Abstract
We examined the longitudinal association between blood pressure (BP) and stroke incidence in young and middle-aged adults. BP measured during 9 examinations of the CARDIA study from 1985–6 to 2015–6 were used to classify participants (n=5,079) according to the 2017 ACC/AHA guideline. We used the highest BP obtained through the 3rd examination (1990–1) to define baseline BP categories; time-dependent categories (accounting for change in BP over time) were determined incorporating follow-up measurements. BP groups at ages 30 and 40 years were also defined. Stroke events were adjudicated until 2018. Mean age at baseline was 29.8 years. Stroke occurred in 100 participants. Stroke incidence (per 100,000 person-years) was higher (P < 0.001) in blacks (120; 95% CI, 95–149) vs. whites (29; 95% CI, 18–46). After adjustment with Cox models for sociodemographic and cardiovascular risk factors, stage 2 hypertension was associated with a higher risk of stroke at baseline (hazard ratio [HR], 3.72; 95% CI, 2.12–6.54), as a time-dependent variable (HR, 5.84; 95% CI, 3.43–9.95), at age 30 (HR, 4.14; 95% CI, 2.19–7.82) and at age 40 (HR, 5.59; 95% CI, 3.35–9.31), compared with normal BP. Elevated BP and stage 1 hypertension showed more modest increases in risk. As a continuous variable, systolic BP ≥90 mm Hg at age 40 was directly associated with stroke risk. These findings call for primordial prevention strategies to reduce population BP levels among young and middle-aged adults, particularly in black young adults given nearly four-fold higher stroke incidence, including within values traditionally considered to be normal.
Keywords: stroke, blood pressure, young adults, cardiovascular risk factors, cohort studies, primary prevention
Graphical Abstract
Introduction
Stroke incidence and hospitalization rates have increased among young adults in recent decades.1–5 Whether this trend is attributable to enhanced case-finding, changes in the burden of standard cardiovascular disease (CVD) risk factors, emerging risk factors such as substance abuse, or other causes remain unclear.6 Regardless of the exact mechanism, the lifetime impact of stroke on young adults is enormous.7, 8
Hypertension is the single most important risk factor for stroke.9, 10 Blood pressure (BP) moderately increased between 1988 and 2000 among young adults11 and has plateaued after the turn of the century.12 The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) BP guideline changed the definitions of BP categories by lowering thresholds, resulting in an estimated 2- to 3-fold increase in the prevalence of hypertension among adults aged 20–44 years.13 To date, little is known about the association of hypertension in the newly defined categories with stroke incidence in young and middle-aged adults. Furthermore, the prognostic importance of long-term BP trajectories starting in young adulthood to midlife stroke risk, and whether the BP-stroke relationship is age-dependent have yet to be determined. Importantly, compared with whites, blacks tend to have an earlier age of onset, a longer duration, and a greater severity of hypertension.14 Not only are blacks more likely to have worse BP control, but they are also more likely to develop stroke, a disparity that persists throughout the lifespan.15 The Coronary Artery Risk Development in Young Adults (CARDIA) study is uniquely positioned to address these gaps in knowledge with its diverse cohort, longitudinal assessment of BP over decades of follow-up, and rigorous ascertainment of stroke incidence.
Methods
Anonymized data and materials have been made publicly available at the National Institutes of Health’s Biologic Specimen and Data Repository Information Coordinating Center (https://biolincc.nhlbi.nih.gov/studies/cardia/).
Study Sample
CARDIA is a multicenter population-based prospective cohort study of the development and determinants of CVD in black and white young adults recruited at 18–30 years of age across 4 U.S. cities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). The study design has been described in detail elsewhere.16 Nine examinations have been completed to date, which were approved by institutional review boards at all sites and informed consent obtained at every examination. After the first examination (year 0 [Y0], 1985–1986), examinations were conducted at follow-up years 2 (Y2), 5 (Y5), 7 (Y7), 10 (Y10), 15 (Y15), 20 (Y20), 25 (Y25) and 30 (Y30). The present study includes participants with available data on BP measurements at Y0 (n = 5,113) who did not drop out of the study before Y5 examination date, yielding data on 5,079 participants. November 7, 1990 (median examination date) was set as the start date for participants who did not attend the Y5 examination.
BP Measurements
The methods of BP measurement have been previously described.17 In brief, during the Y0 to Y15 examinations, trained research staff measured BP 3 times in participants’ right-arm brachial artery at 1-minute intervals after the participant had been sitting in a quiet room for 5 minutes, using a random-zero sphygmomanometer (Hawksley). The average of the second and third measurements were used for the analysis. The standardized BP measurement techniques using validated equipment in this study met the recommendations by the 2017 ACC/AHA BP guideline.13 An automated oscillometric BP monitor (HEM-907XL; Omron) was used from the Y20 examination; all other aspects of measurement remained the same. To minimize potential misclassification, a calibration study was performed at Y20, and values standardized to the sphygmomanometric measures were used for the Y20, Y25, and Y30 BP measurements.18
BP Group Classification
The 2017 ACC/AHA BP guideline defined elevated BP as clinic-measured systolic BP (SBP) of 120 mm Hg to 129 mm Hg and diastolic BP (DBP) less than 80 mm Hg, and stage 1 hypertension as SBP of 130 mm Hg to 139 mm Hg or DBP of 80 mm Hg to 89 mm Hg.13 Accordingly, participants in the present study were categorized as having normal BP, elevated BP, stage 1 hypertension, or stage 2 hypertension (see Table S1 for definitions). Two main strategies for BP group classification were used: the “cumulative” method and the “dynamic” method. The former has been described previously,17 and uses the highest BP measured from the first examination to the examination closest to, but not after, the time point where follow-up begins. In contrast, the dynamic method uses the last examination prior to the time point where follow-up begins. These two strategies served as the basis for different analytical approaches (see Table S2 for detailed description).
Stroke Ascertainment
The primary outcome was fatal and nonfatal stroke events. Stroke was defined as the rapid onset of a headache, meningsmus, or a persistent neurologic deficit attributable to an obstruction or rupture of the arterial system (including stroke occurring during a procedure such as angiography or surgery). Deficits resolving in less than 24 hours accompanied by imaging indicating acute stroke were adjudicated as stroke.19 For the present investigation, the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria for ischemic stroke were applied.20 All stroke hospitalizations and deaths identified from study entry to August 2018 were examined. Mortality (other than stroke) was also examined as a competing event. Research staff collected information on hospitalizations and outpatient medical procedures during examinations and annual contacts with participants or designated proxies. Medical records were requested and used to adjudicate stroke events. Semi-annual contacts were conducted as well to update participants’ contact information and vital status. Vital status was additionally ascertained through periodic searches of the National Death Index. Medical records, death certificates, informant interviews (for outpatient deaths), and autopsy reports, when available, were used to adjudicate stroke events and deaths. Two physician members of the Endpoints Committee independently reviewed medical records to adjudicate each possible stroke event or underlying cause of death using specific definitions and a detailed manual of operations (http://www.cardia.dopm.uab.edu). If disagreement occurred between the primary reviewers, the case was reviewed by the full committee.17 Definite or probable stroke events (including deaths) were used in the analysis. The definitions used in the algorithm are available online (http://www.cardia.dopm.uab.edu/images/more/2020/CARDIA_Endpoint_Events_MOO_v10_09_2017_with_reports_instructions.pdf). Brain imaging studies were used to differentiate between ischemic stroke and intracerebral hemorrhage. Transient ischemic attacks (TIA) were not counted as strokes in this study.
Other Covariates
Standardized protocols for data collection were used across study centers and measurements have previously been described,16 and are available online (https://www.cardia.dopm.uab.edu). Data from all 9 examinations were used. Participants were asked to fast for at least 12h and to avoid smoking and heavy physical activity for at least 2h before each examination. Sociodemographic characteristics and lifestyle habits were assessed through questionnaires. Medication use was reported by participants who also brought in medications for verification. Blood was drawn, separated and plasma frozen to −70°C prior to analysis in a central laboratory. Glucose was assayed using the hexokinase method. Total cholesterol and high-density lipoprotein (HDL) cholesterol levels were measured enzymatically by the Northwest Lipid Laboratory. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation. Cigarette smoking was defined as smoking at least 5 cigarettes per week almost every week. If answered “yes”, the subject was asked if he or she still smoked regularly, and those who responded “no” were considered to be past smokers. Measured height and weight were used to calculate body mass index (BMI) as weight in kilograms divided by the square of height in meters (kg/m2). Leisure-time physical activity was measured in standardized units based on duration and intensity reported through a Physical Activity History questionnaire. Excessive alcohol consumption was defined as ≥ 20 g/day in women and ≥ 30 g/day in men. Diabetes was defined as fasting plasma glucose ≥ 126 mg/dL, oral glucose tolerance test ≥ 200 mg/dL, glycosylated hemoglobin ≥ 6.5% or use of medications (when available).
Statistical Analysis
Analyses were performed using R software, version 3.6.1 (R Development Core Team) and IBM SPSS Statistics, version 25 (IBM SPSS Inc.). Characteristics across baseline BP groups are presented as mean (SD) for continuous variables and as frequencies for categorical variables. Total stroke incidence rates with person-time denominators were calculated for the BP groups; confidence intervals were estimated with Fisher’s exact method. Cox proportional hazards models21 were constructed to estimate the associations (hazard ratios [HRs] and 95% confidence intervals [CIs]) between baseline BP groups (based on measurements performed during Y0, Y2 and Y5 examinations) and all-stroke incidence. Follow-up started at Y5 exam (1990–1991) and lasted through 2018. The time-to-event variable was calculated as the difference between the start date and the date of stroke, death, or last contact (whichever came first). An unadjusted analysis was initially conducted. Subsequently, adjustment was made for sociodemographic variables (age, race, sex, study center and education). Finally, multivariable models additionally adjusted for clinical and behavioral risk factors (smoking, diabetes, LDL cholesterol, HDL cholesterol, BMI, physical activity, and excessive alcohol use). Data obtained during Y0, Y2 and Y5 examinations were used to assess the covariates; average values were used for BMI, LDL cholesterol, HDL cholesterol, alcohol consumption and physical activity (last available information for the remaining covariates). Model covariates were selected a priori if they were well-established stroke risk factors22, 23 or previously shown to be predictive of CVD outcomes in this cohort.17 BP groups were further handled as time-dependent (T-D) variables in extended Cox regression models incorporating data from Y7, Y10, Y15, Y20, Y25 and Y30 exams; this approach is suitable for longitudinal studies as it accounts for changes in exposure levels over time.24 Both the cumulative and dynamic methods (as defined in Table S2) for BP classification were applied.
Cumulative stroke incidence rates across BP groups at ages 30 and 40 years were estimated using the Fine-Gray subdistribution hazard regression model,25 with death treated as a competing event. We used the Fine-Gray model because standard survival models might produce biased estimates of stroke incidence in the presence of competing risks.26 HRs (95% CIs) for incident stroke in BP groups at ages 30 and 40 years were analyzed and compared using Cox proportional hazards models, with age serving as the time scale.27 Participants who experienced stroke or died and those lost to follow-up before the specified ages were excluded. Different strategies for BP group classification (Table S2) were applied and we adjusted for the above-listed covariates (as measured at/last before the specified ages). We used spline methodology to assess and compare the relationship of continuous SBP and DBP levels at age 40 years (or closest before) with subsequent stroke incidence. This was done by adding penalized spline terms for SBP and DBP (separately) to multivariable Cox regression models (as specified above) that also included antihypertensive treatment as a covariate.28 The proportional hazards assumption was tested using Schoenfeld residuals and was met in all models. Missing values did not exceed 1% in any of the baseline variables considered in the analysis. For missing values of covariates repeatedly assessed during follow-up examinations, the last observation carried forward rule was applied. The Harrell C-statistic, which takes into account time to event and censoring, was used as a measure of risk discrimination.29 A P value ≤ 0.05 was considered statistically significant.
Results
We included 5,079 participants with mean age of 29.8 (SD, 3.7) years at the Y5 exam, 55% were female, and 52% were black. The majority of the cohort (82%) participated in all 3 exams (Y0, Y2, and Y5), whereas 13% participated in Y0 and either Y2 or Y5, and 5% in Y0 only. The distribution of BP categories at baseline was 66% normal BP, 10% elevated BP, 18% stage 1 hypertension, and 6% stage 2 hypertension. The mean (SD) SBP and DBP levels were 109 (10) and 68 (8) mm Hg, respectively, and only 102 participants (2%) reported taking antihypertensive medication at any of the 3 exams. On average, higher BP groups were characterized by greater proportions of male and black participants, higher levels of BMI, total and LDL cholesterol, and fasting glucose, and lower levels of HDL cholesterol. Excessive alcohol consumption was more prevalent in hypertensive participants compared to those with normal BP (Table 1). Baseline characteristics across BP groups stratified by race are presented in Table S3.
Table 1.
Characteristics of Participants in the CARDIA Study According to 2017 ACC/AHA Guideline for Blood Pressure Classification
| Characteristics* | Overall (n=5,079) | Baseline Blood Pressure Groups† | |||
|---|---|---|---|---|---|
| Normal (n=3,331) | Elevated (n=516) | Stage 1 HTN (n=934) | Stage 2 HTN (n=298) | ||
| Age, y | 29.8±3.7 | 29.6±3.7 | 29.2±3.7 | 30.1±3.6 | 31.3±3.2 |
| Female, n (%) | 2,778 (54.7) | 2,190 (65.7) | 139 (26.9) | 330 (35.3) | 119 (39.9) |
| Black, n (%) | 2,614 (51.5) | 1,598 (48.0) | 281 (54.5) | 535 (57.3) | 200 (67.1) |
| Education, y | 14.3±2.7 | 14.4±2.8 | 14.0±2.4 | 14.1±2.5 | 13.7±2.5 |
| BMI, kg/m2 | 25.2±5.3 | 24.4±4.7 | 25.9±4.8 | 26.7±6.0 | 28.9±7.4 |
| Smoking, n (%) | |||||
| Current | 1,533 (30.2) | 1,014 (30.5) | 172 (33.3) | 247 (26.4) | 100 (33.7) |
| Past | 706 (13.9) | 475 (14.3) | 75 (14.5) | 130 (13.9) | 26 (8.8) |
| Never | 2,838 (55.9) | 1,841 (55.3) | 269 (52.1) | 557 (59.6) | 171 (57.6) |
| Total cholesterol, mg/dL | 177.2±31.1 | 175.0±30.0 | 178.2±30.4 | 181.1±32.1 | 187.6±37.0 |
| LDL cholesterol, mg/dL | 130.4±31.6 | 127.8±30.5 | 132.0±31.7 | 135.2±32.5 | 141.5±36.7 |
| HDL cholesterol, mg/dL | 53.3±12.6 | 54.3±12.4 | 51.7±12.6 | 51.7±13.0 | 49.6±12.0 |
| SBP, mm Hg | 108.9±9.9 | 103.9±6.5 | 116.7±4.5 | 116.7±7.3 | 126.6±10.3 |
| DBP, mm Hg | 68.4±8.3 | 64.8±5.9 | 68.6±5.4 | 76.2±5.0 | 84.0±8.6 |
| Fasting glucose, mg/dL | 82.6±16.4 | 81.4±13.7 | 84.3±14.1 | 84.5±20.6 | 87.8±27.0 |
| Physical activity, units‡ | 396.1±256.5 | 383.9±249.5 | 470.2±279.1 | 407.6±260.3 | 367.8±257.2 |
| Excessive alcohol use, n (%) | 673 (13.3) | 366 (11.0) | 89 (17.2) | 158 (16.9) | 60 (20.2) |
| eGFR, mL/min/1.73 m² | 122.0±22.9 | 121.2±23.0 | 124.9±24.3 | 123.1±22.0 | 123.3±22.4 |
| No. of clinic visits§ | 2.8±0.5 | 2.7±0.6 | 2.8±0.6 | 2.8±0.5 | 2.9±0.5 |
BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HTN, hypertension; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Data are presented as mean (SD) unless otherwise specified.
Based on cumulative data obtained during CARDIA clinic visits taken place at Y0, Y2 and Y5; the latter defined the follow-up start date for the main analysis. Average values are presented for BMI, total cholesterol, LDL cholesterol, HDL cholesterol, SBP, DBP, and physical activity (last available information for the other variables presented).
Classification based on the highest blood pressure measured during CARDIA Y0, Y2 and Y5 clinic visits.
As assessed with the CARDIA Physical Activity History questionnaire.
Number of clinic visits from Y0 (first CARDIA examination) to Y5 (after which follow-up began).
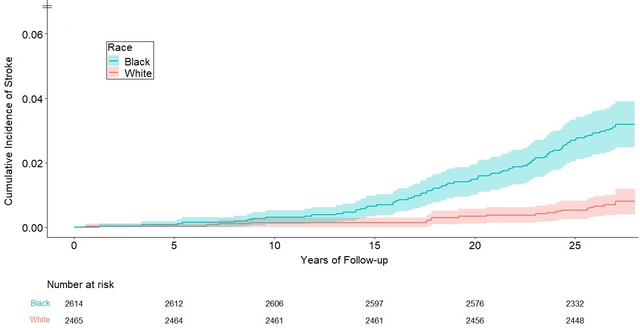
During a mean (SD) follow-up period of 26.1 (3.6) years, 100 individuals experienced fatal/nonfatal incident stroke; 65 were classified as ischemic stroke and 34 as hemorrhagic stroke (1 was unclassified). The median age at stroke occurrence was 49.8 (Q1-Q3, 45.4–54.7) years. The overall incidence of stroke per 100,000 person-years was 76 (95% CI: 62–92); similar between men (71, 95% CI: 51–96) and women (79, 95% CI: 60–103) (P = 0.61); but substantially higher in black (120, 95% CI: 95–149) vs. white (29, 95% CI: 18–46) participants (P < 0.001). There was no evidence of race-specific rates differing by sex (28.9 in white women vs. 29.7 in white men, P = 0.95; 123.7 in black women vs. 113.6 in black men, P = 0.71). The age- and sex-adjusted cumulative incidence rates of stroke at the end of follow-up, treating death as a competing event, were 3.2% (95% CI: 2.5%−3.9%) in blacks vs. 0.8% (0.4%−1.2%) in whites (Figure 1).
Figure 1.
Age- and sex-adjusted cumulative incidence curves for total stroke in black vs. white participants (n = 5,079 subjects; 100 stroke cases), with death treated as a competing event using the Fine and Gray method (P < 0.001 for the race comparison). Colored areas around the curves represent 95% confidence intervals.
Stage 2 hypertension, as measured at baseline, was associated with stroke incidence (unadjusted HR = 5.97, 95% CI: 3.64–9.80, compared with normal BP [Table 2]). Multivariable adjustment for sociodemographic, clinical and behavioral risk factors resulted in a moderate attenuation of the association (HR = 3.72, 95% CI: 2.12–6.54). Elevated BP and stage 1 hypertension were associated with more modest, nonsignificant increases in risk. Repeated BP measurements were performed among 81% (Y7), 78% (Y10), 73% (Y15), 72% (Y20), 72% (Y25), and 71% (Y30) of the baseline study sample, among survivors at each examination. With T-D BP categories, accounting for changes in BP during follow-up, a stronger association was observed between stage 2 hypertension and incident stroke, especially using the dynamic method (multivariable-adjusted HR = 5.84, 95% CI: 3.43–9.95, compared with normal BP [Table 2]). In a sensitivity analysis, using the average (rather than highest) BP measurements during Y0, Y2, and Y5 examinations to define baseline BP groups (restricted to participants with ≥2 measurements; n = 4,808, 97 incident stroke cases), fewer subjects were classified as having hypertension. Relative to the main analysis (shown in Table 2), this analysis (Table S4) yielded a stronger association with stroke for stage 1 hypertension (multivariable-adjusted HR = 2.15, 95% CI: 1.19–3.91) and a more moderate association for stage 2 hypertension (multivariable-adjusted HR = 3.18, 95% CI: 1.52–6.64), compared with normal BP.
Table 2.
Association of 2017 ACC/AHA Blood Pressure Categories with Subsequent Stroke Incidence Among CARDIA Participants
| Exposure Definition | Blood Pressure Categories | C-Statistic (se) | |||
|---|---|---|---|---|---|
| Normal BP | Elevated BP | Stage 1 HTN | Stage 2 HTN | ||
| Baseline classification | |||||
| No. of participants | 3,331 | 516 | 934 | 298 | - |
| No. of events | 50 | 10 | 17 | 23 | - |
| Incidence rate* | 57 (43–75) | 74 (36–136) | 71 (41–113) | 320 (203–480) | - |
| Unadjusted | 1 (ref.) | 1.32 (0.67–2.61) | 1.27 (0.73–2.20) | 5.97 (3.64–9.80) | .613 (.029) |
| Model 1† | 1 (ref.) | 1.43 (0.71–2.87) | 1.17 (0.67–2.07) | 4.45 (2.64–7.49) | .782 (.022) |
| Model 2‡ | 1 (ref.) | 1.42 (0.70–2.86) | 1.21 (0.68–2.15) | 3.72 (2.12–6.54) | .812 (.022) |
| T-D cumulative method | |||||
| Unadjusted | 1 (ref.) | 0.99 (0.34–2.82) | 1.15 (0.54–2.43) | 6.34 (3.74–10.77) | .714 (.025) |
| Model 1† | 1 (ref.) | 0.90 (0.33–2.49) | 1.05 (0.50–2.21) | 4.65 (2.70–8.01) | .800 (.022) |
| Model 2‡ | 1 (ref.) | 0.89 (0.33–2.40) | 1.06 (0.50–2.26) | 4.30 (2.28–7.49) | .824 (.021) |
| T-D dynamic method | |||||
| Unadjusted | 1 (ref.) | 2.08 (0.86–4.99) | 2.05 (0.96–4.38) | 8.65 (5.19–14.42) | .732 (.024) |
| Model 1† | 1 (ref.) | 1.81 (0.76–4.33) | 1.81 (0.85–3.85) | 6.34 (3.6–10.68) | .810 (.021) |
| Model 2‡ | 1 (ref.) | 1.84 (0.79–4.32) | 1.78 (0.83–3.82) | 5.84 (3.43–9.95) | .832 (.021) |
Figures represent hazard ratios (95% confidence intervals) for incident stroke associated with BP groups (unless otherwise specified). The Harrell concordance index (C-statistic) is provided for each model as a measure of risk discrimination. The T-D cumulative method defined BP categories according to the highest BP measured up to each follow-up interval; the T-D dynamic method defined BP categories according to the last BP measurement before each follow-up interval. The number of participants at each BP category in the T-D analysis is not presented because it changes during follow-up.
BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults; HTN, hypertension; T-D, time-dependent.
Incidence density per 100,000 person-years (Fisher's exact 95% confidence intervals).
Model 1: Adjusted for baseline age, race, sex, study center, and education.
Model 2: Model 1 + smoking, diabetes, LDL cholesterol, HDL cholesterol, BMI, physical activity, and excessive alcohol use (as measured at baseline).
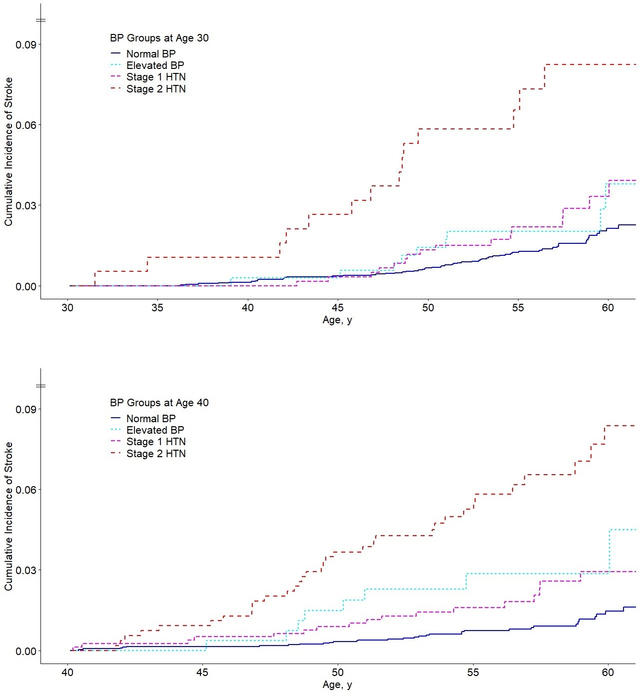
The cumulative incidence curves of total stroke across BP groups at ages 30 and 40 years, with death treated as a competing event and using the last measurements before attaining these ages, are depicted in Figure 2. At age 30, a higher rate of stroke was shown for stage 2 hypertension while the curves for normal BP, elevated BP, and stage 1 hypertension did not differ significantly. A clearer separation of the curves was shown at age 40. Multivariable-adjusted Cox regression models supported these findings (Table 3). High BP groups –as classified using the dynamic method– were more predictive of stroke incidence at age 40 than at age 30. At age 30, the adjusted models revealed a significant association for stage 2 hypertension (HR = 4.14, 95% 2.19–7.82), while ≈50% (nonsignificant) increases in risk were estimated for elevated BP and stage 1 hypertension, compared with normal BP. A stronger graded relationship was demonstrated at age 40, with significant adjusted associations shown for elevated BP (HR = 3.09, 95% CI: 1.35–7.04), stage 1 hypertension (HR = 2.34, 95% CI: 1.30–4.23), and stage 2 hypertension (HR = 5.59, 95% CI: 3.35–9.31), compared with normal BP. Using the same sets of covariates, the discriminatory power of the model at age 40 was superior to that at age 30 (Table 3). Applying the cumulative method, high BP groups at age 40 were consistently more predictive of stroke incidence than at age 30 (Table S5).
Figure 2.
Cumulative incidence curves for total stroke according to blood pressure group at age 30 years (upper panel), based on 5,084 subjects and 99 stroke events, and at age 40 years (lower panel), based on 4,977 subjects and 91 stroke events. Blood pressure groups were determined according to the last examination before attaining 30 and 40 years of age, as appropriate (dynamic method). The cumulative incidence was calculated as a function of age, with death treated as a competing event using the Fine and Gray method (P < 0.001 between BP categories by log-rank test in both panels); BP, blood pressure; HTN, hypertension.
Table 3.
Association of 2017 ACC/AHA Blood Pressure Categories at Different Ages with Subsequent Stroke Incidence Among CARDIA Participants
| Age at start of follow-up | Blood Pressure Groups (Dynamic Method) | C-Statistic (se) | |||
|---|---|---|---|---|---|
| Normal BP | Elevated BP | Stage 1 HTN | Stage 2 HTN | ||
| Age 30 Years | |||||
| No. of subjects | 3,942 | 353 | 601 | 188 | - |
| No. of events | 60 | 9 | 16 | 14 | - |
| Incidence rate* | 59 (45–75) | 99 (45–187) | 104 (60–170) | 309 (169–519) | - |
| Unadjusted | 1 (ref.) | 1.67 (0.83–3.37) | 1.80 (1.04–3.12) | 5.50 (3.05–9.90) | .606 (.028) |
| Model 1† | 1 (ref.) | 1.56 (0.74–3.29) | 1.65 (0.93–2.94) | 4.40 (2.40–8.09) | .783 (.022) |
| Model 2‡ | 1 (ref.) | 1.47 (0.70–3.09) | 1.55 (0.86–2.81) | 4.14 (2.19–7.82) | .803 (.023) |
| Age 40 Years | |||||
| No. of subjects | 3,367 | 271 | 794 | 545 | - |
| No. of events | 32 | 8 | 17 | 34 | - |
| Incidence rate* | 58 (40–82) | 188 (81–371) | 130 (76–208) | 408 (283–570) | - |
| Unadjusted | 1 (ref.) | 3.33 (1.54–7.21) | 2.24 (1.24–4.03) | 7.27 (4.49–11.77) | .712 (.029) |
| Model 1† | 1 (ref.) | 2.98 (1.34–6.64) | 2.12 (1.18–3.80) | 5.23 (3.16–8.66) | .811 (.023) |
| Model 2‡ | 1 (ref.) | 3.09 (1.35–7.04) | 2.34 (1.30–4.23) | 5.59 (3.35–9.31) | .840 (.022) |
Figures represent hazard ratios (95% confidence intervals) for incident stroke associated with BP groups (unless otherwise specified). The Harrell concordance index (C-statistic) is provided for each model as a measure of risk discrimination. BP groups at ages 30 and 40 years were determined according to the last examination before participants attained that specified age (dynamic method).
BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults; HTN, hypertension.
Incidence density per 100,000 person-years (Fisher's exact 95% confidence intervals).
Model 1: Adjusted for race, sex, study center, and education.
Model 2: Model 1 + smoking, diabetes, LDL cholesterol, HDL cholesterol, BMI, physical activity, and excessive alcohol use (as measured at/last before ages 30 or 40 years, as appropriate).
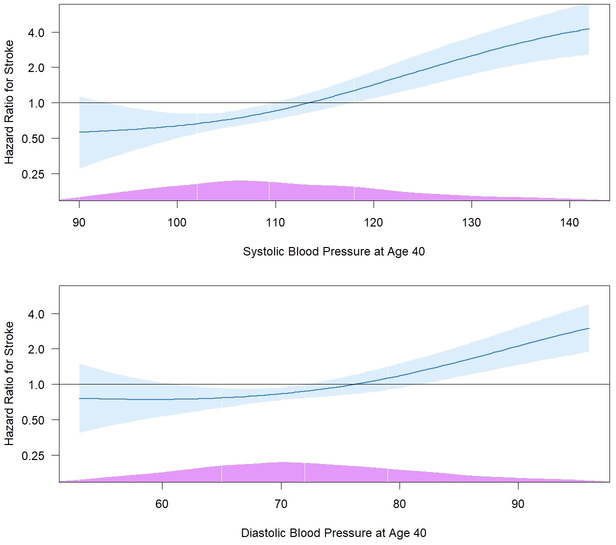
We also evaluated and compared the prognostic importance of SBP and DBP (as continuous variables) at age 40 in subsequent development of stroke. Multivariable Cox regression models were fitted, separately for SBP and DBP, with age as the time scale and a penalized spline term added for SBP/DBP. The relationships of SBP and DBP with stroke incidence were generally linear (tests of nonlinear curves were nonsignificant), with a stronger association observed for SBP (multivariable-adjusted HR = 1.84, 95% CI: 1.46–2.31, per 1 SD increase) compared with DBP (multivariable-adjusted HR = 1.58, 95% CI: 1.25–1.99, per 1 SD increase); for SBP, a graded association began at approximately 90 mm Hg (Figure 3). Restricted to black participants (n = 2,543, 74 stroke cases) and analyzed separately, the multivariable-adjusted HRs for stroke at age 40 were 1.87 (95% CI: 1.45–2.40) for SBP and 1.54 (95% CI: 1.20–1.98) for DBP, per 1 SD increases, similar to estimates in the entire cohort. Spline-based HRs are depicted in Figure S1.
Figure 3.
Spline-based hazard ratios (95% confidence intervals) for stroke incidence associated with systolic blood pressure (upper panel) and diastolic blood pressure (lower panel), in mm Hg, as measured at age 40 years or last before. The curves are based on multivariable Cox models adjusted for covariates assessed at age 40 years or last before, including race, sex, study center, education, antihypertensive medications, smoking, diabetes, LDL cholesterol, HDL cholesterol, BMI, physical activity, and excessive alcohol use. The reference points are 114 and 76 mm Hg (65th percentile) for systolic and diastolic blood pressure, respectively. The histograms at the bottom (purple) show the distribution of blood pressure in the sample. The highest and lowest 2.5% of blood pressure values have been winsorized for visualization purposes.
Discussion
We examined prospectively the association between BP and incident stroke in over 5,000 participants of a population-based cohort study of black and white young adults from 4 U.S. cities. With up to 9 BP measurements between 1985–1986 and 2015–2016, and stroke events identified through 2018, incidence rate of stroke, per 100,000 person-years, was similar between women and men, but 4-fold higher in black vs. white participants. Using the 2017 ACC/AHA BP guideline classifications, stage 2 hypertension in young and later adulthood (up to age 40) was strongly and significantly associated with stroke risk compared to participants with normal BP. Elevated BP and stage 1 hypertension were associated with more modest increases in risk. In a T-D analysis, the last BP measurement before each follow-up interval was more strongly related to stroke risk than the highest measurement up to that point. Analyzed separately, SBP and DBP at age 40 were both linearly associated with adjusted stroke risk throughout the entire range, with SBP showing a stronger and more graded association than DBP.
Approximately 10–15% of all strokes occur in adults aged 18 to 50 years.30, 31 A temporal increase in stroke incidence in younger age groups are concerning. Data from the U.S. Nationwide Inpatient Sample reveal that although hospitalization rates for acute ischemic stroke decreased by 18.4% between 2000 and 2010, trends diverged by age categories. Thus, the decrease observed in individuals aged 65–84 years (−28.5%) and ≥85 years (−22.1%) contrasted with a substantial increase in individuals aged 25–44 years (+43.8%).3 An analysis of the Greater Cincinnati Northern Kentucky Stroke Study suggested that between 1993–4 and 2005 the mean age at stroke decreased by 2 years, while the proportion of stroke contributed by patients aged 20–54 years increased from 12.9% to 18.6%. This increase was significant among both blacks and whites and was primarily seen in ischemic stroke.32 Higher stroke incidence rates have been documented in blacks vs. whites at every age, with the greatest relative risks seen in younger age groups,33–35 a finding supported by our data. About two-thirds of stroke events in our study were classified as ischemic, and one-third as hemorrhagic. This partition is also in line with the literature, showing that among young stroke victims, a greater proportion (≈40%) is due to subarachnoid hemorrhage and intracranial hemorrhage, compared with the general stroke population (≈20%).31
Previous studies have demonstrated increases in traditional stroke risk factors among younger stroke patients.1, 31, 32, 36 Hypertension is particularly prevalent among the latter.32, 37 Moreover, it was recently suggested that young adults have lower awareness, treatment, and control of hypertension compared with adults older than 40 years.12 Our findings highlight the importance of BP control, ideally maintaining normal levels, in primary prevention of stroke in young and middle-aged adults. Hypertension was strongly associated with stroke risk in our study, yet the relationship was dynamic; high BP categories at age 40 were more predictive of stroke risk than the same categories at age 30. Furthermore, our T-D analysis demonstrated that the most recent BP measurement was most strongly associated with stroke. Rather than being a fixed exposure that remains unchanged after a single assessment, BP is a dynamic risk factor that changes over time, for better or for worse. Beyond temporal changes in BP levels, the duration of hypertension also plays a contributory role. Indeed, among ≈240,000 patients with atrial fibrillation from the Republic of Korea, longer duration of hypertension before atrial fibrillation diagnosis was associated with higher risk of subsequent ischemic stroke regardless of baseline SBP levels.38 In support of this concept, it was recently shown that using long-term measures of cumulative BP may improve CVD risk prediction over a single measurement.39 In CARDIA, cumulative BP in young adults (determined by summing the product of average mm Hg and the years between each two consecutive clinic visits from Y0 to Y15) was associated with subsequent CVD risk and improved risk discrimination compared to single BP assessments or changes in BP.40 Previously, data from the Multi-Ethnic Study of Atherosclerosis (MESA) and CARDIA studies were used to examine whether effective treatment of hypertension can reduce CVD risk to that seen in individuals who have always had ideal BP levels. It was found that participants with well-controlled hypertension on antihypertensive medication still constituted a high-risk group for CVD, possibly attributable to a longer period of prior exposure to higher BP levels.41 Hypertension is thought to drive cerebrovascular disease in young adults through mechanisms involving endothelial dysfunction and oxidative stress,42, 43 left atrial structure and function,44 and coronary artery calcification.45
Importantly, in MESA (n=1,457; mean baseline age, 58.1 years), beginning with a SBP level of 90 mm Hg, there was a stepwise increase in the prevalence of classic CVD risk factors, coronary artery calcium, and HRs for CVD events over a 15-year follow-up.46 In CARDIA, cumulative exposure to BP in the prehypertension range (SBP of 120 to 139 mm Hg or DBP of 80 to 89 mm Hg) during young adulthood (up to age 35) was associated with coronary calcium later in life.47 Among 1.3 million adults in a general outpatient population from Kaiser Permanente Northern California (median age [IQR], 53 [40–64] years), both systolic and diastolic hypertension independently influenced the risk of CVD events, with a linear relationship seen for SBP and a J-curve for DBP.48 The present findings regarding SBP at age 40 extend these results to stroke incidence in middle-aged adults by demonstrating a positive graded association that begins at a SBP level as low as 90 mm Hg. Taking into consideration that the vast majority of incident strokes in our study occurred in the normotensive category, these results highlight the importance of primordial prevention to maintain optimal BP levels, including within values traditionally considered to be normal.13
Strengths and Limitations
The findings of this study should be interpreted in the context of several potential limitations. As stroke in young and middle-aged adults is relatively uncommon,49 despite the large biracial population-based cohort and long-term follow-up being used, the small number of incident strokes resulted in reduced statistical precision. This imprecision may explain the greater risk observed for elevated BP compared with stage 1 hypertension in some analyses; indeed, as a continuous variable, the adjusted association with stroke of SBP at age 40 appeared linear. Power considerations also limited subgroup analysis and interaction testing. It remains unclear how BP measurements in this study, collected in a highly controlled research setting, correspond to BP measurements commonly obtained in a typical clinic setting. In addition, research participation effects may have contributed to changes in behaviors or health care provided.50 Also, missing data on variables repeatedly assessed during follow-up may have resulted in an attenuation of regression coefficients caused by infrequent updating. This study also has notable strengths, including the large, diverse and well-characterized cohort, adjudication of suspected stroke events by a panel of physicians using detailed evaluation criteria, high retention rates, and the standardized data collection protocols and rigorous quality control. The young age of participants included in the CARDIA study means that all strokes in this analysis should be considered premature events. BP was repeatedly measured during 9 examinations, allowing assessment of long-term changes. These multiple time-point assessments over a period of 3 decades provide a more accurate picture of true exposure than would be possible from evaluating single points. Furthermore, inclusion of BP categories as T-D variables in the survival models ensures appropriate analysis of these categories as recently defined by the ACC/AHA BP guideline.13
Conclusions
In conclusion, the association of the 2017 ACC/AHA high BP categories with stroke incidence in CARDIA varies with age, strengthening from young adulthood to midlife. The BP-stroke relationship is dynamic, with the last measurement most strongly related to risk. These findings call for primordial prevention strategies to reduce population BP levels among young and middle-aged adults, particularly in black young adults given nearly four-fold higher stroke incidence, including within values traditionally considered to be normal.
Perspectives
The association between high BP and stroke is well known. Our results demonstrate the remarkable increase in stroke risk in black vs. white young adults, and highlight the importance of primordial prevention of stroke by maintaining optimal BP levels in young adulthood, including within values traditionally considered to be normal.
Supplementary Material
Novelty and Significance.
What Is New?
We prospectively examined the association between blood pressure (BP) as longitudinally measured over 30 years and incident stroke in over 5,000 participants of a population-based cohort study of black and white young adults from 4 U.S. cities.
Stroke incidence was 4-fold higher in black vs. white participants.
The association of high BP categories with stroke incidence varied with age, strengthening from young adulthood to midlife.
The BP-stroke relationship was dynamic, with the last BP measurement most strongly related to stroke risk.
At age 40, a stepwise increase in stroke risk was apparent from a systolic BP of 90mm Hg upwards.
What Is Relevant?
These findings call for more careful management of blood pressure amongst younger people and particularly black younger people given nearly four-fold higher incidence of stroke.
Taking into consideration that the majority of incident strokes in our study occurred in participants whose blood pressure levels have traditionally been considered to be normal, an effort to shift the whole population blood pressure levels down should complement efforts to control high blood pressure.
Summary
BP trajectory beginning in young adulthood was associated with long-term incidence of stroke.
Acknowledgments
Source of Funding
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content.
Abbreviations:
- ACC
American College of Cardiology
- AHA
American Heart Association
- BP
blood pressure
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HDL
high-density lipoprotein
- HR
hazard ratio
- LDL
low-density lipoprotein
- MESA
Multi-Ethnic Study of Atherosclerosis
- SBP
systolic blood pressure
- SD
standard deviation
- T-D
time-dependent
Footnotes
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosures
None.
References
- 1.George MG, Tong X, Kuklina EV and Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol 2011;70:713–21. [DOI] [PubMed] [Google Scholar]
- 2.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, Broderick JP and Kleindorfer DO. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez L, Kim-Tenser MA, Sanossian N, Cen S, Wen G, He S, Mack WJ and Towfighi A. Trends in acute ischemic stroke hospitalizations in the United States. Journal of the American Heart Association. 2016;5:e003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tibæk M, Dehlendorff C, Jørgensen HS, Forchhammer HB, Johnsen SP and Kammersgaard LP. Increasing incidence of hospitalization for stroke and transient ischemic attack in young adults: a registry-based study. Journal of the American Heart Association. 2016;5:e003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Béjot Y, Daubail B, Jacquin A, Durier J, Osseby G-V, Rouaud O and Giroud M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. Journal of Neurology, Neurosurgery & Psychiatry. 2014;85:509–513. [DOI] [PubMed] [Google Scholar]
- 6.Béjot Y, Delpont B and Giroud M. Rising Stroke Incidence in Young Adults: More Epidemiological Evidence, More Questions to Be Answered. Journal of the American Heart Association. 2016;5:e003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Synhaeve NE, Arntz RM, Maaijwee NAM, Rutten-Jacobs LCA, Schoonderwaldt HC, Dorresteijn LDA, Kort PLMd, Dijk EJv and Leeuw F-Ed. Poor Long-Term Functional Outcome After Stroke Among Adults Aged 18 to 50 Years. Stroke. 2014;45:1157–1160. [DOI] [PubMed] [Google Scholar]
- 8.Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ and de Leeuw F-E. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309:1136–1144. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G and Yusuf S. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–75. [DOI] [PubMed] [Google Scholar]
- 10.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS and Wilson JA. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajjar I and Kotchen TA. Trends in Prevalence, Awareness, Treatment, and Control of Hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y and Moran AE. Trends in the Prevalence, Awareness, Treatment, and Control of Hypertension Among Young Adults in the United States, 1999 to 2014. Hypertension. 2017;70:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 14.Levine DA, Lewis CE, Williams OD, Safford MM, Liu K, Calhoun DA, Kim Y, Jacobs DR Jr. and Kiefe CI. Geographic and demographic variability in 20-year hypertension incidence: the CARDIA study. Hypertension. 2011;57:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP and Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med 2013;173:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., Liu K and Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 17.Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, Gidding SS, Bress AP, Greenland P, Muntner P and Lloyd-Jones DM. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline With Cardiovascular Events Later in Life. JAMA. 2018;320:1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs DR Jr., Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG and Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E and Sacco RL. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–93. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL and Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society: Series B (Methodological). 1972;34:187–202. [Google Scholar]
- 22.Chambless LE, Heiss G, Shahar E, Earp MJ and Toole J. Prediction of Ischemic Stroke Risk in the Atherosclerosis Risk in Communities Study. American Journal of Epidemiology. 2004;160:259–269. [DOI] [PubMed] [Google Scholar]
- 23.Dufouil C, Beiser A, McLure LA, Wolf PA, Tzourio C, Howard VJ, Westwood AJ, Himali JJ, Sullivan L, Aparicio HJ, Kelly-Hayes M, Ritchie K, Kase CS, Pikula A, Romero JR, D’Agostino RB, Samieri C, Vasan RS, Chêne G, Howard G and Seshadri S. Revised Framingham Stroke Risk Profile to Reflect Temporal Trends. Circulation. 2017;135:1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher LD and Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annual review of public health. 1999;20:145–157. [DOI] [PubMed] [Google Scholar]
- 25.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94:496–509. [Google Scholar]
- 26.Wolbers M, Koller MT, Witteman JC and Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–61. [DOI] [PubMed] [Google Scholar]
- 27.Thiébaut AC and Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med 2004;23:3803–20. [DOI] [PubMed] [Google Scholar]
- 28.Meira-Machado L, Cadarso-Suárez C, Gude F and Araújo A. smoothHR: an R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med 2013;2013:745742–745742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrell FE Jr., Lee KL and Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 30.Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle H, Schroth G, Remonda L, Sturzenegger M and Fischer U. Ischaemic stroke in young adults: predictors of outcome and recurrence. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George MG. Risk Factors for Ischemic Stroke in Younger Adults: A Focused Update. Stroke. 2020;51:729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George MG, Tong X and Bowman BA. Prevalence of Cardiovascular Risk Factors and Strokes in Younger Adults. JAMA Neurol 2017;74:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs BS, Boden-Albala B, Lin IF and Sacco RL. Stroke in the young in the northern Manhattan stroke study. Stroke. 2002;33:2789–93. [DOI] [PubMed] [Google Scholar]
- 34.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, Pancioli A, Jauch E, Shukla R and Broderick J. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–31. [DOI] [PubMed] [Google Scholar]
- 35.Pathak EB and Sloan MA. Recent racial/ethnic disparities in stroke hospitalizations and outcomes for young adults in Florida, 2001–2006. Neuroepidemiology. 2009;32:302–11. [DOI] [PubMed] [Google Scholar]
- 36.von Sarnowski B, Putaala J, Grittner U, Gaertner B, Schminke U, Curtze S, Huber R, Tanislav C, Lichy C, Demarin V, Basic-Kes V, Ringelstein EB, Neumann-Haefelin T, Enzinger C, Fazekas F, Rothwell PM, Dichgans M, Jungehulsing GJ, Heuschmann PU, Kaps M, Norrving B, Rolfs A, Kessler C and Tatlisumak T. Lifestyle risk factors for ischemic stroke and transient ischemic attack in young adults in the Stroke in Young Fabry Patients study. Stroke. 2013;44:119–25. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell AB, Cole JW, McArdle PF, Cheng YC, Ryan KA, Sparks MJ, Mitchell BD and Kittner SJ. Obesity increases risk of ischemic stroke in young adults. Stroke. 2015;46:1690–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TH, Yang PS, Yu HT, Jang E, Shin H, Kim HY, Uhm JS, Kim JY, Sung JH, Pak HN, Lee MH, Joung B and Lip GYH. Effect of hypertension duration and blood pressure level on ischaemic stroke risk in atrial fibrillation: nationwide data covering the entire Korean population. Eur Heart J. 2019;40:809–819. [DOI] [PubMed] [Google Scholar]
- 39.Pool LR, Ning H, Wilkins J, Lloyd-Jones DM and Allen NB. Use of Long-term Cumulative Blood Pressure in Cardiovascular Risk Prediction Models. JAMA Cardiol 2018;3:1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nwabuo CC, Appiah D, Moreira HT, Vasconcellos HD, Yano Y, Reis JP, Shah RV, Murthy VL, Allen NB, Sidney S, Muntner P, Lewis CE, Lloyd-Jones DM, Schreiner PJ, Gidding SS and Lima JA. Long-term cumulative blood pressure in young adults and incident heart failure, coronary heart disease, stroke, and cardiovascular disease: The CARDIA study. Eur J Prev Cardiol 2020:2047487320915342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED and Lloyd-Jones DM. Can Antihypertensive Treatment Restore the Risk of Cardiovascular Disease to Ideal Levels?: The Coronary Artery Risk Development in Young Adults (CARDIA) Study and the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc 2015;4:e002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE and Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650–6. [DOI] [PubMed] [Google Scholar]
- 43.Williamson W, Lewandowski AJ, Forkert ND, Griffanti L, Okell TW, Betts J, Boardman H, Siepmann T, McKean D, Huckstep O, Francis JM, Neubauer S, Phellan R, Jenkinson M, Doherty A, Dawes H, Frangou E, Malamateniou C, Foster C and Leeson P. Association of Cardiovascular Risk Factors With MRI Indices of Cerebrovascular Structure and Function and White Matter Hyperintensities in Young Adults. JAMA. 2018;320:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasconcellos HD, Moreira HT, Ciuffo L, Nwabuo CC, Yared GS, Ambale-Venkatesh B, Armstrong AC, Kishi S, Reis JP, Liu K, Lloyd-Jones DM, Colangelo LA, Schreiner PJ, Sidney S, Gidding SS and Lima JAC. Cumulative blood pressure from early adulthood to middle age is associated with left atrial remodelling and subclinical dysfunction assessed by three-dimensional echocardiography: a prospective post hoc analysis from the coronary artery risk development in young adults study. Eur Heart J Cardiovasc Imaging. 2018;19:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE and Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol 2007;49:2013–20. [DOI] [PubMed] [Google Scholar]
- 46.Whelton SP, McEvoy JW, Shaw L, Psaty BM, Lima JAC, Budoff M, Nasir K, Szklo M, Blumenthal RS and Blaha MJ. Association of Normal Systolic Blood Pressure Level With Cardiovascular Disease in the Absence of Risk Factors. JAMA Cardiol 2020;5(9):1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pletcher MJ, Bibbins-Domingo K, Lewis CE, Wei GS, Sidney S, Carr JJ, Vittinghoff E, McCulloch CE and Hulley SB. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med 2008;149:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB and Bhatt DL. Effect of Systolic and Diastolic Blood Pressure on Cardiovascular Outcomes. N Engl J Med 2019;381:243–251. [DOI] [PubMed] [Google Scholar]
- 49.Aparicio HJ, Himali JJ, Satizabal CL, Pase MP, Romero JR, Kase CS, Beiser AS and Seshadri S. Temporal Trends in Ischemic Stroke Incidence in Younger Adults in the Framingham Study. Stroke. 2019;50:1558–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCambridge J, Witton J and Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014;67:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.