Abstract
The main protease (Mpro) is a major protease having an important role in viral replication of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that caused the pandemic of 2020. Here, active Mpro was obtained as a 34.5 kDa protein by overexpression in E. coli BL21 (DE3). The optimal pH and temperature of Mpro were 7.5 and 37 °C, respectively. Mpro displayed a Km value of 16 μM with Dabcyl-KTSAVLQ↓SGFRKME-Edans. Black garlic extract and 49 polyphenols were studied for their inhibitory effects on purified Mpro. The IC50 values were 137 μg/mL for black garlic extract and 9–197 μM for 15 polyphenols. The mixtures of tannic acid with puerarin, daidzein, and/or myricetin enhanced the inhibitory effects on Mpro. The structure–activity relationship of these polyphenols revealed that the hydroxyl group in C3′, C4′, C5′ in the B-ring, C3 in the C-ring, C7 in A-ring, the double bond between C2 and C3 in the C-ring, and glycosylation at C8 in the A-ring contributed to inhibitory effects of flavonoids on Mpro.
Keywords: main protease, SARS-CoV-2, polyphenol, kinetic, structure–activity relationship, black garlic
1. Introduction
A new coronavirus disease, termed coronavirus disease 2019 (COVID-19), was first identified in Wuhan, China in December 2019 (Hasan et al., 2020). COVID-19 has spread to 235 countries resulting in 81,947,503 confirmed cases and 1,808,041 confirmed deaths by 2 January 2020 according to the World Health Organization (WHO) (covid19.who.int) (accessed on 2 January 2021) and the infection and mortality is increasing day by day. Due to rapid dissemination and deaths, COVID-19 was declared a pandemic for the first time by WHO on 11 March 2020 [1]. COVID-19 has become a global public health crisis, devastating the global economy, and affecting all stages of the education system [2]. Imposing social distancing and self-isolation, restricting travel, closing school, wearing masks, improving immunity, and using air-disinfectant and surface-sanitizing agents are current methods to limit COVID-19 infection [2,3] due to its human-to-human transfer via aerosol transmission [4].
COVID-19 is caused by a novel severe acute respiratory syndrome-related coronavirus (SARS-CoV-2) [5] which belongs to the β-coronavirus type and is closely related to MERS-CoV and SARS-CoV [6,7]. Like other members of coronaviruses, the genome of SARS-CoV-2 is a positive sense, single-stranded RNA (~30 kb) enveloped and contains at least 6 open reading frames (ORFs) [7]. When the virus infects the cell, its genome acts as the mRNA and initiates the synthesis of polyproteins pp1a and pp1b which are essential for viral replication and transcription [8]. These polyproteins encode by two proteases called main protease (Mpro) or 3C-like protease and papain-like protease [8]. Thus, Mpro is considered one of the important targets for blocking viral replication. At present, there are no antiviral drugs on the market for COVID-19 treatment [9]. Broad-spectrum antibiotics such as amoxicillin, azithromycin, and fluoroquinolones, antiviral drugs such as ritonavir, remdesivir, oseltamivir, chloroquine, and lopinavir, corticosteroids such as methylprednisolone, and convalescent plasma are combined to use for COVID-19 treatment [10,11]. More than 200 clinical trials are ongoing (clinicaltrials.gov) (accessed on 11 October 2020).
Nutraceuticals are foods that provide medical or health benefits such as avoiding side effects, protecting human health against chronic diseases, improving human health, and are economically affordable [12,13,14,15]. In addition, herbal medicines have been used for COVID-19 treatment in China [16]. Herbal medicines not only showed the prevention of COVID-19 infection in healthy persons but also improved the health state of treated patients [16]. While screening nutrients and herbal medicines for COVID-19, we found that black garlic showed inhibitory effects on COVID-19 Mpro. Black garlic has a higher total phenolic and total flavonoid content than raw garlic [17]. Black garlic contains various phenolic acids including gallic acid, vanillic acid, chlorogenic acid, caffeic acid, ferulic acid, and coumaric acid), and flavonoids including catechin, epicatechin, epigallocatechin (EGCG), quercitrin, myricetin, resveratrol, morin, and quercetin [17]. Besides the antioxidant, cardioprotection, anti-cancer, anti-inflammatory, anti-aging, and protective activities, polyphenols have antiviral activities against enterovirus, influenza virus, SARS-CoV, Zika, and dengue virus [18,19,20,21]. In our previous study, polyphenols such as ampelopsin, puerarin, quercetin, daidzein, epigallocatechin gallate (EGCG), and gallocatechin gallate (GCG) were shown to inhibit the 3CLpro of SARS-CoV [20]. Mpro sequence identity between SARS-CoV and SARS-CoV-2 was found to be 96% [22]. Also, the inhibitory effects of EGCG and theaflavin on Mpro of SARS-CoV-2 were confirmed [23]. Therefore, it is essential to study the inhibitory effects of each polyphenol on SARS-CoV-2 Mpro and their structure-activity relationship. In this study, the Mpro of SARS-CoV-2 was overexpressed in E. coli BL21(DE3) using IPTG as an inducer and purified using Ni-NTA chromatography. Its proteolytic activity was confirmed by using Dabcyl-KTSAVLQSGFRKME-Edans (FRET) as a substrate. The optimum pH, pH stability, optimum temperature, and temperature stability of SARS-CoV-2′s Mpro were studied. Also, the Michaelis–Menten constant (Km) and the effect of DMSO concentration on Mpro activity were studied. Then, the inhibitory effects of black garlic extract and 49 polyphenols on Mpro and their structure–activity relationships were investigated. The 50% inhibitory concentration (IC50) of black garlic and 15 polyphenols were determined. In addition, the effects of mixtures of polyphenols (tannic acid, puerarin, daidzein, and myricetin) on Mpro activity were studied.
2. Results
2.1. Biochemical Characterization of SARS-CoV-2 Mpro
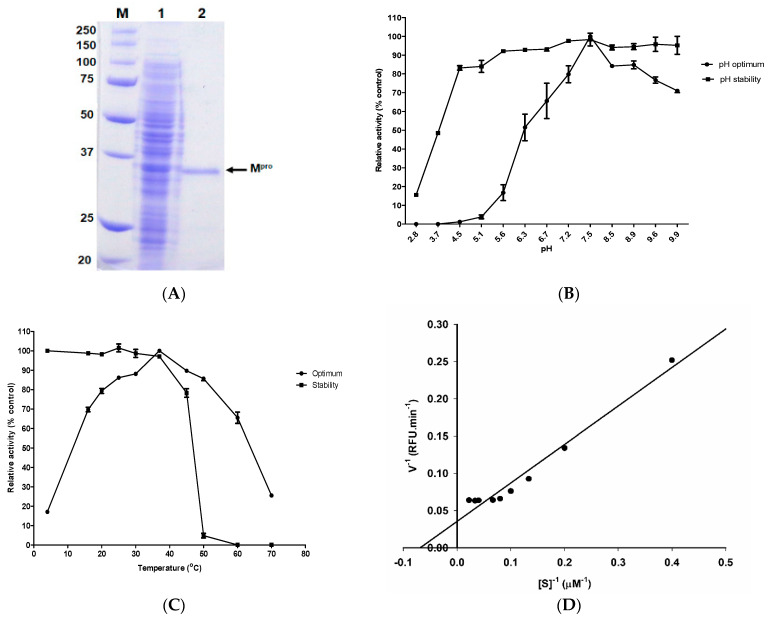
The nucleotide sequences encoding SARS-CoV-2 Mpro were optimized for expression in E. coli through a custom gene synthesis service of Genscript. It was expressed in E. coli BL21(DE3) by using 1.0 mM IPTG at 16 °C for 12 h, purified using Ni-Sepharose chromatography, and confirmed by SDS-PAGE (Figure 1A). A 34.5 kDa protein was observed on 12% SDS-PAGE (Figure 1A). It had activity with Dabcyl-KTSAVLQ↓SGFRKME-Edans, a SARS-CoV 3CLpro substrate (Nguyen et al., 2012) in 50 mM Tris-HCl pH 7.5. The purified Mpro reached maximum activity at pH 7.5 (Figure 1B). Mpro showed over 90% activity at the pH from 5.6 to 9.9 after 24 h incubation at 4 °C. Maximum activity of Mpro was obtained at 37 °C (Figure 1C). A thermos-stability assay showed that Mpro was stable at 37 °C (Figure 1C), then it was left at 76% activity when kept at 45 °C for 24 h. Mpro activity was not detected when kept at 60 °C for 24 h (Figure 1C). The Km value of Mpro calculated from the Lineweaver–Burk double reciprocal plot was 16 μM with Dabcyl-KTSAVLQSGFRKME-Edans (Figure 1D and Figure S1).
Figure 1.
SDS-PAGE analysis (A), optimum pH and pH stability (B), optimum temperature and temperature stability (C), and Lineweaver–Burk plot for determination of Km value of purified SARS-CoV-2 Mpro (D). (A) Lane M: the molecular mass markers, lane 1: cell lysate with 1 mM IPTG induction to overexpress Mpro, lane 2: purified Mpro after using the Ni-NTA column chromatography. (B) Buffers used: glycine-HCl (pH 3.0), sodium acetate buffer (pH 5.0–6.5), Tris buffer (pH 7.0–9.0), and glycine-NaOH buffer (pH 9.5–11.0). For pH stability, the enzyme was kept at 4 °C for 24 h under various pH conditions (pH 3.0–11.0). (C) For thermal stability, the enzyme was kept from 4 to 60 °C for 24 h. The enzyme activity was measured with 20 µM of FRET as a substrate at 37 °C for 30 min. (D) The kinetic parameter of Mpro was added to different concentrations of FREP substrate (2.5–45 μM) to the reaction mixture composed of Mpro in 50 mM Tris-HCl pH 7.5. The reactions were run at 37 °C for 8 min.
2.2. Influence of Dimethylsulfoxide on Mpro Activity
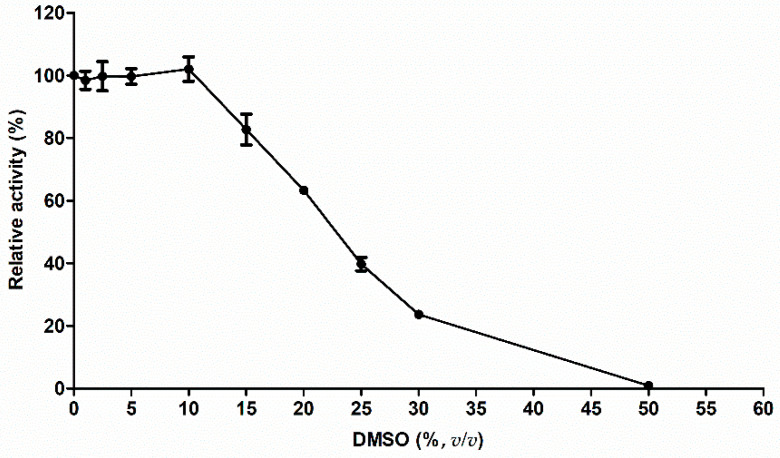
Dimethylsulfoxide (DMSO) is a potent organic solvent that dissolves a variety of organic compounds due to its high dielectric constant and stereochemistry [24]. Although DMSO has been used as an additive, drug carrier to cells, and a cryoprotector, it can affect enzyme activity [24,25]. Therefore, the effect of DMSO on Mpro activity was studied (Figure 2). Mpro was stable in the presence of up to 10% (v/v) DMSO (Figure 2). Then, its activity was reduced when the DMSO concentration increased over 10% (v/v). At 50% (v/v) DMSO, Mpro activity was not detected (Figure 2).
Figure 2.
Effect of DMSO on SARS-CoV-2 Mpro activity. Mpro was incubated for 30 min at 37 °C in 50 mM Tris-HCl buffer pH 7.5 containing 20 µM of FRET with different concentrations of DMSO (0–50%, v/v).
2.3. Inhibitory Effects of Plant Derivative Polyphenols on SARS-CoV-2 Mpro
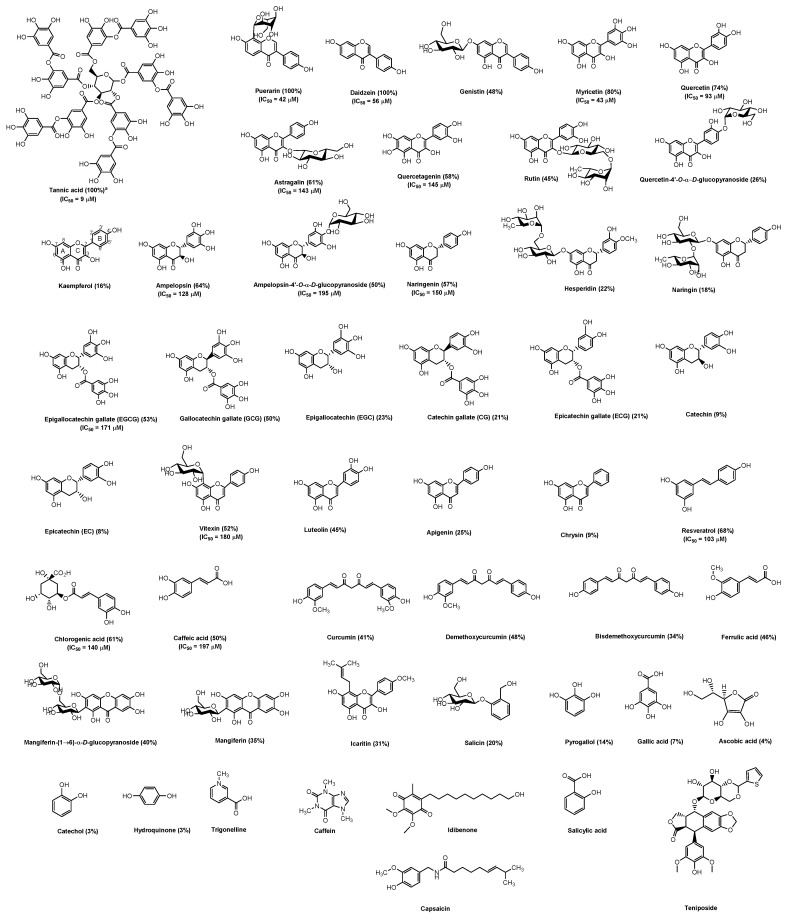
Polyphenols are bioactive compounds found in fruits, vegetables, grains, and herbs and have been widely studied owing to their nutraceutical activity, such as anti-bacterial activities, anti-viral activities, anti-cancer, anti-inflammatory, and anti-diabetes [26]. Polyphenols have been divided into many classes depending on their strength ring. Lignans, phenolic alcohols, stiblins, phenolic acids, and flavonoids are the main polyphenol classes [27]. In this study, we found that black garlic extract inhibited 100% SARS-CoV2 Mpro activity at 0.5 mg/mL. The IC50 value of extracted garlic acid was 137 ± 10 μg/mL (Table 1). Black garlic is produced by heating raw garlic at high temperatures. Black garlic contains different phenolic acids such as gallic acid, caffeic acid, vanillic acid, ferulic acid, and chlorogenic acid, and various flavonoids such as epicatechin, catechin, epigallocatechin gallate, resveratrol, myricetin, and quercetin [17]. Thus, we examined the inhibitory effects of 49 polyphenols from different classes on Mpro. The chemical structures of 49 polyphenols and their inhibitory activity on Mpro are shown in Figure 3 and Table 1. Among them, caffeine, capsaicin, teniposide, and idebenone did not inhibit Mpro at 200 μM. Mpro inhibitory effects of kaempferol, quercetin-4′-O-α-d-glucopyranoside, naringin, epicatechin, catechin, chrysin, trigonelline, ascorbic acid, hydroquinone, gallic acid, pyrogallol, and catechol were less than 20% compared to the control while the other compounds including astragalin, myricein, quercetin, quercetagenin, ampelopsin, ampelopsin-4′-O-α-d-glucopyranoside, naringenin, epigallocatechin gallate (EGCG), vitexin, daidzein, puerarin, resveratrol, tannic acid, chlorogenic acid, and caffeic acid showed over 50% inhibitory activity on Mpro. The compounds exhibiting more than 50% inhibitory activity on Mpro were selected to determine IC50. Their IC50 values were from 9 to 197 μM (Table 1).
Table 1.
The inhibitory effect of polyphenol compounds on SARS-CoV-2 Mpro.
| No | Compound | Group | Source | Inhibition (%) | IC50 (μM) |
|---|---|---|---|---|---|
| 1 | Black garlic extract | Mixtures | SerimFood | 100 | 137 ± 10 μg/mL |
| 2 | Tannic acid | Tannoid | Sigma | 100 | 9 |
| 3 | Puerarin | Isoflavone | Sigma | 100 | 42 ± 2 |
| 4 | Daidzein | Isoflavone | Sigma | 100 | 56 |
| 5 | Genistin | Isoflavone | Sigma | 48 | ND |
| 6 | Myricetin | Flavonol | Sigma | 80 | 43 ± 1 |
| 7 | Quercetin | Flavonol | Sigma | 74 | 93 ± 5 |
| 8 | Astragalin | Flavonol | Amore Pacific | 61 | 143 ± 9 |
| 9 | Quercetagenin | Flavonol | Sigma | 58 | 145 ± 6 |
| 10 | Rutin | Flavonol | Sigma | 45 | ND |
| 11 | Quercetin-4′-O-α-d-glucopyranoside | Flavonol | Synthesized | 26 | ND |
| 12 | Kaempferol | Flavonol | Sigma | 16 | ND |
| 13 | Ampelopsin | Flavanonol | Sigma | 64 | 128 ± 5 |
| 14 | Ampelopsin-4′-O-α-d-glucopyranoside | Flavanonol | Synthesized | 50 | 195 ± 5 |
| 15 | Naringenin | Flavanone | Sigma | 57 | 150 ± 10 |
| 16 | Hesperidin | Flavanone | Sigma | 22 | ND |
| 17 | Naringin | Flavanone | Sigma | 18 | ND |
| 18 | Epigallocatechin gallate (EGCG) | Flavan-3-ols | Sigma | 53 | 171 ± 5 |
| 19 | Gallocatechin gallate | Flavan-3-ols | Sigma | 50 | ND |
| 20 | Epigallocatechin (EGC) | Flavan-3-ols | Sigma | 23 | ND |
| 21 | Catechin gallate (CG) | Flavan-3-ols | Sigma | 21 | ND |
| 22 | Epicatechin gallate (ECG) | Flavan-3-ols | Sigma | 21 | ND |
| 23 | Catechin | Flavan-3-ols | Sigma | 9 | ND |
| 24 | Epicatechin (EC) | Flavan-3-ols | Sigma | 8 | ND |
| 25 | Vitexin | Flavone | Sigma | 52 | 180 ± 6 |
| 26 | Luteolin | Flavone | Sigma | 45 | ND |
| 27 | Apigenin | Flavone | Sigma | 25 | ND |
| 28 | Chrysin | Flavone | Sigma | 9 | ND |
| 29 | Resveratrol | Stilbenoid | Sigma | 68 | 103 ± 6 |
| 30 | Chlorogenic acid | Hydrocinnamic acid | Sigma | 61 | 140 |
| 31 | Caffeic acid | Dihydroxycinnamic acid | Sigma | 50 | 197 ± 1 |
| 32 | Demethoxycurcumin (DMC) | Diarylheptanoid | TCI chemical | 48 | ND |
| 33 | Bisdemethoxycurcumin (BDMC) | Diarylheptanoid | TCI chemical | 34 | ND |
| 34 | Curcumin | Diarylheptanoid | TCI chemical | 41 | ND |
| 35 | Ferulic acid | Hydrocinnamic acid | Sigma | 46 | ND |
| 36 | Mangiferin-(1- > 6)-α-d-glucopyranoside | Xanthonoid | Synthesized | 40 | ND |
| 37 | Mangiferin | Xanthonoid | Sigma | 35 | ND |
| 38 | Icaritin | Favonol | Sigma | 31 | ND |
| 39 | Salicin | Salicylate | Sigma | 20 | ND |
| 40 | Pyrogallol | Benzenetriol | Sigma | 14 | ND |
| 41 | Gallic acid | Sigma | 7 | ND | |
| 42 | Ascorbic acid | Sigma | 4 | ND | |
| 43 | Catechol | Bezenediol | Sigma | 3 | ND |
| 44 | Hydroquinone | Sigma | 3 | ND | |
| 45 | Trigonelline | Alkaloid | Sigma | 1 | ND |
| 46 | Caffein | Alkaloid | Sigma | 0 | ND |
| 47 | Idebenone | 1,4-benzoquinone | TCI chemical | 0 | ND |
| 48 | Salicylic acid | Monohydroxybenzoic acid | Sigma | 0 | ND |
| 49 | Capsaicin | Capsaicinoid | Sigma | 0 | ND |
| 50 | Teniposide | Podophyllotoxin | Sigma | 0 | ND |
Figure 3.
Chemical structure of plant derivate polyphenols against SARS-CoV-2 Mpro.
2.4. Structure-Activity Relationship of Plant Derivative Polyphenols against SARS-CoV-2 Mpro
First, the inhibitory activity of compounds in the sample group was compared. In the flavonol group, the order of Mpro inhibition activity was as follows: kaempferol < quercetin-4′-O-α-d-glucopyranoside < rutin < quercetagenin < astragalin < quercetin < myricetin at 200 μM. Flavonols are a class of flavonoids that has a 3-hydroflavone backbone. The effects of hydroxyl group (OH) substitution in the B-ring on Mpro inhibitory effects were evaluated. The inhibitory effects of quercetin, which has 3′-OH and 4′-OH at the B-ring, and kaempferol, which has 4′-OH group at the B-ring, were lower than that of myricetin which has 3′-OH, 4′-OH, and 5′-OH groups at the B-ring. The absence of 3′-OH and 4′-OH groups at the B-ring was the reason for the lower inhibitory activity of kaempferol and quercetin than myricetin. When quercetin was glycosylated at C4′ in the B-ring (quercetin-4′-O-α-glucopyranoside) and C3 in the C-ring (rutin), its inhibitory effect was decreased. The presence of the OH group at C6 in the A-ring of quercetagenin decreased the inhibitory activity compared to quercetin. Therefore, the OH at C6 in the A-ring, the C3′, C4′, C5′ in the B-ring, and the C3 in C-ring affected Mpro inhibitory activity. Ampelopsin is dihyromyricetin. The existence of double bonds between C2 and C3 in the C-ring of myricetin was the reason for the higher inhibitory activity of myricetin than ampelopsin.
In the flavanone group, the order of Mpro inhibitory activity was as follows: naringin < hesperidin < naringenin. Naringenin is glycosylated naringin. However, its inhibitory activity was 3.2-fold higher than that of naringin. Hesperidin that contained glycosylation at 7-OH at the A-ring like the naringenin and the methoxy group at position 5′ of the B-ring was shown to have higher inhibitory activity than that of naringin but lower inhibitory activity than that of naringenin, indicating that glycosylation at the C7 position enhanced the Mpro inhibitory effect. In contrast, the methoxy group at C5′ in the B-ring reduced its inhibitory activity.
In the flavan-3-ols group, the order of the inhibitory effect was as follows: epicatechin (EC) < catchin < epicatechin gallate (ECG) < catechin gallate (CG) < epigallocatechin (EGC) < gallocatechin gallate (GCG) < epigallocatechin gallate (EGCG) (Table 1). EGCG, GCG, and ECG which contained three OH groups at C3′, C4′, and C5′ in the B-ring showed higher Mpro inhibitory activity than that of the remaining compounds (EC, catechin, CG, and EGC), suggesting that the OH group at C3′, C4′, C5′ in the B-ring increased the inhibitory activity against Mpro, similar to the compounds in the flavonol group. EGCG, GCG, CG, and ECG, which have a galloyl moiety at C3 in the C-ring, showed higher Mpro inhibitory activity, suggesting that galloyl moiety at C3 of the C-ring increased the inhibitory activity on Mpro. Thus, the inhibitory activity of gallic acid on Mpro was studied. Gallic acid inhibited 6.9% Mpro activity at 200 μM. Besides gallic acid, we also tested the inhibitory effects of tannic acid composed of 10 galloyl moieties. Tannic acid inhibited 100% Mpro activity and its IC50 value was 9 μM. Compared to myricetin, EGCG, which has the same three OH groups in the B-ring, galloy moiety at C3 in the C-ring, no double bonds between C2 and C3, and no C4 = O in the C-ring, displayed decreased inhibitory activity on Mpro.
In the flavone group, the order of inhibitor activity on Mpro was as follows: chrysin < apigenin < luteolin < vitexin. Chrysin, lacking the OH group in the B-ring, exhibited the lowest inhibitory activity on Mpro. Compared to the three compounds (luteolin, chrysin, and apigenin), we found that the more the OH group increased in the B-ring, the more the inhibitory effects on Mpro increased. While with attacked glucosyl moiety at C8 in the A-ring, the inhibitory activity on Mpro increased by 2-fold that of apigenin. The difference between flavone and isoflavone is the linked position of B-ring to C-ring. Compared to apigenin, which contained the same OH group in the B-ring but the B-ring was linked in position C3 in the C-ring and lacked the OH group at C5 in the A-ring, daidzein showed an increased inhibition effect on Mpro. Puerarin, which contained the 8-C-glucoside of daidzein, exhibited a slightly higher inhibitory effect on Mpro than that of daidzein. From the inhibitory effect of compounds from flavone and isoflavone, we concluded that the glycosylation at the C8 position increased the inhibitory effect on Mpro.
In the diarylheptanoid group, the order of inhibitory effects was as follows: bisdemethoxycurcumin < curcumin < dimethylcurcumin. In this group, curcumin contained two methoxy groups (C2′ and C4”) and showed higher inhibitory activity on Mpro than bisdemethoxycurcumin which lacked the methoxy group. However, its inhibitory activity was lower than that of dimethylcurcumin, which contained one methoxy group in C2′.
2.5. Combinative Inhibitory Effects on SARS-CoV-2 Mpro of Polyphenols
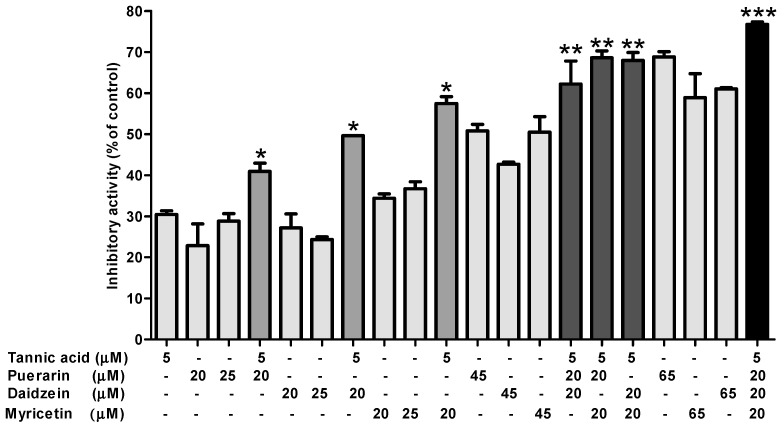
Herbal medicines have been used to treat COVID-19 in China [16]. According to the National Administration of Traditional Chinese Medicine, herbal medicines not only showed the ability to prevent COVID-19 infection in healthy persons but also improved the state of health in treated patients [16]. Most of the clinical studies used herbal extract mixtures [11]. Tannic acid found in red wines, herbaceous, legumes, sorghum, bananas, raspberries, and persimmons, belongs to the tannin family. The concentrations of tannins in red wines were estimated at 5~100 mM. Tannic acid inhibited not only Mpro SARS-CoV2 but also transmembrane protease serin 2 (TMPRSS2) [28]. Although tannic acid inhibits two different SARS-CoV2 enzymes, it has been reported to form complexes with protein, starch, and digestive enzymes causing the nutritional value to decrease [29,30,31]. Therefore, reducing the amount of tannic acid may reduce its side effects. In this study, we tested the effect of different combinations of tannic acid with myricetin, puerarin, and/or daidzein on Mpro activity. The concentration of tannic acid was fixed at 5 μM, while the concentrations of myricetin, puerarin, and daidzein were fixed at 20 μM. The inhibitory effects of mixing tannic acid with myricetin, puerarin, and/or daidzein on Mpro are shown in Figure 4. The inhibitory effects of a single polyphenol on Mpro were 30 ± 1% at 5 μM tannic acid, 23 ± 5% at 20 μM puerarin, 27 ± 3% at 20 μM daidzein, and 34 ± 1% at 20 μM myricetin. The inhibitory effects increased to 41 ± 2% at 5 μM tannic acid with 20 μM puerarin, 50% at 5 μM tannic acid with 20 μM daidzein, and 58 ± 2% at 5 μM tannic acid with 20 μM myricetin. The Mpro inhibitory activities of flavonoids at 45 μM were 50.8 ± 2% for puerarin, 43% for daidzein, and 50.5 ± 4% for myricetin, while they were 62 ± 6% at 5 μM tannic acid with 20 μM puerarin and 20 μM daidzein, 69 ± 2% at 5 μM tannic acid with 20 μM puerarin and 20 μM myricetin, and 69 ± 2% at 5 μM tannic acid with 20 μM puerarin and 20 μM myricetin. The highest inhibitory activity on Mpro (77 ± 1%) was achieved at 5 μM tannic acid with 20 μM puerarin, 20 μM daidzein, and 20 μM myricetin.
Figure 4.
Inhibitory effects on SARS-CoV-2 Mpro of mixtures of tannic acid with puerarin, daidzein, and/or myricetin. All tests were performed in triplicate, and bars indicate the mean and standard deviation. *, **, ***: p < 0.05 compared to 25 μM, 45 μM, 65 μM of each puerarin, daidzein, and myricetin, respectively.
3. Discussion
The SARS-CoV-2 Mpro is one of the best-characterized targets for antiviral drug discovery. It plays an essential role in virus replication by digesting the viral polyproteins at more than 11 sites on the large polyprotein 1ab. The recognition sequence at most sites is LQ↓(S, A, G) (the cleavage site is indicated by ↓) [32] similar to that in SARS-CoV 3CLpro [33,34]. Since inhibiting Mpro activity will block viral replication, we selected SARS-CoV2 Mpro as the target protein and Dabcyl-KTSAVLQ↓SGFRKME-Edans as its commercially available substrate. The biochemical characterization of Mpro showed that the optimum temperature, pH, and Km value were 37 °C, pH 7.5, and 16 μM, respectively. The Km value of the purified Mpro was similar to that of the SARS-CoV 3CLpro expressed in Pichia pastoris with 15 ± 1 μM [20] and to that of the SARS-CoV 3CLpro expressed in E. coli with 17 ± 4 μM [35]. We tested the influence of increasing concentrations of DMSO up to 50% (v/v). At up to 10% (v/v), we found no significant effects on SARS-CoV2 Mpro activity, but over 10% (v/v), DMSO significantly inhibited Mpro activity. At 50% (v/v) DMSO, Mpro lost its activity. No significant effect of 10% (v/v) DMSO on SARS-CoV2 Mpro activity was observed, similar to that on SARS-CoV 3CLpro activity [34].
Polyphenols are a large family of phytochemicals with great chemical diversity as well as potential therapeutic diversity. Many polyphenols act as multi-target agents of high biochemical specificity and chemical diversity with lower cost, more covering mechanism, and minimal side effects. As one of the plant derivate polyphenols, curcumin down-regulated the coactivator of HBV transcription, peroxisome proliferator-activated receptor-gamma coactivator 1-α (PGC-1α), and inhibited HBV gene replication and expression [36]. In another example, tannic acid inhibits two SARS-CoV2 enzymes [28]. Thi et al. reported that epigallocatechin gallate or gallocatechin gallate containing galloyl moiety at 3-OH of the C-ring was required for inhibitory activity on SARS-CoV 3CLpro [20]. Thus, polyphenols may be novel antiviral candidate compounds and lead-structures. In addition, polyphenols are inexpensive with minimal side effects. Polyphenols are known to be bioactive compounds of foods, species, medicinal plants, and nutraceuticals. Polyphenol intake varies within 50–1000 mg/day among different countries such as France (283–1000 mg/day), Spain (500–1100 mg/day), Italy (700 mg/day), Finland (890 mg/day), Brazil (534 mg/day), Japan (1500 mg/day), US (240–350 mg/day), China (50–500 mg/day), and Korea (320 mg/day) [37,38,39]. Thus, polyphenols have potential use in the prevention of SARS-CoV2. Numerous studies on herbal medicines against SARS-CoV-2 have been conducted in vitro, in vivo, and in ovo [16,40,41]. However, most clinical studies were performed with food or herb combinations based on the traditional Chinese formulas. Lianhuaqingwen is a Chinese patent medicine composed of 13 herbs [40,42]. It inhibited SARS-CoV-2 replication in a dose-dependent manner with an IC50 of 411 μg/mL, reduced the production of pro-inflammatory cytokines, and affected particle morphology of the virus [40]; 61 polyphenols were identified in this herbal mixture. Therefore, we aimed to study the effects of bioactive compounds in herbal extracts on SARS-CoV-2 Mpro as well as their structure–activity relationship. Garlic is a popular medicinal food owing to its numerous health benefits (anticancer, antibacterial, antiviral, antidiabetic, antihypertensive, antioxidative, and immunity-enhancing benefits) [43]. However, raw garlic causes gastrointestinal discomfort and has a pungent taste and smell, resulting in a decline in its consumption. Black garlic, with a sweet and sour taste [43], overcame this drawback of raw garlic. Black garlic has more reducing sugar (80-fold), lipids (3.2-fold), organic acids (3.9-fold), total phenolic acids (~7.8-fold), flavonoids (~3.5-fold), polyphenols (1.8-fold), alkaloids (28.6-fold), and vitamins (1.3-fold) than raw garlic [17,44]. Also, chlorogenic acid, vanillic acid, and quercetin were newly synthesized, whereas the amount of other compounds, such as gallic acid, caffeic acid, coumaric acid, ferulic acid, catechin, epicatechin, epigallocatechin gallate, myricetin, resveratrol, and morin increased from 1.1- to 26.2-fold, which contributed to the enhancing of total phenolic acid and total flavonoid content by up to 7.8- and 3.5-fold, respectively [17]. From these results, we found that the inhibitory effect of black garlic on Mpro was possibly owing to its phenolic acids (gallic, chlorogenic, caffeic, and ferulic) and flavonoid compounds (myricetin, quercetin, epigallocatechin gallate, epicatechin, catechin, and resveratrol) [17].
The COVID-19 pandemic started in December 2019 and has extended until now; there are no approved therapeutics for this disease. Although numerous researchers performed computational chemistry [45] and virtual screening of FDA-approved drugs [46] and flavonoid compounds [47,48,49,50] with Mpro, their efficiencies against Mpro need to be confirmed by in vitro and in vivo experiments. To the best of our knowledge, among our tested polyphenols, EGCG and tannic acid have been reported to have inhibitory effects on Mpro with IC50 values of 7.6 μg/mL and 2.1 μM, respectively [23,45]. Here, we found that the EGCG and tannic acid inhibited Mpro with IC50 values of 171 μM and 9 μM, respectively (Table 1). The differences in the results may be due to different substrate and reaction conditions used in each study. For example, Jang et al., (2020) performed the EGCG inhibition assay at 37 °C for 5 h using the same substrate; however, Coelho et al., (2020) conducted the inhibition assay using MCA-AVLQSGFR-K(Dnp)-K-NH2. Although we did not perform experiments to study the binding mode of these compounds by molecular docking, there are several reports that predict the inhibition mode of these compounds [48,49,50]. Molecular docking studies with Mpro protein showed that some compounds, such as rutin (–9.2 kcal/mol), hesperidin (–8.1 kcal/mol), naringin (–8.1 kcal/mol), EGCG (–7.9 kcal/mol), catechin (–5.8 kcal/mol), myricetin (–5.9 kcal/mol), and caffeic acid (–5.1 kcal/mol), had the highest binding energy and hydrogen-bonding interactions with key amino acid residues of Mpro [31,48,50]. Our experiments confirmed that these compounds inhibited Mpro (Table 1). Although the polyphenols assessed in this study showed moderate inhibition against Mpro, they may prevent or at least impede SARS-CoV-2 infection. COVID-19 therapies can be divided into two types depending on their targets [51], one is designed to boost the human immune system or inhibit the inflammatory process, and the other to directly attack the virus by hindering its replication and entry into the human cell. Polyphenols have been reported to affect the level and composition of immunoglobulins, inflammation, and immune cell populations [52]. For example, polyphenols such as curcumin, epigallocatechin gallate, naringenin, kaempferol, apigenin, and resveratrol decrease pro-inflammatory cytokines, according to in vitro and in vivo studies [46]. Thus, polyphenols not only inhibit SARS-CoV2 Mpro activity but also affect the human immune system. In addition, polyphenols are the most common and the largest plant compounds in the human diet. They are extracted from plants and found in foods and beverages. The dietary intake of polyphenols has been estimated to vary from 100 to 1000 mg/day. Further studies of foods containing these polyphenols against SARS-CoV-2 are needed.
4. Materials and Methods
4.1. Preparation of Active SARS-CoV-2 Mpro
SARS-CoV-2 Mpro gene was codon optimized and synthesized for expression in Escherichia coli based on Mpro amino acid sequence (GenBank accession MT483553.1: 3264–3569 aa) by Genscript (Piscataway, NJ, USA). Mpro was inserted into the pET28a vector (pET28a-Mpro) (Novagen, Darmstadt, Germany) for overexpression of Mpro protein with polyhistidine tags. pET28a-Mpro was transformed into E. coli BL21(DE3) using the heat shock method and then spread on LB agar containing (50 μg/mL) (Sigma). The plate was inoculated at 37 °C overnight. LB supplemented with kanamycin was used for a single colony grown at 37 °C. One mM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Sigma) was used to induce Mpro when the optical density (OD600) became 0.5. Induced cells were cultured at 16 °C with shaking (200 rpm) for 12 h. Cells were harvested by centrifugation (8000× g for 30 min at 4 °C), resuspended in 50 mM Tris-HCl pH 7.0, and lysed by sonication (Ultrasonic processor 250, Sonics and Materials Inc., Newtown, CT, USA). After sonication, cell lysate was obtained by centrifugation (12,000× g for 30 min) and then loaded onto 12 mL of Ni-Agarose resin (Qiagen, Hilden, Germany). A buffer containing 50 mM Tris-HCl, 100 mM NaCl, 300 mM imidazole, pH 8.0 was used to elute proteins from the Ni-Agarose column. Fractions containing purified proteins were obtained and dialyzed against a 50 mM Tris-HCl buffer (pH 7.5). Protein concentration was determined using a protein determination kit (Bio-Rad protein assay kit, Bio-Rad Laboratories, Hercules, CA, USA), and 12% SDS-PAGE was used to confirm the purity of the protein.
Proteolytic activity of Mpro was measured by using fluorogenic peptide (Dabcyl-KTSAVLQ↓SGFRKME-Edans) (FRET) as a substrate (Nguyen et al., 2012). The reaction mixtures composed of 20 μM of FRET substrate and 8 μg of Mpro in 50 mM Tris-HCl (pH 7.5) were run at 37 °C for 30 min. Relative fluorescence units (RFUs) were verified with SpectraMax M3 (Molecular Devices, San Jose, CA, USA) at excitation and fluorescence emission wavelengths of 355 and 538 nm, respectively.
4.2. Biochemical Characteristics of the Active SARS-CoV-2 Mpro
4.2.1. Effect of pH on Activity and Stability of SARS-CoV-2 Mpro
The effect of pH on Mpro activity was studied at different pHs ranging from pH 3.0 to 11.0 using the following buffers: glycine-HCl (50 mM, pH 3.0), sodium acetate buffer (50 mM, pH 5.0–6.5), Tris-HCl buffer (50 mM, pH 7.0–9.0), and glycine-NaOH buffer (50 mM, pH 9.5–11.0) [53]. The optimum pH was determined by incubation of Mpro in each buffer for 30 min using 20 µM of FRET as a substrate at 37 °C for 30 min. RFUs were recorded as described above.
The pH stability of Mpro was carried out by incubation of Mpro for 24 h in the chosen buffers at 4 °C. Then, the pH of Mpro was accustomed to 7.5 by membrane dialysis. Mpro activity was carried out with 20 µM of FRET at 37 °C for 30 min. RFUs were recorded as described above.
4.2.2. Effect of Temperature on Activity and Stability of SARS-CoV-2 Mpro
The effect of temperature on Mpro activity was performed by reacting a reaction mixture composed of Mpro enzyme and 20 µM of FRET in 50 mM Tris-HCl (pH 7.5) at various temperatures from 4 to 70 °C. RFUs were recorded as described above.
Mpro temperature stability was performed by keeping the enzyme from 4 to 60 °C for 24 h. Then, the enzyme was added to the reaction digest containing 20 µM of FRET in 50 mM Tris-HCl (pH 7.5). The reactions were done at 37 °C for 30 min. RFUs were verified with SpectraMax M3 as described above.
4.2.3. Effect of DMSO on SARS-CoV-2 Mpro Activity
The effect of dimethyl sulfoxide (DMOS) on Mpro activity was measured by adding different concentrations of DMSO from 0 to 50% (v/v) to the reaction mixture comprising Mpro in 50 mM Tris-HCl pH 7.5. The FRET substrate was added to initiate the reaction. The control was a reaction without adding DMSO. The reactions were conducted at 37 °C for 30 min. RFUs were monitored as described above.
4.2.4. Kinetic Characterization
The kinetic parameter of Mpro was added to different concentrations of FREP substrate (2.5–45 μM) to the reaction mixture composed of Mpro in 50 mM Tris-HCl pH 7.5. The reactions were run at 37 °C for 8 min. Reaction results were linear within this time. The Michaelis–Menten constant (Km) was obtained from the Lineweaver–Burk plot using the Sigmaplot program (Systat Software, San Jose, CA, USA).
4.3. Inhibition Assay
Black garlic extract and 49 compounds that were selected for Mpro inhibitory activity are listed in Table 1. A 16 μM FRET substrate was added to the reaction digest containing Mpro and a 200 μM inhibitor or 0.5 mg/mL of black garlic extract in 50 mM Tris-HCl (pH 7.5) to start a reaction. The reaction was run at 37 °C for 15 min and RFUs were recorded as above. The inhibition activity of Mpro was calculated as follows:
| Inhibition activity (%) = 100 − [(S − So) / (C − Co)] × 100 | (1) |
where Co and C were the RFUs of the control (buffer, enzyme, and substrate) after 0 and 15 min of reaction and So and S were the RFUs of the assay sample (buffer, inhibitor, enzyme, and substrate) after 0 and 15 min of reaction. Fifteen compounds and black garlic extract were selected for the 50% inhibitory concentration (IC50) determination. IC50 was the Mpro inhibitor concentration necessary to reduce 50% Mpro activity compared to the reaction without adding an inhibitor. To determine IC50, 16 μL FRET substrate was added to the reaction digest containing Mpro and different concentrations of inhibitors (1–400 μM) in 50 mM Tris-HCl (pH 7.5) to start the reaction as described above.
4.4. Combinative Inhibitory Effects of Selected Compounds
The combinative inhibitory effects of tannic acid, puerarin, daidzein, and myricetin were performed by adding 5 μM of tannic acid with 20 μM of puerarin, and/or daidzein, and/or myricetin to a reaction mixture containing a 16 μM FRET substrate and Mpro in 50 mM Tris-HCl (pH 7.5) as described above.
4.5. Statistical Analysis
All experiments were performed in triplicate, and results were expressed as the mean ± standard deviation. Statistical comparisons were made by one-way analysis of variance followed by Tukey’s comparison tests on GraphPad Prism 8 (San Diego, CA, USA). Values were considered to be significant when p < 0.05.
5. Conclusions
Here, we reported the overexpression and biochemical characterization of an active SARS-CoV-2 Mpro. The purified Mpro was used to study the inhibitory effect of black garlic extract and 49 polyphenols. Black garlic extract and 15 polyphenols showed the inhibition of Mpro activity with IC50 values of 137 μg/mL and from 9 to 197 μM, respectively. From the structure–activity relationship of these polyphenols, we found that the hydroxyl group in C3′, C4′, C5′ in the B-ring, C3 in the C-ring, C7 in the A-ring, the double bond between C2 and C3 in the C-ring, and glycosylation at C8 in the A-ring contributed to the inhibitory activity of flavonoids on Mpro. Different combinations of tannic acid with puerarin, daidzein, and/or myricetin also increased inhibitory effects on Mpro. Polyphenols are products extracted from plants and found in foods and beverages such as fruits, vegetables, tea, wine. Therefore, further studies of foods containing these polyphenols are needed.
Supplementary Materials
The following are available online, Figure S1: Michaelis-Menten plot for determination of Km value of purified SARS-CoV2 Mpro.
Author Contributions
Conceptualization, T.T.H.N., S.L. and D.K.; Methodology, T.T.H.N.; Validation, T.T.H.N., M.-K.K., S.L., J.-M.C., B.C., D.-W.K., and D.K.; Formal analysis, T.T.H.N., J.-H.J., M.-K.K., J.-M.C., B.C., D.-W.K. and D.K.; Data curation, T.T.H.N.; Writing-original draft preparation, T.T.H.N.; Writing-review and editing, T.T.H.N. and D.K. Supervision, D.K.; Project administration, D.K.; Funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (2018R1D1A1B07049569, T.T.H.N., 2018R1D1A1A09083366, D.-W.K.). This research was partially supported by the Nuclear R&D program of the Ministry of Science and ICT (MSIT), Republic of Korea, and by the Ottogi Corporation through the Research Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are within this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds, black garlic extract, tannic acid, puerarin, daidzein, genistin, myricetin, quercetin, astragalin, quercetagenin, rutin, quercetin-4’-O-α-d-glucopyranoside, kaempferol, ampelopsin, ampelopsin-4’-O-α-d-glucopyranoside, naringenin, hesperidin, naringin, epigallocatechin gallate, gallocatechin gallate, epigallocatechin, catechin gallate, epicatechin gallate, catechin, epicatechin, vitexin, luteolin, apigenin, chrysin, resveratrol, chlorogenic acid, caffeic acid, demethoxycurcumin, bisdemethoxycurcumin, curcumin, ferulic acid, mangiferin-(1->6)-α-d-glucopyranoside, mangiferin, icaritin, salicin, pyrogallol, gallic acid, ascorbic acid, catechol, hydroquinone, trogonelline, caffeine, idebenone, salicylic acid, capsaicin, and teniposide are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cucinotta D., Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenzen M., Li M., Malik A., Pomponi F., Sun Y.Y., Wiedmann T., Faturay F., Fry J., Gallego B., Geschke A., et al. Global socio-economic losses and environmental gains from the Coronavirus pandemic. PLoS ONE. 2020;15:e0235654. doi: 10.1371/journal.pone.0235654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panyod S., Ho C.T., Sheen L.Y. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J. Tradit. Complement. Med. 2020;10:420–427. doi: 10.1016/j.jtcme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanhed A.M., Patel D.V., Teli D.M., Patel N.R., Chhabria M.T., Yadav M.R. Identification of potential Mpro inhibitors for the treatment of COVID-19 by using systematic virtual screening approach. Mol. Divers. 2020;25:383–401. doi: 10.1007/s11030-020-10130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi T., Joshi T., Sharma P., Mathpal S., Pundir H., Bhatt V., Chandra S. In silico screening of natural compounds against COVID-19 by targeting Mpro and ACE2 using molecular docking. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4529–4536. doi: 10.26355/eurrev_202004_21036. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang Q., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005;81:215s–217s. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 13.D’Archivio M., Filesi C., Di Benedetto R., Gargiulo R., Giovannini C., Masella R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita. 2007;43:348–361. [PubMed] [Google Scholar]
- 14.Chauhan B., Kumar G., Kalam N., Ansari S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013;4:4–8. doi: 10.4103/2231-4040.107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee I.C., Ryu C.W., Bae J.S. Novel herbal medicine C-KOK suppresses the inflammatory gene iNOS via the inhibition of p-STAT-1 and NF-kappa B. Biotechnol. Bioprocess Eng. 2020;25:536–542. doi: 10.1007/s12257-020-0126-2. [DOI] [Google Scholar]
- 16.Du H.Z., Hou X.Y., Miao Y.H., Huang B.S., Liu D.H. Traditional Chinese Medicine: An effective treatment for 2019 novel coronavirus pneumonia (NCP) Chin. J. Nat. Med. 2020;18:206–210. doi: 10.1016/S1875-5364(20)30022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.S., Kang O.J., Gweon O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods. 2013;5:80–86. doi: 10.1016/j.jff.2012.08.006. [DOI] [Google Scholar]
- 18.Lim H.J., Nguyen T.T.H., Kim N.M., Park J.S., Jang T.S., Kim D. Inhibitory effect of flavonoids against NS2B-NS3 protease of ZIKA virus and their structure activity relationship. Biotechnol. Lett. 2017;39:415–421. doi: 10.1007/s10529-016-2261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen T.T.H., Kang H.K., Kim Y.M., Jang T.S., Kim D. Inhibition effect of flavonoid compounds against neuraminidase expressed in Pichia pastoris. Biotechnol. Bioprocess Eng. 2014;19:70–75. doi: 10.1007/s12257-013-0599-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T.T., Woo H.J., Kang H.K., Nguyen V.D., Kim Y.M., Kim D.W., Ahn S.A., Xia Y., Kim D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 2012;34:831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Li Y.Y., Gu Z.W., Wang Y.Y., Shi M., Ji Y., Sun J., Xu X.P., Zhang L.R., Jiang J.T., et al. Resveratrol inhibits enterovirus 71 replication and pro-inflammatory cytokine secretion in rhabdosarcoma cells through blocking IKKs/NF-kappa B signaling pathway. PLoS ONE. 2015;10:e0116879. doi: 10.1371/journal.pone.0116879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gahlawat A., Kumar N., Kumar R., Sandhu H., Singh I.P., Singh S., Sjostedt A., Garg P. Structure-based virtual screening to discover potential lead molecules for the SARS-CoV-2 main protease. J. Chem. Inf. Model. 2020;60:5781–5793. doi: 10.1021/acs.jcim.0c00546. [DOI] [PubMed] [Google Scholar]
- 23.Jang M., Park Y.I., Cha Y.E., Park R., Namkoong S., Lee J.I., Park J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid. Based Complement. Alternat. Med. 2020;2020:5630838. doi: 10.1155/2020/5630838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balakin K.V., Savchuk N.P., Tetko I.V. In silico approaches to prediction of aqueous and DMSO solubility of drug-like compounds: Trends, problems and solutions. Curr. Med. Chem. 2006;13:223–241. doi: 10.2174/092986706775197917. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen T.T., Moon Y.H., Ryu Y.B., Kim Y.M., Nam S.H., Kim M.S., Kimura A., Kim D. The influence of flavonoid compounds on the in vitro inhibition study of a human fibroblast collagenase catalytic domain expressed in E. coli. Enzyme Microb. Technol. 2013;52:26–31. doi: 10.1016/j.enzmictec.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Liu A.L., Wang H.D., Lee S.M.Y., Wang Y.T., Du G.H. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008;16:7141–7147. doi: 10.1016/j.bmc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Moga M.A., Dimienescu O.G., Arvatescu C.A., Mironescu A., Dracea L., Ples L. The role of natural polyphenols in the prevention and treatment of cervical cancer-an overview. Molecules. 2016;21:1055. doi: 10.3390/molecules21081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S.C., Chen Y., Wang Y.C., Wang W.J., Yang C.S., Tsai C.L., Hou M.H., Chen H.F., Shen Y.C., Hung M.C. Tannic acid suppresses SARS-CoV-2 as adual inhibitor of the viral main protease and the cellular TMPRSS2 protease. Am. J. Cancer Res. 2020;10:4538–4546. [PMC free article] [PubMed] [Google Scholar]
- 29.Stukelj M., Valencak Z., Krsnik M., Svete A.N. The effect of the combination of acids and tannin in diet on the performance and selected biochemical, haematological and antioxidant enzyme parameters in grower pigs. Acta. Vet. Scand. 2010;52:19. doi: 10.1186/1751-0147-52-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung K.T., Wong T.Y., Wei C.I., Huang Y.W., Lin Y. Tannins and human health: A review. Crit. Rev. Food Sci. 1998;38:421–464. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 31.Al-Hijazeen M., Lee E.J., Mendonca A., Ahn D.U. Effects of Tannic acid on lipid and protein oxidation, color, and volatiles of raw and cooked chicken breast meat during storage. Antioxidants. 2016;5:19. doi: 10.3390/antiox5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan K., Wei P., Feng Q., Chen S., Huang C., Ma L., Lai B., Pei J.F., Liu Y., Chen J., et al. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. J. Biol. Chem. 2004;279:1637–1642. doi: 10.1074/jbc.M310875200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grum-Tokars V., Ratia K., Begaye A., Baker S.C., Mesecar A.D. Evaluating the 3C-like protease activity of SARS-Coronavirus: Recommendations for standardized assays for drug discovery. Virus Res. 2008;133:63–73. doi: 10.1016/j.virusres.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo C.J., Chi Y.H., Hsu J.T.A., Liang P.H. Characterization of SARS main protease and inhibitor assay using afluorogenic substrate. Biochem. Biophys. Res. Commun. 2004;31:862–867. doi: 10.1016/j.bbrc.2004.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rechtman M.M., Har-Noy O., Bar-Yishay I., Fishman S., Adamovich Y., Shaul Y., Halpern Z., Shlomai A. Curcumin inhibits hepatitis Bvirus via down-regulation of the metabolic coactivator PGC-1 alpha. FEBS Lett. 2010;584:2485–2490. doi: 10.1016/j.febslet.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 37.Tresserra-Rimbau A., Arranz S., Vallverdu-Queralt A. New insights into the benefits of polyphenols in chronic diseases. Oxid. Med. Cell Longev. 2017;2017:1432071. doi: 10.1155/2017/1432071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Bo C., Bernardi S., Marino M., Porrini M., Tucci M., Guglielmetti S., Cherubini A., Carrieri B., Kirkup B., Kroon P., et al. systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients. 2019;11:1355. doi: 10.3390/nu11061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grosso G., Stepaniak U., Topor-Madry R., Szafraniec K., Pajak A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition. 2014;30:1398–1403. doi: 10.1016/j.nut.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li R.F., Hou Y.L., Huang J.C., Pan W.Q., Ma Q.H., Shi Y.X., Li C.F., Zhao J., Jia Z.H., Jiang H.M., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? a review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020;26:243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Y.W., Zeng L.J., Li R.F., Chen Q.Y., Zhou B.X., Chen Q.L., Cheng P.L., Wang Y.T., Zheng J.P., Yang Z.F., et al. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017;17:130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryu J.H., Kang D. Physicochemical Properties, biological activity, health benefits, and general limitations of aged black garlic: A review. Molecules. 2017;22:919. doi: 10.3390/molecules22060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu Z.C., Zheng Z.J., Zhang B., Sun-Waterhouse D., Qiao X.G. Formation, nutritional value, and enhancement of characteristic components in black garlic: A review for maximizing the goodness to humans. Compr. Rev. Food Sci. Food Saf. 2020;19:801–834. doi: 10.1111/1541-4337.12529. [DOI] [PubMed] [Google Scholar]
- 45.Coelho C., Gallo G., Campos C.B., Hardy L., Wurtele M. Biochemical screening for SARS-CoV-2 main protease inhibitors. PLoS ONE. 2020;15:e0240079. doi: 10.1371/journal.pone.0240079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandeel M., Al-Nazawi M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020;251:117627. doi: 10.1016/j.lfs.2020.117627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rameshkumar M.R., Indu P., Arunagirinathan N., Venkatadri B., El-Serehy H.A., Ahmad A. Computational selection of flavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: A molecular docking study. Saudi J. Biol. Sci. 2021;28:448–458. doi: 10.1016/j.sjbs.2020.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherrak S.A., Merzouk H., Mokhtari-Soulimane N. Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: A molecular docking and simulation studies. PLoS ONE. 2020;15:e0240653. doi: 10.1371/journal.pone.0240653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abd El-Mordy F.M., El-Hamouly M.M., Ibrahim M.T., Abd El-Rheem G., Aly O.M., Abd El-kader A.M., Youssif K.A., Abdelmohsen U.R. Inhibition of SARS-CoV-2 main protease by phenolic compounds from Manilkara hexandra (Roxb.) Dubard assisted by metabolite profiling and in silico virtual screening. RSC Adv. 2020;10:32148–32155. doi: 10.1039/D0RA05679K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gogoi N., Chowdhury P., Goswami A.K., Das A., Chetia D., Gogoi B. Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease. Mol. Divers. 2020:1–5. doi: 10.1007/s11030-020-10150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tu Y.F., Chien C.S., Yarmishyn A.A., Lin Y.Y., Luo Y.H., Lin Y.T., Lai W.Y., Yang D.M., Chou S.J., Yang Y.P., et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21:2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosseinzade A., Sadeghi O., Naghdipour Biregani A., Soukhtehzari S., Brandt G.S., Esmaillzadeh A. Immunomodulatory effects of flavonoids: Possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front. Immunol. 2019;10:51. doi: 10.3389/fimmu.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regmi S., Choi Y.S., Kim Y.K., Khan M.M., Lee S.H., Cho S.S., Jin Y.Y., Lee D.Y., Yoo J.C., Suh J.W. Endoglucanase produced by Bacillus subtilis Strain CBS31: Biochemical characterization, thermodynamic study, enzymatic hydrolysis, and bio-industrial applications. Biotechnol. Bioprocess Eng. 2020;25:104–116. doi: 10.1007/s12257-019-0338-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are within this manuscript.