Highlights
-
•
Longitudinal study with two waves of imaging data and observational measures of parenting.
-
•
We examined functional connectivity of amygdala and selected large-scale networks.
-
•
We detected developmental effects as a function of parenting.
-
•
Positive parenting was associated with decreased control network connectivity.
-
•
Positive parenting behavior may promote resting state network maturation.
Keywords: Parenting, Brain development, rsfMRI, Amygdala, Resting state networks, Functional connectivity
Abstract
Parenting behavior is associated with internalizing symptoms in children, and cross-sectional research suggests that this association may be mediated by the influence of parenting on the development of frontoamygdala circuitry. However, longitudinal studies are lacking. Moreover, there is a paucity of studies that have investigated parenting and large-scale networks implicated in affective functioning. In this longitudinal study, data from 95 (52 female) children and their mothers were included. Children underwent magnetic resonance imaging that included a 6 min resting state sequence at wave 1 (mean age = 8.4 years) and wave 2 (mean age = 9.9 years). At wave 1, observational measures of positive and negative maternal behavior were collected during mother-child interactions. Region-of-interest analysis of the amygdala, and independent component and dual-regression analyses of the Default Mode Network (DMN), Executive Control Network (ECN) and the Salience Network (SN) were carried out. We identified developmental effects as a function of parenting: positive parenting was associated with decreased coactivation of the superior parietal lobule with the ECN at wave 2 compared to wave 1. Thus our findings provide preliminary longitudinal evidence that positive maternal behavior is associated with maturation of the connectivity between higher-order control networks.
1. Introduction
Childhood experiences have important implications for brain development and mental health outcomes (Teicher et al., 2003), and parenting behaviors are among such influential experiences (Belsky and De Haan, 2011). High levels of negative, and low levels of positive parenting behavior, are associated with depressive and anxiety symptoms in children, pointing to the importance of investigating parental factors as mechanisms in the aetiology of such problems (Yap et al., 2016).
Neurobiological models suggest that this association may be mediated by the influence of parenting on the function of neural circuits underlying emotion regulation, particularly frontoamygdala circuitry (Callaghan and Tottenham, 2015). Neuroimaging studies in youth support this hypothesis by showing that low levels of maternal positive behavior (e.g., warmth), and high levels of maternal negative behavior (e.g., aggression) are associated with increased amygdala reactivity during emotion processing tasks (Butterfield et al., 2020; Pozzi et al., 2020; Romund et al., 2016). Other studies have found that children exposed to negative (i.e., hostile, insensitive) parenting show increased negative amygdala–prefrontal cortex (PFC) functional connectivity compared to unexposed peers during an emotional faces task (Kopala-Sibley et al., 2020) and more positive amygdala-PFC connectivity during rest (Jiang et al., 2020; Thijssen et al., 2017).
Amygdala-PFC connectivity undergoes protracted maturation during the developmental period, and it has been suggested that the transition between childhood and adolescence (around the age of 10) delineates a particular time of change, whereby connectivity undergoes a switch from positive to negative during emotion processing tasks (Gee et al., 2013a) and becomes more positive at rest (Gabard-Durnam et al., 2014). These changes parallel the adoption of more mature strategies of emotion regulation (Gee et al., 2013b). Together, evidence suggests that negative parenting may accelerate the maturation of the frontoamygdala circuit, consistent with the ‘stress acceleration hypothesis’ (Callaghan and Tottenham, 2015). However, inferences so far have been drawn from cross-sectional samples (or longitudinal studies with only one wave of imaging data).
Given the key role of frontoamygdala circuitry in affective functioning, it is not surprising that it has been the focus of most studies to date, with many adopting region of interest (ROI) seed-based approaches. However, a ROI approach is limited to chosen regions. A few recent studies have instead focused on broader networks involved in social and affective processes, such as the default mode network (DMN), which encompasses the precuneus, posterior cingulate, ventromedial PFC and inferior parietal cortices, the executive control network (ECN), comprising medial-frontal areas, and the salience network (SN), comprising insula and cingulate cortex (Smith et al., 2009). Connectivity within networks increases with age (reflecting integration), while connectivity between networks tends to decouple (reflecting segregation) (Sherman et al., 2014). One study found that greater parent–child communication during late childhood predicted greater resting-state functional connectivity within the anterior SN at 25 years of age which, in turn, was associated with lower harmful alcohol use and emotional eating (Holmes et al., 2018). Another study, conducted in the same sample, found that supportive parenting moderated the relationship between socio-economic status (SES) and connectivity in the ECN, whereby youth exposed to both low SES and low, but not high, supportive parenting showed lower connectivity within the ECN (Brody et al., 2019). Others found that positive parenting behavior predicted greater negative connectivity between the ECN and the DMN at 10 years of age (Dégeilh et al., 2018). Together, these results suggest that positive parenting may be associated with more mature (‘adult-like’) (Dégeilh et al., 2018) and adaptive (Brody et al., 2019) connectivity within and between networks. However, the influence of negative parenting behavior on child resting state networks is unclear. Investigating effects of both positive and negative parenting is important because they may not necessarily lie on a continuum (Bhanot et al., 2020). Further, it is unclear how parenting behavior may be related to altered development of resting state network connectivity, including whether high negative and low positive parenting are related to accelerated or delayed development (Callaghan and Tottenham, 2016).
Moreover, previous studies were cross-sectional or included only one time point of imaging. Despite the suggested importance of age-related connectivity changes, there is a lack of longitudinal studies that investigate such changes in children, in addition to a paucity of studies that have investigated the effect of parenting behavior. Therefore, the aims of this study were to 1) investigate changes in connectivity during late childhood (8−10 years), which is an important period for brain maturation (Gee et al., 2013b), 2) examine how maternal positive and negative parenting behavior may influence these changes, and 3) investigate if maternal parenting behavior-related changes in connectivity are associated with internalizing symptoms, to help elucidating whether neurodevelopmental changes are adaptive or maladaptive.
To investigate these aims, we considered two approaches. First, given the importance of the amygdala-PFC circuit, and prior work, we adopted a ROI approach to investigate amygdala connectivity trajectories. Second, we conducted exploratory analyses of resting-state networks, particularly the ECN, DMN and SN. We expected that amygdala connectivity would increase as a sign of maturation from the age of 8–10, and that high negative/low positive parenting behavior would be associated with greater connectivity with age. Regarding resting-state networks, according to previous studies, we expected that within network connectivity would increase with age, while between network connectivity would decrease. While existing ROI based studies of amygdala-PFC circuitry supports the stress acceleration hypothesis (Callaghan and Tottenham, 2016), which posits that exposure to negative caregiving is associated with accelerated brain development, previous RSNs studies (Brody et al., 2019; Dégeilh et al., 2018; Holmes et al., 2018) have found maternal positive behavior to be associated with more mature resting state networks. As such, we considered two hypotheses: high maternal negative/low positive parenting behavior would be associated with 1) increased within and between network connectivity with age, in accordance with the stress acceleration hypothesis; 2) decreased within and between network connectivity with age, in accordance with previous cross-sectional work. Finally, high negative/low positive parenting-related changes in functional connectivity would be positively associated with children’s internalizing symptoms.
2. Materials and methods
2.1. Sample
The current study consisted of a subsample of children and their mothers participating in the Families and Childhood Transitions Study (FACTS), who were recruited from the broader community in Melbourne, Australia, as described elsewhere (Simmons et al., 2017). FACTS consisted of two waves of data collection. During the first wave (N = 163), the child and their mother participated in two Family Interaction Tasks (FIT), described in section 2.2. The child underwent an MRI scan that included a resting state sequence (N = 150). At the 18-months follow up wave, the child underwent a second MRI (N = 127). Measures of internalizing symptoms were collected at both waves. Before the analysis, the imaging data available from both waves underwent quality control (Esteban et al., 2019a). After exclusion of subjects with low quality MRI data (see section 2.4.2.1 for details), the sample consisted of 101 children with imaging data at both waves. One subject had a diagnosis of Attention Deficit Hyperactivity Disorder and was taking psychotropic medication at wave 2, and was therefore excluded from the analysis. Five children were missing parenting data (i.e., the Family Interaction Task (FIT) - see below), leading to a sample of 95 children with complete imaging and parenting data. This sample did not differ from the original sample (N = 163) in socio-economic status, parenting behaviors, maternal depressive symptoms, or child anxiety and depressive symptoms (all p values > 0.05 - see section 2.3 for a description of these variables). None of the children included in the analysis had a psychiatric diagnosis from a health professional, as reported by the parents, at either wave. See Table 1 for demographics and descriptive information of the sample.
Table 1.
Overview of the analyses pipeline.
| Quality control with MRIQC | ||
|---|---|---|
| Preprocessing with fMRIprep | ||
| High-pass filter 0.01 Hz | ||
| 5 mm smoothing | ||
| Data cleaning (ICA FIX) | ||
| Extraction of individual features | ||
| Group analysis | ||
| Seed-based connectivity (amygdala ROI) | Network connectivity (dual regression) | Between network connectivity (FSLnets) |
| Wave 1 - wave 2 | Wave 1 - wave 2 | Wave 1 - wave 2 |
| Amygdala fc maps | RSN fc maps | Z scores partial correlations |
| Wave 2 - wave 1 | Wave 2 - wave 1 | Wave 2 - wave 1 |
| Amygdala fc maps | RSN fc maps | Z scores partial correlations |
Note. fc = functional connectivity.
Predictors: 1) maternal negative behavior during the EPI 1) maternal negative behavior during the PSI 3) maternal positive behavior. Covariates: child sex, SES, maternal depressive symptoms at wave 1.
Ethics approval for this study was granted by the University of Melbourne Human Research Ethics Office (#1339904). Parental informed written consent and child assent were obtained prior to study participation.
2.2. Parenting behaviors: family interaction task (FIT)
Two 15-minute mother-child interactions were performed and video recorded. The first, an Event Planning Interaction (EPI), involved dyads planning two or three pleasant activities to do together, selected from a list in the Pleasant Events Schedule (MacPhillamy and Lewinsohn, 1982). The second, a Problem Solving Interaction (PSI), involved discussing conflictual topics relevant for the dyad, chosen from the Issues Checklist (Prinz et al., 1979). The EPI is designed to elicit positive behaviors, while the PSI elicits negative (Gilboa and Revelle, 1994). Two trained graduate students independently coded the behaviors recorded during the interactions, using a modified version (Richmond et al., 2018) of the Family Interaction Macro-coding System (FIMS) (Holmbeck et al., 2007). Fifty-nine codes were included covering a range of mother and child behaviors that were rated on 5-point Likert scales. A multiple factorial analysis was performed on the FIMS codes on the original wave 1 sample to obtain empirically-derived parenting behavior components (Richmond et al., 2018). Three factors related to maternal affective behavior were identified: 1) maternal negative behavior during the PSI and 2) during the EPI (e.g. frequency and intensity of negative affect: anger) and 3) maternal positive behavior (e.g., frequency and intensity of positive affect: humor, laughter) across both tasks (i.e., in the factor analysis, the maternal positive behavior component comprised codes from both the PSI and the EPI (Richmond et al., 2018)). Maternal negative behavior, especially when displayed in a positive context (EPI), was found to predict onset of depression in adolescents in our previous work (Schwartz et al., 2014). Factor scores for each of the three components were used in subsequent analyses. See Supplementary Material for a list of codes included in each component. Data were checked for outlier values, i.e., values more than 2.2 * InterQuartile Range (IQR) below the first Q or above the third Q (N = 12 for maternal negative behavior during the EPI, N = 1 for maternal positive behavior). After winsorizing the outlier value (i.e., replacing the extreme value with the highest non-outlier value), maternal positive behavior was normally distributed (Shapiro-Wilk test: p = 0.3). Frequencies and intensities of maternal negative behavior during the EPI and PSI were not normally distributed (Shapiro-Wilk test: p < 0.001), even after winsorization and after applying transformation (logarithmic, root-squared). Thus, analyses were run with transformed and not-transformed data and winsorized and not-winsorized to assess the robustness of results. See Figure S1 for plots of the distribution of the parenting variables.
2.3. Questionnaires measures
Children completed the Children's Depression Inventory 2 (CDI-2) (Kovacs, 1992) and the Spence Children Anxiety Scale (SCAS) (Spence, 1998) at both waves. Maternal depressive symptoms at wave 1 were assessed with the Centre for Epidemiologic Studies Depression Scale (CESD) (Radloff, 1977). Socio-economic status (SES) was measured with the neighborhood derived Socio-Economic Indexes for Areas Index of Relative Socio-Economic Disadvantage scale, SEIFA (Australian Bureau of Statistics: Canberra, 2013). See Table 2 for the bivariate correlations between all the variables of interest.
Table 2.
Descriptive statistics of the sample.
| N = 95 |
||
|---|---|---|
| Wave 1 | Wave 2 | |
| Child Sex | ||
| Female | 52 (54.7 %) | – |
| Male | 43 (45.3 %) | |
| Child Age | ||
| Mean (SD) | 8.40 (0.328) | 9.93 (0.346) |
| Median [Min, Max] | 8.38 [7.98, 9.09] | 9.91 [9.41, 10.8] |
| Child SCAS | ||
| Mean (SD) | 25.6 (12.9) | 24.7 (14.2) |
| Median [Min, Max] | 26.0 [3.00, 64.0] | 23.0 [3.00, 76.7] |
| Child CDI | ||
| Mean (SD) | 7.70 (5.57) | 6.96 (6.06) |
| Median [Min, Max] | 6.00 [0, 23.0] | 5.00 [0, 25.0] |
| SES | ||
| Mean (SD) | 38.6 (24.2) | – |
| Median [Min, Max] | 37.0 [1.00, 93.0] | |
| Mat. neg. EPI | ||
| Mean (SD) | −0.08 (0.85) | – |
| Median [Min, Max] | −0.38 [−1.05, 4.45] | |
| Mat. neg. PSI | ||
| Mean (SD) | 0.02 (1.01) | – |
| Median [Min, Max] | −0.21 [−1.37, 3.50] | |
| Mat. pos. PA | ||
| Mean (SD) | 0.03 (0.98) | – |
| Median [Min, Max] | 0.01 [−3.49, 3.10] | |
| Mat. CESD | ||
| Mean (SD) | 20.2 (7.70) | – |
| Median [Min, Max] | 18.0 [12.0, 50.0] | |
Note. Mat. neg. EPI = maternal negative behavior during the EPI; Mat. neg. PSI = maternal negative behavior during the PSI; Mat. pos = maternal positive behavior across tasks; Mat. CESD = maternal depressive symptoms at wave 1 measured with the Centre for Epidemiologic Studies Depression Scale (CESD); SES = Socio-Economic Status measured with the neighborhood derived Socio-Economic Indexes for Areas Index of Relative Socio-Economic Disadvantage scale, SEIFA. A low score indicates relatively greater disadvantage (this measure is a percentage with a mean of 50 across Australia); CDI = Children's Depression Inventory 2; SCAS = Spence Children Anxiety Scale; Raw scores are presented.
2.3.1. Covariates
Although there is evidence for sex differences in brain development (Dennison et al., 2013), in consideration of the sample size and the lack of specific hypotheses about sex effects, the moderating effect of sex was not investigated. Rather, child sex was included as a covariate in all analyses. Maternal depressive symptoms at wave 1 and SES may contribute to meaningful variance related to maternal parenting behavior (Lovejoy et al., 2000). Therefore, all the parenting analyses were first carried without including these variables in the models. In case of significant findings, the models were re-run including these variables as covariates, to understand maternal parenting behavior’s contribution above and beyond maternal depressive symptoms and SES. One participant was missing SES data and two were missing maternal depressive symptoms data at wave 1. Missing data were replaced with the group mean (R version 3.6.1). To investigate the association between maternal parenting behavior-related changes in connectivity and internalizing symptoms at wave 2, internalizing symptoms at wave 1 as entered as a covariate (CDI or SCAS total score).
2.4. MRI
Neuroimaging data were acquired on a 3 T Siemens TIM Trio scanner (Siemens, Erlangen, Germany) at the Murdoch Children’s Research Institute in Melbourne, Australia. Participants lay supine in a 32-channel head coil. Prior to the scan, participants underwent a MRI mock training session where they looked at pictures of the machine, received information about the procedure and practiced staying still in the scanner (Simmons et al., 2017).
2.4.1. MRI parameters
Structural T1-weighted images were acquired as follows: MPRAGE MoCo, repetition time = 2530 msec; echo time1 = 1.74 msec, echo time2 = 3.6 msec, echo time3 = 5.46 msec, echo time4 = 7.32 msec; flip angle = 7°, field of view = 256 × 256 mm2, producing 176 contiguous slices with 1.0 mm3 voxel dimensions. Resting state functional images were acquired as follows: 154 whole-brain T2*-weighted echo-planar volumes, repetition time = 2400 ms, echo time = 35 ms, flip angle = 90°; field of view = 210 × 210 mm2. Thirty-eight interleaved slices were acquired, with 3.3 mm3 voxel dimensions. The total sequence duration was 6.18 min. Participants were asked to keep their eyes open and look at a fixation cross (white cross on a black screen).
2.4.2. Image preprocessing
2.4.2.1. Quality control
MRIQC (Esteban et al., 2017) version 0.15.0 was used to perform quality control before the analysis. Subjects with mean Framewise Displacement (FD) (computed using AFNI 3dvolreg) > 0.5 mm were excluded from further analysis. This led to the exclusion of 13/150 subjects at wave 1 (8.7 %) and 14/127 at wave 2 (11 %). The mean FD at wave 1 did not differ from the mean FD at wave 2 (paired t-test on the whole sample before exclusion: t = −0.996, df = 121, p = 0.321)
2.4.2.2. Preprocessing pipeline and data cleaning
Preprocessing was performed using fMRIPrep 1.5.0 (Esteban et al., 2019b), which is based on Nipype 1.2.2 (Gorgolewski et al., 2011, 2018). The original full report on anatomical and functional data preprocessing as printed by fMRIPrep is reported in Supplementary Material. The Montreal Neurological Institute (MNI)’s unbiased standard MRI template for pediatric data cohort 3 (age 7–11) was selected for spatial normalization (Fonov et al., 2011). All individual reports were visually inspected.
Following preprocessing, a high-pass filter at 0.01 Hz and 5 mm spatial smoothing were applied using FMRIBs Software Library (FSL v6.0; https://fsl.fmrib.ox.ac.uk/fsl/).
Individual-level independent component analysis (ICA) performed with FSL MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components) Version 3.15 combined with FMRIB's ICA-based Xnoiseifier (FIX) (Griffanti et al., 2017; Salimi-Khorshidi et al., 2014) version 1.06 was used to perform data cleaning. First, FIX was trained on 20 subjects from the Imaging brain development in the Childhood to Adolescence Transition Study (iCATS) (Simmons et al., 2014); another longitudinal neuroimaging study that recruited 8−10 year old typically developing children from Melbourne, Australia.
2.4.3. Image analysis
2.4.3.1. Seed-based connectivity analysis
A bilateral amygdala mask was derived from the FSL Harvard-Oxford maximum likelihood subcortical atlas using a probability threshold of 50 % and registered to the MNI’s unbiased standard MRI template for pediatric data cohort 3 (age 7–11) (Fonov et al., 2011), using FSL’s flirt interpolation with nearest neighbor. For each subject, the fMRI signal was averaged over all voxels comprising the bilateral amygdala mask. The averaged signal was then correlated (Pearson correlation) with the fMRI signal for each voxel comprising a gray matter (GM) derived from the pediatric template, using MATLAB 2017Rb (The MathWorks, Natick, MA). This yielded a functional connectivity map for each subject, which characterized the functional connectivity between the amygdala and each GM voxel.
To examine changes in connectivity over-time, each subject’s amygdala functional connectivity maps at both waves were entered into separate paired t-tests (with exchangeability blocks). Of note, this approach is equivalent to calculating a ‘connectivity change map’ per feature (as described below) and conducting a one-sample t-test.
To examine the effect of parenting, for each amygdala-whole brain functional connectivity map, individual ‘connectivity change maps’ were calculated by subtracting the wave 1 spatial map from the wave 2 spatial map (i.e., wave 2 minus wave 1) and vice versa (wave 1 minus wave 2) using FSL’s fslmaths (note that the connectivity maps only contain positive values and thus the subtraction was done in both directions). These change maps were entered into FSL’s one-sample with covariates design (i.e., partial correlation), whereby we examined if maternal positive and negative behavior had a linear relationship with the ‘connectivity change map’. As discussed in section 2.3.1, child sex was included as a covariate of non-interest in all analyses. For significant results, the unique contribution of maternal parenting behavior was explored by adding SES and maternal depressive symptoms at wave 1 as further covariates (see section 2.3.1). Statistical significance was calculated using FSL's randomise (Winkler et al., 2014) permutation-testing tool with 10,000 permutations and corrected with threshold free cluster enhancement (Smith and Nichols, 2009).
For the amygdala connectivity analyses, the critical α for cluster correction was pFWE< 0.016 based on a Bonferroni adjustment for the number of tests (3 parenting components).
2.4.3.2. Exploratory RSNs analyses
2.4.3.2.1. Network connectivity (dual regression)
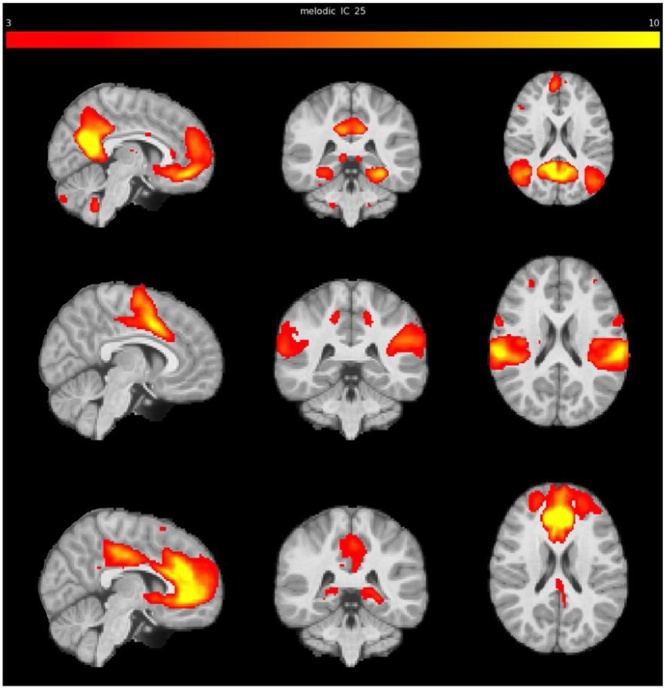
Twenty-five group-level ICs were extracted with MELODIC. Relevant networks (DMN, ECN, and SN – see Fig. 1) were selected based on visual inspection and via cross-correlation with networks templates (Shirer et al., 2011; Smith et al., 2009) (using FSL’s fslcc; DMN: r = 0.57, ECN: r = 0.53, SN: r = 0.24). The networks derived from MELODIC are depicted in Fig. 1. The set of spatial maps from the group-average analysis was used to generate subject-specific versions of the spatial maps, and associated time-series, using dual regression (Beckmann et al., 2009; Nickerson et al., 2017). First, for each subject, the group-average set of spatial maps was regressed (as spatial regressors in a multiple regression) onto the subject's 4D space-time dataset. This resulted in a set of subject-specific time-series, one per group-level spatial map. Next, those time-series were regressed (as temporal regressors, again in a multiple regression) onto the same 4D dataset, resulting in a set of subject-specific spatial maps, one per group-level spatial map.
Fig. 1.
RSNs derived from MELODIC on the FACTS sample. From top to bottom: Default Mode Network (DMN), Salience Network (SN), and Executive Control Network (ECN).
To examine changes in network connectivity over-time, as well as the association between parenting and connectivity change, analyses proceeded exactly as described for amygdala connectivity in section 2.4.3.1 (i.e., individual ‘connectivity change maps’ for each RSN were calculated by subtracting the wave 1 spatial map from the wave 2 spatial map and vice versa).
For the network analyses, the critical α for cluster correction was pFWE< 0.0056 based on a Bonferroni adjustment for the number of tests (i.e., 3 RSNs x 3 parenting components = 9).
2.4.3.2.2. Between network connectivity (FSLnets)
For each wave, partial correlations between networks (following (Smith et al., 2015)) were calculated using FSLnets (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets), using as input the nodes (i.e., spatial maps) from the group ICA map described above and the subject-specific set of time-series from dual regression. For each pair of networks of interest (DMN-CN, DMN-SN and ECN-SN), we calculated r partial correlation coefficients for each subject at both waves.
To compare the magnitude of the correlations of each pair of networks between wave 1 and wave 2 (e.g., DMN at wave 1-ECN at wave 1 vs DMN at wave 2-ECN at wave 2), we used the R package cocor (Diedenhofen and Musch, 2015). Cocor compares pairs of correlations based on dependent groups (i.e., same subjects at both waves) using different tests (e.g., Pearson and Filon's z, Zou’s confidence interval), so that the results can be compared (Meng et al., 1992).
To examine the association with parenting behavior, the same r coefficients were converted to z scores through Fisher's r-to-z transformation. Then, a change score was calculated by subtracting wave 1 z scores from wave 2 z scores. Partial correlations between parenting behavior and network connectivity change scores were calculated, controlling for child sex, as well as SES and maternal depressive symptoms at wave 1 when findings were significant.
2.4.4. Association with symptoms
Associations with depressive and anxiety symptoms were examined for clusters that exhibited associations between changes in connectivity and maternal behavior. To do so, time-series were extracted from peak voxels using FSL’s fslmeants, and correlated with depressive and anxiety symptoms at wave 2, controlling for symptoms at wave 1.
2.4.5. Cross-sectional analyses
See Supplementary Material for a description and results.
3. Results
3.1. Demographic characteristics
See Table 2 for demographics and descriptive information of the sample. See Table S1 for correlation between variables.
3.2. Amygdala-whole brain connectivity
There were no changes between wave 1 and wave 2, and no associations between positive and negative parenting and changes in amygdala-whole brain connectivity. Unthresholded and thresholded statistical maps for amygdala-whole brain connectivity can be accessed on NeuroVault (https://neurovault.org/collections/LFABBRGN/).
3.3. Network connectivity (dual regression)
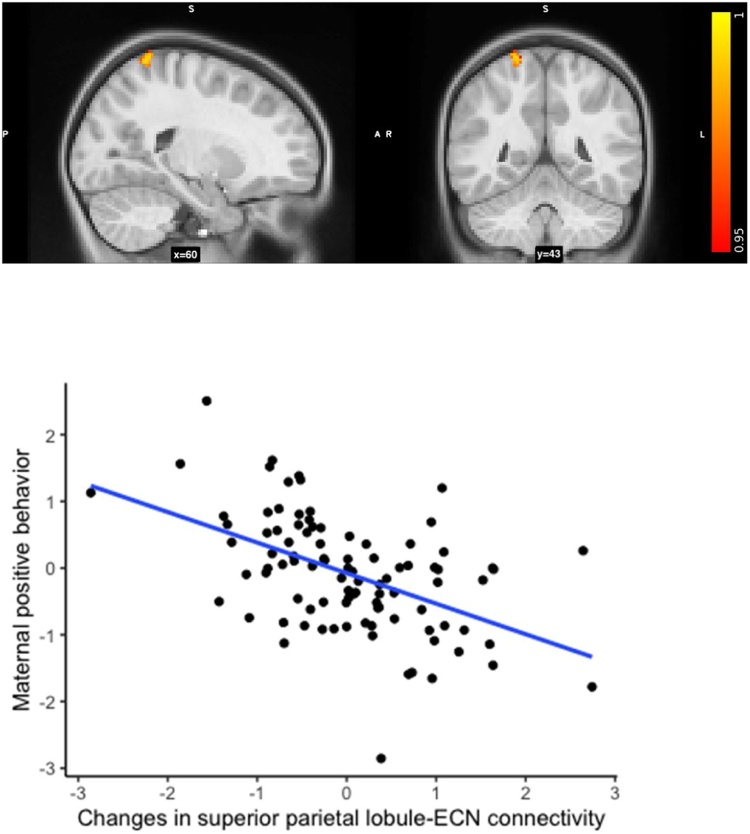
There were no changes between wave 1 and wave 2 and no association between negative parenting and changes in any of the networks connectivity. Maternal positive behavior was associated with decreased coactivation of the right superior parietal lobule with the ECN at wave 2 relative to wave 1 (wave 2 > wave 1). Because dual regression is a whole brain analysis, voxels showing a significant effect outside the given network (i.e., in this case the cluster was located in the superior parietal lobule) are indicative of connectivity between this area and the main network (in this case, the ECN). Including maternal depressive symptoms at wave 1 and SES did not change the results (pFWE<0.0056, MNI x = 20 y=-50 z = 72, k = 4) (Fig. 2).
Fig. 2.
(Top) Maternal positive behavior was associated with decreased coactivation of the superior parietal lobule (depicted in the picture) with the ECN between wave 1 and 2. (Bottom). Scatterplot (for display purposes only) of the association between maternal positive behavior and changes in superior parietal lobule connectivity (MNI x = 20 y = −50 z = 72) with the ECN between wave 1 and wave 2. For plotting, time-series values were extracted from the ECN change maps, using the coordinates of the significant cluster (x = 20 y = −50 z = 72) with fslmaths roi.
Unthresholded and thresholded statistical maps for each RSN can be accessed on NeuroVault (https://neurovault.org/collections/LFABBRGN/).
3.4. Between RSNs connectivity
There were no changes between wave 1 and wave 2 between any of the RSNs connectivity, and no association between parenting variables and changes in between RSN connectivity.
3.5. Association with symptoms
Time-series were extracted (using FSL’s fslmeants) from the voxel in the spatial coordinates (MNI x = 20 y = −50 z = 72) that corresponded to the peak of the superior parietal lobule clusters. To investigate the association between maternal parenting behavior-related changes in connectivity and CDI and SCAS total scores at wave 2, CDI and SCAS total scores at wave 1 were entered as covariates. There were no associations with depressive or anxiety symptoms at wave 2, controlling for symptoms at wave 1.
4. Discussion
In this study, we examined changes in resting state functional connectivity occurring over an 18-month interval in 8–10 year old children. We investigated amygdala connectivity, as well as connectivity of selected large-scale networks (DMN, SN, ECN). Contrary to our hypotheses, we did not detect age-related changes in the connectivity of the amygdala or any of the networks. Maternal positive behavior was associated with decreased connectivity of the superior parietal lobule with the ECN, whereas maternal negative behavior was not associated with any connectivity changes. Connectivity of the superior parietal lobule was not associated with child internalizing symptoms.
The lack of changes in amygdala connectivity with age, particularly with the PFC, was unexpected given prior work suggesting developmental changes in frontoamydala circuitry during late childhood, whereby connectivity is thought to switch from positive to negative during emotion regulation tasks (Gee et al., 2013b) and increase at rest (Gabard-Durnam et al., 2014). However, most of the studies that have identified such a developmental switch, and related effects of early environment, are cross-sectional (Alarcón et al., 2015; Gee et al., 2013a;b; Silvers et al., 2017; Thijssen et al., 2017; Wu et al., 2016). One exception is a recent study that adopted an accelerated longitudinal design and found decreased resting state connectivity between the amygdala and medial PFC with age (Jalbrzikowski et al., 2019). Their sample comprised children/adolescents from 10 to 25 years of age, whilst our study included children aged 8–10 years. It is possible that examining the transition between childhood and adolescence (i.e., after age 10) may be particularly informative of the developmental (Gabard-Durnam et al., 2014). Moreover, the sample included in the analysis covered a narrow age range (8−10) and a relatively short period between the two assessments (18 months). It is possible that a period of 18 months was not sufficient to elicit detectable changes. Finally, most of the studies that have investigated age effects on frontoamygdala circuitry explored connectivity during emotion processing tasks rather than at rest, and there is a lack of consensus on connectivity development at rest (Colich et al., 2020). These considerations point to the need for further longitudinal studies that cover larger age ranges and possibly longer periods of time, to shed light on normative frontoamygdala circuitry development across childhood and adolescence. Such knowledge would have important implications for our understanding of the neural circuits underlying emotion regulatory development that is occurring during the transition between childhood and adolescence (Gullone et al., 2010).
Similarly, we did not detect age-related changes of within and between connectivity of large-scale resting state networks. Previous studies that have investigated resting state connectivity “development” were also cross-sectional (Fair et al., 2007; Supekar et al., 2011) or included samples of adolescents (10–18) assessed over longer periods of time (3–5 years) (Sherman et al., 2014; Teeuw et al., 2019), whereas longitudinal studies that have investigated the transition from childhood to mid-adolescence (8–15) have identified age effects in some (ventral attention network (VAN)) but not other (DMN, SN, frontoparietal network (FPN)) networks (Sylvester et al., 2018). Further research is thus required to understand normative patterns of resting state network connectivity across childhood and adolescence.
Regarding associations between parenting and development of resting state connectivity, we found developmental effects in the absence of group-level changes. In particular, we found that maternal positive behavior predicted decreased connectivity of the superior parietal lobule with the ECN, which comprises medial-frontal areas, and is proposed to play a key role in cognitive tasks as well as emotion processing (Smith et al., 2009). The superior parietal lobe is part of the FPN (as described in (Smith et al., 2009)) and is thought to have a role in attention shifting and orienting (Marlene et al., 2004). Although some evidence (cross-sectional) has suggested that maturation of control networks (i.e., ECN, FPN) involves increased segregation of prefrontal regions from the frontoparietal network (Fair et al., 2007), there is a paucity of longitudinal studies that can speak to developmental trajectories of between and within network connectivity. Previous work found that positive parenting was associated with increased segregation of the DMN and ECN, which was interpreted as reflecting greater maturation (Dégeilh et al., 2018). In line with this interpretation, our result could also suggest positive parenting behavior may promote resting state network maturation (Brody et al., 2019; Dégeilh et al., 2018; Holmes et al., 2018). Earlier maturation of control networks may reflect greater cognitive flexibility, that is the ability to switch attention between tasks or stimuli (Stevens et al., 2009). The ability to shift attention and respond flexibly to the environment, as well as to exert inhibitory control, contributes to emotion regulation (Phillips et al., 2003), whilst impairment in such abilities are associated with affective disorders (Murphy et al., 2012). However, more research into normative development of resting state networks, including between/within the distinct executive control networks, is required to understand the direction of changes and support the hypothesis that positive parenting promotes more adaptive/mature development.
Of important note, the stress acceleration hypothesis postulates that negative experiences lead to accelerated brain development. According to this theory, early maturation is considered adaptive in the short term (e.g., because it results in increased emotion regulation capacities to meet environmental demands) but potentially maladaptive in the long term, because it reduces the plasticity of the system (Callaghan and Tottenham, 2016). Whilst it seems counterintuitive that positive environmental factors would lead to a similar phenotype (i.e., accelerated brain maturation), the stress acceleration hypothesis has focused on cortical thinning and specific biomarkers of development, such as frontoamygdala circuitry (Callaghan and Tottenham, 2016), whereas the extension of the concept to the development of large scale networks has been less explored.
In this study, we did not find an effect of negative parenting on amygdala-PFC or network connectivity. In our previous work, we found that maternal negative behavior was associated with increased amygdala reactivity and connectivity during an emotional faces task (Pozzi et al., 2020). Thus, it is unclear why we only found changes in relation to positive parenting. The discrepancy in results might be related to the different tasks used in this and our previous study (rest vs emotional faces task), pointing to the fact that findings from task-based fMRI data may not be generalizable to resting state studies (and vice versa). This may be particularly important in the context of parenting and children emotion regulation development. Taken together, these considerations highlight once again the need for further longitudinal work to investigate the contribution of both positive and negative experiences to neurodevelopmental trajectories across childhood and adolescence. It may be that both types of parenting behavior impact neurodevelopment, but that these changes only become apparent across a longer time-period. It is also possible that parenting behavior has more or less of an impact at different developmental stages, which can only be discovered by investigation of. Efforts such as the Adolescent Brain Cognitive Development (ABCD) Study (Casey et al., 2018), where children age 9–10 will be followed up over a 10-year period and will undergo a yearly brain scan and complete measures of the family environment, will allow for testing these hypotheses. It is also possible that other environmental factors may contribute to brain development during the period that we studied. For example, it is possible that paternal parenting factors affect child brain development independently or in interaction with maternal parenting. Furthermore, the relationship between parent and child is not unidirectional. Children’s individual differences in temperament and personality, not explored here, may also influence parenting behaviors (Kiff et al., 2011), or moderate the influence of parenting behaviors and neurodevelopment (Guyer et al., 2015).
We did not find an association between maternal parenting behavior-related changes in superior parietal lobule-ECN connectivity and child internalizing symptoms. This is somewhat consistent with our previous work within the same sample (Pozzi et al., 2019, 2020), which we previously interpreted as possibly being related to the low variability and low levels of anxiety and depressive symptoms in the sample. The lack of association with symptoms limits our ability to make inferences about the adaptive or maladaptive nature of changes in functional connectivity. Future research may extend the current findings by investigating factors associated with neurodevelopment in children with greater variability in levels of internalizing symptoms.
It is important to note several limitations of this study. Although the children underwent a mock MRI session before the scan, excessive motion significantly impacted the percentage of data that was retained for the analyses. Children are at higher risk for motion in the scanner compared to adults (Greene et al., 2016), however there are currently no standard guidelines to preprocess and perform appropriate data cleaning in pediatric neuroimaging. Although recently standard pipelines (such as fMRIprep) have begun to include age-appropriate templates (Fonov et al., 2011), to the best of our knowledge there is a lack of standard pediatric atlases (e.g., subcortical parcellation atlases, RSNs atlases) and robust/automated strategies for removing motion artifacts (e.g., ICA-based methods) specific to pediatric populations. Whilst we adopted a data-driven approach (ICA) to investigate RSNs connectivity, alternatives methods that rely on the selection of predefined ROIs, such as ROI-ROI connectivity analysis, could have yielded different results. It is important to note that such inconsistency of methods (e.g., ICA, ROI-ROI, ROI-whole brain) may impact reproducibility of results. The choice of RSNs was performed by visually comparing the networks and correlating them with existing atlases (Shirer et al., 2011; Smith et al., 2009). However, evidence shows there are inconsistencies across studies in the nomenclature of networks, particularly networks related to executive and control functions (Witt et al., 2020). Thus, our results may not be generalizable to other executive function networks. It has been suggested that more than 6 min of scan length are required to obtain reliable resting state data, and this could be particularly important for longitudinal studies (Birna, 2013). Our RSNs analyses were exploratory, and it is important to note that we did not correct for multiple comparisons across network analyses (dual regression and FSLnets), but only within each set of analyses. Thus, our results should be considered preliminary and interpreted with caution.
5. Conclusion
In this study, although we found no evidence for (group-level) age-related changes in connectivity of resting state networks during late childhood, we found that maternal positive behavior was associated with decreased co-activation of the superior parietal lobule with the ECN at wave 2 compared to wave 1, potentially suggesting that positive parenting promotes more mature connectivity between networks (parietal regions and ECN). That our findings were not in line with hypotheses, which were largely based on cross-sectional research, suggests the need for more longitudinal work in order to better understand developmental trajectories of neural networks, and how the caregiving environment impacts them.
Data statement
Participants of this study did not agree for their data to be shared with third parties, so supporting data is not available.
Funding
This study was funded by a Discovery Project grant from the Australian Research Council (ARC; DP130103551). SW was supported by an NHMRC Career Development Fellowship (ID: 1125504). The Authors report no biomedical financial interests or potential conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank The Royal Children’s Hospital, Melbourne, for their assistance in acquiring the brain image data, and the families who participated in the research.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100946.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alarcón G., Cservenkab A., Rudolph M.D., Fair D.A., Nagel B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115(1):235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Bureau of Statistics: Canberra, A . 2013. Statistics, A.B.S.A.B.o.. Building on SEIFA: Finer Levels of Socio-Economic Summary Measures. [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. 2009. Group Comparison of Resting-state FMRI Data Using Multi-subject ICA and Dual Regression, 181. [Google Scholar]
- Belsky J., De Haan M. Annual research review: parenting and children’s brain development: the end of the beginning. J. Child Psychol. Psychiatry. 2011;52(4):409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Bhanot S., Bray S., Mcgirr A., Lee K., Kopala-Sibley D.C. 2020. Parenting and Offspring Brain Development: What Do We Know? pp. 2–64. Preprint, (October) [DOI] [Google Scholar]
- Birna R.M. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Yu T., Nusslock R., Barton A.W., Miller G.E., Chen E. The protective effects of supportive parenting on the relationship between adolescent poverty and resting-state functional brain connectivity during adulthood. Psychol. Sci. 2019;30:1040–1049. doi: 10.1177/0956797619847989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield R.D., Silk J.S., Lee K.H., Siegle G.S., Dahl R.E., Forbes E.E. 2020. Parents Still Matter! Parental Warmth Predicts Adolescent Brain Function and Anxiety and Depressive Symptoms 2 Years Later; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology. 2015;41(1):163–176. doi: 10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., Rosen M.L., Williams E.S., McLaughlin K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. Psychol. Bull. 2020;146(9):721. doi: 10.1037/bul0000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégeilh F., Bernier A., Leblanc É., Daneault V., Beauchamp M.H. Quality of maternal behaviour during infancy predicts functional connectivity between default mode network and salience network 9 years later. Dev. Cogn. Neurosci. 2018;34(November 2017):53–62. doi: 10.1016/j.dcn.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison M., Whittle S., Yucel M., Vijayakumar N., Kline A., Simmons J., Allen N.B. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev. Sci. 2013;16(5):772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Diedenhofen B., Musch J. Cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One. 2015:1–12. doi: 10.1371/journal.pone.0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Birman D., Schaer M., Koyejo O.O., Poldrack A., Gorgolewski K.J. Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12(9):1–21. doi: 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Ciric R., Finc K., Blair R., Markiewicz C.J., Moodie C.A. Analysis of task-based functional MRI data preprocessed with fMRIPrep. bioRxivorg. 2019:1–17. doi: 10.1038/s41596-020-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16(January):111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. U.S.A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95(15):193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H. Early developmental emergence of human amygdala – prefrontal connectivity after maternal deprivation. PNAS. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Revelle W. Personality and the structure of affective responses. In: Van Goozen S.H.M., Van De Poll N.E., Sergeant J.A., editors. Emotions: Essays on Emotion Theory. Erlbaum; Hillsdale, NJ: 1994. [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O. 2011. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. 5(August) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Esteban O., Markiewicz C.J., Ziegler E., Gage Ellis D., Philipp Notter M., Jarecka D. 2018. “Nipype.” Software. Zenodo. [DOI] [Google Scholar]
- Greene D.J., Black K.J., Schlaggar B.L. Considerations for MRI study design and implementation in pediatric and clinical populations. Dev. Cogn. Neurosci. 2016;18:101–112. doi: 10.1016/j.dcn.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Douaud G., Bijsterbosch J., Evangelisti S., Alfaro-almagro F., Glasser M.F. Hand classification of fMRI ICA noise components. NeuroImage. 2017;154(December 2016):188–205. doi: 10.1016/j.neuroimage.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullone E., Hughes E.K., King N.J., Tonge B. The normative development of emotion regulation strategy use in children and adolescents: a 2-year follow-up study. J. Child Psychol. Psychiatry. 2010;5:567–574. doi: 10.1111/j.1469-7610.2009.02183.x. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Jarcho J.M., Pérez-Edgar K., Degnan Ka., Pine D.S., Fox Na., Nelson E.E. Temperament and parenting styles in early childhood differentially influence neural response to peer evaluation in adolescence. J. Abnorm. Child Psychol. 2015;43(5):863–874. doi: 10.1007/s10802-015-9973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G.N., Zebracki K., Johnson S.Z., Belvedere M., Hommeyer J.S. Loyola University.; Chicago: 2007. Parent- Child Interaction Macro-coding Manual. [Google Scholar]
- Holmes C.J., Barton A.W., MacKillop J., Galván A., Owens M.M., McCormick M.J. Parenting and salience network connectivity among african americans: a protective pathway for health-risk behaviors. Biol. Psychiatry. 2018;84(5):365–371. doi: 10.1016/j.biopsych.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M., Ph D., Murty V.P., Ph D., Tervo-clemmens B., Foran W. 2019. Age-Associated Deviations of Amygdala Functional Connectivity in Youths with Psychosis Spectrum Disorders: Relevance to Psychotic Symptoms. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Xu J., Li X., Wang Y., Zhuang L., Qin S. Negative parenting affects adolescent internalizing symptoms through alterations in amygdala-prefrontal circuitry: a longitudinal twin study. Biol. Psychiatry. 2020 doi: 10.1016/j.biopsych.2020.08.002. [DOI] [PubMed] [Google Scholar]
- Kiff C.J., Lengua L.J., Zalewski M. Nature and nurturing: parenting in the context of child temperament. Clin. Child Fam. Psychol. Rev. 2011;14(3):251. doi: 10.1007/s10567-011-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala-Sibley D.C., Cyr M., Finsaas M.C., Orawe J., Huang A., Klein D.N. Early childhood parenting predicts late childhood brain functional connectivity during emotion perception and reward processing. Child Dev. 2020;91(1):110–128. doi: 10.1111/cdev.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Multi-Health Systems, Inc; North Tonawanda, NY: 1992. Children’s Depression Inventory. [Google Scholar]
- Lovejoy M.C., Graczyk Pa., O’Hare E., Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin. Psychol. Rev. 2000;20(5):561–592. doi: 10.1016/S0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- MacPhillamy D.J., Lewinsohn P.M. The pleasant events schedule: studies on reliability, validity, and scale intercorrelation. J. Consult. Clin. Psychol. 1982 doi: 10.1037/0022-006X.50.3.363. [DOI] [Google Scholar]
- Marlene B., Geng J.J., Shomstein S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Meng X.-L., Rosenthal R., Rubin D.B. Comparing correlated correlation coefficients. Psychol. Bull. 1992;111:172–175. [Google Scholar]
- Murphy F.C., Michael A., Sahakian B.J. Emotion modulates cognitive flexibility in patients with major depression. Psychol. Med. 2012;42(7):1373–1382. doi: 10.1017/S0033291711002418. [DOI] [PubMed] [Google Scholar]
- Nickerson L.D., Smith S.M., Öngür D., Beckmann C.F. 2017. Using Dual Regression to Investigate Network Shape and Amplitude in Functional Connectivity Analyses; pp. 1–18. 11(March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion percepition. Biol. Psychiatry. 2003;54:504–515. doi: 10.1016/S0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pozzi E., Bousman C.A., Simmons J.G., Vijayakumar N., Schwartz O., Seal M. Interaction between hypothalamic-pituitary-adrenal axis genetic variation and maternal behavior in the prediction of amygdala connectivity in children. NeuroImage. 2019;197(October 2018):493–501. doi: 10.1016/j.neuroimage.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Pozzi E., Simmons J.G., Bousman C.A., Vijayakumar N., Bray K.O., Dandash O. The influence of maternal parenting style on the neural correlates of emotion processing in children. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59(2):274–282. doi: 10.1016/j.jaac.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Prinz J.R., Sharon F., Ronald K.N., O’Leary K.D. Multivariate assessment of conflict in distressed and nondisressed mother-adolescent dyads. J. Appl. Behav. Anal. 1979;4(12):691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: a self-report depression scale for use in general populations. Appl. Psychol. Meas. 1977;1:385–400. [Google Scholar]
- Richmond S., Schwartz O.S., Johnson Ka., Seal M.L., Bray K., Dean C. Exploratory factor analysis of observational parent–Child interaction data. Assessment. 2018 doi: 10.1177/1073191118796557. [DOI] [PubMed] [Google Scholar]
- Romund L., Raufelder D., Flemming E., Lorenz R.C., Pelz P., Gleich T. Maternal parenting behavior and emotion processing in adolescents—an fMRI study. Biol. Psychol. 2016;120:120–125. doi: 10.1016/j.biopsycho.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O.S., Byrne M.L., Simmons J.G., Whittle S., Dudgeon P., Yap M.B.H. Parenting during early adolescence and adolescent-onset major depression: a 6-Year prospective longitudinal study. Clin. Psychol. Sci. 2014;1(4):1–15. doi: 10.1177/2167702613505531. [DOI] [Google Scholar]
- Sherman L.E., Rudie J.D., Pfeifer J.H., Masten C.L., Mcnealy K., Dapretto M. Development of the Default Mode and Central Executive Networks across early adolescence: a longitudinal study. Dev. Cogn. Neurosci. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W., Ryali S., Rykhlevskaia E., Menon E., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex. 2011;22 doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R. The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Dev. Cogn. Neurosci. 2017;25:128–137. doi: 10.1016/j.dcn.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.G., Whittle S.L., Patton G.C., Dudgeon P., Olsson C., Byrne M.L. Study protocol: imaging brain development in the Childhood to Adolescence Transition Study (iCATS) BMC Pediatr. 2014;14(115):1–10. doi: 10.1186/1471-2431-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.G., Schwartz O.S., Bray K., Deane C., Pozzi E., Richmond S. Study protocol: families and childhood transitions study (FACTS) – a longitudinal investigation of the role of the family environment in brain development and risk for mental health disorders in community based children. BMC Pediatr. 2017;17(153):1–14. doi: 10.1186/s12887-017-0905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E., Vidaurre D., Winkler A.M., Glasser M.F., Ugurbil K. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat. Neurosci. 2015;18(11):1565–1567. doi: 10.1038/nn.4125.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S.H. A measure of anxiety symptoms among children. Behav. Res. Ther. 1998;36(5):545–566. doi: 10.1016/S0005-7967(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30(8):2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2011;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009.Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Whalen D.J., Belden A.C., Sanchez S.L., Luby J.L., Barch D.M. Shyness and trajectories of functional network connectivity over early adolescence. Child Dev. 2018;89(3):734–745. doi: 10.1111/cdev.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeuw J., Brouwer R.M., Guimarães J.P.O.F.T., Brandner P., Koenis M.M.G., Swagerman S.C. Genetic and environmental influences on functional connectivity within and between canonical cortical resting-state networks throughout adolescent development in boys and girls. NeuroImage. 2019;202(June) doi: 10.1016/j.neuroimage.2019.116073. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Polcari A., Anderson C.M., Navalta C.P., Kim D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27(1–2):33–44. doi: 10.1016/S0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C. Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29(02):505–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S.T., Ettinger-veenstra H..Van, Salo T., Riedel M.C. What executive function network is that? An image-based meta-analysis of network labels. bioRxivorg. 2020:1–16. doi: 10.1007/s10548-021-00847-z. [DOI] [PubMed] [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D. Age-related changes in Amygdala – frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 2016;37:1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M.B.H., Morgan A.J., Cairns K., Jorm A.F., Hetrick S.E., Merry S. Parents in prevention: a meta-analysis of randomized controlled trials of parenting interventions to prevent internalizing problems in children from birth to age 18. Clin. Psychol. Rev. 2016;50:138–158. doi: 10.1016/j.cpr.2016.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.