Abstract
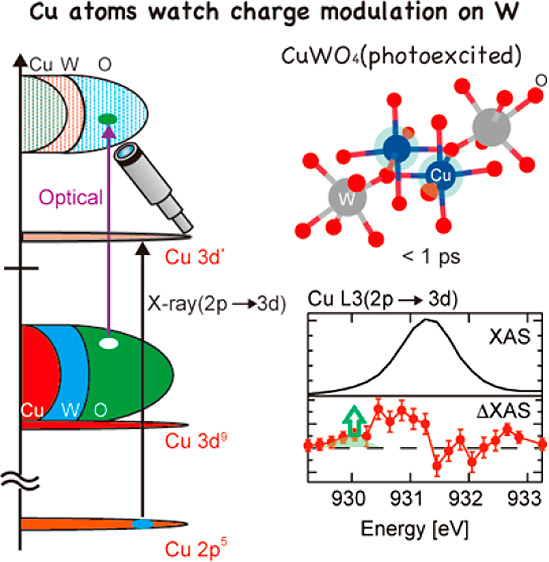
Copper tungstate (CuWO4) is an important semiconductor with a sophisticated and debatable electronic structure that has a direct impact on its chemistry. Using the PAL-XFEL source, we study the electronic dynamics of photoexcited CuWO4. The Cu L3 X-ray absorption spectrum shifts to lower energy upon photoexcitation, which implies that the photoexcitation process from the oxygen valence band to the tungsten conduction band effectively increases the charge density on the Cu atoms. The decay time of this spectral change is 400 fs indicating that the increased charge density exists only for a very short time and relaxes electronically. The initial increased charge density gives rise to a structural change on a time scale longer than 200 ps.
1. Introduction
Copper tungstate (CuWO4) is an important functional material that has gained a lot of attention in materials science because of its small optical band gap and electrical conductivity. In particular, it is a promising candidate for photocatalysis1 and a potential photoelectrode for the water-splitting reaction.2−6 CuWO4 has a triclinic distorted wolframite-type structure due to the Jahn–Teller distortion around the Cu2+ ions.7 It is known as an n-type semiconductor and its valence band is believed to be composed mainly of oxygen 2p states that are hybridized with tungsten and copper s, p, and d states.8,9 The conduction band contains mainly W 5d states hybridized with the oxygen 2p states. In addition, it has been claimed that the single Cu 3d hole contributes to the bottom of the conduction band, which shifts the conduction band minimum in CuWO4 downward compared to WO3.10−14 However, the origin of charge carriers and their behavior in CuWO4 are not well understood due to the scarcity of the experimental studies that probe the relaxation dynamics of the optically excited Cu electronic states, particularly in the fs/ps time domain.15,16 Therefore, we believe that understanding the ultrafast electronic transitions in CuWO4 in this time domain could provide a clearer insight on the nature of the electronic structure of CuWO4, which is highly debatable in literature8,10,11,14,17
In this work, we conducted the first investigation of the ultrafast charge carrier dynamics in CuWO4 using time-resolved optical and X-ray spectroscopies in the 100 fs to 200 ps time scale with a focus on the role of the copper 3d states in the charge carrier transport process. Time-resolved X-ray absorption spectroscopy (XAS) provides detailed information on the ultrafast charge carrier dynamics and recombination in several metal oxide semiconductors.18−20 Charge carrier dynamics of water splitting semiconductors (TiO2,21−23 α-Fe2O3,24−27 BiVO4,28,29 WO3,30−32 ZnO,33 and CsPbBr3 and CsPb(ClBr)3 perovskites34) were successfully observed using ultrafast XAS. Herein, we present transient Cu L3 XAS studies on the photoexcited state of CuWO4. Cu L3 XAS is attributed to electron transition from Cu 2p orbitals to Cu 3d orbitals, which reflects the chemical state of the Cu atoms directly. The optical laser excitation triggers a transition from the oxygen 2p valence band to the W 5d conduction band.8 The empty Cu 3d state is not active in the optical transition and, as such, plays the role of a spectator state. During the X-ray excitation process, an electron is excited to the Cu 3d band and one can observe its reaction to the optical excitation. This role as a spectator state makes it attractive to study its response to the optical excitation, in order to determine the model of the electronic structure and the dynamics in electronic states of CuWO4.
2. Experiments
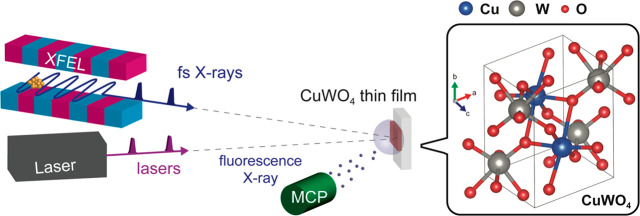
The CuWO4 sample was a polycrystalline thin film with a thickness of 50 nm synthesized using pulsed laser deposition (PLD) supported on a conductive glass substrate (more details of the sample preparation and physical characterizations are described in the Supporting Information). Pump–probe Cu L3 XAS experiments were conducted in the soft X-ray spectroscopy and scattering (SSS) beamline at PAL-XFEL.35−37 A simple illustration of the experimental setup is shown in Figure 1. The sample was placed at the focal point of the X-rays (the focal size of the X-rays was less than 50 μm (H) × 50 μm (V)). The 400 nm optical pump laser was transferred to the focal position of the X-rays. The focal size of the optical laser was ∼210 μm (H) × 210 μm (V). XAS signals were obtained using a michrochannel plate (MCP) detector. A more detailed experimental setup is shown in Figure S3. For the observation of transient XAS at longer delay time (>80 ps), pump–probe experiments were conducted at BL07LSU, SPring-8.38 The detailed experimental setup at SPring-8 as well as other experimental details (optical pump-probe experiments and static Cu K-edge XAS) are described in the Supporting Information.
Figure 1.
Time-resolved X-ray absorption spectroscopy (XAS) on CuWO4: experimental setup of the pump–probe XAS in PAL-XFEL and the crystal structure of CuWO4.
The X-ray intensity fluctuates shot-by-shot in PAL-XFEL, and some X-ray shots have too large or too small intensities to obtain XAS signals. Therefore, such X-ray shots were removed from data sets since they worsen the signal to noise ratio (S/N). Details of analyzing the X-ray data to calculate XAS are described in Supporting Information. In addition to the shot-by-shot fluctuations, variations in the intensity of XFEL pulses were observed over several hours, which might affect the XAS signal and result in artificial differential spectra. To avoid these artifacts, the XAS at each delay time and those at the reference were measured in sequence. That is to say, a reference XAS spectrum at a delay time of −10 ps, i.e., an excitation laser pulse arrives at the sample 10 ps after an X-ray pulse arrives, was measured for each measurement in order to calculate a difference spectrum for each delay time. Each difference XAS spectrum (ΔXAS) at a delay (Δt) was calculated by subtracting XAS at −10 ps from XAS at Δt, i.e.,
At SPring-8, the laser pump repetition rate was set to half of the X-ray probe repetition rate. XAS signals with or without laser shots were accumulated consecutively.
3. Results
3.1. Transient Cu L3 XAS of CuWO4
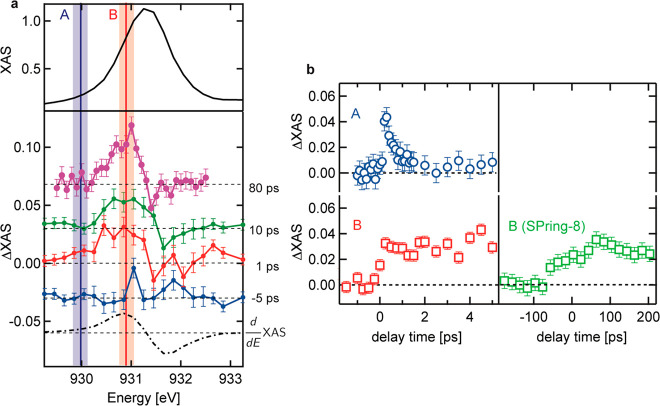
The transient XAS spectra at several delays are displayed in Figure 2. The difference XAS spectra at delays of −5 ps, 1 ps, and 10 ps were measured at PAL-XFEL, whereas the difference XAS spectrum at a delay of 80 ps was measured at SPring-8. After optical excitation, one observes an absorption increase at energies below the static L3 edge maximum of 931.4 eV and in addition there is a minor absorption decrease at energies above the L3 edge maximum. We note that the observed spectral changes do not agree with the first derivative of Cu L3 XAS (dashed line). Figure 2b (right panels) shows the kinetic traces of the L3 edge at 930.0 eV (position A) and 930.8 eV (position B). The kinetic trace at position B shows a rise related to the time resolution of the experiment followed by a constant intensity increase over the whole range of 5 ps. The absorption change at position B was measured up to 200 ps, but at a delay time of 200 ps, and was not recovered to its original ground state value at this time delay (Figure 2b, SPring-8). On the other hand, the kinetic trace of peak A (930.0 eV) decays on a much shorter time scale, where the kinetic constant of this fast process was found to be 400 ± 160 fs (Figure S4).
Figure 2.
(a) Normalized Cu L3 XAS (top panel) and the transient X-ray absorption spectroscopy (XAS) data of Cu L3 edge in CuWO4 at several delay times (−5, 1, 10, and 80 ps with respect to −10 ps) (bottom panel). The transient XAS spectra were normalized according to the reference Cu L3 XAS. (b) Kinetic traces of the Cu L3 edge at peaks A and B measured at PAL-XFEL and the kinetic trace of Cu L3 edge XAS at peak B for longer delay times measured at SPring-8. The intensities were normalized according to the reference Cu L3 XAS. The error bars for the transient XAS were estimated from the standard deviation of the transient XAS at a negative delay time. Due to shot-by-shot variations of the X-ray intensity, there remains a point-by-point error that can be larger than the standard deviation. The error bars for the kinetic traces were estimated from the standard deviation of the unpumped XAS intensity.
3.2. Pump–Probe Optical Spectroscopy and Transient Cu K-Edge XAS of CuWO4
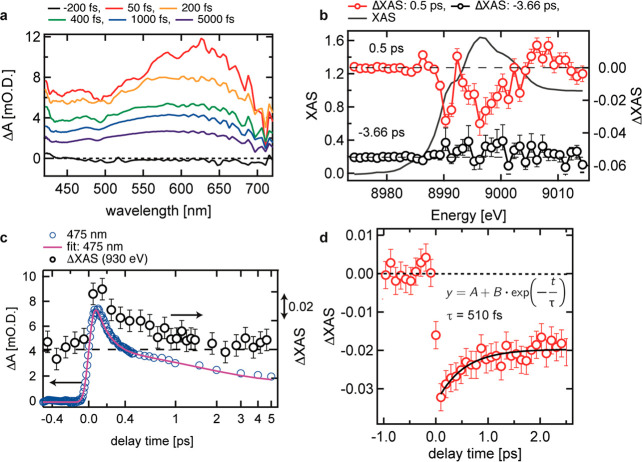
A similar fast decay process was also observed by optical pump–probe spectroscopy shown in Figure 3a and Figure 3c and by pump–probe Cu K-edge XAS conducted at SPring-8 angstrom compact free electron laser (SACLA)39−43 shown in Figure 3b and Figure 3d . In the optical spectroscopy study, a broad transient absorption was observed between 420 and 720 nm. This broad absorption should be mainly attributed to excited electrons distributed in the conduction band. Since excited electrons have different energies and transition to different final states, a broad absorption can be observed after photoexcitation. As described in Figure S2, there were four time constants extracted from a rate-equation model. The Cu K-edge XAS after photoexcitation is interpreted by the shift of the Cu K-edge XAS to lower energy. This result agrees with the increase of the electron density at the Cu sites. A fast kinetic process was observed at 8990.3 eV, which decays by a delay of 2 ps. The kinetic constant of this process was estimated to be 510 ± 150 fs. This value is consistent with the kinetic constant estimated by Cu L3 edge within the error limits. This excess decrease of the X-ray intensity could reflect the excess charge density at the Cu atoms in agreement with the Cu L3 edge data.
Figure 3.
(a) Transient optical absorbance of CuWO4 from 420 to 720 nm. (b) Transient Cu K-edge XAS of CuWO4 before photoexcitation (−3.66 ps) and after photoexcitation (0.5 ps). The difference spectra were normalized according to the Cu K-edge XAS in the optical ground state. (c) Kinetic trace of the transient optical absorbance of CuWO4 at 475 nm. (d) Kinetic trance of transient Cu K-edge XAS of CuWO4 at 8980 eV. The intensity was normalized according to the difference XAS shown in part b.
3.3. Interpretation of the Transient Cu L3 XAS
We first note that the peak position of Cu2+ in the Cu L3 XAS is lower than that of both Cu1+ and Cu3+ (for example, refs (44−46)), which is in contrast to all other transition metal ions. The reason is that the ground state of Cu2+ is 3d9 and the only allowed final state is 2p53d10; hence there is only a single peak observed at the L3 edge. The peak position of Cu3+ appears at higher energy position due to the larger effective charge of the copper ion. Also, the first L3 peak of Cu1+ appears at higher energy since the 3d band of Cu1+ is full and the intensity is given by the transition to a 4s orbital that forms an exciton.47 Interestingly neither a change to Cu1+ or Cu3+ will allow a peak at an energy lower than Cu2+. In other words, the spectral change at 930.0 eV cannot be assigned to change of the valence state of the copper ion.
Instead, we assign this feature to Cu2+ that has a modified local electronic structure. The optical excitation moves an electron from the oxygen 2p valence band to the tungsten 5d conduction band, while Cu2+ is essentially a spectator ion. Since the charge density of the copper s and p states is larger in the W 5d conduction band than in the oxygen 2p valence band, the effective charge density on the Cu2+ ions will increase creating a Cu2+ 3d9 ion that has enhanced electron density, which will shift the L3 edge to lower energy. This enhanced charge density is contained within band states with copper s and p character, and being a delocalized band state, this extra charge will rapidly delocalize, faster than the time constant of 400 fs. The change in charge distribution can affect the vibrational potential in the excited system due to electron–lattice coupling.48 Under such situation, low-frequency modes move toward this new origin with the modified interatomic distances and made additional modification to charge distribution. The experiment thus uses the copper L3 edge spectrum as an indicator of the local charge density changes (and phonon modes) on the copper site following optical laser excitation.
3.4. Photoexcitation Dynamics of CuWO4
Two main changes were observed by transient Cu L3 XAS upon optical excitation of CuWO4: (i) An initial photoexcited state is created by optical excitation, creating enhanced charge density at the copper sites, decaying with a time constant of 400 fs. The enhanced charge density is related to copper s and p states, and the valence remains Cu2+. (ii) The photoexcited state undergoes a decay process to form a metastable state that has a lifetime of more than 200 ps. The metastable state has increased charge density with respect to the optical ground state but reduced with respect to the initial excited state. This metastable state must involve structural reorganization of atoms related to a polaronic state while the valence remains Cu2+. It should be noted that local structural changes can also shift the Cu L3 edge XAS to lower energy; i.e., if the Cu–O distance is elongated by 10%, calculations show that the Cu L3 XAS shifts by ∼0.2 eV.49 The local structural change affects more directly the spectral features of the Cu K-edge XAS in higher energy, which is visible at ∼9000 eV at delays of 2.3 and 8.3 ps (see Figure S5). Local structural changes are related to the formation of polaronic states. Regarding the polaron state formation in CuWO4, Hoang et al. suggested that an electron hole should be more localized on the O atoms while an electron would be more localized on metal sites.14 Considering their results, we conclude that the effective negative charge on the O atoms would be decreased by +δ while the effective positive charge on W atoms and Cu atoms would also decrease by −δ, reducing the charge difference and thereby increasing the bond length.
4. Discussion
4.1. Model of the Electronic Structure of CuWO4
As mentioned above, the electronic structure of CuWO4 is still an open question and there have been models suggested by several authors. For instance, Tian et al.8 reported that the bottom of the conduction band of CuWO4 comes from Cu 3d orbitals while the top of the valence band is formed by Cu 3d orbitals and O 2p orbitals. Hoang et al.14 pointed out that O 2p orbitals are dominant in the valence band of CuWO4 while its conduction band is formed mainly by Cu 3d orbitals and W 5d orbitals. Khyzhun et al.11 suggested that the conduction band of CuWO4 is a W 5d-like state while its valence band is an O 2p-like state and they also assume the existence of Cu3+.10 Considering the previous reports and our transient XAS results, we assume a combined electronic structure of WO3-type structure with an oxygen 2p valence band and a W 5d conduction band. The 3d-electrons of the Cu2+ ion contribute to the oxygen 2p valence band, while its occupied 3d electrons are positioned at lower energies, in line with all other Cu2+ oxides that are charge transfer insulators.17
In order to discuss the electronic structure of CuWO4 in detail, we first introduce the electronic structure of the binary parent oxides WO3 and CuO. WO3 is a semiconductor with a valence band mainly composed of oxygen 2p character. The oxygen 2p state hybridizes strongly with the tungsten 5d, 6s, and 6p states that yield a considerable amount of W character in the valence band. The conduction band consists of the antibonding combinations of oxygen 2p states with tungsten 5d, 6s, and 6p states. For simplicity, this could be called the tungsten 5d band, but it also contains significant contributions of tungsten s and p character. The electronic structure of CuO is described as a charge transfer insulator,17 where the Cu 3d states are split between the lower Hubbard band below the oxygen 2p band and the empty upper Hubbard band that has one hole state.50 CuWO4 can be considered as a combination of both oxides. The (top of the) valence band contains mainly oxygen 2p character mixed with W 5d and sp states plus Cu 3d and sp states. The conduction band is dominated by W 5d but also contains oxygen 2p and copper 3d and sp states. In between, the upper Hubbard band of Cu 3d sits in the band gap (see Figure 4a).
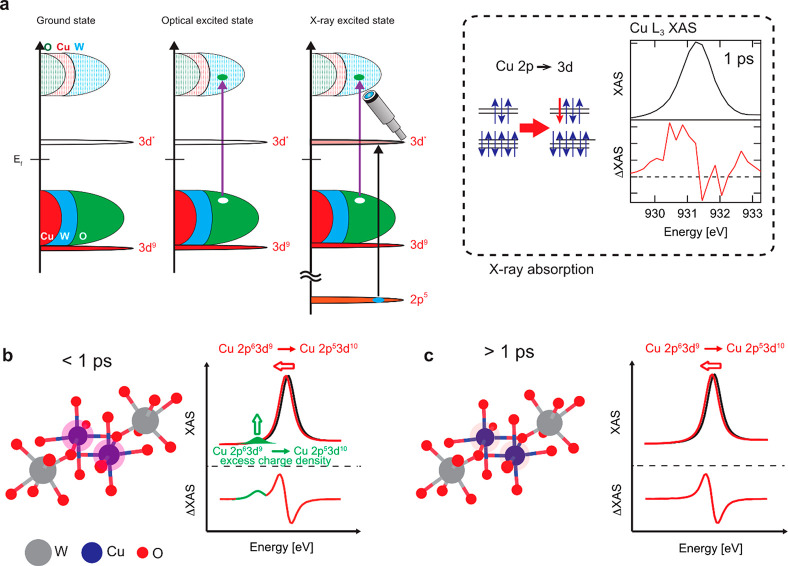
Figure 4.
(a) A sketch that explains the optical and X-ray excitation processes in CuWO4 and the Cu L3-edge transient XAS spectra at a delay time of 10 ps. Illustrations of the charge density at Cu sites in CuWO4 after photoexcitation below a delay of 1 ps (b) and above a delay of 1 ps (c).
4.2. The 400 fs Metastable Charge Density Modulation
The laser excitation of CuWO4 involves a transition from the oxygen 2p valence band to the tungsten 5d conduction band. This creates a ligand hole plus an electron in the W 5d band. The Cu 3d states are spectators to this laser excitation since the Cu 3d states are not influenced by the photoexcitation as described (section 3.3). The electronic structure of the copper sites is however affected because the excited electron in the conduction band has partly copper 4s/4p character. This additional charge at the Cu site will decrease the binding energy of the 2p core states, following the general rule that the lower is the number of electrons at a metal site (i.e., a higher valency) the higher is its binding energy. Thus, the laser excitation creates a “spectator” copper site with a lower binding energy of its 2p core states.
The Cu 2p XAS spectrum of CuWO4 shows essentially one sharp peak and at higher energy some weaker intensity. The sharp peak is related to the 3d9 → 2p53d10 transition. The laser excited CuWO4 sample will also have a single 3d9 → 2p53d10 transition, but due to the reduced binding energy of the 2p core state, this transition will be shifted to lower excitation energy. The small absorption appears due to this shifted 3d9 → 2p53d10 transition (the green small peak in Figure 4b). In other words, we can track the charge density change of the Cu spectator state by measuring the time evolution of the transient signal at an excitation energy below the copper L3 edge (see Figure 4b and Figure 4c). The Cu state with the modified charge density has a lifetime of only 400 fs. The 400 nm laser excites the electrons ∼3.1 eV up in energy, and with a band gap of 2.3 eV they are excited in the bottom 0.8 eV of the conduction band. The corresponding modified charge density can lose energy via carrier cooling and electronic decay channels to the lowest states of the conduction band. Potentially this electronic picture could be modified by phonons affecting the charge density on copper via a changing Cu–O distance.
4.3. Effect on Photocatalysis
The observations above provide insights on the photocatalytic behavior of CuWO4 compared to WO3 and CuO. Adding Cu to WO3 modifies the band gap of the system, making it capable of absorbing optical light in the visible range of the solar spectrum which enhances its photocatalytic performance. The system still requires a large overpotential to drive photocatalytic processes such as water splitting. This is possibly due to the formation of a polaronic state. The initial optical excitation creates a modified charge density that is viewed from the Cu site in our study. After the decay of the original optical excitation within 400 fs, the system does not return to its original state, which is a clear indication of the formation of a polaronic state. It is believed that polarons are formed due to lattice distortion around the light-absorbing atom, resulting in trapping and hence reducing the mobility of electrons, which effects the photocatalytic efficiency of the system. The formation of this polaronic state has been observed in several metal oxide systems.26,51,52
Furthermore, it is important to note that in order to create an effective photocatalyst, one needs to optimize the system of photon capture, which is mainly governed by the band gap. The latter is determined by the overlap between tungsten and oxygen. Copper remains Cu2+ throughout the whole process of the optical excitation and the 400 fs decay. In that sense the Cu state is described as a spectator that is not redox active but rather creates a better (electronic and structural) environment for the WO3 system that receives the optical photon.
Concluding Remarks
In summary, by tracking a 50 fs, 3.1 eV optical laser excitation in CuWO4 via a pump–probe delayed X-ray excitation 1.0 eV before the Cu L3 edge, we have identified an increased charge density at the Cu site that has a decay time of 400 fs. This fast decay implies that the modified charge density decays electronically via carrier cooling to a longer-lived electronic excited state. It would be very interesting to investigate if this type of charge modulation with decay times of a few hundred femtoseconds is a general phenomenon in transition metal oxides and if this phenomenon is correlated with photocatalytic activity that can be modified by sample preparation and by the parameters in the laser excitation.
Acknowledgments
This work was financially supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreement 340279), The Netherlands Center for Multiscale Catalytic Energy Conversion (MCEC), a Gravitation Program from The Netherlands Organisation for Scientific Research (NWO), a grant for collaborative research in the Institute for Catalysis, Hokkaido University (Grant 18A1005), a Grant-in-Aid for Scientific Research (A) (Grant 15H02173, JSPS), and a basic science research program funded by the Ministry of Education of Korea (Grants NRF-2020R1A2C1007416 and 2018R1D1A1B07046676). N. Huse and N. Höppel acknowledge funding by the collaborative research center SFB 925 of the German Science Foundation (DFG), project 170620586. The experiment at SACLA was performed with an approval of Japan Synchrotron Radiation Research Institute (JASRI; Proposal 2018A8049). We thank Prof. Thomas Elsasser (Max-Born Institute/Humboldt Universität zu Berlin) and Prof. Kiyotaka Asakura (Hokkaido University) for useful comments and suggestions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcc.0c10525.
Physical characterizations of the CuWO4 thin films (SEM, XRD, XPS, AFM, UV–Vis spectrum, and a Tauc plot), details of the transient optical spectroscopy, experimental details of transient XAS spectroscopy (PAL-XFEL, SPring-8 and SACLA), and supplemental results of transient XAS at SACLA (PDF)
Author Present Address
◆ Y.U.: Laboratory for Environmental Chemistry (LUC), Energy and Environmental Research Division (ENE), Paul Scherrer Institute PSI, Forschungsstrasse 111, 5232 Villigen, Switzerland.
Author Present Address
∞ Y.Z.: Institute of High Energy Physics, Chinese Academy of Sciences, Yuquan Road 19B, Shijingshan District, Beijing, 100049, China.
Author Contributions
△ Y.U. and A.S.M.I. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Momeni M. M. Fabrication of copper decorated tungsten oxide–titanium oxide nanotubes by photochemical deposition technique and their photocatalytic application under visible light. Appl. Surf. Sci. 2015, 357, 160–166. 10.1016/j.apsusc.2015.09.015. [DOI] [Google Scholar]
- Lhermitte C. R.; Bartlett B. M. Advancing the Chemistry of CuWO4 for Photoelectrochemical Water Oxidation. Acc. Chem. Res. 2016, 49, 1121–1129. 10.1021/acs.accounts.6b00045. [DOI] [PubMed] [Google Scholar]
- Valenti M.; Dolat D.; Biskos G.; Schmidt-Ott A.; Smith W. A. Enhancement of the Photoelectrochemical Performance of CuWO4 Thin Films for Solar Water Splitting by Plasmonic Nanoparticle Functionalization. J. Phys. Chem. C 2015, 119, 2096–2104. 10.1021/jp506349t. [DOI] [Google Scholar]
- Yourey J. E.; Pyper K. J.; Kurtz J. B.; Bartlett B. M. Chemical Stability of CuWO4 for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2013, 117, 8708–8718. 10.1021/jp402048b. [DOI] [Google Scholar]
- Hill J. C.; Ping Y.; Galli G. A.; Choi K.-S. Synthesis, photoelectrochemical properties, and first principles study of n-type CuW1–xMoxO4 electrodes showing enhanced visible light absorption. Energy Environ. Sci. 2013, 6, 2440–2446. 10.1039/c3ee40827b. [DOI] [Google Scholar]
- Benko F. A.; MacLaurin C. L.; Koffyberg F. P. CuWO4 and Cu3WO6 as anodes for the photoelectrolysis of water. Mater. Res. Bull. 1982, 17, 133–136. 10.1016/0025-5408(82)90194-5. [DOI] [Google Scholar]
- Schofield P. F.; Knight K. S.; Redfern S. A. T.; Cressey G. Distortion Characteristics Across the Structural Phase Transition in (Cu1–xZnx)WO4. Acta Crystallogr., Sect. B: Struct. Sci. 1997, 53, 102–112. 10.1107/S0108768196010403. [DOI] [Google Scholar]
- Tian C. M.; Jiang M.; Tang D.; Qiao L.; Xiao H. Y.; Oropeza F. E.; Hofmann J. P.; Hensen E. J. M.; Tadich A.; Li W.; et al. Elucidating the electronic structure of CuWO4 thin films for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 2019, 7, 11895–11907. 10.1039/C8TA12070F. [DOI] [Google Scholar]
- Kihlborg L.; Gebert E. CuWO4, a distorted Wolframite-type structure. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1970, 26, 1020–1026. 10.1107/S0567740870003515. [DOI] [Google Scholar]
- Khyzhun O. Y.; Strunskus T.; Cramm S.; Solonin Y. M. Electronic structure of CuWO4: XPS, XES and NEXAFS studies. J. Alloys Compd. 2005, 389, 14–20. 10.1016/j.jallcom.2004.08.013. [DOI] [Google Scholar]
- Khyzhun O. Y.; Bekenev V. L.; Solonin Y. M. First-principles calculations and X-ray spectroscopy studies of the electronic structure of CuWO4. J. Alloys Compd. 2009, 480, 184–189. 10.1016/j.jallcom.2009.01.119. [DOI] [Google Scholar]
- Lalić M. V.; Popović Z. S.; Vukajlović F. R. Ab initio study of electronic, magnetic and optical properties of CuWO4 tungstate. Comput. Mater. Sci. 2011, 50, 1179–1186. 10.1016/j.commatsci.2010.11.018. [DOI] [Google Scholar]
- Gaillard N.; Chang Y.; Braun A.; DeAngelis A. Copper Tungstate (CuWO4)-Based Materials for Photoelectrochemical Hydrogen Production. MRS Proc. 2012, 1446, 31–36. 10.1557/opl.2012.952. [DOI] [Google Scholar]
- Hoang K.; Oh M.; Choi Y. Electronic structure, polaron formation, and functional properties in transition-metal tungstates. RSC Adv. 2018, 8, 4191–4196. 10.1039/C7RA13436C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J.; Müller S.; Peeters D.; Sadlo A.; Mai L.; Reyes O. M.; Friedrich D.; Mitoraj D.; Devi A.; Beranek R.; et al. Comparative Study of Photocarrier Dynamics in CVD-deposited CuWO4, CuO, and WO3 Thin Films for Photoelectrocatalysis. Z. Phys. Chem. (Muenchen, Ger.) 2020, 234, 699–717. 10.1515/zpch-2019-1485. [DOI] [Google Scholar]
- Peeters D.; Mendoza Reyes O.; Mai L.; Sadlo A.; Cwik S.; Rogalla D.; Becker H. W.; Schütz H. M.; Hirst J.; Müller S.; et al. CVD-grown copper tungstate thin films for solar water splitting. J. Mater. Chem. A 2018, 6, 10206–10216. 10.1039/C7TA10759E. [DOI] [Google Scholar]
- Zaanen J.; Sawatzky G. A.; Allen J. W. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 1985, 55, 418–421. 10.1103/PhysRevLett.55.418. [DOI] [PubMed] [Google Scholar]
- Kraus P. M.; Zürch M.; Cushing S. K.; Neumark D. M.; Leone S. R. The ultrafast X-ray spectroscopic revolution in chemical dynamics. Nat. Rev. Chem. 2018, 2, 82–94. 10.1038/s41570-018-0008-8. [DOI] [Google Scholar]
- Chergui M.; Collet E. Photoinduced Structural Dynamics of Molecular Systems Mapped by Time-Resolved X-ray Methods. Chem. Rev. (Washington, DC, U. S.) 2017, 117, 11025–11065. 10.1021/acs.chemrev.6b00831. [DOI] [PubMed] [Google Scholar]
- Milne C. J.; Penfold T. J.; Chergui M. Recent experimental and theoretical developments in time-resolved X-ray spectroscopies. Coord. Chem. Rev. 2014, 277–278, 44–68. 10.1016/j.ccr.2014.02.013. [DOI] [Google Scholar]
- Obara Y.; Ito H.; Ito T.; Kurahashi N.; Thürmer S.; Tanaka H.; Katayama T.; Togashi T.; Owada S.; Yamamoto Y.-i.; et al. Femtosecond time-resolved X-ray absorption spectroscopy of anatase TiO2 nanoparticles using XFEL. Struct. Dyn. 2017, 4, 044033 10.1063/1.4989862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santomauro F. G.; Lübcke A.; Rittmann J.; Baldini E.; Ferrer A.; Silatani M.; Zimmermann P.; Grübel S.; Johnson J. A.; Mariager S. O.; et al. Femtosecond X-ray absorption study of electron localization in photoexcited anatase TiO2. Sci. Rep. 2015, 5, 14834. 10.1038/srep14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmann-Frank M. H.; Milne C. J.; Rittmann J.; Reinhard M.; Penfold T. J.; Chergui M. Mapping of the Photoinduced Electron Traps in TiO2 by Picosecond X-ray Absorption Spectroscopy. Angew. Chem., Int. Ed. 2014, 53, 5858–5862. 10.1002/anie.201310522. [DOI] [PubMed] [Google Scholar]
- Ismail A. S. M.; Uemura Y.; Park S. H.; Kwon S.; Kim M.; Elnaggar H.; Frati F.; Niwa Y.; Wadati H.; Hirata Y.; et al. Direct observation of the electronic states of photoexcited hematite with ultrafast 2p3d X-ray absorption spectroscopy and resonant inelastic X-ray scattering. Phys. Chem. Chem. Phys. 2020, 22, 2685–2692. 10.1039/C9CP03374B. [DOI] [PubMed] [Google Scholar]
- Leshchev D.; Harlang T. C. B.; Fredin L. A.; Khakhulin D.; Liu Y.; Biasin E.; Laursen M. G.; Newby G. E.; Haldrup K.; Nielsen M. M.; et al. Tracking the picosecond deactivation dynamics of a photoexcited iron carbene complex by time-resolved X-ray scattering. Chem. Sci. 2018, 9, 405–414. 10.1039/C7SC02815F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro L. M.; Cushing S. K.; Liu C.; Su Y.; Yang P.; Alivisatos A. P.; Leone S. R. Excitation-wavelength-dependent small polaron trapping of photoexcited carriers in α-Fe2O3. Nat. Mater. 2017, 16, 819–825. 10.1038/nmat4936. [DOI] [PubMed] [Google Scholar]
- Vura-Weis J.; Jiang C.-M.; Liu C.; Gao H.; Lucas J. M.; de Groot F. M. F.; Yang P.; Alivisatos A. P.; Leone S. R. Femtosecond M2,3-Edge Spectroscopy of Transition-Metal Oxides: Photoinduced Oxidation State Change in α-Fe2O3. J. Phys. Chem. Lett. 2013, 4, 3667–3671. 10.1021/jz401997d. [DOI] [Google Scholar]
- Uemura Y.; Kido D.; Koide A.; Wakisaka Y.; Niwa Y.; Nozawa S.; Ichiyanagi K.; Fukaya R.; Adachi S.-i.; Katayama T.; et al. Capturing local structure modulations of photoexcited BiVO4 by ultrafast transient XAFS. Chem. Commun. 2017, 53, 7314–7317. 10.1039/C7CC02201H. [DOI] [PubMed] [Google Scholar]
- Ravensbergen J.; Abdi F. F.; van Santen J. H.; Frese R. N.; Dam B.; van de Krol R.; Kennis J. T. M. Unraveling the Carrier Dynamics of BiVO4: A Femtosecond to Microsecond Transient Absorption Study. J. Phys. Chem. C 2014, 118, 27793–27800. 10.1021/jp509930s. [DOI] [Google Scholar]
- Koide A.; Uemura Y.; Kido D.; Wakisaka Y.; Takakusagi S.; Ohtani B.; Niwa Y.; Nozawa S.; Ichiyanagi K.; Fukaya R.; et al. Photoinduced anisotropic distortion as the electron trapping site of tungsten trioxide by ultrafast W L1-edge X-ray absorption spectroscopy with full potential multiple scattering calculations. Phys. Chem. Chem. Phys. 2020, 22, 2615–2621. 10.1039/C9CP01332F. [DOI] [PubMed] [Google Scholar]
- Uemura Y.; Kido D.; Wakisaka Y.; Uehara H.; Ohba T.; Niwa Y.; Nozawa S.; Sato T.; Ichiyanagi K.; Fukaya R.; et al. Dynamics of Photoelectrons and Structural Changes of Tungsten Trioxide Observed by Femtosecond Transient XAFS. Angew. Chem., Int. Ed. 2016, 55, 1364–1367. 10.1002/anie.201509252. [DOI] [PubMed] [Google Scholar]
- Uemura Y.; Uehara H.; Niwa Y.; Nozawa S.; Sato T.; Adachi S.; Ohtani B.; Takakusagi S.; Asakura K. In Situ Picosecond XAFS Study of an Excited State of Tungsten Oxide. Chem. Lett. 2014, 43, 977–979. 10.1246/cl.140144. [DOI] [Google Scholar]
- Penfold T. J.; Szlachetko J.; Santomauro F. G.; Britz A.; Gawelda W.; Doumy G.; March A. M.; Southworth S. H.; Rittmann J.; Abela R.; et al. Revealing hole trapping in zinc oxide nanoparticles by time-resolved X-ray spectroscopy. Nat. Commun. 2018, 9, 478. 10.1038/s41467-018-02870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santomauro F. G.; Grilj J.; Mewes L.; Nedelcu G.; Yakunin S.; Rossi T.; Capano G.; Al Haddad A.; Budarz J.; Kinschel D.; et al. Localized holes and delocalized electrons in photoexcited inorganic perovskites: Watching each atomic actor by picosecond X-ray absorption spectroscopy. Struct. Dyn. 2017, 4, 044002 10.1063/1.4971999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H.; Kim H.-D.; Kim M.; Park S. H.; Kwon S.; Lee J. Y.; Park S.-Y.; Park G.; Kim S.; Hyun H.; et al. Time-resolved resonant elastic soft x-ray scattering at Pohang Accelerator Laboratory X-ray Free Electron Laser. Rev. Sci. Instrum. 2020, 91, 083904 10.1063/5.0016414. [DOI] [PubMed] [Google Scholar]
- Park S. H.; Kim M.; Min C.-K.; Eom I.; Nam I.; Lee H.-S.; Kang H.-S.; Kim H.-D.; Jang H. Y.; Kim S.; et al. PAL-XFEL soft X-ray scientific instruments and X-ray optics: First commissioning results. Rev. Sci. Instrum. 2018, 89, 055105 10.1063/1.5023557. [DOI] [PubMed] [Google Scholar]
- Kang H.-S.; Min C.-K.; Heo H.; Kim C.; Yang H.; Kim G.; Nam I.; Baek S. Y.; Choi H.-J.; Mun G.; et al. Hard X-ray free-electron laser with femtosecond-scale timing jitter. Nat. Photonics 2017, 11, 708–713. 10.1038/s41566-017-0029-8. [DOI] [Google Scholar]
- Takubo K.; Yamamoto K.; Hirata Y.; Yokoyama Y.; Kubota Y.; Yamamoto S.; Yamamoto S.; Matsuda I.; Shin S.; Seki T.; et al. Capturing ultrafast magnetic dynamics by time-resolved soft x-ray magnetic circular dichroism. Appl. Phys. Lett. 2017, 110, 162401. 10.1063/1.4981769. [DOI] [Google Scholar]
- Katayama T.; Nozawa S.; Umena Y.; Lee S.; Togashi T.; Owada S.; Yabashi M. A versatile experimental system for tracking ultrafast chemical reactions with X-ray free-electron lasers. Struct. Dyn. 2019, 6, 054302 10.1063/1.5111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T.; Hirano T.; Morioka Y.; Sano Y.; Osaka T.; Owada S.; Togashi T.; Yabashi M. X-ray optics for advanced ultrafast pump-probe X-ray experiments at SACLAThis article will form part of a virtual special issue on X-ray free-electron lasers. J. Synchrotron Radiat. 2019, 26, 333–338. 10.1107/S1600577518018362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T.; Owada S.; Togashi T.; Ogawa K.; Karvinen P.; Vartiainen I.; Eronen A.; David C.; Sato T.; Nakajima K.; et al. A beam branching method for timing and spectral characterization of hard X-ray free-electron lasers. Struct. Dyn. 2016, 3, 034301 10.1063/1.4939655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tono K.; Togashi T.; Inubushi Y.; Sato T.; Katayama T.; Ogawa K.; Ohashi H.; Kimura H.; Takahashi S.; Takeshita K.; et al. Beamline, experimental stations and photon beam diagnostics for the hard x-ray free electron laser of SACLA. New J. Phys. 2013, 15, 083035 10.1088/1367-2630/15/8/083035. [DOI] [Google Scholar]
- Ishikawa T.; Aoyagi H.; Asaka T.; Asano Y.; Azumi N.; Bizen T.; Ego H.; Fukami K.; Fukui T.; Furukawa Y.; et al. A compact X-ray free-electron laser emitting in the sub-ångström region. Nat. Photonics 2012, 6, 540–544. 10.1038/nphoton.2012.141. [DOI] [Google Scholar]
- Huang M. J.; Deng G.; Chin Y. Y.; Hu Z.; Cheng J. G.; Chou F. C.; Conder K.; Zhou J. S.; Pi T. W.; Goodenough J. B.; et al. Determination of hole distribution in Sr14-xCaxCu24O41 using soft x-ray absorption spectroscopy at the Cu L3 edge. Phys. Rev. B: Condens. Matter Mater. Phys. 2013, 88, 014520 10.1103/PhysRevB.88.014520. [DOI] [Google Scholar]
- Choudhury D.; Rivero P.; Meyers D.; Liu X.; Cao Y.; Middey S.; Whitaker M. J.; Barraza-Lopez S.; Freeland J. W.; Greenblatt M.; et al. Anomalous charge and negative-charge-transfer insulating state in cuprate chain compound KCuO2. Phys. Rev. B: Condens. Matter Mater. Phys. 2015, 92, 201108. 10.1103/PhysRevB.92.201108. [DOI] [Google Scholar]
- Wang Y.; Lany S.; Ghanbaja J.; Fagot-Revurat Y.; Chen Y. P.; Soldera F.; Horwat D.; Mücklich F.; Pierson J. F. Electronic structures of Cu2O, Cu4O3, and CuO: A joint experimental and theoretical study. Phys. Rev. B: Condens. Matter Mater. Phys. 2016, 94, 245418. 10.1103/PhysRevB.94.245418. [DOI] [Google Scholar]
- Grioni M.; Goedkoop J. B.; Schoorl R.; de Groot F. M. F.; Fuggle J. C.; Schäfers F.; Koch E. E.; Rossi G.; Esteva J. M.; Karnatak R. C. Studies of copper valence states with Cu L3 x-ray absorption spectroscopy. Phys. Rev. B: Condens. Matter Mater. Phys. 1989, 39, 1541–1545. 10.1103/PhysRevB.39.1541. [DOI] [PubMed] [Google Scholar]
- Ruiz-Fuertes J.; Segura A.; Rodríguez F.; Errandonea D.; Sanz-Ortiz M. N. Anomalous High-Pressure Jahn-Teller Behavior in CuWO4. Phys. Rev. Lett. 2012, 108, 166402. 10.1103/PhysRevLett.108.166402. [DOI] [PubMed] [Google Scholar]
- Ye X.; Schmidt J. E.; Wang R.-P.; van Ravenhorst I. K.; Oord R.; Chen T.; de Groot F.; Meirer F.; Weckhuysen B. M. Deactivation of Cu-Exchanged Automotive-Emission NH3-SCR Catalysts Elucidated with Nanoscale Resolution Using Scanning Transmission X-ray Microscopy. Angew. Chem. 2020, 132, 15740–15747. 10.1002/ange.201916554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyżyk M. T.; Sawatzky G. A. Local-density functional and on-site correlations: The electronic structure of La2CuO4 and LaCuO3. Phys. Rev. B: Condens. Matter Mater. Phys. 1994, 49, 14211–14228. 10.1103/PhysRevB.49.14211. [DOI] [PubMed] [Google Scholar]
- Mohamed M.; May M. M.; Kanis M.; Brützam M.; Uecker R.; van de Krol R.; Janowitz C.; Mulazzi M. The electronic structure and the formation of polarons in Mo-doped BiVO4 measured by angle-resolved photoemission spectroscopy. RSC Adv. 2019, 9, 15606–15614. 10.1039/C9RA01092K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor J.; Ambrosio F.; Pasquarello A. Role of Polarons in Water Splitting: The Case of BiVO4. ACS Energy Lett. 2018, 3, 1693–1697. 10.1021/acsenergylett.8b00938. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.