Abstract
Background.
Childhood obsessive-compulsive symptoms (OCS) are common and can be an early risk marker for Obsessive-Compulsive Disorder (OCD). The Adolescent Brain and Cognitive Development (ABCD) Study provides a unique opportunity to characterize OCS in a large, normative sample of school-age children and to explore cortico-striatal and task-control circuits implicated in pediatric OCD.
Method.
The ABCD Study acquired data from 9–10-year-olds (N=11,876). Linear mixed-effects models probed associations between OCS (Child Behavior Checklist) and cognition (NIH Toolbox), brain structure (subcortical volume, cortical thickness), white matter (diffusion tensor imaging), and resting-state functional connectivity.
Results.
OCS scores showed good psychometric properties, high prevalence, and related to familial/parental factors, including family conflict. Higher OCS related to better cognitive performance (b=0.06, t(9966.60)=6.28, p<.001, η2p=0.01), particularly verbal, when controlling for ADHD, which related to worse performance. OCS did not significantly relate to brain structure but did relate to lower superior cortico-striate tract fractional anisotropy (b=−0.03, t=−3.07, p=.002, η2p=0.02). Higher OCS related to altered functional connectivity, including weaker within dorsal attention network connectivity (b=−0.04, t(7262.87)=−3.71, p<.001, η2p=0.002) and weaker dorsal attention-default mode anti-correlation (b=0.04, t(7251.95)=3.94, p<.001, η2p=0.002). Dorsal attention-default mode connectivity predicted OCS at 1-year (b=−0.04, t(2407.61)=−2.23, p=.03, η2p=0.03).
Conclusions.
OCS are common and may persist throughout childhood. Cortico-striatal and attention network connectivity are likely mechanisms in the subclinical-to-clinical spectrum of OCS. Understanding correlates and mechanisms of OCS may elucidate their role in childhood psychiatric risk and suggest potential utility of neuroimaging, e.g. dorsal attention-default mode connectivity, for identifying children at increased risk for OCD.
Keywords: OCD, MRI, DTI, dorsal attention network, children, ABCD
Introduction
Subclinical obsessive-compulsive symptoms (OCS) in childhood predict increased risk for Obsessive-Compulsive Disorder (OCD) diagnosis in adulthood (odds ratio [OR]~5–10) (1). Critically, OCS are common in children and adolescents and, even below clinical threshold, associate with impairment and comorbidity (1–7). Childhood OCS exhibit moderate stability, with some ebb and flow within-individuals over development (4, 8). Cross-sectional research generally suggests greater OCS prevalence in mid vs. early childhood, (3) whereas longitudinal work indicates weak persistence of OCS during this developmental period (4, 8). Uncovering neural and cognitive mechanisms underlying OCS and their variable course over childhood OCS could help improve early identification of children most at risk for OCD.
Few pediatric studies have examined neural or cognitive mechanisms underlying OCS or subsequent OCD risk. Several relatively small studies in healthy adults associate OCS with executive function deficits, e.g. cognitive flexibility (9, 10), spatial problem (11, 12), and response inhibition (13); c.f. (14). Likewise, few pediatric studies have related OCS to brain structure and function. Among 255 healthy 8–12-year-olds, overall OCS scores did not associate with voxel-based morphometry, whereas subscales showed differential associations, e.g. ordering symptoms vs. doubt-checking symptoms (15). Among 1,938 healthy adolescents from the IMAGEN study, compulsive behaviors (including OCD and eating disorder symptoms) associated with greater ventral striatal, orbitofrontal, and dorsolateral prefrontal cortex gray matter volume (16).
While much work suggests that disruptions in cortico-striatal-thalamo-cortical (CSTC) circuits are central to OCD pathophysiology, e.g. (17), leading to deficits in controlling obsessions and urges to perform rituals, emerging evidence highlights broader circuit alterations, e.g. (18). Particularly, meta-analytic work suggests OCD-related alterations in connectivity within and between executive, salience, and default mode networks (DMN) (19) in line with a proposed triple network model of psychopathology. (20) Furthermore, reviews examining research on pediatric OCD highlight structural, functional, and connectivity alterations in both fronto-striatal and task-control neural circuits (21–23). For example, findings from our group suggest alterations in cingulo-opercular structural connectivity (24) and functional connectivity between default mode network (DMN) and task-positive network regions in pediatric OCD (25). The ENIGMA OCD consortium noted larger thalamic volumes (26) (N=201 healthy controls vs. N=103 unmedicated OCD) and thinner parietal cortices (27) in youth with OCD compared to healthy controls.
Large, normative studies are a critical and powerful approach to study childhood OCS in the population. The Adolescent Brain and Cognitive Development (ABCD) Study is one such study, which provides a unique opportunity to characterize OCS in 9–10-year-old children (N=11,876). ABCD is the largest, publicly accessible, prospective study of school-age child sampled with minimally restrictive exclusion criteria across the USA to collect harmonized neuroimaging data. This study yields several advantages in addressing our questions of interest, including sufficient power to identify even small effect sizes and to address heterogeneity in a normative population, compared to studies like the Pediatric Imaging, Neurocognition, and Genomics Study (PING) or Healthy Brain Network (HBN), both of which sample restricted geographic areas and less focal age ranges, or the ENIGMA Consortium which conducts meta-/mega-analyses on unharmonized data collected across multiple sites.
Given prior data, we expected that OCS in the ABCD sample would exhibit a ~5–10% prevalence above established cutoff scores (28, 29), minimal sex differences or a slight male preponderance (3, 4, 6, 8, 28), significant familiality (8), and greater longitudinal risk for poor psychiatric outcomes (1). We further expected that higher OCS scores would relate to lower executive functioning (9–13), smaller thalamic volumes (26) and thinner parietal cortices (27), as well as altered structural and functional connectivity within task-control circuits and between task-control networks and the DMN (21–25). In longitudinal analyses, we examined whether neural and cognitive factors associated with OCS at baseline also predicted change in OCS over development. Finally, we explored effects of comorbid ADHD on brain and behavioral correlates of OCS given the high prevalence of ADHD in the ABCD sample and data suggesting both shared and unique neural mechanisms across pediatric ADHD and OCD (30–33).
Materials and Method
Overview.
The ABCD Study is a multi-site study (21 sites across the USA) with the overall goals of: (a) assessing variability in adolescent brain and cognitive development and (b) understanding factors that influence development (34). Using a school-based recruitment strategy (public and private elementary schools), the ABCD Study has collected baseline clinical, questionnaire, behavioral, and neuroimaging data from 9–10-years-olds with ongoing longitudinal assessments (35). Exclusion criteria included lack of English fluency, major medical or neurological conditions, premature birth, MRI contraindication, history of traumatic brain injury, current diagnosis of schizophrenia, moderate/severe autism spectrum disorder, intellectual disability, or alcohol/substance use disorder. The present study examined the second public ABCD data release (version 2.0.1, released July 2019; http://dx.doi.org/10.15154/1503209), which included baseline clinical, questionnaire, cognitive, and neuroimaging data and questionnaire data from the 1-year follow-up assessment.
Assessment.
Children and their parent/guardian completed an extensive battery of clinical interviews, self- and parent-report measures, and neurocognitive tests (36). Measures examined in the current analyses are briefly summarized below (see Table 1 and Supplement). Questionnaires included parent-reported demographics, brief assessments of parent- and child-reported pubertal status (37), and parental/familial measures are summarized in the Supplement (38, 39)(40, 41)(42).
Table 1:
Demographic Characteristics of the ABCD Sample
| Full Sample (N=11876) | OCD Absent (n=10584) | OCD Present (n=1099) | Group Difference | p | Effect Size | |
|---|---|---|---|---|---|---|
| Age | 118.94 (7.46) | 118.97 (7.47) | 118.61 (7.36) | t=−1.55 | .12 | d=−0.05 |
| Sex (F) *** | 5681 (47.86%) | 5138 (48.56%) | 440 (40.11%) | χ2=28.13 | <.001 | OR=0.71 |
| Pubertal Status | 1.68 (0.72) | 1.68 (0.72) | 1.69 (0.75) | t=0.38 | .70 | d=0.01 |
| Race-White | 8803 (74.13%) | 7879 (74.44%) | 789 (71.79%) | χ2=3.51 | .06 | OR=0.87 |
| Race-Black *** | 2515 (21.18%) | 2186 (20.65%) | 293 (26.66%) | χ2=21.13 | <.001 | OR=1.40 |
| Hispanic | 9308 (79.44%) | 8312 (79.59%) | 863 (79.69%) | χ2=0.00 | .97 | OR=1.01 |
| Parents Marital Status (together/married) *** | 8679 (73.69%) | 7822 (74.5%) | 732 (67.47%) | χ2=24.79 | <.001 | OR=0.71 |
| Parental Education (completed some college) *** | 9812 (82.77%) | 8818 (83.45%) | 850 (77.55%) | χ2=23.91 | <.001 | OR=0.69 |
| Parental Income *** | 7.22 (2.42) | 7.31 (2.37) | 6.45 (2.76) | t=−9.33 | <.001 | d=−0.33 |
| NIH Toolbox - Cognition Total *** | 100.37 (17.96) | 100.7 (17.89) | 97.3 (18.48) | t=−5.68 | <.001 | d=−0.19 |
| Height (inches) | 55.26 (3.22) | 55.27 (3.21) | 55.13 (3.36) | t=−1.31 | .19 | d=−0.04 |
| Parental Monitoring *** | 4.38 (0.52) | 4.39 (0.51) | 4.3 (0.57) | t=−5.28 | <.001 | d=−0.18 |
| Parent 1 Acceptance ** | 2.78 (0.3) | 2.78 (0.3) | 2.75 (0.33) | t=−2.88 | .004 | d=−0.10 |
| Parent 2 Acceptance ** | 2.69 (0.39) | 2.69 (0.38) | 2.65 (0.42) | t=−2.79 | .01 | d=−0.10 |
| Family Conflict - Child Report *** | 2.05 (1.95) | 2.02 (1.94) | 2.27 (2.03) | t=3.92 | <.001 | d=0.13 |
| Family Conflict – Parent Report *** | 2.54 (1.96) | 2.47 (1.93) | 3.12 (2.09) | t=9.93 | <.001 | d=0.33 |
| Usable Structural Data | 10534 (88.7%) | 9405 (88.86%) | 962 (87.53%) | χ2=1.62 | .20 | OR=0.88 |
| CBCL OCS sum *** | 1.34 (1.82) | 1.13 (1.56) | 3.41 (2.66) | t=27.84 | <.001 | d=1.04 |
| CBCL OCS T-score *** | 53.70 (6.12) | 52.97 (5.2) | 60.69 (9.22) | t=27.27 | <.001 | d=1.03 |
Note. Demographic characteristics of the sample are summarized here for the full ABCD baseline sample and split by the presence/absence of a lifetime K-SADS OCD diagnosis. Continuous and categorical variables are characterized respectively by mean and (standard deviation) or N and (percent) with group differences based on OCD diagnosis presence are compared by t-test (Cohen’s d effect size) or chi-squared test (odds ratio [OR] effect size). Pubertal status range=1–4. Income range=1–10. Conflict score range=0–9. Monitoring score range=1–5. Acceptance score range=1–3.
N=193 missing K-SADS OCD diagnosis. Additionally, n=1 missing Age, n=7 missing Sex, n=1 missing Pubertal Status and Race, n=159 missing ethnicity, n=99 missing Parents Marital Status, n=21 missing Parental Education, n=1019 missing Parental Income, n=413 missing NIH Toolbox - Cognition Total, n=13 missing height, n=24 missing parental monitoring behavior, n=37 missing parent 1 acceptance behavior, n=919 missing parent 2 acceptance behavior, n=27 missing family conflict - child report, n=6 missing family conflict - parent report, n=8 missing CBCL OCS sum, n=9 missing CBCL OCS T-score.
Group difference:
p<.05,
p<.01,
p<.001
Children and their parent/guardian completed modules of a self-administered, computerized Kiddie-Schedule for Affective Disorders (K-SADS COMP; Table 2) (43–45) to assess children’s lifetime (past, present, or partial remission) DSM-5 diagnoses (36). OCD was assessed solely by parent-report on the K-SADS COMP. Composite variables were created to examine lifetime diagnoses of ADHD (parent-report only), depressive disorders (parent- or child-report of major depressive disorder, dysthymia, or an unspecified depressive disorder), anxiety disorders (parent- or child-report of separation anxiety disorder, social anxiety disorder, or generalized anxiety disorder), and externalizing disorders (parent-report of conduct or oppositional defiant disorder).
Table 2:
Clinical Characteristics of the ABCD Sample
| Full Sample (N=11876) | OCD Absent (n=10584) | OCD Present (n=1099) | Group Difference | Effect Size | |
|---|---|---|---|---|---|
| CBCL T-score | |||||
| DSM Anxiety | 53.49 (6.13) | 52.89 (5.35) | 59.21 (9.29) | t=22.16 | d=0.83 |
| DSM Depression | 53.6 (5.73) | 53.13 (5.21) | 58.19 (8.02) | t=20.45 | d=0.75 |
| Thought Problems | 53.8 (5.9) | 53.13 (5.14) | 60.19 (8.45) | t=27.17 | d= 1.01 |
| DSM ADHD | 53.23 (5.64) | 52.79 (5.13) | 57.55 (8.04) | t= 19.18 | d=0.71 |
| Internalizing | 48.45 (10.64) | 47.46 (10.12) | 57.88 (10.8) | t=30.58 | d=1.00 |
| Externalizing | 45.73 (10.33) | 44.98 (9.92) | 52.94 (11.41) | t=22.25 | d=0.75 |
| Total | 45.85 (11.34) | 44.78 (10.78) | 56.17 (11.4) | t=31.63 | d=1.03 |
| K-SADS lifetime diagnosis | |||||
| Any Depressive Disorder | 1272 (10.9%) | 1014 (9.61%) | 247 (22.6%) | χ2=171.62 | OR=2.75 |
| MDD | 614 (5.27%) | 475 (4.5%) | 137 (12.53%) | χ2=126.64 | OR=3.04 |
| Dysthymia | 24 (0.21%) | 18 (0.17%) | 6 (0.55%) | χ2=5.17 | OR=3.23 |
| Depression NOS | 697 (5.97%) | 570 (5.4%) | 117 (10.7%) | χ2=49.19 | OR=2.10 |
| Any Anxiety Disorder | 1730 (14.83%) | 1307 (12.39%) | 419 (38.23%) | χ2=523.32 | OR=4.38 |
| Separation Anxiety | 1047 (8.95%) | 783 (7.4%) | 263 (23.93%) | χ2=331.82 | OR=3.94 |
| Social Anxiety | 619 (5.31%) | 477 (4.52%) | 140 (12.8%) | χ2=133.59 | OR=3.10 |
| GAD | 579 (4.96%) | 382 (3.62%) | 195 (17.81%) | χ2=420.87 | OR=5.76 |
| ADHD | 2428 (20.76%) | 1921 (18.15%) | 506 (46.04%) | χ2=468.92 | OR=3.85 |
| ODD/CD | 1782 (15.24%) | 1397 (13.2%) | 385 (35.03%) | χ2=365.45 | OR=3.55 |
| PTSD | 231 (1.98%) | 141 (1.33%) | 90 (8.19%) | χ2=238.01 | OR=6.60 |
| No diagnoses | 7348 (61.99%) | 6898 (65.17%) | 296 (26.93%) | χ2=613.74 | OR=0.20 |
| Unmedicated | 10739 (90.43%) | 9704 (91.69%) | 859 (78.16%) | χ2=208.52 | OR=0.32 |
Note. Clinical characteristics of the sample are summarized here for the full ABCD baseline sample and split by the presence/absence of a lifetime K-SADS OCD diagnosis. Continuous and categorical variables are characterized respectively by mean and (standard deviation) or N and (percent) with group differences based on OCD diagnosis presence are compared by t-test (Cohen’s d effect size) or chi-squared test (odds ratio [OR] effect size). All group differences were p<.001 significant, except for dysthymia (p=.02). No diagnoses indicated that none of the listed disorders were diagnosed past or present on the K-SADS.
N=193 missing K-SADS OCD diagnosis. Additionally, n=10 missing CBCL T-scores, n=42 missing MDD and Dysthymia, n=40 missing Depression NOS, n=41 missing Social Anxiety, n=40 missing GAD.
OCS Measure.
Parents/guardians completed the Child Behavior Checklist (CBCL) (46) to assess their child’s emotional and behavioral functioning. Age- and sex-normed T-scores were used for analyses (for high correspondence with raw scores, see Figure S1). Our primary predictor of interest was the 8-item OCS subscale (Table 3) (28, 29, 47–50), c.f. (51, 52). N=4937 participants completed the CBCL at 1-year follow-up as of the 2.0.1 release. We confirmed good psychometrics of the 8-item CBCL OCS subscale (see Supplement), including good fit of a one-factor/unidimensional model (lavaan package) (53, 54) and moderate/good internal consistency (psych package, standardized Cronbach’s alpha=.71, omega=.87) (55). For item-level inter-correlation, see Figure S2. See Supplement and Figure S3 for receiver operator curve analyses (pROC package) (56, 57).
Table 3:
Item-wise CBCL OCS Characterization
| CBCL OCS Item | Score | Total (N=11876) | OCD Absent (n=10584) | OCD Present (n=1099) |
|---|---|---|---|---|
| 9 - Can’t get his/her mind off certain thoughts; obsessions | 0 | 8830 (74.4) | 8259 (78.1) | 434 (39.5) |
| 1 | 2534 (21.3) | 2017 (19.1) | 472 (43.0) | |
| 2 | 505 (4.3) | 304 (2.9) | 192 (17.5) | |
| 66 - Repeats certain acts over and over; compulsions | 0 | 11050 (93.1) | 10142 (95.9) | 733 (66.8) |
| 1 | 687 (5.8) | 393 (3.7) | 282 (25.7) | |
| 2 | 131 (1.1) | 44 (0.4) | 83 (7.6) | |
| 84 - Strange behavior | 0 | 11374 (95.8) | 10289 (97.3) | 904 (82.3) |
| 1 | 456 (3.8) | 270 (2.6) | 177 (16.1) | |
| 2 | 38 (0.3) | 20 (0.2) | 17 (1.5) | |
| 85 - Strange ideas | 0 | 11054 (93.1) | 10003 (94.6) | 871 (79.3) |
| 1 | 770 (6.5) | 549 (5.2) | 210 (19.1) | |
| 2 | 44 (0.4) | 27 (0.3) | 17 (1.5) | |
| 31 - Feels he/she might think or do something bad | 0 | 10862 (91.5) | 9834 (93.0) | 857 (78.1) |
| 1 | 932 (7.9) | 701 (6.6) | 212 (19.3) | |
| 2 | 74 (0.6) | 44 (0.4) | 29 (2.6) | |
| 32 - Feels he/she has to be perfect | 0 | 8620 (72.6) | 7896 (74.6) | 587 (53.5) |
| 1 | 2775 (23.4) | 2342 (22.1) | 387 (35.2) | |
| 2 | 473 (4.0) | 341 (3.2) | 124 (11.3) | |
| 52 - Feels too guilty | 0 | 11093 (93.5) | 10008 (94.6) | 902 (82.1) |
| 1 | 708 (6.0) | 527 (5.0) | 173 (15.8) | |
| 2 | 67 (0.6) | 44 (0.4) | 23 (2.1) | |
| 112 - Worries | 0 | 7945 (66.9) | 7396 (69.9) | 420 (38.3) |
| 1 | 3418 (28.8) | 2864 (27.1) | 499 (45.4) | |
| 2 | 505 (4.3) | 319 (3.0) | 179 (16.3) | |
| OCS > 0 | 6611 (55.7%) | 5539 (52.4%) | 962 (87.6%) | |
| OCS > 1 | 3880 (32.7%) | 3010 (28.5%) | 802 (73.04%) | |
| OCS ≥ 5 | 807 (6.8%) | 464 (4.4%) | 328 (29.9%) |
Note. Item-level data are presented for the CBCL OCS subscale. The number (percent) of participants endorsing each CBCL item response option (0, 1, or 2) presented for the full sample and split by the presence/absence of a K-SADS lifetime OCD diagnosis. The first four items are drawn from the Thought Problems subscale (#9, 66, 84, 85) and the latter four items are drawn from the Anxious/Depressed subscale (#31, 32, 52, 112). The number (percent) of participants with a raw sum score>0, >1, or ≥ 5 are also presented. All differences between those with and without lifetime OCD are p<.001 significant in this table.
Cognition.
Neurocognitive performance was assessed using the NIH Toolbox (58, 59). Total Cognition age-corrected T-scores (M=100, SD=15) were examined as proxy for commonly used full-scale IQ measures (59, 60). This is derived from two verbal measures (Picture Vocabulary, Oral Reading Recognition) and six executive functioning measures (Dimensional Change Card Sort, Flanker, Picture Sequence Memory, List Sorting Working Memory, Pattern Comparison Processing Speed).
Structural Magnetic Resonance Imaging (MRI).
Children completed neuroimaging on GE, Siemens, or Phillips scanners (61). High-resolution T1- and T2-weighted structural MR images (1mm isotropic, prospective motion correction) were processed through ABCD pipelines (61) (including registration, intensity normalization, bias field correction). Structural data that did not pass quality control (QC) procedures performed by ABCD (visual inspection of T1 images and FreeSurfer outputs) (62) were excluded (see Supplement). Herein, we focused on ABCD summary of standard FreeSurfer v5.3.0 (http://surfer.nmr.mgh.harvard.edu/) (63–66) subcortical volumes and cortical thickness from Destrieux atlas (67) regions of interest (ROI).
Diffusion Tensor Imaging (DTI).
High angular resolution diffusion imaging (1.7mm isotropic) was collected using multiband acquisition (factor=3), 96 diffusion directions, and four b-values (61). Standardized preprocessing included eddy current, head motion, and distortion correction (62). Major white matter tracts were automatically segmented using AtlasTrack (68). Participants whose DTI and structural MRI data did not pass ABCD QC (62) were excluded. Herein, we focused on tract-average factional anisotropy (FA). Supplementary analyses examined mean, longitudinal (or axial), and transverse (or radial) diffusivity (MD, LD, and TD) as well as Restriction Spectrum Imaging (RSI) model parameters (69).
Resting-State Functional Connectivity (RSFC).
Children completed resting-state acquisition during high-resolution (2.4 mm isotropic, TR=800ms) multi-band (factor=6) scanning. Standardized fMRI preprocessing included registration, distortion correction, and normalization. RSFC post-processing included regression of 24 temporally filtered motion parameters, frame-wise displacement (FD)>0.3mm outliers, as well as white matter, cerebral spinal fluid, and whole brain signal (62). Within- and between-network connectivity was extracted from the cortical ribbon parcellated using the Gordon atlas (70), i.e. averaging all connections between ROIs assigned to given networks. Participants whose resting-state and structural MRI data did not pass ABCD QC (62) were excluded. Further, per ABCD suggestions, we excluded participants with <375 frames of good data after motion/outlier regression and excluded data from Philips scanners given a preprocessing issue affecting only functional MRI (identified 12/05/2019). Given our hypotheses, we focused on 28 network-level RSFC averages within and between task-control circuits (cingulo-opercular [CO], cingulo-parietal [CP], dorsal attention network [DAN], fronto parietal [FP], salience network [SN], ventral attention network [VAN]) and the DMN.
Analysis.
Analyses were performed in R v4.0.0 (71). Sample characteristics and group differences as a function of K-SADS lifetime OCD diagnosis were summarized using the scipub package (72). Specifically, two-sample t-tests were used for continuous variables and χ2 tests for categorical variables with Cohen’s d or OR effect sizes, respectively. SOLAR (73) polygenic analyses estimated familiality of OCS scores (see Supplement).
Linear mixed-effects (LME) models (lme4 package) (74) were used to examine OCS scores (or OCD diagnosis) as predictors of our outcomes of interest, similar to prior work (75). All models included random effects for family nested within acquisition site (or MRI serial number for brain analyses) and fixed-effects covariates for age, sex, race, ethnicity, family income, parental education, parental marital status, pubertal status, and Total Cognition T-scores (except when cognition was the outcome). Structural analyses additionally covaried child height (accounting for overall body size/development), T1 image signal-to-noise, and intracranial volume (ICV) in subcortical analyses. DTI analyses additionally covaried mean FD. RSFC analyses additionally covaried number of frames retained after processing. Inter-correlations among all covariates noted in Table S1. Standardized beta coefficients and partial eta-squared (η2p) (76) effect sizes are presented for all models.
False discovery rate (FDR) was used to correct for multiple comparisons across measures/ROIs within each analysis. For each analysis, participants were excluded for missing data using list-wise deletion. Within the useable sample for each cognition, structural, and DTI analysis, outcome variable outliers >3SD from the mean were Winsorized to the next non-outlier value (trimmed 0.25% most extreme RSFC values instead, per ABCD; see Supplement).
Follow-up Analyses.
Follow-up tests were run to confirm significant effects and address comorbidity. LME models were conducted again with psychotropic medication status and K-SADS depressive, anxious, and ADHD diagnoses as covariates. Given the high rate of ADHD in the ABCD sample (see Results, Table 2) and potential shared/distinct neurocognitive mechanisms between OCD and ADHD (30–33), we also ran separate LME models covarying CBCL DSM-oriented ADHD T-scores. Further analyses comparing children with and without OCD and ADHD as well as propensity-matched (77, 78) groups (from full sample and an unmedicated subset) comparing children with OCD to healthy and clinical controls are detailed in the Supplement.
Longitudinal Analysis.
Longitudinal analyses examined whether cognitive or neural factors associated with baseline OCS also predicted change in OCS at 1-year follow-up. Specifically, we used similar LME models to test whether these factors significantly associated with follow-up OCS over and above baseline OCS and our standard fixed and random effects.
Results
Participants.
Table 1–2 summarize demographic and clinical characteristics of the full sample and comparing by K-SADS OCD diagnosis (see Tables S2–3 comparing by OCS≥5). N=1099 (9.41%) children had a lifetime parent-reported diagnosis of OCD, including n=898 (7.68%) current and n=501 (4.29%) past. Lifetime OCD prevalence varied by site (χ2(21)=59.29, p<.001) from 5.79–13.77%. Yet, site differences (n=10,137, F(21)=1.49, p=.07) were largely accounted for by our demographic covariates in a logistic regression. OCD was more common among children with other psychiatric diagnoses (Table 2, Table S4, OR>1.50, Supplement).
Clinical Correlates of OCS:
A full range of CBCL OCS scores was observed (Figure 1; Table 3); N=5866 (55.7%) children exhibited non-zero scores and n=807 (6.8%) surpassed the suggested ≥5 threshold for clinically significant symptoms (28, 29). Obsessions, perfectionism, and worries were most commonly endorsed (Table 3). Baseline and 1-year follow-up OCS T-scores were strongly correlated (r=.61, t(4935)=53.53, 95%CI=[.59,.62], p<.001). OCS T-scores also correlated moderately with counts of current K-SADS OCD symptoms (r=0.42, 95%CI=[.40,.43], t(11851)=50.22, p<.001). See Table S5 for K-SADS OCD symptom endorsement.
Figure 1: CBCL OCS T-scores split by the presence/absence of lifetime OCD.
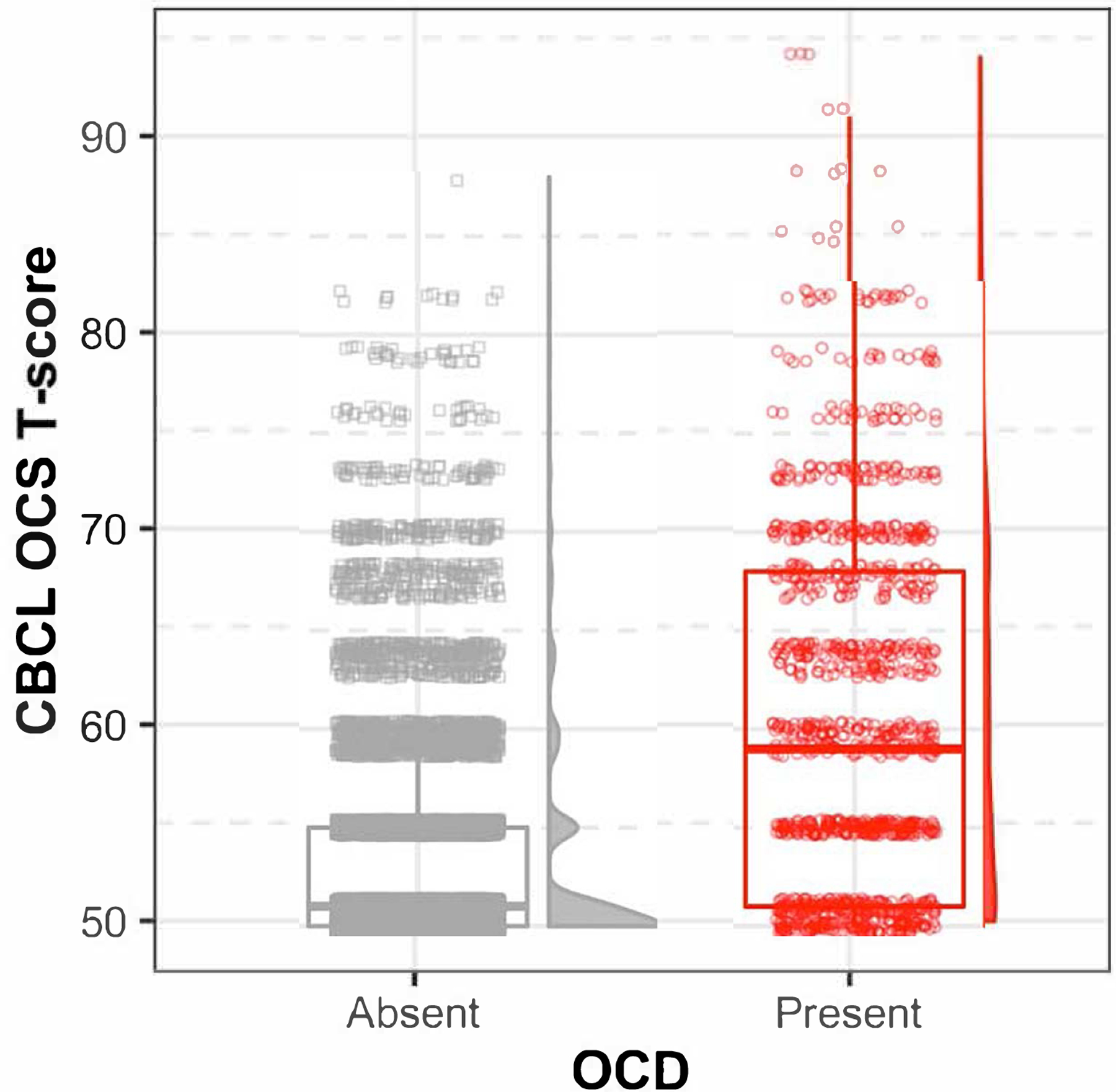
Scatter plot, boxplot, and histograms indicate the distribution of Child Behavior Checklist (CBCL) obsessive-compulsive symptom (OCS) T-scores for the full ABCD study sample. Scores are split based on the absence (gray square) or presence (red circle) of a lifetime Obsessive-Compulsive Disorder (OCD) diagnosis.
As expected, OCS scores were higher among children with vs. without a lifetime OCD diagnosis (Table 1, Figure 1). Supra-threshold OCS≥5 were much more common among youth with vs. without an OCD diagnosis (Table 3; N=328/1098 [29.87%] vs. N=770/10,579 [4.39%]; z=23.56, p<.001, OR=8.54), over and above our standard covariates. Similar to predictors of OCD diagnosis (Table S4), higher OCS scores were also observed among participants who were male, White, lower income, or with college-educated parents (Table S2, S6). OCS scores correlated strongly with other CBCL subscales, particularly internalizing and thought problems (all r>.70, t(11862)>106.00, p<.001) likely due to item overlap, relative to ADHD (r=.50, t(11862)=62.38, p<.001) and externalizing subscales (r=.48, t(11862)=60.25, p<.001). Analysis of OCS heritability (~70%) and associations between child OCS and elevated parent self-report OCS, parenting monitoring, and family conflict are detailed in the Supplement.
OCS and NIH Toolbox Cognition:
Higher OCS scores weakly associated with worse performance on the NIH Toolbox Flanker, Card Sort, Working Memory, and Episodic Memory tasks and better Picture Vocabulary performance (all p<.05, FDR-p<.08; Table S7–8). Analyses covarying CBCL ADHD T-scores revealed FDR-corrected associations between higher OCS and better Cognition Total scores (b=0.06, t(9966.60)=6.28, p<.001, η2p=0.01; Table S7–8), driven by Working Memory, Picture Vocabulary, and Oral Reading scores, whereas ADHD associated with worse performance (b=−0.14, t(9967.11)=−13.90, p<.001, η2p=0.03; Table S8). Similar results were observed when controlling for K-SADS lifetime ADHD or medication use (Tables S7–8). See Supplement for results of additional follow-up tests, including better performance among children with lifetime OCD vs. those with ADHD or ADHD and OCD.
OCS and Brain Structure:
No FDR-corrected associations emerged between OCS or lifetime OCD and subcortical volumes or cortical thickness (Tables S9–10; Figure S4; Supplementary Results). Note that effects were similarly non-significant controlling for CBCL ADHD (which was itself a non-significant predictor), though thalamic and hippocampal volumes differed between children with OCD vs. comorbid OCD + ADHD (see Supplement). Among unmedicated children, higher OCS scores related to smaller right accumbens volume (b=−0.02, t(8233.19)=−2.75, p<.001, p-FDR=.08, η2p=0.001) but no FDR-corrected effects of OCS or OCD diagnosis were observed on subcortical volumes or cortical thickness.
OCS and White Matter Integrity (DTI):
Examining the 35 AtlasTrack tracts, FDR-corrected results indicated higher OCS scores related to lower FA in in the left superior cortico-striatal tract (SCS; Table S11), particularly in the parietal portion of the left SCS (n=8585, b=−0.03, t(8546.23)=−3.31, p=.001, FDR-p=.03, η2p=0.02 Table S12), including when controlling for ADHD scores or medication status. No significant effects of lifetime OCD were observed on FA in the full sample. However, children with lifetime OCD exhibited lower parietal SCS FA than propensity-matched healthy controls and children with current OCD exhibited higher FA than those with current ADHD (See Supplement, Figure S5).
OCS and Functional Connectivity:
Examining 28 within- and between-network RSFC averages of interest, FDR-corrected associations were detected between OCS and four connections (Figure 2; Table S13). Specifically, higher OCS scores associated with weaker/less positive within-DAN RSFC and weaker/less negative DAN-VAN and DAN-DMN connectivity (Table S11). Higher OCS also related to stronger positive CO-VAN connectivity (which averaged near zero in the full sample, Figure S6). These four FDR-corrected effects were generally robust to follow-up covariates, including ADHD T-scores or medication status (see Supplement). Weaker within-DAN RSFC was also detected in children with OCD relative to healthy and clinical control groups in follow-up analyses (see Supplement). No OCD vs. ADHD or OCD+ADHD subgroup effects were noted for these four connections.
Figure 2: OCS Associations with Resting-State Functional Connectivity.
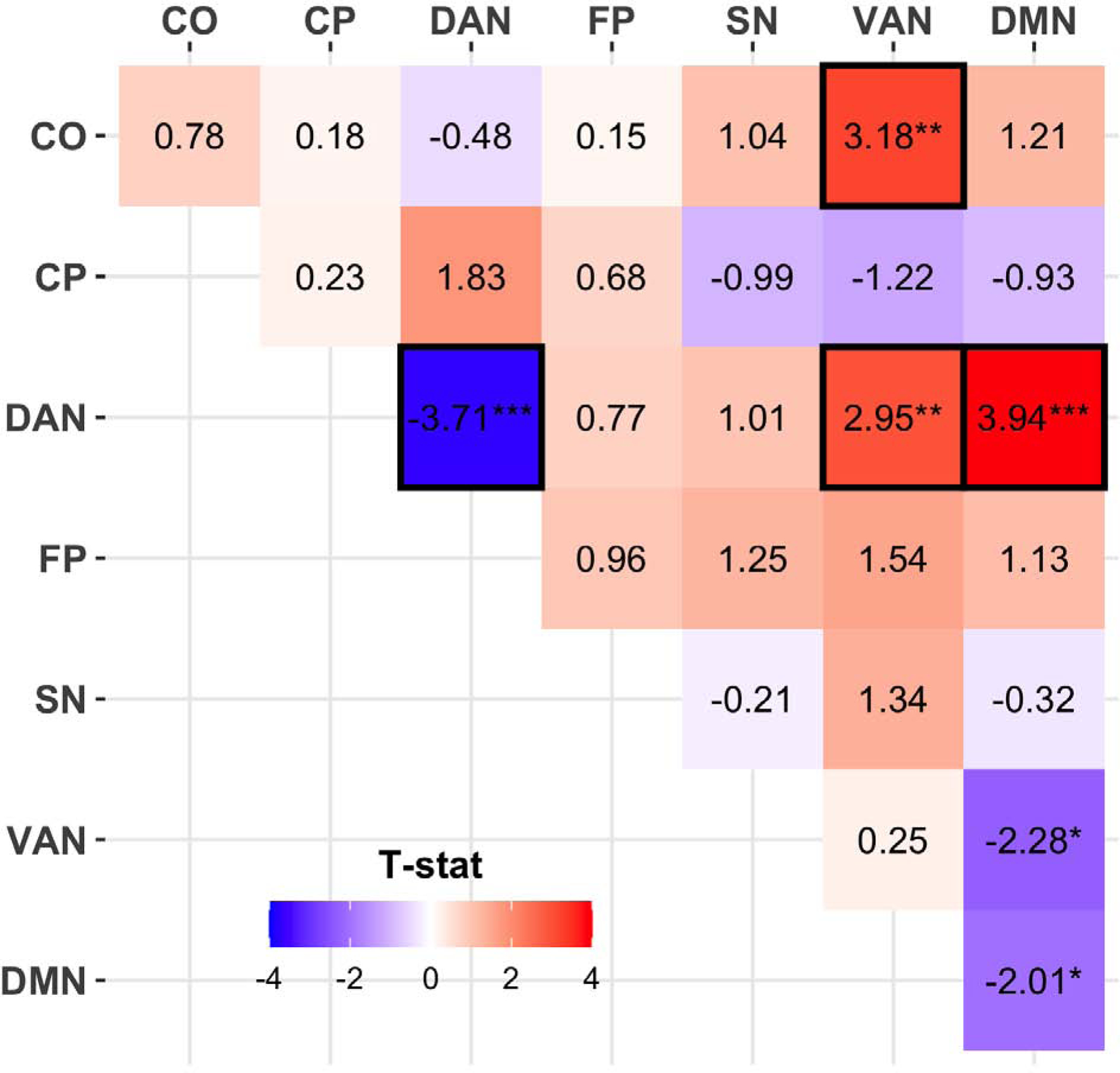
Child Behavior Checklist (CBCL) obsessive-compulsive symptom (OCS) were related to resting-state functional connectivity within and between task-control and default mode networks. T-statistics from linear mixed-effects models are presented here for this association between OCS and connectivity. Red squared with positive t-statistics indicate positive associations such that greater OCS related to higher connectivity. Effects passing false discovery rate correction for multiple comparisons are outlined in black.
Networks: CO=cingulo-opercular, CP=cingulo-parietal, DAN=dorsal attention network, FP=fronto parietal, SN=salience network, VAN=ventral attention network, DMN=default mode network
* p<.05, ** p<.01, *** p<.001
OCS at 1-Year Longitudinal Follow-up:
NIH Toolbox cognition measures and SCS FA, which related to baseline OCS, did not significantly predict change in OCS by 1-year follow-up (p>.05; See Supplement). Of the four RSFC connections significantly associating with baseline OCS, more negative DAN-DMN connectivity predicted worsening OCS at 1-year follow-up (n=3040, b=−0.04, t(2407.61)=−2.23, p=.03, η2p=0.03), above and beyond baseline OCS (b=0.61, t(2946,42)=38.89, p<.001, η2p=0.36). Note that DAN-DMN connectivity did not significantly predict change in CBCL Internalizing, Thought, or ADHD T-scores at 1-year follow-up (|t<1.64, p>.10, η2p<.001), above and beyond corresponding baseline scores.
Discussion
Herein, we leveraged the large, normative ABCD Study dataset to probe obsessive-compulsive symptoms among 9–10-year-old children across the United States. Complex associations with cognitive performance were detected, specifically higher OCS scores related to better cognitive performance, particularly verbal, only when covarying for ADHD. Though we did not detect associations with brain structure, OCS did relate to differences in structural and functional connectivity of cortico-striatal and attentional circuits implicated in the pathophysiology of OCD. Particularly, altered DAN-DMN connectivity may serve as a marker for longitudinal worsening OCS over childhood. Thus, ABCD allowed for unprecedented analysis of continuous associations between OCS and brain structure/function in youth and our findings highlight the potential for ABCD to allow the identification of circuit-based neuroimaging markers of OCS/OCD risk.
Supporting prior work, the CBCL OCS subscale showed promising psychometrics, including internal consistency, high heritability (that can be further refined when greater genetic relatedness information is available from ABCD), convergence with K-SADS OCD symptom count, and potential longitudinal stability (8, 28, 29, 47–50). With this measure, OCS were prevalent among 9–10-year-olds (6.80% with OCS≥5; 25.6% with parent-reported obsessions), consistent with prior estimates of 2–10% of youth exhibiting CBCL OCS≥5 (28, 29). We noted slightly higher OCS scores among boys than girls (~0.2 raw points, ~0.8 T-score). This has been noted in some prior pediatric work (3, 4, 6, 28) though OCS are more prevalent among females in adulthood (79, 80). As expected, OCS were elevated among children with a diagnosis of OCD, as well as other mood and anxiety disorders, similar to prior adult work (79, 80). Importantly, greater information about longitudinal stability, risk, and comorbidity can be determined when later waves of follow-up are available from ABCD. Furthermore, supplemental analyses linked OCS to familial conflict, a potential indicator of impairment and reduced quality of life that is often associated with OCS (1, 2, 81). Future longitudinal ABCD analyses could better parse parental/familial factors as potential causes or consequences of OCS.
Next, we identified associations between OCS and cognitive performance, though complicated by comorbid ADHD symptoms. ADHD severity related to worse performance on NIH Toolbox measures; given strong correlations with OCS, ADHD likely masked/confounded associations between OCS and cognition. Note that this is difficult to parse fully as ADHD-OCS correlations likely reflect both method variance (i.e. both parent-report CBCL) and actual underlying symptom comorbidity. Nonetheless, when covarying (or excluding) ADHD, OCS related to better Total Cognition scores, driven by verbal performance, though effect sizes were small. OCS was unassociated with executive function deficits, inconsistent with prior work typically examining small samples of young adults (n<40 with OCS) (9, 11–13), potentially due to differences in sample characteristics (e.g., undergraduate sampling) or the types of executive functions measured, e.g. more in-depth behavioral assessments of goal-directed behavior (two-step reinforcement learning task) related to compulsivity dimensionally in adults (82). Though the NIH Toolbox measures provide robust and reliable estimates of executive function, scores generally represent a scaled composite of accuracy and reaction time (58–60, 83). This approach may not probe differential cognitive functions with optimal specificity, e.g. examining rule-switch vs. rule-repeat trials on the Card Sort to examine cognitive inflexibility (84). Additionally, as prior young adult OCS studies generally did not control for attentional problems (9, 11–13), our findings may underscore the need for thorough evaluation of potential transdiagnostic symptoms (e.g., inattention due to ADHD vs. due to intrusive obsessions).
Significant associations between OCS and brain structure were not detected. Expected associations with thalamic volumes, given ENIGMA findings in pediatric OCD (26), did not emerge from examination of OCS continuously or OCD diagnosis categorically, perhaps due to differences in MRI acquisition or sampling as ENIGMA meta-/mega-analyzed data collected from varying protocols across 1.5T and 3T studies with different demographic and clinical inclusion/exclusion criteria. In addition, the pediatric participants included in the ENIGMA analyses were older than the 9–10-year-olds in ABCD (age means across studies=11.2–15.7 years). Thus, structural correlates of OCS should indeed be examined in the ABCD sample in future longitudinal waves as the children get older. Expected effects in parietal cortices (27) were also not replicated. Instead, we detected other significant but uncorrected cortical effects of OCS in the ABCD data, e.g. thinner ACC, thicker inferior frontal gyrus. Effects were small in magnitude but could be examined further with vertex-wise analyses and over development when longitudinal MRI data is available.
We detected significant associations between OCS and white matter microstructure, specifically, lower FA in the left superior cortico-striatal (SCS) tract. This finding is consistent with models highlighting CSTC circuits as central to OCS pathophysiology (85, 86) and with our prior work using a streamline-based approach in youth with OCD, suggesting reduced CSTC connectivity between ACC and both the thalamus and putamen (24). DTI studies in pediatric OCD have been sparse and small (generally n<40 patients), but often suggest greater FA in several tracts, including the cingulum (87–89) and corpus callosum (87–91) cf. (92) and greater axial and radial diffusivity in the anterior thalamic radiations (91). Herein, the right SCS, parahippocampal cingulum, anterior thalamic radiation, and fornix showed only uncorrected, small effects towards greater FA with higher OCS. Future work could parse specificity within cortico-striatal tracts given previously suggested dissociable roles of cortico-striatal tracts in balancing habitual and goal-direction action, potentially underlying vulnerability for OCS (93).
Finally, we detected significant associations between OCS and resting-state functional connectivity. Particularly, higher OCS related to less positive within-DAN and less negative DAN-VAN and DAN-DMN connectivity, as well as more positive CO-VAN connectivity. The DAN is a network of frontal and partial regions, including the frontal eye fields and intraparietal parietal sulcus, related to top-down, goal directed processing (94) and to selective attention in children (95). Altered connectivity between DAN and DMN regions has been implicated previously in adult (96) and pediatric OCD (25) and suggests that an imbalance between task positive and negative networks may underlie OCD pathophysiology. Critically, more negative DAN-DMN connectivity predicted worsening OCS at 1-year follow-up, possibly representing a marker of OCS risk; stability and specificity of this can be further probed with later waves of ABCD.
ABCD characterizes a normative, heterogenous sample of children with acquisition harmonized across sites/scanners, all strengthening the generalizability of results. Yet, several limitations and caveats to the current analyses should be noted. First, only one measure of OCS (and OCD) was available from one parent/guardian. Parent-report of symptoms is inherently limited to observable signs (e.g., behaviors or observable expressions of emotion), whereas internal/subjective experience may be better captured by self-report, e.g. experience of intrusive thoughts. Focusing on parent-report from the computerized, self-administered K-SADS may also relate to the relatively high rates of lifetime OCD diagnosis (5.79–13.77% across sites) relative to clinician interviews in epidemiological studies; alternatively, this may reflect some aspect of study sampling. In the future, having more in-depth assessments of OCS from multiple informants would be highly informative, particularly self- and clinician-report. Having multiple measures and informants could also relieve method variance issues and help disentangle OCS and ADHD effects. Second, large sample sizes increase power to detect small effects as statistically significant (e.g. >80% power to detect r>.025 with N=11,876). Note that a partial eta-squared (η2p)~0.01–0.02 is considered small (97)–characterizing the variance explained by a given variable (of variance unexplained by other predictors, converging on R2 in a one-predictor model). For example, OCS related to higher (b=0.06, η2p=0.006), whereas ADHD scores related lower NIH cognition scores (b=−0.14, η2p=0.028), yet both were p<5×10−10. Though both effects were small, we can determine that cognition associated with ADHD at several times the magnitude but in the opposite direction of OCS. Whereas current effect sizes may be hard to detectable in smaller, normative samples, they may emerge more readily in clinically severe samples. Relatedly, though we focused on OCS, certain covariates had larger effects of note, e.g. age strongly related to SCS FA (η2p=0.130 vs. η2p=0.002 for OCS). A large number of covariates/other measures of interest that can potentially influence brain volume, including psychotropic medication status, which we examined herein. Finally, there is a great deal of flexibility in analyzing MRI data (98), with diverse pre-/post-processing options having a potential large effect on the outcome, e.g. (99–101). Global signal regression (GSR) is a particularly contentious issue for RSFC, e.g. (102–104), with potential benefits to confound removal and potential issues in anticorrelation induction. For reproducibility and transparency, we have focused on the released ABCD data which includes GSR. Thus, the current RSFC results must be interpreted in this context while future work could aim to replicate them without GSR.
Overall, we characterized OCS and associated clinical, cognitive, and neural correlates in a large, normative sample of 9–10-year-olds. Results highlight structural and functional connectivity of cortico-striatal and attentional circuits as potential mechanisms of interest and DAN-DMN connectivity as a potential predictor of longitudinal risk for worsening OCS. Future work can expand upon this to provide greater characterization of these circuits over development with forthcoming longitudinal ABCD data and to probe the utility of targeting these circuits in future clinical trials aimed at preventing the development of OCD in youth.
Supplementary Material
Acknowledgements.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from http://dx.doi.org/10.15154/1503209.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures.
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Fullana MA, Mataix-Cols D, Caspi A, Harrington H, Grisham JR, Moffitt TE, et al. (2009): Obsessions and compulsions in the community: prevalence, interference, help-seeking, developmental stability, and co-occurring psychiatric conditions. Am J Psychiatry. 166:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarenga PG, do Rosario MC, Cesar RC, Manfro GG, Moriyama TS, Bloch MH, et al. (2016): Obsessive-compulsive symptoms are associated with psychiatric comorbidities, behavioral and clinical problems: a population-based study of Brazilian school children. Eur Child Adolesc Psychiatry. 25:175–182. [DOI] [PubMed] [Google Scholar]
- 3.Alvarenga PG, Cesar RC, Leckman JF, Moriyama TS, Torres AR, Bloch MH, et al. (2015): Obsessive-compulsive symptom dimensions in a population-based, cross-sectional sample of school-aged children. J Psychiatr Res. 62:108–114. [DOI] [PubMed] [Google Scholar]
- 4.Voltas N, Hernandez-Martinez C, Arija V, Aparicio E, Canals J (2014): A prospective study of paediatric obsessive-compulsive symptomatology in a Spanish community sample. Child Psychiatry Hum Dev. 45:377–387. [DOI] [PubMed] [Google Scholar]
- 5.Flament MF, Whitaker A, Rapoport JL, Davies M, Berg CZ, Kalikow K, et al. (1988): Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry. 27:764–771. [DOI] [PubMed] [Google Scholar]
- 6.Canals J, Hernandez-Martinez C, Cosi S, Voltas N (2012): The epidemiology of obsessive--compulsive disorder in Spanish school children. J Anxiety Disord. 26:746–752. [DOI] [PubMed] [Google Scholar]
- 7.Valleni-Basile LA, Garrison CZ, Jackson KL, Waller JL, McKEOWN RE, Addy CL, et al. (1994): Frequency of obsessive-compulsive disorder in a community sample of young adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 33:782–791. [DOI] [PubMed] [Google Scholar]
- 8.van Grootheest DS, Bartels M, Cath DC, Beekman AT, Hudziak JJ, Boomsma DI (2007): Genetic and environmental contributions underlying stability in childhood obsessive-compulsive behavior. Biol Psychiatry. 61:308–315. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Jang KM, Kim BN (2009): The neuropsychological profile of a subclinical obsessive-compulsive sample. J Int Neuropsychol Soc. 15:286–290. [DOI] [PubMed] [Google Scholar]
- 10.Sternheim L, van der Burgh M, Berkhout LJ, Dekker MR, Ruiter C (2014): Poor cognitive flexibility, and the experience thereof, in a subclinical sample of female students with obsessive-compulsive symptoms. Scand J Psychol. 55:573–577. [DOI] [PubMed] [Google Scholar]
- 11.Mataix-Cols D (2003): Declarative and procedural learning in individuals with subclinical obsessive-compulsive symptoms. J Clin Exp Neuropsychol. 25:830–841. [DOI] [PubMed] [Google Scholar]
- 12.Mataix-Cols D, Junque C, Sanchez-Turet M, Vallejo J, Verger K, Barrios M (1999): Neuropsychological functioning in a subclinical obsessive-compulsive sample. Biol Psychiatry. 45:898–904. [DOI] [PubMed] [Google Scholar]
- 13.Abramovitch A, Shaham N, Levin L, Bar-Hen M, Schweiger A (2015): Response inhibition in a subclinical obsessive-compulsive sample. J Behav Ther Exp Psychiatry. 46:66–71. [DOI] [PubMed] [Google Scholar]
- 14.Hamo N, Abramovitch A, Zohar A (2018): A computerized neuropsychological evaluation of cognitive functions in a subclinical obsessive-compulsive sample. J Behav Ther Exp Psychiatry. 59:142–149. [DOI] [PubMed] [Google Scholar]
- 15.Sunol M, Contreras-Rodriguez O, Macia D, Martinez-Vilavella G, Martinez-Zalacain I, Subira M, et al. (2018): Brain Structural Correlates of Subclinical Obsessive-Compulsive Symptoms in Healthy Children. J Am Acad Child Adolesc Psychiatry. 57:41–47. [DOI] [PubMed] [Google Scholar]
- 16.Montigny C, Castellanos-Ryan N, Whelan R, Banaschewski T, Barker GJ, Buchel C, et al. (2013): A phenotypic structure and neural correlates of compulsive behaviors in adolescents. PLoS One. 8:e80151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena S, Rauch SL (2000): Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatric Clinics of North America. 23:563–586. [DOI] [PubMed] [Google Scholar]
- 18.Milad MR, Rauch SL (2012): Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 16:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gursel DA, Avram M, Sorg C, Brandl F, Koch K (2018): Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 87:151–160. [DOI] [PubMed] [Google Scholar]
- 20.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15:483–506. [DOI] [PubMed] [Google Scholar]
- 21.Abramovitch A, Mittelman A, Henin A, Geller D (2012): Neuroimaging and neuropsychological findings in pediatric obsessive-compulsive disorder: a review and developmental considerations. Neuropsychiatry. 2:313–329. [Google Scholar]
- 22.Liu Y, Bilek EL, Fitzgerald KD (2016): Developmental Neuroimaging in Pediatric Obsessive-Compulsive Disorder. Current Behavioral Neuroscience Reports. 3:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brem S, Hauser TU, Iannaccone R, Brandeis D, Drechsler R, Walitza S (2012): Neuroimaging of cognitive brain function in paediatric obsessive compulsive disorder: a review of literature and preliminary meta-analysis. J Neural Transm (Vienna). 119:1425–1448. [DOI] [PubMed] [Google Scholar]
- 24.Pagliaccio D, Cha J, He X, Cyr M, Yanes-Lukin P, Goldberg P, et al. (2019): Structural neural markers of response to cognitive behavioral therapy in pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cyr M, Pagliaccio D, Yanes-Lukin P, Fontaine M, Rynn MA, Marsh R (2020): Altered network connectivity predicts response to cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. (2017): Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta- and Mega-Analysis. Am J Psychiatry. 174:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boedhoe PS, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. (2017): Cortical Abnormalities Associated With Pediatric and Adult Obsessive-Compulsive Disorder: Findings From the ENIGMA Obsessive-Compulsive Disorder Working Group. Am J Psychiatry.appiajp201717050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudziak JJ, Althoff RR, Stanger C, van Beijsterveldt CE, Nelson EC, Hanna GL, et al. (2006): The Obsessive Compulsive Scale of the Child Behavior Checklist predicts obsessive-compulsive disorder: a receiver operating characteristic curve analysis. J Child Psychol Psychiatry. 47:160–166. [DOI] [PubMed] [Google Scholar]
- 29.Saad LO, do Rosario MC, Cesar RC, Batistuzzo MC, Hoexter MQ, Manfro GG, et al. (2017): The Child Behavior Checklist-Obsessive-Compulsive Subscale Detects Severe Psychopathology and Behavioral Problems Among School-Aged Children. J Child Adolesc Psychopharmacol. 27:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. (2016): Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-analysis. JAMA Psychiatry. 73:815–825. [DOI] [PubMed] [Google Scholar]
- 31.Norman LJ, Carlisi CO, Christakou A, Murphy CM, Chantiluke K, Giampietro V, et al. (2018): Fronto-striatal dysfunction during decision-making in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brem S, Grunblatt E, Drechsler R, Riederer P, Walitza S (2014): The neurobiological link between OCD and ADHD. Atten Defic Hyperact Disord. 6:175–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vloet TD, Marx I, Kahraman-Lanzerath B, Zepf FD, Herpertz-Dahlmann B, Konrad K (2010): Neurocognitive performance in children with ADHD and OCD. J Abnorm Child Psychol. 38:961–969. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. (2018): The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci. 32:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, et al. (2018): Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci. 32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. (2018): Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci. 32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen AC, Crockett L, Richards M, Boxer A (1988): A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of youth and adolescence. 17:117–133. [DOI] [PubMed] [Google Scholar]
- 38.Stattin H, Kerr M (2000): Parental knowledge: A reinterpretation. Child Development. 71:1072–1085. [DOI] [PubMed] [Google Scholar]
- 39.Karoly HC, Callahan T, Schmiege SJ, Feldstein Ewing SW (2016): Evaluating the Hispanic paradox in the context of adolescent risky sexual behavior: The role of parent monitoring. Journal of pediatric psychology. 41:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schludermann E, Schludermann S (1988): Children’s Report on Parent Behavior (CRPBI-108, CRPBI-30) for older children and adolescents. Winnipeg, MB, Canada: University of Manitoba. [Google Scholar]
- 41.Schaefer ES (1965): A configurational analysis of children’s reports of parent behavior. Journal of consulting psychology. 29:552. [DOI] [PubMed] [Google Scholar]
- 42.Moos RH (1994): Family environment scale manual: Development, applications, research. Consulting Psychologists Press. [Google Scholar]
- 43.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman J, Townsend LD, Kobak K (2017): The Computerized Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS): Development and Administration Guidelines. 64th Annual Meeting: AACAP. [Google Scholar]
- 45.Townsend L, Kobak K, Kearney C, Milham M, Andreotti C, Escalera J, et al. (2020): Development of Three Web-Based Computerized Versions of the Kiddie Schedule for Affective Disorders and Schizophrenia Child Psychiatric Diagnostic Interview: Preliminary Validity Data. J Am Acad Child Adolesc Psychiatry. 59:309–325. [DOI] [PubMed] [Google Scholar]
- 46.Achenbach Rescorla (2001): Manual for the Achenbach system of empirically based assessment school-age forms profiles. Burlington, VT: Aseba. [Google Scholar]
- 47.Nelson EC, Hanna GL, Hudziak JJ, Botteron KN, Heath AC, Todd RD (2001): Obsessive-compulsive scale of the child behavior checklist: specificity, sensitivity, and predictive power. Pediatrics. 108:E14. [DOI] [PubMed] [Google Scholar]
- 48.Althoff RR, Rettew DC, Boomsma DI, Hudziak JJ (2009): Latent class analysis of the Child Behavior Checklist Obsessive-Compulsive Scale. Compr Psychiatry. 50:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geller DA, Doyle R, Shaw D, Mullin B, Coffey B, Petty C, et al. (2006): A quick and reliable screening measure for OCD in youth: reliability and validity of the obsessive compulsive scale of the Child Behavior Checklist. Compr Psychiatry. 47:234–240. [DOI] [PubMed] [Google Scholar]
- 50.Andersen PA, Bilenberg N (2012): Comparison of Child Behavior Checklist subscales in screening for obsessive-compulsive disorder. Dan Med J. 59:A4523. [PubMed] [Google Scholar]
- 51.Storch EA, Murphy TK, Bagner DM, Johns NB, Baumeister AL, Goodman WK, et al. (2006): Reliability and validity of the Child Behavior Checklist Obsessive-Compulsive Scale. J Anxiety Disord. 20:473–485. [DOI] [PubMed] [Google Scholar]
- 52.Ivarsson T, Larsson B (2008): The Obsessive-Compulsive Symptom (OCS) scale of the Child Behavior Checklist: A comparison between Swedish children with Obsessive-Compulsive Disorder from a specialized unit, regular outpatients and a school sample. Journal of anxiety disorders. 22:1172–1179. [DOI] [PubMed] [Google Scholar]
- 53.Rosseel Y (2012): Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). J Stat Softw. 48:1–36. [Google Scholar]
- 54.Rosseel Y, Oberski D, Byrnes J, Vanbrabant L, Savalei V, Merkle E, et al. (2017): Package ‘lavaan’. Retrieved June. 17:2017. [Google Scholar]
- 55.Revelle W (2014): psych: Procedures for psychological, psychometric, and personality research. Northwestern University, Evanston, Illinois. 165:1–10. [Google Scholar]
- 56.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. (2018): pROC: display and analyze ROC curves. R package version. 1. [Google Scholar]
- 57.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. (2011): pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC bioinformatics. 12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. (2013): Cognition assessment using the NIH Toolbox. Neurology. 80:S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, et al. (2013): VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 78:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slotkin J, Nowinski C, Hays R, Beaumont J, Griffith J, Magasi S, et al. (2012): NIH Toolbox scoring and interpretation guide. Washington (DC): National Institutes of Health.6–7. [Google Scholar]
- 61.Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. (2018): The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagler DJ, Hatton SN, Makowski C, Cornejo MD, Fair DA, Dick AS, et al. (2018): Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dale AM, Fischl B, Sereno MI (1999): Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- 64.Fischl B, Sereno MI, Tootell RB, Dale AM (1999): High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- 66.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex. 14:11–22. [DOI] [PubMed] [Google Scholar]
- 67.Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hagler DJ, Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, et al. (2009): Automated white‐matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Human brain mapping. 30:1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White NS, Leergaard TB, D’Arceuil H, Bjaalie JG, Dale AM (2013): Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Human brain mapping. 34:327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE (2016): Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 26:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Team RC (2015): R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 72.Pagliaccio D (2020): scipub: Summarize Data for Scientific Publication.
- 73.Blangero J, Almasy L (1996): Solar: sequential oligogenic linkage analysis routines. Population Genetics Laboratory Technical Report. 6. [Google Scholar]
- 74.Bates D, Mächler M, Bolker B, Walker SJapa (2014): Fitting linear mixed-effects models using lme4. [Google Scholar]
- 75.Pagliaccio D, Alqueza KL, Marsh R, Auerbach RP (2019): Brain Volume Abnormalities in Youth at High Risk for Depression: Adolescent Brain and Cognitive Development Study. J Am Acad Child Adolesc Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lüdecke D, Lüdecke MD (2017): Package ‘sjstats’.
- 77.Ho D, Imai K, King G, Stuart E, Whitworth A (2018): Package ‘MatchIt’. Version. [Google Scholar]
- 78.Stuart EA, King G, Imai K, Ho D (2011): MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. [Google Scholar]
- 79.Fullana MA, Vilagut G, Rojas-Farreras S, Mataix-Cols D, de Graaf R, Demyttenaere K, et al. (2010): Obsessive-compulsive symptom dimensions in the general population: results from an epidemiological study in six European countries. J Affect Disord. 124:291–299. [DOI] [PubMed] [Google Scholar]
- 80.Grabe HJ, Meyer C, Hapke U, Rumpf HJ, Freyberger HJ, Dilling H, et al. (2001): Lifetime-comorbidity of obsessive-compulsive disorder and subclinical obsessive-compulsive disorder in Northern Germany. Eur Arch Psychiatry Clin Neurosci. 251:130–135. [DOI] [PubMed] [Google Scholar]
- 81.Grabe HJ, Meyer C, Hapke U, Rumpf HJ, Freyberger HJ, Dilling H, et al. (2000): Prevalence, quality of life and psychosocial function in obsessive-compulsive disorder and subclinical obsessive-compulsive disorder in northern Germany. Eur Arch Psychiatry Clin Neurosci. 250:262–268. [DOI] [PubMed] [Google Scholar]
- 82.Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND (2016): Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Conway KP, et al. (2014): NIH Toolbox Cognition Battery (CB): validation of executive function measures in adults. J Int Neuropsychol Soc. 20:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gruner P, Pittenger C (2017): Cognitive inflexibility in Obsessive-Compulsive Disorder. Neuroscience. 345:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graybiel AM, Rauch SL (2000): Toward a neurobiology of obsessive-compulsive disorder. Neuron. 28:343–347. [DOI] [PubMed] [Google Scholar]
- 86.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET (2008): Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience & Biobehavioral Reviews. 32:525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gruner P, Vo A, Ikuta T, Mahon K, Peters BD, Malhotra AK, et al. (2012): White matter abnormalities in pediatric obsessive-compulsive disorder. Neuropsychopharmacology. 37:2730–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarei M, Mataix-Cols D, Heyman I, Hough M, Doherty J, Burge L, et al. (2011): Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 70:1083–1090. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald KD, Liu Y, Reamer EN, Taylor SF, Welsh RC (2014): Atypical frontal-striatal-thalamic circuit white matter development in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 53:1225–1233, 1233 e1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silk T, Chen J, Seal M, Vance A (2013): White matter abnormalities in pediatric obsessive-compulsive disorder. Psychiatry Res. 213:154–160. [DOI] [PubMed] [Google Scholar]
- 91.Jayarajan RN, Venkatasubramanian G, Viswanath B, Janardhan Reddy YC, Srinath S, Vasudev MK, et al. (2012): White matter abnormalities in children and adolescents with obsessive-compulsive disorder: a diffusion tensor imaging study. Depress Anxiety. 29:780–788. [DOI] [PubMed] [Google Scholar]
- 92.Rosso IM, Britton JC, Stewart SE, Papamiditriou G, Killgore WDS, Makris N, et al. (2013): Brain White Matter Integrity and Association with Age at Onset in Pediatric Obsessive-Compulsive Disorder. Biological Psychiatry. 73:75s–75s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR (2012): Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 32:12066–12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Corbetta M, Shulman GL (2002): Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3:201–215. [DOI] [PubMed] [Google Scholar]
- 95.Rohr CS, Vinette SA, Parsons KAL, Cho IYK, Dimond D, Benischek A, et al. (2017): Functional Connectivity of the Dorsal Attention Network Predicts Selective Attention in 4–7 year-old Girls. Cereb Cortex. 27:4350–4360. [DOI] [PubMed] [Google Scholar]
- 96.Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C (2014): Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 205:376–382. [DOI] [PubMed] [Google Scholar]
- 97.Miles J, Shevlin M (2001): Applying regression and correlation: A guide for students and researchers. Sage. [Google Scholar]
- 98.Carp J (2012): On the plurality of (methodological) worlds: estimating the analytic flexibility of FMRI experiments. Frontiers in neuroscience. 6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aurich NK, Alves Filho JO, Marques da Silva AM, Franco AR (2015): Evaluating the reliability of different preprocessing steps to estimate graph theoretical measures in resting state fMRI data. Frontiers in neuroscience. 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009): Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 47:1408–1416. [DOI] [PubMed] [Google Scholar]
- 101.Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. (2017): Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 154:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chai XJ, Castañón AN, Öngür D, Whitfield-Gabrieli S (2012): Anticorrelations in resting state networks without global signal regression. Neuroimage. 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murphy K, Fox MD (2017): Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 154:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


