Abstract
Objective
Single-use duodenoscopes have been recently developed to eliminate risk of infection transmission from contaminated reusable duodenoscopes. We compared performances of single-use and reusable duodenoscopes in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP).
Design
Patients with native papilla requiring ERCP were randomised to single-use or reusable duodenoscope. Primary outcome was comparing number of attempts to achieve successful cannulation of desired duct. Secondary outcomes were technical performance that measured duodenoscope manoeuvrability, mechanical-imaging characteristics and ability to perform therapeutic interventions, need for advanced cannulation techniques or cross-over to alternate duodenoscope group to achieve ductal access and adverse events.
Results
98 patients were treated using single-use (n=48) or reusable (n=50) duodenoscopes with >80% graded as low-complexity procedures. While median number of attempts to achieve successful cannulation was significantly lower for single-use cohort (2 vs 5, p=0.013), ease of passage into stomach (p=0.047), image quality (p<0.001), image stability (p<0.001) and air–water button functionality (p<0.001) were significantly worse. There was no significant difference in rate of cannulation, adverse events including mortality (one patient in each group), need to cross-over or need for advanced cannulation techniques to achieve ductal access, between cohorts. On multivariate logistic regression analysis, only duodenoscope type (single-use) was associated with less than six attempts to achieve selective cannulation (p=0.012), when adjusted for patient demographics, procedural complexity and type of intervention.
Conclusion
Given the overall safety profile and similar technical performance, single-use duodenoscopes represent an alternative to reusable duodenoscopes for performing low-complexity ERCP procedures in experienced hands.
Trial registration number
Clinicaltrials.gov number: NCT04143698
Keywords: therapeutic endoscopy, endoscopic retrograde pancreatography
Significance of this study.
What is already known about this subject?
Single-use duodenoscopes have been recently developed to eliminate the risk of infection transmission from contaminated reusable duodenoscopes. However, there are currently no studies comparing the performance of single-use and reusable duodenoscopes.
What are the new findings?
In this randomised trial, the overall technical performance and safety profile were similar between single-use and reusable duodenoscopes.
However, while there was no significant difference in cannulation rates, the median number of attempts to achieve successful cannulation was significantly lower for single-use duodenoscopes.
How might it impact on clinical practice in the foreseeable future?
Given the overall safety profile and similar technical performance, single-use duodenoscopes represent an alternative to reusable duodenoscopes for performing low-complexity ERCP procedures in experienced hands.
Introduction
Outbreaks of duodenoscope-related infection have been reported with pathogenic organisms that include Klebsiella pneumoniae, Pseudomonas aeruginosa and more recently carbapenem-resistant Enterobacteriaceae (CRE).1 2 Unlike a gastroscope or colonoscope, the duodenoscope is a complex instrument with unique mechanical features incorporated at the distal tip. This includes a recessed space containing an elevator, a wire cable that moves the elevator, working channel, and most recently, a seal that prevents contamination of the elevator wire channel. This complex design creates hard-to-reach areas that make optimal mechanical cleaning and disinfection difficult. Persistent bacterial growth in duodenoscopes allows the development of biofilm that protects microorganisms from gas or liquid disinfection.3 Only the prevention or complete removal of a biofilm can prevent infection transmission from the duodenoscope. While it has been presumed that following manufacturers’ reprocessing instructions or following high-level disinfection or sterilisation practices will eliminate the risk for infection transmission during endoscopic retrograde cholangiopancreatography (ERCP), recent evidence suggests otherwise.4 5 The United States Food and Drug Administration (FDA) postmarket surveillance communication reported duodenoscope culture results demonstrating contamination rates of up to 3.6% for low and moderate-concern organisms and up to 5.4% for high-concern organisms in reprocessed reusable duodenoscopes.6 Consequently, the FDA has mandated that endoscope manufacturers transition away from fixed endcap duodenoscopes to those with features that significantly improve cleaning and disinfection or eliminate the need for reprocessing altogether.
A single-use duodenoscope has been developed in the USA and approved by the FDA for clinical use in December 2019. While this new design should reduce or eliminate transmission of infection from the duodenoscope, if the functionality is suboptimal, it may simply create a trade-off to reduce one complication while increasing others. It is therefore critically important to ascertain whether the newly developed duodenoscopes function equally well, or perhaps better, than the older, reusable duodenoscopes.
The primary objective of this randomised trial was to evaluate performance of the single-use duodenoscope by comparing the number of attempts to achieve successful cannulation of the desired duct between the single-use and reusable duodenoscopes. Secondary outcomes were technical performance, need for advanced cannulation techniques or cross-over to the alternate duodenoscope cohort to achieve ductal access, time to achieve cannulation, total procedural duration and adverse events.
Methods
Participants
After approval of the study by the Institutional Review Board (IRB) at our institution (Approval No. 1388902), consecutive patients with symptomatic pancreatic–biliary disorders requiring an ERCP were recruited from the inpatient ward service or outpatient referrals. Written informed consents were obtained from all patients in conjunction with IRB approval prior to enrolment in the study and the procedure selection criteria are shown in box 1. Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy. All authors had full access to the study data and have reviewed and approved the final manuscript.
Box 1. Patient selection criteria.
Inclusion criteria
Age ≥18 years
Biliary or pancreatic duct disorder, based on clinical symptoms and radiological findings at CT or magnetic resonance cholangiopancreatography
Native papilla
Exclusion criteria
Age <18 years
Pregnancy
Altered upper GI surgical anatomy
Patients with percutaneous transhepatic biliary drainage catheters
Prior history of ERCP
Inability to provide informed consent
Randomisation and masking
Patients were enroled in this clinical trial by interventional endoscopists who evaluated the study subjects in the inpatient wards or preprocedure consultation rooms. Computer-generated randomisation assignments were provided by the statistician using a block randomisation method and placed in sequentially numbered, sealed, opaque envelopes. Once the inclusion criteria were met, the randomisation envelope was opened by one of the study investigators just prior to the procedure to determine treatment allocation and patients were randomised equally (1:1 allocation) to both treatment arms. Given the differences in the type of duodenoscopes used, endoscopists performing the procedure and assessing its performance could not be blinded to the allocation and therefore, the outcome measure assessments. However, a dedicated research coordinator was allocated to perform only follow-up phone calls for adverse events. This coordinator had information only on patient demographics including contact details but not information on the type of duodenoscope used, interventions undertaken or access to procedure-related case report forms. The follow-up information was transcribed to the electronic database only at the time of data analysis, after conclusion of the clinical trial.
Procedures
All ERCPs were performed using a single-use or reusable duodenoscope under general anaesthesia by one of three endoscopists (JYB, RH, SV) with a lifetime experience of performing >2000 procedures. The procedural steps performed during the ERCP were left to the discretion of the endoscopist, depending on the procedure indication. Rectal indomethacin was administered in all patients intraprocedurally and prophylactic intravenous antibiotics were administered as needed per established criteria.7
Single-use duodenoscope
The single-use duodenoscope used in this study was EXALT Model D (Boston Scientific Corporation, Marlborough, Massachusetts, USA), which is lightweight, made of recyclable plastic and has a four-way bending capability at the distal tip with guidewire locking capability for the elevator (made of titanium). An image capture button on the scope handle records pictures that can be integrated within endoscopy reports. The working length of the duodenoscope is 1240 mm, with insertion tube outer diameter of 11.3 mm, working channel inner diameter of 4.2 mm, up–down angulation of 120°–90° and right–left range of 110°–90°. The duodenoscope is delivered in a completely sterile package that is opened only prior to use. As with reusable duodenoscopes, the water bottle and suction are connected to dedicated ports. The duodenoscope is plugged into a dedicated EXALT processor (Boston Scientific Corporation), which provides the operational power (figure 1).
Figure 1.

The EXALT Model D (Boston scientific Corporation, Marlborough, Massachusetts, USA) single-use duodenoscope with EXALT processor.
Reusable duodenoscope
The reusable duodenoscopes used in this study were Olympus TJF-180 (Olympus America Inc, Center Valley, Pennsylvania, USA). The working length of the duodenoscope is 1240 mm, with insertion tube outer diameter of 11.3 mm, working channel diameter of 4.2 mm and is equipped with narrow band imaging. The four-way angulation (120° up, 90° down, 110° right and 90° left) facilitates approach to the papilla of Vater. The forceps elevator has a locking mechanism to secure guidewires. The duodenoscope is compatible with the Olympus CV-160 and 140 processors (Olympus America Inc).
Patient follow-up
All patient follow-up was obtained by telephone calls at 7 and 30 days after ERCP by research coordinators who were blinded to the type of duodenoscope used.
Definitions
Successful cannulation was defined as the ability to achieve deep access to the desired ductal system. Cannulation time was defined as duration from first contact of the papilla using a wire-guided sphincterotome until ductal access was achieved. Total procedure duration was measured from the time the duodenoscope was passed into the oesophageal lumen until the end of the ERCP procedure. Advanced cannulation technique was defined as manoeuvres required to achieve deep ductal access when standard wire-guided cannulation failed. Cross-over was defined as the need to change to the alternate duodenoscope type when requisite interventions could not be performed successfully using the assigned duodenoscope. Technical performance measured duodenoscope manoeuvrability, mechanical-imaging characteristics and the ability to perform therapeutic interventions based on a recently developed assessment tool (online supplemental file 1).8 ERCP procedural complexity and adverse events were categorised based on the American Society for Gastrointestinal Endoscopy (ASGE) criteria.9–11
gutjnl-2020-321836supp001.pdf (62.4KB, pdf)
Outcome measures
The main outcome measure was to compare the total number of attempts required to achieve successful cannulation of the desired duct. In order to minimise the risk of bias, an attempt at cannulation was defined as a sustained contact with the papilla for at least 1 s. Secondary outcome measures were technical performance, need for advanced cannulation techniques, cross-over to the alternate duodenoscope group to achieve ductal access, time to cannulate, total procedural duration, adverse events (including procedure-related infections) and to identify factors associated with fewer than six attempts to achieve successful cannulation, which is a surrogate marker for lower risk of post-ERCP pancreatitis.12–14
Sample size calculation
The sample size was based on the mean number of attempts to achieve successful cannulation. A two-sided sample size calculation performed at 85% power and alpha 0.05 to detect a difference of one in the mean number of attempts at cannulation (5 for reusable vs 6 for single-use) and SD of 1.5 for both groups. We assumed that the reusable duodenoscopes may be functionally superior and hence the number of cannulation attempts may be lower compared with the single-use duodenoscope.12–15 This resulted in a sample size estimation of 42 patients for each group and hence was set at 48 patients per group to account for a 15% drop out rate (PASS 15 Power Analysis and Sample Size Software, NCSS, LLC, Kaysville, Utah, USA).
Statistical analysis
Continuous data were summarised as means with SD and medians with IQR and range and were compared using the Wilcoxon rank-sum test or Student’s t-test as indicated. Categorical data were summarised as frequencies with percentages and were compared using the χ2 or Fisher’s exact test as indicated. In order to identify factors associated with the outcome variable of fewer than six attempts at cannulation (which was in turn taken as surrogate marker for difficult cannulation and procedural difficulty), multiple logistic regression and reverse multivariate logistic regression analyses were performed.16 17 Clinically important predictor variables were utilised in the regression analyses, which included patient age (in years), gender (male vs female), race (Non-Caucasian vs Caucasian), ASGE grade of procedure difficulty (grades 2, 3 and 4 vs grade 1), procedure type (biliary vs non-biliary procedure) and duodenoscope type (single-use vs reusable duodenoscopes). A two-sided p value of 0.05 was used as the criterion for leaving the model. All analyses were performed using the intention-to-treat principle, with the analysis of the outcome data for all patients according to their originally assigned duodenoscope group, regardless of cross-over to the alternate arm or loss to follow-up.18 19 Statistical significance was determined at p<0.05 and two-sided p values were reported for comparison of all outcome measures. All statistical analyses were performed using Stata V.14 (Stata Corp, College Station, Texas, USA).
Results
Study enrolment and termination
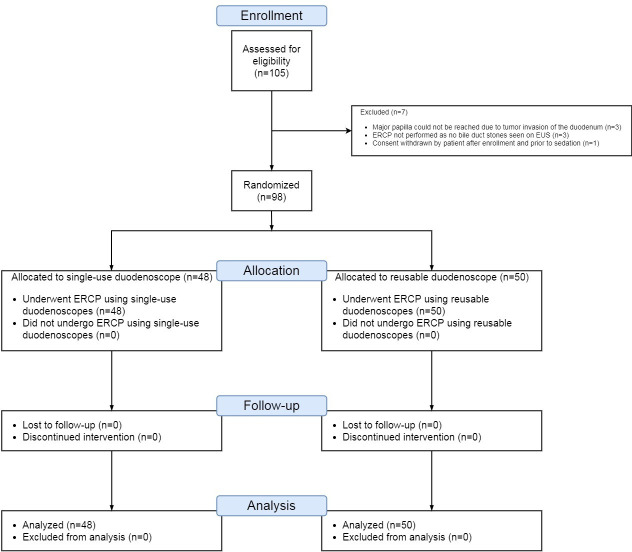
Between January and March 2020, 105 patients were enroled and 98 underwent randomisation: 50 patients in the reusable group and 48 patients in the single-use duodenoscope group (figure 2). Seven patients did not undergo randomisation after study enrolment as the major papilla could not be reached due to tumour infiltration of the duodenum in three patients, no bile duct stones were visualised on endoscopic ultrasound (EUS) examination performed prior to ERCP in three patients and one patient withdrew study consent after enrolment but prior to sedation. All patients were followed up for a minimum duration of 30 days and no patients were lost to follow-up with no missing information on any patient.
Figure 2.
Consolidated Standards of Reporting Trials flow diagram of patients recruited for participation in the randomised trial.
Baseline patient characteristics and procedural details
In the single-use duodenoscope cohort, the median patient age was 70 years, 54.2% were male and 87.5% were Caucasian or African–American. In the reusable duodenoscope cohort, the median patient age was less by 5.5 years, comprised 8% more female patients and twice the proportion of patients belonged to race other than Caucasian or African–American as compared with the single-use cohort. There was no significant difference in the baseline patient demographics between the two groups (table 1).
Table 1.
Baseline patient characteristics and procedure details
| Single-use duodenoscope (n=48) |
Reusable duodenoscope (n=50) |
P value | |
| Age (years) | |||
| Mean (SD) | 67.2 (14.4) | 60.8 (18.2) | 0.063 |
| Median | 70 | 64.5 | |
| IQR | 58–78 | 47–72 | |
| Range | 22–88 | 26–93 | |
| Gender, n (%) | |||
| Female | 22 (45.8) | 27 (54.0) | 0.419 |
| Male | 26 (54.2) | 23 (46.0) | |
| Race, n (%) | |||
| Black | 8 (16.7) | 5 (10.0) | 0.193 |
| White | 34 (70.8) | 32 (64.0) | |
| Other | 6 (12.5) | 13 (26.0) | |
| Indication for procedure, n (%) | |||
| Biliary* | 44 (91.7) | 43 (86.0) | 0.374 |
| Pancreatic† | 3 (6.3) | 6 (12.0) | |
| Ampullectomy | 1 (2.1) | 1 (2.0) | |
| ASGE grade for procedural difficulty, n (%) | |||
| 1 | 9 (18.8) | 12 (24.0) | 0.761 |
| 2 | 31 (64.6) | 31 (62.0) | |
| 3 | 4 (8.3) | 2 (4.0) | |
| 4 | 4 (8.3) | 5 (10.0) | |
*Indications for biliary interventions: single-use duodenoscope—biliary stricture (n=20), bile duct stones (n=22), bile leak (n=2). Reusable duodenoscope—biliary stricture (n=18), bile duct stones (n=21), bile leak (n=3), elevated liver tests (n=1).
†Indications for pancreatic interventions: single-use duodenoscope—pancreatic duct stricture/leak (n=3). Reusable duodenoscope—pancreatic duct stricture/leak (n=4), minor papilla interventions (n=1), idiopathic acute recurrent pancreatitis (n=1).
ASGE, American Society for Gastrointestinal Endoscopy.
In the single-use duodenoscope cohort, more than 90% of the procedures were performed for biliary indications and 81.3% of the cases were ASGE complexity grade 2 and above. In the reusable duodenoscope cohort, more than 85% of the procedures were performed for biliary indications and 76% were ASGE grade 2 and above. There was no significant difference in procedure indication or complexity between the two cohorts (table 1).
Outcome measures
In the single-use duodenoscope cohort, successful cannulation was achieved in 46 of 48 patients with the need for advanced techniques in 14.6%. Cannulation failed in two patients with malignant distal biliary obstruction despite adopting advanced manoeuvres (needle-knife sphincterotomy over a pancreatic duct stent) and were subsequently crossed over to reusable duodenoscopes. Cannulation was also not successful with reusable duodenoscopes in both patients. While one patient was successfully treated by EUS-guided choledochoduodenostomy, the rescue procedure failed in the other patient as the common bile duct was more than 25 mm away from the EUS transducer and therefore required percutaneous transhepatic biliary drainage catheter placement with interventional radiology. Per intention-to-treat principle, the outcome data on these two single-use duodenoscope patients who underwent cross-over were analysed within their originally assigned group of single-use duodenoscopes. In the reusable duodenoscope cohort, successful cannulation was achieved in all patients, with need for advanced techniques in 22% (table 2). While there was no significant difference in overall rates of successful cannulation, the median number of attempts to achieve selective ductal access was significantly lower for the single-use cohort (2 vs 5, p=0.013). There was no significant difference in total procedural duration, need to cross-over to alternate treatment arm or the need for advanced cannulation techniques to achieve ductal access between the two cohorts (table 3, online supplemental figure 1).
Table 2.
Comparison of procedure outcomes between the duodenoscope types
| Single-use duodenoscope | Reusable duodenoscope | P value | |
| (n=48) | (n=50) | ||
| Time taken to reach the papilla (s) | |||
| Mean (SD) | 34.2 (55.6) | 32.7 (81.9) | 0.714 |
| Median | 20 | 20 | |
| IQR | 20–20.5 | 20–22 | |
| Range | 15–380 | 15–600 | |
| Successful cannulation achieved, n (%) | 46 (95.8) | 50 (100) | 0.237 |
| Total number of attempts at cannulation | |||
| Mean (SD) | 6.7 (10.9) | 12.7 (15.7) | 0.013 |
| Median | 2 | 5 | |
| IQR | 1–5.5 | 2–22 | |
| Range | 1–43 | 1–75 | |
| Advanced cannulation technique used, n (%)* | 7 (14.6) | 11 (22.0) | 0.343 |
| Cross-over to alternate duodenoscope, n (%) | 2 (4.2) | 0 | 0.237 |
| Time taken for cannulation (s) | |||
| Mean (SD) | 239.5 (650.2) | 359.5 (524.2) | 0.010 |
| Median | 35 | 99 | |
| IQR | 10–150 | 30–510 | |
| Range | 1–4220 | 5–2400 | |
| Total procedure duration (min) | |||
| Mean (SD) | 22.7 (19.2) | 23.2 (15.0) | 0.310 |
| Median | 14.8 | 18.3 | |
| IQR | 9.9–28.2 | 12–32.3 | |
| Range | 6.1–82 | 6.5–75 | |
*Advanced cannulation techniques: single-use duodenoscope—cannulation over pancreatic stent (n=4), needle knife sphincterotomy over pancreatic stent (n=2), transpancreatic biliary sphincterotomy (n=1). Reusable duodenoscope—cannulation over pancreatic stent (n=4), freehand needle knife sphincterotomy (n=2), needle knife sphincterotomy over pancreatic stent (n=1), transpancreatic biliary sphincterotomy (n=4).
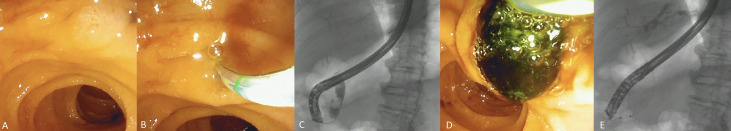
Figure 3.
(A) Single-use duodenoscope enface to the major duodenal papilla on endoscopic view. (B) Endoscopic image showing the position of the single-use duodenoscope in relation to the major papilla and (C) the corresponding fluoroscopic image. (D) Bile duct stone extraction via the major duodenal papilla as seen on endoscopic view and (E) the corresponding fluoroscopic image (note the relatively straight-scope position).
gutjnl-2020-321836supp002.pdf (226.4KB, pdf)
There was no significant difference in most elements of manoeuvrability and the ability to undertake therapeutic interventions between the two duodenoscope types. However, the ease of duodenoscope passage into the stomach (p=0.047), image quality (p<0.001), image stability (p<0.001) and air–water button functionality (p<0.001) were significantly worse for single-use duodenoscopes (online supplemental tables 1–4).
gutjnl-2020-321836supp003.pdf (122.4KB, pdf)
On multivariate logistic regression analysis, only the duodenoscope type (single-use) was significantly associated with fewer than six attempts to achieve selective ductal cannulation (OR=3.0, 95% CI, 1.27 to 7.07; p=0.012) when adjusted for patient demographics, procedural complexity and type of intervention (table 3).
Table 3.
Multiple logistic regression and reverse multivariate logistic regression analyses to identify factors associated with fewer than six cannulation attempts
| Variable | OR | 95% CI | P value |
| Multiple logistic regression analysis | |||
| Patient age (years) | 0.98 | 0.95 to 1.01 | 0.106 |
| Patient gender: male vs female | 1.39 | 0.57 to 3.40 | 0.463 |
| Race: other vs white | 0.95 | 0.37 to 2.45 | 0.909 |
| ASGE grade of procedure difficulty | |||
| 2 vs 1 | 1.70 | 0.57 to 5.04 | 0.338 |
| 3 vs 1 | 1.27 | 0.17 to 9.76 | 0.816 |
| 4 vs 1 | 1.09 | 0.12 to 10.1 | 0.939 |
| Procedure type: biliary vs non-biliary interventions | 0.52 | 0.076 to 3.55 | 0.505 |
| Type of duodenoscope: single-use vs reusable | 3.58 | 1.44 to 8.94 | 0.006 |
| Reverse multivariate logistic regression analysis | |||
| Type of duodenoscope: single-use vs reusable | 3.00 | 1.27 to 7.07 | 0.012 |
ASGE, American Society for Gastrointestinal Endoscopy.;
In the single-use duodenoscope cohort, adverse events were observed in two patients. The first patient had cholangitis, Escherichia coli bacteremia and sepsis from bile duct stones and decompensated despite undergoing ERCP for bile duct stone removal and died 2 days postprocedure. Decompensation of this patient was attributed to an ongoing endogenous infection rather than a duodenoscope-related exogenous infection as the repeat of blood cultures post-ERCP was negative for microorganisms. The second patient developed post-ERCP pancreatitis of moderate severity—this patient was hospitalised for 7 days and was managed conservatively. In the reusable duodenoscope cohort, adverse events were observed in four patients that included postsphincterotomy bleeding of mild severity in one patient that was managed conservatively, post-ERCP pancreatitis in two patients (one mild and one moderate severity) who were both managed conservatively and death in one patient who developed atrial fibrillation with rapid ventricular response and cardiogenic shock following ERCP. There was no significant difference in the overall rate of adverse events between the two cohorts (p=0.429) and no duodenoscope-associated infection was observed in any patient in this study (online supplemental table 5).
Discussion
Given the overall safety profile and similar technical performance, we believe that single-use duodenoscopes can represent an alternative to reusable duodenoscopes for performing ERCP procedures. Although acceptable, technical refinements to avoid image flickering and to arrest water leakage due to valve dysfunction will make the single-use duodenoscopes more user friendly.
Three important clinical observations are worth reporting. One, despite worse performance scores for scope stiffness, image quality and image stability, the number of attempts to achieve ductal access was significantly fewer with the single-use duodenoscopes. On review of fluoroscopy images, we observed that the inherent scope stiffness facilitates a straight but stable scope position when enface to the major duodenal papilla (figure 3A–E). Consequently, the papilla is engaged from a superior or horizontal angle than from below-upwards (as with reusable duodenoscopes). Another potential advantage is the shaft stiffness that provides firm anchorage for pulling retrieval balloons in line with the bile duct axis thereby making stone extraction easier (online supplemental video 1). Regardless, there was no significant difference in the rate of successful cannulation between the two duodenoscope types and a larger randomised trial is required to confirm these observations. Two, the passage of accessories such as biopsy forceps and laser fibres during single-operator cholangioscopy (SOC) procedures was technically easier with single-use duodenoscopes compared with reusable duodenoscopes. The inherent stiffness of single-use duodenoscope shaft straightens the rubber tubing of the cholangioscope and facilitates easier passage of accessories through its working channel (online supplemental video 2). Three, we encountered a case of postprocedure cholangitis in the single-use duodenoscope cohort that resulted in death. While the single-use duodenoscope is a timely and innovative option to improve exogenous infection control, it plays no role in the control of endogenous infections, which are likely the predominant cause of postprocedure cholangitis. Therefore, standard infection control measures and safe techniques such as selective opacification and complete drainage of obstructed ductal systems must be practiced judiciously to avoid infection.
gutjnl-2020-321836supp004.mp4 (135.8MB, mp4)
gutjnl-2020-321836supp005.mp4 (56.3MB, mp4)
There are several limitations of this study. One, as minor papilla interventions were not performed using the single-use duodenoscopes in this study, the findings may not be pertinent to patients with pancreas divisum. Also, pancreatic interventions and procedures of ASGE difficulty grades 3 and 4 comprised only 15% of the cases in this study. Therefore, technical performance of the single-use duodenoscope in this patient cohort requires further validation. Two, the procedures were performed by experienced investigators at a high-volume centre and hence the results may not be generalisable to all endoscopists. Finally, the primary outcome measure, the number of attempts to achieve successful cannulation, was based on procedural difficulty and its impact on outcomes (post-ERCP pancreatitis). A complete assessment should ideally include randomisation based on procedural difficulty grading and overall technical success of the procedure. Although the single-use duodenoscope was developed to overcome the risk of infection transmission, a study evaluating this outcome is impractical given a rate of 0.4% to 1% in clinical practice.2 20 21 Consequently, assuming a 0% risk for single-use duodenoscopes versus 0.4% for reusable duodenoscopes, the sample size would be a total of 5234 patients (2617 patients in each group) at 90% power and alpha of 0.05 (assuming no patient loss to follow-up). At an approximate cost of US$2900 per single-use duodenoscope, it would cost US$15 178 600 to purchase sufficient number of single-use duodenoscopes for the trial. This level of cost is prohibitive and consequently the study is unlikely to be funded by any national agency or institution.
However, one burning question in the mind of every endoscopist is, does the development of single-use duodenoscope represent a significant advancement in the field of ERCP? This question can be addressed from three perspectives—clinical, financial and innovation.
From a clinical perspective, the risk of transmitting virulent microbes such as CRE by a contaminated duodenoscope may be eliminated with single-use duodenoscopes and this will improve patient care. A recent study showed that biliary stent placement in the setting of cholangiocarcinoma was associated with an increased risk of CRE transmission.2 One could speculate that the use of a single-use duodenoscope may be preferred in such high-risk cases, in immunocompromised patients, or in known carriers of multidrug-resistant organisms. Also, procedures that may have to be performed on an emergent basis outside of the endoscopy unit, such as in the operating room, emergency room or in the intensive care unit, are likely to benefit given the ease of mobility and elimination of the need to reprocess the duodenoscope, which is often delayed after an offsite procedure. They may also be useful in the event that reusable duodenoscopes are unavailable due to repairs or quarantined awaiting culture results.
From a financial perspective, presently, the price point for a single-use duodenoscope is US$2500–US$2900 based on hospital contractual agreements with Boston Scientific Corporation. The cost per procedure for a reusable duodenoscope varies but we previously published it to be US$612 at our centre (ERCP annual volume of 1850), based on an assumed infection rate of 0.4%.22 The difference in cost is hence approximately US$1888–US$2288. A major factor determining usage will be whether or not the United States Centers for Medicare and Medicaid Services and private insurance carriers will fully cover the cost of single-use duodenoscopes, especially as the cross-over rate from single use to reusable duodenoscopes was 4.2% in this study. It is possible that small volume institutions that do not want to invest in capital equipment but have the requisite technical expertise to perform ERCP may invest in this technology.
From an innovation perspective, the reusable endoscope is currently designed with a one-size-fits-all concept. Studies have shown that endoscopists suffer from injuries, likely related to the endoscope design. The current single-use duodenoscope is lighter and more importantly, given the nature and manufacturing process of this product, there is great flexibility for refinement in design that can occur at a rapid pace. Therefore, we speculate that it may be possible for an ergonomically designed duodenoscope tailored to specific hand sizes to meet individual needs could be manufactured. However, because endoscope-related injuries take place over years of repetitive use, proving that a new design would prevent these injuries will be difficult.
In summary, given the overall safety profile and similar technical performance, single-use duodenoscopes may represent an alternative to reusable duodenoscopes for performing low-complexity ERCP procedures in experienced hands and therefore this innovation is a significant advancement in the field of ERCP.
Footnotes
Contributors: JYB helped in the study design, endoscopist performing procedures in the study, statistical analysis, interpretation of data, drafting of the manuscript and critical revision of the manuscript. SV helped in study concept and design, endoscopist performing procedures in the study, interpretation of data, drafting of the manuscript and critical revision of the manuscript. RH helped in endoscopist performing procedures in the study and critical revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JYB: Consultant for Olympus America Inc, Boston Scientific Corporation. SV: Consultant for Boston Scientific Corporation, Olympus America Inc, Covidien, Creo Medical. RH: Consultant for Boston Scientific Corporation, Olympus America Inc, Covidien, Creo Medical, Nine Points Medical, Cook Medical.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Rauwers AW, Voor In 't Holt AF, Buijs JG, et al. High prevalence rate of digestive tract bacteria in duodenoscopes: a nationwide study. Gut 2018;67:1637–45. 10.1136/gutjnl-2017-315082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim S, Russell D, Mohamadnejad M, et al. Risk factors associated with the transmission of carbapenem-resistant Enterobacteriaceae via contaminated duodenoscopes. Gastrointest Endosc 2016;83:1121–9. 10.1016/j.gie.2016.03.790 [DOI] [PubMed] [Google Scholar]
- 3. Verfaillie CJ, Bruno MJ, Voor in 't Holt AF, et al. Withdrawal of a novel-design duodenoscope ends outbreak of a VIM-2-producing Pseudomonas aeruginosa. Endoscopy 2015;47:493–502. 10.1055/s-0034-1391886 [DOI] [PubMed] [Google Scholar]
- 4. Ross AS, Baliga C, Verma P, et al. A quarantine process for the resolution of duodenoscope-associated transmission of multidrug-resistant Escherichia coli. Gastrointest Endosc 2015;82:477–83. 10.1016/j.gie.2015.04.036 [DOI] [PubMed] [Google Scholar]
- 5. Naryzhny I, Silas D, Chi K. Impact of ethylene oxide gas sterilization of duodenoscopes after a carbapenem-resistant Enterobacteriaceae outbreak. Gastrointest Endosc 2016;84:259–62. 10.1016/j.gie.2016.01.055 [DOI] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration: Medical Devices; Medical Device Safety; Safety Communications . The FDA continues to remind facilities of the importance of following duodenoscope reprocessing instructions: FDA safety communication, 2019. Available: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm635828.htm [Accessed 12 Apr 2019].
- 7. ASGE Standards of Practice Committee, Khashab MA, Chithadi KV, et al. Antibiotic prophylaxis for Gi endoscopy. Gastrointest Endosc 2015;81:81–9. 10.1016/j.gie.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 8. Bang JY, Rösch T, Robalino Gonzaga ES, et al. Su1513 technical evaluation of duodenoscope performance using a newly developed assessment tool. Gastrointest Endosc 2020;91:AB358. 10.1016/j.gie.2020.03.2228 [DOI] [Google Scholar]
- 9. Cotton PB, Eisen G, Romagnuolo J, et al. Grading the complexity of endoscopic procedures: results of an ASGE Working Party. Gastrointest Endosc 2011;73:868–74. 10.1016/j.gie.2010.12.036 [DOI] [PubMed] [Google Scholar]
- 10. Chandrasekhara V, Khashab MA, Muthusamy VR, et al. Adverse events associated with ERCP. Gastrointest Endosc 2017;85:32–47. 10.1016/j.gie.2016.06.051 [DOI] [PubMed] [Google Scholar]
- 11. NIH . National cancer Institute common terminology criteria for adverse events (CTCAE) v5.0. Available: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 12. Elmunzer BJ, Serrano J, Chak A, et al. Rectal indomethacin alone versus indomethacin and prophylactic pancreatic stent placement for preventing pancreatitis after ERCP: study protocol for a randomized controlled trial. Trials 2016;17:120. 10.1186/s13063-016-1251-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of gastrointestinal endoscopy (ESGE) clinical guideline. Endoscopy 2016;48:657–83. 10.1055/s-0042-108641 [DOI] [PubMed] [Google Scholar]
- 14. Liao W-C, Angsuwatcharakon P, Isayama H, et al. International consensus recommendations for difficult biliary access. Gastrointest Endosc 2017;85:295–304. 10.1016/j.gie.2016.09.037 [DOI] [PubMed] [Google Scholar]
- 15. Buxbaum J, Leonor P, Tung J, et al. Randomized trial of Endoscopist-Controlled vs. Assistant-Controlled wire-guided cannulation of the bile duct. Am J Gastroenterol 2016;111:1841–7. 10.1038/ajg.2016.268 [DOI] [PubMed] [Google Scholar]
- 16. Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd edn. John Wiley & Sons Inc., 2013. [Google Scholar]
- 17. Afifi A, May S, Donatello RA, et al. Practical multivariate analysis. 6th edn. Taylor & Francis Group, LLC, 2020. [Google Scholar]
- 18. Montori VM, Guyatt GH. Intention-To-Treat principle. CMAJ 2001;165:1339–41. [PMC free article] [PubMed] [Google Scholar]
- 19. Sedgwick P. Intention to treat analysis versus per protocol analysis of trial data. BMJ 2015;350:h681. 10.1136/bmj.h681 [DOI] [PubMed] [Google Scholar]
- 20. Rauwers AW. Voor In 't Holt AF, Buijs JG, et al. High prevalence rate of digestive tract bacteria in duodenoscopes: a nationwide study. Gut 2018;67:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alrabaa SF, Nguyen P, Sanderson R, et al. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control 2013;41:562–4. 10.1016/j.ajic.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 22. Bang JY, Sutton B, Hawes R, et al. Concept of disposable duodenoscope: at what cost? Gut 2019;68:1915–7. 10.1136/gutjnl-2019-318227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-321836supp001.pdf (62.4KB, pdf)
gutjnl-2020-321836supp002.pdf (226.4KB, pdf)
gutjnl-2020-321836supp003.pdf (122.4KB, pdf)
gutjnl-2020-321836supp004.mp4 (135.8MB, mp4)
gutjnl-2020-321836supp005.mp4 (56.3MB, mp4)