Abstract
Rationale & Objective
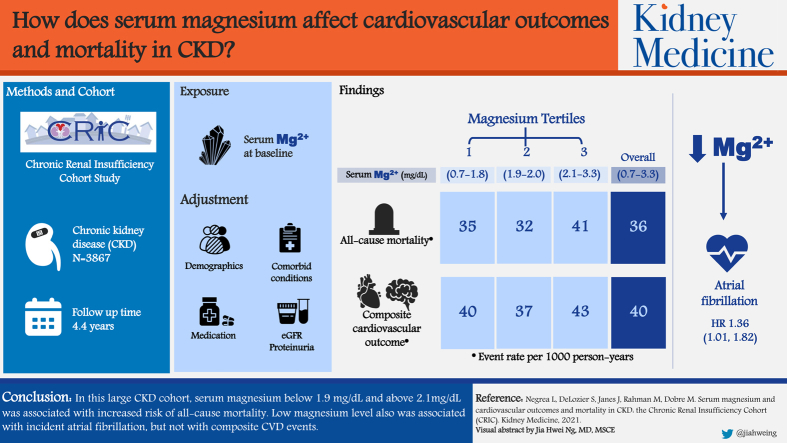
Low serum magnesium level has been shown to be associated with increased mortality, but its role as a predictor of cardiovascular disease is unclear. This study evaluates the association between serum magnesium level and cardiovascular events and all-cause mortality in a large cohort of individuals with chronic kidney disease (CKD).
Study Design
Prospective cohort study.
Setting & Participants
3,867 participants with CKD, enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study.
Exposures
Serum magnesium measured at study baseline.
Outcomes
Composite cardiovascular events (myocardial infarction, cerebrovascular accident, heart failure, and peripheral arterial disease) and all-cause mortality.
Analytical Approach
Cox proportional hazards models adjusted for demographic, clinical, and laboratory characteristics.
Results
During the 14.6 (4.4) years (standard deviation) of follow-up, 1,384 participants died (36/1,000 person-years), and 1,227 (40/1,000 person-years) had a composite cardiovascular event. There was a nonlinear association between serum magnesium level and all-cause mortality. Low and high magnesium levels were associated with greater rates of all-cause mortality after adjusting for demographics, comorbid conditions, medications including diuretics, estimated glomerular filtration rate, and proteinuria (P < 0.001). No significant associations were observed between serum magnesium levels and the composite cardiovascular events. Low serum magnesium level was associated with incident atrial fibrillation (HR, 1.36; 95% CI, 1.01-1.82; P = 0.04).
Limitations
Single measurement of serum magnesium.
Conclusions
In this large CKD cohort, serum magnesium level < 1.9 mg/dL and >2.1 mg/dL was associated with increased risk for all-cause mortality. Low magnesium level was associated with incident atrial fibrillation but not with composite cardiovascular disease events. Further studies are needed to determine the optimal range of serum magnesium in CKD to prevent adverse clinical outcomes.
Index Words: Serum magnesium, all-cause mortality, cardiovascular outcomes, CK
Graphical abstract
Plain-Language Summary.
Chronic kidney disease (CKD)-specific risk factors predispose to hypomagnesemia, including long-term use of certain medications, diabetes, proteinuria, hyperaldosteronism, volume expansion, and metabolic acidosis. Low serum magnesium level is associated with increased mortality but its role as a predictor of cardiovascular disease is unclear. This study evaluates the association between serum magnesium level and cardiovascular disease and all-cause mortality in a large CKD cohort. We found that serum magnesium level < 1.9 and >2.1 mg/dL was associated with increased all-cause mortality. No significant associations were observed between serum magnesium level and composite cardiovascular disease. Low serum magnesium level associated with incident atrial fibrillation. Interventional trials are needed to establish the optimal range of serum magnesium levels to prevent adverse clinical outcomes in CKD.
Editorial, p. 162
Magnesium, the second most common intracellular ion in the human body, is involved in more than 300 enzymatic reactions and is essential for life. Total body magnesium is approximately 24 g and is distributed mainly in bone and muscle. Serum magnesium represents only 0.3% of total body magnesium, and in healthy individuals, the serum concentration range is about 1.8 to 2.8 mg/dL (with 1 mmol/L = 2 mEq/L = 2.4 mg/dL).1 Despite some limitations, serum magnesium is used as the standard for evaluating magnesium homeostasis.2
In chronic kidney disease (CKD) stages 1-3, serum magnesium level is maintained within normal limits by the compensatory increase of the renal fractional excretion of magnesium.3 Hypermagnesemia becomes apparent at an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 and particularly at eGFRs < 15 mL/min/1.73 m2. However, certain CKD-specific risk factors predispose to hypomagnesemia, including long-term use of certain medications (diuretics, proton pump inhibitors, calcineurin inhibitors, etc), the presence of diabetes, proteinuria, hyperaldosteronism, volume expansion, and metabolic acidosis.3, 4, 5, 6 Hypomagnesemia may portend poor outcomes.7, 8, 9, 10
Observational studies and small intervention trials show that magnesium may improve vascular tone and endothelial function, reduce platelet aggregation, increase high-density lipoprotein cholesterol level, improve glucose homeostasis, and favorably affect metabolic syndrome parameters.5,11,12 A recent open-label randomized controlled trial in patients with stage 3-4 CKD and risk factors for coronary artery calcification showed a significantly smaller change in the median coronary artery calcification score for individuals taking magnesium oxide versus controls (11.3% vs 39.5%) at 2 years.13 In dialysis patients, magnesium supplementation has been reported to reduce carotid intima media thickness.14 A meta-analysis of serum magnesium, including 20 studies and 200,934 participants with CKD and end-stage kidney disease, showed that compared with individuals with normal or high magnesium levels, those with low levels had increased risk for all-cause mortality (hazard ratio [HR], 1.32; 95% CI, 1.19-1.47; P < 0.001).15
By and large, available studies associate low serum magnesium levels with various poor clinical outcomes in CKD, but the exact mechanistic link is unknown. It is suggested that the beneficial effects of magnesium are mediated by the inhibition of inflammation, aortic osteogenic signaling, and extracellular matrix remodeling pathways, thereby leading to reduced vascular calcification in CKD.16,17
The high cardiovascular morbidity and mortality in CKD remains far from explained by current traditional risk factors, and the discovery of novel nontraditional risk factors may contribute to improved clinical outcomes. Because hypomagnesemia in CKD may be more frequent than previously estimated, better understanding of the association between low serum magnesium levels and adverse cardiovascular outcomes in well-characterized CKD populations warrants further investigations.
In this study, we sought to determine the association between serum magnesium level and clinical outcomes in the Chronic Renal Insufficiency Cohort (CRIC) Study. We hypothesize that low serum magnesium level is associated with an increased risk for all-cause mortality and cardiovascular events.
Methods
Study Design
The CRIC Study is a prospective multicenter observational cohort study of participants with CKD. The study design and participants’ baseline characteristics have been previously described.18,19 Between 2003 and 2015, a total of 5,499 participants aged 21 to 74 years with eGFRs of 20 to 70 mL/min/1.73 m2 were enrolled in the study. Exclusion criteria included a diagnosis of polycystic kidney disease, active immunosuppression for glomerulonephritis, cirrhosis, class III/IV heart failure, HIV infection, cancer, and pregnancy. The study protocol was approved by the institutional review board of each participating site, and written informed consent was obtained from all participants.
Data Collection
Primary Exposure
Magnesium was measured at study baseline using colorimetric reflectance spectrophotometry; the normal range for age older than 18 years defined as 1.7 to 2.8 mg/dL20 at the University of Pennsylvania Core Laboratory. The current analysis examined serum magnesium level as a nonlinear function and categorically by the following tertiles: low (0.70-1.89 mg/dL), mid (1.90-2.09 mg/dL; reference group), and high (2.10-3.30 mg/dL) tertile group.
Outcomes
Incident cardiovascular events (myocardial infarction [MI], cerebrovascular accident, heart failure, and peripheral arterial disease) were ascertained during the course of the study by asking participants about hospitalizations during the clinic or telephone visit. Hospital records were then obtained and were adjudicated using predefined event-specific guidelines by 2 clinicians. Heart failure events were determined on the basis of clinical symptoms, radiographic evidence of pulmonary edema, physical examination of the heart and lungs, central venous hemodynamic monitoring data, and echocardiographic imaging in hospitalized patients using criteria consistent with previous studies.21 Diagnosis of probable or definite MI was made on the basis of symptoms consistent with acute ischemia, cardiac biomarker levels, and electrocardiograms as recommended by a consensus statement on the universal definition of MI.22 Two neurologists reviewed all hospitalizations and emergency department visits suggestive of stroke and adjudicated the stroke outcome based on predefined criteria.23 Incident atrial fibrillation (AF) was determined by identification of hospitalizations or emergency department visits involving AF during study follow-up. At least 2 study physicians adjudicated all possible AF events by manual review of relevant medical records and electrocardiograms, if available, using standardized criteria. All discordances were discussed by the 2 reviewers and resolved.
Mortality was ascertained through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Mortality Master File. Participants’ follow-up was censored as of end of year 2018 when the data set was locked for analysis, loss to follow-up, when they achieved the event of interest, or death, whichever occurred first.
Two primary outcomes were analyzed: (1) all-cause mortality and (2) cardiovascular events (a composite of MI, cerebrovascular accident, heart failure, and peripheral arterial disease). Secondary outcomes included incident MI, incident congestive heart failure, and incident AF.
Covariates
At each study visit, demographic and physical measures, medical history, medication use, and serum and urine for laboratory assessments were collected. Participants were followed up annually with in-person clinic visits and also contacted by telephone calls approximately 6 months apart.
All antihypertensive medications were categorized into drug classes, and the total number of antihypertensive drug classes was calculated. Serum creatinine was measured at the CRIC Central Laboratory using an enzymatic method (Ortho Clinical Diagnostics) through October 2008 and by the Jaffé method (Beckman Coulter) thereafter and standardized to isotope-dilution mass spectrometry–traceable values.24,25 Twenty-four–hour urinary total protein excretion was measured using a turbidometric method (Roche Diagnostics) through October 2008 and spectrophotometric quantitation (Beckman Coulter) thereafter. Glucose was detected using a coupled enzymatic method (Ortho Clinical Diagnostics) through October 2008 and by an oxygen-depletion method (Beckman Coulter) thereafter. Diabetes was determined as at least 1 of the following: self-reported insulin or oral hypoglycemic medication, fasting blood glucose level ≥ 126 mg/dL or nonfasting level ≥ 200 mg/dL, glycated hemoglobin level ≥ 6.5%, or use of insulin or other antidiabetic medication.26
GFR was estimated using the CRIC Study equation.27 Self-reported history of any cardiovascular disease at baseline included previous MI, coronary revascularization, heart failure, stroke, or peripheral arterial disease.
Statistical Analysis
Standard descriptive statistics were used to characterize the study cohort at CRIC Study baseline, stratified by serum magnesium tertiles. Continuous variables were expressed as mean with standard deviation or median with interquartile range (IQR) and compared using t tests or Wilcoxon rank sum tests as appropriate. Categorical variables were expressed as frequency or proportion. Differences in covariates between magnesium level tertiles were examined using χ2 tests or 1-way analyses of variance with Bonferroni corrections.
Kaplan-Meier curves were used to depict the incidence of clinical outcomes by serum magnesium strata. Cox proportional hazards regression models were used to analyze time to composite cardiovascular events, death, MI, and congestive heart failure for both the first and third magnesium tertiles using tertile 2 as the reference group. We estimated the effect of serum magnesium as a categorical variable, by tertiles, and as a continuous predictor to allow for nonlinear effects using restricted cubic spline models. Covariates used for adjustment in the models included age, sex, race, clinical center, diabetes status, eGFR, 24-hour urinary protein excretion, and baseline cardiovascular, systolic blood pressure, and antihypertensive medications (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, β-blocker, and diuretic use). Participants with cardiovascular disease at study baseline were excluded from the survival analyses.
Sensitivity analyses were modeled to assess whether the association between serum magnesium levels and clinical outcomes of interest is modified by age (<70 and >70 years), sex, diuretic use, presence of diabetes, eGFR (<45 and >45 mL/min/1.73 m2), and fibroblast growth factor 23 (FGF-23) level (below or above median), using an interaction between serum magnesium level and each potential modifier. All models were tested for linearity of continuous variables of interest. Given the small number of missing covariate data and the large data set, imputation was not performed; participants with missing data were excluded from the multivariable analyses. Model assumptions were tested using residual values versus fitted plots, normal Q-Q plots of standardized residuals, plots of standardized residuals versus leverage, scale-location plots, and Cook’s distance. Age was not normally distributed and was log transformed. For all analyses, 2-sided P = 0.05 was considered statistically significant.
All analyses were performed using IBM Corp, released 2017, IBM SPSS Statistics for Windows, version 25.0, and the R Statistical Computing Environment (R Foundation for Statistical Computing), version 3.6.1.
Results
A total of 3,867 participants with available serum magnesium values at study baseline were included in these analyses. Mean age was 58±11 years, 45% were women, 42% were non-Hispanic White, and 48% had diabetes at study baseline. Average eGFR was 44.9±16.7 mL/min/1.73 m2, and average magnesium level was 1.96±0.28 mg/dL. Magnesium distribution is depicted in Figure S1. Compared with the mid and high magnesium tertiles, patients in the low tertile were more likely to be younger, be female, and have diabetes at study baseline and higher body mass index. Baseline characteristics of study participants are depicted in Table 1. CRIC participants with missing magnesium levels tended to be older and have more prevalent diabetes, but otherwise were similar to the study cohort (Table S1).
Table 1.
Baseline Characteristics of Chronic Renal Insufficiency Cohort Participants by Serum Magnesium Tertiles
| Characteristic | Magnesium Tertile |
||||
|---|---|---|---|---|---|
| All Participants (N = 3,867) | 1 (n = 1,289) | 2 (n = 1,289) | 3 (n = 1,289) | P | |
| Serum magnesium, mg/dL | 0.70-3.30 | 0.70-1.89 | 1.90-2.09 | 2.10-3.30 | <0.001 |
| Demographic data | |||||
| Age, y | 58 (11) | 56 (11) | 57 (11) | 59 (10) | <0.001 |
| Women | 1,738 (45%) | 650 (50%) | 536 (42%) | 552 (43%) | <0.001 |
| Race, n (%) 0.18 | |||||
| Non-Hispanic White | 1,610 (42%) | 500 (39%) | 548 (43%) | 562 (44%) | |
| Non-Hispanic Black | 1,615 (42%) | 578 (45%) | 518 (40%) | 519 (40%) | |
| Hispanic | 491 (13%) | 161 (12%) | 170 (13%) | 160 (12%) | |
| Other | 151 (4%) | 50 (4%) | 53 (4%) | 48 (4%) | |
| Hypertension | 3,325 (86%) | 1,117 (87%) | 1,099 (85%) | 1,109 (86%) | 0.59 |
| Diabetes | 1,875 (48%) | 687 (53%) | 584 (45%) | 604 (47%) | <0.001 |
| Any cardiovascular disease | 1,289 (33%) | 389 (30%) | 409 (32%) | 491 (38%) | <0.001 |
| Current smoking | 499 (13%) | 151 (12%) | 190 (15%) | 158 (12%) | 0.05 |
| Body mass index, kg/m2 | 32.1 (7.8) | 32.9 (8.2) | 31.8 (7.6) | 31.6 (7.6) | <0.001 |
| Systolic blood pressure, mm Hg | 128 (22) | 129 (22) | 129 (22) | 127 (22) | 0.07 |
| Diastolic blood pressure, mm Hg | 72 (13) | 73 (13) | 72 (13) | 70 (12) | <0.001 |
| LDL cholesterol, mg/dL | 102.7 (35.5) | 101.9 (35.4) | 104.0 (34.5) | 102.2 (35.5) | 0.25 |
| HDL cholesterol, mg/dL | 47.5 (15.5) | 47.2 (14.8) | 47.1 (15.8) | 48.3 (15.9) | 0.09 |
| Medications | |||||
| Aspirin | 1,645 (43%) | 509 (40%) | 523 (41%) | 613 (48%) | <0.001 |
| β-Blockers | 1,891 (49%) | 640 (50%) | 585 (46%) | 666 (52%) | 0.005 |
| Statins | 2,116 (55%) | 674 (53%) | 711 (56%) | 731 (57%) | 0.08 |
| ACE inhibitor/ARB | 1,884 (49%) | 668 (52%) | 631 (49%) | 585 (46%) | 0.004 |
| Proton pump inhibitors | 879 (23%) | 294 (23%) | 299 (23%) | 286 (22%) | 0.84 |
| Any diuretic | 2,282 (59%) | 745 (58%) | 709 (55%) | 828 (65%) | <0.001 |
| Laboratory data: | |||||
| eGFR, mL/min/1.73 m2 | 44.9 (16.7) | 46.8 (16.3) | 46.7 (16.9) | 41.1 (16.3) | <0.001 |
| Creatinine, mg/dL | 1.8 (0.6) | 1.7 (0.6) | 1.8 (0.6) | 2.0 (0.6) | <0.001 |
| 24-h urinary protein, g/24 h | 0.2 [0.1-0.9] | 0.2 [0.1-1.1] | 0.2 [0.1-0.9] | 0.2 [0.1-0.8] | 0.01 |
| Calcium, mg/dL | 9.2 (0.5) | 9.2 (0.6) | 9.2 (0.5) | 9.2 (0.5) | 0.68 |
| Phosphorus, mg/dL | 3.73 (0.7) | 3.7 (0.7) | 3.7 (0.6) | 3.8 (0.7) | <0.001 |
| Bicarbonate, mg/dL | 24.4 (3.2) | 24.2 (3.3) | 24.5 (3.2) | 24.6 (3.2) | 0.02 |
| Albumin, g/dL | 3.9 (0.5) | 3.9 (0.5) | 4.0 (0.5) | 4.0 (0.5) | <0.001 |
| Hemoglobin, g/dL | 12.6 (1.8) | 12.5 (1.7) | 12.7 (1.8) | 12.6 (1.8) | 0.002 |
| Fibroblast growth factor 23, RU/mL | 145 [96-238] | 149 [99-247] | 135 [91-214] | 156 [98-253] | 0.01 |
| High-sensitivity C-reactive protein, mg/L | 5.6 (9.8) | 5.6 (10.4) | 5.4 (8.2) | 5.8 (10.8) | 0.62 |
| Aldosterone, pmol/L | 102 [72-153] | 95 [66-95] | 100 [71-147] | 112 [79-167] | 0.008 |
| Dietary protein, g/kg/d | 0.8 (0.4) | 0.8 (0.4) | 0.8 (0.4) | 0.8 (0.4) | 0.06 |
Note: Values expressed as mean (standard deviation), number (percent), or median (interquartile range; 25th-75th percentiles). Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; calcium in mg/dL to mmol/L, ×0.2495; phosphorus in mg/dL to mmol/L, ×0.3229.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Serum Magnesium Level and All-Cause Mortality
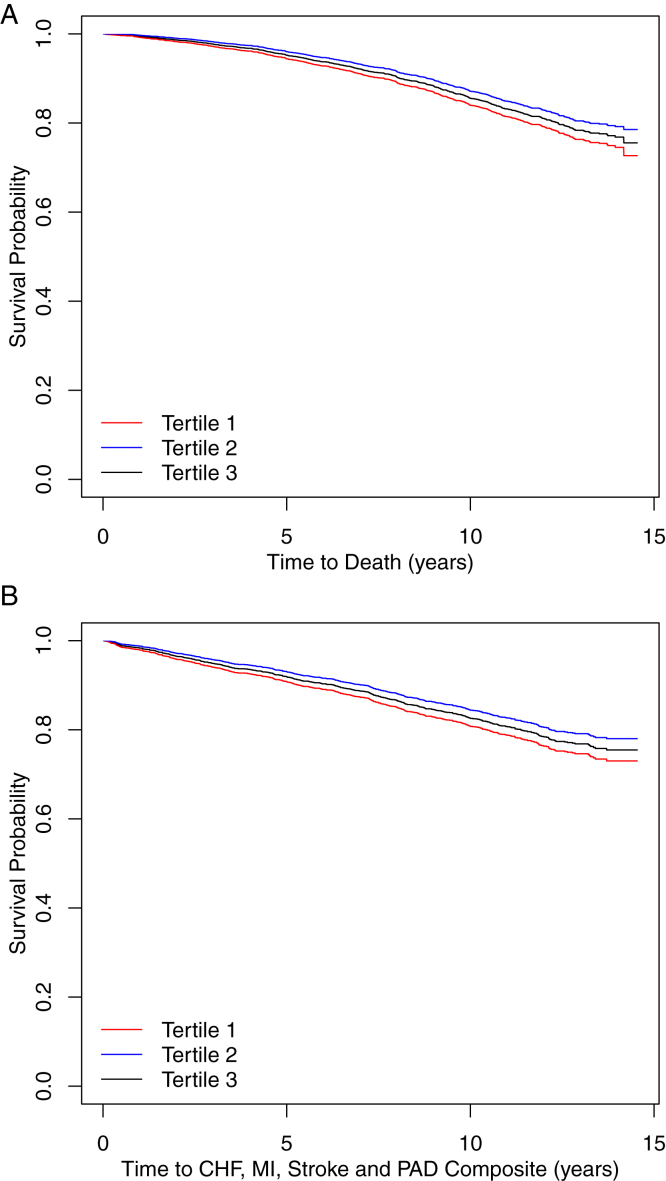
Participants in the low and high magnesium tertiles had 2.9% and 9.6% higher crude mortality rates, respectively, compared with participants in the mid tertile (Table 2). Adjusted survival curves showed an increased risk for all-cause mortality for both the low and high tertiles compared with the mid tertile, P < 0.001 (Fig 1).
Table 2.
Cardiovascular Events and Mortality Rates by Baseline Serum Magnesium Level in the Chronic Renal Insufficiency Cohort Study
| Events | Overall | Magnesium Tertilea | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| <1.90 | 1.90-2.09 | ≥2.10 | ||
| All-cause mortality | ||||
| No. of events/N | 1,384/3,867 | 446/1,289 | 419/1,289 | 519/1,289 |
| Event rate/1,000 person-y | 35.9 | 34.7 | 31.8 | 41.3 |
| Composite cardiovascular eventsb | ||||
| No. of events/N | 1,227/3,864 | 411/1,288 | 388/1,287 | 428/1,289 |
| Event rate/1,000 person-y | 40.0 | 40.1 | 37.2 | 42.7 |
| Myocardial infarction | ||||
| No. of events/N | 379/3,864 | 129/1,288 | 122/1,287 | 128/1,289 |
| Event rate/1,000 person-y | 11.0 | 11.2 | 10.4 | 11.3 |
| Incident congestive heart failure | ||||
| No. of events/N | 650/3866 | 216/1,289 | 187/1,288 | 247/1,289 |
| Event rate/1,000 person-y | 19.4 | 19.3 | 16.3 | 22.7 |
| Atrial fibrillation | ||||
| No. of events/N | 639/3,864 | 202/1,288 | 190/1,288 | 247/1,288 |
| Event rate/1,000 person-y | 18.5 | 17.6 | 16.2 | 21.8 |
The distribution of magnesium by tertiles was tertile 1, 0.70 to 1.89 mg/dL; tertile 2, 1.90 to 2.09 mg/dL (reference group); and tertile 3, 2.10 to 3.30 mg/dL.
Composite cardiovascular events include congestive heart failure, myocardial infarction, stroke, and peripheral arterial disease.
Figure 1.
Adjusted Kaplan-Meier survival curve for (A) all-cause mortality and (B) composite cardiovascular events (congestive heart failure [CHF], myocardial infarction [MI], stroke, and peripheral arterial disease [PAD]) by magnesium tertiles. P < 0.001 for both survival curves. Models adjusted for age, sex, race, clinical center, diabetes status, estimated glomerular filtration rate, 24-hour urinary protein excretion, and baseline cardiovascular, systolic blood pressure, and antihypertensive medications.
Participants in the low magnesium tertile had 21% higher risk for death compared with those in the mid tertile (HR, 1.21 [95% CI, 0.99-1.48]; P = 0.06; Table 3).
Table 3.
Multivariable-Adjusted HRs for All-Cause Mortality and Cardiovascular Events by Baseline Serum Magnesium Levels in the Chronic Renal Insufficiency Cohort Study
| Unadjusted Model | Adjusted Modela | |
|---|---|---|
| Hazard Ratio (95% CI), P | Hazard Ratio (95% CI), P | |
| All-cause mortality (reference: tertile 2; magnesium 1.9-2.1) | ||
| Tertile 1b | 1.18 (0.97-1.42), 0.09 | 1.21 (0.99-1.48), 0.06 |
| Tertile 3 | 1.33 (1.10-1.60), 0.003c | 1.17 (0.96-1.42), 0.12 |
| Composite cardiovascular eventsd (reference: tertile 2; magnesium 1.9-2.1) | ||
| Tertile 1 | 1.16 (0.95-1.42), 0.14 | 1.11 (0.90-1.38), 0.32 |
| Tertile 3 | 1.18 (0.97-1.45), 0.11 | 1.07 (0.86-1.33), 0.55 |
| Incident myocardial infarction (reference: tertile 2; magnesium 1.9-2.1) | ||
| Tertile 1 | 1.25 (0.89-1.75), 0.19 | 1.17 (0.82-1.66), 0.39 |
| Tertile 3 | 0.94 (0.85-1.36), 0 .75 | 0.92 (0.63-1.36), 0.69 |
| Incident congestive heart failure (reference: tertile 2; magnesium 1.9-2.1) | ||
| Tertile 1 | 1.28 (0.96-1.72), 0.09 | 1.15 (0.84-1.57), 0.40 |
| Tertile 3 | 1.43 (1.06-1.91), 0.02c | 1.20 (0.88-1.64), 0.26 |
| Incident atrial fibrillation (reference: tertile 2; magnesium 1.9-2.1) | ||
| Tertile 1 | 1.36 (1.03-1.80), 0.03c | 1.36 (1.01-1.82), 0.04c |
| Tertile 3 | 1.47 (1.11-1.95), 0.007c | 1.27 (0.95-1.70), 0.10 |
Models adjusted for age, sex, race, clinical center, diabetes status, estimated glomerular filtration rate, 24-hour urinary protein excretion, and baseline cardiovascular, systolic blood pressure, and antihypertensive medications.
The distribution of magnesium by tertiles was tertile 1, 0.70 to 1.89 mg/dL; tertile 2, 1.90 to 2.09 mg/dL (reference group); and tertile 3, 2.10 to 3.30 mg/dL.
Statistically significant.
Composite cardiovascular events include incident congestive heart failure, myocardial infarction, stroke, and peripheral arterial disease.
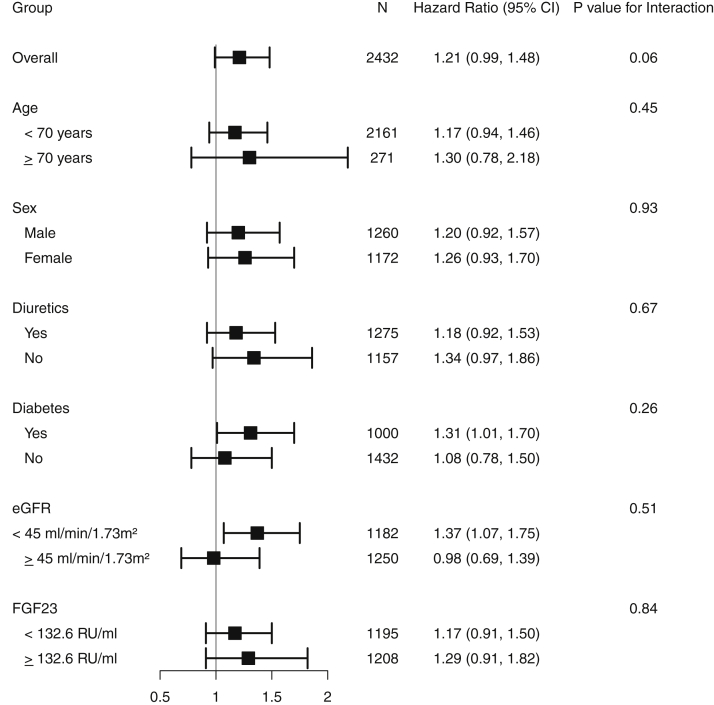
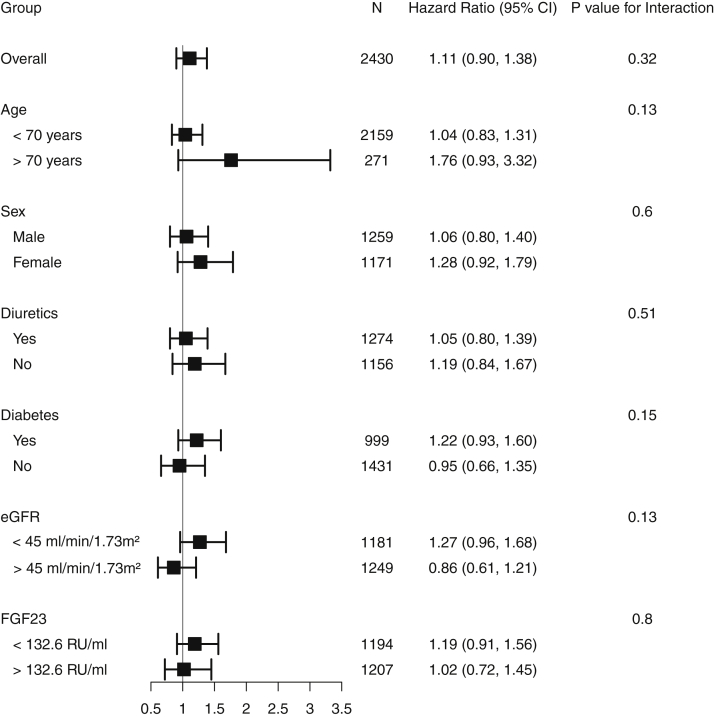
Results were consistent in subgroups by sex (HR, 1.06 [95% CI, 0.80-1.40] and HR, 1.28 [95% CI, 0.92-1.79], for men and women, respectively), diabetes (HR, 1.22 [95% CI, 0.93-1.60], and HR, 0.95 [95% CI, 0.66-1.35] for participants with and without diabetes, respectively), any diuretic use (HR, 1.05 [95% CI, 0.80-1.39] and HR, 1.19 [95% CI, 0.84-1.67] for participants taking diuretics and not taking diuretics, respectively), and eGFR (HR, 1.27 [95% CI, 0.96-1.68], and HR, 0.85 [95% CI, 0.61-1.21] for eGFR < 45 or ≥45 mL/min/1.73 m2, respectively; Fig 2).
Figure 2.
Forest plot for risk for all-cause mortality overall and by subgroups for the low magnesium tertile compared with the mid tertile. Abbreviations: eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23.
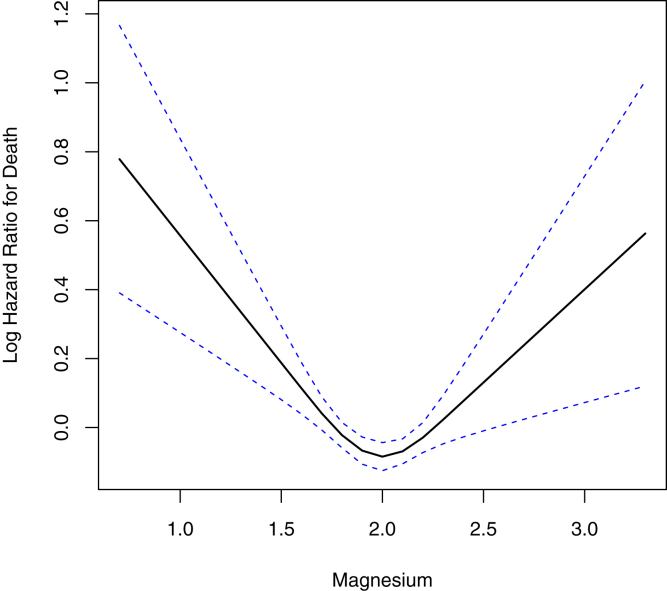
To assess for potential nonlinearity, a multivariable-adjusted restricted cubic spline model was built, showing consistent results. Low magnesium level was associated with greater rates of all-cause mortality, P < 0.001. Likewise, high magnesium level was statistically significant associated with all-cause mortality, P < 0.001 (Fig 3).
Figure 3.
Restricted cubic splines for the hazard of death by serum magnesium levels. Solid lines indicate the log-hazard ratio; dotted lines indicate standard error of the mean. The 2 knots are placed at the 1.9- and 2.1-mg/dL magnesium level (P < 0.001).
Serum Magnesium Level and Composite Cardiovascular Events
Participants in the low and high magnesium tertiles had 2.9% and 5.5% higher crude composite cardiovascular event rates, respectively, compared with those in the mid tertile (Table 2). Adjusted Kaplan-Meier plots showed increased risk for composite cardiovascular events for both the low and high tertiles compared with the mid tertile, P <0.001 (Fig 1).
Serum magnesium levels were not associated with risk for composite cardiovascular events in adjusted Cox regression models (HR, 1.11 [95% CI, 0.90-1.38], P = 0.32 for the low compared with the mid tertile; Table 3). Results were consistent in subgroup analyses defined by age, sex, diabetes, diuretic use, eGFR, and FGF-23 level (Fig 4). This is likely due to a reduction in the predictive power derived from categorization of magnesium level.
Figure 4.
Forest plot for the risk for composite cardiovascular events (congestive heart failure, myocardial infarction, stroke, and peripheral arterial disease) overall and by subgroups for the low magnesium tertile compared with the mid tertile. Abbreviations: eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23.
Serum Magnesium Level and Incident MI
Serum magnesium levels were not associated with risk for incident MI in adjusted Cox regression models (HR, 1.17 [95% CI, 0.82-1.66]; P =0.39 for the low compared with the mid tertile (Table 3).
Serum Magnesium Level and Incident Congestive Heart Failure Events
Participants in the low and high magnesium tertiles had 3.0% and 6.3% higher crude composite congestive heart failure events rates, respectively, compared with those in the mid tertile (Table 2).
Serum magnesium levels were not associated with risk for incident congestive heart failure for the low compared with the mid magnesium tertile (HR, 1.15 [95% CI, 0.84-1.57]; P = 0.40[ Table 3).
Serum Magnesium Level and AF
Participants in the low and high magnesium tertiles had 1.3% and 5.6% higher crude new-onset AF event rates, respectively, compared with those in the mid tertile (Table 2).
Participants in the low magnesium tertile had 36% higher risk for new-onset AF compared with those in the mid tertile; HR, 1.36 (95% CI, 1.01-1.82; P = 0.04; Table 3).
Discussion
In a large cohort of adults with CKD, participants with serum magnesium levels < 1.9 mg/dL and >2.1 mg/dL had increased risk for all-cause mortality when accounting for demographics, comorbid conditions, medications, and kidney function. Low magnesium level was associated with incident AF. Serum magnesium level was not independently associated with composite cardiovascular events. The findings were consistent in subgroups defined by age, sex, diabetes status, diuretic use, eGFR, and FGF-23 level.
The study suggests that low serum magnesium level is a risk factor for all-cause mortality in individuals with CKD. Magnesium balance is dependent on intestinal absorption and kidney excretion, without any identified specific hormonal regulator of its homeostasis. Multiple factors can contribute to hypomagnesemia in CKD, including medication use (diuretics, proton pump inhibitors, or calcineurin inhibitors), diabetes, hyperaldosteronism, volume expansion, and metabolic acidosis. In this large well-characterized CKD cohort, a third of participants had a magnesium levels < 1.9 mg/d, with a mean value of 1.7 mg/dL, suggesting that hypomagnesemia is prevalent in CKD. Experimental studies have demonstrated that low magnesium level can have detrimental health consequences leading to increased mortality in several ways.
Magnesium modulates the activity of both cardiac myocytes and pacemaker cells by regulating a number of potassium and calcium channels (ie, sodium–potassium adenosine triphosphatase [Na/K-ATPase], inwardly rectifying potassium channels [Kir], and L-type calcium channels).28 Magnesium depletion could cause depolarization of the transmembrane potential at rest, as well as prolong the depolarization time during action potential (phase 3), making the heart more vulnerable to arrhythmia and sudden cardiac death. Magnesium influences cardiomyocyte contractility by acting as a natural antagonist to calcium (competes for binding to troponin C and calmodulin) and also limits intracellular calcium overload during myocardial ischemia.29 Further, magnesium can favorably affect cardiac remodeling through its inhibitory effect on production of matrix metalloproteinase-2 in cardiac fibroblasts.30
Because cause-specific mortality was unable to be assessed, the proportion of cardiovascular deaths, and especially arrhythmia-related deaths, is unknown in our study. It is reasonable to speculate that most deaths were related to cardiovascular disease because this is recognized as the leading cause of death in CKD.31
In addition to being proarrhythmogenic, magnesium deficiency promotes a preatherogenic phenotype by increasing oxidative stress and inflammation in cardiac tissue and vascular smooth muscle cells.32,33 The relationship between low magnesium level and oxidative stress is bidirectional, and oxidative stress may also exacerbate magnesium deficiency.34 An inflammatory marker, high-sensitivity C-reactive protein, was simultaneously measured at study baseline and found to be the high in participants with serum magnesium levels in the lowest and highest tertiles, suggesting that abnormal magnesium levels may be associated with a higher degree of inflammation. In the current study, we did not observe a statistically significant association between serum magnesium level and incident MI, potentially explained by residual confounding due to incomplete ascertainment of the severity or duration of exposure to traditional atherosclerotic risk factors.
Compared with participants with serum magnesium levels in the mid tertile, those with serum magnesium levels < 1.9 mg/dL had 15% higher risk for incident heart failure events. Though this association did not reach statistical significance (P =0.05), magnesium may be linked to heart failure in at least 3 ways. First, low magnesium level is inversely associated with vascular calcifications. In vitro, magnesium administration prevents vascular smooth muscle cell calcification through the magnesium transporter transient receptor potential cation channel subfamily M member 7 (TRPM7), it inhibits the Wnt/β-catenin pathway that mediates high phosphorus–induced calcifications,35 and can interfere directly with calcium and phosphorus crystallization into hydroxyapatite.36 Magnesium may prevent vascular calcifications through activation of the calcium-sensing receptor.37 In a rat model of uremic vascular calcification, moderately increased dietary magnesium and also intraperitoneal magnesium reduced vascular calcifications.38
Second, low magnesium level is associated with high blood pressure. Magnesium has a substantial vasodilatory effect by being a natural calcium channel antagonist, inhibiting endothelin 1 expression,and increasing nitric oxide production and prostacyclin 2 levels.39
Third, magnesium also decreases directly aldosterone production,40 decreasing sodium reabsorption and thus limiting volume overload.
Magnesium deficiency can cause increased mortality by altering the mineral metabolism.37 In normal parathyroid glands under conditions of moderate hypocalcemia, magnesium decreases parathyroid hormone levels by activating the calcium-sensing receptor and upregulating the vitamin D receptor and the FGF receptor 1/klotho co-receptor. However, hypomagnesemia impairs parathyroid hormone secretion and lowers 1,25 dihydroxyvitamin D levels. In addition, certain enzymes on the parathyroid hormone–vitamin D axis are also magnesium dependent and have decreased activity in hypomagnesemia.
Serum FGF-23 levels have not been studied extensively in hypomagnesemic states. In a mice model of CKD fed a high-phosphorus diet, a low-magnesium diet was associated with lower FGF -23 levels than the normal-magnesium diet group. In our study, subgroup analyses by FGF-23 categories showed that participants in the lower magnesium tertile had significantly increased risk for mortality compared with participants in the mid tertile, suggesting that low FGF-23 level has an additive negative effect in hypomagnesemic states. A high-magnesium diet can bind dietary phosphorus, resulting in less hyperphosphatemia in CKD. In a large cohort of hemodialysis patients, increasing serum magnesium largely altered the mortality risk associated with hyperphosphatemia.41
Although the definition of low magnesium level varied in the cited studies, magnesium sufficiency appears beneficial in multiple systems. Higher predialysis magnesium levels were associated with a lower incidence rate of arrhythmia in patients with continuous electrocardiogram monitoring.42
In the general population, many, though not all, epidemiologic studies and clinical trials have demonstrated an association between low serum magnesium levels and hypertension, type 2 diabetes, and several cardiovascular events (ischemic heart disease, cardiovascular mortality, and left ventricular hypertrophy).11,43,44 Recently, low serum magnesium level was also associated with incident kidney disease in the Atherosclerosis Risk in Communities (ARIC) Study.45 Although the main analysis in this study was based on a 1-time measurement of serum magnesium, using the 3-year study visit serum magnesium level as the baseline resulted in a similar association between magnesium level and incident CKD.45
A meta-analysis in patients without CKD46 demonstrated an inverse association between serum magnesium concentration and risk for total cardiovascular events (stroke, coronary heart disease, and cardiovascular death). Also, low serum magnesium level has been associated with the development of AF in the ARIC Study47 and in the Framingham Offspring Study.48 In the latter study, baseline serum magnesium level was associated with the development of AF in individuals without heart disease. Moreover, the baseline serum potassium level in that study was not associated with incident AF and secondary analyses adjusting for serum potassium did not change the serum magnesium–AF association. The development of AF years after the baseline data raises the possibility that hypomagnesemia may be a marker of unmeasured risk factors. Importantly, our study extends the association of low magnesium level and new-onset AF to patients with CKD. With AF remaining the most common cause of arrhythmic death in the end-stage kidney disease population, a more consistent examination of serum magnesium levels across the spectrum of CKD and its relation with arrhythmic events is warranted.
This study has a few important strengths: it represents a comprehensive analysis of the association between serum magnesium level and rigorously adjudicated cardiac events and mortality in CKD. The large and racially diverse patient population, long duration of follow-up, comprehensive covariate measurements, and large subgroup sizes to allow robust subgroup analyses are also important strengths.
However, a few limitations need to be considered. First, it is an observational study and therefore a certain degree of residual confounding and selection bias cannot be excluded and causality cannot be inferred. Second, these analyses are based on a single measurement of serum magnesium; notably, after the first year of follow-up, magnesium level was not significantly different with median values of 2.0 (IQR, 1.8-2.1) and 2.1 (IQR, 1.9-2.2) mg/dL at baseline and study year 1, respectively. Whether changes in serum magnesium levels over time add incremental predictive value will need further study.
In this large CKD cohort, serum magnesium level < 1.9 mg/dL and >2.1 mg/dL was associated with increased risk for death from any cause. Low magnesium level was associated with incident AF but not with composite cardiovascular disease events. Further studies are needed to determine the optimal range of serum magnesium levels in CKD to prevent adverse clinical outcomes.
Article Information
Authors’ Full Names and Academic Degrees
Lavinia Negrea, MD, Sarah J. DeLozier, PhD, Jessica L. Janes, MA, Mahboob Rahman, MD, and Mirela Dobre, MD.
Authors’ Contributions
Research idea and study design LN, MD, MR; data acquisition: MR, MD; data analysis/interpretation: LN, SJD, MD, MR; statistical analysis: SJD, JLJ, supervision and mentorship: MD, MR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Dr Rahman is supported by 5U01DK061021. Dr Dobre is supported by R01HL141846.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received May 15, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form October 25, 2020.
Footnotes
Complete author and article information provided before references.
Figure S1: Magnesium distribution at study baseline
Table S1: Selected baseline characteristics of participants with missing magnesium levels
Supplementary Material
Figure S1, Table S1.
References
- 1.Navarro-Gonzalez J.F., Mora-Fernandez C., Garcia-Perez J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial. 2009;22(1):37–44. doi: 10.1111/j.1525-139X.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes . National Academies Press (US); Washington, DC: 1997. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. [PubMed] [Google Scholar]
- 3.Cunningham J., Rodriguez M., Messa P. Magnesium in chronic kidney disease stages 3 and 4 and in dialysis patients. Clin Kidney J. 2012;5(suppl 1):i39–i51. doi: 10.1093/ndtplus/sfr166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander R.T., Hoenderop J.G., Bindels R.J. Molecular determinants of magnesium homeostasis: insights from human disease. J Am Soc Nephrol. 2008;19(8):1451–1458. doi: 10.1681/ASN.2008010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danziger J., William J.H., Scott D.J. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692–699. doi: 10.1038/ki.2012.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oka T., Hamano T., Sakaguchi Y. Proteinuria-associated renal magnesium wasting leads to hypomagnesemia: a common electrolyte abnormality in chronic kidney disease. Nephrol Dial Transplant. 2019;34(7):1154–1162. doi: 10.1093/ndt/gfy119. [DOI] [PubMed] [Google Scholar]
- 7.Van Laecke S., Nagler E.V., Verbeke F., Van Biesen W., Vanholder R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med. 2013;126(9):825–831. doi: 10.1016/j.amjmed.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Silva A.P., Fragoso A., Silva C., Tavares N., Santos N. Magnesium and mortality in patients with diabetes and early chronic kidney disease. J Diabetes Metab. 2014;5 347. [Google Scholar]
- 9.Kanbay M., Yilmaz M.I., Apetrii M. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2012;36(3):228–237. doi: 10.1159/000341868. [DOI] [PubMed] [Google Scholar]
- 10.Azem R., Daou R., Bassil E. Serum magnesium, mortality and disease progression in chronic kidney disease. BMC Nephrol. 2020;21(1):49. doi: 10.1186/s12882-020-1713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shechter M. Magnesium and cardiovascular system. Magnes Res. 2010;23(2):60–72. doi: 10.1684/mrh.2010.0202. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Moran M., Simental-Mendia L.E., Gamboa-Gomez C.I., Guerrero-Romero F. Oral magnesium supplementation and metabolic syndrome: a randomized double-blind placebo-controlled clinical trial. Adv Chronic Kidney Dis. 2018;25(3):261–266. doi: 10.1053/j.ackd.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi Y., Hamano T., Obi Y. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol. 2019;30(6):1073–1085. doi: 10.1681/ASN.2018111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turgut F., Kanbay M., Metin M.R., Uz E., Akcay A., Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40(4):1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 15.Xiong J., He T., Wang M. Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: a systematic review and meta-analysis. J Nephrol. 2019;32(5):791–802. doi: 10.1007/s40620-019-00601-6. [DOI] [PubMed] [Google Scholar]
- 16.Ter Braake A.D., Smit A.E., Bos C. Magnesium prevents vascular calcification in Klotho deficiency. Kidney Int. 2020;97(3):487–501. doi: 10.1016/j.kint.2019.09.034. [DOI] [PubMed] [Google Scholar]
- 17.Kaesler N., Goettsch C., Weis D. Magnesium but not nicotinamide prevents vascular calcification in experimental uraemia. Nephrol Dial Transplant. 2020;35(1):65–73. doi: 10.1093/ndt/gfy410. [DOI] [PubMed] [Google Scholar]
- 18.Feldman H.I., Appel L.J., Chertow G.M. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(7):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 19.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chernecky C.C., Berger B.J. Magnesium - serum. In: Chernecky C.C., Berger B.J., editors. Laboratory Tests and Diagnostic Procedures. 6th ed. Elsevier Saunders; St Louis, MO: 2013. pp. 750–751. [Google Scholar]
- 21.Ho K.K., Anderson K.M., Kannel W.B., Grossman W., Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K., Alpert J.S., White H.D. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond W.D., Folsom A.R., Chambless L.E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 24.Joffe M., Hsu C.Y., Feldman H.I. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010;31(5):426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey A.S., Coresh J., Greene T. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(suppl 1):S43–S48. [PubMed] [Google Scholar]
- 27.Anderson A.H., Yang W., Hsu C.Y. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leenders N.H.J., Vervloet M.G. Magnesium: a magic bullet for cardiovascular disease in chronic kidney disease? Nutrients. 2019;11(2):455. doi: 10.3390/nu11020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangvoraphonkchai K., Davenport A. Magnesium and cardiovascular disease. Adv Chronic Kidney Dis. 2018;25(3):251–260. doi: 10.1053/j.ackd.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Yue H., Uzui H., Lee J.D., Shimizu H., Ueda T. Effects of magnesium on matrix metalloproteinase-2 production in cultured rat cardiac fibroblasts. Basic Res Cardiol. 2004;99(4):257–263. doi: 10.1007/s00395-004-0472-9. [DOI] [PubMed] [Google Scholar]
- 31.Navaneethan S.D., Schold J.D., Arrigain S., Jolly S.E., Nally J.V., Jr. Cause-specific deaths in non-dialysis-dependent CKD. J Am Soc Nephrol. 2015;26(10):2512–2520. doi: 10.1681/ASN.2014101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier J.A. Endothelial cells and magnesium: implications in atherosclerosis. Clin Sci (Lond) 2012;122(9):397–407. doi: 10.1042/CS20110506. [DOI] [PubMed] [Google Scholar]
- 33.Altura B.M., Shah N.C., Shah G.J. Short-term Mg deficiency upregulates protein kinase C isoforms in cardiovascular tissues and cells; relation to NF-kB, cytokines, ceramide salvage sphingolipid pathway and PKC-zeta: hypothesis and review. Int J Clin Exp Med. 2014;7(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 34.Kolisek M., Montezano A.C., Sponder G. PARK7/DJ-1 dysregulation by oxidative stress leads to magnesium deficiency: implications in degenerative and chronic diseases. Clin Sci (Lond) 2015;129(12):1143–1150. doi: 10.1042/CS20150355. [DOI] [PubMed] [Google Scholar]
- 35.Montes de Oca A., Guerrero F., Martinez-Moreno J.M. Magnesium inhibits Wnt/beta-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louvet L., Bazin D., Buchel J., Steppan S., Passlick-Deetjen J., Massy Z.A. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS One. 2015;10(1) doi: 10.1371/journal.pone.0115342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massy Z.A., Drueke T.B. Magnesium and cardiovascular complications of chronic kidney disease. Nat Rev Nephrol. 2015;11(7):432–442. doi: 10.1038/nrneph.2015.74. [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Tocados J.M., Peralta-Ramirez A., Rodriguez-Ortiz M.E. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017;92(5):1084–1099. doi: 10.1016/j.kint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 39.de Baaij J.H., Hoenderop J.G., Bindels R.J. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 40.Jin K., Kim T.H., Kim Y.H., Kim Y.W. Additional antihypertensive effect of magnesium supplementation with an angiotensin II receptor blocker in hypomagnesemic rats. Korean J Intern Med. 2013;28(2):197–205. doi: 10.3904/kjim.2013.28.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi Y., Fujii N., Shoji T. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tumlin J.A., Roy-Chaudhury P., Koplan B.A. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol. 2019;20(1):80. doi: 10.1186/s12882-019-1212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.M de Francisco A.L., Rodriguez M. Magnesium - its role in CKD. Nefrologia. 2013;33(3):389–399. doi: 10.3265/Nefrologia.pre2013.Feb.11840. [DOI] [PubMed] [Google Scholar]
- 44.Song Y., He K., Levitan E.B., Manson J.E., Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23(10):1050–1056. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 45.Tin A., Grams M.E., Maruthur N.M. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015;87(4) doi: 10.1038/ki.2014.331. 820-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu X., Jin F., Hao Y. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0057720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misialek J.R., Lopez F.L., Lutsey P.L. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in African Americans--Atherosclerosis Risk in Communities (ARIC) study. Circ J. 2013;77(2):323–329. doi: 10.1253/circj.cj-12-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan A.M., Lubitz S.A., Sullivan L.M. Low serum magnesium and the development of atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2013;127(1):33–38. doi: 10.1161/CIRCULATIONAHA.111.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Table S1.