Abstract
Evidence for the effectiveness and safety of the third-generation beta-blockers other than atenolol in hypertension remains scarce. We assessed the effectiveness and safety of beta-blockers as first-line treatment for hypertension using three databases in the United States: two administrative claim databases and one electronic health record-based database from 2001 to 2018. In each database, comparative effectiveness of beta-blockers for the risks of acute myocardial infarction, stroke, and hospitalization for heart failure was assessed, using large-scale propensity adjustment and empirical calibration. Estimates were combined across databases using random-effects meta-analyses. Overall, 118,133 and 267,891 patients initiated third-generation beta-blockers (carvedilol and nebivolol) or atenolol, respectively. The pooled hazard ratios of acute myocardial infarction, stroke, hospitalization for heart failure, and most metabolic complications were not different between the third-generation beta-blockers versus atenolol after propensity score matching and empirical calibration (hazard ratio 1.07, 95% CI 0.74 to 1.55 for acute myocardial infarction; hazard ratio 1.06, 95% CI 0.87 to 1.31 for stroke; hazard ratio 1.46, 95% CI 0.99 to 2.24 for hospitalized heart failure). Third-generation beta-blockers were associated with significantly higher risk of stroke than angiotensin-converting enzyme inhibitors (hazard ratio 1.29, 95% CI 1.03 to 1.72), and thiazide diuretics (hazard ratio 1.56, 95% CI 1.17 to 2.20). In conclusion, this study found many patients with first-line beta-blocker monotherapy for hypertension and no statistically significant differences in the effectiveness and safety comparing atenolol with third-generation beta-blockers. Patients on third-generation beta-blockers had a higher risk of stroke than those on angiotensin-converting enzyme inhibitors and thiazide diuretics.
Keywords: Hypertension, Blood pressure, Antihypertensive agents, Beta-adrenergic receptor blocker, Stroke
INTRODUCTION
Beta-blockers have been a mainstay for anti-hypertensive treatment over the past half a century.1 Historically, beta-blockers had been recommended as one of the first-line treatment options for primary hypertension by the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC) from its first report in 1977 through its seventh report in 2003.2,3 However, Messerli et al4,5 raised concerns about this preference due to paucity of the evidence for beta-blockers to reduce morbidity or mortality in patients with uncomplicated hypertension. Several meta-analyses have concluded that efficacy of beta-blockers in hypertension is inferior compared with other classes of anti-hypertensive drugs: angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blocker (ARBs), calcium-channel blockers (CCBs), and thiazide or thiazide-like diuretics (TDs).6–10 As such, the American College of Cardiology/American Heart Association (ACC/AHA) and Japanese Society of Hypertension (JSH) guidelines no longer recommend beta-blockers as first-line therapy for hypertensive patients without compelling indications.11,12
One explanation for the inferior efficacy of beta blockers compared with other classes of first line anti-hypertensive drugs has been that three-quarters of randomized clinical trials employed by previous meta-analyses used second-generation beta blockers without vasodilating effect, mostly atenolol.7,10 Beta-blockers are not a homogeneous class, differing both in their β1/β2-receptor selectivity and vasodilatory effect.13 Theoretically, third-generation beta-blockers with vasodilating properties, such as nebivolol and carvedilol, may have more favorable efficacy compared with second-generation beta-blockers in reducing cardiovascular events.14,15 Nonetheless, evidence for the effectiveness and safety of third-generation beta-blockers for treating hypertension in comparison with atenolol and with other classes of anti-hypertensive medications is lacking, especially regarding clinical outcomes.
We developed the previously reported methods for large-scale evidence generation and evaluation across a network of databases for hypertension (LEGEND-HTN) study to provide comprehensive evidence for comparative effectiveness and safety among first-line anti-hypertensives.16–19 Here, we investigated the effectiveness and safety of third-generation beta-blockers as first-line treatment for hypertension compared with atenolol and other anti-hypertensive medications, including ACEIs, ARBs, CCBs, and TDs.
METHODS
Data Source and Overall Study Design
LEGEND-HTN is a distributed network study across the Observational Health Data Science and Informatics (OHDSI) network.20,21 Details were described in previous papers.16–18 All materials have been made publicly available at the (https://github.com/ohdsi/Legend/). All LEGEND-HTN study outcomes are available at a dedicated website (https://data.ohdsi.org/LegendBasicViewer/).
We included three databases that had at least 2500 patients with exposures to atenolol, nebivolol, and carvedilol who met the eligibility criteria described below (Methods in the Data Supplement). The included databases were the MarketScan Commercial Claims and Encounters database (CCAE, US employer-based private payer; 2001 to 2018), the deidentified Optum Clinformatics Data Mart Database (Optum, US private payer; 2001 to 2017), and Optum De-Identified Electronic Health Record Dataset (PanTher; US deidentified electronic health record dataset; 2007 to 2017).18 All of these databases have been converted into the Observational Medical Outcomes Partnership common data model version 5 with standardized data schema and semantics.22 This research was approved by the Institutional Review Board of Ajou University Hospital (IRB number: AJIRB-MED-EXP-17–054). Because the databases were de-identified, informed consent was not required.
Within each database, we performed a retrospective comparative new-user cohort study to generate propensity-score-adjusted and systematic-error-calibrated hazard ratios (HRs) for all pairwise comparison between the first-line use of anti-hypertensive drugs, including beta-blockers, against a panel of 55 health outcomes.23,24
Exposure and Outcomes
We identified patients who initiated monotherapy for hypertension with any medication within the five drug classes: ACEI, ARB, dihydropyridine CCB (dCCB), TD, and beta-blocker. The index date was defined as the first observed prescription for one of these medications in an individual with a prior or concurrent diagnosis of hypertension. We excluded patients who initiated an anti-hypertensive drug other than the originally prescribed drug within 7 days of the index date. If a patient initiated a different anti-hypertensive drug after 7 days, then the patient remained in the cohort. To address left censoring, we required patients to have at least 1-year of continuous observation in the database before the index date. We compared atenolol versus the third-generation beta-blockers, carvedilol and nebivolol, and we compared atenolol and third-generation beta-blockers versus drugs in other recommended classes of antihypertensives (ACEIs, ARBs, dCCBs, and TDs). Because most comparisons including carvedilol failed to pass the diagnostics described below, we additionally present results from the comparison of nebivolol versus atenolol or other classes of anti-hypertensives. Furthermore, the effectiveness of metoprolol was investigated compared with atenolol, carvedilol, and nebivolol.
The prespecified primary outcomes were acute myocardial infarction, stroke, and hospitalization for heart failure based on the 2017 ACC/AHA Guidelines systematic review.25 Six secondary effectiveness outcomes include ischemic stroke, hemorrhagic stroke, heart failure, sudden cardiac death, sudden cardiac death, and cardiovascular event (a composite endpoint of three primary outcomes and sudden cardiac death). The other 46 outcomes are safety outcomes, including angioedema, cardiac arrhythmia, syncope, fall, impotence, and all-cause mortality. All of these outcomes were constructed based on previously published phenotypes.16
For each outcome, we excluded patients with a history of the outcome before the date of treatment initiation from the cohort. The on-treatment outcome risk window started on the first day after treatment initiation and ended at the first cessation of continuous exposure of the initiating drug allowing a 30-day gap. Additionally, we performed an intention-to-treat (ITT) analysis, in which outcome risk windows extended to the end of the medical records or observation. We reported on-treatment results in this article unless otherwise specified, consistent with a priori specification of primary analysis and previous reports.
Statistical analysis
The detailed process generating systematic-error calibrated and PS-adjusted HRs was described previously.16,17,19 We built large-scale PS models to adjust for differences in baseline characteristics between each treatment pair through regularized regression based on a data-driven process.26 The HRs were estimated using Cox proportional hazard models after PS matching or stratification for each pair of anti-hypertensive monotherapies in each database. The results after PS matching rather than PS stratification are reported in the main article unless otherwise specified. We judged sufficient balance when every absolute standardized mean of difference was less than 0.1 after PS matching or stratification. We defined empirical equipoise as when the majority of patients in each comparison pair had preference scores (a transformation of PS adjusted for prevalence differences between populations) from 0.25 to 0.75.17,27
HRs were combined through a random-effect meta-analysis to produce a composite effect estimate of comparison.28 For example, the HRs of carvedilol versus atenolol and nebivolol versus atenolol from each database were aggregated for the third-generation beta-blockers versus atenolol comparison. We included only results from comparisons that passed the diagnostics, including sufficient balance and empirical equipoise in the meta-analysis. To measure residual bias due to systematic sources or unmeasured confounders, we employed 76 negative control outcomes, such as ingrowing nail and nicotine dependence, using a data-rich algorithm;29 these outcomes are not considered to be caused by anti-hypertensive treatments (i.e. outcomes where the true HR is assumed 1). Then, HRs were empirically calibrated based on the results from negative control and synthetic positive control outcomes, without correcting for multiplicity.30 A P value of less than 0.05 was considered statistically significant for all two-sided tests. Since this study did not conduct a correction for multiplicity other than empirical calibration, results for secondary outcomes should be interpreted as exploratory owing to the potential for type I error.
Post hoc analyses
Because of the potential confounding effect of baseline cardiovascular comorbidities and blood pressure, we performed two post hoc sensitivity analyses for comparison between third-generation beta-blockers and atenolol. First, we estimated the hazard ratios after excluding patients with previous heart failure, ischemic heart disease, or atrial fibrillation on three databases. Second, we repeated the analysis on the PanTher, the only database containing blood pressure information, to include last systolic and diastolic blood pressure within 1 year from the index date in the propensity score model using cubic splines.
RESULTS
The effectiveness of third-generation beta-blockers compared to atenolol
From CCAE, 45,262 and 92,155 individuals received the third-generation beta-blockers and atenolol as first-line therapy for hypertension, respectively; from Optum 31,618 and 82,127, received third-generation beta-blockers and atenolol, respectively; from PanTher 41,253 and 93,609 received third-generation beta-blockers and atenolol, respectively. The baseline characteristics of carvedilol and nebivolol versus atenolol are provided in Table S1–2. The maximum standardized mean difference of over 20,000 covariates was less than 0.1 from all three databases after PS matching (Figure S1–2). The preference score distribution is shown in Figure S3–4. The result from Optum comparing atenolol and carvedilol was excluded in the meta-analysis because empirical equipoise was not achieved. Before and after calibration, nominal 95% CIs covered in 71 of 74 (95.9%) negative control estimates across comparisons between third-generation beta-blockers and atenolol (Figure S5).
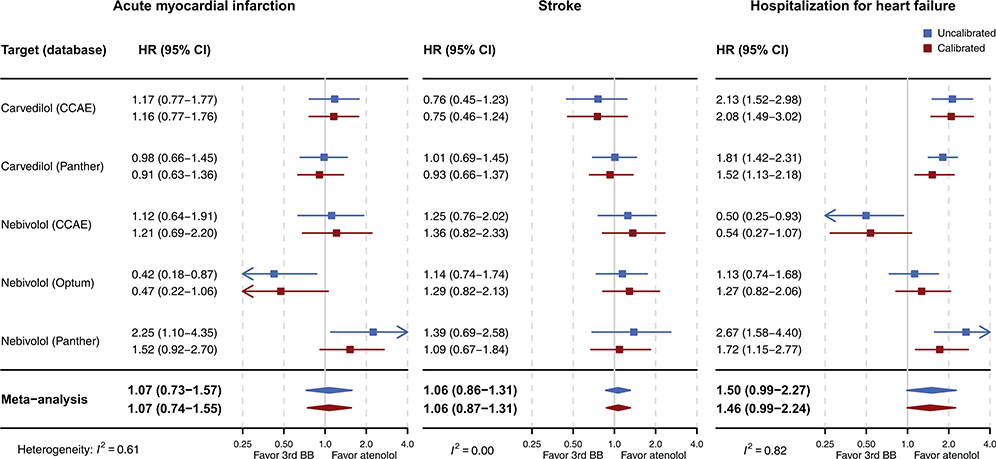
Table 1 describes the number of patients in each group with their follow-up durations, incidence rates of the events, and absolute risk differences for the three primary outcomes after PS matching. The Kaplan–Meier plots for three primary outcomes from the three databases are depicted in Figure S6–8. When comparing third-generation beta-blockers to atenolol, the aggregated risk of acute myocardial infarction (uncalibrated HR [uHR] 1.07 [95% CI 0.73–1.57]; calibrated HR [cHR] 1.07, 95% CI 0.74–1.55), stroke (uHR 1.06 [95% CI 0.86–1.31]; cHR 1.06 [95% CI 0.87–1.31]), and hospitalization for heart failure (uHR 1.50 [95% CI 0.99–2.27]; cHR 1.46 [95% CI 0.99–2.24]) were not statistically significantly different after PS matching (Figure 1). The findings from meta-analyses in other analytic setting (PS stratification or ITT) and the meta-analyses, including results without sufficient balance or empirical equipoise, were consistent, which did not favor third-generation beta-blockers over atenolol (Figures S9–10 in the Data Supplement).
Table 1.
Cohort size, follow-up duration, incidence rate, and the absolute risk difference between the third-generation beta-blockers and atenolol (Propensity score matching, on-treatment)
| Target drug | Outcome / database | Patients, n* | Follow-up duration, PYs (year per person) | Incidence rate, /1000 PYs | ARD,/1000 PYs | |||
|---|---|---|---|---|---|---|---|---|
| Target drug | Atenolol | Target drug | Atenolol | Target drug | Atenolol | |||
| Carvedilol | Acute myocardial infarction | |||||||
| CCAE | 14,003 | 87,844 | 8,658 (0.6) | 69,057 (0.8) | 5.2 | 3.2 | 2.0 | |
| Optum† | 11,981 | 80,622 | 8,596 (0.7) | 69,535 (0.9) | 14.1 | 6.8 | 7.3 | |
| PanTher | 22,246 | 92,3 32 | 8,016 (0.4) | 35,031 (0.4) | 8.6 | 4.0 | 4.6 | |
| Stroke | ||||||||
| CCAE | 14,191 | 87,900 | 8,857 (0.6) | 69,183 (0.8) | 4.0 | 2.6 | 1.4 | |
| Optum† | 12,134 | 80,630 | 8,789 (0.7) | 69,368 (0.9) | 12.4 | 7.9 | 4.5 | |
| PanTher | 22,286 | 92,142 | 8,004 (0.4) | 34,953 (0.4) | 9.4 | 5.2 | 4.2 | |
| Hospitalization for heart failure | ||||||||
| CCAE | 14,198 | 88,242 | 8,846 (0.6) | 69,504 (0.8) | 12.7 | 2.8 | 9.9 | |
| Optum† | 11,915 | 80,624 | 8,496 (0.7) | 69,388 (0.9) | 35.5 | 11.1 | 24.4 | |
| PanTher | 22,135 | 92,209 | 7,874 (0.4) | 34,924 (0.4) | 32.5 | 8.2 | 24.3 | |
| Nebivolol | Acute myocardial infarction | |||||||
| CCAE | 22,314 | 49,736 | 14,639 (0.7) | 33,252 (0.7) | 2.5 | 2.9 | 0.4 | |
| Optum | 11,579 | 35,527 | 7,896 (0.7) | 27,286 (0.8) | 2.0 | 5.9 | 3.9 | |
| PanTher | 17,270 | 86,235 | 2,322 (0.1) | 33,054 (0.4) | 6.0 | 3.8 | 2.2 | |
| Stroke | ||||||||
| CCAE | 22,270 | 49,674 | 14,605 (0.7) | 33,205 (0.7) | 3.0 | 3.0 | 0.0 | |
| Optum | 11,560 | 35,311 | 7,870 (0.7) | 26,972 (0.8) | 5.5 | 9.1 | −3.7 | |
| PanTher | 17,279 | 86,252 | 2,320 (0.1) | 33,048 (0.4) | 6.0 | 5.2 | 0.8 | |
| Hospitalization for heart failure | ||||||||
| CCAE | 22,357 | 49,878 | 14,699 (0.7) | 33,401 (0.7) | 1.4 | 2.8 | −1.4 | |
| Optum | 11,574 | 35,470 | 7,891 (0.7) | 27,136 (0.8) | 5.5 | 11.2 | −5.7 | |
| PanTher | 17,268 | 86,307 | 2,322 (0.1) | 33,014 (0.4) | 11.2 | 7.9 | 3.3 | |
Number of patients exposed varies by outcome owing to differences in whether databases had hospitalization information and outcome-specific preexposure exclusions.
The results from Optum comparing carvedilol and atenolol were not included in the meta-analysis owing to lack of empirical equipoise
Abbreviations: PY, person-year; ARD, absolute rate difference; CCAE, Truven MarketScan Commercial Claims and Encounters; Optum, Optum ClinFormatics; PanTher, Optum de-identified Electronic Health Record Dataset
Figure 1.
The meta-analytic HR estimates and their 95% CIs comparing the relative risk of acute myocardial infarction, stroke, and hospitalization for heart failure between third-generation beta-blockers (carvedilol and nebivolol) versus atenolol (PS matching, on-treatment)
Abbreviations: HR, hazard ratio; CI, confidence interval; 3rd BB, third-generation beta-blocker; PS, propensity score; CCAE, Truven MarketScan Commercial Claims and Encounters; Optum, Optum ClinFormatics; Panther, Optum de-identified Electronic Health Record Dataset
A post hoc analysis also revealed that there were no statistically significant differences in primary outcomes risks between the third-generation beta-blockers and atenolol in patients without previous history of heart failure, ischemic heart disease, or atrial fibrillation (Table S3). Furthermore, another post hoc analysis including baseline blood pressure in the propensity model showed consistent results, of which confidence intervals completely overlap the confidence intervals estimated by the original analysis in the PanTher database (Table S4).
Secondary outcomes and safety of third-generation beta-blockers compared to atenolol
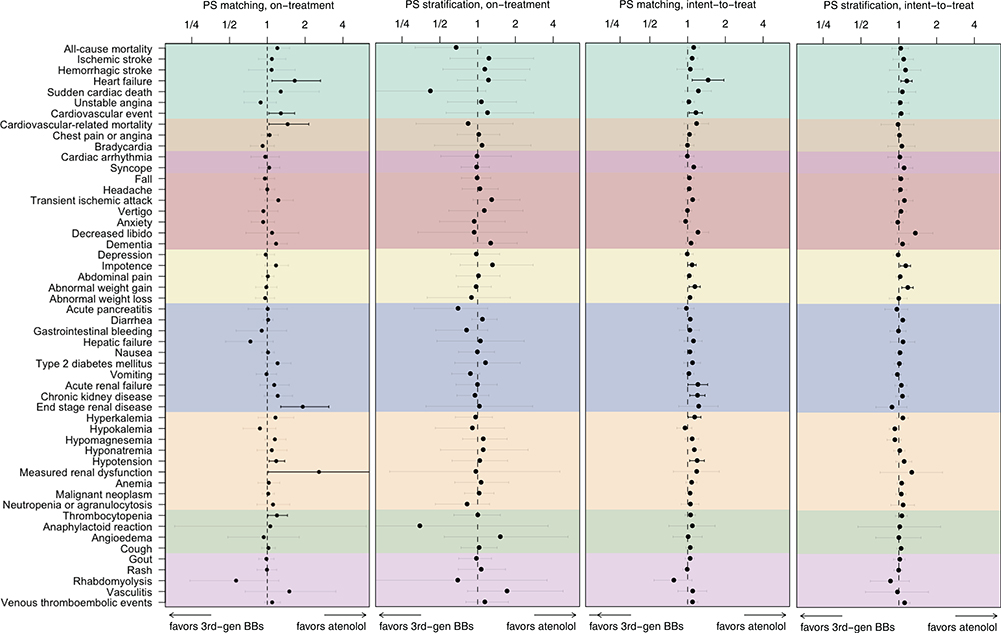
Figure 2 shows the differences in risks for the six secondary outcomes and safety profiles between third-generation beta-blockers versus atenolol. In the primary analysis (PS matching, on-treatment), there were significantly higher risks for the third-generation beta-blocker group compared with atenolol for heart failure (cHR 1.65 [95% CI 1.09–2.65]), cardiovascular-related mortality (cHR 1.45 [95% CI 1.03–2.14]), end-stage renal disease (cHR 1.91 [95% CI 1.28–3.09]), and measured renal dysfunction (cHR 2.58 [95% CI 1.02–7.25]).
Figure 2.
Meta-analytic safety profiles comparing third-generation beta-blockers (carvedilol and nebivolol) to atenolol across six secondary outcomes (all-cause mortality, ischemic stroke, hemorrhagic stroke, heart failure, sudden cardiac death, unstable angina, and cardiovascular event) and 46 safety outcomes listed on product labels.
Points and lines identify calibrated HR estimates with their 95% CIs, respectively. Outcomes in grey signify that the CI covers an HR of 1 (null hypothesis of no differential risk). The cardiovascular event outcome includes acute myocardial infarction, stroke, hospitalization for heart failure, and sudden cardiac death.
Abbreviations: HR, hazard ratio; CI, confidence interval; PS, propensity score; 3rd-gen BBs, third-generation beta-blockers
Other safety profiles including all-cause mortality (cHR 1.20 [95% CI 0.98–1.51]), cardiac arrhythmia (cHR 0.96 [95% CI 0.74–1.25]), bradycardia (cHR 0.92 [95% CI 0.73–1.13]), syncope (cHR 1.03 [95% CI 0.86–1.26]), depression (cHR 0.97 [95% CI 0.82–1.14]), impotence (cHR 1.17 [95% CI 0.95–1.17]), and type 2 diabetes mellitus (cHR 1.21 [95% CI 0.97–1.54]), were not significantly different between third-generation beta-blockers and atenolol after PS matching. There were no consistent differences in risks of secondary outcomes or safety outcomes between third-generation beta-blockers and atenolol across different statistical adjustment and time-at-risk settings.
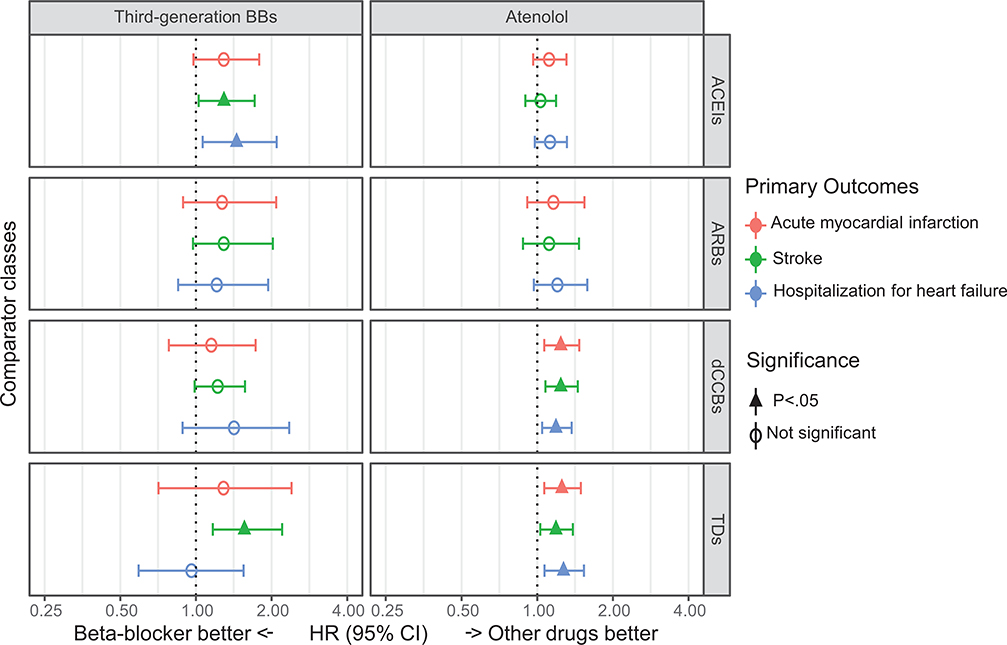
Comparison between beta-blockers and other recommended drug classes
The comprehensive comparative effectiveness of beta-blockers and other anti-hypertensive drugs is shown in Figure 3. After PS matching and empirical calibration, there were no significant differences in risks for primary outcomes of atenolol versus ACEIs or ARBs (all calibrated CIs include 1). Compared with dCCBs, atenolol was associated with higher risk of acute myocardial infarction (cHR 1.24 [95% CI 1.07–1.47]), stroke (cHR 1.24 [95% CI 1.08–1.45]), and hospitalization for heart failure (cHR 1.19 [95% CI 1.05–1.37]). Compared with TDs, atenolol was associated with significantly higher risks of acute myocardial infarction (cHR 1.26 [95% CI 1.07–1.49]), stroke (cHR 1.19 [95% CI 1.03–1.39]), and hospitalization for heart failure (cHR 1.27 [95% CI 1.07–1.54]).
Figure 3.
The meta-analytic calibrated HR estimates and their 95% CIs comparing the relative risk of cardiovascular diseases in new users of anti-hypertensive drugs.
Abbreviations: HR, hazard ratio; CI, confidence interval; BBs, beta-blockers; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blocker; dCCBs, dihydropyridine calcium-channel blocker; TDs, thiazide, or thiazide-like diuretics
Third-generation beta-blockers were associated with a significantly higher risk of stroke than ACEIs (cHR 1.29 [95% CI 1.03–1.72]) and thiazide-diuretics (cHR 1.56 [95% CI 1.17–2.20]). Most risk estimates were higher in third-generation beta-blockers compared with other classes without statistical significance. The detailed meta-analyses are depicted in Figure S11–12 in the Data Supplement.
Comparative effectiveness of metoprolol versus atenolol, carvedilol, and nebivolol
The risk of hospitalization for heart failure was higher in patients initiating metoprolol than patients initiating atenolol (cHR 1.30 [95% CI 1.15–1.50]), while the risks of acute myocardial infarction (cHR 1.01 [95% CI 0.86–1.20]) and stroke (cHR 1.06 [95% CI 0.92–1.23]) were not significantly different between metoprolol and atenolol (Figure S13a). Compared with carvedilol, metoprolol use was associated with lower risk of hospitalization for heart failure (cHR 0.80 [95% CI 0.71–0.89]; Figure S13b). The risks for primary outcomes were not significantly different between metoprolol and nebivolol (all calibrated CIs include 1; Figure S13c).
Comparison between nebivolol and atenolol or other recommended drug classes
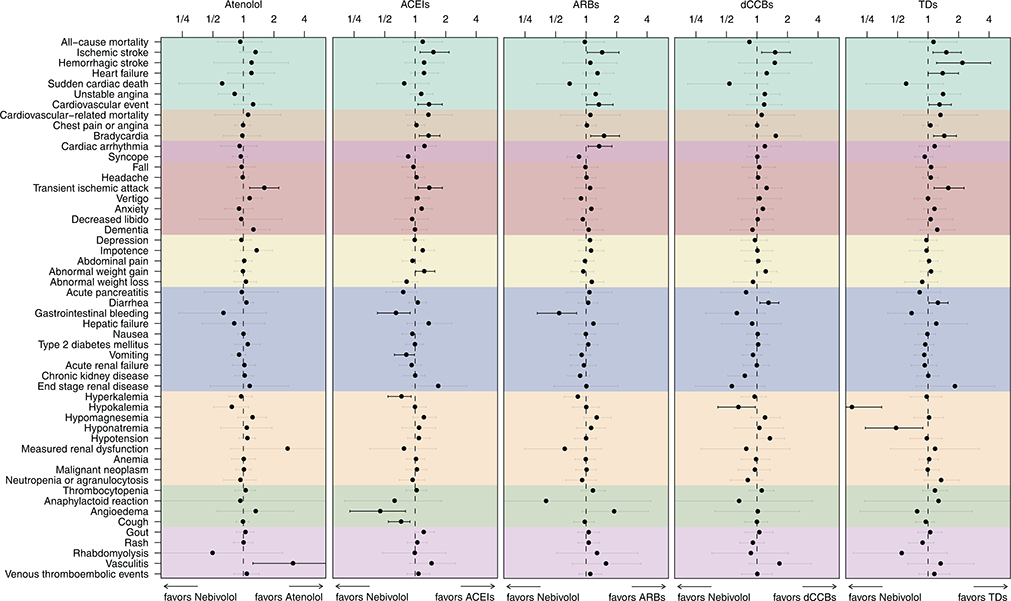
The risks for primary outcomes were not significantly different between nebivolol and atenolol (all calibrated CIs include 1; Table S5). Nebivolol was associated with a higher risk for stroke compared to ACEIs (cHR 1.39 [95% CI 1.04–1.98]), dCCBs (cHR 1.40 [95% CI 1.05–1.97]), and TDs (cHR 1.56 [95% CI 1.17–2.20]). The risks for acute myocardial infarction and hospitalization for heart failure were not significantly different between nebivolol and other classes.
Figure 4 summarizes the comparison between nebivolol and atenolol or other classes of anti-hypertensives. First-line nebivolol use was associated with a higher risk for transient ischemic attack than first-line atenolol use in hypertensive patients (cHR 1.59 [95% CI 1.15–2.23]). Except for increased vasculitis risk for atenolol (cHR 3.05 [95% CI 1.24–7.77]), no significant differences in the safety profile of nebivolol versus atenolol were observed. First-line use of nebivolol increased the risk for ischemic stroke compared to ACEIs (cHR 1.50 [95% CI 1.12–2.16]), ARBs (cHR 1.43 [95% CI 1.03–2.11]), dCCBs (cHR 1.49 [95% CI 1.11–2.14]), and TDs (cHR 1.50 [95% CI 1.12–2.12]), which is consistent with the results from other analytic settings (Figure S14 in the Data Supplement). Nebivolol was also associated with a higher risk for bradycardia compared with ACEIs (cHR 1.34 [95% CI 1.10–1.75]), ARBs (cHR 1.49 [95% CI 1.11–2.14]), and TDs (cHR 1.43 [95% CI 1.13–1.90]). Compared to ACEIs, nebivolol was associated with decreased risk of gastro-intestinal (GI) bleeding (cHR 0.64 [95% CI 0.42–0.89]), vomiting (cHR 0.81 [95% CI 0.62–0.98], hyperkalemia (cHR 0.73 [95% CI 0.54–0.91]), angioedema (cHR 0.45 [95% CI 0.23–0.80]), and cough (cHR 0.72 [95% CI 0.54–0.89]). Risks for hemorrhagic stroke (cHR 2.16 [95% CI 1.21–4.13], heart failure (cHR 1.38 [95% CI 1.00–2.00]), cardiovascular events (cHR 1.28 [95% CI 1.02–1.70]), and transient ischemic attack (cHR 1.57 [95% CI 1.15–2.27]) were significantly higher in nebivolol compared with TDs, while risks for hypokalemia (cHR 0.18 [95% CI 0.08–0.35]) and hyponatremia (cHR 0.48 [95% CI 0.24–0.89]) were lower in nebivolol compared with TDs.
Figure 4.
Meta-analytic safety profiles comparing nebivolol to atenolol and other classes (ACEI, ARB, dCCB, and TD) across six secondary outcomes (all-cause mortality, ischemic stroke, hemorrhagic stroke, heart failure, sudden cardiac death, unstable angina, and cardiovascular event) and 46 safety outcomes listed on product labels (PS-matching, on-treatment). Points and lines identify calibrated HR estimates with their 95% CIs, respectively. Outcomes in gray signify that the CI covers HR of 1 (null hypothesis of no differential risk). The cardiovascular event outcome includes acute myocardial infarction, stroke, hospitalization for heart failure, and sudden cardiac death.
Abbreviations: HR, hazard ratio; CI, confidence interval; PS, propensity score; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blocker; dCCBs, dihydropyridine calcium-channel blocker; TDs, thiazide, or thiazide-like diuretics
DISCUSSION
We found 118,133 and 267,891 patients initiating third-generation beta-blockers or atenolol, respectively, while 741,340 patients initiated TDs for hypertension across the three databases.16 Many patients were being initiated with third-generation beta-blockers, but we found no comparative effectiveness or safety advantage for third-generation beta-blockers, specifically carvedilol and nebivolol, as first-line treatment for hypertension compared with atenolol. Third-generation beta-blockers was not superior to metoprolol in terms of prevention of future cardiovascular events in hypertensive patients. First-line third-generation beta-blockers were associated with a higher risk of cardiovascular events, especially stroke, than other classes of anti-hypertension medication.
A Cochrane review revealed the inferiority of first-line beta-blockers in prevention of death compared with CCBs and stroke compared with CCBs or renin-angiotensin system inhibitors for hypertension.10 In another meta-analysis of 147 randomized trials by Law et al., beta-blockers were associated with an 18% higher risk for stroke compared with other anti-hypertensive drugs.31 In a meta-analysis of 123 studies that included 613,815 people, beta-blockers, mostly atenolol, were inferior to other anti-hypertensive drugs in reducing major cardiovascular events, stroke, and renal failure.32 The latest meta-analysis revealed that beta-blockers were less protective for stroke and all-cause death in 24 trials including 103,764 patients, compared with other agents.33 This study also confirmed the inferiority of atenolol in cardiovascular disease prevention, especially compared to dCCB and TDs in routine clinical practice. However, there are no randomized trials or large-scale observational studies to demonstrate the effectiveness of third-generational beta-blockers in hypertension compared with atenolol or other anti-hypertensives. The 2017 ACC/AHA guidelines went as far as recommending not using atenolol for the treatment of hypertension.11
This study identified signals for most well-known adverse events of anti-hypertensives, such as increased risk for bradycardia in beta-blockers, angioedema or cough in ACEIs, and electrolyte disturbance in TDs. Of note, nebivolol was associated with decreased risk for GI bleeding compared with ACEIs or ARBs. This might be attributable to the effect of beta-blockers on the prevention of bleeding from esophageal varices or their pleiotropic effects on gastric mucosal blood flow and gastrin production.34 Nonetheless, the results for secondary outcomes or safety outcomes should be considered as exploratory, since this study did not adjust for multiple testing.
Third-generation beta-blockers are known to have favorable effects on endothelial function and metabolic profiles compared with atenolol.14,15 The CAFE study demonstrated that atenolol was less effective in reducing central blood pressure compared to other classes despite similar brachial blood pressure effects.35 Another randomized study reported nebivolol decreased central blood pressure significantly while metoprolol did not.36 Therefore, in theory, third-generation beta-blockers with vasodilating properties could be superior to the second-generation beta-blockers in reducing cardiovascular outcomes for hypertensive patients due to their pleiotropic effects and better efficacy in reducing central blood pressure. Ironically, only these theoretical benefits, together with lack of clinical evidence, supplied the basis for the argument that the third-line beta-blockers may have better efficacy than conventional beta-blockers such as atenolol for treatment of hypertension. However, in a recent meta-analysis comparing atenolol and the third-generation beta-blockers, there were no significant differences in central blood pressure changes after accounting for differences in heart rate changes.37
Beta-blockers remain as an option for first-line anti-hypertensive treatment in several guidelines.38,39 Nationwide researches revealed that the use of beta-blockers monotherapy for hypertensive patients is still prevalent in US and Korea.40,41 Our study extends the prior literature substantially as it is the largest and most comprehensive evidence for the comparative effectiveness and safety of initial beta-blocker monotherapy in hypertensive patients under routine medical practice and identifies key opportunities to improve current practice.
Still, this study has several limitations. First, potential residual confounding due to observational nature of the study may bias our findings. Nonetheless, the rigorous features of this study, including new-user cohort design with active comparators and empirical equipoise, all helped to reduce the potential for confounding by indication and unmeasured characteristics.27,42,43 We applied large-scale PS models to adjust more than 20,000 covariates in each site. We successfully matched and balanced all these covariates including index years to cover different epochs of treatment choice. We confirmed that this approach is insensitive to the hidden confounders such as baseline BPs through post hoc analysis, which has also been demonstrated in previous reports.16,17 A large set of negative and positive controls were employed to quantify and mitigate residual bias by empirical calibration of confidence intervals. The cHRs were close to the uHRs, which revealed that the possibility of severe systematic errors was likely small. Since all the study protocols were prespecified before execution and all the study results were made public, there is little chance of publication bias or p-hacking.
Second, the results across drugs and databases were considerably heterogeneous. The random-effects meta-analysis of this study averaged over observed differences and might fail to detect true effects of the third-generation beta-blockers.44 However, we argue for more caution concerning type I error rather than type II error when investigating the effectiveness of anti-hypertensive medications using large real-world data because there is an established initial pharmacologic treatment in hypertension. Third, since we did not exclude those who added second-line antihypertensives after 7 days of index date, it is possible that selective secondary drug choices could have influenced the different effectiveness between beta-blockers. Fourth, due to the relatively short duration of maintenance of the allocated medication in on-treatment design, we could not determine whether long-term treatment with this drug might result in differences in cardiovascular events. However, we found that the results from the ITT design, which followed the patients during much longer period, were consistent with the results from the on-treatment design. Sixth, since this study identified most of the clinical outcomes through the reported diagnosis, outcomes including sudden cardiac death might be over- or under-detected due to diagnostic or coding errors. Seventh, we did not stratify the study population into young and old patients. Since majority of included patients were younger than 60, caution is needed in extrapolating these results to old hypertensive patients. Eighth, since this study focused on treatment-naive hypertensive patients without stratification according to the patient’s concomitant diseases, the effectiveness of beta-blockers in patients with certain diseases should be investigated in the future research. Nineth, certain drugs might have disproportionately large impact on the drug class comparison, such as the majority of patients used lisinopril in the ACEI group. Nevertheless, this reflects the actual percentage of drug use in routine clinical practice, which, in turn, can reflect the real-world comparativeness effectiveness at class level. Finally, the results related with metoprolol should be interpreted in caution, this study did not distinguish metoprolol succinate and metoprolol tartrate.
Perspectives
We found that the use of beta-blockers as an initial pharmacological therapy for hypertension is prevalent in routine clinical practice. This study found no significant differences in effectiveness between atenolol and third-generation beta-blockers, specifically carvedilol and nebivolol. We also found that these third-generation beta-blockers were less effective in preventing stroke than ACEIs and TDs. Our findings support the current ACC/AHA and the JSH guidelines, which recommend not using beta-blockers as first-line treatment in hypertensive patients without compelling indications. Nonetheless, further study is warranted given the potential for residual confounding and the short duration of medication.
Supplementary Material
Novelty and Significance.
What is new?
We found no statistically significant differences in the effectiveness and safety comparing atenolol with third-generation beta-blockers, specifically carvedilol and nebivolol.
Patients on third-generation beta-blockers had a higher risk of stroke than those on angiotensin-converting enzyme inhibitors and or thiazide or thiazide-like diuretics.
What is Relevant?
Evidence for the effectiveness and safety of the third-generation beta-blockers other than atenolol in hypertension remains scarce.
Prescription of beta-blockers for hypertension is still prevalent in routine clinical practice.
Summary.
Despite their widespread use in hypertension, both atenolol and third-generation beta-blockers showed inferior effectiveness when compared to other recommended medications for first-line pharmacological treatment of hypertension in routine clinical practice.
Acknowledgments:
The analysis is based in part on work from the Observational Health Sciences and Informatics collaborative. OHDSI (http://ohdsi.org) is a multi-stakeholder, interdisciplinary collaborative to create open-source solutions that reveal the value of observational health data through large-scale analytics.
Sources of Funding
This work was supported by the Bio Industrial Strategic Technology Development Program (20001234) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea [grant number: HI16C0992]; a faculty research grant from Yonsei University College of Medicine [6–2019-0170 to S.H.P]; and from the Korean Centers for Disease Control and Prevention [2018-ER6302–01 to S.H.P.].
Disclosures
The authors declare the following disclosures: Dr. Schuemie, and Dr. Ryan are employees of Janssen Research & Development, a subsidiary of Johnson & Johnson. Dr. Reich is an employee of IQVIA. Dr. Krumholz works under contract with the Centers for Medicare & Medicaid Services to develop publicly reported quality measures; was a recipient of a research grant, through Yale, from Medtronic and the U.S. Food and Drug Administration to develop methods for post-market surveillance of medical devices; is a recipient of a research grant with Medtronic and Johnson & Johnson, through Yale, to develop methods of clinical trial data sharing; was a recipient of a research agreement, through Yale, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; received payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation and from the Ben C. Martin Law Firm for work related to the Cook IVC filter litigation; receives payment from the Siegfried & Jensen Law Firm for work related to Vioxx litigation; chairs a Cardiac Scientific Advisory Board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science, the Advisory Board for Facebook, and the Physician Advisory Board for Aetna; and is the co-founder of HugoHealth, a personal health information platform and a co-founder of Refactor Health, an enterprise healthcare artificial intelligence-augmented data management company. Dr. Madigan has testified as an expert in litigation related to a number of drugs and devices but not related to any of the products considered in this study. Drs. Hripcsak and Suchard have received grant funding from Janssen through their universities to support methods research not directly related to this study. Dr. Suchard has also received contracts from Janssen and IQVIA for work not directly related to this study. Dr. Sungha Park has received grant funding and consultation fee from Daiichi Sankyo, which is not directly related to this study. Dr. Sungha Park has received speaking honoraria from Servier, Daiichi Sankyo, Hanmi, Daewoong, Pfizer, Takeda, Donga, and Boryoung, which is not directly related to this study. None of sponsors and funders above including Janssen and IQVIA had input in the design, execution, interpretation of results, or decision to publish. The rest of the authors declare no conflict of interest.
References
- 1.Prichard BN, Gillam PM. Use of propranolol (Inderal) in treatment of hypertension. Br Med J. 1964;2:725–727. doi: 10.1136/bmj.2.5411.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Report of the joint national committee on detection, evaluation, and treatment of high blood pressure: a cooperative study. JAMA. 1977;237:255–261. doi: 10.1001/jama.1977.03270300059008 [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 4.Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? a systematic review. JAMA. 1998;279:1903–1907. doi: 10.1001/jama.279.23.1903 [DOI] [PubMed] [Google Scholar]
- 5.Messerli FH, Beevers DG, Franklin SS, Pickering TG. β-Blockers in hypertension-the emperor has no clothes: an open letter to present and prospective drafters of new guidelines for the treatment of hypertension. Am J Hypertens. 2003;16:870–873. doi: 10.1016/s0895-7061(03)01017-3 [DOI] [PubMed] [Google Scholar]
- 6.Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet. 2004;364:1684–1689. doi: 10.1016/s0140-6736(04)17355-8 [DOI] [PubMed] [Google Scholar]
- 7.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? a meta-analysis. Lancet. 2005;366:1545–1553. doi: 10.1016/s0140-6736(05)67573-3 [DOI] [PubMed] [Google Scholar]
- 8.Bradley HA, Wiysonge CS, Volmink JA, Mayosi BM, Opie LH. How strong is the evidence for use of beta-blockers as first-line therapy for hypertension? systematic review and meta-analysis. J Hypertens. 2006;24:2131–2141. doi: 10.1097/01.hjh.0000249685.58370.28 [DOI] [PubMed] [Google Scholar]
- 9.Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ. 2006;174:1737–1742. doi: 10.1503/cmaj.060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta‐blockers for hypertension. Cochrane Database Syst Rev. 2017;1:CD002003. doi: 10.1002/14651858.CD002003.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/hyp.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 12.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9 [DOI] [PubMed] [Google Scholar]
- 13.Mann SJ. Redefining beta-blocker use in hypertension: selecting the right beta-blocker and the right patient. J Am Soc Hypertens. 2017;11:54–65. doi: 10.1016/j.jash.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 14.Cheng JW. Nebivolol: a third-generation beta-blocker for hypertension. Clin Ther. 2009;31:447–462. doi: 10.1016/j.clinthera.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Manrique C, Giles TD, Ferdinand KC, Sowers JR. Realities of newer beta-blockers for the management of hypertension. J Clin Hypertens (Greenwich). 2009;11:369–375. doi: 10.1111/j.1751-7176.2009.00140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suchard MA, Schuemie MJ, Krumholz HM, You SC, Chen R, Pratt N, Reich CG, Duke J, Madigan D, Hripcsak G, Ryan PB. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394:1816–1826. doi: 10.1016/s0140-6736(19)32317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hripcsak G, Suchard MA, Shea S, Chen R, You SC, Pratt N, Madigan D, Krumholz HM, Ryan PB, Schuemie MJ. Comparison of cardiovascular and safety outcomes of chlorthalidone vs hydrochlorothiazide to treat hypertension. JAMA Intern Med. 2020;180:542–551. doi: 10.1001/jamainternmed.2019.7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuemie M, Ryan P, Pratt N, Chen R, You S, Krumholz H, Madigan D, Hripcsak G, Suchard M. Principles of large-scale evidence generation and evaluation across a network of databases (LEGEND). J Am Med Inform Assoc. 2020;0:1331–1337. doi: 10.1093/jamia/ocaa103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuemie M, Ryan P, Pratt N, Chen R, You S, Krumholz H, Madigan D, Hripcsak G, Suchard M. Large-scale evidence generation and evaluation across a network of databases (LEGEND): assessing validity using hypertension as a case study. J Am Med Inform Assoc. 2020;0:1268–1277. doi: 10.1093/jamia/ocaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bate A, Chuang-Stein C, Roddam A, Jones B. Lessons from meta-analyses of randomized clinical trials for analysis of distributed networks of observational databases. Pharm Stat. 2019;18:65–77. doi: 10.1002/pst.1908 [DOI] [PubMed] [Google Scholar]
- 21.Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, Suchard MA, Park RW, Wong IC, Rijnbeek PR, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–578. doi: 10.3233/978-1-61499-564-7-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc. 2012;19:54–60. doi: 10.1136/amiajnl-2011-000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan PB, Schuemie MJ, Gruber S, Zorych I, Madigan D. Empirical performance of a new user cohort method: lessons for developing a risk identification and analysis system. Drug Saf. 2013;36:59–72. doi: 10.1007/s40264-013-0099-6 [DOI] [PubMed] [Google Scholar]
- 24.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EPR 3rd, Polonsky T, Thompson-Paul AM, Vupputuri S. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e116–e135. doi: 10.1161/hyp.0000000000000067 [DOI] [PubMed] [Google Scholar]
- 26.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47:2005–2014. doi: 10.1093/ije/dyy120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker A, Patrick A, Lauer M, Hornbrook M, Marin M, Platt R, Roger V, Stang P, Schneeweiss S. A tool for assessing the feasibility of comparative effectiveness research. Comp Eff Res (Auckl). 2013;3:11–20. doi: 10.2147/CER.S40357 [DOI] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 29.Voss EA, Boyce RD, Ryan PB, van der Lei J, Rijnbeek PR, Schuemie MJ. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform. 2017;66:72–81. doi: 10.1016/j.jbi.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci U S A. 2018;115:2571–2577. doi: 10.1073/pnas.1708282114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/s0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 33.Thomopoulos C, Bazoukis G, Tsioufis C, Mancia G. Beta-blockers in hypertension: overview and meta-analysis of randomized outcome trials. J Hypertens. 2020;38(9):1669–1681. doi: 10.1097/HJH.0000000000002523 [DOI] [PubMed] [Google Scholar]
- 34.Suissa S, Bourgault C, Barkun A, Sheehy O, Ernst P. Antihypertensive drugs and the risk of gastrointestinal bleeding. Am J Med. 1998;105(3):230–235. doi: 10.1016/s0002-9343(98)00239-3 [DOI] [PubMed] [Google Scholar]
- 35.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/circulationaha.105.595496 [DOI] [PubMed] [Google Scholar]
- 36.Kampus P, Serg M, Kals J, Zagura M, Muda P, Karu K, Zilmer M, Eha J. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension. 2011;57:1122–1128. doi: 10.1161/hypertensionaha.110.155507 [DOI] [PubMed] [Google Scholar]
- 37.Pucci G, Ranalli MG, Battista F, Schillaci G. Effects of β-blockers with and without vasodilating properties on central blood pressure: systematic review and meta-analysis of randomized trials in hypertension. Hypertension. 2016;67:316–324. doi: 10.1161/hypertensionaha.115.06467 [DOI] [PubMed] [Google Scholar]
- 38.Lee HY, Shin J, Kim GH, Park S, Ihm SH, Kim HC, Kim KI, Kim JH, Lee JH, Park JM, Pyun WB, Chae SC. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20. doi: 10.1186/s40885-019-0124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, Harris KC, Nakhla M, Cloutier L, Gelfer M, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506–525. doi: 10.1016/j.cjca.2018.02.022 [DOI] [PubMed] [Google Scholar]
- 40.Derington CG, King JB, Herrick JS, Shimbo D, Kronish IM, Saseen JJ, Muntner P, Moran AE, Bress AP. Trends in antihypertensive medication monotherapy and combination use among US adults, National Health and Nutrition Examination Survey 2005–2016. Hypertension. 2020;75:973–981. doi: 10.1161/hypertensionaha.119.14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SH, Shin DW, Kim S, Han K, Park SH, Kim YH, Jeon SA, Kwon YC. Prescribing patterns of antihypertensives for treatment-naïve patients in South Korea: from Korean NHISS claim data. Int J Hypertens. 2019;2019:4735876. doi: 10.1155/2019/4735876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2:221–228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneeweiss S, Patrick AR, Stürmer T, Brookhart MA, Avorn J, Maclure M, Rothman KJ, Glynn RJ. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45:S131–S142. doi: 10.1097/MLR.0b013e318070c08e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.