Abstract
Purpose
Our purpose was to report outcomes of a novel palliative radiation therapy protocol that omits computed tomography simulation and prospectively collects electronic patient-reported outcomes (ePROs).
Methods and Materials
Patients receiving extracranial, nonstereotactic, linear accelerator-based palliative radiation therapy who met inclusion criteria (no mask-based immobilization and a diagnostic computed tomography within 4 weeks) were eligible. Global pain was scored with the 11-point numerical pain rating scale (NPRS). Patients were coded as having osseous or soft tissue metastases and no/mild versus severe baseline pain (NPRS ≥ 5). Pain response at 4 weeks was measured according to the international consensus (no analgesia adjustment). Transition to ePRO questionnaires was completed in 3 phases. Initially, pain assessments were collected on paper for 11 months, then pilot ePROs for 1 month and then, after adjustments, revised ePROs from 1 year onwards. ePRO feasibility criteria were established with reference to the paper-based process and published evidence.
Results
Between May 2018 and November 2019, 542 consecutive patients were screened, of whom 163 were eligible (30%), and 160 patients were successfully treated. The proportion of patients eligible for the study improved from approximately 20% to 50% by study end. Routine care pain monitoring via ePROs was feasible. One hundred twenty-seven patients had a baseline NPRS recording. Ninety-five patients had osseous (61% severe pain) and 32 had soft tissue (25% severe pain) metastases. Eighty-four patients (66%) were assessable for pain response at 4 weeks. In the 41 patients with severe osseous pain, overall and complete pain response was 78% and 22%, respectively.
Conclusions
By study completion, 50% of patients receiving palliative extracranial radiation therapy avoided simulation, streamlining the treatment process and maximizing patient convenience. Pain response for patients with severe pain from osseous lesions was equivalent to published evidence.
Introduction
Radiation therapy (RT) has an important role in the symptomatic relief and improvement in the quality of life for patients with bony and soft tissue metastases,1 and approximately 40% of all RT courses are palliative.2 Bone pain is the most common indication for palliative RT with the strongest evidence base.3 Even a single RT treatment can be very effective in treating pain or other symptoms such as bleeding.4,5 Historically, 2-dimensional planning was common for palliative RT and same-day treatment was possible without delays or extra resources required. Today, most departments use 3-dimensional (3D) planning after a dedicated computed tomography (CT) simulation scan, which has made palliative RT more resource and time-intensive.
Patient inconvenience and access issues between referral and treatment are key reasons why some referrers may decide against palliative RT.6,7 Inconvenience relating to the extra visit for CT simulation is a contributor to these barriers. Each additional attendance to the RT department is a major logistical event for an unwell patient, which can destabilize symptom control or cause anxiety. Furthermore, these symptomatic patients are often reliant on others for support and transport, further complicating the logistics of attending for RT. From a cancer caregiver perspective, arranging or providing extra transport is also an inconvenience or may be impossible in an overall stressful time. Finally, timely completion of RT is required to reduce unnecessary suffering and derive the maximum benefit in the context of limited life expectancy.8
A well-described strategy for overcoming these issues is rapid access clinics that combine patient assessment, CT simulation-based planning, and RT delivery on a single day.9 However, these services are logistically challenging, particularly for smaller departments without a dedicated palliative program.10,11 Even with a well-designed and optimally staffed rapid access process it is not unusual for a patient to be in a RT department for over 5 hours.12 Thus, from a patient perspective, although an improvement on the traditional model, attending a rapid access clinic plus travel can be a long and arduous experience.13
Routinely collecting patient-generated data such as electronic patient-reported outcomes (ePROs) provides major benefits for palliative patients including better symptom control and improved survival.14 It is also a foundational ingredient of a learning health system15 that uses research-quality routine care data to generate new evidence, aids safe implementation of new insights, and provides guidance for clinical decisions.16 Collecting ePROs in a routine care setting is challenging, particularly in unwell palliative patients. A study from the Dana-Farber Cancer Institute in Boston demonstrated a limited completion rate of only 20% after introducing tablet-based ePRO collection in the waiting room of an adult palliative care clinic.17
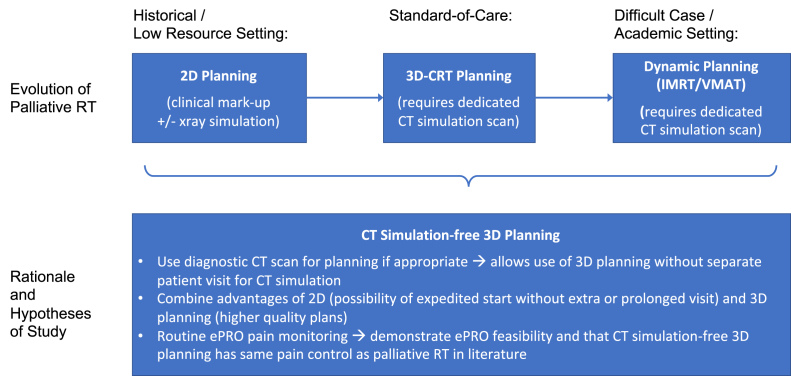
This report describes clinical outcomes and our experience with ePRO introduction after treatment of 160 consecutive routine care patients without CT simulation. Figure 1 provides a summary of the rationale and hypotheses of this study.
Figure 1.
Conceptual model: Evolution of palliative RT and rationale of study. Abbreviations: 3D-CRT = 3D conformal RT; IMRT = intensity modulated RT; RT = radiation therapy; VMAT = volumetric modulated arc RT.
Methods and Materials
Our clinical pathway allowing planning and delivery of palliative RT without formal CT simulation has been previously described.18 Briefly, palliative cases with lesion(s) of chest (except lung), abdomen, lumbar and thoracic spine, pelvis, or sacrum suitable for doses below stereotactic body RT (SBRT) range were included (head and neck including cervical spine and extremity regions were excluded). Patients were required to have a diagnostic CT or positron emission tomography/CT with sufficient field-of-view within 4 weeks of referral. We aimed to replicate the diagnostic CT position during patient set-up for treatment and verified this using cone beam CT (CBCT) before the first fraction. Unless setup concerns existed, daily planar kilovoltage imaging was used from the second fraction onwards for multifraction courses.
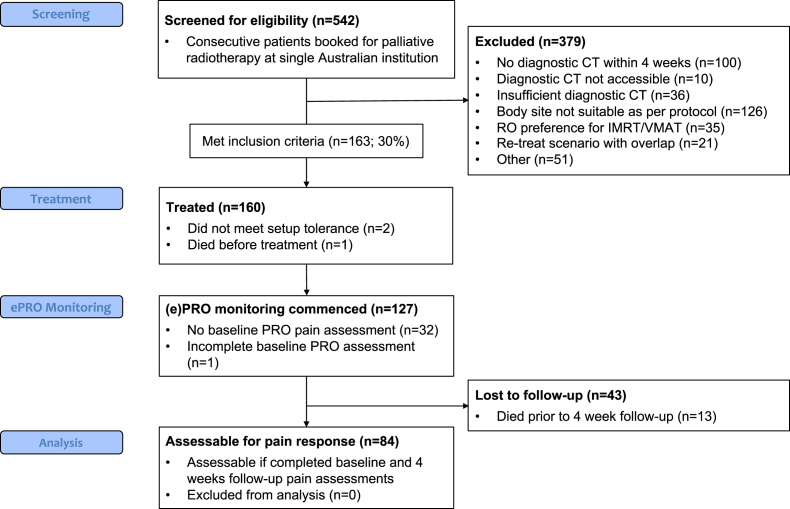
The Strengthening the Reporting of Observational Studies in Epidemiology flow diagram in Figure 2 describes number of patients screened, recruited, and in follow-up. Initially, only 3D-conformal (3D-CRT) techniques were used. After demonstration of acceptable dose coverage with dynamic techniques in a retrospective planning study of previously treated 3D-CRT patients on this pathway, the approach was expanded to allow intensity modulated RT (IMRT) from July 2019 onwards.
Figure 2.
Strengthening the Reporting of Observational Studies in Epidemiology flow diagram of screening, treatment, electronic patient-reported outcomes (ePROs) monitoring, and analysis stages.
All palliative cases in our department were considered and, if eligible, treated on this CT simulation–free pathway. Patient and treatment data were recorded in an institutional review board–approved palliative database. Pain response was measured by collecting patient-reported outcomes (PROs) at baseline and 4 weeks post-RT completion using the 11-point numerical pain rating scale (NPRS), where 0 = no pain and 10 = worst pain imaginable.19 Patients were coded as (1) osseous or (2) soft tissue based on site of treatment. Each cohort was divided into no/mild pain (NPRS 0-4) and severe pain (NPRS ≥ 5) at baseline.
Pain response assessment
Pain response was measured at 4 weeks, with collection cut-off at 42 days after last RT treatment. We implemented ePROs in 3 phases. Initially, the proposed PRO NPRS ratings were recorded on paper either in person or via phone. During this paper-based phase these responses were predominantly collected by 2 radiation therapists (S.W., K.G.). Phase 2 transitioned paper questionnaires to the ePRO using the Research Electronic Data Capture platform.20,21 As part of this transition a larger team of radiation therapists approached patients before the first treatment to collect baseline assessments. Our strategy to overcome ePRO completion rate issues described by others17 was to offer remote ePRO collection, include automated reminders, involve caregivers for assistance,22 and, as a fallback mechanism, provide staff-based ePRO assistance.23 After a planned 1-month review of the ePRO pilot phase 2, a refined ePRO process was established for phase 3.
At baseline, ePRO surveys were completed on a tablet device with or without assistance from a staff member. Follow-up ePRO surveys were completed by the patient remotely after invitation via short message service or e-mail including up to 2 automated reminders. The automated invitation using the patient’s preferred delivery mechanism (short message service or email) and recipient (patient or caregiver) was set up by the staff member after the baseline ePRO was completed. In cases where there was no response to either invitation or reminders, patients were offered assistance either by phone or in person (staff-based ePRO response monitoring and assistance) to encourage them to respond.
Endpoints
The primary outcome was the proportion of palliative patients eligible for the CT simulation–free pathway out of all consecutively screened palliative patients. Global pain response, that is, not reporting pain at treated index site(s) only, at 4 weeks, was collected following the international consensus definition,24 except that no analgesia adjustment was performed. Response was coded as partial (≤2 point NPRS improvement) or complete (NPRS score improved by at least 2 points to 0 ie, pain-free) and overall (sum of previous 2 pain response types). We planned a 2-sided X2 test with an alpha of 0.05 to compare our results with published randomized3 and nonrandomized25 outcomes. Other endpoints were ePRO completion (on-site vs remote and paper vs ePRO using X2 test statistics with an alpha of 0.05); overall patient survival and median follow-up using Kaplan-Meier and reverse Kaplan-Meier statistics, respectively26; and feasibility of the ePRO in a challenging palliative patient population during routine care. The latter was defined as similar ePRO completion rates to paper-based surveys, ≤20% staff assistance needed during completion of ePRO surveys, and ≥50% ePRO completeness after adjusting for death before follow-up. The statistical package R (v 3.6.1) was used for Kaplan-Meier and X2 test statistics.27
Results
Eligibility
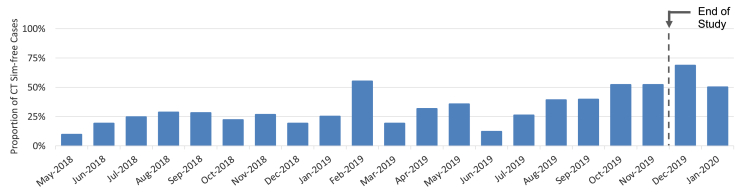
From May 2018 to November 2019, 542 patients were prescribed palliative RT by a radiation oncologist (RO) in our department. One hundred and sixty-three (30%) were eligible for the CT simulation-free pathway (see Appendix E1 for ineligibility reasons and their distribution). Figure 3 displays the temporal recruitment to our CT simulation-free pathway.
Figure 3.
Proportion of palliative patients treated on computed tomography (CT) simulation-free (Sim-free) pathway by month.
Of 163 eligible patients, 2 patients were excluded because they required secondary CT simulation (body outline changed significantly within 1 week of diagnostic CT due to generalized edema; significant tumor size progression), and 1 patient died before treatment. Therefore, 160 patients were treated per protocol.
Baseline characteristics
Table 1 lists patient characteristics including performance status, disease site, fractionation, treatment technique, number of sites irradiated, and distribution of treated patients by group and baseline pain. In the osseous group (n = 95), 61% had severe pain at baseline, whereas this was only the case for 25% on the nonosseous group patients (n = 32). Baseline pain was missing for 33 patients in total.
Table 1.
Baseline patient characteristics of treated patients
| Osseous group |
Nonosseous group |
No baseline NPRS recorded |
All patients |
|||
|---|---|---|---|---|---|---|
| NPRS 0-4 |
NPRS 5-10 |
NPRS 0-4 |
NPRS 5-10 |
(n = 33) | (n = 160) | |
| (n = 37) | (n = 58) | (n = 24) | (n = 8) | |||
| Age | ||||||
| Min. | 42 | 28 | 54 | 61 | 21 | 21 |
| P25 | 64 | 62 | 71 | 68 | 54 | 62.8 |
| Median | 72 | 71 | 76 | 73 | 69 | 71.5 |
| P75 | 79 | 77 | 79 | 77 | 80 | 78 |
| Max. | 96 | 91 | 94 | 90 | 98 | 98 |
| Sex | ||||||
| Female | ||||||
| n (%) | 20 (54) | 35 (60) | 16 (67) | 4 (50) | 22 (67) | 97 (61) |
| Male | 17 (46) | 23 (40) | 8 (33) | 4 (50) | 11 (33) | 63 (39) |
| Cancer type | ||||||
| Lung | ||||||
| n (%) | 4 (11) | 17 (29) | 6 (25) | 0 (0) | 10 (30) | 37 (23) |
| Prostate | 11 (30) | 10 (17) | 1 (4) | 0 (0) | 5 (15) | 17 (17) |
| Colorectal | 5 (14) | 5 (9) | 3 (13) | 1 (13) | 3 (9) | 17 (11) |
| Breast | 7 (19) | 7 (12) | 0 (0) | 0 (0) | 2 (6) | 16 (10) |
| Other | 10 (27) | 19 (33) | 14 (58) | 7 (88) | 13 (39) | 63 (39) |
| Performance status | ||||||
| ECOG 0 | ||||||
| n (%) | 9 (24) | 14 (24) | 5 (21) | 1 (13) | 6 (18) | 35 (22) |
| ECOG 1 | 16 (43) | 27 (47) | 6 (25) | 4 (50) | 13 (39) | 66 (41) |
| ECOG 2 | 8 (22) | 15 (26) | 11 (46) | 0 (0) | 11 (33) | 45 (28) |
| ECOG 3 | 3 (8) | 1 (2) | 2 (8) | 2 (25) | 2 (6) | 10 (6) |
| ECOG 4 | 1 (3) | 1 (2) | 0 (0) | 1 (13) | 1 (3) | 4 (3) |
| Baseline pain (NPRS score) | ||||||
| Min. | 0 | 5 | 0 | 5 | - | 0 |
| P25 | 0 | 6 | 0 | 6 | - | 1 |
| Median | 2 | 7 | 0 | 7 | - | 5 |
| P75 | 3 | 8 | 1 | 7 | - | 7 |
| Max. | 4 | 10 | 4 | 10 | - | 10 |
| Dose/fractionation | ||||||
| 8 Gy/1# | ||||||
| n (%) | 22 (59) | 31 (53) | 8 (33) | 2 (25) | 16 (48) | 80 (50) |
| 20 Gy/4-5# | 11 (30) | 16 (28) | 11 (46) | 3 (38) | 10 (30) | 51 (32) |
| 25 Gy/5# | 3 (8) | 4 (7) | 1 (4) | 1 (13) | 4 (12) | 13 (8) |
| Other | 1 (3) | 7 (12) | 4 (17) | 2 (25) | 3 (9) | 16 (10) |
| Treatment technique | ||||||
| 3D-CRT | ||||||
| n (%) | 23 (62) | 44 (76) | 16 (67) | 2 (25) | 26 (79) | 111 (69) |
| IMRT | 14 (38) | 14 (24) | 8 (33) | 6 (75) | 7 (21) | 49 (31) |
| Number of treatment sites | ||||||
| One (1) site | ||||||
| n (%) | 30 (81) | 46 (79) | 23 (96) | 8 (100) | 30 (91) | 137 (86) |
| Two (2) sites | 5 (14) | 9(16) | 1 (4) | 0 (0) | 1 (3) | 16 (10) |
| Three (3) sites | 2 (5) | 3 (15) | 0 (0) | 0 (0) | 2 (6) | 7 (4) |
Abbreviations: 3D-CRT = 3D conformal radiation therapy; ECOG = Eastern Cooperative Oncology Group; Gy = Gray; IMRT = intensity modulated radiation therapy; NPRS = numerical pain rating scale; P25 = 25th percentile; P75 = 75th percentile; # = fraction(s).
Pain response and attrition
At the 1-month follow-up, 84 patients were assessable for pain analysis. The remaining patients did not complete a baseline pain assessment (n = 33), were deceased (n = 13), were too unwell to complete the follow-up survey, or were otherwise noncontactable (n = 30). This constitutes an overall attrition rate of 43 of 127 or 34% after a successfully completed baseline pain assessment.
Table 2 shows the change in pain scores for assessable patients at 4-weeks follow-up (refer to Appendix E2 for intention-to-treat results), including a comparison of our largest cohort with severe osseous pain at baseline to published results. This cohort is most relevant for comparison (for details see 'Discussion' section). There was 78% overall response (OR) and 22% complete response in this cohort. We achieved similar results in terms of pain response for both OR (P = .644) and complete response (P = .446); however, attrition was significantly worse (34% vs 19%, P < .001) compared with randomized evidence.3 Compared with nonrandomized evidence,25 our results in this cohort were statistically significant for OR (78% vs 55%; P = .005) and attrition (34% vs 46%; P = .009). As a sensitivity analysis we used Fisher exact test to compare the response rates, resulting in similar P values.
Table 2.
Pain response as per international consensus without analgesia adjustment, with comparison of severe osseous pain response outcomes against published results3,25
| Nonosseous cohort |
Osseous cohort |
Comparison of outcomes of osseous cohort with severe pain (NPRS 5-10) against published evidence |
||||||
|---|---|---|---|---|---|---|---|---|
| NPRS 0-4 | NPRS 5-10 | NPRS 0-4 | NPRS 5-10 | Randomized evidence3 | P value | Nonrandomized evidence25 | P value | |
| No. of assessable patients | n = 19 | n = 3 | n = 21 | n = 41 | ||||
| OR | 21% | 100% | 24% | 78% | 74% | .644 | 55% | .005∗ |
| CR | 21% | 33% | 14% | 22% | 29% | .446 | 15% | .245 |
| Partial response | 0% | 67% | 10% | 56% | ||||
| Indeterminate response | 53% | 0% | 38% | 15% | ||||
| Pain progression | 26% | 0% | 38% | 7% | ||||
Abbreviations: CR = complete response; NPRS = numerical pain rating scale; OR = overall response.
Bold values are the part of comparison with published evidence.
P < .05.
ePRO completion
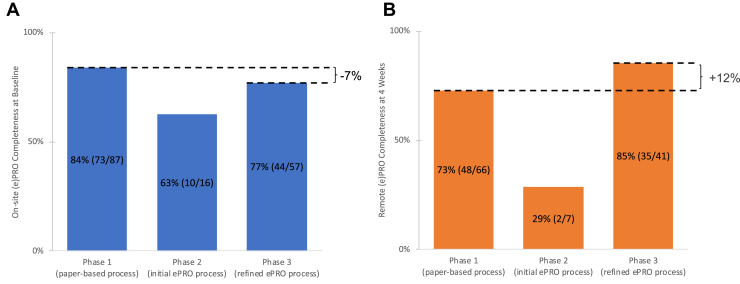
Figure 4 displays a comparison of PRO completion rates by collection location and timepoint (on-site at baseline vs remote at follow-up) and by collection mechanism (paper [phase 1] and ePRO [phase 2/3]). We transitioned from paper to ePRO in 2 iterations, labeled “phase 2” and “phase 3,” respectively. Questionnaires were provided at baseline and at 4-weeks follow-up. The 1-month review revealed a marked deterioration of ePRO completions in phase 2. After process improvements (staff education and scheduling a shared patient-specific ePRO collection task for radiation therapists via the ARIA oncology information management system by Varian Medical Systems, Inc, Palo Alto, CA), the ePRO completion rate improved in phase 3. We disregarded phase 2 in the remaining ePRO completion analysis. For baseline surveys there was an absolute 7% reduction (P = .122) in completion rate between paper-based phase 1 (84%) and ePRO-based phase 3 (77%). After adjusting for patients who didn’t complete the baseline survey, that is, who were never commenced on ePRO monitoring or died before the repeat PRO survey, the corresponding rates for the 4-week follow-up survey were 73% and 85%, an absolute 12% increase from phase 1 to phase 3 (P = .429). During on-site ePRO (phase 3 baseline) the staff assistance rate was 12%. This was not measured for remote ePRO (phase 3 follow-up). All ePRO feasibility criteria were met.
Figure 4.
Patient-reported outcome (PRO) completeness at (A) baseline and (B) 4-weeks follow-up by phase.
Overall survival
At a median follow-up of 8.8 months the median survival was 6.5 months. As per expectations, baseline performance status predicted for survival with a log-rank test P value < .001 (for Kaplan-Meier survival plots refer to Supplementary Materials).
Discussion
We have demonstrated the clinical feasibility of CT simulation–free palliative RT combined with routine ePRO monitoring of pain response in a real-world setting. The ePRO completion rates were high compared with a similar palliative setting,17 and pain response outcomes for our patients with severe osseous pain were statistically equivalent to randomized evidence.3
Eligibility
Our eligibility rate was 30% over the whole study period. However, over the last 5 months it gradually reached approximately 50% and was maintained at this level for the 2 months after the study period (see Fig 3). The main factors driving increased eligibility were improvements in access to systems of external radiology providers to download diagnostic CT scans (DICOM format) and the protocol extension to include IMRT.
Pain response
Uncomplicated malignant bone pain is the most common indication for palliative RT, and consequently most randomized evidence regarding palliative radiation pertains to this population. The systematic review by Chow et al,28 including its 2 updates,3,29 summarizes a significant part of this evidence base. We reviewed these systematic reviews as well as a recent systematic review of nonrandomized studies25 regarding pain response for assessable patients and attrition rate. Pain response to palliative RT has been mostly described in patients with severe osseous pain. We compared our pain response outcomes for this subgroup and found them to be similar to the aggregate results of the 29 randomized controlled trials (RCTs). Our OR results in this subgroup were better than the aggregate result from the 12 nonrandomized studies published by Saito et al.25 The equivalence to the RCT results and superiority to the nonrandomized evidence may be due to fact that we, like most studies in the RCT meta-analysis, did not adjust for analgesia. This is in contrast to the work by Saito et al,25 who also raised this issue when they discussed the discrepancy between their results and the previously mentioned systematic reviews of RCT data.3,28,29 Both our study and the nonrandomized studies did include complicated bone pain (eg, incident or neuropathic pain, unlike the RCT data), so this is not a factor that would explain our superior OR pain outcomes compared with the nonrandomized studies.
Baseline ePRO completion and attrition
For the first 87 patients, paper-based survey management was handled by 2 staff members with a strong interest in the study. Despite this, there was attrition of 16% before performing the baseline PRO. Key drivers of missed baseline PROs were logistical issues such as communication between staff members, staff holidays, or patients declining completion of a survey. A minority of patients struggled because English was not their primary language or they were deemed to be too unwell. The ePRO mode itself has unlikely contributed to this deterioration as staff would assist in-person as required (up to a level where staff would ask the survey questions verbally and record the answers for the patient), which occurred in 12% of encounters.
At first glance, our rate of assessable patients (84 of 160 or 53%) appears low. However, unlike the randomized3 and nonrandomized25 studies reviewed, we treated all eligible routine care patients in a consecutive fashion, which is a strength of this study, as it makes it more “real world.” Thus, adjusting for patients who never filled out a baseline PRO makes comparison more realistic (84 of 127 or 66%). Correspondingly, our attrition of patients with a completed baseline PRO (paper-based or ePRO) was 34%, which compares favorably to the 46% overall attrition rate in the Saito et al25 systematic review of nonrandomized studies. However, we acknowledge that half the studies reviewed by Saito et al25 had a longer follow-up interval (up to 8 weeks). Not unexpectedly, the attrition rate was lower in the RCTs (19%), which can be explained by selection and volunteer bias between participants in an RCT and nonrandomized studies or even routine care. Apart from death before follow-up (13 of 43 or 30%), the biggest reason for attrition was the patient not being able to be contacted, presumably at least partially due to having deteriorated or dying out-of-area and therefore without our knowledge.
Remote ePRO completeness
Using phase 1 (paper-based) as a reference, the transition to ePRO improved the remote follow- up completion rate by an absolute 12% from 73% to 85%. Although not statistically significant due to the modest cohort sizes, it demonstrates at least similar completeness with fewer staff needed to call patients. Further, this may be an early indicator that remote ePRO collection, which foremost relied on automated send out of invitation and reminders, appeared to be less susceptible to logistical issues on behalf of involved staff. Based on the experience by Chua et al17 we prespecified a conservative ePRO completeness goal of ≥ 50%, which we achieved both for on-site and remote collection.
Workflow and resource implications
Although in a palliative setting it is clear that there is value in saving the patient an extra attendance and starting treatment earlier, are there trade-offs? Our experience is that simulation-free RT is at least resource neutral compared with conventional CT simulation-based RT. Extra time sourcing, assessing, and registering the diagnostic scan and replicating its setup is offset by not having to attend a dedicated simulation CT session. From a departmental view it frees up CT simulation time and a same-day-treatment workflow is much less disruptive than the rapid access clinic or on-couch planning paradigms. It requires well-trained radiotherapists that can interpret CBCT. The simulation-free RT approach can be used for simple 3DCRT techniques or IMRT. Thirty-one percent of delivered courses in this study used IMRT. This was purely at the discretion of the prescribing RO based on clinical grounds. In our department 3DCRT and IMRT have a similar workload and resource effect, as it is department policy that all patients get contoured, and we use in-house and third-party software such as knowledge-based planning and quality assurance check tools to semiautomate processes.
Other related work
The tomotherapy-based palliative RT developed by Read et al30,31 is similar to our pathway, using a diagnostic CT for precontouring. However, the plan is then created from a same-day megavoltage CT scan while the patient remains on the couch. With the emergence of magnetic resonance imaging linear accelerators, there is increased interest in using on-couch adaptive treatment strategies for palliation32 and again diagnostic imaging is used for precontouring. Several groups have described using the linear accelerator’s on-board CBCT for 1-step simulation, planning, and delivery of palliative RT.33, 34, 35 Compared with our pathway these strategies have the advantage of planning directly off the treatment scan, thus eliminating interfraction motion, which allows reduced margins. There is also less dependence on having to set-up the patient in a similar position to the diagnostic scan. Overall, on-couch adaptive planning likely increases the percentage of patients who a simulation-free palliative RT pathway can be offered to. However, this comes with a large logistical cost, relying on on-couch planning and quality assurance (presence of RO and physicist) and likely requires the patient to remain longer on the treatment couch, which is a problem for some palliative patients and most departments. Our approach is more generalizable as the required technology is available in most radiation oncology departments, unlike tomotherapy or magnetic resonance-based linear accelerators. The on-board CBCT units, megavoltage and kilovoltage, currently don’t achieve the same image quality as a diagnostic CT. Thus, planning off a diagnostic CT is an advantage in this respect.
Limitations
Limitations of our study include the modest sample size, particularly for nonosseous and no/mild pain cohorts, and the single institution design. We did not adjust pain response for analgesia. This is similar to most of the RCTs we compared our results to, but we acknowledge that this is likely the reason for our superior OR rate compared with the nonrandomized studies. As explained previously, in our real-world study we had a larger attrition rate than in the randomized evidence, which is reflected in our intention-to-treat analysis (see Appendix E2). This needs to be taken into consideration when interpreting these data, as missing data are likely not random in terms of pain outcome.36 Another limitation is that we didn’t collect PRO toxicity information. We deliberately opted to focus on PRO pain response and analgesia to reduce reporting burden for potentially unwell palliative RT patients. However, based on our experience in this study, we think using acute toxicity self-reporting in the modular Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events37 system in combination with intelligent branching logic is feasible. Anatomic region-specific Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events toxicity reporting by the patient and/or caregiver has been designed into our current palliative RT study.
Future directions
We will further improve our process with a focus on reducing the attrition rate before baseline assessment by providing extra staff training regarding the importance of ePRO completion, considering it as a mandatory step before being able to deliver RT (unless explicitly overwritten) and by clarifying staff accountabilities. We plan to run regular audits to support this change management process.
We feel that there is a role for on-couch adaptive RT with same-day planning to expand the proportion of patients to whom a simulation-free palliative pathway can be offered (eg, for SBRT techniques or body regions such as brain metastases that were excluded in the current pathway and which together represented around 50% of all noneligible cases). We are planning to build on the skills that our team has gained during this project when moving into CT linear accelerator-based on-couch adaptive planning using the Ethos (Varian Medical Systems, Inc) solution. This will include determining which palliative patients will benefit from the extra precision and SBRT-dose range features that on-couch adaptive planning offers over the less-technology intensive solution presented in this paper.
Conclusions
To our knowledge, this study represents the largest published series of palliative patients treated with 3D-planned RT in the absence of a dedicated CT simulation scan. We demonstrated the feasibility of this novel pathway during routine care and successfully implemented a remote ePRO strategy in this challenging group of unwell patients. We achieved high ePRO completion rates and our documented pain response for patients with severe pain from bony lesions was equivalent to the published evidence, while sparing patients an extra visit to the department for CT simulation.
Acknowledgments
The authors would like to thank the Luan and Yoong Foundation for their generous support of radiation oncology research in our department.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are not available at this time.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.100632.
Supplementary Materials
References
- 1.Lutz S.T., Jones J., Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol. 2014;32:2913–2919. doi: 10.1200/JCO.2014.55.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radiotherapy in Australia 2017–18, Report editions Australian Institute of Health and Welfare. https://www.aihw.gov.au/reports/cancer/radiotherapy-in-australia-2017-18/contents/radiotherapy-courses Available at:
- 3.Rich S.E., Chow R., Raman S. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol. 2018;126:547–557. doi: 10.1016/j.radonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ryu S., Deshmukh S., Timmerman R.D. Radiosurgery compared to external beam radiotherapy for localized spine metastasis: Phase III results of NRG Oncology/RTOG 0631. Int J Radiat Oncol Biol Phys. 2019;105:S2–S3. [Google Scholar]
- 5.Fareed M.M., Krishnan M., Balboni T.A., Yu H.-H.M. Indications, barriers and paths to advancement in palliative radiation oncology. Appl Radiat Oncol. 2018;7:18–25. [Google Scholar]
- 6.Eastman B., Mayr N.A., Kane G.M., Tseng Y.D. Importance of timely patient evaluation for palliative radiotherapy: A survey-based study of referring oncologists’ expectations for palliative radiotherapy. Int J Radiat Oncol. 2019;105:S150–S151. [Google Scholar]
- 7.Parker S.M., Wei R.L., Jones J.A., Mattes M.D. A targeted needs assessment to improve referral patterns for palliative radiation therapy. Ann Palliat Med. 2019;8:516–522. doi: 10.21037/apm.2019.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt T.R., Yau V.K.Y. Innovative program for palliative radiotherapy in Australia. J Med Imaging Radiat Oncol. 2010;54:76–81. doi: 10.1111/j.1754-9485.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 9.Dennis K., Linden K., Balboni T., Chow E. Rapid access palliative radiation therapy programs: An efficient model of care. Future Oncol. 2015;11:2417–2426. doi: 10.2217/FON.15.153. [DOI] [PubMed] [Google Scholar]
- 10.Danjoux C., Chow E., Drossos A. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14:38–43. doi: 10.1007/s00520-005-0822-7. [DOI] [PubMed] [Google Scholar]
- 11.Job M., Holt T., Bernard A. Reducing radiotherapy waiting times for palliative patients: The role of the advanced practice radiation therapist. J Med Radiat Sci. 2017;64:274–280. doi: 10.1002/jmrs.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colbert L.E., Gomez M., Todd S. Implementation of a rapid access multidisciplinary bone metastases clinic to improve access to care at a large cancer center. J Clin Oncol. 2018;36(34_suppl):79. [Google Scholar]
- 13.Fairchild A., Pituskin E., Rose B. The rapid access palliative radiotherapy program: Blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17:163–170. doi: 10.1007/s00520-008-0468-3. [DOI] [PubMed] [Google Scholar]
- 14.Basch E., Deal A.M., Kris M.G. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abernethy A.P., Ahmad A., Zafar S.Y., Wheeler J.L., Reese J.B., Lyerly H.K. Electronic patient-reported data capture as a foundation of rapid learning cancer care. Med Care. 2010;48(6 Suppl):S32–S38. doi: 10.1097/MLR.0b013e3181db53a4. [DOI] [PubMed] [Google Scholar]
- 16.Abernethy A.P., Etheredge L.M., Ganz P.A. Rapid-learning system for cancer care. J Clin Oncol. 2010;28:4268–4274. doi: 10.1200/JCO.2010.28.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chua I.S., Siegel J., Hayne V., McCleary N.J. Improving PROMs collection rate in an outpatient palliative care clinic. J Clin Oncol. 2018;36(34_suppl):105. [Google Scholar]
- 18.Anonymous 2020 - Details omitted for peer review
- 19.Brunelli C., Zecca E., Martini C. Comparison of numerical and verbal rating scales to measure pain exacerbations in patients with chronic cancer pain. Health Qual Life Outcomes. 2010;8:42. doi: 10.1186/1477-7525-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bausewein C., Schildmann E., Rosenbruch J., Haberland B., Tänzler S., Ramsenthaler C. Starting from scratch: Implementing outcome measurement in clinical practice. Ann Palliat Med. 2018;7:S253–S261. doi: 10.21037/apm.2018.06.08. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen L.K., King M., Möller S. Strategies to improve patient-reported outcome completion rates in longitudinal studies. Qual Life Res. 2020;29:335–346. doi: 10.1007/s11136-019-02304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow E., Hoskin P., Mitera G. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol. 2012;82:1730–1737. doi: 10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Saito T., Toya R., Oya N. Pain response rates after conventional radiation therapy for bone metastases in prospective nonrandomized studies: A systematic review. Pract Radiat Oncol. 2019;9:81–88. doi: 10.1016/j.prro.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team . R Foundation for Statistical Computing; 2019. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 28.Chow E., Harris K., Fan G., Tsao M., Sze W.M. Palliative radiotherapy trials for bone metastases: A systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 29.Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol. 2012;24:112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Wilson D., Sheng K., Yang W., Jones R., Dunlap N., Read P. STAT RAD: a potential real-time radiation therapy workflow. In: Modern Practices in Radiation Therapy. London, UK: IntechOpen. 2012 [Google Scholar]
- 31.Muller D.A., Wilson D.D., Wages N.A. Comparison of multi-fraction versus single-fraction stereotactic body radiation therapy (SBRT) for symptomatic bone metastasis: Results of the STAT RT and STAT RAD Phase I/II prospective trials. Int J Radiat Oncol Biol Phys. 2019;105:S48. [Google Scholar]
- 32.Mittauer K.E., Hill P.M., Geurts M.W. STAT-ART: The promise and practice of a rapid palliative single session of mr-guided online adaptive radiotherapy (ART) Front Oncol. 2019;9:1013. doi: 10.3389/fonc.2019.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong R.K.S., Letourneau D., Varma A. A one-step cone-beam ct-enabled planning-to-treatment model for palliative radiotherapy-from development to implementation. Int J Radiat Oncol. 2012;84:834–840. doi: 10.1016/j.ijrobp.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Le A.H., Stojadinovic S., Timmerman R. Real-time whole-brain radiation therapy: A single-institution experience. Int J Radiat Oncol. 2018;100:1280–1288. doi: 10.1016/j.ijrobp.2017.12.282. [DOI] [PubMed] [Google Scholar]
- 35.Dyer B.A., Nair C.K., Deardorff C.E., Wright C.L., Perks J.R., Rao S.S. Linear accelerator-based radiotherapy simulation using on-board kilovoltage cone-beam computed tomography for 3-dimensional volumetric planning and rapid treatment in the palliative setting. Technol Cancer Res Treat. 2019;18 doi: 10.1177/1533033819865623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito T., Toya R., Tomitaka E. Predictors of the predominance of nonindex pain after palliative radiation therapy for painful tumors. Adv Radiat Oncol. 2019;4:118–126. doi: 10.1016/j.adro.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandler K.A., Mitchell S.A., Basch E. Content validity of anatomic site-specific Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) item sets for assessment of acute symptomatic toxicities in radiation oncology. Int J Radiat Oncol. 2018;102:44–52. doi: 10.1016/j.ijrobp.2018.04.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.