectrum of neurologic and neurobehavioral abnormalities. Similar neurologic effects have been demonstrated in rodent models of SGA, utilizing either maternal undernutrition or uterine artery ligation (Vickers et al., 2000; Desai et al., 2005; Coupe et al., 2009; Goodspeed et al., 2015).
Our laboratory has utilized a model of maternal undernutrition during the second half of rat pregnancy, which consistently results in the birth of SGA newborns (Desai et al., 2005). Despite the low birth weight, the SGA newborns demonstrate a rapid catch-up growth when nursed by ad libitum fed dams, and exhibit hyperphagia and obesity as adults (Desai et al., 2005; Desai et al., 2007a). We have confirmed that the increased food intake is a result of central dysregulation of appetite/satiety function within the hypothalamic arcuate nucleus (Fukami et al., 2012). Reduced responses to leptin (Desai et al., 2007b) and insulin (Fukami et al., 2013) with increased sensitivity to ghrelin (Yousheng et al., 2008) are among the physiologic associations, with cellular studies demonstrating a dysregulation of cellular energy sensors and epigenetic signal factors (Desai et al., 2011a). The hypothalamic arcuate nucleus of SGA offspring contains an increased ratio of appetite to satiety neurons suggesting an imbalance in early neural development (Desai et al., 2014).
We have further explored the process of neural proliferation and neural differentiation using an ex-vivo neural progenitor cell (NPC) culture model. When removed from the SGA somatic environment, NPCs from SGA newborns exhibit a marked reduction in neural proliferation, altered neural differentiation, and ultimately a reduced increased ratio of appetite to satiety neurons, replicating the in-vivo results (Desai et al., 2011a; Desai et al., 2014). Thus, the developmental origins of adult obesity associated with SGA newborns may be a result in part of a programmed dysfunctional neurogenesis. In view of the significance of altered neurogenesis for both appetite regulation and the diversity of potential neural programming, we sought to examine mechanisms by which SGA neurodevelopment is altered, focusing on epigenetic regulation.
DNA methylation plays an important role in neurogenesis and DNA methyltransferases (DNMT1, 3a, 3b) are spatially and temporally expressed during neurogenesis (Hu et al., 2012; Wang et al., 2016). Specifically, DNMT1 an enzyme responsible for the maintenance of DNA methylation, is highly expressed in proliferative cells (Goto et al., 1994; Noguchi et al., 2015) and regulates neurogenesis by maintaining NSC proliferation and suppressing premature differentiation (Fan et al., 2005; Stricker & Gotz, 2018). Once differentiation ensues, DNMT1 preferentially promotes astroglial and inhibits neuronal fate (Fan et al., 2005; Namihira et al., 2009; Stricker & Gotz, 2018). We hypothesized that the programmed dysfunction of NSC proliferation and differentiation in SGA offspring is epigenetically mediated via DNMT1. Using both DNMT1 silencing and pharmacologic inhibition approaches, the results demonstrate a critical role of SGA-associated reduced DNMT1 as a putative etiologic factor that may have widespread consequences.
EXPERIMENTAL PROCEDURES
SGA model
Studies were in accordance with the American Association for Accreditation of Laboratory Care (AALC) and National Institutes of Health (NIH) guidelines, and were approved by the Animal Research Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA (LABioMed). All animals were housed in a facility with constant temperature and humidity and controlled 12:12 hour light/dark cycle. The rat model utilized for maternal food restriction during pregnancy and lactation has been previously described (Desai et al, 2005). Briefly, first time pregnant Sprague Dawley rats were obtained from Charles River Laboratories, Hollister, California and provided standard laboratory chow (protein 23%, fat 4.5%, metabolizable energy 3030 kcal/kg; Lab Diet 5001, Brentwood, MO). At day 10 of pregnancy, Control rats (N=17) were provided an ad libitum diet to term (21 days) whereas study group (N=16) were 50% food restricted diet to create small for gestation age newborns (SGA). The food restriction was determined by quantification of normal intake in ad libitum fed rats. All dams delivered normally and at 1 day of age, all litters were culled to four males and four females (to standardize nursing) and all offspring nursed by Control dams till 21 days of age. Separate litters were used for in vivo and in vitro studies as stated below.
In Vivo Studies
NPC Cell Proliferation:
N=4 pregnant dams per group were used for BrdU (5-bromodeoxyuridine; incorporated during S-phase of cell cycle) labeling. Pregnant dams from gestational age e17-e19 (N=4 per group) were injected with BrdU (50mg/kg/day, i.p.) (Desai et al., 2011b). After birth at 1 day of age, four male newborns from each litter were euthanized for cell proliferation assessment (detailed below). Three sections per brain (5μm thickness) were immunostained for BrdU and PCNA (proliferating cell nuclear antigen; expression increases during G1-phase, peaks at the S-phase, and declines during G2/M-phases of the cell cycle). Secondary antibodies used were donkey anti-rabbit IgG conjugated with Alexa 488 (Invitrogen, Carlsbad, CA). Florescence microscopy (Zeiss, Axioskop 40) was used to capture photomicrographs at ×20 magnification and Image PRO software (version 5.1) was used to for counting PCNA/BrdU positive cells in mid-line region per field (Mosmann, 1983).
Hypothalamus Tissue Protein Expression:
Separate litters (Controls, N=6; food-restricted, N=5) were used for determining offspring hypothalamic protein expression (Western Blot) at two ages (1 and 21 days). Excess 1 day old male pups during litter culling were euthanized by decapitation and brains collected. At 21 days of age, one male offspring per litter was anesthetized by 5 % isoflurane/2 % oxygen by mask, subsequently euthanized by an overdose of pentobarbital (200 mg/kg i.p.) and brain collected. Hypothalami were obtained by dissecting semi-spheres adjunct to two sides of hypothalamus, the dorsal part removed, and the ventral part (~2 mm) used.
Western Blot was performed as previously reported by our group (Desai et al, 2008). Data were normalized to GAPDH (1:10,000, Chemicon). Primary antibodies used were DNMT1 (1:500, Santa Cruz), rabbit anti-Hes1 (1:1000, Santa Cruz), rabbit anti-Tuj1 (1:5000, Sigma) and rabbit anti-GFAP (1:10,000, DAKO).
In Vitro Studies
Neurosphere cultures, DNMT1 silencing (siRNA) and treatment with DNMT1 inhibitor:
Separate litters (Controls, N=5; food-restricted, N=5) were used for NPC culture studies. From each litter, N=4 males at 1 day of age were euthanized and hypothalami pooled. NPC cultures were prepared as previously reported (Desai et al, 2011b; Erickson et al, 2008; Kelly et al, 2009). In brief, dissected hypothalamus in DMEM/F12 medium was subjected to trypsin, centrifuged and dissociated cells seeded at 5x104cell/ml in complete medium composing of NeurobasalTM medium with 1% anti-anti (Invitrogen), 2% B27 (GIBCO, Cat#17504-044), 20ng/ml FGF2 (Sigma), 20ng/ml EGF (Sigma), 1μg/ml heparin (Lylli), and 2.5μg/ml L-glutamine (Invitrogen). Cultures were maintained for 8 days (passage 0), after which neurospheres were trypsinized and dissociated single-cells were reseeded at same cell density (passage 1) in complete medium. To induce differentiation, dissociated cells were re-suspended in differentiating medium which lacked FGF2, EGF and heparin and seeded in culture dishes pre-coated with 0.01% poly-L-lysine (Sigma).
For DNMT1 silencing (siRNA), hypothalamic NPCs were cultured in complete medium. At day 1 of passage 1 NPC seeding, the NPCs were transfected with rat DNMT1-specific siRNA (20 nM) or control siRNA (negative control) using siPORT™ NeoFX™ Reagent (Ambion). The transfected cells were reseeded in complete or differentiating medium. At day 5 of siRNA transfection, protein expression (Western blot) was determined as described above, and cell proliferation and immunostaining undertaken as described below.
For treatment with DNMT1 inhibitor, hypothalamic NPCs were cultured in complete or differentiating medium. At day 1 of passage 1 NPC seeding, the NPCs were treated with two doses of specific inhibitor for DNMT1 (5, 10 μM 5-Aza; 5-Azacytidine) for 5 days. Following treatment at day 5, protein expression (Western blot) was determined as described above, and cell proliferation and immunostaining undertaken as described below. .
Hypothalamic NPC cell proliferation:
NPC cultured in complete medium were used for MTT (3-(4,5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide; Sigma) colorimetric assay (McLenachan et al, 2009). Cell proliferating index was expressed as value of OD 570nm.
For in vitro labeling, BrdU (3 μg/ml) was added to NPCs in complete medium and incubated at 37°C for 24h in CO2 incubator (Groszer et al., 2001; Fujimoto et al., 2009). Cells were fixed in 4% paraformaldehyde in PBS for 30 min and processed for immunostaining using anti-BrdU (Sigma).
Hypothalamic Immunostaining of Neurons and Astrocyte:
Disassociated Control NPCs in differentiating medium were seeded in 24 well plates with pre-placed cover glasses (Fisher, catalogue number: 12-545-84 18CIR-1D). Following 5 day treatments with DNMT siRNA and 5-Aza, cells were fixed in 4% paraformaldehyde in PBS for 30 min and stained using rabbit anti-Tuj1 (1:500, Sigma), rabbit anti-GFAP (1:500, DAKO) and secondary antibody donkey anti-rabbit IgG-Alexa 488 (1:250). Secondary antibodies were donkey anti-rabbit IgG-Alexa 488 (1:250) or donkey antimouse IgG-Alexa 568 (1:250).
Data Analysis
For in vivo BrdU proliferation studies, N=4 litters of Controls and N-4 litters of SGA were studied. The average of BrdU-labeled cell numbers of three sections represented one brain and the average of four brain cell numbers represented one litter. For hypothalamic tissue studies, N=6 litters for Control and N=5 litters for SGA were studied. For NPC cultures, N=5 litters per group were studied and from each litter, hypothalami from 4 male pups were pooled. For protein expression, data are presented as fold change or percentage of Control. Differences between SGA and the Control males were compared using either unpaired t-test or two-way ANOVA with Dunnett’s post hoc tests.
RESULTS
Body Weights:
Male offspring born to undernourished dams had reduced birth weight as compared to controls (6.6 ± 0.2 vs 7.0 ± 0.2 g, P<0.01), as previously reported (Desai et al, 2005). However when nursed by Control dams, SGA male offspring at 21 days of age were significantly heavier than the Controls (51 ± 3 vs 43 ± 2 g, t value=4.038; P<0.01). There was no difference in gestational age at birth, litter size and/or gender distribution between SGA and control offspring.
In Vivo Cell Proliferation:
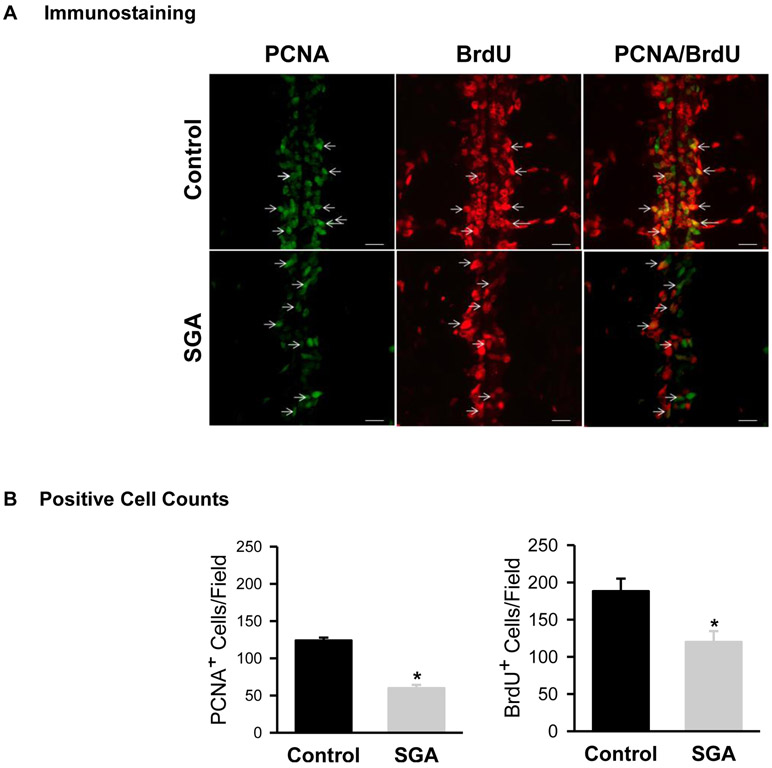
BrdU and PCNA staining was used to assess in vivo NPC proliferation in brain sections obtained from 1 day old newborns which demonstrated co-localization of BrdU and PCNA staining. Brain sections from SGA newborns showed significantly decreased BrdU and PCNA positive cells near the midline region (Figure 1A, B).
Figure 1: In Vivo Cell Proliferation.
Mid-line immunostaining of PCNA (green) and BrdU (red) incorporation in 1 day old Control (■) and SGA (■) males. (B) Quantification of PCNA and BrdU positive cells of 4 litters in each group and N=4 male pups per litter were studied. PCNA: t-value = 11.226, *P<0.001; BrdU: t-value = 3.208; *P<0.01 vs Control.
Hypothalamic Tissue Protein Expression:
At one day of age, SGA newborn demonstrated significantly decreased hypothalamic protein expression of DNMT1, Hes1 and Tuj1 with increased expression of GFAP (Figure 2). This pattern persisted and was evident in the hypothalamus of 21 day old male offspring (Figure 3).
Figure 2: Hypothalamic Tissue Protein Expression in 1 Day Old Newborns.
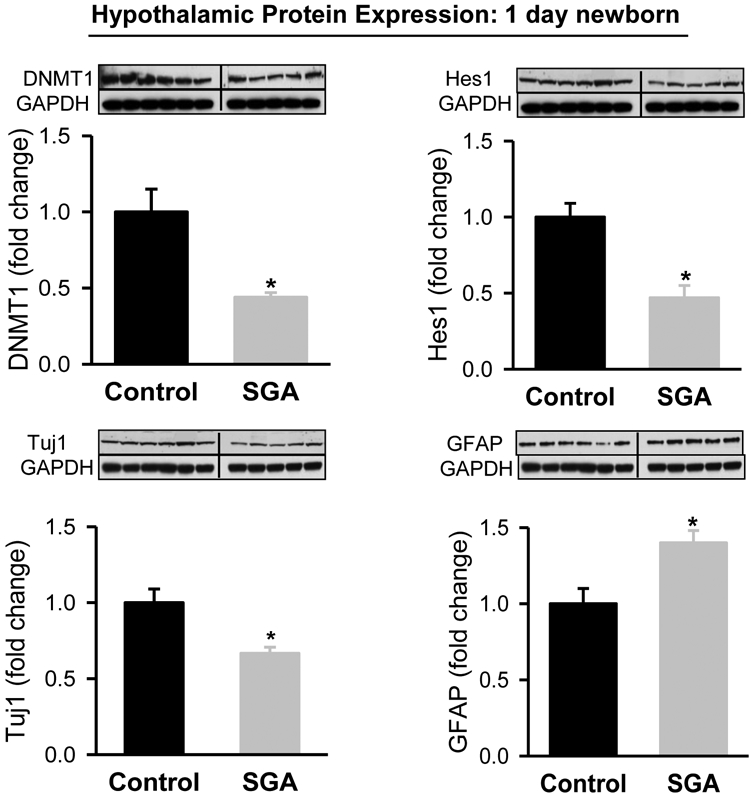
Hypothalamic protein expression of DNMT1 and Hes1 from 1 day old Control (■) and SGA (■) males. Control: N=6 male pups from 6 litters; SGA: N=5 male pups from 5 litters. DNMT1: t-value=3.401, P<0.001; Hes1: t-value=4.373, P<0.001; Tuj1: t value=4.2514, P<0.002; GFAP: t-value=2.548, P<0.05 vs Control.
Figure 3: Hypothalamic Tissue Protein Expression in 3 Week Old Offspring.
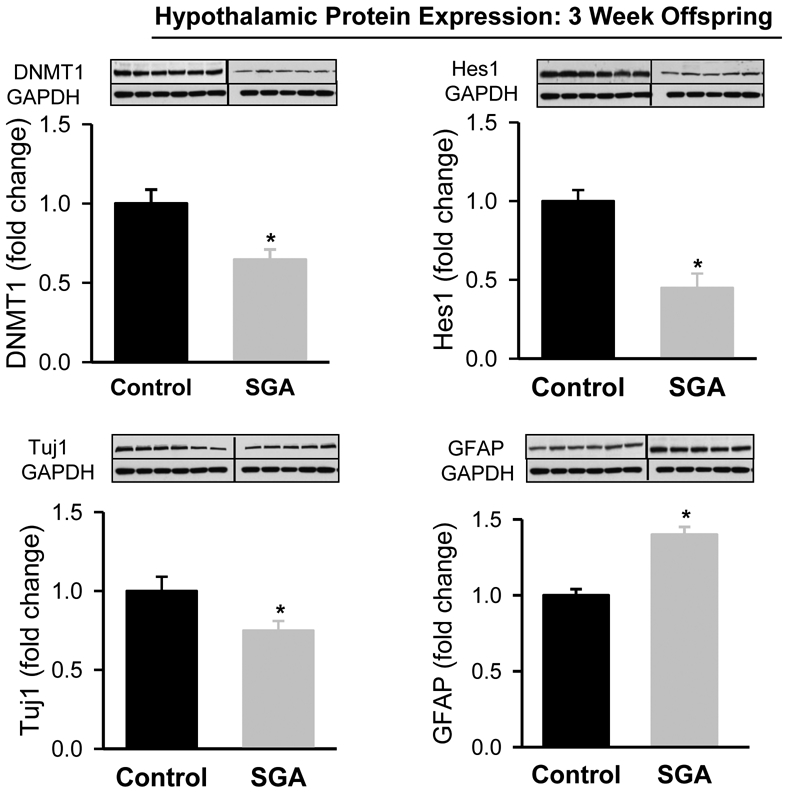
Hypothalamic protein expression of DNMT1 and Hes1 from 3 week old Control (■) and SGA (■) males. Control: N=6 male pups from 6 litters; SGA: N=5 male pups from 5 litters. DNMT1: t-value=3.122, P<0.01; Hes1: t value=4.297; P<0.002; Tuj1: t value=3.661; P<0.005; GFAP: t-value=6.538, P<0.001 vs Control.
Control and SGA NPC culture:
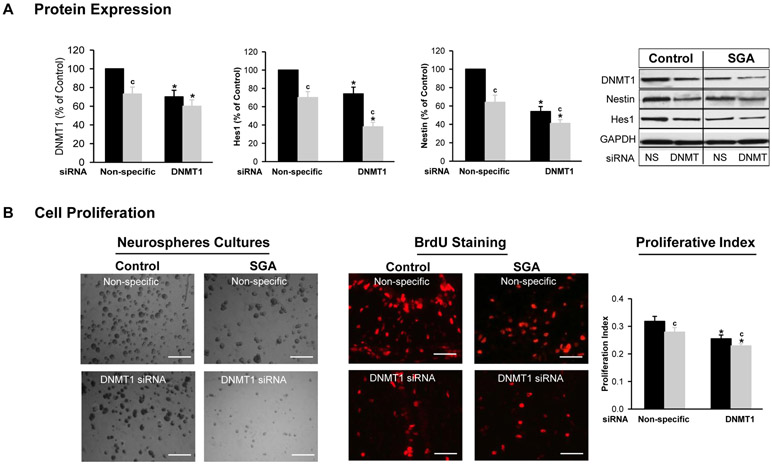
Under basal conditions, SGA NPCs in complete media exhibited significantly decreased DNMT1 expression (Figure 4A) though with unchanged DNMT3a expression (data not shown) as compared to Controls. In addition, the expression of cell proliferative measures Nestin (NPC cell marker) and Hes1 (proliferative transcription factor) was significantly less in SGA as compared to Control NPCs under basal conditions (Figure 4A). Consistent with these changes, SGA NPCs exhibited visually reduced colony density and BrdU staining with significantly decreased cell proliferation index as compared to Control NPCs (Figure 4B).
Figure 4: DNMT1 Silencing (siRNA) Effect on Neurosphere Proliferation.
NPCs from Control (■) and SGA (■) were transfected with non-specific or DNMT1-specific siRNA (20 nM) in complete media. (A) Protein expression of DNMT1, Hes1 and Nestin, and Immunoblots. (B) Images of neurospheres (x20), BrdU staining and proliferation index. (NS=non-specific siRNA). 5 litters were studied in each group. From each litter, hypothalami from N=4 male pups were pooled representing N=1. F-values: DNMT1=15.35; Hes1=28.45; Nestin=15.47; Proliferative Index=23.4. * p<0.001 vs non-specific siRNA and c p<0.01 SGA vs Control.
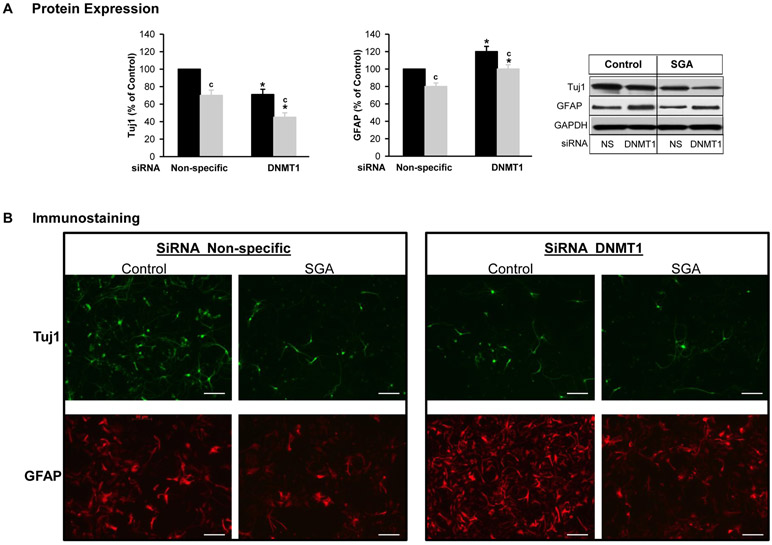
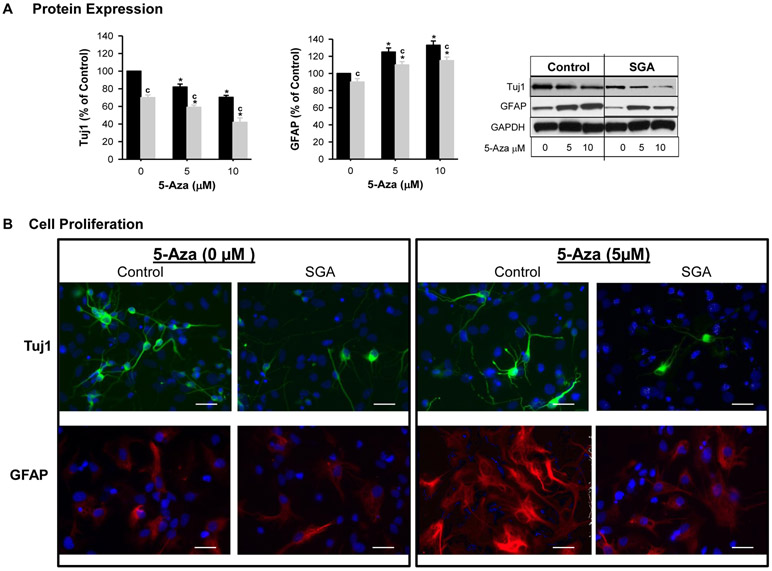
In differentiating media under basal conditions, SGA NPCs exhibited significantly decreased Tuj1 and GFAP expression as compared to Controls. Of note in SGA NPCs, the reduction in Tuj1 was 14% greater as compared to GFAP (Figure 5).
Figure 5: DNMT1 Silencing (siRNA) Effect on Neurosphere Differentiation.
NPCs from Control (■) and SGA (■) were transfected with non-specific or DNMT1-specific siRNA (20 nM) in differentiating media. (A) Protein expression of Tuj1 and GFAP. (B) Immunostaining of Tuj1 and GFAP. (NS=non-specific siRNA). 5 litters were studied in each group. From each litter, hypothalami from N=4 male pups were pooled representing N=1. F-values: Tuj1=31.36 and GFAP=21.47. * p<0.001 vs non-specific siRNA and c p<0.001 SGA vs Control.
Effects of DNMT Silencing on NPC Proliferation and Differentiation:
When both Control and SGA NPC’s were transfected with DNMT1 specific siRNA, DNMT1 expression decreased as expected, with Control NPCs showing suppression of DNMT1 to the levels equivalent to that seen in SGA NPCs (Figure 4A).
In Control NPCs, DNMT1 specific siRNA significantly decreased Nestin and Hes1 expression including cell proliferative index. In SGA NPCs, there was a further reduction in all factors and the proliferation remained below that of Controls in both non-specific and DNMT1 specific siRNA (Figures 4A, B). This was consistent with visual images of reduced colony density and BrdU staining in both Control and SGA DNMT1 siRNA (Figures 4B).
In differentiating media, DNMT1 siRNA decreased expression of Tuj1 but increased GFAP in both Control and SGA (Figure 5A). The immunostaining images of Tuj1 and GFAP showed similar changes (Figure 5B).
Effects of DNMT1 Inhibition on NPC Proliferation and Differentiation:
DNMT1 pharmacologic inhibition with 5-Aza resulted in similar responses to DNMT1 siRNA.
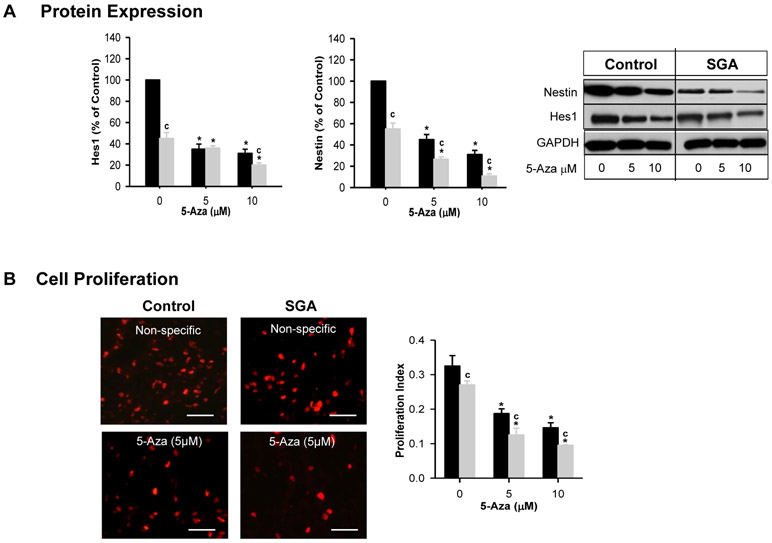
Both Control and SGA NPCs cultured in complete media showed a progressive decline in protein expression of Nestin and Hes1 with increasing dose of 5-Aza. Consistent with this, both control and SGA NPCs demonstrated a dose-dependent reduction of NPC proliferation index and BrdU staining (Figure 6A, B). Overall in SGA NPCs, Nestin, Hes1 and proliferation remained significantly less than that of Controls.
Figure 6: Effects of Pharmacologic Inhibitor on NPC Proliferation.
Control (■) and SGA (■) p1 hypothalamic neurospheres were cultured in complete media and treated for 4 days with DNMT1 inhibitor 5-Aza. (A) Protein expression of Hes1 and Nestin, and Immunoblots. (B) Images of BrdU staining and proliferation index. (NS=non-specific siRNA). 5 litters were studied in each group. From each litter, hypothalami from N=4 male pups were pooled representing N=1. F-values: Hes1=36.84; Nestin=46.4; Proliferative Index=21.35. * p<0.001 vs non-specific siRNA and c p<0.001 SGA vs Control.
5-Aza exposure in differentiation media significantly decreased Tuj1 though increased GFAP expression above respective untreated NPCs. SGA continued to show reduced Tuj1 and GFAP (Figure 6A). The immunostaining images of Tuj1 and GFAP showed similar changes (Figure 6B).
DISCUSSION:
Early embryogenesis is the most critical period for the establishment of the epigenome and any disruption leads to dysregulation of gene expression (Sebert et al., 2011; Desai et al., 2015). Importantly, this period is especially vulnerable to environmental cues (e.g., nutrition) that can alter epigenetic marks and ultimately influence outcomes. Whilst previous studies have demonstrated the effects of early nutrition on offspring DNA methylation with respect to specific genes, metabolism, including brain cognitive abilities and disorders of the brain, (Lillycrop, 2011; Weaver, 2014; Desai et al., 2015) this is the first study to document changes in newborn hypothalamic NPCs as a result of in utero exposure to maternal undernutrition.
Maternal undernutrition produced the expected reduced birth weight of newborns with postnatal catch-up growth and increased body weight at 21 days of age as we have previously reported. (Desai et al., 2005) Although sacrificed at an earlier age in the present study, prior studies have demonstrated offspring hyperphagia and the development of obesity, when nursed by ad libitum fed dams (Desai et al., 2007b). In the present study, newborn and 21 day old SGA male offspring had significantly reduced expression of hypothalamic DNMT1, suggesting a reduced potential for gene silencing. Although there was no change in levels of DNMT3A, these DNA methyltransferases are primarily involved in de novo methylation during embryogenesis, whereas DNMT1 primarily functions to maintain demethylation. Prior studies have demonstrated effects of induced or spontaneous SGA on DNMT1 in a diversity of organs. In intrauterine growth-restricted piglets, DNMT1 was significantly lower in the jejunum in association with mucosal atrophy and reduced villous height (Liu et al., 2011). In comparison, ileum DNMT1 in growth-restricted piglets and testes DNMT1 from growth-restricted bores were reported to be increased (Hu et al., 2015; Lin et al., 2017). Despite this variation of results, these findings clearly demonstrate that the developmental nutrient environment may have marked impact on DNMT1. Notably, maternal obesity also reduces hypothalamic DNMT1 expression in the newborns (Desai et al., 2016). Further, early exposure to environmental toxins (polychlorinated biphenyls) or stress reduces Dnmt1 mRNA abundance levels in the hypothalamus of rats (Desaulniers et al., 2005; Kashimoto et al., 2016).
The reduction in hypothalamic DNMT1 was associated with a reduced expression of the basic helix-loop-helix factor (bHLH) Hes1, a neuroproliferative transcription factor. The reduction in Hes1 associated with decreased DNMT1 would suggest an indirect effect of gene methylation, perhaps mediated by increased expression of Hes1 suppressive factors. Of note, prenatal alcohol exposure has been demonstrated to reduce expression of both NPC DNMT1 and Hes1 (Tyler & Allan, 2014).
To explore the association of reduced DNMT with programmed neurogenesis, we utilized NPC cell culture, as previously reported. When grown in complete media, NPCs proliferate whereas when select growth factors are removed from the media (differentiating media), NPCs differentiate into neurons and glia, ultimately expressing more specific neurophenotypes. As demonstrated previously, there was a visually reduced colony density and BrdU staining in SGA as compared to control NPCs in complete media, (Desai et al., 2011a) a finding confirmed by quantification of Nestin and proliferative index. Similar to the reduction in Hes1 in hypothalamic tissue, SGA NPCs similarly demonstrated a reduction in Hes1 in neurosphere culture. Previous studies have shown that Hes1 promotes NPC self-renewal and inhibits differentiation by repressing neuronal differentiation genes Mash1, neurogenin (Kageyama et al., 2007) and as such, Hes1 levels decrease as neural differentiation proceeds (Hisahara et al., 2008). Notably, in Hes1-deficient brains, NPCs prematurely differentiate (Hatakeyama & Kageyama, 2006; Kageyama et al., 2007) reducing the NPC pool.
When grown in differentiating media and controlled for the number of cultured cells, SGA NPCs demonstrated significantly decreased TUJ1 and GFAP expression, suggesting reduced differentiation to both neurons and astrocytes, respectively. Importantly, the reduction in TUJ1 was greater than that seen in GFAP. Although not demonstrated by the current study, we speculate that there may be a preferential differentiation towards GFAP in SGA NPCs.
The in vitro NPC data corresponded with in vivo data in which SGA newborns showed reduced cell proliferation consistent with decreased expression of hypothalamic Hes1, increased neurons as evident by decreased Tuj1expression and decreased astrocytes as evident by increased GFAP. Notably, these changes were persistent in 21 day old SGA offspring.
We sought to determine if DNMT was a putative factor in the perinatal nutrient programming. Both control and SGA NPCs transfected with DNMT1-specific siRNA showed a reduction in DNMT1. Although not completely silenced, we achieved a reduction in control NPC DNMT1 to the level of SGA NPC DNMT1. Consistent with our findings in SGA versus control NPC, DNMT1 silencing resulted in a reduction in visual colony counts and BrdU staining in both control and SGA NPCs and further decreased Nestin,Hes1 and proliferative index in both groups. Within differentiation media DNMT1 silencing resulted in a marked shift in the neuronal to astrocyte ratio, decreasing the expression of the neuronal factor TUJ1, but increasing GFAP in both control and NPCs. The pharmacologic inhibition of DNMT with 5-AZA confirmed the responses to DNMT1 siRNA, demonstrating reduced proliferation in complete media and evidence of increasing astrocytes as compared to neurons in differentiation media.
The similarities of in-vivo and ex-vivo results support a putative role of DNMT1 in neural programming. The mechanism by which maternal and thus fetal undernutrition reduces the DNMT1 is unclear, but may involve a reduced supply of methyl donors. For example, in IUGR piglets, folic acid supplementation has been demonstrated to increase DNMT1 expression (Liu et al., 2011). Furthermore, folate has been demonstrated to increase DNMT1 and NPC proliferation in cell culture with upregulation of Hes1 (Zhang et al., 2008; Li et al., 2013; Yu et al., 2014). Whether methyl donor supplementation (e.g., folate, choline, B vitamins) may reverse the epigenetic and neural differentiation findings associated with SGA would be of tremendous interest.
Consistent with their role in maintaining DNA methylation patterns throughout cell replication, DNMT1 is ubiquitously expressed in both dividing neural precursor cells and post-mitotic neurons in mouse brains (Goto et al., 1994). In contrast, DNMT3b is expressed in the subventricular zone (SVZ) between E10.5 and E13.5, and then gradually diminishes, becoming undetectable after E15.5 (Feng et al., 2005). DNMT3a is expressed in the SVZ of NPCs from E10.5 to E17.5 and is also detected in postnatal neurons in all regions of the brain (Feng et al., 2005; Yao & Jin, 2014). Neurogenesis which precedes astrogliosis occurs mainly during early to mid-gestational period. To facilitate this process, astrocytic gene promoters are hypermethylated (Takizawa et al., 2001). The mechanism for demethylation of astrogenic genes is due in part to the loss of methyltransferase activity of DNMT1. Conditional deletion of DNMT1 from mouse neural lineage shows precocious shift toward astroglial differentiation (Fan et al., 2005) and demethylation of GFAP promoter regions (Katai et al., 1997).
The alteration in the neuronal to glial ratio raises significant implications for programming of neural disorders associated with SGA. Reduced DMNT1 associated with BrdU administration has been demonstrated to increase GFAP expression and glial differentiation in NPC culture (Schneider & d'Adda di Fagagna, 2012). Similarly, folate mediated increased DNMT1 results in an increase in neuronal differentiation and reduced astrocyte differentiation, effects which are blocked by DNMT inhibition (Luo et al., 2013). Although glial cells represent nearly ten-fold the number of neurons in the human brain, the hypothalamic region and cortical gray matter are neuron dense. Clearly, a reduction in neuronal differentiation and the neuron/astrocyte ratio can raise significant concerns for neural programming.
Maternal nutrition-modified developmental neurogenesis in the offspring extends well beyond hypothalamic NPCs, to effects on cortical NPCs which influence behavior, cognition and diverse neurologic functions (Gould et al., 2018). Studies show that early nutrition alters neurogenesis in the hippocampus, cerebellum, and likely the cerebral cortex (Hoeijmakers et al., 2014; Gould et al., 2018).
In summary, the present findings confirm a significant fetal/newborn epigenetic effect of maternal nutrient restriction, which results in reduced neural proliferation and altered neurogenesis, evidenced by a reduced neuron/astrocyte ratio. DNMT1 molecular and/or pharmacologic inhibition simulates the results of maternal undernutrition, suggesting a putative role for DNA methylation. Additional clarification of the mechanism of nutrient neural programming and potential therapeutic approaches may have marked translational value.
Figure 7: Effects of Pharmacologic Inhibitor on NPC Differentiation.
Control (■) and SGA (■) p1 hypothalamic neurospheres were cultured in differentiating media and treated for 4 days with DNMT1 inhibitor 5-Aza. (A) Protein expression of Tuj1 and GFAP. (B) Immunostaining of Tuj1 and GFAP. (NS=non-specific siRNA). 5 litters were studied in each group. From each litter, hypothalami from N=4 male pups were pooled representing N=1. F-values: Tuj1=52.7 and GFAP=31.62. * p<0.0001 vs non-specific siRNA and c p<0.001 SGA vs Control.
Acknowledgements:
The authors thank Stacy Behare for assistance with animal work.
Funding: This work was supported by the National Institutes of Health Grants R01HD054751 (MGR); R01DK081756 (MD); and LA BioMed Research Committee Bridge and Institutional Use Grant Program 30970-01 (MD).
ABBREVIATIONS
- SGA
Small-for-gestational age
- DNMT1
DNA methyltransferase 1
- NPCs
neuroprogenitor cells
- Hes1
hairy and enhancer of split-1
- Tuj1
Neuron-specific class III beta-tubulin
- GFAP
Glial fibrillary acidic protein
- 5-Aza
5-Azacytidine
- MTT
3-(4,5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide.
REFERENCES
- Arcangeli T, Thilaganathan B, Hooper R, Khan KS & Bhide A (2012) Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol, 40, 267–275. [DOI] [PubMed] [Google Scholar]
- Coupe B, Grit I, Darmaun D & Parnet P (2009) The timing of "catch-up growth" affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol, 297, R813–824. [DOI] [PubMed] [Google Scholar]
- Desai M, Babu J & Ross MG (2007a) Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol, 293, R2306–2314. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Babu J & Ross MG (2005) Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol, 288, R91–96. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Han G & Ross MG (2007b) Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci, 14, 329–337. [DOI] [PubMed] [Google Scholar]
- Desai M, Han G & Ross MG (2016) Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite, 99, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Jellyman JK & Ross MG (2015) Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes (Lond), 39, 633–641. [DOI] [PubMed] [Google Scholar]
- Desai M, Li T, Han G & Ross MG (2014) Programmed hyperphagia secondary to increased hypothalamic SIRT1. Brain Res, 1589, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Li T & Ross MG (2011a) Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: neurotrophic effects of leptin and insulin. Brain Res, 1378, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Li T & Ross MG (2011b) Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: Neurotrophic effects of leptin and insulin. Brain Research, 1378, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaulniers D, Xiao GH, Leingartner K, Chu I, Musicki B & Tsang BK (2005) Comparisons of brain, uterus, and liver mRNA expression for cytochrome p450s, DNA methyltransferase-1, and catechol-o-methyltransferase in prepubertal female Sprague-Dawley rats exposed to a mixture of aryl hydrocarbon receptor agonists. Toxicol Sci, 86, 175–184. [DOI] [PubMed] [Google Scholar]
- Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H, ten Hoeve J, Shuai K & Sun YE (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development, 132, 3345–3356. [DOI] [PubMed] [Google Scholar]
- Feng J, Chang H, Li E & Fan G (2005) Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res, 79, 734–746. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Negishi M & Katoh H (2009) RhoG promotes neural progenitor cell proliferation in mouse cerebral cortex. Mol Biol Cell, 20, 4941–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami T, Sun X, Li T, Desai M & Ross MG (2012) Mechanism of programmed obesity in intrauterine fetal growth restricted offspring: paradoxically enhanced appetite stimulation in fed and fasting states. Reprod Sci, 19, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami T, Sun X, Li T, Yamada M, Desai M & Ross MG (2013) Mechanism of programmed obesity: altered central insulin sensitivity in growth-restricted juvenile female rats. J Dev Orig Health Dis, 4, 239–248. [DOI] [PubMed] [Google Scholar]
- Goodspeed D, Seferovic MD, Holland W, McKnight RA, Summers SA, Branch DW, Lane RH & Aagaard KM (2015) Essential nutrient supplementation prevents heritable metabolic disease in multigenerational intrauterine growth-restricted rats. FASEB J, 29, 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Numata M, Komura JI, Ono T, Bestor TH & Kondo H (1994) Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation, 56, 39–44. [DOI] [PubMed] [Google Scholar]
- Gould JM, Smith PJ, Airey CJ, Mort EJ, Airey LE, Warricker FDM, Pearson-Farr JE, Weston EC, Gould PJW, Semmence OG, Restall KL, Watts JA, McHugh PC, Smith SJ, Dewing JM, Fleming TP & Willaime-Morawek S (2018) Mouse maternal protein restriction during preimplantation alone permanently alters brain neuron proportion and adult short-term memory. Proc Natl Acad Sci U S A, 115, E7398–E7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X & Wu H (2001) Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science, 294, 2186–2189. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J & Kageyama R (2006) Notch1 expression is spatiotemporally correlated with neurogenesis and negatively regulated by Notch1-independent Hes genes in the developing nervous system. Cereb.Cortex, 16 Suppl 1, i132–i137. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M & Horio Y (2008) Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl.Acad.Sci U.S.A, 105, 15599–15604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Lucassen PJ & Korosi A (2014) The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Front Mol Neurosci, 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Liu Y, Yan C, Peng X, Xu Q, Xuan Y, Han F, Tian G, Fang Z, Lin Y, Xu S, Zhang K, Chen D, Wu D & Che L (2015) Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br J Nutr, 114, 53–62. [DOI] [PubMed] [Google Scholar]
- Hu XL, Wang Y & Shen Q (2012) Epigenetic control on cell fate choice in neural stem cells. Protein Cell, 3, 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet D, Deghmoun S, Chevenne D, Collin D, Czernichow P & Levy-Marchal C (2005) Dynamic change in adiposity from fetal to postnatal life is involved in the metabolic syndrome associated with reduced fetal growth. Diabetologia, 48, 849–855. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T & Kobayashi T (2007) The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development, 134, 1243–1251. [DOI] [PubMed] [Google Scholar]
- Kashimoto RK, Toffoli LV, Manfredo MHF, Volpini VL, Martins-Pinge MC, Pelosi GG & Gomes MV (2016) Physical exercise affects the epigenetic programming of rat brain and modulates the adaptive response evoked by repeated restraint stress. Behav Brain Res, 296, 286–289. [DOI] [PubMed] [Google Scholar]
- Katai S, Maruyama T, Nakamura A, Tokuda T, Shindo M & Yanagisawa N (1997) [A case of corticobasal degeneration presenting with primary progressive aphasia]. Rinsho Shinkeigaku, 37, 249–252. [PubMed] [Google Scholar]
- Kyle UG & Pichard C (2006) The Dutch Famine of 1944-1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care, 9, 388–394. [DOI] [PubMed] [Google Scholar]
- Li W, Yu M, Luo S, Liu H, Gao Y, Wilson JX & Huang G (2013) DNA methyltransferase mediates dose-dependent stimulation of neural stem cell proliferation by folate. J Nutr Biochem, 24, 1295–1301. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA (2011) Effect of maternal diet on the epigenome: implications for human metabolic disease. Proc Nutr Soc, 70, 64–72. [DOI] [PubMed] [Google Scholar]
- Lin Y, Cheng X, Sutovsky P, Wu, Che LQ, Fang ZF, Xu SY, Ren B & Dong HJ (2017) Effect of intra-uterine growth restriction on long-term fertility in boars. Reprod Fertil Dev, 29, 374–382. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen D, Mao X & Yu B (2011) Effects of maternal folic acid supplementation on morphology and apoptosis-related gene expression in jejunum of newborn intrauterine growth retarded piglets. Arch Anim Nutr, 65, 376–385. [DOI] [PubMed] [Google Scholar]
- Luo S, Zhang X, Yu M, Yan H, Liu H, Wilson JX & Huang G (2013) Folic acid acts through DNA methyltransferases to induce the differentiation of neural stem cells into neurons. Cell Biochem Biophys, 66, 559–566. [DOI] [PubMed] [Google Scholar]
- Meas T, Deghmoun S, Alberti C, Carreira E, Armoogum P, Chevenne D & Levy-Marchal C (2010) Independent effects of weight gain and fetal programming on metabolic complications in adults born small for gestational age. Diabetologia, 53, 907–913. [DOI] [PubMed] [Google Scholar]
- Namihira M, Kohyama J, Semi K, Sanosaka T, Deneen B, Taga T & Nakashima K (2009) Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell, 16, 245–255. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Kimura A, Murao N, Matsuda T, Namihira M & Nakashima K (2015) Expression of DNMT1 in neural stem/precursor cells is critical for survival of newly generated neurons in the adult hippocampus. Neurosci Res, 95, 1–11. [DOI] [PubMed] [Google Scholar]
- O'Neill SM, Hannon G, Khashan AS, Hourihane JO, Kenny LC, Kiely M & Murray DM (2017) Thin-for-gestational age infants are at increased risk of neurodevelopmental delay at 2 years. Arch Dis Child Fetal Neonatal Ed, 102, F197–F202. [DOI] [PubMed] [Google Scholar]
- Painter RC, Roseboom TJ & Bleker OP (2005) Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol, 20, 345–352. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV & de Rooij SR (2011) Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas, 70, 141–145. [DOI] [PubMed] [Google Scholar]
- Ross MG & Desai M (2014) Developmental programming of appetite/satiety. Ann Nutr Metab, 64 Suppl 1, 36–44. [DOI] [PubMed] [Google Scholar]
- Ross MG, Desai M, Khorram O, McKnight RA, Lane RH & Torday J (2007) Gestational programming of offspring obesity: a potential contributor to Alzheimer's disease. Curr Alzheimer Res, 4, 213–217. [DOI] [PubMed] [Google Scholar]
- Schneider L & d'Adda di Fagagna F (2012) Neural stem cells exposed to BrdU lose their global DNA methylation and undergo astrocytic differentiation. Nucleic Acids Res, 40, 5332–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebert S, Sharkey D, Budge H & Symonds ME (2011) The early programming of metabolic health: is epigenetic setting the missing link? Am J Clin Nutr, 94, 1953S–1958S. [DOI] [PubMed] [Google Scholar]
- Stricker SH & Gotz M (2018) DNA-Methylation: Master or Slave of Neural Fate Decisions? Front Neurosci, 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M & Taga T (2001) DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell, 1, 749–758. [DOI] [PubMed] [Google Scholar]
- Tyler CR & Allan AM (2014) Prenatal alcohol exposure alters expression of neurogenesis-related genes in an ex vivo cell culture model. Alcohol, 48, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL & Gluckman PD (2000) Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab, 279, E83–87. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tang B, He Y & Jin P (2016) DNA methylation dynamics in neurogenesis. Epigenomics, 8, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC (2014) Integrating early life experience, gene expression, brain development, and emergent phenotypes: unraveling the thread of nature via nurture. Adv Genet, 86, 277–307. [DOI] [PubMed] [Google Scholar]
- Yao B & Jin P (2014) Unlocking epigenetic codes in neurogenesis. Genes Dev, 28, 1253–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousheng J, Nguyen T, Desai M & Ross MG (2008) Programmed alterations in hypothalamic neuronal orexigenic responses to ghrelin following gestational nutrient restriction. Reprod Sci, 15, 702–709. [DOI] [PubMed] [Google Scholar]
- Yu M, Li W, Luo S, Zhang Y, Liu H, Gao Y, Wang X, Wilson JX & Huang G (2014) Folic acid stimulation of neural stem cell proliferation is associated with altered methylation profile of PI3K/Akt/CREB. J Nutr Biochem, 25, 496–502. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu H, Cong G, Tian Z, Ren D, Wilson JX & Huang G (2008) Effects of folate on notch signaling and cell proliferation in neural stem cells of neonatal rats in vitro. J Nutr Sci Vitaminol (Tokyo), 54, 353–356. [DOI] [PubMed] [Google Scholar]