Abstract
Knowledge on the mechanisms of acid and base secretion in airways has progressed recently. The aim of this review is to summarize the known mechanisms of airway surface liquid (ASL) pH regulation and their implication in lung diseases. Normal ASL is slightly acidic relative to the interstitium, and defects in ASL pH regulation are associated with various respiratory diseases, such as cystic fibrosis. Basolateral bicarbonate (HCO3−) entry occurs via the electrogenic, coupled transport of sodium (Na+) and HCO3−, and, together with carbonic anhydrase enzymatic activity, provides HCO3− for apical secretion. The latter mainly involves CFTR, the apical chloride/bicarbonate exchanger pendrin and paracellular transport. Proton (H+) secretion into ASL is crucial to maintain its relative acidity compared to the blood. This is enabled by H+ apical secretion, mainly involving H+/K+ ATPase and vacuolar H+-ATPase that carry H+ against the electrochemical potential gradient. Paracellular HCO3− transport, the direction of which depends on the ASL pH value, acts as an ASL protective buffering mechanism. How the transepithelial transport of H+ and HCO3− is coordinated to tightly regulate ASL pH remains poorly understood, and should be the focus of new studies.
Keywords: pH, CFTR, SLC26A4, ATP12A, lung
The airway epithelium is central to the defenses of the lung. It constitutes an interface between the internal milieu and the external environment, acting as a barrier against particles deposited in the larger airways, and inactivating infectious microorganisms in the lower airways [1]. The luminal side of airway epithelia is lined by a thin layer of fluid called airway surface liquid (ASL) [2,3]. ASL arises mainly from submucosal gland secretions and transepithelial hydro-osmotic movements. Its composition is finely tuned, more specifically its pH. Increasing data have provided evidence that normal ASL is slightly acidic relative to the interstitium, and that defects in its pH regulation are associated with various respiratory diseases [4]. Research on the mechanisms of acid and base secretion in airways has progressed significantly recently. The aim of this review was to summarize the known mechanisms of ASL pH regulation in airway physiology and their implication in lung diseases, as well as to highlight gaps in knowledge.
1. Methods
A literature review was performed according to PRISMA guidelines [5]. An exhaustive systematic review was performed based on the following keywords: airway surface liquid pH, bicarbonate, CFTR, SLC26A4, SLC26A9, shunt pathway, SLC4A4, ATP12A, carbonic anhydrase, SLC9A1, and V-ATPase. Experimental models and methods of pH assessment were specifically considered. The flow diagram of the studies we selected is shown in Figure 1.
Figure 1.
PRISMA flow diagram.
2. Surface Liquid of the Airway Bronchial Epithelium, an Important Player in Airway Physiology
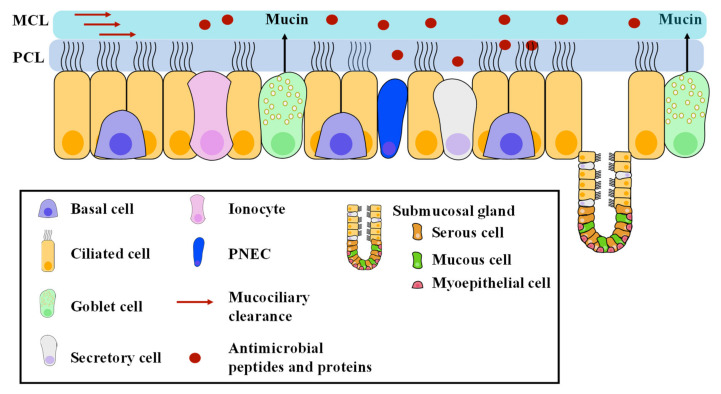
The airway bronchial epithelium is composed of a variety of cell types, described in Figure 2 [1,6].
Figure 2.
Pseudo-stratified airway epithelium and physiological role of main cells.
In the large airways, the ciliated, goblet, and basal cells are predominant, whereas in the small airways, the secretory cells are found more frequently. Ciliated cells account for at least 50% of all epithelial cells within human airways, and are terminally differentiated. They express up to 300 cilia per cell, whose coordinated beating enables mucous clearance out of the airways. Goblet cells contain acidic-mucin granules. They can self-renew, as well as transdifferentiate into ciliated cells. Secretory (clara/club) cells secrete surfactants and specific antiproteases. Basal cells display stem cell-like properties and give rise to secretory and ciliated cells in response to epithelial injury. Ionocytes are a rare cell type, recently identified in the adult murine and human trachea, and in human proximal bronchi; they express cystic fibrosis transmembrane regulator (CFTR) protein at high levels [6,7]. Pulmonary neuroendocrine cells (PNEC) are ubiquitous in human adult airway epithelium, and located between epithelial cells adjacent to the basement membrane. Submucosal glands build invaginations throughout the cartilaginous airways in the trachea and large human airways. They are only present in the uppermost part of the mouse trachea. They are composed of mucous cells, serous cells, and myoepithelial cells. Thus, luminal mucus originates from both mucous and goblet cells. The two main mucins in human airways are MUC5AC, mainly produced in the goblet cells of the surface epithelium, and MUC5B, mainly produced in the mucous cells of the submucosal glands. MUC5AC is thought to be secreted as an acute response to environmental insults, while MUC5B is involved in the response to chronic infection and inflammation.
Regulation of ASL composition and mucociliary clearance is critical for normal airway function. The ASL is a ~10 µm bilayer made up of a periciliary liquid (PCL) and a mucus layer (MCL). The PCL is a watery layer which bathes the cilia, and is in direct contact with the epithelial cells. The MCL is a gel-like layer sitting over the tips of the cilia. The PCL allows the cilia to beat and strike at the underside of the MCL. Whereas the PCL is composed of 96% water, 1% salts, 1% lipids, 1% proteins, and 1% mucus [2,8], the MCL layer is a heterogeneous mixture of polypeptides and cellular debris tethered together on the PCL surface by MUC5AC and MUC5B complexes [9,10,11]. It is thought that the mucous layer acts as a fluid reservoir to maintain ASL hydration [12].
The ASL pH and bicarbonate (HCO3−) concentration are critical for several key functions of the airway epithelium.
At the cellular level, pH is involved in the regulation of ion transporters, and therefore in trans- and para-cellular salt and water movement and ASL homoeostasis. Indeed, the equilibrium between different redundant pathways for net chloride (Cl−) secretion and net sodium (Na+) absorption ensures the extremely precise regulation of ASL volume [13]. Chloride secretion mainly involves the cystic fibrosis transmembrane regulator protein (CFTR), both directly and indirectly, because CFTR also promotes the activity of other Cl− transporters, including Cl−/HCO3− exchangers and ANO1 (a Ca2+ activated Cl− channel) [14]. Importantly, the luminal concentration of HCO3− directly modulates the level of CFTR expression by stimulating soluble adenylate cyclase (sAC) and cAMP cellular production, leading to nuclear accumulation of the CREB transcription factor [15]. Similarly, ASL pH affects the membrane expression of ENaC, the main pathway for Na+ reabsorption, by regulating the activity of short palate lung and nasal epithelial clone 1 (SPLUNC1) antimicrobial peptide. This protein binds to αβγ-ENaC and causes the internalization of αγ-ENaC, thus preventing activation of the channel by serine proteases [16,17,18]. Importantly, this peptide fails to function at pH values below 7.0 due to pH-sensitive salt bridges, resulting in abnormal Na+ and water absorption [16,17].
At the epithelial level, pH and HCO3− are crucial regulators of innate airway defense [19,20]. This is due, at least partially, to a reduction in the killing properties of antimicrobial peptides, such as LL-37, because their cationic electric charge is modified at pH values below 7 [21]. The physiological relevance of this observation has been demonstrated in vivo by showing that lowering the pH of lung porcine epithelium ASL from 7.58 to 6.35 decreased its bacterial killing capacity [22]. This combines with the intrinsic effects of HCO3− on bacterial growth, airway colonization, and biofilm formation inhibition [23,24,25,26,27,28].
Finally, at the organ level, ASL pH modulates mucociliary and cough clearance, a process that enables continuous drainage of the airways, and thus proper air conduction. Mucociliary clearance is a complex phenomenon involving different factors: the mucus which traps exogenous particles and pathogens, the cilia whose beating moves up secretions, and the periciliary fluid which protects the epithelium against dehydration and bathes the cilia [29]. Mucus rheology and the viscoelastic properties of ASL are finely regulated by HCO3−. Indeed, mucins are condensed by calcium in secretory granules due to their acidic groups [30]. When they are secreted, they undergo expansion by as much as 1000-fold [30,31], triggered by the chelation of calcium by HCO3− [32,33,34]. Additionally, pH values below 7.0 decrease ciliary beating in vitro (50% at pH = 5.5 and almost 100% at pH = 3.5) [35]. This is due to the fact that sAC activity is decreased at low intracellular HCO3− concentrations (≤10 mM), thus decreasing local cAMP concentration necessary for cilia beating [36,37]. As a whole, defective ciliary beating and increased mucus viscosity combine to decrease mucociliary clearance (MCC) at acidic pH.
3. ASL pH in Physiology and Disease
3.1. Methods of Measurements
There are two classes of methods for measuring pH values.
Optical methods use the pH-dependent color changes of organic dye molecules (indicators) that are weak bases or acids. For the purpose of ASL pH measurements, the dextran-coupled (cell impermeant) pH fluorescent dyes BCECF, Fluorescein, HCC, or HPTS are employed, by adding them to the ASL directly [16,22,38,39,40,41].
The potentiometric methods measure the electrical voltage between a reference electrode and a pH electrode. Ionophore-filled home-made microelectrodes or commercial miniaturized pH electrodes have been used for determination of ASL pH in samples from human bronchial epithelial (HBE) cells [16,42,43]. However, these measurements in small samples are subjected to multiple confounding factors, including the immersion depth of the measuring electrode, placement of the reference electrode, disturbance of the epithelial layer, change in hygrometry, carbon dioxide concentration, and temperature in the vicinity of the measurement. Rapid developments in the field of sensors have enabled pH measurements in vivo, using mobidium pH probes [44,45,46], monocrystalline antimony catheters, or in-gold combined pH-glass electrodes [47]. More recently, Schultz et al. used a pH-sensitive luminescent dye-based fiber-optic probe. The dye was embedded in a hydrogel matrix, which limits interactions between the dye and ASL proteins that could potentially alter the measured pH values, and provides a high signal-to-noise ratio allowing for accurate measurements [48].
3.2. ASL pH Values in Physiological Conditions
The studies listed in Table 1, Table 2, Table 3 and Table 4 have shown that ASL pH in normal airways ranges in vivo between 5.6 and 6.7 in the nasal mucosa, and is around 7.0 in bronchia. Indeed, studies by McShane et al. showed that ASL pH was more alkaline in lower airways (7.1 ± 0.1) than in upper airways (6.6 ± 0.1) [47]. Similar ranges of values were observed in murine and porcine animal models. Such a wide range of ASL pH values may be explained by technical limitations. For example, obtaining stable recordings in vivo is challenging because of CO2 variations during breathing, which can skew pH measurements [48,49,50]. Moreover, applying an electrode to the epithelium immediately changes the transport equilibrium and therefore alters ASL pH.
Table 1.
Airway surface liquid (ASL) pH in animal models.
| Sample/Model | pH Value | Method Reference | ||
|---|---|---|---|---|
| WT | CF | |||
| Mouse | 6.95 ± 0.03 | 6.84 ± 0.07 | BCECF-dextran | [51] |
| 7.14 ± 0.01 (in vivo) | BCECF-dextran | [52] | ||
| 6.98 ± 0.16 | pH electrode | [50] | ||
| 7.28 | BCECF-dextran | [8] | ||
| Rat | 7.25 ± 0.05 | 6.42 ± 0.12 | Not specified | [53] |
| Ferret | 6.84 ± 0.03 | pH electrode | [54] | |
| Rabbit * | 6.92 ± 0.01 | pH electrode | [55] | |
| Cow | 6.81 ± 0.04 (25 mM bicarbonate) | BCECF-dextran | [52] | |
| 6.98 ± 0.05 (no bicarbonate) | BCECF-dextran | [52] | ||
| Pig | 7.14 ± 0.04 | 6.94 ± 0.05 | Optode (in vivo) | [22] |
| Primary Bronchial epithelia | 7.37 ± 0.05 | 7.05 ± 0.03 | SNARF pH indicator | [22] |
| 6.93 ± 0.04 | pH electrode | [56] | ||
| ~7.1 | 7.2 | pH electrode | [50] | |
| Gland fluid | 6.9 ± 0.06 | BCECF-dextran | [57] | |
* alveolar subphase; WT: Wild Type; CF: Cystic Fibrosis.
Table 2.
ASL pH in vitro measurements of human samples.
| Sample/Model | pH Value | Method | Reference | |
|---|---|---|---|---|
| WT | CF | |||
| Cell lines | ||||
| CFBE41σ | 7.42 ± 0.02 * | 7.15 ± 0.01 * | pH electrode | [58] |
| 7.24 ** | X Ray microanalysis | [59] | ||
| 16HBE14 σ | 7.14 ± 0.02 | pH electrode | [60] | |
| 7.16 ** | pH electrode | [59] | ||
| Calu3 | ~7.2 | BCECF-dextran | [61] | |
| 7.55 ± 0.04 | 7.28 ± 0.02 | pH electrode | [62] | |
| NuLi-1/CuFi-1 | 7.52 ± 0.07 | 6.88 ± 0.02 | SNARF pH indicator | [63] |
| C38/IB3-1/ | 7.32 ± 0.08 | 7.02 ± 0.04 | pH electrode | [64] |
| Primary cells (bronchi) | ||||
| 6.81 ± 0.20 | BCECF-dextran | [65] | ||
| 6.6 ± 0.1 | SNARF-1 | [66] | ||
| ΔpH = −0.096 ± 0.029 * | ΔpH = −0.146 ± 0.011 * | pH electrode | [42] | |
| ~7.4 | ~7.1 | BCECF-dextran | [67] | |
| 7.77 | 7.31 | pH electrode | [68] | |
| 7.35 ± 0.09 | pH electrode | [43] | ||
| 7.43 ± 0.06 * | 7.26 ± 0.02 * | pH electrode | [58] | |
| 7.35 ± 0.05 | 6.70 ± 0.03 | pH electrode | [69] | |
| Submucosal gland secretions | ||||
| 7.18 ± 0.06 | 6.57 ± 0.09 | BCECF-dextran | [70] | |
| 6.97 ± 0.06 | BCECF-dextran | [65] | ||
* after 6 h Ringer incubation; ** after 3 h Ringer incubation; WT: Wild Type; CF: Cystic Fibrosis.
Table 3.
ASL pH in vivo measurements of human samples in healthy controls and patients with cystic fibrosis.
| Sample/Model | pH Value | Method | Reference | |
|---|---|---|---|---|
| WT | CF | |||
| Nose | ||||
| Edge of nostril/adults | 5.5 ± 0.1 | 5.6 ± 0.1 | Monocrystalline antimony catheter | [47] |
| 4 cm from nares/adults | 6.7 ± 0.13 | 6.2 ± 0.1 | Monocrystalline antimony catheter | [47] |
| 6.6 ± 0.1 | 6.8 ± 0.10 | Gold probe | [47] | |
| 5 to 7.2 * | Mobidium pH probe | [44] | ||
| Neonates | 6.4 ± 0.2 | 5.2 ± 0.3 (4.5–6.9) | Mobidium pH probe | [44,45] |
| Lower airway/children | ||||
| 7.1 ± 0.1 | 7.1 ± 0.2 | Gold probe | [47] | |
| 7.00 ± 0.12 | 6.98 ± 0.15 | Fiberoptic probe | [48] | |
*: different genotypes; WT: Wild Type; CF: Cystic Fibrosis.
Table 4.
ASL pH in vivo measurements of human samples in patients with diseases other than cystic fibrosis.
| pH Value | Method | Reference | |
|---|---|---|---|
| Pneumonia | 6.62 ± 0.07 | pH electrode | [71] |
| 6.72 | pH electrode | [72] | |
| Chronic lung diseases | 6.64 ± 0.08 | pH electrode | [71] |
| COPD | 6.21 ± 0.37 | pH test strip | [73] |
| Acute exacerbation of COPD (AECOPD) | 6.89 ± 0.53 | pH test strip | [73] |
| Chronic rhinosinusitis | 6.7 ± 0.6 | pH electrode | [74] |
| Pulmonary tuberculosis (sputum) | 7.00 (range 5.50–8.37) | pH electrode | [75] |
| Chronic bronchitis | 7.59 (mucoid) | pH electrode | [76] |
| 7.83 (purulent) | pH electrode | [76] | |
| Rhinitis | 7.2–8.3 | pH electrode | [77] |
COPD: Chronic Obstructive Broncho Pulmonary Disease.
To avoid problems with in vivo measurements, ASL pH may be measured in vitro. Most studies focused on HCO3− transport have been performed in monolayers of Calu3, a cell line derived from a bronchial adenocarcinoma. Even though Calu3 cells are representative of submucosal cells, they are often considered to be a convenient model for epithelial airway cells, because they display strong cAMP-stimulated HCO3− transport and they express CFTR. They are clearly different from other ciliated epithelial cell lines or native tissue cells (as listed in Table 2), since they display characteristics of both serous and mucus cells [78]. Fewer studies have been performed in ciliated epithelial cell lines or primary airway 2D cultures using fluorescent indicators or ion selective electrodes under controlled conditions (temperature, pCO2, and humidity) [58,68]. Interestingly, these studies show an ASL pH close to 7.4 in human bronchial primary cells. Whether these in vitro observations can translate to in vivo physiology is still unknown [22,50,58]. In vitro studies in primary bronchial cells also have a number of limitations, including the fact that the relative proportion of different cells may differ according to the samples, and transporter expression levels according to the culture medium, as recently pointed out by Saint Cricq et al. [79].
3.3. ASL pH Values in Disease
In 1930, Hilding et al. reported “acidic” nasal secretions (i.e., below the blood pH of 7.4) in inflammatory acute lung diseases, and “alkaline” pH in the common cold [80]. It was thus hypothesized that airway pH may be an indicator of airway disease, either as a cause or a consequence. Since then, various studies have shown changes in ASL pH in specific diseases. Inflammation and infection seem to raise pH above the values observed in healthy airways, including pneumonia (7.2–7.4) [81], rhinitis (7.2–8.3) [77], and chronic bronchitis (7.6–7.8) [76], with the exception of one study on active bacterial infection (5.6–6.2) [49].
Many studies have suggested decreased pH in the airways of asthmatic [82,83,84] and Chronic Obstructive Broncho Pulmonary Disease (COPD) patients [85,86]. This is mainly based on the observed reductions in the pH value of exhaled breath “condensate” compared to healthy controls. This may reflect more intense airways inflammation, but obviously cannot be extrapolated to ASL pH values [84,86].
The main relevant studies are summarized in Table 1, Table 2, Table 3 and Table 4. In vitro studies have shown that ASL pH is decreased in the context of CFTR mutations, as compared to epithelia carrying WT CFTR [8,22,42,43,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. The majority of studies focused on the most frequent CFTR mutation, p. Phe508delCFTR (F508del). In 2011, Cho et al. observed that the rate of cAMP-dependent base secretion in CF nasal tissues was significantly lower than that in WT cells (11.8 ± 2.4 nmol min−1 cm−2, versus 57.2 ± 9.2 nmol min−1 cm−2) [87]. Interestingly, Coakley et al. showed in F508del cell lines exposed to a luminal acid challenge that the lack of HCO3− secretion resulted in a defective re-alkalinization of ASL [42].
Few groups have focused on submucosal glands. Jayaraman et al. found that submucosal gland secretions in CF patients exhibited a pH of 6.97 ± 0.06 but high viscosity [51]. In contrast, Song et al. reported a pH that was 0.6 pH units higher in healthy subjects than in CF patients, corresponding to a three-fold increase in HCO3− concentration [70]. This observation suggests that submucosal glands might contribute to ASL pH homeostasis.
In vivo data are discordant. Lower pH values were observed within the nasal epithelium of CF patients in comparison to healthy controls [44,47], while other studies did not report differences [48]. To date, the debate on ASL pH values in the context of CFTR mutations has not been resolved. If proved true, this abnormally low pH, or at least the defective buffering of acidic load, could explain a number of physiopathological aspects in lung disease, such as increased mucus thickness, defective innate defense, bacterial colonization, and the inflammation observed in CF epithelium [88,89].
The ASL pH value is the result of transepithelial acid and base transport and buffering power. The studies describing the main transporters involved in these processes are reported below.
4. Bicarbonate Transport in Airway Cells
Several important findings on the mechanisms underpinning HCO3− transmembrane transport were obtained by Devor et al. in 1999 [90]. Measurements of short-circuit current and labeled fluxes in Calu3 cell lines provided convincing functional evidence that basolateral HCO3− entry involves the electrogenic, coupled transport of Na+ and HCO3−, and that apical HCO3− secretion involves CFTR and other transporters.
4.1. Apical HCO3− Transport
4.1.1. CFTR
CFTR is a channel that belongs to the ATP binding cassette (ABC) superfamily. The channel is gated by ATP binding/hydrolysis at its two transmembrane domains and by the phosphorylation of its large intracellular regulatory (R) domain. R phosphorylation is mostly achieved by the cAMP/PKA pathway (and less efficiently by the PKC phosphorylation pathway) [91]. Patch-clamp experiments in transfected cells have shown that CFTR exhibits a high anionic selectivity (PNa/PCl ~0.03) and a small linear conductance (~10 pS) [92]. Various anions may flow across the activated channel, down their favorable transmembrane electrochemical potential gradients [93]. Chloride is the favorite substrate among halide ions, as both its transmembrane electrochemical gradient and its permeability are the most favorable (the halide permeability sequence is Br− > Cl− > I− > F−). Many other anions can be transported by CFTR, among them glutathione and HCO3− (PCl/PHCO3 ~ 4) [94,95]. This supports the role of CFTR, not only in Cl− transport, but also in pH regulation.
CFTR is expressed at the apical membrane of airway ciliated epithelial cells, in submucosal glands, and in the recently described ionocytes [7,96,97]. Even though ionocyte-type cells are rare (1–2% of epithelial cells), single-cell RNA sequencing and Ussing chamber experiments in human bronchial epithelial cells (HBECs) demonstrated that they express 50% of CFTR transcripts, causing 60% of CFTR-mediated current, whereas the common ciliated epithelial cells, thought to be the major location of CFTR, express only 1.5% of CFTR transcripts and mediate 4% of the mean channel current [97]. However, the precise role of ionocytes in Cl− secretion (and a fortiori in HCO3− secretion) in the native epithelium remains unclear. Secretory cells might also play a predominant role in the expression and function of CFTR in the lungs, as recently reported [98].
At least three points increase the complexity of understanding Cl− vs. HCO3− transport by CFTR. First, CFTR is not only an anionic channel but also a regulator of other transport systems, including HCO3− transporters [99,100,101]. Physical and/or functional interactions between CFTR and several transporters from the SLC26 family (A3, A6, A8, A9…) have been well documented [102,103,104]. It has been proposed that CFTR itself can act as a Cl−/HCO3− exchanger [105], but this hypothesis is debated [106]. Second, the respective selectivity of the CFTR channel for Cl− and HCO3− appears to be dynamically regulated. The transport of Cl− increases at intracellular pH (pHi) values of 6.6 and 6.3, whereas alkaline pHi inhibits Cl− flow [107]. Moreover, the amount of CFTR is rate-limiting for HCO3− transport in contrast to Cl− secretion, which nearly reaches the WT level in CFTR+/F508del pig epithelia [28]. In addition, a low intracellular Cl− concentration may switch CFTR from a predominant Cl− channel to a predominant HCO3− channel, by activating intracellular Cl−-sensitive kinases (with-no-lysine kinase WNK1, oxidative stress-responsive kinase 1, OSR1, and sterile 20/SPS1-related proline/alanine-rich kinase, SPAK) [108,109]. Although this has been shown in pancreatic cells, this may be a mechanism that is also in place in the lung, as WNK1 and OSR1 are largely present in the lung [110]. Interestingly some CFTR mutations located in transmembrane domains selectively decrease the transport of HCO3− by hindering the physical association between CFTR and WNK1 [109,111,112]. Third, HCO3− transport is often assessed indirectly, i.e., by measuring pH changes in experimental protocols. It is in this context very difficult to differentiate between the effects of CFTR on pH values, and those of other transporters, which renders the interpretation of results difficult [113].
All these observations indicate the importance of CFTR-mediated HCO3− transport in the pulmonary epithelium. This is reinforced by the recent observation that correctors of defective CFTR increase the permeability of the mutated channel to HCO3− more than to halide ions [114].
4.1.2. ANO1
Studies in HEK293 cells have shown that the Cl− channel ANO1 becomes highly permeable to HCO3− at high intracellular [Ca2+] [100]. Interestingly, in epithelial ciliated cells, a cross-activation between CFTR and ANO1 (TMEM16A) has been demonstrated, involving compartmentalized Ca2+ and cAMP crosstalk in specific plasma membrane domains containing GPCRs, CFTR, and TMEM16A [115].
4.1.3. Apical Cl−/HCO3− Exchangers
SLC26A4 and A9 belong to the solute carriers SLC26 transporter family, which is composed of a N-terminal transmembrane domain (TMD) connected to a C-terminal sulfate transporter anti-sigma factor antagonist (STAS) domain [116]. Multiple studies have shown physical, biochemical, and functional interactions between SLC26 anion exchangers and CFTR, leading to reciprocal activation [101,102,117]. This has especially been described for SLC26A3 and A6 [102,118], and more recently for SLC26A9 [103]. Interestingly, mutations in the STAS domain abolished this functional activation [102,103,119], suggesting a physical interaction between the STAS domain of the SLC26 transporters and the R domain of CFTR [102,117].
4.1.4. SLC26A4 (Pendrin/PDS)
Pendrin is expressed preferentially in goblet cells, and at low levels in ciliated epithelial cells, but not in ionocytes [120]. Its expression at the apical membrane is increased by the pro-inflammatory cytokines IL-4, IL-13, and IL-17A [121,122]. The role of SLC26A4 in HCO3− transport is still a matter of debate.
Garnett et al. showed that in Calu3 cells, increases in pendrin activity by cAMP/PKA can induce ASL alkalinization up to pH 7.9 (corresponding to a final concentration of 75 mM HCO3−) [101]. Pendrin remained active after the addition of GlyH-101 (a CFTR inhibitor) and basolateral DIDS (a Cl− channel blocker), which suggests a mechanism independent of CFTR-mediated HCO3− secretion. This might not be, however, physiological, as lower pH values are more frequently reported (Table 2).
Indeed, Shan et al. [62] reported lower HCO3− concentrations in forskolin-stimulated fluid secretions (up to pH 7.55, corresponding to a final concentration of 31 mM), and a reduced HCO3− flux after inhibition of CFTR, similarly to cells that do not express CFTR.
These observations are consistent with electroneutral Cl−/HCO3− exchange by pendrin working in parallel with electrogenic Cl− secretion mediated by CFTR [101,123]. In this model, the coupled Cl−/HCO3− transport is mediated by the cAMP signaling pathway via protein phosphatase 1 (PP1), which inhibits HCO3−/Cl− exchange by basolateral AE2 and activates CFTR, thereby enhancing the electrogenic efflux of Cl−, which is further recycled by SLC26A4, leading ultimately to HCO3− secretion [101]. According to this model, CFTR acts mainly as a Cl− transporter that fuels SLC26A4 to transport HCO3− into the ASL. A similar mechanism has been described in pancreatic ductular cells between SLC26A6 and CFTR [101,124].
Several studies have supported the hypothesis of CFTR-pendrin coupling. In primary cultures of differentiated human airway epithelia, and in secretory cells where both proteins were co-expressed, Rehman et al. observed that the combination of TNFα+IL-17 increased CFTR and pendrin expression. This was associated with elevated ASL pH stemming from increased HCO3− secretion [120]. Simonin et al. [58] found that inhibition of pendrin by a specific inhibitor decreased ASL pH. However, this is in contrast to Haggie et al. [67], who observed no effect on ASL pH. A possible explanation for these contradictory results is that pendrin expression may vary according to inflammation and culture conditions.
Indeed upregulation of pendrin transcription has been shown in chronic rhinosinusitis and in rodent models of inflammatory lung diseases [125,126,127]. More recently, Bajko et al. [128] showed that stimulation with interleukins IL-4, IL-13, or IL-17a increased pendrin levels in human bronchial epithelial cells from CF patients (CF HBECs) and non-CF donors (HBECs) [121,129]. Indeed, modulation of pendrin expression/activity by inflammation may represent a protective mechanism for re-alkalinization of ASL and epithelium defense during disease.
4.1.5. SLC26A9
SLC26A9 is widely expressed in the luminal membranes of HBE cells, where it contributes to constitutive Cl− secretion [130,131]. Patch clamp studies, on both cell lines and transfected HEK cells, showed that SLC26A9 is a highly selective Cl− channel with linear current-voltage characteristics and minimal HCO3− permeability [99,132]. Additionally, electrophysiological studies on different models (HEK, Xenopuslaevis oocytes, and animal models) showed that SLC26A9 may act as a Cl−/HCO3− exchanger working in tandem with an apical Cl− transporter, such as CFTR [133,134]. More speculatively, the channel might also switch its permeability from Cl− to HCO3−, similarly to CFTR [108,135,136]. To date, the contribution of SLC26A9 to HCO3− transport in airways remains unknown, and may be cell/tissue dependent.
4.2. Basolateral HCO3− Transport
4.2.1. Cl−/HCO3− Exchanger (AE2)
The anion exchanger type 2 (AE2, or SLC4A2) is expressed at the basolateral membrane of airway epithelium and thought to participate in fluid secretion [137]. AE2 performs the reversible electroneutral exchange of Cl− for HCO3−, as directed by the electrochemical potential gradients, and is in part responsible for the regulation of cytosolic pH and cell volume [138]. Its activity is downregulated by cAMP agonists, via a PKA-independent mechanism involving Ca2+, calmodulin, and the protein kinase CK2. This mechanism was confirmed in primary human nasal epithelia, where CK2 inhibition abolished the activity of AE2. In Calu3 cells, the involvement of AE2 in anion secretion [139,140] entails the coupled inhibition of AE2 and the activation of CFTR and pendrin by cAMP agonists, as described above [101].
4.2.2. Electrogenic Na-Coupled Bicarbonate Co-Transport (NBCe, SLC4A4)
Na+-coupled bicarbonate symports belong to the large SLC4 family (see for review [141]). Among them, NBCe are electrogenic transporters, mediating the co-transport of 1 Na+ with 2 or 3 HCO3−, consequently transporting a net negative electrical charge. NBCe1-B and NBCe2 (aka pNBC1 and NBC4) were identified at the basolateral cell membrane of Calu3 cells [142], confirming an earlier conclusion that an electrogenic, inwardly directed coupled transport of Na+ and HCO3− was the basolateral step of transcellular base secretion, intimately related to Cl− and HCO3− apical secretion [90]. It is noteworthy that the regulation of NBCe1-B is finely coupled to that of CFTR [143,144]. First, NBCe1-B has a consensus phosphorylation site for PKA located in the N-terminus and is stimulated by cAMP. This is in line with the forskolin-induced stimulation of net transepithelial HCO3− transport in Calu3 cells [90]. Second, phosphorylated IRBIT (Inositol-1,4,5-trisphosphate receptor-binding protein released with IP3), a known activator of CFTR, also stimulates the activity of NBCe1-B [145], and antagonizes the inhibition of both NBCe1-B and CFTR by the WNK-SPAK pathway [108,146]. Finally, in transfected HeLa cells, NBCe1-B is sensitive to the intracellular Cl− concentration [147]. Further studies are needed to confirm the above regulations in other cell models.
4.2.3. Carbonic Anhydrases (CA)
Carbonic anhydrases (cytosolic, mitochondrial, or membrane-bound CAs) catalyze the hydration of CO2 and H2O into carbonic acid (H2CO3), which immediately equilibrates with HCO3− and H+, thereby leading to a quasi-immediate equilibrium between CO2 and HCO3−. In the lungs, CA activity was demonstrated thanks to the effect of acetazolamide on the conversion of H14CO3− to 14CO2 [148]. The two fastest CAs, i.e., the cytosolic CAII and the membrane-bound CAIV (which may also contribute to cytosolic CA activity) [149], are expressed in the lung. It has been proposed that the association of CAII and CA IV to various acid–base transporters (AE1, NHE1, NBCe1) forms a metabolon that greatly accelerates the transcellular transport of acid equivalents [150,151,152,153]. Such a functional complex in Calu3 cells is supported by the acetazolamide-induced decrease in HCO3− secretion [90,154], which suggests that some fraction of the transported HCO3− results from metabolic cell production. At the ASL interface, if CA catalyzes the immediate equilibrium of CO2 with HCO3− and H+, large and rapid pH variations should be observed at each inspiration/expiration [155]. However, only slow and acetazolamide-insensitive pH variations were measured, indicating minimal CA activity in ASL, thus preventing large and rapid changes of ASL pH upon CO2 variations during inspiration/expiration [156]. This means that a disequilibrium pH (i.e., a discrepancy between the measured pH, and the pH value if CO2-HCO3−-H+ reactions were at equilibrium) may prevail in the ASL. This implies that one can infer HCO3− concentrations from measured pH values only in steady-state conditions, an important matter of concern during laboratory experiments, and most importantly, in vivo.
Several arguments suggest that CA expression/function plays a role in CF. First, CA IV targeting may be altered in CF cells [157]. Second, the genetic loss of CA XII function is associated with CF-like features [158]. Third, in co-cultured Calu3 cells/WT murine lymphocytes, CAII and IV are up-regulated, and HCO3− secretion is enhanced, whereas this defense mechanism against infection is impaired in Calu3/CF lymphocytes co-culture [159].
4.3. Paracellular HCO3− Transport
The paracellular pathway has mostly been explored to assess the transport of medicinal drugs and various molecules (such as cytokines or allergens) in the pulmonary epithelium [160,161]. Few studies have focused on the ionic permeation properties of the shunt barrier, which depend largely on the expressed claudins [162]. In human airway primary cultures, a preferential cationic selectivity was evidenced by measurements of dilution potentials under open circuit conditions [163]. Recent studies confirmed this selectivity, and showed that the permeabilities of the shunt pathway to Cl− and HCO3− are equal, but that their conductances differ due to their different luminal concentrations [164]. According to the authors’ calculations, the paracellular HCO3− flux is secretory under basal conditions (ASL pH < basolateral pH), but it reverses to absorption when ASL pH is >7.0, a condition reached when transcellular HCO3− secretion is enhanced by the presence of pro-inflammatory cytokines [120,164].
5. Acid Transport in Airway Cells
Different observations point to H+ secretion into ASL. Even though both transcellular and paracellular routes across the airway epithelium are permeable to HCO3−, normal steady-state ASL pH has been found to be below blood pH. Moreover, ASL pH re-acidifies at an initial rate of ~0.2 pH/hour after mild alkalinization, which is equivalent to a net acid secretion of 3.5 nmol h−1 cm−2 (as per the calculation of Fisher et al. in a ASL of volume 2.5 µL cm−2 and buffer capacity of 7 mM/UpH) [165]. We describe below the main transporters involved in acid transport.
5.1. Basolateral Secretion: Na+/H+ Exchangers
Na+/H+ exchangers form a family of transporters that regulate pH homeostasis, with nine known members: NHE1–9 (SLC9A1 to A9). NHEs mediate the entry into the cell of extracellular Na+ in exchange for cytosolic H+, with a 1:1 stoichiometry, as dictated by the chemical gradients of Na+ and H+ [166]. Al-Bazzaz et al. showed by transcript expression that NHE1 is expressed along the entire human respiratory tract. In particular, the relative abundance of NHE1 mRNA was found to be higher in the trachea and distal airways in humans [167].
At the functional level, Paradiso et al. observed a Na+-dependent re-alkalization upon cytosolic acidification (NH4Cl prepulse method); the pHi recovery was blocked by serosal but not mucosal amiloride in human nasal epithelial cell monolayers [168]. This suggests a basolateral localization of NHE1.
Other studies, however, have suggested the potential localization of a Na+/H+ exchanger isoform at the apical membrane, based on decreased acid secretion after treatment with mucosal amiloride in primary human airway epithelia, intact distal airways from pigs, and tracheal tubes from sheep [56,169,170,171]. This potential NHE-mediated H+ secretion into the ASL might explain the association between SLC9A3 variants and the susceptibility to airway infections in CF patients, but this hypothesis needs experimental support [172,173,174,175]. As a whole, the localization of NHEs, their role in regulating ASL pH, and their clinical relevance need to be further investigated.
5.2. Apical Secretion
5.2.1. H+/K+ ATPase
HKA2 (ATP12A) belongs to the P2-type ATPase family and shares sequence homologies with both the gastric H,K-ATPase (ATP4A) and the Na,K-ATPase (ATP1A). HKA2 mediates the electroneutral exchange of H+ for potassium (K+) but may also function in a Na+/K+ exchange mode [176,177,178]. HKA2 is inhibited by ouabain (previously thought to be specific to the Na,K-ATPase) but its sensitivity to SCH28080 (a potent inhibitor of the gastric H,K-ATPase) is debated. HKA2 is located on the apical side of respiratory epithelia, particularly in the goblet cells [68,179]. Its trafficking to the apical cell membrane is triggered by its association with the β subunit of Na,K-ATPase, ATP1B1, which is increased in inflammatory conditions, whereas the Na,K-ATPase (composed of a αcatalytic subunit associated with ATP1B1) remains strictly located to the basolateral cell membrane [68]. Of note, HKA2 expression is low in murine airways, and this may partly explain the very mild pulmonary CF phenotype in this species [40]. In human primary cell cultures and fresh tissue, the contribution of ATP12A to ASL pH was thoroughly investigated by Coakley et al. [42]. They measured a K+-dependent, ouabain-sensitive, SCH28080-insensitive ASL acidification, a result that supports an acidifying process mediated by HKA2. However, even though ASL pH was lower in CF cultures than in WT cultures, the acidification rate was not different. The authors concluded that HCO3− secretion mediated by CFTR buffers HKA2-mediated H+ secretion, but that the activity of this pump is the same in CF and WT cells. This thus would explain the “hyper acidity” of the CF ASL [42]. Interestingly, similar to CFTR, HKA2 is upregulated by an increase in intracellular cAMP [180]. Supporting the role of HKA2 in the lower ASL pH observed in CF airway epithelia, Simonin et al. showed that using ouabain to inhibit HKA2 restored the pH value of CF ASL to that of WT [58]. The deleterious involvement of HKA2-induced acidification of ASL during infectious episodes was demonstrated by Shah et al. in pigs and humans. Shah et al. concluded that in non-CF epithelia, HCO3− secretion by CFTR balanced H+ secretion by HKA2, but that in CF epithelia, unbalanced proton secretion occurs, and impairs the host defenses against bacteria [28]. This observation is reinforced by the upregulation of HKA2 by several interleukins in the context of inflammation [68]. Inhibition of HKA2 in lungs is therefore expected to be beneficial during CF, as it might increase ASL pH. Up to now, only ouabain has been proven to inhibit HKA2, but its toxicity precludes its use for therapy.
5.2.2. Hydrogen Voltage-Gated Channel 1 (HVCN1)
The fundamental function of voltage-gated H+ channels is acid extrusion from the cells. Their open probability depends only on pH or membrane depolarization [181]. H+ channels were found in airway epithelia, both in primary and immortalized cell lines, and their activity was localized to the apical membrane [171,182,183]. Ivovannisci et al. demonstrated that the HVCN1 channel contributes to ASL pH by opening its transmembrane pore when extracellular pH exceeds 7.0 [184]. Since the membrane potential of airway epithelia is stable, H+ channel gating must be primarily regulated by the pH gradient across the apical membrane (increased extracellular pH rather than decreased intracellular pH). This gradient may be enhanced by DUOX oxidase (nicotinamide adenine dinucleotide phosphate-oxidase) [182] and/or mitochondria tightly packed at the apical membrane of airways [185], which can contribute to intracellular H+ production. Fischer proposed a model with a passive feedback system controlling ASL pH, which combines CFTR alkalizing the ASL by secreting HCO3− and voltage-gated H+ channels secreting H+ [186]. It might be disrupted in CF, as suggested by a recent study showing that HVCN1 protein levels in lysates of nasal cells were significantly lower in CF patients than in healthy subjects [187].
5.2.3. Vacuolar H+-ATPase
Vacuolar (V-type) H+-ATPases play an important role in regulating pH, by pumping H+ across membranes against the pH gradient, using the energy from ATP hydrolysis [188]. The contribution of V-ATPase to ASL pH remains unclear. Whereas some groups observed that bafilomycin, a specific V-ATPase blocker, raises ASL pH in Calu3 cells and in distal pig bronchi [56,62,189], others found that bafilomycin has minimal or no effects in human, pig, and cow airway epithelia [70,171,182,190]. Of note, a study on primary nasal epithelial cells and cultured lung showed that Pseudomonas aeruginosa inactivates V-ATPase, thereby reducing the expression and trafficking of CFTR [191].
6. Proposed Model
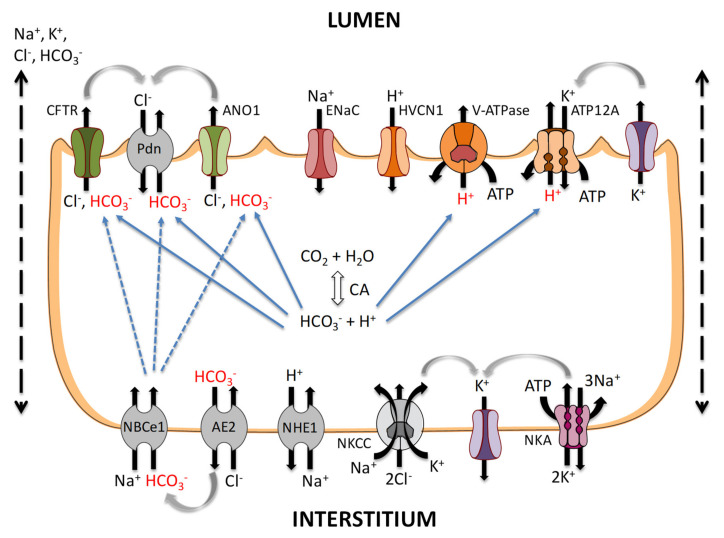
Altogether, the studies described above indicate that the airways constitutively secrete both acid and base at substantial rates, which concur to tightly regulate ASL pH. The model shown in Figure 3 recapitulates our current understanding of acid–base transport across airway epithelia. Apical HCO3− secretion is mediated by CFTR, pendrin, and possibly ANO1, and may be facilitated by the coupling between Cl− secretion via CFTR (and possibly other Cl− channels) and Cl− recycling through Cl−/HCO3− exchangers. Apical H+ secretion is mediated by ATP-driven H+ transporters, such as ATP12A and V-ATPase, that carry H+ against the electrochemical potential gradient. In contrast, HVCN1-mediated H+ secretion is passive, and can only occur when the electrochemical potential H+ gradient is reversed.
Figure 3.
Distribution of the currently known acid and base transporters of airway epithelial cells.
Basolateral NBC activity coupled with CA-mediated HCO3− formation provide HCO3− for apical secretion. The protons that are concomitantly formed are reabsorbed by basolateral NHE1, or secreted into the ASL. In the basal state, AE2, acting in concert with Na+-HCO3− cotransporters, enables basolateral Cl− loading and HCO3− recycling, which reduces the electrochemical driving force for HCO3− transport across the apical membrane. Under cAMP-stimulated conditions, AE2 is inhibited whereas NBCe1 is activated, both of which enhance the driving force for apical HCO3− secretion. Paracellular HCO3− transport, the direction of which depends on the ASL pH value, acts as an ASL protective buffering mechanism.
7. Current Gaps in Knowledge
Many aspects of the model proposed above remain to be elucidated, including the contribution of each epithelial cell type, the role of specific transporters, and regulatory pathways.
Airway secretion is a very complex biofluid, containing ASL, as well as mucins, cell micro-organisms, and their byproducts.
Mucins are acidic with a low isoelectric point, and should therefore contribute to low pH buffering, an additional protective factor in inflammatory disease, where their amount is greatly increased. Indeed, mucins have an excellent acid buffer capacity. This has been well documented in the intestine [192]. In the lung, Holma et al. studied induced sputum from smokers, and determined a pH of 6.8 with a buffering capacity by around 7 µM/pH unit of sputum; they also found that the patients whose sputum contained less mucin were more prone to pollution induced lung damage [193,194]. A more recent study on sub mucosal gland (SMG) secretions from the Verkman laboratory yielded a relatively acidic pH of 7.0–7.2 [65]. Finally, Song, et al. reported in human gland secretions induced by pilocarpine a buffer capacity of 12 mM/pH unit, decreasing to ~3.7 mM/pH unit, in HCO3− free conditions, thereby confirming that HCO3− is a substantial contributor to mucus pH [70].
Those observations are of the utmost importance. Indeed, in vitro measurements in excised porcine bronchi suggested that airway fluid originates primarily from the SMGs [195]. Moreover, in vitro optical studies from individual SMGs from pigs and ferrets showed that cAMP agonists induced a combination of Cl− and HCO3− secretion in SMG fluid/mucus secretion [196]. This process was reduced in CF ferrets and pigs [197]. This is consistent with the known high level of CFTR expression in SMG serous cells [198] and the fact that CFTR-Inh 172 inhibits pilocarpine- and forskolin-induced airway SMG secretion in WT, but not CF pigs and humans [57]. The relative contribution of SMGs, goblet cells, and epithelial ciliated cells to ASL pH buffering at basal state and during acid load is still unclear. This is, at least partly, because most of the mechanistic studies were done on specific cell lines and not on full tissue explant, which would allow better dissecting of the different pathways of H+ and HCO3− secretion, and better understanding the physiopathology of diseases that alter ASL pH. The pH of ASL is also intimately related to its cellular and bacterial content. Pulmonary diseases can cause accumulation of inflammatory cells, which in turn release reactive oxygen species and other byproducts of aerobic metabolism that cause damage to lipids, proteins, and DNA, and modify the buffering capacity of airway secretions. Pulmonary diseases also induce microbiota dysbiosis, as well as alterations of the structure of the airways and the formation of niches, that favor the growth and increase of common commensal bacteria. The relationship between microbiota and pH is complex. Indeed, Ratske et al. [199] showed that, bacteria can change the pH of the microenvironment by producing metabolites, namely indoles and organic acids. Organic acids lower lung mucus pH, which in turn favors anaerobic fermentation and the production of propionic acid. In the lung, this could contribute to a further decrease in the buffering capacity of airway secretions and low sputum pH [200].
Our understanding of the function of each cell type remains very limited. Which cells contribute significantly to HCO3− and/or H+ secretion? Do ciliated cells play a role in the process of pH homeostasis? What is the role of secretory cells? Does this change along the airways? Is substantial acid and base secretion made possible by large H+ and HCO3− fluxes across a small number of cells, or by small fluxes across a large number of cells?
The respective roles of the different transporters, and how their action is coordinated are still unclear. The following issues can be highlighted.
Regarding HCO3− transport, the relative contribution of CFTR and pendrin to apical HCO3− secretion remains unclear. Some investigators have reported that CFTR is the main, if not the only, apical HCO3− transporter in Calu3 and native airway epithelial cells [62,201]. However, several recent studies have suggested that pendrin-mediated HCO3− secretion plays a more important role than CFTR-mediated HCO3− secretion in regulating ASL pH, as observed in porcine airway epithelial cells [50], human nasal, and bronchial epithelial cells pre-treated with IL-4 [129], and cAMP-activated Calu3 cells [101]. Recently, SNPs in the SLC26A9 gene were found to be associated with CF-related disease onset, suggesting that SLC26A9 acts as a CFTR regulator [202], and may be involved in the response to CFTR-directed therapeutics [172,203]. Whether this might involve modification of pH ASL is unknown.
The mechanisms underlying basolateral HCO3− transport have not been fully deciphered. NBCe1-B and NBCe2 have been identified on the basolateral side of the pulmonary epithelium. Both are electrogenic, but whether they operate as a 1Na+:2HCO3−, 1Na+:3 HCO3−, or 1Na+:1HCO3−:1CO32− cotransporter is not yet established [141,204]. Aside from a heuristic viewpoint, the stoichiometry of these symports has physiological relevance. Depending on the value of the basolateral membrane potential and their respective reversal potential, the direction of the NBC-mediated transport may reverse, switching from basolateral HCO3− influx into the cell, to efflux out of the cell.
Regarding H+ transport, the physiological role of HKA2 and its therapeutic target potential remain to be clarified. Whether apical K+ recycling via K+ channels acts to enhance H+ secretion by HKA2 is unknown. Indeed, upregulation of the K+ channel KCNMB4a by IL-4 has been shown by microarray analysis in bronchial epithelial cells (BE37), but this was not characterized at the protein level [179]. Interestingly, Coakley et al. showed that the K+ channel blocker barium did not alter ASL acidification rates, arguing against a significant K+ conductance on the apical cell membrane [42]. Moreover, several studies indicate that HKA2 might switch from a H+/K+-ATPase to a Na+/K+-ATPase [176,178]. In the native renal collecting duct, this mode leads to luminal Na+ secretion [205]. Interestingly, a recent study showed that the Na+/K+ exchange mode of HKA2 is induced by increases in intracellular cGMP [206], and thus indirectly related to NO, whose level is decreased in CF epithelial cells. Importantly, there have been conflicting findings regarding the sensitivity of HKA2 to the inhibitors of the gastric H/K-ATPase (see for review [207]). These discrepancies are possibly explained by a tissue- (or species-) dependence of HKA2 sensitivity. The search for non-toxic HKA2 inhibitors is under active investigation.
Finally, as described above, the binding of CAII and CAIV to acid–base transporters may form a “metabolon” [150,151,153]. As reviewed by Pushkin and Kurtz, this functional complex would greatly enhance transcellular HCO3− fluxes across transporters from the SLC4 family [152], but this remains a matter of debate [208]. Further studies on the physical/functional link between CA and acid–base transporters in the different cell types in normal and in CF conditions are necessary, and may identify novel therapeutic targets.
8. Conclusions
There is increasing evidence that ASL pH is linked to airway function, and that the tight regulation of ASL pH is crucial to airway homeostasis. Recent progress has led to a better understanding of acid and base transporters in airway epithelium. However, knowledge on how the transepithelial transport of H+ and HCO3− is coordinated to tightly regulate ASL pH is clearly limited. This should be the focus of new studies to improve our understanding of physiology and disease, and identify therapeutic targets.
Author Contributions
All authors performed literature review, writing, review and editing; I.S.-G.: conceptualization of the review. All authors have read and agreed to the published version of the manuscript.
Funding
Miroslaw Zajac: funded by research grant no. 2018/02/X/NZ4/00304 from the National Science Centre (NCN, Poland) and partially by Wlasny Fundusz Stypendialny SGGW. Elise Dreano was funded by Vertex Innovation Award.
Conflicts of Interest
I.S.-G. declares the following funding sources from Vertex Pharmaceuticals: Innovation Award funding program 2016 and 2017. She was the PI in clinical trials from Vertex Pharmaceuticals and took part in scientific advisory board meetings organized by Vertex Pharmaceuticals, Eloxx and Proteostasis. G.P. declares the following funding sources from Vertex Pharmaceuticals: Innovation Award funding program 2017. The authors declare no conflict of intrest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tam A., Wadsworth S., Dorscheid D., Man S.F.P., Sin D.D. The airway epithelium: More than just a structural barrier. Ther. Adv. Respir. Dis. 2011;5:255–273. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- 2.Widdicombe J.H. Regulation of the depth and composition of airway surface liquid. J. Anat. 2002;201:313–318. doi: 10.1046/j.1469-7580.2002.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahy J.V., Dickey B.F. Airway Mucus Function and Dysfunction. N. Engl. J. Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widdicombe J.H., Wine J.J. Airway gland structure and function. Physiol. Rev. 2015;95:1241–1319. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 5.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scudieri P., Musante I., Venturini A., Guidone D., Genovese M., Cresta F., Caci E., Palleschi A., Poeta M., Santamaria F., et al. Ionocytes and CFTR Chloride Channel Expression in Normal and Cystic Fibrosis Nasal and Bronchial Epithelial Cells. Cells. 2020;9:2090. doi: 10.3390/cells9092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montoro D.T., Haber A.L., Biton M., Vinarsky V., Birket S., Yuan F., Chen S., Leung H.M., Villoria J., Rogel N., et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y., Thiagarajah J., Verkman A.S. Sodium and Chloride Concentrations, pH, and Depth of Airway Surface Liquid in Distal Airways. J. Gen. Physiol. 2003;122:511–519. doi: 10.1085/jgp.200308866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams O.W., Sharafkhaneh A., Kim V., Dickey B.F., Evans C.M. Airway mucus: From production to secretion. Am. J. Respir. Cell Mol. Biol. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henke M.O., John G., Germann M., Lindemann H., Rubin B.K. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am. J. Respir. Crit. Care Med. 2007;175:816–821. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- 11.Rose M.C., Voynow J.A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 12.Tarran R., Grubb B.R., Gatzy J.T., Davis C.W., Boucher R.C. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J. Gen. Physiol. 2001;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korbmacher J.P., Michel C., Neubauer D., Thompson K., Mizaikoff B., Frick M., Dietl P., Wittekindt O.H. Amiloride-sensitive fluid resorption in NCI-H441 lung epithelia depends on an apical Cl- conductance. Physiol. Rep. 2014;2:1–10. doi: 10.1002/phy2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruffin M., Voland M., Marie S., Bonora M., Blanchard E., Blouquit-Laye S., Naline E., Puyo P., Le Rouzic P., Guillot L., et al. Anoctamin 1 dysregulation alters bronchial epithelial repair in cystic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2013;1832:2340–2351. doi: 10.1016/j.bbadis.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Baudouin-Legros M., Hamdaoui N., Borot F., Fritsch J., Ollero M., Planelles G., Edelman A. Control of basal CFTR gene expression by bicarbonate-sensitive adenylyl cyclase in human pulmonary cells. Cell. Physiol. Biochem. 2008;21:75–86. doi: 10.1159/000113749. [DOI] [PubMed] [Google Scholar]
- 16.Garland A.L., Walton W.G., Coakley R.D., Tan C.D., Gilmore R.C., Hobbs C.A., Tripathy A., Clunes L.A., Bencharit S., Stutts M.J., et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc. Natl. Acad. Sci. USA. 2013;110:15973–15978. doi: 10.1073/pnas.1311999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim C.S., Ahmad S., Wu T., Walton W.G., Redinbo M.R., Tarran R. SPLUNC1 is an allosteric modulator of the epithelial sodium channel. FASEB J. 2018;32:2478–2491. doi: 10.1096/fj.201701126R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Caballero A., Rasmussen J.E., Gaillard E., Watson M.J., Olsen J.C., Donaldson S.H., Stutts M.J., Tarran R. SPLUNC1 regulates airway surface liquid volume by protecting ENaC from proteolytic cleavage. Proc. Natl. Acad. Sci. USA. 2009;106:11412–11417. doi: 10.1073/pnas.0903609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bals R., Hiemstra P.S. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 20.Ridley C., Thornton D.J. Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 2018;46:1099–1106. doi: 10.1042/BST20170402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alaiwa M.H.A., Reznikov L.R., Gansemer N.D., Sheets K.A., Horswill A.R., Stoltz D.A., Zabner J., Welsh M.J. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. USA. 2014;111:18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pezzulo A.A., Tang X.X., Hoegger M.J., Abou Alaiwa M.H., Ramachandran S., Moninger T.O., Karp P.H., Wohlford-Lenane C.L., Haagsman H.P., Eijk M.V., et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobay O., Laub K., Stercz B., Kéri A., Balázs B., Tóthpál A., Kardos S., Jaikumpun P., Ruksakiet K., Quinton P.M., et al. Bicarbonate inhibits bacterial growth and biofilm formation of prevalent cystic fibrosis pathogens. Front. Microbiol. 2018;9:1–12. doi: 10.3389/fmicb.2018.02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gawande P.V., Lovetri K., Yakandawala N., Romeo T., Zhanel G.G., Cvitkovitch D.G., Madhyastha S. Antibiofilm activity of sodium bicarbonate, sodium metaperiodate and SDS combination against dental unit waterline-associated bacteria and yeast. J. Appl. Microbiol. 2008;105:986–992. doi: 10.1111/j.1365-2672.2008.03823.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaushik K.S., Stolhandske J., Shindell O., Smyth H.D., Gordon V.D. Tobramycin and bicarbonate synergise to kill planktonic Pseudomonas aeruginosa, but antagonise to promote biofilm Survival. NPJ Biofilms Microbiomes. 2016;2:1–12. doi: 10.1038/npjbiofilms.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratten J., Wiecek J., Mordan N., Lomax A., Patel N., Spratt D., Middleton A.M. Physical disruption of oral biofilms by sodium bicarbonate: An in vitro study. Int. J. Dent. Hyg. 2016;14:209–214. doi: 10.1111/idh.12162. [DOI] [PubMed] [Google Scholar]
- 27.Xie C., Tang X., Xu W., Diao R., Cai Z., Chan H.C. A host defense mechanism involving CFTR-mediated bicarbonate secretion in bacterial prostatitis. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah V.S., Ernst S., Tang X.X., Karp P.H., Parker C.P., Ostedgaard L.S., Welsh M.J. Relationships among CFTR expression, HCO3− secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc. Natl. Acad. Sci. USA. 2016;113:5382–5387. doi: 10.1073/pnas.1604905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowles M.R., Boucher R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 2002;109:571–577. doi: 10.1172/JCI0215217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdugo P. Goblet Cells Secretion And Mucogenesis. Annu. Rev. Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- 31.Verdugo P. Mucin Exocytosis. Am. Rev. Respir. Dis. 1991;144:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 32.Quinton P.M. Cystic fibrosis: Impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen E.Y.T., Yang N., Quinton P.M., Chin W.C. A new role for bicarbonate in mucus formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299 doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdullah L.H., Evans J.R., Wang T.T., Ford A.A., Makhov A.M., Nguyen K., Coakley R.D., Griffith J.D., Davis C.W., Ballard S.T., et al. Defective postsecretory maturation of MUC5B mucin in cystic fibrosis airways. JCI Insight. 2017;2:e89752. doi: 10.1172/jci.insight.89752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clary-Meinesz C., Mouroux J., Cosson J., Huitorel P., Blaive B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur. Respir. J. 1998;11:330–333. doi: 10.1183/09031936.98.11020330. [DOI] [PubMed] [Google Scholar]
- 36.Schmid A., Sutto Z., Nlend M.C., Horvath G., Schmid N., Buck J., Levin L.R., Conner G.E., Fregien N., Salathe M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J. Gen. Physiol. 2007;130:99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid A., Sutto Z., Schmid N., Novak L., Ivonnet P., Horvath G., Conner G., Fregien N., Salathe M. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J. Biol. Chem. 2010;285:29998–30007. doi: 10.1074/jbc.M110.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delpiano L., Thomas J.J., Yates A.R., Rice S.J., Gray M.A., Saint-Criq V. Esomeprazole increases airway surface liquid ph in primary cystic fibrosis epithelial cells. Front. Pharmacol. 2018;9:1–15. doi: 10.3389/fphar.2018.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang X.X., Ostedgaard L.S., Hoegger M.J., Moninger T.O., Karp P.H., McMenimen J.D., Choudhury B., Varki A., Stoltz D.A., Welsh M.J. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Investig. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah V.S., Meyerholz D.K., Tang X.X., Reznikov L., Abou Alaiwa M., Ernst S.E., Karp P.H., Wohlford-Lenane C.L., Heilmann K.P., Leidinger M.R., et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lennox A.T., Coburn S.L., Leech J.A., Heidrich E.M., Kleyman T.R., Wenzel S.E., Pilewski J.M., Corcoran T.E., Myerburg M.M. ATP12A promotes mucus dysfunction during Type 2 airway inflammation. Sci. Rep. 2018;8:1–13. doi: 10.1038/s41598-018-20444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coakley R.D., Grubb B.R., Paradiso A.M., Gatzy J.T., Johnson L.G., Kreda S.M., O’Neal W.K., Boucher R.C. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. USA. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanova R., Benton D.C.H., Munye M.M., Rangseesorranan S., Hart S.L., Moss G.W.J. A Nanosensor Toolbox for Rapid, Label-Free Measurement of Airway Surface Liquid and Epithelial Cell Function. ACS Appl. Mater. Interfaces. 2019;11:8731–8739. doi: 10.1021/acsami.8b14122. [DOI] [PubMed] [Google Scholar]
- 44.Abou Alaiwa M.H., Beer A.M., Pezzulo A.A., Launspach J.L., Horan R.A., Stoltz D.A., Starner T.D., Welsh M.J., Zabner J. Neonates with cystic fibrosis have a reduced nasal liquid pH; A small pilot study. J. Cyst. Fibros. 2014;13:373–377. doi: 10.1016/j.jcf.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abou Alaiwa M.H., Launspach J.L., Grogan B., Carter S., Zabner J., Stoltz D.A., Singh P.K., McKone E.F., Welsh M.J. Ivacaftor-induced sweat chloride reductions correlate with increases in airway surface liquid pH in cystic fibrosis. JCI Insight. 2018;3:10–13. doi: 10.1172/jci.insight.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alaiwa M.H.A., Launspach J.L., Sheets K.A., Rivera J.A., Gansemer N.D., Taft P.J., Thorne P.S., Welsh M.J., Stoltz D.A., Zabner J. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight. 2016;1:1–11. doi: 10.1172/jci.insight.87535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McShane D., Davies J.C., Davies M.G., Bush A., Geddes D.M., Alton E.W.F.W. Airway surface pH in subjects with cystic fibrosis. Eur. Respir. J. 2003;21:37–42. doi: 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 48.Schultz A., Puvvadi R., Borisov S.M., Shaw N.C., Klimant I., Berry L.J., Montgomery S.T., Nguyen T., Kreda S.M., Kicic A., et al. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat. Commun. 2017;8:1–8. doi: 10.1038/s41467-017-00532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerrin F., Voisin C., Macquet V., Robin H., Lequien P. Apport de la pH metrie bronchique in situ. Progr. Resp. Res. 1971;6:372–383. [Google Scholar]
- 50.Benedetto R., Centeio R., Ousingsawat J., Schreiber R., Janda M., Kunzelmann K. Transport properties in CFTR−/− knockout piglets suggest normal airway surface liquid pH and enhanced amiloride-sensitive Na+ absorption. Pflugers Arch. Eur. J. Physiol. 2020;472:1507–1519. doi: 10.1007/s00424-020-02440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayaraman S., Song Y., Vetrivel L., Shankar L., Verkman A.S. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J. Clin. Investig. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayaraman S., Song Y., Verkman A.S. Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am. J. Physiol. Cell Physiol. 2001;281 doi: 10.1152/ajpcell.2001.281.5.C1504. [DOI] [PubMed] [Google Scholar]
- 53.Birket S., Tuggle K., Oden A., Fernandez C., Chu K., Tearney G., Fanucchi M., Sorscher E., Rowe S. Poster Session Abstracts. Pediatr. Pulmonol. 2016;51:S194–S485. doi: 10.1002/ppul.23576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kyle H., Ward J.P.T., Widdicombe J.G. Control of pH of airway surface liquid of the ferret trachea in vitro. J. Appl. Physiol. 1990;68:135–140. doi: 10.1152/jappl.1990.68.1.135. [DOI] [PubMed] [Google Scholar]
- 55.Nielson D.W., Goerke J., Clements J.A. Alveolar subphase pH in the lungs of anesthetized rabbits. Proc. Natl. Acad. Sci. USA. 1981;78:7119–7123. doi: 10.1073/pnas.78.11.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inglis S.K., Wilson S.M., Olver R.E. Secretion of acid and base equivalents by intact distal airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:855–862. doi: 10.1152/ajplung.00348.2002. [DOI] [PubMed] [Google Scholar]
- 57.Thiagarajah J.R., Song Y., Haggie P.M., Verkman A.S. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J. 2004;18:875–877. doi: 10.1096/fj.03-1248fje. [DOI] [PubMed] [Google Scholar]
- 58.Simonin J., Bille E., Crambert G., Noel S., Dreano E., Edwards A., Hatton A., Pranke I., Villeret B., Cottart C.H., et al. Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-42751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kozlova I., Nilsson H., Henriksnäs J., Roomans G.M. X-ray microanalysis of apical fluid in cystic fibrosis airway epithelial cell lines. Cell. Physiol. Biochem. 2006;17:13–20. doi: 10.1159/000091455. [DOI] [PubMed] [Google Scholar]
- 60.Zając M., Lewenstam A., Stobiecka M., Dołowy K. New ISE-Based Apparatus for Na+, K+, Cl−, pH and Transepithelial Potential Difference Real-Time Simultaneous Measurements of Ion Transport across Epithelial Cells Monolayer–Advantages and Pitfalls. Sensors. 2019;19:1881. doi: 10.3390/s19081881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo J.B., Moody M., Koh D.S. Epithelial monolayer culture system for real-time single-cell analyses. Physiol. Rep. 2014;2:1–16. doi: 10.14814/phy2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shan J., Liao J., Huang J., Robert R., Palmer M.L., Fahrenkrug S.C., O’Grady S.M., Hanrahan J.W. Bicarbonate-dependent chloride transport drives fluid secretion by the human airway epithelial cell line Calu-3. J. Physiol. 2012;590:5273–5297. doi: 10.1113/jphysiol.2012.236893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muraglia K.A., Chorghade R.S., Kim B.R., Tang X.X., Shah V.S., Grillo A.S., Daniels P.N., Cioffi A.G., Karp P.H., Zhu L., et al. Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia. Nature. 2019;567:405–408. doi: 10.1038/s41586-019-1018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valdivieso Á.G., Clauzure M., Massip-Copiz M.M., Cancio C.E., Asensio C.J.A., Mori C., Santa-Coloma T.A. Impairment of CFTR activity in cultured epithelial cells upregulates the expression and activity of LDH resulting in lactic acid hypersecretion. Cell. Mol. Life Sci. 2019;76:1579–1593. doi: 10.1007/s00018-018-3001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayaraman S., Joo N.S., Reitz B., Wine J.J., Verkman A.S. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc. Natl. Acad. Sci. USA. 2001;98:8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kreindler J.L., Bertrand C.A., Lee R.J., Karasic T., Aujla S., Pilewski J.M., Frizzell R.A., Kolls J.K. Interleukin-17A induces bicarbonate secretion in nrmal human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:257–266. doi: 10.1152/ajplung.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haggie P.M., Phuan P.W., Tan J.A., Zlock L., Finkbeiner W.E., Verkman A.S. Inhibitors of pendrin anion exchange identified in a small molecule screen increase airway surface liquid volume in cystic fibrosis. FASEB J. 2016;30:2187–2197. doi: 10.1096/fj.201600223R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scudieri P., Musante I., Caci E., Venturini A., Morelli P., Walter C., Tosi D., Palleschi A., Martin-Vasallo P., Sermet-Gaudelus I., et al. Increased expression of ATP12A proton pump in cystic fibrosis airways. JCI Insight. 2018;3:1–14. doi: 10.1172/jci.insight.123616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gianotti A., Capurro V., Delpiano L., Mielczarek M., García-valverde M., Carreira-barral I., Ludovico A., Fiore M., Baroni D., Moran O., et al. Small molecule anion carriers correct abnormal airway surface liquid properties in cystic fibrosis airway epithelia. Int. J. Mol. Sci. 2020;21:1488. doi: 10.3390/ijms21041488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song Y., Salinas D., Nielson D.W., Verkman A.S. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am. J. Physiol. Cell Physiol. 2006;290:669–672. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 71.Bodem C.R., Lampton L.M., Miller D.P., Tarka E.F., Everett E.D. Endobronchial pH. Relevance to aminoglycoside activity in gram-negative bacillary pneumonia. Am. Rev. Respir. Dis. 1983;127:39–41. doi: 10.1164/arrd.1983.127.1.39. [DOI] [PubMed] [Google Scholar]
- 72.Karnad D.R., Mhaisekar D.G., Moralwar K.V. Respiratory mucus pH in tracheostomized intensive care unit patients. Crit. Care Med. 1990;18:699–701. doi: 10.1097/00003246-199007000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Lozo Vukovac E., Miše K., Gudelj I., Perić I., Duplančić D., Vuković I., Vučinović Z., Lozo M. Bronchoalveolar pH and inflammatory biomarkers in patients with acute exacerbation of chronic obstructive pulmonary disease. J. Int. Med. Res. 2019;47:791–802. doi: 10.1177/0300060518811560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim B.G., Kim J.H., Kim S.W., Kim S.W., Jin K.S., Cho J.H., Kang J.M., Park S.Y. Nasal pH in patients with chronic rhinosinusitis before and after endoscopic sinus surgery. Am. J. Otolaryngol. Head Neck Med. Surg. 2013;34:505–507. doi: 10.1016/j.amjoto.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 75.Masuda M., Sato T., Sakamaki K., Kudo M., Kaneko T., Ishigatsubo Y. The effectiveness of sputum pH analysis in the prediction of response to therapy in patients with pulmonary tuberculosis. PeerJ. 2015;2015:1–14. doi: 10.7717/peerj.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adler K., Wooten O., Philippoff W., Lerner E., Dulfano M.J. Physical Properties of Sputum. Am. Rev. Respir. Dis. 1972;106:86–96. doi: 10.1164/arrd.1972.106.1.86. [DOI] [PubMed] [Google Scholar]
- 77.England R.J.A., Homer J.J., Knight L.C., Ell S.R. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryngol. Allied Sci. 1999;24:67–68. doi: 10.1046/j.1365-2273.1999.00223.x. [DOI] [PubMed] [Google Scholar]
- 78.Shan J., Huang J., Liao J., Robert R., Hanrahan J.W. Anion secretion by a model epithelium: More lessons from Calu-3. Acta Physiol. 2011;202:523–531. doi: 10.1111/j.1748-1716.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- 79.Saint-Criq V., Delpiano L., Casement J., Onuora J.C., Lin J.H., Gray M.A. Choice of Differentiation Media Significantly Impacts Cell Lineage and Response to CFTR Modulators in Fully Differentiated Primary Cultures of Cystic Fibrosis Human Airway Epithelial Cells. Cells. 2020;9:2137. doi: 10.3390/cells9092137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hilding A. The Common Cold. Arch. Otolaryngol. Head Neck Surg. 1930;12:133–150. doi: 10.1001/archotol.1930.03570010161001. [DOI] [Google Scholar]
- 81.Steinmann E. La secretion bronchique et le pH. Bronches. 1956;6:126–129. [Google Scholar]
- 82.Hunt J.F., Fang K., Malik R., Snyder A., Malhotra N., Platts-Mills T.A.E., Gaston B. Endogenous Airway Acidification. Am. J. Respir. Crit. Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 83.Carraro S., Folesani G., Corradi M., Zanconato S., Gaston B., Baraldi E. Acid-base equilibrium in exhaled breath condensate of allergic asthmatic children. Allergy Eur. J. Allergy Clin. Immunol. 2005;60:476–481. doi: 10.1111/j.1398-9995.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 84.Kostikas K., Papatheodorou G., Ganas K., Psathakis K., Panagou P., Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am. J. Respir. Crit. Care Med. 2002;165:1364–1370. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 85.Antus B., Barta I., Kullmann T., Lazar Z., Valyon M., Horvath I., Csiszer E. Assessment of exhaled breath condensate ph in exacerbations of asthma and chronic obstructive pulmonary disease: A longitudinal study. Am. J. Respir. Crit. Care Med. 2010;182:1492–1497. doi: 10.1164/rccm.201003-0451OC. [DOI] [PubMed] [Google Scholar]
- 86.Papaioannou A.I., Loukides S., Minas M., Kontogianni K., Bakakos P., Gourgoulianis K.I., Alchanatis M., Papiris S., Kostikas K. Exhaled breath condensate pH as a biomarker of COPD severity in ex-smokers. Respir. Res. 2011;12:67. doi: 10.1186/1465-9921-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho D.Y., Hwang P.H., Illek B., Fischer H. Acid and base secretion in freshly excised nasal tissue from cystic fibrosis patients with ΔF508 mutation. Int. Forum Allergy Rhinol. 2011;1:123–127. doi: 10.1002/alr.20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hiemstra P.S., McCray P.B., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015;45:1150–1162. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ng A.W., Bidani A., Heming T.A. Innate host defense of the lung: Effects of lung-lining fluid pH. Lung. 2004;182:297–317. doi: 10.1007/s00408-004-2511-6. [DOI] [PubMed] [Google Scholar]
- 90.Devor D.C., Singh A.K., Lambert L.C., DeLuca A., Frizzell R.A., Bridges R.J. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J. Gen. Physiol. 1999;113:743–760. doi: 10.1085/jgp.113.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Z., Liu F., Chen J. Molecular structure of the ATP-bound, phosphorylated human CFTR. Proc. Natl. Acad. Sci. USA. 2018;115:12757–12762. doi: 10.1073/pnas.1815287115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tabcharani J.A., Linsdell P., Hanrahan J.W. Halide permeation in wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels. J. Gen. Physiol. 1997;110:341–354. doi: 10.1085/jgp.110.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linsdell P., Evagelidis A., Hanrahan J.W. Molecular determinants of anion selectivity in the cystic fibrosis transmembrane conductance regulator chloride channel pore. Biophys. J. 2000;78:2973–2982. doi: 10.1016/S0006-3495(00)76836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poulsen J.H., Fischer H., Illek B., Machen T.E. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linsdell P., Hanrahan J.W. Glutathione permeability of CFTR. Am. J. Physiol. Cell Physiol. 1998;275:323–326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- 96.Kreda S.M., Mall M., Mengos A., Rochelle L., Yankaskas J., Riordan J.R., Boucher R.C. Characterization of wild-type and ΔF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol. Biol. Cell. 2005;16:2154–2167. doi: 10.1091/mbc.e04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plasschaert L.W., Žilionis R., Choo-Wing R., Savova V., Knehr J., Roma G., Klein A.M., Jaffe A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okuda K., Dang H., Kobayashi Y., Carraro G., Nakano S., Chen G., Kato T., Asakura T., Gilmore R.C., Morton L.C., et al. Secretory Cells Dominate Airway CFTR Expression and Function in Human Airway Superficial Epithelia. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202008-3198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bertrand C.A., Zhang R., Pilewski J.M., Frizzell R.A. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J. Gen. Physiol. 2009;133:421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung J., Nam J.H., Park H.W., Oh U., Yoon J.-H., Lee M.G. Dynamic modulation of ANO1/TMEM16A HCO3- permeability by Ca2+/calmodulin. Proc. Natl. Acad. Sci. USA. 2013;110:360–365. doi: 10.1073/pnas.1211594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garnett J.P., Hickman E., Burrows R., Hegyi P., Tiszlavicz L., Cuthbert A.W., Fong P., Gray M.A. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J. Biol. Chem. 2011;286:41069–41082. doi: 10.1074/jbc.M111.266734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ko S.B.H., Zeng W., Dorwart M.R., Luo X., Kim K.H., Millen L., Goto H., Naruse S., Soyombo A., Thomas P.J., et al. Gating of CFTR by the STAS domain of SLC26 transporters. Nat. Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bakouh N., Bienvenu T., Thomas A., Ehrenfeld J., Liote H., Roussel D., Duquesnoy P., Farman N., Viel M., Cherif-Zahar B., et al. Characterization of SLC26A9 in patients with CF-like lung disease. Hum. Mutat. 2013;34:1404–1414. doi: 10.1002/humu.22382. [DOI] [PubMed] [Google Scholar]
- 104.Rode B., Dirami T., Bakouh N., Rizk-rabin M., Norez C., Lhuillier P., Lorès P., Jollivet M., Melin P., Zvetkova I., et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: A potential role during sperm capacitation. Hum. Mol. Genet. 2012;21:1287–1298. doi: 10.1093/hmg/ddr558. [DOI] [PubMed] [Google Scholar]
- 105.Choi J.Y., Lee M.G., Ko S., Muallem S. Cl--dependent HCO3- transport by cystic fibrosis transmembrane conductance regulator. J. Pancreas. 2001;2:243–246. [PubMed] [Google Scholar]
- 106.Massey M.K., Reiterman M.J., Mourad J., Luckie D.B. Is CFTR an exchanger?: Regulation of HCO3−Transport and extracellular pH by CFTR. Biochem. Biophys. Rep. 2021;25:100863. doi: 10.1016/j.bbrep.2020.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J.H., Cai Z., Sheppard D.N. Direct sensing of intracellular pH by the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. J. Biol. Chem. 2009;284:35495–35506. doi: 10.1074/jbc.M109.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park H.W., Nam J.H., Kim J.Y., Namkung W., Yoon J.S., Lee J.S., Kim K.S., Venglovecz V., Gray M.A., Kim K.H., et al. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Kim Y., Jun I., Shin D.H., Yoon J.G., Piao H., Jung J., Park H.W., Cheng M.H., Bahar I., Whitcomb D.C., et al. Regulation of CFTR Bicarbonate Channel Activity by WNK1: Implications for Pancreatitis and CFTR-Related Disorders. Cmgh. 2020;9:79–103. doi: 10.1016/j.jcmgh.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.The Human Protein Atlas. [(accessed on 13 January 2021)]; Available online: v14.proteinatlas.org/ENSG00000060237-WNK1/tissue/lung.
- 111.Choi J.Y., Muallem D., Kiselyov K., Lee M.G., Thomas P.J., Muallem S. Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LaRusch J., Jung J., General I.J., Lewis M.D., Park H.W., Brand R.E., Gelrud A., Anderson M.A., Banks P.A., Conwell D., et al. Mechanisms of CFTR Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hug M.J., Clarke L.L., Gray M.A. How to measure CFTR-dependent bicarbonate transport: From single channels to the intact epithelium. Methods Mol. Biol. 2011;741:489–509. doi: 10.1007/978-1-61779-117-8_30. [DOI] [PubMed] [Google Scholar]
- 114.Fiore M., Picco C., Moran O. Correctors modify the bicarbonate permeability of F508del-CFTR. Sci. Rep. 2020;10:1–7. doi: 10.1038/s41598-020-65287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lérias J., Pinto M., Benedetto R., Schreiber R., Amaral M., Aureli M., Kunzelmann K. Compartmentalized crosstalk of CFTR and TMEM16A (ANO1) through EPAC1 and ADCY1. Cell. Signal. 2018;44:10–19. doi: 10.1016/j.cellsig.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 116.Geertsma E.R., Chang Y.N., Shaik F.R., Neldner Y., Pardon E., Steyaert J., Dutzler R. Structure of a prokaryotic fumarate transporter reveals the architecture of the SLC26 family. Nat. Struct. Mol. Biol. 2015;22:803–808. doi: 10.1038/nsmb.3091. [DOI] [PubMed] [Google Scholar]
- 117.Gray M.A. Bicarbonate secretion: It takes two to tango. Nat. Cell Biol. 2004;6:292–294. doi: 10.1038/ncb0404-292. [DOI] [PubMed] [Google Scholar]
- 118.Shcheynikov N., Wang Y., Park M., Ko S.B.H., Dorwart M., Naruse S., Thomas P.J., Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J. Gen. Physiol. 2006;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wheat V.J., Shumaker H., Burnham C., Shull G.E., Yankaskas J.R., Soleimani M. CFTR induces the expression of DRA along with Cl-/HCO3- exchange activity in tracheal epithelial cells. Am. J. Physiol. Cell Physiol. 2000;279:62–71. doi: 10.1152/ajpcell.2000.279.1.C62. [DOI] [PubMed] [Google Scholar]
- 120.Rehman T., Thornell I.M., Pezzulo A.A., Thurman A.L., Romano Ibarra G.S., Karp P.H., Tan P., Duffey M.E., Welsh M.J. TNFα and IL-17 Alkalinize Airway Surface Liquid through CFTR and Pendrin. Am. J. Physiol. Physiol. 2020 doi: 10.1152/ajpcell.00112.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pedemonte N., Caci E., Sondo E., Caputo A., Rhoden K., Pfeffer U., Di Candia M., Bandettini R., Ravazzolo R., Zegarra-Moran O., et al. Thiocyanate Transport in Resting and IL-4-Stimulated Human Bronchial Epithelial Cells: Role of Pendrin and Anion Channels. J. Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- 122.Adams K.M., Abraham V., Spielman D., Kolls J.K., Rubenstein R.C., Conner G.E., Cohen N.A., Kreindler J.L. IL-17A induces Pendrin expression and chloride-bicarbonate exchange in human bronchial epithelial cells. PLoS ONE. 2014;9:e103263. doi: 10.1371/journal.pone.0103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garnett J.P., Turner M.J. Controversies surrounding the role of CFTR in airway bicarbonate secretion. J. Physiol. 2013;591:2241–2242. doi: 10.1113/jphysiol.2013.251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y., Soyombo A.A., Shcheynikov N., Zeng W., Dorwart M., Marino C.R., Thomas P.J., Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3- secretion: Relevance to cystic fibrosis. EMBO J. 2006;25:5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Valentin E., Crahay C., Garbacki N., Hennuy B., Guéders M., Noël A., Foidart J.M., Grooten J., Colige A., Piette J., et al. New asthma biomarkers: Lessons from murine models of acute and chronic asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296 doi: 10.1152/ajplung.90367.2008. [DOI] [PubMed] [Google Scholar]
- 126.Ishida A., Ohta N., Suzuki Y., Kakehata S., Okubo K., Ikeda H., Shiraishi H., Izuhara K. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol. Int. 2012;61:589–595. doi: 10.2332/allergolint.11-OA-0370. [DOI] [PubMed] [Google Scholar]
- 127.Nakagami Y., Favoreto S., Zhen G., Park S.-W., Nguyenvu L.T., Kuperman D.A., Dolganov G.M., Huang X., Boushey H.A., Avila P.C., et al. The Epithelial Anion Transporter Pendrin Is Induced by Allergy and Rhinovirus Infection, Regulates Airway Surface Liquid, and Increases Airway Reactivity and Inflammation in an Asthma Model. J. Immunol. 2008;181:2203–2210. doi: 10.4049/jimmunol.181.3.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bajko J., Duguid M., Altmann S., Hurlbut G.D., Kaczmarek J.S. Pendrin stimulates a chloride absorption pathway to increase CFTR-mediated chloride secretion from Cystic Fibrosis airway epithelia. FASEB BioAdvances. 2020 doi: 10.1096/fba.2020-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim D., Huang J., Billet A., Abu-Arish A., Goepp J., Matthes E., Tewfik M.A., Frenkiel S., Hanrahan J.W. Pendrin mediates bicarbonate secretion and enhances cystic fibrosis transmembrane conductance regulator function in airway surface epithelia. Am. J. Respir. Cell Mol. Biol. 2019;60:705–716. doi: 10.1165/rcmb.2018-0158OC. [DOI] [PubMed] [Google Scholar]
- 130.Bertrand C.A., Mitra S., Mishra S.K., Wang X., Zhao Y., Pilewski J.M., Madden D.R., Frizzell R.A. The CFTR trafficking mutation F508del inhibits the constitutive activity of SLC26A9. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L12–L925. doi: 10.1152/ajplung.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lohi H., Kujala M., Mäkelä S., Lehtonen E., Kestilä M., Saarialho-Kere U., Markovichand D., Kere J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J. Biol. Chem. 2002;277:14246–14254. doi: 10.1074/jbc.M111802200. [DOI] [PubMed] [Google Scholar]
- 132.Salomon J.J., Spahn S., Wang X., Füllekrug J., Bertrand C.A., Mall M.A. Generation and functional characterization of epithelial cells with stable expression of SLC26A9 Cl- channels. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;310:L593–L602. doi: 10.1152/ajplung.00321.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu J., Henriksnäs J., Barone S., Witte D., Shull G.E., Forte J.G., Holm L., Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl-/HCO3- exchange, and is inhibited by NH4+ Am. J. Physiol. Cell Physiol. 2005;289:C493–C505. doi: 10.1152/ajpcell.00030.2005. [DOI] [PubMed] [Google Scholar]
- 134.Demitrack E.S., Soleimani M., Montrose M.H. Damage to the gastric epithelium activates cellular bicarbonate secretion via SLC26A9 Cl-/HCO3- exchange. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G255–G264. doi: 10.1152/ajpgi.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dorwart M.R., Shcheynikov N., Wang Y., Stippec S., Muallem S. SLC26A9 is a Cl- channel regulated by the WNK kinases. J. Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chang M.-H.H., Plata C., Zandi-Nejad K., Sinđić A., Sussman C.R., Mercado A., Broumand V., Raghuram V., Mount D.B., Romero M.F., et al. Slc26a9-Anion exchanger, channel and Na+ transporter. J. Membr. Biol. 2009;228:125–140. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alper S.L. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J. Exp. Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stewart A.K., Chernova M.N., Shmukler B.E., Wilhelm S., Alper S.L. Regulation of AE2-mediated Cl- transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. J. Gen. Physiol. 2002;120:707–722. doi: 10.1085/jgp.20028641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang J., Shan J., Kim D., Liao J., Evagelidis A., Alper S.L., Hanrahan J.W. Basolateral chloride loading by the anion exchanger type 2: Role in fluid secretion by the human airway epithelial cell line Calu-3. J. Physiol. 2012;590:5299–5316. doi: 10.1113/jphysiol.2012.236919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ibrahim S.H., Turner M.J., Saint-Criq V., Garnett J., Haq I.J., Brodlie M., Ward C., Borgo C., Salvi M., Venerando A., et al. CK2 is a key regulator of SLC4A2-mediated Cl−/HCO3− exchange in human airway epithelia. Pflugers Arch. Eur. J. Physiol. 2017;469:1073–1091. doi: 10.1007/s00424-017-1981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parker M.D., Boron W.F. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol. Rev. 2013;93:803–959. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kreindler J.L., Peters K.W., Frizzell R.A., Bridges R.J. Identification and membrane localization of electrogenic sodium bicarbonate cotransporters in Calu-3 cells. Biochim. Biophys. Acta Mol. Basis Dis. 2006;1762:704–710. doi: 10.1016/j.bbadis.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 143.Pushkin A., Abuladze N., Newman D., Lee I., Xu G., Kurtz I. Two C-terminal variants of NBC4, a new member of the sodium bicarbonate cotransporter family: Cloning, characterization, and localization. IUBMB Life. 2000;50:13–19. doi: 10.1080/15216540050176539. [DOI] [PubMed] [Google Scholar]
- 144.Barbuskaite D., Pedersen F.D., Christensen H.L., Johnsen L., Praetorius J., Damkier H.H. NBCe2 (Slc4a5) Is Expressed in the Renal Connecting Tubules and Cortical Collecting Ducts and Mediates Base Extrusion. Front. Physiol. 2020;11:1–13. doi: 10.3389/fphys.2020.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shirakabe K., Priori G., Yamada H., Ando H., Horita S., Fujita T., Fujimoto I., Mizutani A., Seki G., Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3- cotransporter 1 (pNBC1) Proc. Natl. Acad. Sci. USA. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang D., Shcheynikov N., Zeng W., Ohana E., So I., Ando H., Mizutani A., Mikoshiba K., Muallem S. IRBIT coordinates epithelial fluid and HCO3- secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J. Clin. Investig. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shcheynikov N., Son A., Hong J.H., Yamazaki O., Ohana E., Kurtz I., Shin D.M., Muallem S. Intracellular Cl-as a signaling ion that potently regulates Na+/Hco3—Transporters. Proc. Natl. Acad. Sci. USA. 2015;112:E329–E337. doi: 10.1073/pnas.1415673112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Effros R., Chang R., Silverman P. Acceleration of plasma bicarbonate conversion to carbon dioxide by pulmonary carbonic anhydrase. Science. 1978;199:427–429. doi: 10.1126/science.413195. [DOI] [PubMed] [Google Scholar]
- 149.Schneider H.P., Alt M.D., Klier M., Spiess A., Andes F.T., Waheed A., Sly W.S., Becker H.M., Deitmer J.W. GPI-anchored carbonic anhydrase IV displays both intra- and extracellular activity in cRNA-injected oocytes and in mouse neurons. Proc. Natl. Acad. Sci. USA. 2013;110:1494–1499. doi: 10.1073/pnas.1221213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vince J.W., Reithmeier R.A.F. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte Cl-/HCO3- exchanger. J. Biol. Chem. 1998;273:28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 151.Li X., Alvarez B., Casey J.R., Reithmeier R.A.F., Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 2002;277:36085–36091. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- 152.Pushkin A., Kurtz I. SLC4 base (HCO3−, CO32−) transporters: Classification, function, structure, genetic diseases, and knockout models. Am. J. Physiol. Ren. Physiol. 2006;290:580–599. doi: 10.1152/ajprenal.00252.2005. [DOI] [PubMed] [Google Scholar]
- 153.Becker H.M., Deitmer J.W. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3- cotransporter. J. Biol. Chem. 2007;282:13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- 154.Inglis S.K., Finlay L., Ramminger S.J., Richard K., Ward M.R., Wilson S.M., Olver R.E. Regulation of intracellular pH in Calu-3 human airway cells. J. Physiol. 2002;538:527–539. doi: 10.1113/jphysiol.2001.012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Effros R.M. Carbonic anhydrase and alveolar fluid absorption. Am. J. Respir. Cell Mol. Biol. 2008;39:124. doi: 10.1165/ajrcmb.39.1.124. [DOI] [PubMed] [Google Scholar]
- 156.Thornell I.M., Li X., Tang X.X., Brommel C.M., Karp P.H., Welsh M.J., Zabner J. Nominal carbonic anhydrase activity minimizes airway-surface liquid pH changes during breathing. Physiol. Rep. 2018;6:1–10. doi: 10.14814/phy2.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fanjul M., Salvador C., Alvarez L., Cantet S., Hollande E. Targeting of carbonic anhydrase IV to plasma membrane is altered in cultured human pancreatic duct cells expressing a mutated (ΔF508) CFTR. Eur. J. Cell Biol. 2002;81:437–447. doi: 10.1078/0171-9335-00264. [DOI] [PubMed] [Google Scholar]
- 158.Lee M., Vecchio-Pagán B., Sharma N., Waheed A., Li X., Raraigh K.S., Robbins S., Han S.T., Franca A.L., Pellicore M.J., et al. Loss of carbonic anhydrase XII function in individuals with elevated sweat chloride concentration and pulmonary airway disease. Hum. Mol. Genet. 2016;25:1923–1933. doi: 10.1093/hmg/ddw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tang X.X., Fok K.L., Chen H., Chan K.S., Tsang L.L., Rowlands D.K., Zhang X.H., Da Dong J., Ruan Y.C., Jiang X., et al. Lymphocyte CFTR promotes epithelial bicarbonate secretion for bacterial killing. J. Cell. Physiol. 2012;227:3887–3894. doi: 10.1002/jcp.24101. [DOI] [PubMed] [Google Scholar]
- 160.Coyne C.B., Vanhook M.K., Gambling T.M., Carson J.L., Boucher R.C., Johnson L.G. Regulation of Airway Tight Junctions by Proinflammatory Cytokines. Mol. Biol. Cell. 2002;13:3218–3234. doi: 10.1091/mbc.e02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vinhas R., Cortes L., Cardoso I., Mendes V.M., Manadas B., Todo-Bom A., Pires E., Veríssimo P. Pollen proteases compromise the airway epithelial barrier through degradation of transmembrane adhesion proteins and lung bioactive peptides. Allergy Eur. J. Allergy Clin. Immunol. 2011;66:1088–1098. doi: 10.1111/j.1398-9995.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 162.Günzel D., Yu A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Flynn A.N., Itani O.A., Moninger T.O., Welsh M.J. Acute regulation of tight junction ion selectivity in human airway epithelia. Proc. Natl. Acad. Sci. USA. 2009;106:3591–3596. doi: 10.1073/pnas.0813393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thornell I.M., Rehman T., Pezzulo A.A., Welsh M.J. Paracellular bicarbonate flux across human cystic fibrosis airway epithelia tempers changes in airway surface liquid pH. J. Physiol. 2020:JP280120. doi: 10.1113/JP280120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fischer H., Widdicombe J.H. Mechanisms of acid and base secretion by the airway epithelium. J. Membr. Biol. 2006;211:139–150. doi: 10.1007/s00232-006-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aronson P.S., Nee J., Suhm M.A. Modifier role of internal H+ in activating the Na +-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982;299:161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- 167.Al-Bazzaz F.J., Hafez N., Tyagi S., Gailey C.A., Toofanfard M., Alrefai W.A., Nazir T.M., Ramaswamy K., Dudeja P.K. Detection of Cl--HCO3- and Na+-H+ exchangers in human airways epithelium. J. Pancreas. 2001;2:285–290. [PubMed] [Google Scholar]
- 168.Paradiso A.M. ATP-activated baselateral Na+/H+ exchange in human normal and cystic fibrosis airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997;273 doi: 10.1152/ajplung.1997.273.1.L148. [DOI] [PubMed] [Google Scholar]
- 169.Acevedo M., Steele L. Na(+)−H+ exchanger in isolated epithelial tracheal cells from sheep. Involvement in tracheal proton secretion. Exp. Physiol. 1993;78:383–394. doi: 10.1113/expphysiol.1993.sp003692. [DOI] [PubMed] [Google Scholar]
- 170.Willumsen N.J., Boucher R.C. Intracellular pH and its relationship to regulation of ion transport in normal and cystic fibrosis human nasal epithelia. J. Physiol. 1992;455:247–269. doi: 10.1113/jphysiol.1992.sp019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fischer H., Widdicombe J.H., Illek B. Acid secretion and proton conductance in human airway epithelium. Am. J. Physiol. Cell Physiol. 2002;282:736–743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- 172.Pereira S.V.N., Ribeiro J.D., Bertuzzo C.S., Marson F.A.L. Association of clinical severity of cystic fibrosis with variants in the SLC gene family (SLC6A14, SLC26A9, SLC11A1 and SLC9A3) Gene. 2017;629:117–126. doi: 10.1016/j.gene.2017.07.068. [DOI] [PubMed] [Google Scholar]
- 173.Dorfman R., Taylor C., Lin F., Sun L., Sandford A., Paré P., Berthiaume Y., Corey M., Durie P., Zielenski J. Modulatory effect of the SLC9A3 gene on susceptibility to infections and pulmonary function in children with cystic fibrosis. Pediatr. Pulmonol. 2011;46:385–392. doi: 10.1002/ppul.21372. [DOI] [PubMed] [Google Scholar]
- 174.Corvol H., Blackman S.M., Boëlle P.Y., Gallins P.J., Pace R.G., Stonebraker J.R., Accurso F.J., Clement A., Collaco J.M., Dang H., et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li W., Soave D., Miller M.R., Keenan K., Lin F., Gong J., Chiang T., Stephenson A.L., Durie P., Rommens J., et al. Unraveling the complex genetic model for cystic fibrosis: Pleiotropic effects of modifier genes on early cystic fibrosis-related morbidities. Hum. Genet. 2014;133:151–161. doi: 10.1007/s00439-013-1363-7. [DOI] [PubMed] [Google Scholar]
- 176.Cougnon M., Bouyer P., Planelles G., Jaisser F. Does the colonic H,K-ATPase also act as an Na,K-ATPase? Proc. Natl. Acad. Sci. 1998;95:6516–6520. doi: 10.1073/pnas.95.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Burnay M., Crambert G., Kharoubi-Hess S., Geering K., Horisberger J.D. Bufo marinus bladder H-K-ATPase carries out electroneutral ion transport. Am. J. Physiol. Ren. Physiol. 2001;281:869–874. doi: 10.1152/ajprenal.2001.281.5.F869. [DOI] [PubMed] [Google Scholar]
- 178.Grishin A.V., Caplan M.J. ATP1AL1, a member of the non-gastric H,K-ATPase family, functions as a sodium pump. J. Biol. Chem. 1998;273:27772–27778. doi: 10.1074/jbc.273.43.27772. [DOI] [PubMed] [Google Scholar]
- 179.Gorrieri G., Scudieri P., Caci E., Schiavon M., Tomati V., Sirci F., Napolitano F., Carrella D., Gianotti A., Musante I., et al. Goblet Cell Hyperplasia Requires High Bicarbonate Transport to Support Mucin Release. Sci. Rep. 2016;6:1–15. doi: 10.1038/srep36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Laroche-Joubert N., Marsy S., Luriau S., Imbert-Teboul M., Doucet A. Mechanism of activation of ERK and H-K-ATPase by isoproterenol in rat cortical collecting duct. Am. J. Physiol. Ren. Physiol. 2003;284:948–954. doi: 10.1152/ajprenal.00394.2002. [DOI] [PubMed] [Google Scholar]
- 181.Cherny V.V., Markin V.S., Decoursey T.E. The Voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schwarzer C., Machen T.E., Illek B., Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J. Biol. Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 183.Cho D.Y., Hajighasemi M., Hwang P.H., Illek B., Fischer H. Proton secretion in freshly excised sinonasal mucosa from asthma and sinusitis patients. Am. J. Rhinol. Allergy. 2009;23:1–11. doi: 10.2500/ajra.2009.23.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Iovannisci D., Illek B., Fischer H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J. Gen. Physiol. 2010;136:35–46. doi: 10.1085/jgp.200910379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ribeiro C.M.P., Paradiso A.M., Livraghi A., Boucher R.C. The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia. J. Gen. Physiol. 2003;122:377–387. doi: 10.1085/jgp.200308893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fischer H. Function of proton channels in lung epithelia. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012;1:247–258. doi: 10.1002/wmts.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Davis M.D., Abdul Rahman R., Khalid F., Cotton C., Smith L., Boyne K., Marozkina N., Ramsey I.S., Chmiel J., Gaston B.M. Airway Epithelial Voltage-Gated Proton Channel Expression and pH in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020;201:A7452. doi: 10.1164/ajrccm-conference.2020.201.1_meetingabstracts.a7452. [DOI] [Google Scholar]
- 188.Futai M., Nakanishi-Matsui M., Okamoto H., Sekiya M., Nakamoto R.K. Rotational catalysis in proton pumping ATPases: From E. coli F-ATPase to mammalian V-ATPase. Biochim. Biophys. Acta Bioenerg. 2012;1817:1711–1721. doi: 10.1016/j.bbabio.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 189.Li X., Thornell I., Villacreses Rada R., Brommel C., Lu L., Mather S., Ehler A., Karp P., Welsh M., Zabner J. V-Type ATPase Mediates Airway Surface Liquid Acidification in Cultured Pig Small Airway Epithelial Cells. Am. J. Respir. Crit. Care Med. 2019;199:A2573. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Poulsen J.H., Machen T.E. HCO3-dependent pH(i) regulation in tracheal epithelial cells. Pflugers Arch. Eur. J. Physiol. 1996;432:546–554. doi: 10.1007/s004240050168. [DOI] [PubMed] [Google Scholar]
- 191.Kong F., Young L., Chen Y., Ran H., Meyers M., Joseph P., Cho Y.H., Hassett D.J., Lau G.W. Pseudomonas aeruginosa pyocyanin inactivates lung epithelial vacuolar ATPase-dependent cystic fibrosis transmembrane conductance regulator expression and localization. Cell. Microbiol. 2006;8:1121–1133. doi: 10.1111/j.1462-5822.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 192.Allen A., Flemström G. Gastroduodenal mucus bicarbonate barrier: Protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 2005;288:C1–C19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 193.Holma B., Hegg P.O. pH- and protein-dependent buffer capacity and viscosity of respiratory mucus. Their interrelationships and influence of health. Sci. Total Environ. 1989;84:71–82. doi: 10.1016/0048-9697(89)90371-9. [DOI] [PubMed] [Google Scholar]
- 194.Holma B. Influence of buffer capacity and pH-dependent rheological properties of respiratory mucus on health effects due to acidic pollution. Sci. Total Environ. 1985;41:101–123. doi: 10.1016/0048-9697(85)90181-0. [DOI] [PubMed] [Google Scholar]
- 195.Ballard S.T., Trout L., Bebök Z., Sorscher E.J., Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999;277:3–8. doi: 10.1152/ajplung.1999.277.4.L694. [DOI] [PubMed] [Google Scholar]
- 196.Cho H.J., Joo N.S., Wine J.J. Mucus secretion from individual submucosal glands of the ferret trachea. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:124–136. doi: 10.1152/ajplung.00049.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Joo N.S., Cho H.J., Khansaheb M., Wine J.J. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J. Clin. Investig. 2010;120:3161–3166. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Engelhardt J.F., Yankaskas J.R., Ernst S.A., Yang Y., Marino C.R., Boucher R.C., Cohn J.A., Wilson J.M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat. Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 199.Ratzke C., Gore J. Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 2018;16:e2004248. doi: 10.1371/journal.pbio.2004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Quinn R.A., Comstock W., Zhang T., Morton J.T., Da Silva R., Tran A., Aksenov A., Nothias L.F., Wangpraseurt D., Melnik A.V., et al. Niche partitioning of a pathogenic microbiome driven by chemical gradients. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aau1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Smith J.J., Welsh M.J. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J. Clin. Investig. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu X., Li T., Tuo B. Physiological and pathophysiological relevance of the anion transporter Slc26a9 in multiple organs. Front. Physiol. 2018;9:1–7. doi: 10.3389/fphys.2018.01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Strug L.J., Gonska T., He G., Keenan K., Ip W., Böelle P.Y., Lin F., Panjwani N., Gong J., Li W., et al. Cystic fibrosis gene modifier SLC26A9 modulates airway response to CFTR-directed therapeutics. Hum. Mol. Genet. 2016;25:4590–4600. doi: 10.1093/hmg/ddw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kurtz I., Petrasek D., Tatishchev S. Molecular Mechanisms of Electrogenic Sodium Bicarbonate Cotransport: Structural and Equilibrium Thermodynamic Considerations. J. Membr. Biol. 2004;197:77–90. doi: 10.1007/s00232-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 205.Morla L., Doucet A., Lamouroux C., Crambert G., Edwards A. The renal cortical collecting duct: A secreting epithelium? J. Physiol. 2016;594:5991–6008. doi: 10.1113/JP272877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheval L., Bakouh N., Walter C., Tembely D., Morla L., Escher G., Vogt B., Crambert G., Planelles G., Doucet A. ANP-stimulated Na+ secretion in the collecting duct prevents Na+ retention in the renal adaptation to acid load. Am. J. Physiol. Ren. Physiol. 2019;317:F435–F443. doi: 10.1152/ajprenal.00059.2019. [DOI] [PubMed] [Google Scholar]
- 207.Crambert G. H-K-ATpase type 2: Relevance for renal physiology and beyond. Am. J. Physiol. Ren. Physiol. 2014;306 doi: 10.1152/ajprenal.00605.2013. [DOI] [PubMed] [Google Scholar]
- 208.Moss F.J., Boron W.F. Carbonic anhydrases enhance activity of endogenous Na-H exchangers and not the electrogenic Na/HCO3 cotransporter NBCe1-A, expressed in Xenopus oocytes. J. Physiol. 2020 doi: 10.1113/JP280143. [DOI] [PMC free article] [PubMed] [Google Scholar]