Abstract
Background:
Most cohort studies have only a single physical activity (PA) measure and are thus susceptible to reverse causation and measurement error. Few studies have examined the impact of these potential biases on the association between PA and mortality.
Methods:
A total of 133,819 participants from Nurses’ Health Study and Health Professionals Follow-up Study (1986–2014) reported PA through biennial questionnaires. Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for PA and mortality using different analytic approaches comparing single (baseline, simple update=most recent) vs. repeated (cumulative average) measures of PA and applying various lag times separating PA measurement and time at risk.
Results:
Over 3.2 million person-years, we documented 47,273 deaths. The pooled multivariable-adjusted HR (95% CI) of all-cause mortality per 10 MET-hours/week was 0.95 (0.94–0.96) for baseline PA, 0.78 (0.77–0.79) for simple updated PA and 0.87 (0.86–0.88) for cumulative average PA. Simple updated PA showed the strongest inverse association, suggesting larger impact of reverse causation. Application of 2-year lag substantially reduced reverse causation, and 4–12-year lags had minimal additional effects. In the dose-response analysis, baseline or simple updated PA showed a J or U-shaped association with all-cause mortality while cumulative average PA showed an inverse association across a wide range of PA (0–150 MET-hour/week). Similar findings were observed for different specific mortality causes.
Conclusion:
PA measured at baseline or with short lag time was prone to bias. Cumulative average PA showed robust evidence that PA is inversely associated with mortality in a dose-response manner.
Keywords: physical activity, mortality, measurement error, reverse causation, bias
INTRODUCTION
Numerous prospective cohort studies reported beneficial associations of physical activity on the prevention of many diseases and mortality.(1–3) However, most of these studies used a single measure of physical activity at baseline to examine the association with subsequent disease events occurring over various times of follow-up period. These studies require a strong assumption that individuals’ physical activity measured at baseline does not change over time. Thus, use of single measure of physical activity is prone to measurement error, which may also affect the dose-response shape.(4–6) In recent years, very large cohort studies including over 0.5 million participants (e.g., UK/China Biobank, Million Women Study) have been initiated and evaluated the association of lifestyle factors including physical activity with health outcomes.(7–9) The large sample size of these studies allowed the ascertainment of sufficient numbers of outcomes in short period of follow-up times (~5 years). However, these large studies with a short follow-up time are susceptible to reverse causation bias because participants with preexisting or undiagnosed diseases who are at higher risk of subsequent disease or death may be more likely to become inactive. Reverse causation can be especially problematic for mortality studies, and it tends to overestimate the inverse association between physical activity and health outcomes.(10) Moreover, use of single physical activity assessment, particularly measured close to diagnosis or death, may also result in spurious relationships. Several physical activity studies have reported a reverse J or U-shaped association, suggesting an increased risk of disease or mortality at the higher range of physical activity.(11, 12) The biases of measurement error due to use of only one questionnaire and reverse causation from short follow-up periods may have contributed to unreliable results.
These potential biases have generally been under-appreciated or only briefly mentioned as limitations of the studies. We found only one recent UK Biobank study that evaluated the influence of reverse causation on the association between physical activity and health outcomes by different follow-up periods and modeling approaches.(10) This study found a stronger inverse association between physical activity and health outcomes in multivariable-adjusted models not excluding those with prevalent diseases and with shorter follow-up periods than that did not use these approaches. However, this study had a single measure of self-reported physical activity and relatively short period of follow-up time (median of 7 years) and thus limited ability to assess comprehensively both reverse causation and measurement error over a long period of time.
In the current study, we leveraged biennially collected physical activity questionnaires over 28 years in two large prospective cohort studies, Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS), to examine the association between physical activity and all-cause and cause-specific mortality. To assess the impact of reverse causation and measurement error, we used several analytic approaches including comparison of single vs. repeated measures of physical activity and application of different lag times (between physical activity assessment and time at risk).
METHODS
Study population
The NHS was initiated in 1976 when 121,701 female nurses aged 30–55 years were enrolled. The HPFS was initiated in 1986 when 51,529 male health professionals aged 40–76 years were enrolled. Participants completed questionnaires on demographic, lifestyle and medical history at enrollment, which were updated every two years. The follow-up rate exceeded 90% for all questionnaire cycles for both cohorts. In the current study, we included all participants who reported information on detailed physical activity first assessed in 1986 (82,951 women and 50,868 men). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required.
Physical activity assessment
In both cohorts, detailed information on physical activity was measured in 1986 and updated biennially using a validated questionnaire. Participants were asked to report the average hours they spent per week during the past year on each of the following activities: walking, jogging, running, swimming, bicycling, calisthenics and other aerobic exercises, squash/racquetball, tennis, lower-intensity exercise, weight lifting, and outdoor work. For each physical activity, we assigned a metabolic equivalent task (MET), which indicates metabolic rates for a specific activity divided by metabolic rates at rest, based on a compendium of physical activity(13) to quantify the intensity of physical activities. Total physical activity was calculated by summing all physical activities in MET-hours per week. Distributions of physical activity are shown in Supplementary Figure 1.
The reproducibility and validity of the physical activity questionnaire have been described previously.(14, 15) The correlations between physical activity reported on the questionnaire and those recorded in four single-week diaries administered across four different seasons were 0.62 for NHS and 0.58 for HPFS. Moreover, the physical activity questionnaire had good predictability of resting heart rate (Supplementary Tables 1–2) and inflammatory biomarkers.(16, 17)
Ascertainment of death
We identified deaths from the National Death Index, next of kin and postal system.(18, 19) Over 98% of deaths were ascertained using these methods for both cohorts. The cause of death was determined primarily by physician review of medical records and by death certificates when records were not available. The International Classification of Diseases (ICD-8) codes were used to classify deaths from cardiovascular disease (ICD-8 code: 390–459 and 795), cancer (ICD-8 code: 140–239), respiratory disease (ICD-8 code: 460–519), and other causes.
Covariate assessment
Information on covariates such as age, race, body weight, height, family history of diseases, medical history of diseases, and smoking were assessed from the biennial questionnaires. Dietary information (including alcohol) was assessed using a validated semiquantitative food frequency questionnaire every four years.(20–22) Body mass index was calculated by body weight in kg divided by height in meter squared. Alternate Healthy Eating Index was calculated to assess overall diet quality.(23)
Statistical analysis
Person-time was calculated from the time when physical activity was first available in 1986 until the time of death or the end of study (June 2014 for NHS and January 2014 for HPFS). Cox proportional regression models with age as time scale with stratifying by calendar time were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The primary outcome of interest was all-cause mortality and secondary outcomes were cause-specific mortality due to cardiovascular disease, cancer, respiratory disease or other causes. Potential confounders were selected a priori based on literature(1–3) and we fitted three models: (1) Model 1: age-adjusted model; (2) Model 2: multivariable-adjusted model including race (white or non-white), family history of cardiovascular disease (yes or no), family history of cancer (yes or no), postmenopausal hormone use (premenopausal, postmenopausal current user, or postmenopausal never/past user), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or 15.0+ g/day), total energy intake (quintiles), smoking status (never, ever, 1–14, 15–24, ≥25 cigs/day), Alternate Healthy Eating Index (quintiles), and body mass index (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, or ≥40 kg/m2); and (3) Model 3: multivariable-adjusted model excluding participants with prevalent major chronic disease at baseline (i.e., cardiovascular disease and cancer).
Total physical activity was modeled using different approaches: (1) physical activity measured once at baseline in 1986 (referred as ‘baseline’), (2) physical activity based on most recent updated single measure (referred as ‘simple update’), (3) average of repeated measures of physical activity from baseline to time at risk (referred as ‘cumulative average’). Total physical activity was included in the models as a continuous variable (10 MET-hour/week increment). To examine the influence of reverse causation on the association between physical activity and mortality, we applied different lag time periods between physical activity assessment and time at risk (2, 4, 8, and 12-year lag). For single measure of physical activity analysis with lag times, we used a single physical activity information measured 2, 4, 8 or 12 years prior to death for 2, 4, 8 or 12-year lag analyses, respectively. For repeated measures of physical activity analysis with lag times, we used the same lag time approaches but averaged all repeated measures of physical activity information measured from baseline to 2, 4, 8 or 12 years prior to death.
To flexibly model the shape of the association between physical activity and mortality, we conducted restricted cubic spline models with 3 knots.(24) For this dose-response analysis, we selected the fully-adjusted model (Model 3) and applied the same other approaches used for the continuous analyses above to evaluate the impact of reverse causation and measurement error. To examine whether the influence of the biases on the association between physical activity and mortality differs by age, we also conducted stratified analysis by three age groups based on the distribution of age at death (<70, 70–79 or ≥80 years).
Lastly, we conducted several sensitivity analyses. We performed additional models further excluding current and past (quitted smoking within 10 years) smokers or adjusting for physical limitation factors (e.g., health limits in daily, working, leisure activities). To further validate the physical activity data, we used self-reported resting heart rate data available in 1992 from HPFS to examine the correlation between resting heart rate and physical activity and medians of resting heart rate across physical activity levels. Proportional hazard assumption was tested by including a cross product term of physical activity and time (P>0.05). We pooled the data of two cohorts (NHS and HPFS) after testing for heterogeneity by cohort (sex) (P>0.05). All statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC).
RESULTS
The mean ages of participants over the follow-up were 63.8 years for women (NHS) and 64.0 years for men (HPFS). Participants with higher physical activity had lower body mass index and higher calorie intake, alcohol intake and overall diet quality. Moreover, they were less likely to be current smokers (Table 1).
Table 1.
Age-standardized characteristics of participants according to physical activity (Health Professionals Follow-up Study and Nurses’ Health Study, 1986–2014)a
| Physical activityc | ||||||
|---|---|---|---|---|---|---|
| NHS (82,951 women) | HPFS (50,868 men) | |||||
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| Age, yearsb | 63.9 (10.2) | 63.7 (10.0) | 63.8 (9.9) | 64.6 (11.4) | 64.2 (11.2) | 63.1 (11.0) |
| Body mass index, kg/m2 | 27.4 (5.7) | 25.9 (4.6) | 24.6 (4) | 26.6 (3.9) | 25.7 (3.2) | 25.2 (3.1) |
| White, % | 97.0 | 97.8 | 97.7 | 93.5 | 95.3 | 95.5 |
| Family history of CVD, % | 37.9 | 38.6 | 38.4 | 33.2 | 33.5 | 32.2 |
| Family history of cancer, % | 38.8 | 40.3 | 39.9 | 14.9 | 16.2 | 16.3 |
| Smoking status, % | ||||||
| Never smoker | 44.4 | 46.2 | 44.5 | 45.8 | 48.3 | 52.4 |
| Past smoker | 39.9 | 43.1 | 46.1 | 43.9 | 45.7 | 43.0 |
| Current smoker | 15.7 | 10.7 | 9.4 | 10.3 | 6.1 | 4.5 |
| Postmenopausal hormone use, % | 21.0 | 25.8 | 27.0 | |||
| Calorie intake, kcal/d | 1702 (461) | 1747 (449) | 1789 (462) | 1892 (567) | 1963 (560) | 2077 (586) |
| Alcohol intake, g/d | 5.4 (10) | 5.7 (9.1) | 6.8 (9.4) | 10.2 (15.2) | 11.1 (13.9) | 11.9 (13.7) |
| Alternate Fleathy Eating Index | 48.1 (9.1) | 51.7 (9.4) | 55.3 (9.8) | 50.2 (10.4) | 54.1 (10.5) | 56.9 (10.8) |
Values are presented as means (SD) for continuous variables and percentages for categorical variables.
Updated information over the study follow-up was used
Value is not age adjusted
Median, interquartile range, and range of total physical activity: 14, 6.3–26.4, and 0–150 MET-hour/week
During 3,229,449 person-years of follow-up, we identified 47,273 total deaths. In the pooled analysis of NHS and HPFS cohorts, higher physical activity was significantly associated with reduced mortality (Table 2). Adjustment for potential confounding variables and exclusion of those with prevalent diseases slightly attenuated the magnitude of the associations. In the fully adjusted models (Model 3), HRs (95% CI) of death per 10 MET-hour/week were 0.98 (0.97–0.98) for ‘baseline’ physical activity, 0.88 (0.87–0.88) for ‘simple update’ (most recent) physical activity, 0.91 (0.90–0.91) for ‘cumulative average’ (repeated measure) of physical activity measures. Simple updated (most recent) physical activity showed the strongest inverse association with mortality. Application of 2-year lag time (use of physical activity 2 years prior to risk period for death) substantially reduced the magnitude of the association for the simple updated physical activity measure. HRs (95% CI) of death per 10 MET-hour/week were 0.92 (0.91–0.92) for simple update with 2-year lag and 0.93 (0.93–0.94) for cumulative average with 2-year lag. Application of longer lag times (use of physical activity 4–12 years prior to death) further attenuated the associations though all remained statistically significant.
Table 2.
Association between physical activity (PA) and all-cause mortality using different analytical approaches (pooled results of Nurses’ Health Study and Health Professionals Follow-up Study, 1986–2014)
| Hazard ratio (95% Cl) per 10 MET-hour/week | |||
|---|---|---|---|
| Physical activity | Model la | Model 2b | Model 3c |
| Single measure of PA | |||
| Baseline1 | 0.95 (0.95–0.96) | 0.97 (0.97–0.98) | 0.98 (0.97–0.98) |
| Simple update2 | 0.85 (0.84–0.85) | 0.87 (0.86–0.87) | 0.88 (0.87–0.88) |
| 2-yr lag3 | 0.90 (0.89–0.90) | 0.91 (0.90–0.91) | 0.92 (0.91–0.92) |
| 4-yr lag3 | 0.90 (0.89–0.90) | 0.91 (0.90–0.91) | 0.92 (0.91–0.93) |
| 8-yr lag3 | 0.90 (0.90–0.91) | 0.91 (0.90–0.91) | 0.93 (0.92–0.94) |
| 12-yr lag3 | 0.91 (0.90–0.91) | 0.92 (0.91–0.92) | 0.94 (0.93–0.94) |
| Repeated measures of PA | |||
| Cumulative average4 | 0.87 (0.86–0.87) | 0.90 (0.89–0.90) | 0.91 (0.90–0.91) |
| 2-yr lag5 | 0.91 (0.90–0.91) | 0.92 (0.91–0.93) | 0.93 (0.93–0.94) |
| 4-yr lag5 | 0.91 (0.90–0.91) | 0.92 (0.92–0.93) | 0.93 (0.93–0.94) |
| 8-yr lag5 | 0.91 (0.91–0.92) | 0.93 (0.92–0.93) | 0.94 (0.93–0.95) |
| 12-yr lag5 | 0.92 (0.92–0.93) | 0.94 (0.93–0.94) | 0.95 (0.94–0.96) |
Model 1: 47,273 deaths and 3,229,449 person-years, Age-adjusted model used Cox regression model using age (month) as time scale with stratification by calendar time (year) and cohort
Model 2: 47,273 deaths and 3,229,449 person-years, Model 1 additionally adjusted for race (white or non-white), family history of cardiovascular disease (yes or no), family history of cancer (yes or no), postmenopausal hormone use (premenopausal, postmenopausal current user, or postmenopausal never/past user)alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or 15.0+ g/day), total energy intake (quintiles), smoking status (never, ever, 1–14, 15–24, ≥25 cigs/day), Alternate Healthy Eating Index (quintiles), and body mass index (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, or ≥40 kg/m2).
Model 3: 39,293 deaths and 3,003,673 person-years, Model 2 excluding prevalent diseases (cardiovascular disease and cancer).
Physical activity measured at baseline in 1986;
Physical activity measured closest (most recent) to death;
Physical activity measured 2, 4, 8 or 12 years prior to death;
Average of repeated measures of physical activity from baseline to death;
Average of repeated measures of physical activity measured 2, 4, 8 or 12 years prior to death.
In Figure 1, we conducted restricted cubic spline models to examine the dose-response relationship between physical activity and all-cause and cause-specific mortality. For a single measure of physical activity, baseline physical activity showed a U-shaped association with all-cause mortality, while simple updated (most recent) physical activity showed a reverse J-shaped association with all-cause mortality. On the other hand, cumulative average (repeated measures) of physical activity showed a consistently inverse association with all-cause mortality, showing large reduction of risk until around 50 MET-hour/week and a weak inverse trend after 50 MET-hour/week, especially when considering time lag. Application of 2-year lag substantially reduced the magnitude of the association when using both single and repeated measures of physical activity, particularly in the lower range of physical activity. Longer lag times only slightly further attenuated the associations. At the higher range of physical activity, single measures of physical activity showed no additional benefits or slight upwards trend. The association between PA and mortality had different patterns in the 0–50 MET-hour/week and 50–150 MET-hour/week ranges for simple updating and cumulative average (Table 3). With a 2-year lag, the magnitude of the inverse association for all-cause mortality (per 10 MET-hour/week) was stronger for simple updating (HR = 0.85) than for cumulative updating (HR = 0.90), but in the 50–150 MET-hour/week range, there was no significant association for simple updating (HR = 0.99) and a moderate inverse association for cumulative updating (HR = 0.97).
Figure 1. Dose-response relationship between physical activity and all-cause and cause-specific mortality using different analytic approaches (pooled results of Nurses’ Health Study and Health Professionals Follow-up Study, 1986–2014).
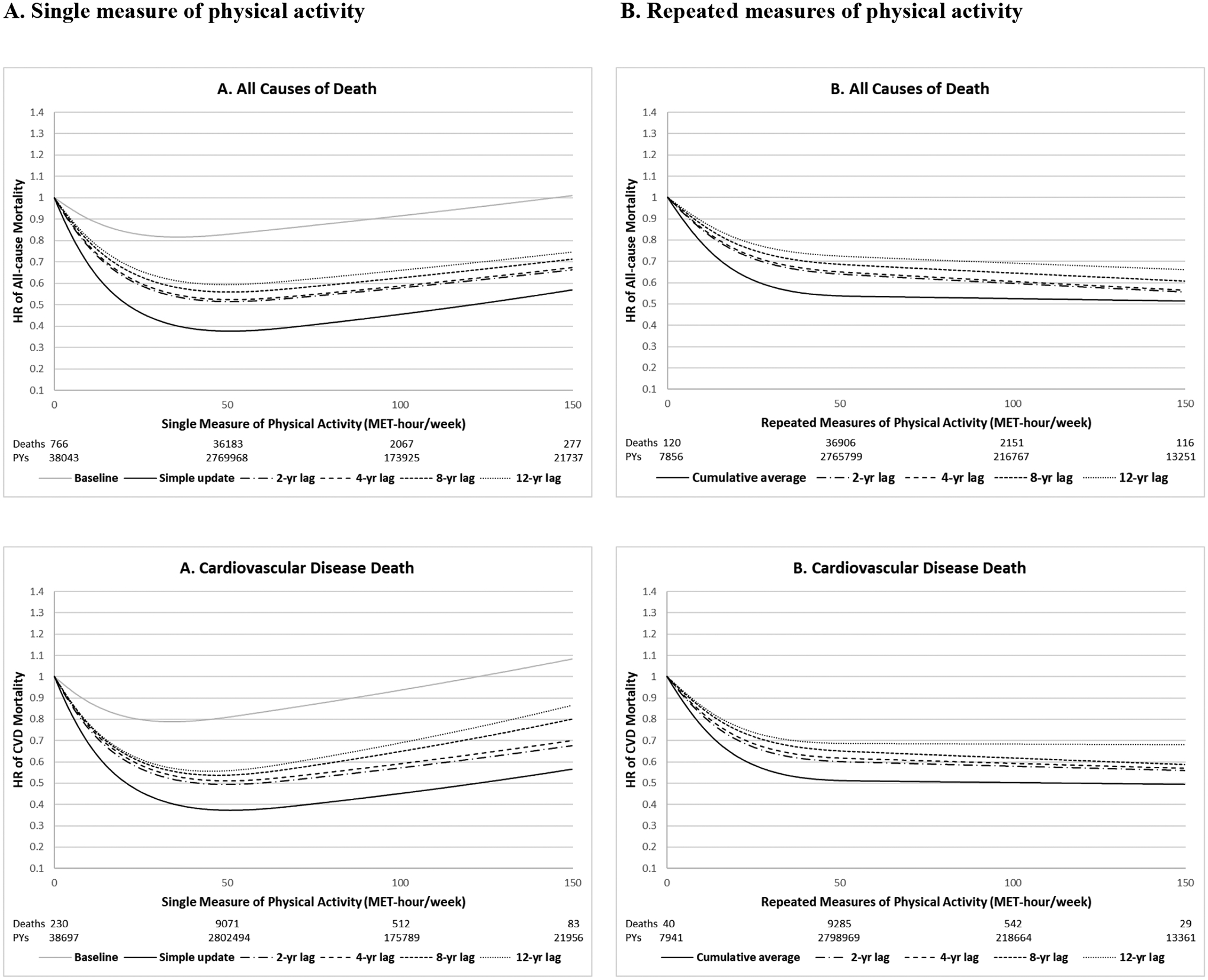
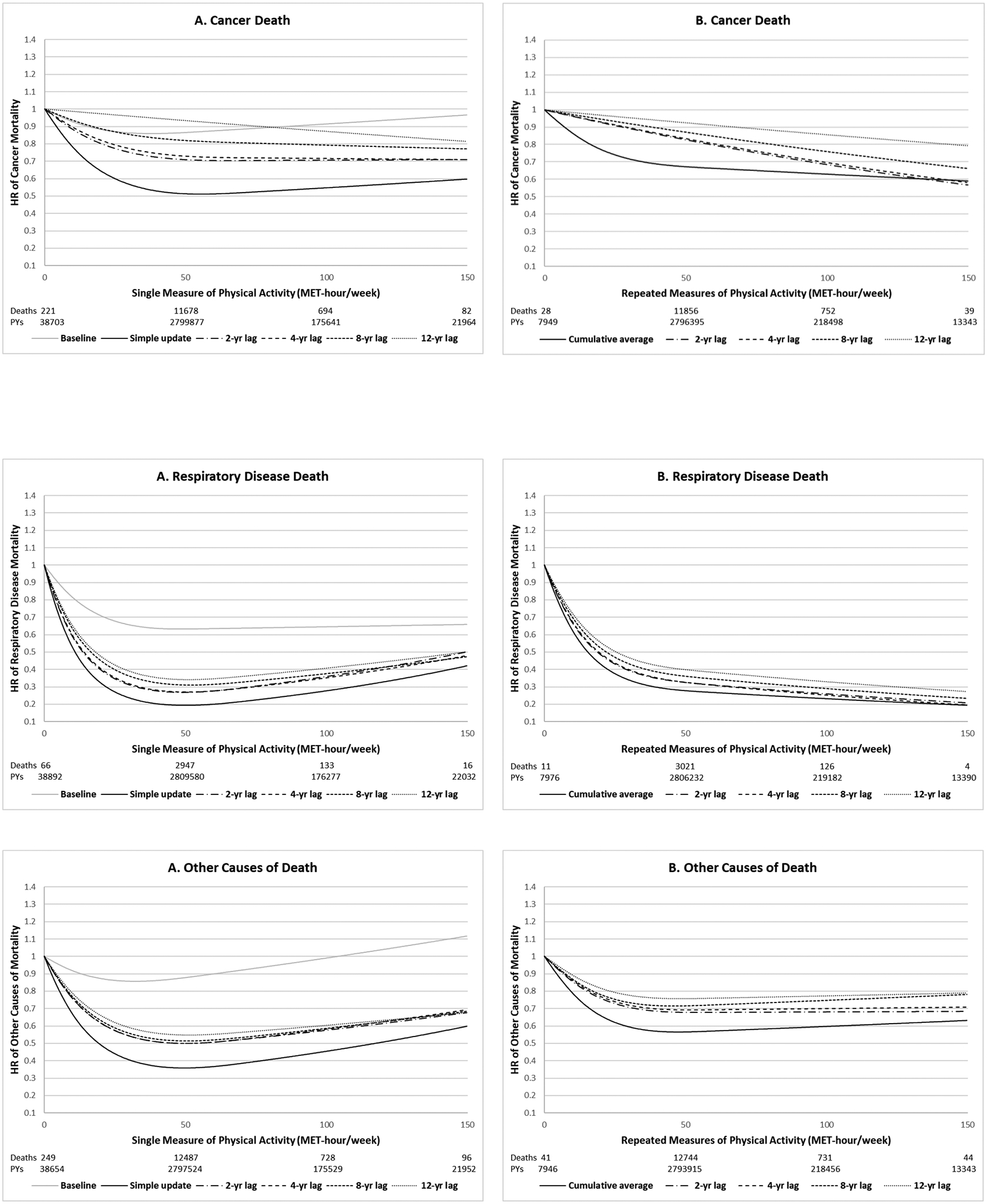
All models adjusted for the same covariates for model 3 (fully adjusted model) in Table 2. P for non-linearity<0.001 for all. Number of deaths: 39,293 total deaths including 9,896 CVD deaths, 12,675 cancer deaths, 3,162 respiratory deaths and 13,560 other causes of deaths.
Table 3.
Association between physical activity (PA) and all-cause mortality using different analytical approaches stratified by PA levels (pooled results of Nurses’ Health Study and Health Professionals Follow-up Study, 1986–2014)
| Hazard ratio (95% Cl) per 10 MET-hour/week | ||
|---|---|---|
| Physical activity | 0–50 MET-hour/week | 50–150 MET-hour/week |
| Single measure of PA | ||
| Baseline1 | 0.95 (0.94–0.96) | 1.00 (0.98–1.02) |
| Simple update2 | 0.78 (0.77–0.79) | 1.00 (0.99–1.02) |
| 2-yr lag3 | 0.85 (0.84–0.86) | 0.99 (0.98–1.01) |
| 4-yr lag3 | 0.85 (0.84–0.86) | 1.00 (0.98–1.02) |
| 8-yr lag3 | 0.86 (0.85–0.87) | 0.99 (0.97–1.01) |
| 12-yr lag3 | 0.88 (0.87–0.89) | 0.99 (0.97–1.01) |
| Repeated measures of PA | ||
| Cumulative average4 | 0.87 (0.86–0.88) | 0.99 (0.96–1.02) |
| 2-yr lag5 | 0.90 (0.89–0.91) | 0.97 (0.94–0.99) |
| 4-yr lag5 | 0.91 (0.90–0.92) | 0.97 (0.94–1.00) |
| 8-yr lag5 | 0.92 (0.91–0.93) | 0.96 (0.93–0.99) |
| 12-yr lag5 | 0.93 (0.92–0.94) | 0.96 (0.93–1.00) |
All models adjusted for the same covariates for model 3 (fully adjusted model) in Table 2.
Physical activity measured at baseline in 1986;
Physical activity measured closest (most recent) to death;
Physical activity measured 2, 4, 8 or 12 years prior to death;
Average of repeated measures of physical activity from baseline to death;
Average of repeated measures of physical activity measured 2, 4, 8 or 12 years prior to death.
For cause-specific mortality, we observed similar patterns with regard to comparison of single vs. repeated measures of physical activity and application of lag times (Figure 1 and Supplementary Table 3). The shape of the associations was similar for all-cause death, cardiovascular disease death, respiratory disease death and other causes of death. However, physical activity showed the largest reduction of risk for mortality due to respiratory disease in the lower range of physical activity. Unlike the other three causes of death, which showed a plateau after 50 MET-hour/week, physical activity was more linearly inversely associated with cancer-specific mortality extending up to 150 MET-hours/week.
When stratified by age, older people tended to have more linear association between physical activity and all-cause mortality, compared to younger people (Figure 2). Application of 2-year lag time appeared to adjust for reverse causation and unexpected increase of mortality in the high end of physical activity, especially for younger people. In the sensitivity analyses, stratified analysis by sex (Supplementary Figure 2), exclusion of current/past smokers (Supplementary Table 2) or adjustment for physical limitation-related factors did not change the results materially (data not shown). Exclusion of respiratory deaths from all-cause mortality showed slightly attenuated but similar shape of the associations (Supplementary Figure 3). Spearman correlations of resting heart rate were −0.187 for baseline physical activity, −0.169 for simple updated physical activity and −0.209 for cumulative average physical activity (Supplementary Table 1). Higher correlations were observed for cumulative average then baseline or simple updated physical activity in both lower and higher range of physical activity. Similar patterns were shown when examined medians of resting heart rate across physical activity (Supplementary Table 2).
Figure 2. Dose-response relationship between physical activity and all-cause mortality stratified by age (pooled results of Nurses’ Health Study and Health Professionals Follow-up Study, 1986–2014).
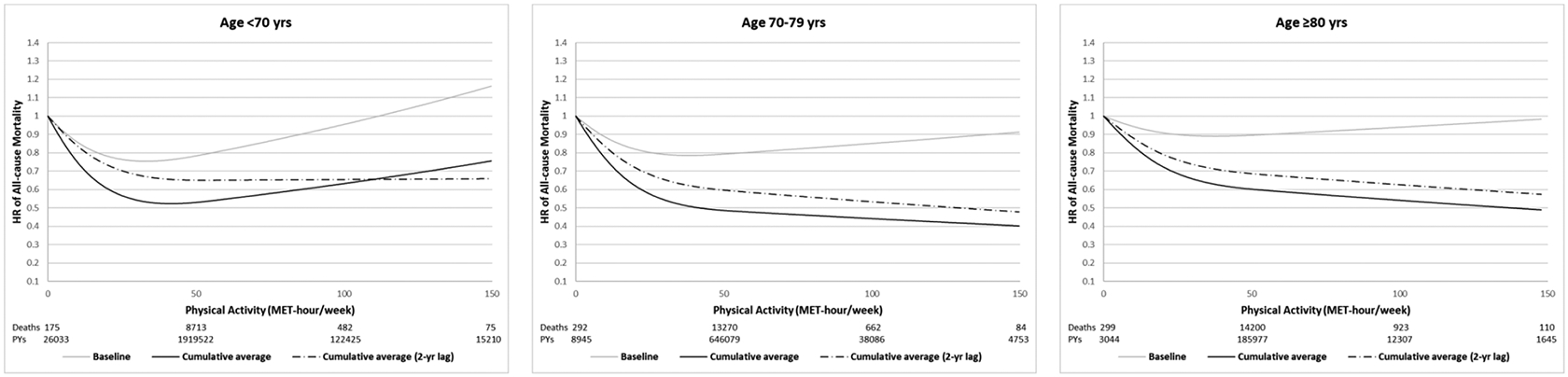
All models adjusted for the same covariates for model 3 (fully adjusted model) in Table 2. P for non-linearity<0.001 for all. Number of deaths: 9,445 for age<70, 14,308 for age 70–79, and 15,540 for ≥80 years.
DISCUSSION
In two large prospective cohort studies, we examined how the use of single physical activity measurement versus repeated measures and how different time lags affected the association between physical activity and mortality. A single measure of physical activity at baseline without updating showed a weak and somewhat U-shaped dose-response relationship with mortality. Simple updated physical activity, essentially examining short-term associations with morality, showed a strong inverse association up to 50 MET-hours/week and no further association above this level. In contrast, cumulative average of repeated measures of physical activity showed a consistent inverse association with mortality across all range of physical activity levels, but with stronger inverse associations at the lower end of physical activity (0–50 MET-hour/week). Compared to the simple-updated, the cumulative average measure showed a less steep inverse association below 50 MET-hours/week. Moreover, physical activity with no or short lag time showed the strongest association with mortality, and application of a 2-year lag between physical activity assessment and period of risk attenuated the magnitude of the association, while longer time lags greater than 2 years only minimally further changed the association.
Extensive evidence suggests that high level of physical activity is associated with decreased risk of many diseases and mortality.(1–3) While a true benefit of physical activity remains relatively uncontroversial, questions remain regarding the magnitude of the association, the timing, and the dose-response and type of activities. Most of the evidence is based on a single measure of physical activity, which limits the ability to address these features. In particular, smaller size cohorts tend to have long follow-up times to allow adequate time to accumulate disease or death, which may be prone to regression dilution bias (measurement error). In contrast, some recent very large cohorts can accumulate sufficient cases in a relatively short time period, which can be prone to reverse causation bias. Because of repeated measures in our two cohorts over 3 decades of follow-up, we were able to address the effects of using a single versus repeated measure of physical activity on the dose-response.
In our analysis, we found large differences in the magnitude of association between a single measure of physical activity at baseline vs. repeated measures (cumulative average) of physical activity in relation to mortality. A single measure of physical activity at baseline with 28 years of follow-up showed an approximately 2–3 fold weaker association than the use of repeated measures. Furthermore, the relationship appeared somewhat U-shaped. As participants may change their physical activity over time,(4, 5) the use of a single measure will likely induce non-differential measurement error in regards to outcome ascertainment, which underestimates the true effect of physical activity on health outcomes, particularly for studies with longer period follow-up times.(6) On the other hand, with the repeated measures of physical activity in our cohort, we estimated a stronger association, possibly by reducing regression dilution bias. Furthermore, we found no evidence of a reversal of benefit at higher doses of physical activity up to 150 MET-hours/week, suggesting that previous findings showing a U-shape relationship between physical activity and mortality might be an artifact of bias.(11, 12)
Using single and repeated measures of physical activity, when we applied different lag times (0, 2, 4, 8, 12 years) between physical activity assessment and time at risk, we found a stronger inverse association with shorter lag times, especially with lag <2 years. For cumulative average, in the range of 0–50 MET-hours/week, the HR (for a 10 MET-hours/week increment) for 0 lag was 0.87, which reduced to 0.90 with 2-year lag and incrementally reduced the HR to 0.93 with 12-year lag. Interestingly, at the higher range of physical activity (>50 MET-hours/week), there seemed less of a lowering of the benefit with greater lags, and perhaps even an increasing trend towards the null. A potential explanation of these findings is that those with severe preexisting or undiagnosed health conditions are more likely to decrease their physical activity (or become completely inactive) and these people are at higher risk of mortality. Therefore, it is likely that some of the benefits observed from engaging in some vs. no physical activity in large prospective cohort studies with short follow-up may partially reflect reverse causation bias. A recent UK Biobank study reported consistent results as in the current study, suggesting potential reverse causation in the lower range of physical activity for analysis of short follow-up periods (1–2 years).(10)
There is inconclusive evidence in respect to the dose-response relationship between physical activity and health outcomes, particularly at the higher range of physical activity. Several studies suggested that high amount of physical activity over 30–40 MET-hour/week (approximately 3–5 times the physical activity recommendation) does not provide additional benefits(2, 3) or can be even deleterious.(11, 12, 25) In our study, both single and repeated approaches showed large reductions in mortality until around 50 MET-hour/week but after 50 MET-hour/week, single measure of physical activity showed some increasing trends while repeated measure of physical activity showed a weak but gradually decreasing trend even at the high end of physical activity (50–150 MET-hour/week). Our study using cumulative average suggests that the inflection point where benefit of activity tapers off or even reverses is much higher than suggested by some of the studies and that being physically active consistently (long-term) is more important than being physically active sporadically. It also indicates that studies that reported increased mortality (attenuated benefits) in participants with high amount of physical activity may be due to measurement error and/or reverse causation in using a single measure rather than true biologic effects. Being extremely active and then becoming inactive could be harmful physiologically or is a sign of reverse causation.
In recent years, there has been increasing number of published papers using very large cohort studies with a relatively short follow-up period (~5 years) that examined the association between physical activity in relation to health outcomes. Some of these studies have received great attention, especially for assessing physical activity through accelerometer, which may substantially reduce measurement error compared to questionnaires.(26–29) Importantly, all accelerometer studies have used a single measure of physical activity over time, and our study suggest that repeated measures provides a considerably stronger inverse association between physical activity and mortality likely due to lower measurement error. In addition, to reduce reverse causation, previous studies often excluded deaths occurred in the first year of follow-up, but our study shows that exclusion of 1-year follow-up may not be enough to address reverse causation. Future prospective studies should consider applying at least 2-year lag period to minimize the impact of reverse causation on the association between physical activity and mortality, and to provide more conservative estimates. Accelerometer studies may also need to consider applying repeated measures of physical activity to reduce within-person variability and capture long-term habitual physical activity of cohort participants.
Compared to the other causes of deaths, respiratory disease death showed a markedly stronger inverse association in the lower range of physical activity. Previous studies showed some evidence that high physical activity was associated with improved lung capacity and reduced risk of lung diseases.(30–32) However, the substantial reduction of respiratory disease mortality may also reflect large degree of reverse causation because poor lung function due to preexisting or undiagnosed lung diseases, which may last over a decade, can hinder physical activity. In our lag analyses, there was suggestion of reduction in association, especially in the cumulative average physical activity, even from 2–12 years, though it was not as steep as 0–2 years.
Strengths of our study include a large sample of participants with 28 years of follow-up period. We had sufficient statistical power and wide variations in physical activity levels (0–150 MET-hour/week) to precisely examine the dose-response relationship between physical activity and mortality. Moreover, repeated measures of physical activity and covariates allowed us to utilize different analytical approaches comparing single vs. repeated measures of physical activity and various lag analyses. There are several limitations as well. First, our cohorts collected self-reported data thus some measurement error is inevitable, though our questionnaires were validated. However, to our knowledge, there are no cohort studies with objective physical activity information measured at multiple times over a long follow-up period. Given that the majority of cohort studies rely on self-reported questionnaires, our findings may provide more practical implications for future studies. Second, although we adjusted for multiple known potential confounding variables in our analyses, we cannot rule out residual or unmeasured confounding. Lastly, our cohorts predominantly consisted of white health professionals which may limit the generalizability of our findings but it strengthens internal validity.
In conclusion, we found that the impact of measurement error and reverse causation can be considerable for studies with only a single measure of physical activity or with a short lag time. Use of repeated measures of physical activity was more robust to such biases and showed evidence that a high level of physical activity is associated with reduced risk of all-cause and cause-specific mortality in a dose-response manner. Our study suggests that the optimal benefits of physical activity can be reached around 50 MET-hours per week which corresponds to approximately 10 hours of moderate activities per week and there was no evidence of reverse association (J or U-shape) but an inverse trend even at very high physical activity levels.
Supplementary Material
Acknowledgement:
We would like to thank the participants and staff of the NHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding: This work was supported by the National Institutes of Health (UM1 CA186107, U01 CA167552 and P01 CA87969). NK was supported by grants from the National Research Foundation of Korea (NRF-2018R1C1B6008822; NRF-2018R1A4A1022589).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: The authors have declared no conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Current opinion in cardiology. 2017;32(5):541–56. doi: 10.1097/hco.0000000000000437 [DOI] [PubMed] [Google Scholar]
- 2.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA internal medicine. 2015;175(6):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blond KA-Ohoo, Brinklov CF, Ried-Larsen MA-Ohoo, Crippa A, Grontved A. Association of high amounts of physical activity with mortality risk: a systematic review and meta-analysis. Br J Sports Med. 2019;bjsports-2018–100393. doi: 10.1136/bjsports-2018-100393 [DOI] [PubMed] [Google Scholar]
- 4.Andersen LB. Relative risk of mortality in the physically inactive is underestimated because of real changes in exposure level during follow-up. American journal of epidemiology. 2004;160(2):189–95. [DOI] [PubMed] [Google Scholar]
- 5.Paffenbarger RS Jr., Kampert JB, Lee IM, Hyde RT, Leung RW, Wing AL. Changes in physical activity and other lifeway patterns influencing longevity. Med Sci Sports Exerc. 1994;26(7):857–65. [PubMed] [Google Scholar]
- 6.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. Bmj. 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Du H, Clarke R, et al. Association of physical activity with risk of major cardiovascular diseases in Chinese men and women. JAMA cardiology. 2017;2(12):1349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celis-Morales CA, Lyall DM, Anderson J, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. European heart journal. 2017;38(2):116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celis-Morales CA, Lyall DM, Welsh P, et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: prospective cohort study. Bmj. 2017;357:j1456. [DOI] [PubMed] [Google Scholar]
- 10.Strain T, Wijndaele K, Sharp SJ, Dempsey PC, Wareham N, Brage S. Impact of follow-up time and analytical approaches to account for reverse causality on the association between physical activity and health outcomes in UK Biobank. International journal of epidemiology. 2020;49(1):162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Keefe JH, Lavie CJ. Run for your life… at a comfortable speed and not too far. BMJ Publishing Group Ltd and British Cardiovascular Society; 2013. [DOI] [PubMed] [Google Scholar]
- 12.O’Keefe JH, Patil HR, Lavie CJ, Magalski A, Vogel RA, McCullough PA, editors. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clinic proceedings; 2012: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Medicine and science in sports and exercise. 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 14.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 15.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996:81–6. [DOI] [PubMed] [Google Scholar]
- 16.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure- time physical activity and reduced plasma levels of obesity- related inflammatory markers. Obesity research. 2003;11(9):1055–64. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, de Rezende LFM, Eluf- Neto J, Wu K, Tabung FK, Giovannucci EL. Association of type and intensity of physical activity with plasma biomarkers of inflammation and insulin response. International journal of cancer. 2019;145(2):360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and Equifax nationwide death search. American journal of epidemiology. 1994;140(11):1016–9. [DOI] [PubMed] [Google Scholar]
- 19.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. American journal of epidemiology. 1984;119(5):837–9. [DOI] [PubMed] [Google Scholar]
- 20.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International journal of epidemiology. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. American journal of epidemiology. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 23.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. Journal of clinical epidemiology. 2009;62(5):511–7. e1. [DOI] [PubMed] [Google Scholar]
- 25.Eijsvogels TM, Fernandez AB, Thompson PD. Are There Deleterious Cardiac Effects of Acute and Chronic Endurance Exercise? Physiological reviews. 2016;96(1):99–125. doi: 10.1152/physrev.00029.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsey PC, Strain T, Khaw K-T, Wareham NJ, Brage S, Wijndaele K. Prospective Associations of Accelerometer-Measured Physical Activity and Sedentary Time With Incident Cardiovascular Disease, Cancer, and All-Cause Mortality. Circulation. 2020;141(13):1113–5. [DOI] [PubMed] [Google Scholar]
- 27.Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. Bmj. 2019;366:l4570. doi: 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilchrist SC, Howard VJ, Akinyemiju T, et al. Association of Sedentary Behavior With Cancer Mortality in Middle-aged and Older US Adults. JAMA Oncology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saint-Maurice PF, Troiano RP, Bassett DR, et al. Association of daily step count and step intensity with mortality among US adults. Jama. 2020;323(12):1151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner DR, Yannitsos DH, Farris MS, Johansson M, Friedenreich CM. Leisure-time physical activity and lung cancer risk: a systematic review and meta-analysis. Lung Cancer. 2016;95:17–27. [DOI] [PubMed] [Google Scholar]
- 31.Cordova-Rivera L, Gibson PG, Gardiner PA, McDonald VM. A systematic review of associations of physical activity and sedentary time with asthma outcomes. The Journal of Allergy and Clinical Immunology: In Practice. 2018;6(6):1968–81. e2. [DOI] [PubMed] [Google Scholar]
- 32.Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respiratory Soc; 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


