Abstract
Background:
Pharmacological management of migraine can be ineffective for some patients. We previously demonstrated that exposure to green light resulted in antinociception and reversal of thermal and mechanical hyper-sensitivity in rodent pain models. Given the safety of green light emitting diodes, we evaluated green light as a potential therapy in patients with episodic or chronic migraine.
Material and methods:
We recruited (29 total) patients of whom 7 had episodic migraine (EM) and 22 had chronic migraine (CM). We used a one-way cross-over design consisting of exposure for 1–2 hours daily to white light emitting diodes (WLED) for 10 weeks followed by a 2 week washout period followed by exposure for 1–2 hours daily to green light emitting diodes (GLED) for 10 weeks. Patients were allowed to continue current therapies and to initiate new treatments as directed by their physicians. Outcomes consisted of patient-reported surveys. The primary outcome measure was the number of headache days per month. Secondary outcome measures included patient reported changes in the intensity and frequency of the headaches over a two week period and other quality of life measures including ability to fall and stay asleep, ability to perform work. Changes in pain medications were obtained to assess potential reduction.
Results:
When 7 EM and 22 CM patients were analyzed as separate cohorts, WLED produced no significant change in headache days in either EM or CM patients. Combining data from the EM and CM groups showed that WLED produced a small, but statistically significant reduction in headache days from (days ± SEM) 18.2 ± 1.8 to 16.5 ± 2.01 days. GLED resulted in a significant decrease in headache days from 7.9 ± 1.6 to 2.4 ± 1.1 and from 22.3 ± 1.2 to 9.4 ± 1.6 in EM and CM patients, respectively. While some improvement in secondary outcomes was observed with WLED, more secondary outcomes with significantly greater magnitude including assessments of quality of life, Short-Form McGill Pain Questionnaire, Headache Impact Test-6, and Five-level version of the EuroQol five-dimensional survey without reported side effects were observed with GLED. Conclusions regarding pain medications reduction with GLED exposure were not possible. No side effects of light therapy were reported. None of the patients in the study reported initiation of new therapies.
Discussion:
GLED significantly reduced the number of headache days in people with EM or CM. Additionally, GLED significantly improved multiple secondary outcome measures including quality of life and intensity and duration of the headache attacks. As no adverse events were reported, GLED may provide a treatment option for those patients who prefer non-pharmacological therapies or may be considered in complementing other treatment strategies. Limitations of this study are the small number of patients evaluated. The positive data obtained support implementation of larger clinical trials to determine possible effects of GLED therapy.
Keywords: Green light, migraine, non-pharmacological therapy, light therapy
INTRODUCTION
Migraine is a multiphasic neurological disorder associated with multiple symptoms including moderate to severe headache, photophobia, phonophobia, nausea and vomiting (1). It is a very common disorder with an estimated global prevalence of 14.7%, with annual and lifetime prevalence of 18% and 33% in women, respectively, and 6% and 13% in men, respectively (1). Migraine is listed among the leading non-fatal disabling consequences of common illnesses and the number one cause of years lived with disability among patients under the age of 50 (2, 3). Migraine is classified by headache frequency with chronic migraine (CM) defined as 15 or more headache days a month for 3 or more months (4) while episodic migraine (EM) defined as occurring on fewer than 15 headache days per month. Migraine patients frequently have one or more associated comorbidities including chronic pain disorders, respiratory disorders, cardiovascular and cerebrovascular disorders, psychiatric disorders, and digestive disorders with comorbidities more prevalent among those with chronic migraine as compared to episodic migraine (5). Patients suffering from migraine frequently report decreased quality of life, decreased work productivity, higher rates of work absences, more use and overuse of both over-the-counter and prescription medications including opioids, and more healthcare usage including emergency department visits (5, 6).
Current strategies to manage migraine rely on acute and preventive therapies as well as non-pharmacological approaches. Acute treatments include single (acetaminophen, NSAIDs) and combination analgesics, migraine-specific treatments such as triptans, ergotamine, and more recently gepants and ditans (7–12). Preventive medications include beta blockers, antidepressants, calcium channel blockers, and angiotensin-converting enzyme inhibitors and receptor blockers (1). Recent clinical trials have also demonstrated that anti-CGRP peptide and receptor monoclonal antibodies reduce the migraine frequency by up to 50% in both episodic and chronic migraineurs (13–15). Additionally, small molecule CGRP receptor antagonists were approved for the acute treatment of migraine (7–12).
Despite the above pharmacological strategies for managing migraine, some patients do not achieve adequate pain control or may not tolerate their side effects (16, 17). Also, some patients may prefer non-pharmacological approaches to manage their pain. Therefore, offering a non-pharmacological therapeutic option could be useful as a standalone or as a complement to pharmacotherapies.
We recently reported that exposure to lights of different wavelengths can affect nociception in rodents(18, 19). For example, exposure to green light emitting diode (GLED) resulted in antinociception in naïve rats and antihyperalgesia in rats with neuropathic pain that was dependent on engagement of the visual system. Moreover, GLED exposure was accompanied by anxiolysis in rats (18). Given this pain modulating effect of light, in particular the antinociceptive effect GLED, we hypothesized that GLED therapy results in reduction of headache-days/month in migraineurs with concomitant improvement in quality of life measures.
MATERIALS AND METHODS
Study Approval
This study has been conducted according to Declaration of Helsinki principles. All study subjects provided informed written consent prior to enrolling and the study was approved by the University of Arizona Institutional Review Board (IRB) protocol number 1604514512. This study is registered with clinicaltrials.gov under NCT03677206.
Study Design
We employed a one-way cross-over design. Patients were divided into episodic (EM) and chronic (CH) migraine groups based on the number of headache-days/month from retrospective history at enrollment.
Setting
Patients were recruited individually from the University of Arizona/Banner Medical Center (Tucson, AZ) chronic pain clinic between August 2016 and October 2019.
Inclusion/exclusion criteria
Inclusion Criteria
Eighteen years or older
Meets the diagnostic criteria for EM or CH according to the International Headache Society (4).
Average headache pain intensity of migraine episodes of 5 out of 10 or greater using the numeric pain scale (NPS) over the 10 weeks prior to enrolling in the study.
Not satisfied with the migraine treatment(s) they currently receive whether they are preventative or acute in nature.
Exclusion Criteria
Serious mental illness. This was assessed during the screening interview.
Inability to read or understand English.
Light Emitting Diodes (LED)
All LED strips were purchased from ledsupply.com (VT, USA). The specifications of the LEDs were: #LS-AC60-6-GR, 525 nanometer wavelength (i.e., green), 8 watts, 120 Volts, 120-degree beam angle; #LS-AC60-66-WH, (white), 9.6 watts, 120 Volts, 120-degree beam angle. A luxmeter (Tondaj LX1010B, Amazon.com) was used to determine the illuminance and luminous emittance of the LED strips.
Light exposure protocol
Patients were consented individually to undergo the GLED therapy. Patients were free to continue or discontinue their migraine treatments as recommended by their physicians throughout the study, but were required to report any changes of their treatments. Patients were provided with a white light emitting diode (WLED) strip that was approximately 2 meters long. We used tape to sequentially cover two diodes and leave one diode uncovered for the length of the LED strip (Supplementary Figure 1) to achieve a light intensity of between 4 and 100 lux measured at approximately 2 and 1 meters from a luxmeter, respectively. This range of intensities was based on our previously published preclinical study (18). Patients were free to change the distance of the light source between 1 and 2 meters from their eyes. Patients were instructed to take the LED strip home and use it in a dark room for a minimum of 1 hour/day with the option to increase the exposure time to 2 hours/day for 10 weeks. Patients were instructed to keep the LED strips in their fields of vision and to not fall asleep during the therapy. The patients were encouraged to participate in activities that did not require external light sources such as listening to music, reading books, exercise or any similar activities. We asked the patients to undergo the light therapy at the same time everyday all at once. They were encouraged to blink at a normal rate and not to stare directly at the strips. After WLED completion, they were crossed over to the GLED after a two week “washout” period. Patients were provided with GLED of 525 ± 10 nm wavelength with similar exposure parameters. All patients had regular access to their physicians and were free to undergo any procedure or treatment their physicians saw fit. Figure 1 represents the study design.
Figure 1.
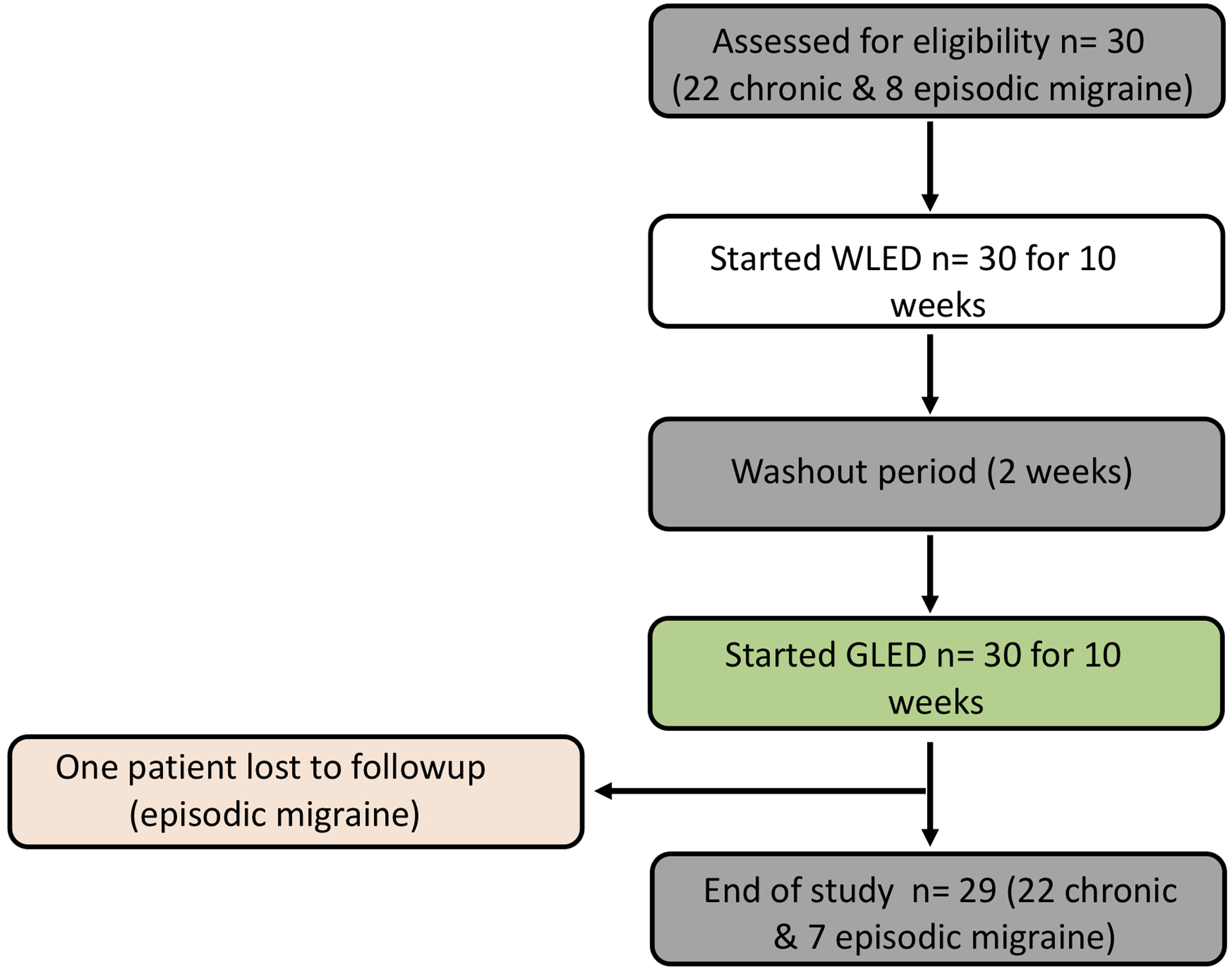
Study design. One-way cross-over clinical trial design. Patients were divided into episodic and chronic migraine groups based on the number of headache days per month. Patients with migraine were assigned to the control white light emitting diode (WLED) first then crossed over to the green light emitting diode (GLED) therapy. After 10 weeks of daily WLED, patients underwent a 2 week “washout” period and were then crossed over to the GLED.
Surveys for data collections
Patients were provided with seven paper surveys to complete at baseline and every two weeks thereafter except for the Time log, the Daily Medications, and the Migraine Diary surveys which were completed daily. The first survey (Time Log) documented the number of hours/day exposure to the LED strips. The second survey (Daily Medications) documented their daily analgesic(s). The third survey was a migraine daily dairy documenting headache-days/month (primary outcome). The fourth survey was a modified University of Arizona pain clinic follow-up questionnaire documenting the headache phase NPS. Patients were asked to subjectively rate the intensity of their headaches during the headache phase of their migraine episodes every two weeks and report them as a number from 0 to 10, where 0 is no pain and 10 is the worst possible pain.
Patients were asked to report their perceived change in the duration and frequency of headache phase of migraine episodes, improvement of the ability to fall asleep and stay sleep, ability to perform work, and daily activity (Supplementary Figure 2). The headache phase frequency of the migraine episodes was assessed with two different outcome measures – daily, and every two weeks, and then these were compared. The fifth survey was the Short Form McGill Pain Questionnaire (SFMPQ) (20). The sixth survey was the HIT-6 Headache Questionnaire (21). The seventh survey was the Five-level version of the EuroQol five-dimensional survey (EQ-5D-5L), designed to evaluate a global quality of life of patients with pain (22). The patients were contacted once every 2–3 weeks by a research team member. At the end of the 10 weeks, patients returned all the surveys. Any data not reported (NR) by patients were not part of the analysis.
Outcome measures
The primary outcome measure was the number of headache-days/month. We defined a headache day as a day with moderate to severe headache pain that lasted for at least 4 hours (23). Secondary outcome measures included: subjective reduction in the perceived intensity of the headache phase of the migraine episodes assessed every two weeks as measured by the NPS, perceived decrease in duration of the headache phase of the migraine episodes, improvement of ability to fall and stay sleep, ability to perform work and daily activity, quality of life, and reduction of pain medications. We analyzed the data separately for EM and CH patents and then combined the two groups for additional analysis.
Statistical analyses
All patients finished the WLED treatment and crossed over to GLED. One patient with EM was lost to followup after receiving the GLED. All the returned survey data were analyzed. If a survey was not completed or if the answer was not legible and we were not able to contact the patient for clarification, we excluded that survey from analysis. If a patient did not have one of the parameters affected by migraine, they wrote Not Applicable (N/A) next to the question and we did not include this particular parameter in the analysis. To analyze our results, we chose to use rank tests as data corresponded to qualitative ordinal data. We utilized Mann-Whitney tests to determine statistical significance comparing groups that were not paired. For paired groups (before and after), we utilized Wilcoxon matched-pairs signed rank tests. Statistical significance was considered when the P-value was measured at 0.05 or less. We used GraphPad Prism 8 for statistical analysis.
Data availability
The data from this study will be made available upon request.
RESULTS
Demographic and baseline pain data
Unless otherwise specified, all data are presented as mean ± SEM. The average age was 52.2 ± 3 years old at the time of enrollment (age ranged from 24 to 72 years old). All but two patients recruited were females. Twenty-two patients suffered from migraine more than 15 days/month and were classified as CM patients while 8 patients had fewer than 15 migraines/month and were classified as EM patients. The patients’ baseline pain during migraine attacks on the NPS was 8.52 ± 0.25 (out of 10). Supplementary Table 1 illustrates the demographics. While patients were free to start new medications recommended by their physicians, no patients reported additional new medications or procedures during the study period. Additionally, six CM patients were receiving botulinum toxin injections that had been initiated more than 3 years prior to enrolling in this study These six patients had an average headache-days/month of 21± 2.2 days (range 16 to 30 headache-days/month) prior to initiating the study.
Compliance
To monitor compliance, patients were contacted every 2–3 weeks to assess if they missed any days of light exposure and to answer any questions related to the study. Seven patients in the WLED group reported missing exposures with an average of 2.7 ± 0.7 days (range 1 to 6 days) compared to nine patients in the GLED group missing an exposure with an average of 4.3 ± 0.7 days (range 1 to 7 days) (p=0.1269, Mann Whitney test).
Primary outcome
The number of headache-days/month of the EM and CM groups before and after WLED decreased from 7.8 ± 1.4 to 5.9 ± 1.3 and 23.1 ± 1.4 to 21.5 ± 1.9, respectively; these changes were not significant. When the data from both the EM and CM groups were combined, WLED significantly decreased the number of migraine days from 18.2 ± 1.8 to 16.5 ± 2.0 days. (Figure 2A & Supplementary Table 2).
Figure 2.
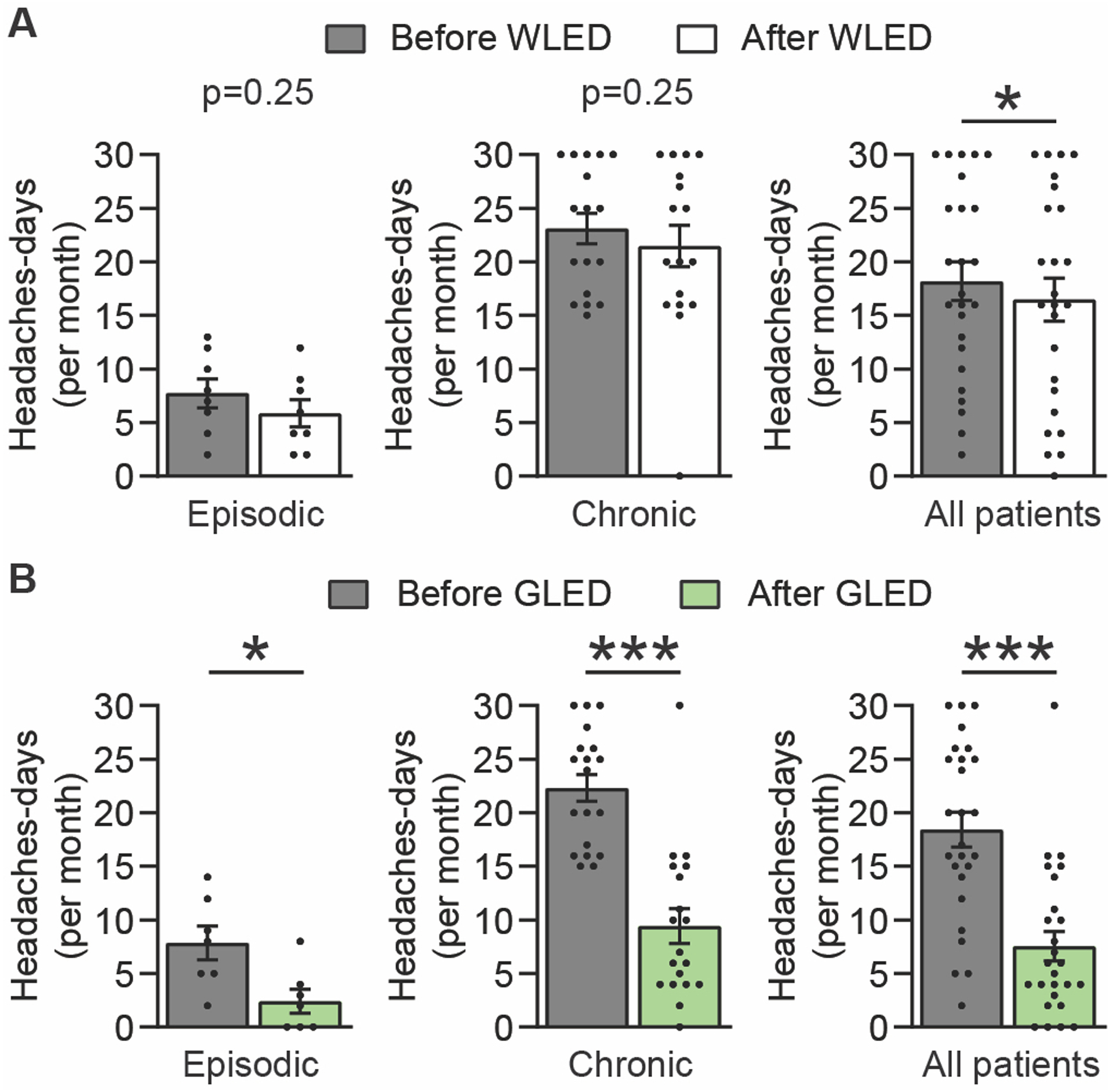
GLED exposure decreased headache days/month. (a) Number of headache days/month before and after patients were exposed to WLED. Results are presented for all patients and divided in two cohorts: Episodic and chronic migraine patients (n[WLED]epi = 8, n[WLED]chro = 17). WLED did not decrease the number of headache days/month in both episodic and chronic migraine patients, but a small significant reduction was observed when both cohorts were pooled. (b) GLED exposure produced a statistically significant reduction in the number of headache days/ month in both episodic and chronic migraine groups (n[GLED]epi = 7, n[GLED]chro = 19). Scatter plots, with every point representing a study subject: The bars represent the average and the error bars represent the standard error of the mean (Wilcoxon matched-pairs signed rank test, *p<0.05, ***p<0.001).
GLED significantly reduced the number of headache-days/month in the EM and CM groups from 7.9 ± 1.6 to 2.4 ± 1.1 and 22.3 ± 1.3 to 9.4 ± 1.6 days, respectively. When data from EM and CM patients were combined, there was a significant reduction in headache-days/month for GLED from 18.4 ± 1.6 to 7.4 ± 1.3 days, a reduction of ~60% (Figure 2B & Supplementary Table 2). Post-hoc analysis was performed to determine the responder rate defined as the percentage of patients who experienced greater than 50% reduction in headache-days/month. We found an 86% responder rate (6 out of 7 patients) for EM patients and 63% responder rate (12 out of 19) for CM patients.
Secondary outcomes
NPS:
WLED did not significantly reduce the NPS in the headache phase of the EM group with scores of 8.4 ± 0.5 to 8.1 ± 0.5. WLED produced a small, but significant, reduction in NPS of the headache phase in the CM group from 8.6 ± 0.3 to 7.8 ± 0.4. When the data from groups were combined, WLED produced a statistically significant reduction in NPS of the headache phase from 8.5 ± 0.3 to 7.9 ± 0.4 (Figure 3A & Supplementary Table 3).
Figure 3.
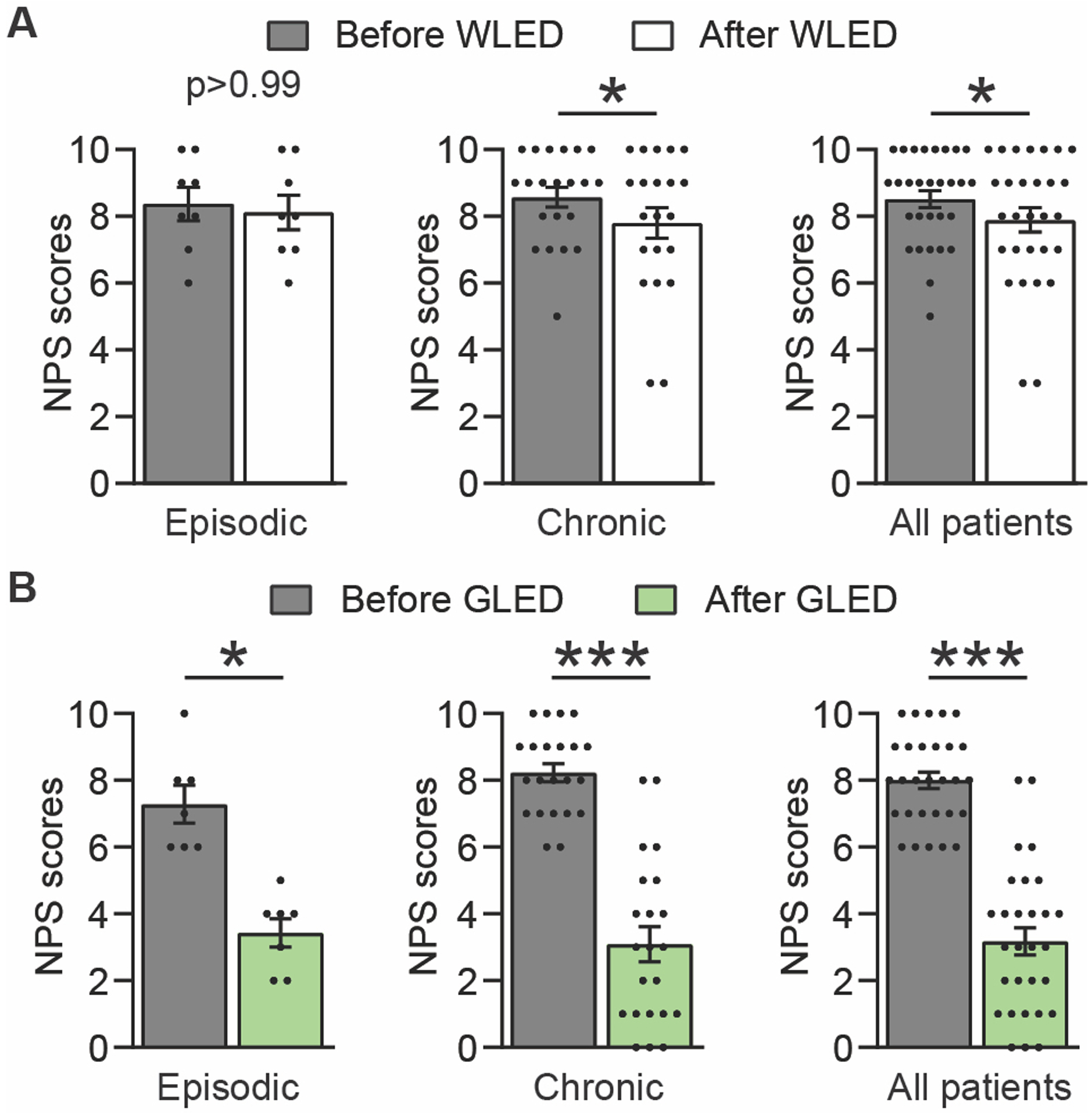
GLED exposure decreased the numeric pain scale (NPS). (a) WLED did produce a slight reduction in the NPS for the chronic migraine group (n[WLED]epi = 8, n[WLED]chro = 21). (b) GLED exposure produced a greater and statistically significant reduction in the NPS in both episodic and chronic migraine groups (n[GLED]epi = 7, n[GLED]chro = 22). Data presented as Average ± SEM, standard error of the mean (Wilcoxon matched-pairs signed rank test, *p<0.05, ***p<0.001).
GLED significantly reduced NPS of the headache phase of the EM group from 7.3 ± 0.6 to 3.4 ± 0.4. GLED treatment significantly reduced NPS of the headache phase in the CM group from 8.2 ± 0.3 to 3.1 ± 0.5. When the data from both groups were combined, there was a significant reduction in NPS of the headache phase from 8 ± 0.3 to 3.2 ± 0.4 (Figure 3B & Supplementary Table 3).
HIT-6:
Regarding functional capacity, WLED did not improve the HIT-6 values in any of the migraine groups (Figure 4A & Supplementary Table 4). GLED exposure produced a significant reduction in the HIT-6 survey in all migraine groups (Figure 4B & Supplementary Table 4).
Figure 4.
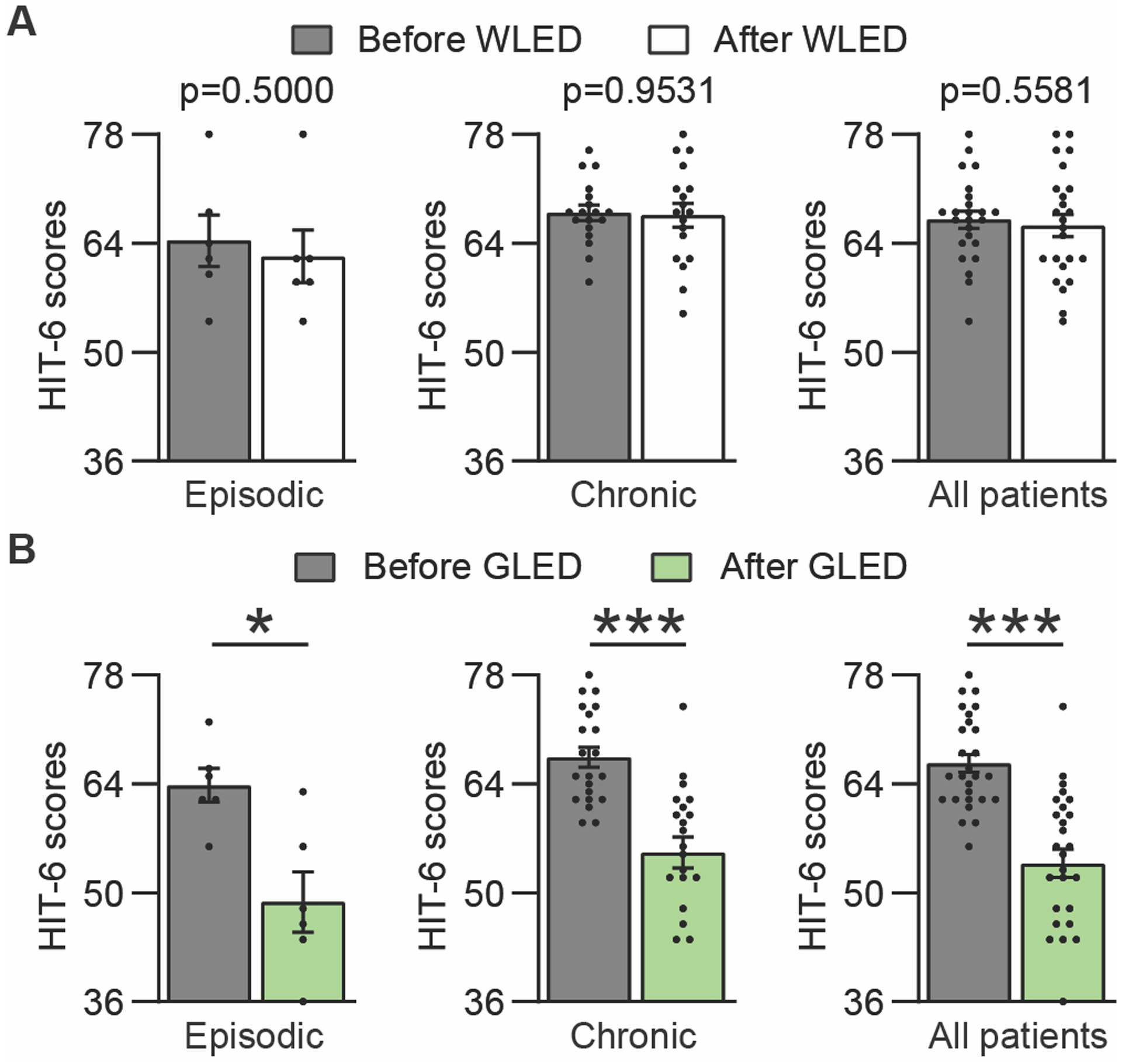
GLED exposure improved the Headache Impact Test-6 (HIT-6) results. (a) WLED exposure did not produce a significant reduction in HIT-6 scores (scale 36–78, where 36 = No impact from migraine, 78 = worst possible impact from migraine) (n[WLED]epi = 6, n[WLED]chro = 18). (b) GLED exposure produced a statistically significant reduction in the HIT-6 scores in all migraine groups (n[GLED]epi = 6, n[GLED]chro = 21). Data presented as average ± SEM, standard error of the mean (Wilcoxon matched pairs signed rank test, *p<0.05, ***p<0.001).
Modified University of Arizona pain clinic follow-up questionnaire:
WLED had a minor reduction in the perceived intensity of the headache phase of the migraine episodes in all migraine groups. WLED exposure also produced modest improvement of (a) headache frequency; (b) duration of the headache phase; (c) patients’ ability to fall and stay asleep; (d) ability to perform chores; (e) exercise and (f) work in all migraine groups.
GLED exposure also significantly improved all of these outcomes in all patient groups (Figure 5A, B, C & Supplementary Table 5). The combined EM and CM patients perceived a 69.3% reduction in their headache days/month after GLED therapy when they assessed their improvement every two weeks.
Figure 5.
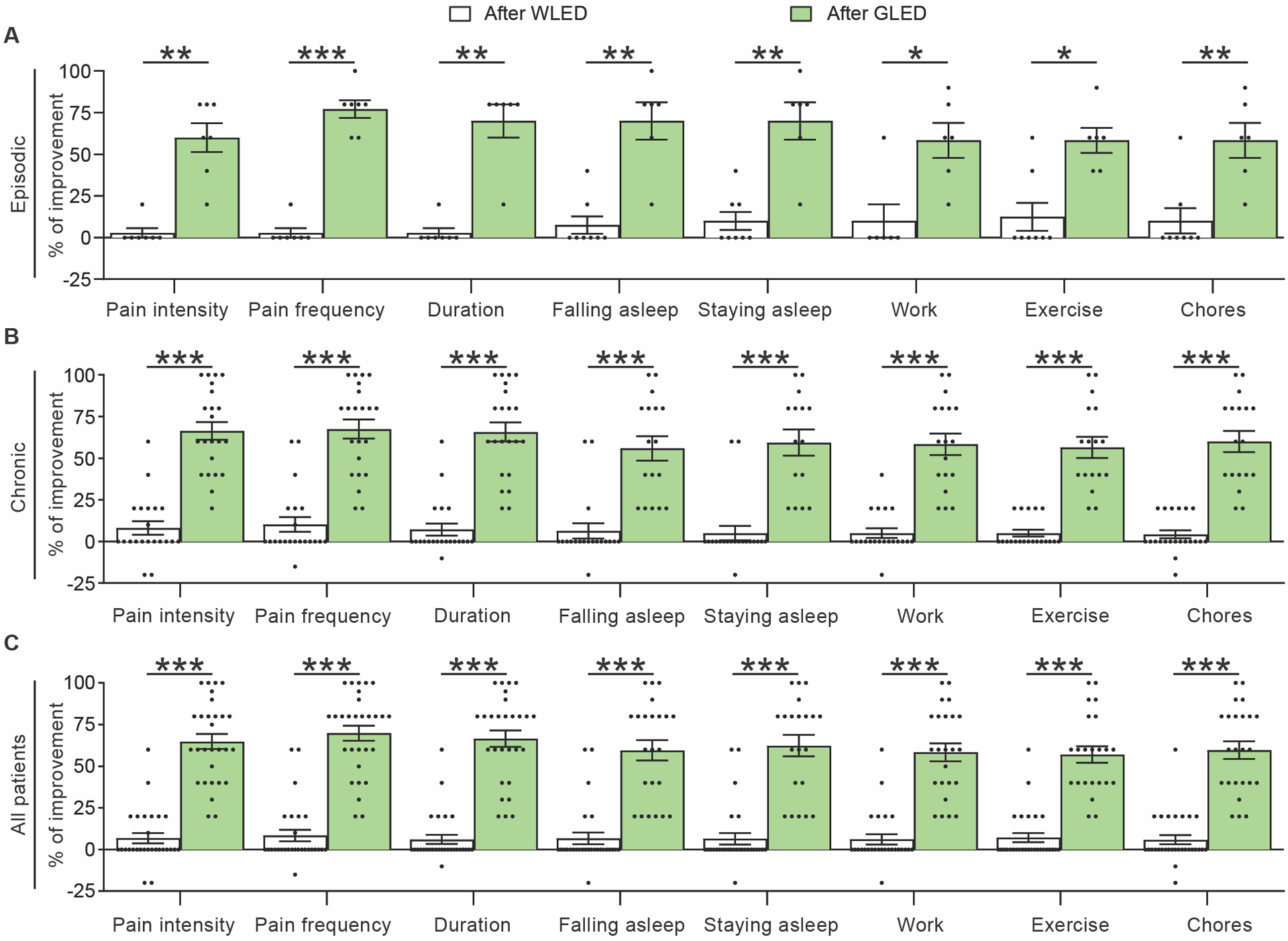
GLED exposure improved all quality of life parameters reported using the modified University of Arizona pain clinic follow up questionnaire. WLED exposure had minimal improvement of several parameters in episodic (a), chronic (b), and all migraine patients (c). The following criteria were evaluated: Perceived percent improvement of migraine pain intensity, migraine headache frequency, pain duration, ability to fall asleep, ability to stay asleep, work, exercise and doing chores, after completion of WLED therapy compared with baseline (n[WLED]epi = 6–8, n[WLED]chro = 19–21). On the contrary, GLED exposure demonstrated significantly greater improvements in all measured parameters in episodic, chronic and combined migraine groups. (n[GLED]epi = 6–7, n[GLED]chro = 15–22). Data presented as average ± SEM, standard error of the mean (Mann-Whitney test, *p<0.05, **p<0.01, ***p<0.001).
EQ-5D-5L:
Quality of life, evaluated using the EQ-5D-5L survey, demonstrated significant improvement following exposure to both WLED and GLED in both EM and CM and combined migraine groups. This improvement was more pronounced and observed in all groups after GLED exposure (Figure 6A, B, C & Supplementary Table 6). Patients did not notice significant improvement of their perception of their own health following WLED exposure. However, patients reported significant improvement of their perception of their own health following GLED exposure in the EM, CM and combined migraine groups (Figure 6A, B, C & Supplementary Table 6).
Figure 6.
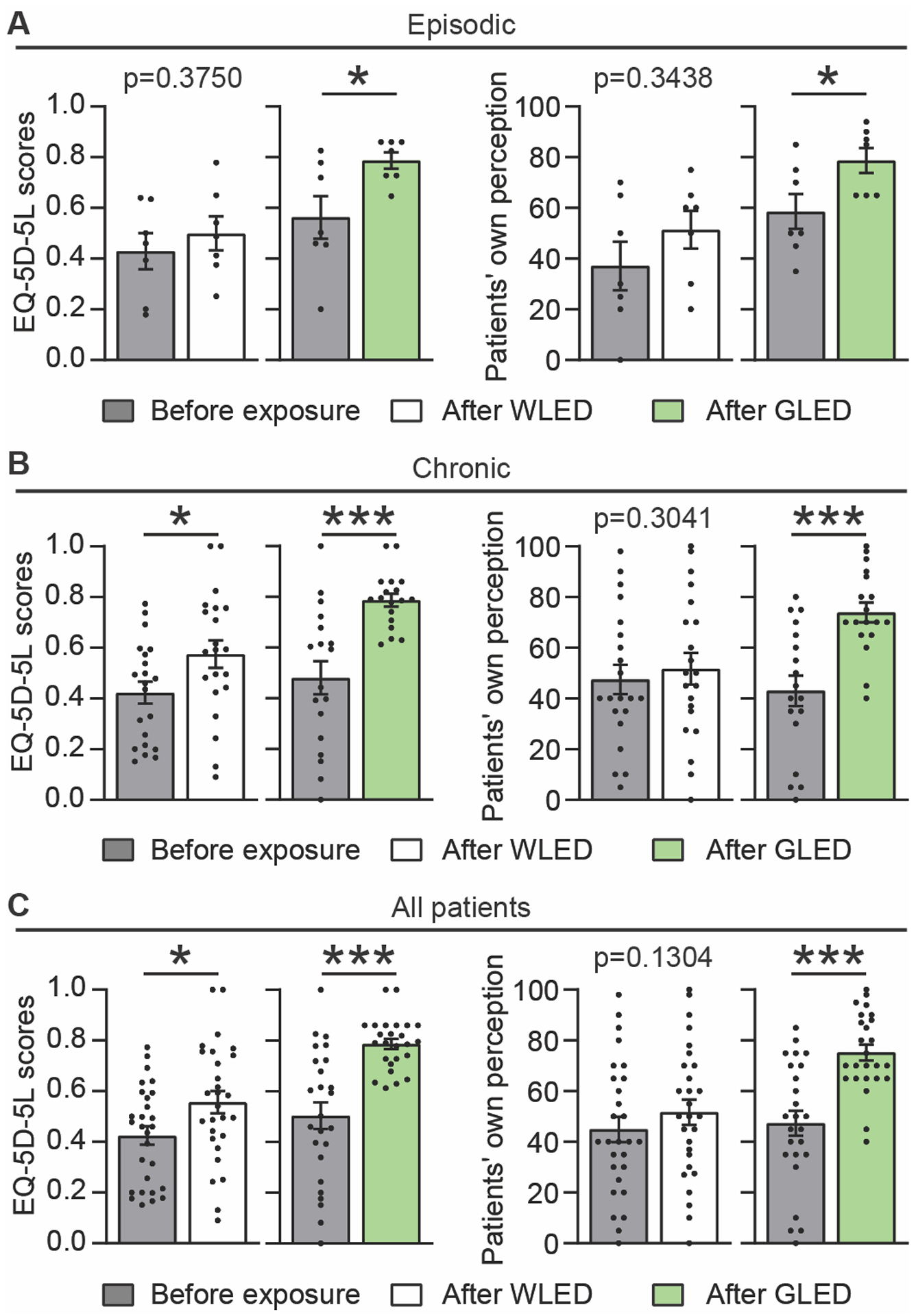
Five-level version of the EuroQol five-dimensional survey (EQ-5D-EL) evaluation after WLED or GLED exposure. EQ-5D-5L index and patients’ own perception of health before and after WLED or GLED, in episodic (a), chronic (b) and combined (c) groups of patients. Index scale 0–1 where 0.worst quality of life, 1.best quality of life. Health perception scale 0–100, where 0 = worst imagined health, 100 = best imagined health. WLED had a statistically significant improvement of the EQ-5D-5L index in both combined and chronic migraine groups. WLED exposure did not produce any improvement of the patients’ own perceived health using the EQ-5D-5L survey (n[WLED]epi = 7, n[WLED]chro = 21). GLED exposure had a statistically more significant improvement of the EQ-5D-5L index in episodic, chronic, and combined migraine groups. GLED exposure also produced a statistically significant improvement of the patients’ own perceived health in both all groups (n[GLED]epi = 7, n[GLED]chro = 18). Data presented as average ± SEM, standard error of the mean (Wilcoxon matched-pairs signed rank test, *p<0.05, ***p<0.001).
SFMPQ:
Fifteen descriptors reflecting intensity and quality of pain were measured using the Short-Form McGill Pain Questionnaire (SFMPQ). WLED exposure did not improve any of the 15 pain parameters of the SFMPQ in either the EM (Supplementary Figure 3 & Supplementary Table 7), CM (Supplementary Figure 4 and Supplementary Table 7), or the combined migraine groups (Supplementary Figure 5 and Supplementary Table 7). GLED also did not improve these parameters in the EM group. (Supplementary Figure 6 and Supplementary Table 8). GLED produced significant improvement in 14 of the 15 parameters of the SFMPQ of the CM group as well as when data from both groups were combined (Supplementary Figure 7–8 & Supplementary Table 8).
Daily Medications:
Conclusions regarding the reduction in pain medication after WLED or GLED treatment were not possible as patients reported using different types of pain medication for reasons unrelated to migraine.
Adverse events
No adverse events were reported.
DISCUSSION
We investigated the possible effects of WLED and GLED in a pilot one-way cross-over design in EM and CM patients. This study is the first to investigate the effect of light as a preventive therapy for patients with migraine. While patients were free to start new medications or procedures as suggested by their physicians, no patient reported additional new medications or procedures during the study period including biological agents or devices. Additionally, while it was not stated as an exclusion criterion, none of the recruited patients had ocular disease or color blindness nor did they wear tinted contact lenses.
Effects of light therapy on primary outcome measures:
Analysis of combined data from patients with EM and CM demonstrated a small but significant reduction in their number of headache-days/month after finishing the WLED treatment. WLED therapy reduced the number of headache-days/month by 2 days, which may be clinically meaningful. The potential contribution of a placebo response to the benefits seen with WLED in some outcome measures cannot be discounted. Additionally, we had asked patients to employ the light therapy in a relatively calm environment. Calm environments may in themselves lead to improvement in migraine (24).
When compared to WLED, GLED therapy resulted in a much larger and significant reduction in the number of headache-days/month of approximately 5.4 and 12.9 days in the EM and CM group, respectively. The reduction in headache-days with GLED was highly significant and comparable to reported decreases in headache-days with pharmacological therapies including 2.4, 3.6, 4.6–8, days with propranolol (25), topiramate (26) and CGRP peptide/receptor antibodies (27), respectively. As is the case with WLED, the contributions of placebo response and calm environments cannot be discounted. Additionally, our data are based on a small sample size and it remains to be determined if similar outcomes would be achieved in a larger trial.
Effects of light therapy on secondary outcome measures:
Our secondary outcome measures were reduction in the perceived pain intensity of the headache phase of the migraine episodes as measured by NPS, reduction in the migraine impact on social or work-related functions as measured by HIT-6, as well as decrease in duration and frequency of the headache phase of migraine episodes. Patients also assessed possible improvement of ability in falling and staying asleep, ability to perform work and daily activity, and improvement of quality of life using the EQ-5D-5L surveys, and some patients reported reduction of pain medications. Finally, SFMPQ results assessed possible improvement of pain descriptors in either affective or sensory-related categories.
There were no clinically significant improvements of the measured secondary outcomes after finishing the WLED treatment except for a small, but statistically significant reduction in NPS of the headache phase and the EQ-5D-5L index score for CM groups. GLED resulted in improvement of all the secondary outcomes measured in the CM group except for the feeling of “Gnawing pain” in SFMPQ. GLED did not improve any secondary outcomes in the EM group. The reasons for improvement in secondary outcome measures in CM, but not EM, patients is not clear but may have to do with the small number of patients studied. None of the recruited patients reported any side effects associated with WLED or GLED exposure.
While the primary outcome was the reduction of the number of headache days/month as reported using the migraine dairies, we also investigated the patients’ own perception of the reduction of the number of headache-days/month. This comparison allows us to better understand how patients view the GLED therapy which may be a surrogate of their satisfaction with the GLED therapy. For example, if the patients’ perceived reduction in headache days/month were less than their reported reduction using the migraine diaries, it may suggest that the patients did not consider the GLED favorably even if migraine actually improved. We found that the patients perceived a greater reduction in their headache-days/month after GLED therapy than they actually reported. This suggests that the patients viewed the GLED favorably. An alternate interpretation is that the therapy significantly shortened the duration of the headache phase (e.g., hours to mins) and/or that the patient did not believe that a mild, short-lasting headache represented a headache day. On many occasions, patients reported that they wanted “non-invasive” or “non-chemicals” methods to improve their migraine. It is possible that the patients expressed higher satisfaction with the GLED therapy because it was not associated with side effects and was relatively simple and “non-chemical”. To further illustrate this point, all patients were instructed to return the GLED upon completion of the study, but we offered them the option of keeping the GLED at the end. All but one patient kept the GLED. The person who returned the GLED indicated that her headache-days/month decreased from 5 to zero per month (100% reduction) using the migraine diary. However, she reported her perceived reduction in headache-days/month as 20%. This suggests that this particular patient did not view the GLED as a favorable therapy, which may explain why she did not wish to keep the GLED.
Previous studies have shown that light of different colors may modulate the perception of pain. Noseda and colleagues reported that exposure to green light during a migraine attack was less aversive than other colors and additionally, about 20% of patients reported an improvement of migraine pain intensity of about 15% (28). In addition to reducing pain during migraine, light therapy may offer preventive benefits. CM patients who were asked to wear special filter glasses which block out certain spectrums during all waking hours for two weeks reported that blocking either red or blue light lowered the intensity of pain experienced by migraine patients (29). While that study focused on blocking certain colors, it is important to note that the glasses allowed the green spectrum to pass through. Our study focused on the use of GLED as a preventive strategy. The mechanism of the GLED pain relief remains unknown. We have shown that when we fitted rats with clear contact lenses that did not impede or change the wavelength of GLED, they experienced antinociception when exposed to GLED. However, when the rats were fitted with opaque contact lenses which did not allow light to pass to the retina, they did not develop antinociception (18). Therefore, GLED appears to require the visual system, at least in our rodent studies. It is unclear which part of the visual system might mediate the effects of GLED. The intrinsically photosensitive retinal ganglion cells (IPRGCs) project to the midbrain and are involved in triggering photophobia in migraine patients (30). It is therefore possible that GLED is mediating its effect through the IPRGCs. Future studies will be required to elucidate the mechanisms of GLED and the components of the visual system involved. Other pain modulatory mechanisms may also play a role. For example, in rats GLED significantly increased the levels of proenkephalin mRNA in the spinal cord and decreased entry of calcium through the voltage-gated N-type (CaV2.2) calcium channel (18). It is possible that GLED produces its effects through several mechanisms acting in harmony. For example, while it has long been established that pain and sleep are intimately related (31, 32), whether GLED improved pain which then led to an improvement in sleep or vice versa could not be determined in this study. The effect of light on sleep has been documented previously with blue light having profound effects on melatonin and sleep (33, 34). Therefore, it is possible that GLED therapy may have independently improved both sleep and pain by different mechanisms.
Study limitations:
This study assessed the effectiveness of GLED as a preventive treatment in migraine patients and was designed as a proof of concept investigation. The number of the recruited EM and CM patients is small and thus a limitation of our study. Therefore, a larger study is being planned to recruit a greater number of patients in future studies. For ethical reasons, we only recruited patients who were not satisfied with their current or previous migraine therapies. This may have introduced an element of selection bias in this study. Another limitation of the study is blinding patients to the treatment. Given the nature of the study, it was not possible to blind patients to the color they received. However, to minimize bias and expectations, the patients were not told which color was the treatment and which was the control.
In conclusion, the safety and efficacy observed coupled with the simplicity of this method merit further investigations to fully investigate the role of GLED for migraine prevention.
Supplementary Material
CLINICAL IMPLICATIONS.
Green light exposure decreased the number of headache-days/month in both episodic and chronic migraine patients.
Green light exposure improved the quality of life for both episodic and chronic migraine patients.
No patient reported side effects after being exposed to green light.
Given the safety, affordability, and efficacy of green light exposure, there is merit to conduct a larger study.
Green light therapy may offer an additional therapy for prevention of episodic and chronic migraine.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Vangie Steinbrenner for her valuable assistance with obtaining the IRB for this study. We also would like to thank Debbie Schaab, RN, for her tireless efforts to follow-up with patients and her overall help with this study. We thank Mr. Scott Derigne for his help with the LED strips.
FUNDING
This study was funded by support from NCCIH R01AT009716 (to M.M.I), the Comprehensive Chronic Pain and Addiction Center-University of Arizona, and the University of Arizona CHiLLi initiative.
Footnotes
This study is registered with clinicaltrials.gov under NCT03677206
DECLARATION OF CONFLICTING INTERESTS
Drs. Ibrahim and Khanna have a patent pending through the University of Arizona for using green light therapy for the management of chronic pain. All other authors have no conflict of interest to report. None of the authors of the manuscript received any remuneration or any reimbursement or honorarium in any other manner. The authors are not affiliated with any vendor or pharmaceutical company associated with this study. Dr. Ibrahim was involved in recruiting and analyzing data.
REFERENCES
- [1].Dodick DW. Migraine. Lancet. 2018;391(2018):1315–30. [DOI] [PubMed] [Google Scholar]
- [2].Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(2012):2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ceriani CEJ, Wilhour DA, Silberstein SD. Novel Medications for the Treatment of Migraine. Headache. 2019;59(2019):1597–608. [DOI] [PubMed] [Google Scholar]
- [4].Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(2018):1–211. [DOI] [PubMed] [Google Scholar]
- [5].Lipton RB, Fanning KM, Buse DC, Martin VT, Reed ML, Manack Adams A, Goadsby PJ. Identifying Natural Subgroups of Migraine Based on Comorbidity and Concomitant Condition Profiles: Results of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2018;58(2018):933–47. [DOI] [PubMed] [Google Scholar]
- [6].Diener HC, Solbach K, Holle D, Gaul C. Integrated care for chronic migraine patients: epidemiology, burden, diagnosis and treatment options. Clin Med (Lond). 2015;15(2015):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dodick DW, Lipton RB, Ailani J, Lu K, Finnegan M, Trugman JM, Szegedi A. Ubrogepant for the Treatment of Migraine. N Engl J Med. 2019;381(2019):2230–41. [DOI] [PubMed] [Google Scholar]
- [8].Lipton RB, Dodick DW, Ailani J, Lu K, Finnegan M, Szegedi A, Trugman JM. Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine: The ACHIEVE II Randomized Clinical Trial. JAMA. 2019;322(2019):1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M, et al. Rimegepant, an Oral Calcitonin Gene-Related Peptide Receptor Antagonist, for Migraine. N Engl J Med. 2019;381(2019):142–9. [DOI] [PubMed] [Google Scholar]
- [10].Croop R, Goadsby PJ, Stock DA, Conway CM, Forshaw M, Stock EG, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(2019):737–45. [DOI] [PubMed] [Google Scholar]
- [11].Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB, Group CM-S. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology. 2018;91(2018):e2222–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK, Gaul C. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(2019):1894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bigal ME, Walter S, Rapoport AM. Fremanezumab as a preventive treatment for episodic and chronic migraine. Expert Rev Neurother. 2019;19(2019):719–28. [DOI] [PubMed] [Google Scholar]
- [14].Dodick DW, Lipton RB, Silberstein S, Goadsby PJ, Biondi D, Hirman J, et al. Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia. 2019;39(2019):1075–85. [DOI] [PubMed] [Google Scholar]
- [15].Xu D, Chen D, Zhu LN, Tan G, Wang HJ, Zhang Y, Liu L. Safety and tolerability of calcitonin-gene-related peptide binding monoclonal antibodies for the prevention of episodic migraine - a meta-analysis of randomized controlled trials. Cephalalgia. 2019;39(2019):1164–79. [DOI] [PubMed] [Google Scholar]
- [16].Lipton RB, Munjal S, Buse DC, Alam A, Fanning KM, Reed ML, et al. Unmet Acute Treatment Needs From the 2017 Migraine in America Symptoms and Treatment Study. Headache. 2019;59(2019):1310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 2018;75(2018):1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ibrahim MM, Patwardhan A, Gilbraith KB, Moutal A, Yang X, Chew LA, et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain. 2017;158(2017):347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khanna R, Patwardhan A, Yang X, Li W, Cai S, Ji Y, et al. Development and Characterization of An Injury-free Model of Functional Pain in Rats by Exposure to Red Light. J Pain. 2019(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Melzack R The short-form McGill Pain Questionnaire. Pain. 1987;30(1987):191–7. [DOI] [PubMed] [Google Scholar]
- [21].Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6) across episodic and chronic migraine. Cephalalgia. 2011;31(2011):357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(2011):1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tassorelli C, Diener HC, Dodick DW, Silberstein SD, Lipton RB, Ashina M, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38(2018):815–32. [DOI] [PubMed] [Google Scholar]
- [24].Tonelli ME, Wachholtz AB. Meditation-based treatment yielding immediate relief for meditation-naive migraineurs. Pain Manag Nurs. 2014;15(2014):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stovner LJ, Linde M, Gravdahl GB, Tronvik E, Aamodt AH, Sand T, Hagen K. A comparative study of candesartan versus propranolol for migraine prophylaxis: A randomised, triple-blind, placebo-controlled, double cross-over study. Cephalalgia. 2014;34(2014):523–32. [DOI] [PubMed] [Google Scholar]
- [26].Dodick DW, Freitag F, Banks J, Saper J, Xiang J, Rupnow M, et al. Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migraineurs. Clin Ther. 2009;31(2009):542–59. [DOI] [PubMed] [Google Scholar]
- [27].Akhtar A The Role of Anti-calcitonin Gene-related Peptide in Migraine and its Implication in Developing Countries: A Reasonable Option to Consider Despite Higher Cost. Cureus. 2019;11(2019):e4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Noseda R, Bernstein CA, Nir RR, Lee AJ, Fulton AB, Bertisch SM, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain. 2016;139(2016):1971–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hoggan RN, Subhash A, Blair S, Digre KB, Baggaley SK, Gordon J, et al. Thin-film optical notch filter spectacle coatings for the treatment of migraine and photophobia. J Clin Neurosci. 2016;28(2016):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Benarroch EE. The melanopsin system: Phototransduction, projections, functions, and clinical implications. Neurology. 2011;76(2011):1422–7. [DOI] [PubMed] [Google Scholar]
- [31].Cheatle MD, Foster S, Pinkett A, Lesneski M, Qu D, Dhingra L. Assessing and Managing Sleep Disturbance in Patients with Chronic Pain. Sleep Med Clin. 2016;11(2016):531–41. [DOI] [PubMed] [Google Scholar]
- [32].Yang CP, Wang SJ. Sleep in Patients with Chronic Migraine. Curr Pain Headache Rep. 2017;21(2017):39. [DOI] [PubMed] [Google Scholar]
- [33].Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(2001):6405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Esaki Y, Kitajima T, Ito Y, Koike S, Nakao Y, Tsuchiya A, et al. Wearing blue light-blocking glasses in the evening advances circadian rhythms in the patients with delayed sleep phase disorder: An open-label trial. Chronobiol Int. 2016;33(2016):1037–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study will be made available upon request.


