Abstract
Background
Immediate contraceptive initiation, including start of a method before abortion completion, is a convenient option for women seeking abortion care.
Objectives
To evaluate the effect of systemic hormonal contraception initiation on medical abortion effectiveness and the safety of hormonal contraceptive methods following abortion.
Data sources
PubMed, Popline, Cochrane Library, and Clinicaltrials.gov.
Study eligibility criteria
Studies that assessed medical abortion effectiveness after systemic hormonal contraception initiation and the safety of hormonal contraception initiation after abortion.
Participants
Pregnant persons undergoing or who had recently undergone an abortion.
Interventions
Initiation of systemic hormonal contraception post abortion or on the day of the first pill of the medical abortion.
Study appraisal and synthesis methods
We assessed study quality using the US Preventive Services Task Force evidence grading system. We created narrative summaries and calculated pooled relative risks when appropriate.
Results
We identified 16 studies for inclusion, 7 randomized controlled trials, and 9 cohorts. Nine studies assessed medical abortion effectiveness with hormonal contraception initiation and generally found no decreased risk of abortion success or increased risk of additional treatment. One fair-quality study reported a small increase in ongoing pregnancy rate with immediate depot medroxyprogesterone (DMPA) compared with delayed DMPA initiation (3.6% vs 0.9%, risk difference 2.7%, 90% confidence interval 0.4–5.6). We identified no bleeding-related safety concerns following hormonal contraception initiation after medical or surgical abortion. Pooled results were too imprecise to draw firm conclusions.
Limitations
Included studies were poor or fair quality and primarily in high-income or upper-middle-income settings.
Conclusions
Abortion effectiveness did not differ between immediate vs delayed initiation of most systemic hormonal contraceptive methods after a first trimester medical abortion. However, immediate DMPA initiation did show increased ongoing pregnancy. Bleeding effects with hormonal contraception initiation postabortion appeared minimal.
Implications
Initiating a hormonal contraceptive method after an abortion and as early as the same day as the first pill of the medical abortion is an option if contraception is desired. The slight increase in ongoing pregnancy with immediate DMPA initiation highlights the importance of information provision during contraceptive counseling.
Keywords: Abortion, Contraception, Delayed, Hormonal, Immediate, Medical abortion effectiveness, Systematic review
1. Introduction
Contraceptive initiation after abortion is important for those who desire delayed or no future pregnancy. Following an induced or spontaneous abortion, ovulation can return as early as 8 to 10 days and usually within 1 month [1], [2], [3], [4]. Reports of intercourse within 2 weeks after an induced abortion are common [5]. Moreover, contraceptive counseling and services are considered a human right and should be available and included as a routine component of postabortion care [6], [7], [8].
Currently, recommendations in the World Health Organization's (WHO) Medical eligibility criteria for contraceptive use state that there are no restrictions for the use of combined estrogen-progestogen (combined hormonal) or progestogen-only methods (not including intrauterine devices [IUDs]) immediately following a first trimester abortion, second trimester abortion, or septic abortion (Category 1) [9]. However, current medical abortion regimens that include mifepristone, an antiprogestogen, could have decreased effectiveness with concurrent administration of progestogen-containing contraceptive methods. Additionally, immediate use of hormonal contraceptive methods after an abortion could cause less predictable bleeding patterns, which could affect satisfaction and continuation [10].
The 2 objectives of this systematic review are to assess the effect of hormonal contraception initiation on medical abortion effectiveness (abortion success) and to assess the safety of hormonal contraceptive methods following an induced (medical or surgical), spontaneous, or septic abortion. We focus on non-IUD hormonal methods and will evaluate the effect of timing of hormonal contraception initiation (immediate vs delayed) and hormonal contraception initiation (hormonal method vs non-hormonal/no method).
2. Methods
2.1. Inclusion and exclusion criteria
We included studies that assessed medical abortion effectiveness after hormonal contraception initiation and the safety of hormonal contraception initiation after abortion (Table 1). We included pregnant persons undergoing or who had recently undergone an induced abortion (medical or surgical in the first or second trimester), spontaneous abortion (treated surgically, medically, or expectantly managed), or septic abortion. We focused on systemic hormonal contraception, including combined hormonal methods (oral, patch, ring, or injectable) and progestogen-only methods (oral, injectable, or implant). Nonhormonal comparison groups included individuals using any nonhormonal contraceptive method (e.g., copper intrauterine device [Cu-IUD], barrier method, sterilization) or no contraceptive method. We defined immediate initiation of contraception as: (1) for medical abortion, day of first or second medication use prior to abortion success; (2) for surgical aspiration, the day of or day prior to surgical evacuation for induced, spontaneous, or septic abortion; or (3) for medically or expectantly managed spontaneous or septic abortion, the time of presentation prior to diagnosis of successful outcome. We defined delayed contraceptive initiation as initiation at follow-up (usually between 1 and 4 weeks) after abortion success (all types).
Table 1.
Inclusion criteria of studies to assess medical abortion effectiveness after systemic hormonal contraception (HC)* initiation and safety of systemic HC initiation after abortion
| Population | Intervention | Comparison | Outcome(s) | Included studies |
|---|---|---|---|---|
| Medical abortion | ||||
| Individuals undergoing medical abortion | Immediate HC initiation | Delayed HC initiation | Abortion success Adverse events |
5 (4 RCTS, 1 cohort) |
| Individuals undergoing medical abortion | HC initiation at any time | No HC initiation | Abortion success Adverse events |
5 (3 RCTs, 2 cohort) |
| Surgical abortion | ||||
| Individuals undergoing surgical abortion | Immediate HC initiation | Delayed HC initiation | Adverse events | 1 RCT |
| Individuals undergoing surgical abortion | HC initiation at any time | No HC initiation | Adverse events | 6 cohort |
| Spontaneous abortion | ||||
| Individuals with spontaneous abortion | Immediate HC initiation | Delayed HC initiation | Adverse events | None |
| Individuals with spontaneous abortion | HC initiation at any time | No HC initiation | Adverse events | None |
| Septic abortion | ||||
| Individuals with septic abortion | Immediate HC initiation | Delayed HC initiation | Adverse events | None |
| Individuals with septic abortion | HC initiation at any time | No HC initiation | Adverse events | None |
RCT, randomized controlled trial.
*HC, hormonal contraception is defined as systemic hormonal contraception and includes combined hormonal methods (oral, patch, ring, or injectable) and progestogen-only methods (oral, injectable, or implant).
The main outcomes of interest included medical abortion effectiveness following systemic hormonal contraception initiation and safety of systemic hormonal contraception with medical, surgical, spontaneous, or septic abortion. We accepted any measurement of abortion success for medical abortion effectiveness (e.g., surgery to complete abortion, additional surgical or medical treatment, ongoing pregnancy). Safety outcomes included adverse health events: thromboembolic events (by self-report or clinical diagnosis), bleeding outcomes up to 12 weeks after abortion (changes in hemoglobin/hematocrit, bleeding related method discontinuation, or other self-reporting methods), and pelvic infection up to 12 weeks after abortion.
2.2. Sources
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidance [11]. We searched PubMed, Popline, Cochrane Library, and Clinicaltrials.gov for all primary peer-reviewed research from database inception through January 6, 2020 and included randomized controlled trials [RCTs], cohort studies, and case-control studies in any language (Appendix A). We examined previously published systematic reviews and key review reference lists for relevant articles.
2.3. Study selection
Three authors (CK, AN, and EBB) independently screened all titles, abstracts and full texts identified from the initial search to determine eligibility for inclusion. We used a standard template for data abstraction (Tables 2 and 3).
Table 2.
Safety and effectiveness of systemic hormonal contraception initiated after medical abortion
| Author, year, location, funding | Study design, follow-up, study quality* | Population, abortion regimen | Intervention (I) | Comparison (C) | Outcome(s) and results | Strengths | Weaknesses |
|---|---|---|---|---|---|---|---|
| Raymond, 2016 [26] Mexico United States Anonymous donor |
Noninferiority RCT Visit or phone f/u at 4 or 7 months, assessed with UPT and 86%–91% had US Fair quality |
Women eligible for outpatient medical abortion (up to 75 days GA) and desired DMPA (n = 461) -GA of 64 d or greater: 12% Abortion: mifepristone 200mg PO, misoprostol 800µg buccal 1–2 days later |
Immediate DMPA: on day of mifepristone (n = 225, n = 220 analyzed) |
Delayed DMPA: once abortion completed >6 days after mifepristone (n = 236, n = 226 analyzed) |
Abortion Success Surgery to complete abortion (%): I: 14/220 (6.4%) C: 12/226 (5.3%) Difference (90% CI): 1.1 (−2.8, 4.9) Additional surgical or medical treatment (%): I: 25/220 (11.4%) C: 26/226 (11.5%) Difference (90% CI): −0.1 (−5.2, 4.9) Ongoing pregnancy (%): I: 8/220 (3.6%) C: 2/226 (0.9%) Difference (90% CI): 2.7 (0.4, 5.6) Bleeding: self-reported within 1, 4, and 7 months Median bleeding days: I: 0-7 – 21.8% 8-14 – 53.2% 15+ – 25.0% C: 0-7 – 26.1% 8-14 – 57.8% 15+ – 16.1% p = 0.01 Bleeding heavier than menses: I: 159/220 (74%) C: 171/226 (78.4%) p = 0.27 |
Adequate randomization and allocation concealment Low attrition (immediate: 2.2%, delayed: 4.2%); Masked record review by independent clinician blinded to group assignments for those who received extra treatment Adequate sample size powered to detect difference in medical abortion failure |
Timing of DMPA administration in delayed group not described US assessment not reported as blinded |
| Raymond, 2016 [27] Mexico United States Anonymous donor |
Non-inferiority RCT Visit or phone f/u at 4 or 7 months, assessed with UPT and 90-92% had US Fair quality |
Women eligible for outpatient medical abortion and desired ENG implant (n = 476) -GA of 64 d or greater: 14-18% Abortion: mifepristone 200mg PO, misoprostol 800µg buccal 1-2 days later |
Immediate ENG implant: inserted on day of mifepristone (n = 236, n = 229 analyzed) |
Delayed ENG implant: inserted once abortion completed >6 days after mifepristone (n = 240, n = 234 analyzed) |
Abortion Success Surgery to complete abortion (%): I: 9/229 (3.9%) C: 9/234 (3.9%) Difference (90% CI): 0.08 (-3.06, 3.25) Additional surgical or medical treatment (%): I: 21/229 (9.2%) C: 26/234 (11.1%) Difference (90% CI): -1.94 (-6.68, 2.77) Ongoing pregnancy (%): I: 2/229 (0.9%) C: 2/234 (0.9%) Difference (90% CI): 0.02 (-1.8, 1.85) Bleeding: self-reported within 1, 4, and 7 months Median bleeding days: I: 12 C: 10p = 0.03 Bleeding heavier than menses: I: 155/229 (68.3%) C:158/234 (68.1%)p = 0.91 |
Adequate randomization and allocation concealment Low attrition (immediate: 3.0%, delayed: 2.5%) Masked record review by independent clinician blinded to group assignments for those who received extra treatment Adequate sample size powered to detect difference in medical abortion failure |
Timing of implant insertion in delayed group not described US assessment not reported as blinded |
| Hognert, 2016 [25] Sweden Scotland Swedish Research County, Stockholm City County, Karolinska Institutet ENG implants funded by MSD/Merck |
Equivalence RCT Visit or phone f/u at 2-3 weeks, assessed with low sensitivity UPT; use of US NR; phone f/u at 3 and 6 months Fair quality |
Women who had a medical abortion at ≤ 63 days GA based on ultrasound and desired ENG implant (n = 538) -Median GA: 46 days Abortion: mifepristone 200mg PO, misoprostol 800µg PV 1-2 days later, in Sweden additional misoprostol 400 µg if no bleeding by 3 hours |
Immediate ENG implant: inserted on day of mifepristone (n = 282, n = 277 ITT, n = 274 per protocol analyzed) |
Delayed ENG implant: inserted 2-4 weeks after mifepristone (n = 268, n = 261 ITT, n = 249 per protocol analyzed) |
Abortion Success Surgery to complete abortion (%) ITT: I: 16/277 (5.7%) C: 10/261 (3.8%) Difference (95% CI): 1.3 (-0.9, 4.1) Additional medical treatment needed (extra dose of misoprostol) (%): I: 19/277 (6.8%), 6 women also had surgery C: 9/261 (3.4%), 3 women also had surgeryp = 0.083 Bleeding Bleeding as reason for implant removal: I: 6/275 (2.2%) at 3 months 12/275 (4.3%) at 6 months C: 4/187 (2.1%) at 3 months 11/187 (5.9%) at 6 months |
Adequate randomization and allocation concealment Pregnancy dating by ultrasound Access to patient records for all regions to account for those who did not follow up Sensitivity analysis performed Standardized timing of implant placement Adequate sample size powered to establish equivalence between 2 groups for successful completion of abortion |
Reason for surgery not detailed, including reporting of ongoing pregnancy Reporting of additional misoprostol to those who did not bleed within 3 hours after mifepristone |
| Barros Pereira, 2015 [24] Portugal Source of support NR |
Prospective cohort Visit at 14 days, assessed with US; phone f/u at 6 months, data verified by hospital records Poor quality |
Women who had a medical abortion at ≤ 70 days GA and desired ENG implant (n = 129, n = 119 analyzed) -GA results are not detailed Abortion: mifepristone 200mg PO, misoprostol 800µg PO 48h later |
Immediate ENG implant: inserted on day of mifepristone (n = 61, n = 57 analyzed) |
Delayed ENG implant: clinic visit at 4 weeks (n = 68, n = 62 analyzed) |
Abortion Success No need for vacuum aspiration to complete abortion (incomplete abortion defined as >15 mm endometrial thickness on US) No surgery to complete abortion (%): I: 55/57 (96.5%) C: 61/62 (98.4%)p = 0.47 |
Groups appear similar at baseline Cleary defined abortion completion and assessment was objective Low attrition (immediate: 6.6%, delayed: 8.8%) |
Small sample size; not powered to detect difference for outcomes of interest No confounding assessment Some in delayed group used “other groups” (n = 29) |
| Martin, 1998 [19] Scotland No financial disclosures |
RCT F/u at 2 and 6 weeks Poor quality |
Women who had a medical abortion at ≤ 63 days GA in a hospital (n = 40) -mean GA for all four groups: 48-49 days Abortion: mifepristone 200 mg PO, 0.5 mg gemeprost pessary 48 hours later |
Immediate COC (Group A): initiated after abortion success confirmed and prior to discharge from hospital (n = 20, n = 19 analyzed) |
Delayed COC (Group B): initiated on 1st day of next menses (n = 20, n = 19 analyzed) |
Abortion Success Incomplete abortion requiring surgery (%): I: 0/19=0% C: 0/19=0% Bleeding Days of bleeding after abortion (median, range): I: 14 (9-45) C: 17 (6-34) |
Adequate randomization Low loss to follow up (total of 2 women from the 80) Bleeding measured with daily bleeding diaries |
Allocation concealment not detailed Outcome assessment (blinding and criteria used) not detailed No information on participation rates, COC adherence Large ranges of bleeding duration around median |
| Lang, 2018 [31] Scotland No financial disclosures |
Retrospective cohort Low-sensitivity UPT at home at 2 weeks Fair quality |
Women who had a medical abortion at ≤ 63 days GA (n = 5122) -GA≥8 weeks: 18% Abortion: mifepristone 200 mg PO, misoprostol 800 mcg PV or SL 24-48 hours later in clinic |
Hormonal method: initiated with misoprostol DMPA (n = 475) COC (n = 1268) POP (n = 485) Implant (n = 797) |
No hormonal method/none: condoms, diaphragm, IUD, sterilization or no method (n = 1813) |
Abortion Success Database review for subsequent visit for abortion services, ongoing pregnancy or maternity care Continuing pregnancy (RR [95% CI]): DMPA: 0.48 (0.06, 3.81) COC: 0.90 (0.29, 2.73) POP : 1.40 (0.37, 5.76) Implant: 0.85 (0.23, 3.21) No method: 1.0 (reference) |
Population-based sample Large study Complete participation and follow-up, due to record review and robust efforts made to capture all continuing pregnancies Contraceptive exposure well-defined and measured |
Details of timing and duration of method not provided No adjustment for potential confounders Wide 95% confidence intervals and lack of precision |
| Douthwaite, 2016 [28] Mexico City Marie Stopes International |
Retrospective cohort Phone f/u at 8 and 30 days with UPT at 30 days or visit f/u at 3 weeks with UPT/US Poor quality |
Women who had a medical abortion at ≤ 63 days GA (n = 2482, n = 2204 analyzed) -GA 7-9 weeks: 30% Abortion: mifepristone 200mg PO, misoprostol 800mcg buccal 24-48h later |
POC: Net-EN, DMPA, LNG implant, or ENG implant initiated within 15 minutes of mifepristone (n = 511, n = 448 analyzed) |
None: no method initiated (n = 1971, n = 1756 analyzed) |
Abortion Success Rate of complete uterine evacuation without additional interventions Complete abortion, overall (%): POC: 385/448 (87.9%) No method: 1505/1756 (85.7%)p = 0.56 Complete abortion, progestin subgroups (%): DMPA: 294/346 (85.0%) Non-DMPA POC: 91/102 (89.2%) No method: 1505/1756 (85.7%)p = 0.56 |
Large sample size powered for outcome of interest Clearly defined outcome Low attrition (POC: 12%, no method: 11%); analysis excluded those loss to follow-up who were similar at baseline to those with follow-up |
Record review Groups differed by type of follow-up and parity – no confounding assessment Small sample size of POC users, other than DMPA users, could not be analyzed separately |
| Tang, 2002 [22] Hong Kong Shanghai WHO HRP |
RCT Visits at day 15 (exam, US, Hgb), day 43 (exam), day 67 if persistent bleeding or no menses Fair quality |
Women with menstrual delay ≤ 35 days presenting for medical abortion, GA confirmed with US -Mean menstrual delay: 17 days Abortion: mifepristone 200mg PO, misoprostol 400µg miso PV in hospital 48h later |
COC (30µg EE/0.15mg LNG): initiated 1 day after misoprostol x 21 days (n = 50) |
Placebo: initiated after misoprostol x 21 days (n = 50) |
Abortion Success No emergency/elective surgery in the interval up to first menses No need for surgery (%): COC: 49/50 (98%) Placebo: 46/50 (92%) NS (p-value not reported) Bleeding: Hgb testing and bleeding diaries Measured blood loss, mL (median, range): COC: 69.9 (4.4-429.6) Placebo: 72.8 (5.2-855.0) NS (p-value not reported) Hgb level, g/dL (mean, SD): COC: Day 1 12.0 (0.9) Day 15 11.5 (1.1)p = 0.002 Placebo: Day 1 11.7 (1.0) Day 15 11.6 (0.9) NS (p-value not reported) Median bleeding days: COC: 17 Placebo: 15 NS (p-value not reported) |
Adequate sample powered to detect difference in blood loss of 65mL Adequate randomization Multiple methods to assess blood loss including 2 objective blood loss measurements Well-defined outcomes Low attrition: COC: 0% Placebo: 2/50 (4%) |
Allocation not defined Methods for blinding not reported |
| Tang, 1999 [20] Hong Kong Shanghai WHO HRP |
RCT Visits at day 15 (exam, US, Hgb), day 43 (exam), day 67 if persistent bleeding or no menses Fair quality |
Women with menstrual delay ≤ 21 days presenting for medical abortion, GA confirmed with exam +/- US -Mean menstrual delay: 12 days Abortion: mifepristone 200mg PO, misoprostol 400µg PV in hospital 48h later |
COC (30µg EE/ 0.15mg LNG): initiated 1 day after misoprostol x 21 days (n = 100) |
Placebo: initiated after misoprostol x 21 days (n = 100) |
Abortion Success No emergency/elective surgery in the interval up to first menses No need for surgery (%, 95% CI): COC: 98% (93.0, 99.8) Placebo: 99% (94.6, 100.0) NS (p-value not reported) Bleeding: Hgb testing and bleeding diaries Hgb level, g/L (mean, SD): COC: Day 1 122.1 (9.8) Day 15 116.8 (10.4p < 0.001 Placebo: Day 1 123.5 (10.0) Day 15 123.1 (0.9) NS (p-value not reported) Median bleeding days (median, range): COC: 17 (5-57) Placebo: 16 (6-55) No significance testing reported Bleeding more than menses (%): COC: 53% Placebo: 59% No significance testing reported |
Adequate randomization Multiple methods to assess blood loss including objective Hgb measurements Well defined outcomes Low attrition (varied by outcome but highest is 12%) |
Allocation not described Methods for blinding not described No significance testing reported for several outcomes |
| Martin, 1998 [19] Scotland No financial disclosures |
RCT, indirect comparison F/u at 2 and 6 weeks Poor quality |
Women who had a medical abortion at ≤ 63 days GA in hospital (n = 80) -mean GA for all four groups: 48-49 days Abortion: mifepristone 200 mg PO, 0.5 mg gemeprost pessary 48 hours later |
COC (Group A): initiated after abortion completion confirmed and prior to discharge from hospital (n = 20, n = 19 analyzed) |
Placebo (Group D): injection was given after abortion complete confirmed and prior to discharge from hospital (n = 20, n = 20 analyzed) |
Bleeding Days of bleeding after abortion (median, range): COC: 14 (9-45) Placebo: 15 (7-35) Days of heavy bleeding after abortion (median, range) COC: 4 (1-10) Placebo: 4 (0-8) |
Low loss to follow up (total of 2 women from the 80) Bleeding measured through daily diaries |
Allocation concealment was not mentioned Indirect comparison No information on participation rates No analysis of potential confounders Large ranges of bleeding days around median |
AE, adverse events; CI, confidence interval; COC, combined oral contraception; DMPA, depot medroxyprogesterone acetate; EE, ethinyl estradiol; ENG, etonogestrel; F/U, follow-up; GA, gestational age; Hgb, hemoglobin; ITT, intention to treat; LNG, levonorgestrel; Net-EN, norethisterone enanthate; NR, not reported; NS, not significant; PO, oral route; POC, progestogen-only contraception; POP, progestogen-only pill; PV, vaginal route; RCT, randomized controlled trial; SE, side effects; SD, standard deviation; SL, sublingual route; UPT, urine pregnancy test; US, ultrasound; WHO, World Health Organization.
Table 3.
Safety of systemic hormonal contraception initiated after surgical abortion
| Author, year, location, funding | Study design, follow-up, study quality* | Population | Intervention | Comparison | Outcome & results | Strengths | Weaknesses |
|---|---|---|---|---|---|---|---|
| Steinauer, 2014 [23] United States Anonymous donor |
RCT Phone f/u at 2 and 6 months Poor quality |
Women who had a surgical abortion (1st and 2nd trimester) Median weeks GA at time of abortion: Immediate: 15.9 wks delayed: 15.6 wks (n = 298, n = 212 analyzed at 2 month) |
Immediate contraceptive patch: observed initiation in clinic after abortion (n = 154, n = 108 analyzed at 2 month) |
Delayed contraceptive patch: initiated on first Sunday after abortion (n = 144, n = 104 analyzed at 2 month) |
Bleeding Pattern Changes Days of bleeding requiring a pad, self-reported at 2 months Median days of bleeding: Immediate: 5 Delayed: 5p = 0.94 |
Adequate randomization and allocation concealment Blinded outcome assessment by research assistant conducting follow-up Acceptable attrition (immediate: 29.9%, delayed: 27.8%) |
Power calculation rates based on continuation rates Self-reported outcome with potential for recall bias High attrition (29%) at 2 months and at 6 months (47%) Delayed group may have varying times to Sunday start, potentially affecting bleeding outcome |
| Hou, 2017 [30] China Shanghai Population and Family Planning Commission Of PR China |
Prospective cohort Phone f/u at 1, 3, and 6 months Poor quality |
Women who had a vacuum aspiration at 42-70 days GA (n = 705) |
COC (EE/ desogestrel): initiated immediately after abortion (n = 230) |
Non-hormonal: initiated immediately after abortion Cu-IUD (n = 240) Condoms |
Bleeding Pattern Changes Bleeding days after abortion (median, range): COC: 5 (2-7) Cu-IUD: 7 (3-10) Condom: 5.5 (3-8)p = 0.041 |
Outcomes clearly defined | Day of COC initiation was not detailed nor confirmed Non-comparable groups (COC users were younger and had a higher education level; IUD users were older) Self-report of bleeding duration |
| Wang, 2017 [29] China Source of funding NR |
Prospective cohort Clinic f/u on day 21 following abortion Poor quality |
Women who had a surgical abortion, GA was not clearly stated (n = 726) |
COC (30µg EE/3 mg drosperinone): initiated immediately after abortion x 21 days (n = 312) |
Non-hormonal: no COC x 21 days after abortion, recommended condom use (n = 414) |
Bleeding Pattern Changes Bleeding days after abortion (%): <7 days COC: 286/312=91.7% No COC: 294/414=71.0% p < 0.01 >15 days COC: 4/312=1.3% No COC: 27/414=6.5% p < 0.01 Bleeding volume more than menses (%): COC: 13/312=4.2% No COC: 24/414=5.8% p < 0.01 |
No loss to follow-up | GA not specified Day of COC initiation not specified |
| Ortayli, 2001 [21] Turkey Population Council Nobel Co. |
Prospective cohort Clinic f/u at 2 weeks, 6 weeks, 6 and 12 months (Hct at 6 weeks, 6 and 12 months) Menstrual diaries collected at 2 and 6 weeks Poor quality |
Women who had a surgical abortion at ≤ 10 weeks GA by vacuum or electric aspiration (n = 150) Mean GA age Implant: 54.2 days Nonhormonal: 49.4 days |
LNG implant: inserted immediately after abortion (n = 50) |
Non-hormonal/none: withdrawal method or no method (n = 50) |
Bleeding Pattern Changes Hct change from baseline to 6 weeks (mean, SD): Implant: 1.0 ± 3.8 No hormonal/none: 1.7 ± 4.3p = NS Bleeding days, 2 weeks (mean, SD): Implant: 5.0 ± 3.2 No hormonal/none: 6.2 ± 4.6p < 0.05 Bleeding days, 6 weeks (mean, SD): Implant: 6.3 ± 5.7 No hormonal/none: 5.7 ± 6.7p = NS |
Groups similar at baseline by education, pregnancy and abortion history, and use of recent contraception, experience with IUDs or condoms, and BP, Hct and GA Long follow up time Well defined outcomes |
Groups differed by age, time since last pregnancy and experience with OCs, and weight Attrition not reported Some women switched methods during follow-up period No power calculations |
| Querido, 1985 [18] Netherlands Organon Schering |
Multi-site prospective cohort Clinic f/u at 6 weeks Poor quality |
Women who had a surgical abortion, GA was not clearly stated (n = 423) |
OC: initiated after abortion, unclear timing (n = 123) |
Non-hormonal: Cu-IUD inserted immediately after abortion (n = 300) |
Bleeding Pattern Changes Bleeding days after abortion (%): 1-7 days of bleeding: OC: 56.6% Cu-IUD: 29.7% >14 days of bleeding: OC: 9.8% Cu-IUD: 19.9% p < 0.0001 |
OC group was overrepresented with younger, unmarried, nullip, without prior abortion | OC type not specified OC initiation not detailed Groups differed demographically Range of follow-up times (range of less to more than 6 weeks) Unclear randomization of OC group |
| Kurunmaki, 1983 [17] Finland International Development Research Center of Canada, Ford Foundation, Rockefeller Foundation |
Prospective cohort Clinic f/u at 3, 6, 12 months (measured Hgb, BP, weight; daily bleeding records) Poor quality |
Women who had at surgical abortion in the first trimester (n = 68) |
LNG implant: inserted immediately after abortion (n = 38 assigned, n = 36 inserted) |
Non-hormonal: Cu-IUD inserted immediately after abortion (n = 30 assigned, n = 23 inserted) |
Bleeding Pattern Changes Bleeding days after abortion (mean, SD): Implant: 15.1 ± 6.9 Cu-IUD: 14.3 ± 6.9p = NS |
Measured bleeding by both objective and subjective measures Outcomes clearly defined |
Small study size with no power calculations Assignment of contraception type not described Groups only described and compared by assignment not insertions at baseline Attrition not reported No confounding assessment |
| Peterson, 1974 [16] United States Wyeth Labs |
Prospective cohort Clinic f/u at 6 weeks or form filled out by non-study physician at 6 weeks and mailed to clinic Poor quality |
Women who had a surgical abortion, GA was not clearly stated (n = 978, n = 823 analyzed) |
COC: initiated on day of abortion (n = 479 analyzed) |
Non-hormonal/none: diaphragms, condoms, no method (n = 198 analyzed) |
Bleeding Pattern Changes Self-reported at clinic visit or by form at 6 weeks Stopped bleeding by day 7 (%): COC: 58% Control: 59% Stopped bleeding by day 14 (%): COC: 86% Control: 90% Stopped bleeding by day 21 (%): COC: 94% Control: 96% Stopped bleeding by day 28 (%): COC: 97% Control: 98% No significance testing at any time points |
Two control groups using non-hormonal contraceptives Acceptable attrition (overall 15.8%) |
Groups not assessed at baseline or by outcome for potential confounders Outcome ascertainment not described No significance testing to detect outcome differences Unclear what GA included in study Only outcome is a self-reported outcome with potential for recall bias |
BP, blood pressure; COC, combined oral contraception; Cu, copper; EE, ethinyl estradiol; F/U, follow-up; GA, gestational age; Hct, hematocrit; Hgb, hemoglobin; IUD, intrauterine device; LNG, levonorgestrel; LTFU, lost to follow up; NR, not reported; NS, not significant; OC, oral contraception; RCT, randomized controlled trial.
2.4. Quality assessment
Two authors (CK and AN) assessed the quality of each individual study using the United States Preventive Services Task Force grading criteria [12,13]. We rated study quality as good, fair, or poor based on evaluation criteria that included random sequence generation and allocation concealment (for RCTs), selection of participants (for cohort and case-control studies), maintenance of comparable groups, participation rate, extent of loss to follow-up, rigor and completeness of exposure and outcome measurements, adjustment for potential confounders, sample size and precision, and clear definition of interventions and consideration of all important outcomes. We resolved any disagreements between authors for selection, abstraction, or quality assessment by discussion.
2.5. Data synthesis
All authors participated in summarizing and systematically assessing the evidence. We synthesized findings descriptively. We pooled study results when we found comparable study aims, design and outcomes with little statistical heterogeneity. We performed the meta-analysis using Review Manager 5.3 Software (Cochrane Collaboration, Oxford, UK). We pooled data from RCTs on the number of abortion outcome events and number of participants assigned to each treatment group and used a Peto fixed‐effect model to calculate pooled odds ratios and 95% confidence intervals (95% CI). We used the standard Cochrane χ2 to assess statistical heterogeneity and I2 to evaluate magnitude of heterogeneity (greater than 75% indicates considerable heterogeneity) [14,15].
3. Results
We identified 7860 articles (duplicates removed) in our initial search and review of abstracts led to further review of 116 full-text articles (Fig. 1). A total of 16 studies met inclusion criteria: 9 focused on medical abortion and 7 on surgical abortion (Tables 2 and 3) [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. We did not identify any studies of hormonal contraception use following spontaneous or septic abortion that met our inclusion criteria. Six studies addressed timing of hormonal contraception initiation, and 11 studies compared hormonal contraception initiation with nonhormonal or no contraception. Nine studies reported on success of medical abortion after hormonal contraception initiation. Thirteen studies reported on adverse events and side effects.
Fig. 1.
Identification of included studies.
3.1. Medical abortion
We identified 9 medical abortion studies that used a regimen of mifepristone 200 mg orally followed by a prostaglandin analogue 24 to 48 hours later (8 studies used misoprostol and 1 study used gemeprost) and included pregnancies <70 days gestation (Table 2) [19,20,22,[24], [25], [26], [27], [28],31]. Six studies were RCTs [19,20,22,[25], [26], [27]] and 3 were prospective cohort studies [24,28,31]. Nine studies assessed medical abortion effectiveness and 6 evaluated bleeding outcomes. The quality of the studies was fair or poor.
3.1.1. Immediate vs delayed initiation of hormonal contraception
Five studies compared immediate vs delayed initiation of hormonal contraception following medical abortion [19,[24], [25], [26], [27]]. All 5 studies reported abortion success outcomes [19,[24], [25], [26], [27]] and 4 reported bleeding changes or bleeding- related method discontinuation [19,[25], [26], [27]]. One study assessed the initiation of depot medroxyprogesterone (DMPA) [27], 3 studies assessed the etonorgestrel implant [24], [25], [26], and 1 study assessed combined oral contraception (COC) [19]. Immediate contraception initiation in all 5 studies was the day of mifepristone administration.
A fair-quality, noninferiority RCT assigned participants up to 75 days gestation to DMPA initiation at the time of mifepristone (n = 220) or after abortion completion (n = 226) [27]. The investigators defined abortion success as not needing surgery to complete abortion, assessed by urine pregnancy test (UPT) and/or ultrasound. They collected follow-up data within 1 month and at 4 and 7 months. The investigators found no significant differences between the groups in the proportion needing surgery to complete the abortion (risk difference 1.1%, 90% CI −2.8 to 4.9) or the proportion needing any additional treatment (risk difference −0.1%, 90% CI −5.2 to 4.9). Ongoing pregnancy before additional treatment, however, was significantly higher with immediate DMPA compared with delayed DMPA (3.6% vs 0.9%, risk difference 2.7%, 90% CI 0.4–5.6). Those in the immediate DMPA group were more likely to have ≥15 median days of bleeding compared to delayed DMPA group (p = 0.01) but no difference in self-reported bleeding heavier than menses (74% vs 78%, p = 0.27).
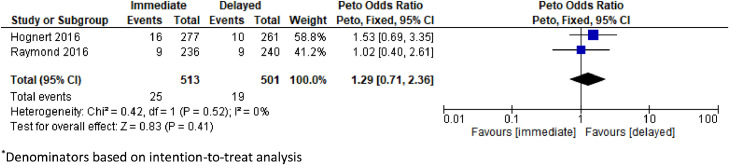
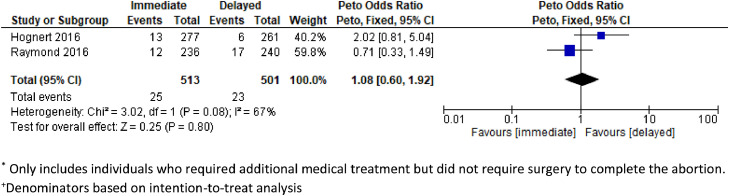
Two fair-quality RCTs and 1 poor-quality prospective cohort study assessed etonorgestrel implants and none demonstrated a significant difference in abortion success rates, rates of surgery required to complete the abortion, or rates of additional treatment [24], [25], [26]. All 3 studies defined abortion success as not needing surgical evacuation. In a noninferiority RCT with a similar design as the DMPA trial, the etonorgestrel implant insertion was on the same day as mifepristone (n = 236) or after the abortion (n = 240) [26]. The investigators assessed abortion success by UPT and/or ultrasound. The study included those who were eligible for an outpatient medical abortion, and approximately 14% to 18% of included participants had a gestational age greater than 64 days. There were no differences between the immediate and delayed initiation groups in the proportion needing surgery to complete abortion (risk difference 0.08%, 90% CI −3.1 to 3.3), the proportion needing any additional treatment (risk difference −1.9%, 90% CI −6.7 to 2.8), or the proportion of ongoing pregnancies (risk difference 0.02%, 90% CI −1.8 to 1.9). Those in the immediate etonorgestrel implant group had a median of 12 days of bleeding after the abortion compared with 10 days for the delayed implant group (p = 0.03) but no difference in self-reported bleeding heavier than menses (68% vs 68%, p = 0.91). A second RCT compared 277 participants at ≤63 days gestational age who had an etonorgestrel implant inserted within 1 hour of mifepristone with 261 participants with insertion at 2 to 4 weeks after mifepristone [25]. There was equivalence in the need for surgery to complete the abortion (risk difference 1.8%, 95% CI 0.4–4.1) and similar implant discontinuation rates due to bleeding at three and six months in the immediate and delayed initiation groups (3 months: 2.2% vs 2.1%; 6 months: 4.3% vs 5.9%, no significance testing reported) [25]. A pooled analysis from these 2 RCTs [25,26] indicated no significant increased risk of requiring surgery to complete the abortion (OR 1.29, 95% CI 0.71–2.36, n = 1014, I2 = 0%) or requiring additional medical treatment (OR 1.08, CI 0.60–1.92, n = 1014, I2 = 66%) with immediate insertion compared to delayed insertion (Figs. 2 and 3). Finally, a prospective cohort study of women ≤70 days compared immediate etonorgestrel implant (n = 68) with etonorgestrel implant insertion at a 4-week follow-up visit (n = 68) [24]. At the 2-week visit, assessment of abortion success by transvaginal ultrasound showed no difference between the immediate and delayed initiation groups (96.5% vs 98.4%, p = 0.47).
Fig. 2.
Pooled estimate of unadjusted odds ratio for need of surgery to complete abortion among individuals* initiating immediate vs delayed etonogestrel implant insertion after a medical abortion.
*Denominators based on intention-to-treat analysis.
Fig. 3.
Pooled estimate of unadjusted odds ratio for need of additional medical treatment* among individuals+ initiating immediate vs delayed etonogestrel implant insertion after a medical abortion.
*Only includes individuals who required additional medical treatment but did not require surgery to complete the abortion.
+Denominators based on intention-to-treat analysis.
One poor-quality RCT assessed COC initiation after medical abortion by randomizing participants at ≤63 days gestational age into immediate COC initiation after abortion success confirmed (n = 20) vs initiation on the first day of next menses (n = 20) [19]. The study defined abortion success as need for surgical evacuation due to retained products of conception (POC) and assessed at 2 and 6 weeks postabortion. There were no cases of incomplete abortion in either group and no differences in bleeding duration between the groups.
3.1.2. Hormonal contraception vs nonhormonal or no contraception
Five studies compared hormonal contraception vs nonhormonal or no contraception following a medical abortion [19,20,22,28,31]. Four studies reported abortion success outcomes, which all found no significant difference in abortion success rates [20,22,28,31]. Three studies reported on bleeding changes [19,20,22]. Three studies assessed the initiation of COC [19,20,22] and 2 studies assessed the initiation of multiple methods: DMPA, COC, progestogen-only pill (POP), and progestogen-only implant [28,31].
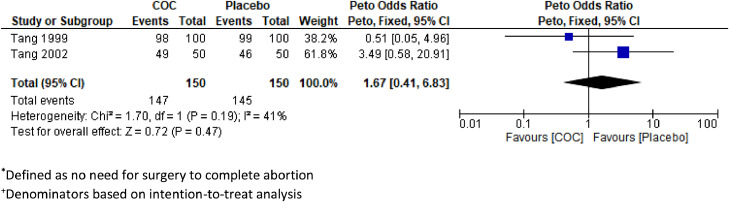
Two fair-quality RCTs randomized participants to either COCs or placebo and compared rates of abortion success, defined as no need for curettage prior to first menses [20,22]. Follow-up in both trials was at days 15 and 43 after misoprostol. The first RCT enrolled 100 participants with menstrual delay ≤21 days in each group, and there were similar rates of abortion success among COC users (98%, 95% CI 93.0–99.8) and placebo (99%, 95% CI 94.6–100.0) [20]. The second RCT enrolled 50 participants with menstrual delay ≤35 days in each group, and the abortion success rate among COC users (98%) was not different from those taking placebo (92%; CI and p value not reported) [22]. In both studies, participants recorded all bleeding and other side effects in diaries and had hemoglobin measurements at days 1 and 15 following mifepristone. The RCT that assessed median blood loss extracted from sanitary pads reported no difference between groups (COC: 69.9 mL, range [4.4–429.6]; placebo: 72.8 mL, range [5.2–855.0]; no p values reported) [22]. For self-reported outcomes, neither study reported a difference in median duration of bleeding, and 1 study did not find a difference in bleeding described as heavier than normal menses. We performed a pooled analysis of data from these 2 RCTs [20,22] and found no significantly different rate of abortion success, defined as no need for curettage prior to first menses, among the group that initiated COC compared with placebo (OR 1.67, 95% CI 0.41–6.83, n = 300, I2 = 40%; Fig. 4). A third poor-quality RCT compared side effects of COC initiation to placebo using a daily bleeding diary for 6 weeks [19]. The bleeding duration and median heavy bleeding days was similar between the 2 groups.
Fig. 4.
Pooled estimate of unadjusted odds ratios for abortion success* among individuals+ initiating combined oral contraception (COC) vs placebo after a medical abortion.
*Defined as no need for surgery to complete abortion.
+Denominators based on intention-to-treat analysis.
A fair-quality retrospective cohort study included 5122 people at ≤63 days who either initiated a hormonal method (DMPA, COC, POP, or implant) at the time of misoprostol or had no hormonal method. The 2-week follow-up involved a home low-sensitivity UPT [31]. For all 4 methods, there were no differences in continuing pregnancy rates compared with no hormonal method. A second poor-quality retrospective cohort study compared 511 people at ≤63 days receiving a progestogen-only method (injectables or implants) to 1971 people receiving no contraception [28]. Follow-up was by phone or at a 3-week clinic visit. The study defined abortion success as complete uterine evacuation without need for additional intervention. Among the progestogen-only subgroups (DMPA and all other progestogen-only methods) and the no contraception group, there was no difference in the abortion success rates (overall progestogen-only: 87.9%, DMPA: 85%, all other progestogen-only: 89.2%, no contraception: 85.7%, p = 0.56).
3.2. Surgical abortion
We identified seven surgical abortion studies: one RCT [23] and 6 cohort studies [[16], [17], [18],21,29,30] (Table 3). Gestational ages included both first and second trimester pregnancies. All 7 studies reported bleeding outcomes [[16], [17], [18],21,29,30], and 2 reported bleeding-related method discontinuation rates [17,18]. We did not identify any studies that assessed other adverse events. The quality of the studies was fair or poor.
3.2.1. Immediate vs delayed initiation of hormonal contraception
One poor-quality RCT compared immediate vs delayed initiation of the contraceptive patch following a surgical abortion and assessed bleeding outcomes [23]. Participants (n = 298) underwent a first- or second-trimester surgical abortion and initiated a contraceptive patch immediately in clinic (n = 154) or on the following first Sunday (n = 144). The self-reported median days of bleeding was 5 in both groups (p = 0.94).
3.2.2. Hormonal contraception vs nonhormonal or no contraception
Six prospective cohort studies compared hormonal contraception vs nonhormonal or no contraception following surgical abortion and assessed bleeding days, comparison to menstrual flow, and hematocrit change [[16], [17], [18],21,29,30]. Three studies assessed COC initiation [16,29,30], 1 study assessed the initiation of oral contraception (OC) [18], and 2 studies the initiation of levonorgestrel implant [17,21].
Three poor-quality studies assessed bleeding duration among COC users compared with nonhormonal/no contraception users, of which 2 studies found significantly shorter durations for COC users [16,29,30]. The earliest study included 823 people who had a surgical abortion at unspecified gestational ages with a 6-week follow-up clinic visit. The investigators assessed self-reported bleeding cessation following the abortion among those who initiated COC on the day of the surgical abortion (n = 479) compared with participants using diaphragm, condom, or no contraception (n = 198). Over the first 28 days, COC users and nonusers had similar proportions who had stopped bleeding (no significance testing reported). A second cohort study included 705 people who had a surgical abortion at 6 to 10 weeks gestation and initiated COC (n = 230), Cu-IUD (n = 240), or condoms (n = 235) on the day of the surgical abortion [30]. The COC group exhibited shorter duration of bleeding (COC: 5 days [2–7], Cu-IUD: 7 days [2–10], condom: 5.5 days [3–8]; p = 0.041) on telephone follow-up at 1, 3, and 6 months. The third study assessed 726 people after surgical abortion at unspecified gestation and compared COC users (n = 312) with condoms users (n = 414) for 21 days after the abortion [29]. Almost 92% of COC users had bleeding duration <7 days, and only 1.3% of COC users had bleeding for more than 15 days compared with 6.5% of condom users (p < 0.01). COC users reported lower proportions of more than usual menstrual volume compared with condom users (4.2% vs 5.8%, p < 0.01).
One poor-quality study assessed bleeding patterns of OC users compared with Cu-IUD users [18]. In this multisite cohort study, 423 people had a surgical abortion at unspecified gestation and immediately initiated OC (n = 123) or Cu-IUD (n = 300). Follow-up at a 6-week clinic visit demonstrated differences in duration of bleeding. The majority of OC users experienced 1 to 7 days of bleeding (57%) while 40% of Cu-IUD users had >7 days of bleeding (p < 0.0001).
Two poor-quality studies assessed bleeding patterns of levonorgestrel implant users compared with nonhormonal or nonusers [17,21]. One study of 150 people who had a surgical abortion at ≤10 weeks gestation assessed hematocrit levels among levonorgestrel implant users (n = 50) and participants using withdrawal or no contraception [21]. Follow-up visits were at 2 weeks, 6 weeks, 6 months, and 12 months. Between levonorgestrel implant users and comparison group, there was no difference in change from baseline to 6 weeks (levonorgestrel implant: 1.0 ± 3.8, control: 1.7 ± 4.3; not significant). This study assessed mean bleeding days as well as a second study which included 68 people who initiated either levonorgestrel implant or Cu-IUD immediately after a first-trimester surgical abortion [17,21]. In the first study, levonorgestrel implant users reported more mean bleeding days at 2 weeks than the comparison group (levonorgestrel implant: 5.0 ± 3.2, control: 4.4 ± 4.5; p < 0.05) [21]. However, in both studies, there was no difference in mean bleeding days at 4 to 6 weeks when comparing levonorgestrel implant users to nonhormonal/nonusers [21] or to Cu-IUD users [17].
4. Discussion
We assessed the effect of non-IUD hormonal contraception initiation on medical abortion effectiveness and the safety of non-IUD hormonal contraceptive methods following abortion. We evaluated the effect of hormonal contraception initiation as well as its timing. We found no studies that met our inclusion criteria for spontaneous or septic abortion nor did we find studies reporting on adverse outcomes of thrombosis and infection. Following a first trimester medical abortion, we found that hormonal contraception initiation (hormonal method vs nonhormonal/no method) and the timing of initiation (immediate vs delayed) generally had little effect on abortion effectiveness or bleeding outcomes. However, evidence was very limited, and estimation of effects was imprecise. One study did report an increase in ongoing pregnancy rate with immediate vs delayed DMPA initiation. Following a surgical abortion, there was mostly no difference in self-reported bleeding outcomes, but there was some variation depending on the method initiated.
We were particularly interested in the timing of systemic hormonal contraception initiation and medical abortion effectiveness given concerns of progestogen-containing methods interfering with mifepristone. Three studies of etonorgestrel implants, including 2 RCTs, found no difference in medical abortion success with immediate vs delayed insertion, which was reiterated by meta-analysis [24], [25], [26]. However, the overall confidence intervals are wide implying inadequate power to make definitive conclusions that success is not altered by implant use. One study of DMPA showed no increase in risk of surgery after medical abortion with a slight, statistically significant increase in ongoing pregnancy with immediate vs delayed DMPA use [27]. This difference may be due to the increased potency of DMPA compared to other progestogen-containing methods, where animal model studies have shown medroxyprogesterone exhibiting a higher concentration to produce half the maximal response [32]. Another plausible explanation is the pharmacologic profile of DMPA where serum MPA concentrations steadily peak to effective concentrations within the first 24 hours of injection and are at high levels the first 30 days [33,34]. In addition, the DMPA study reported on equivalent DMPA utilization rates at 6-month follow-up, thus implying no long-term benefit to immediate DMPA initiation [27]. Nevertheless, immediate initiation of most systemic hormonal methods can be an option and those who opt for immediate DMPA initiation should be informed of the slight increased risk of ongoing pregnancy.
When assessing the 2 studies that compared systemic hormonal contraception initiation to nonhormonal contraception following medication abortion, there was limited evidence to draw conclusions. Results were inconsistent; one study reported slightly fewer women with abortion success in the COC group, but differences were not statistically significant and event rates were very low. A pooled analysis of the 2 trials was similarly imprecise, with wide confidence intervals around the estimated effect. We require larger studies to evaluate whether there is no difference in success rates with COC initiation.
The safety concern with initiating hormonal contraception after abortion revolve around the theoretical concerns of the increased risk of thromboembolism after pregnancy [35] and with combined hormonal contraception use [36], [37], [38]. We did not identify any data that addressed this outcome. In addition, we identified no safety concerns in terms of bleeding following systemic hormonal contraception initiation after medical or surgical abortion. In terms of method discontinuation, bleeding was a main reason for implant removal in immediate and delayed groups at 3- and 6-month follow-up [25], which is consistent with other studies that found bleeding changes was a main reason for implant discontinuation [39,40].
Our study has several weaknesses. We were only able to pool results from four RCTs for 2 contraceptive methods. All included studies were of poor or fair quality. Primary reasons for these low-quality ratings were lack of blinding in abortion success assessment, mixed clinical and self-reporting of bleeding assessment. Currently, there are no standardized measurements for abortion success or postabortion bleeding. Efforts in standardizing abortion outcomes have been published (MARE guidelines, PAIRS framework STAR project) and immediate implementation by researchers will be critical in strengthening study designs and outcomes [41], [42], [43]. In particular, continued pregnancy as a reason for additional treatment is an important indicator of whether an abortion was successful, which not all studies reported. Further research is needed to confirm the risk of ongoing pregnancy with immediate DMPA initiation after medical abortion. All included studies were conducted in high-income or upper-middle-income settings. Future studies should explore hormonal contraception initiation in low-income and lower-middle income settings, where follow-up of adverse outcomes, including abortion failure, may be more challenging. Additional research gaps identified include the need for further investigation of hormonal contraception initiation prior to surgical abortion (e.g., at preprocedure visit or with osmotic dilator insertion) including the benefits of immediate initiation and hormonal contraception initiation with abortion at later gestational ages. The included studies focused on early medical abortions up to 70 days gestation and surgical abortions in the first-trimester or at unspecified gestational ages. We need robust data for medical and surgical abortion beyond 70 days and late second trimester gestation. Different safety concerns may arise with abortions at later gestations, such as risk of thromboembolism.
Initiating non-IUD hormonal contraceptive methods immediately after a medical or surgical abortion and as early as the same day as the first pill of the medical abortion did not result in appreciably different abortion success rates or more adverse bleeding and side effects compared to delayed initiation. While there was no increase in incomplete abortions requiring surgical intervention, there is evidence of slightly higher ongoing pregnancy rates with immediate DMPA use on day of mifepristone administration for medical abortion. Further research on immediate contraception provision after second trimester abortion is important to better inform practices, protocols and patient counseling. Individuals should be informed of potential risks and benefits and engaged in shared decision-making with their providers during contraceptive counseling and provision, which should be a routine component of abortion care for those who desire it.
Declaration of competing interest
None.
Funding
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP).
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.contraception.2021.01.017.
Appendix A. Search strategy
| Database | Strategy |
|---|---|
| PubMed | ((((((((((((("Contraceptives, Oral, Combined"[Mesh] OR "Contraceptives, Oral"[Mesh] OR "Contraceptives, Oral, hormonal"[Mesh] OR "Contraceptives, Oral, Combined"[Pharmacological Action]) OR (contracept* AND (oral OR pill OR tablet)) OR ((combined hormonal) OR (combined oral) AND contracept*) OR (contracept* AND (ring OR patch)) OR "ortho evra" OR nuvaring))) OR (((progestin* OR progestins[MeSH] OR Progesterone[MeSH] OR progestogen* OR progestagen* OR "Levonorgestrel"[Mesh] OR levonorgestrel OR "Norgestrel"[Mesh] OR norgestrel OR etonogestrel) AND contracept*) OR ((depo* AND medroxyprogesterone) OR dmpa OR (net en) OR (norethisterone enant*) AND (contracept* OR inject* OR depo*)) OR (norplant* OR ((levonorgestrel OR etonogestrel) AND implant) OR implanon OR nexplanon OR jadelle OR uniplant OR sino-implant OR (levonorgestrel-releasing two-rod implant))))))) OR (injectable AND contracept))) AND (((((((("Abortion, Induced"[Mesh] OR "Abortion, Spontaneous"[Mesh] OR abortion[tw] OR abortions[tw] OR "Extraction, Obstetrical"[Mesh] OR "Dilatation and Curettage"[Mesh] OR "Vacuum"[Mesh] OR "Suction"[Mesh] OR surgical[tw] OR dilation and evacuation[tw] OR suction[tw] OR vacuum[tw] OR extraction[tw] OR aspiration[tw] OR curettage[tw] OR “D and C”[tw] OR "menstrual regulation" OR mifepristone[tw] OR mifeprex[tw] OR mifegyne[tw] OR RU486[tw] OR misoprostol[tw] OR medical abortion[tw] OR surgical abortion[tw])))))))))) |
| Popline | "Oral Contraceptives" OR "Oral Contraceptive" OR (contracept* AND (oral OR pill OR tablet)) OR (("combined hormonal" OR "combined oral") AND contracept*) OR (contracept* AND (ring OR patch)) OR "ortho evra" OR nuvaring OR ((progestin* OR progestogen* OR progestagen* OR levonorgestrel OR norgestrel OR etonogestrel) AND contracept*) OR (((depo* AND medroxyprogesterone) OR dmpa OR "net en" OR norethisteron*) AND (contracept* OR inject* OR depo*)) OR norplant* OR ((levonorgestrel OR etonogestrel) AND implant) OR implanon OR nexplanon OR jadelle OR uniplant OR sino-implant OR "levonorgestrel-releasing two-rod implant" OR (injectable AND contracept*) AND abortion OR abortions OR "Dilatation and Curettage" OR surgical OR "dilation and evacuation" OR suction OR vacuum OR extraction OR aspiration OR curettage OR "D and C" OR "menstrual regulation" OR mifepristone OR mifeprex OR mifegyne OR RU486 OR misoprostol |
| Cochrane Library | ("Oral Contraceptives" OR "Oral Contraceptive" OR (contracept* AND (oral OR pill OR tablet)) OR (("combined hormonal" OR "combined oral") AND contracept*) OR (contracept* AND (ring OR patch)) OR "ortho evra" OR nuvaring OR ((progestin* OR progestogen* OR progestagen* OR levonorgestrel OR norgestrel OR etonogestrel) AND contracept*) OR (((depo* AND medroxyprogesterone) OR dmpa OR "net en" OR norethisteron*) AND (contracept* OR inject* OR depo*)) OR norplant* OR ((levonorgestrel OR etonogestrel) AND implant) OR implanon OR nexplanon OR jadelle OR uniplant OR sino-implant OR "levonorgestrel-releasing two-rod implant" OR (injectable AND contracept*)):ti,ab AND (abortion OR abortions OR "Dilatation and Curettage" OR surgical OR "dilation and evacuation" OR suction OR vacuum OR extraction OR aspiration OR curettage OR "D and C" OR "menstrual regulation" OR mifepristone OR mifeprex OR mifegyne OR RU486 OR misoprostol):ti,ab |
| Clinicaltrials.gov | Contraception OR contraceptive OR contraceptives OR ortho evra OR nuvaring OR depo* OR medroxyprogesterone OR dmpa OR net en OR norethisterone OR norplant OR implanon OR nexplanon OR jadelle OR uniplant OR sino-implant OR levonorgesterel OR birth control AND Abortion OR abortions |
Appendix. Supplementary materials
References
- 1.Lahteenmaki P, Luukkainen T. Return of ovarian function after abortion. Clin Endocrinol (Oxf) 1978;8:123–132. doi: 10.1111/j.1365-2265.1978.tb02160.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyd Jr EF, Holmstrom EG. Ovulation following therapeutic abortion. Am J Obstet Gynecol. 1972;113:469–473. doi: 10.1016/s0002-9378(15)32496-0. [DOI] [PubMed] [Google Scholar]
- 3.Donnet ML, Howie PW, Marnie M, Cooper W, Lewis M. Return of ovarian function following spontaneous abortion. Clin Endocrinol (Oxf) 1990;33:13–20. doi: 10.1111/j.1365-2265.1990.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber CA, Sober S, Ratcliffe S, Creinin MD. Ovulation resumption after medical abortion with mifepristone and misoprostol. Contraception. 2011;84:230–233. doi: 10.1016/j.contraception.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Boesen HC, Rorbye C, Norgaard M, Nilas L. Sexual behavior during the first eight weeks after legal termination of pregnancy. Acta Obstet Gynecol Scand. 2004;83:1189–1192. doi: 10.1111/j.0001-6349.2004.00494.x. [DOI] [PubMed] [Google Scholar]
- 6.Alegre HdCdP. Misoprostol 400 µg versus 200 µg for cervical ripening in 1st trimester miscarriage. Available at: https://ClinicalTrials.gov/show/NCT02957305; 2016. Accessed 15 May 2020.
- 7.Cansino C, Lichtenberg ES, Perriera LK, Hou MY, Melo J, Creinin MD. Do women want to talk about birth control at the time of a first-trimester abortion? Contraception. 2018;98:535–540. doi: 10.1016/j.contraception.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Flink-Bochacki R, Hamm ME, Borrero S, Chen BA, Achilles SL, Chang JC. Family planning and counseling desires of women who have experienced miscarriage. Obstet Gynecol. 2018;131:625–631. doi: 10.1097/AOG.0000000000002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amsterdam AMC-Uv. Misoprostol for Second Trimester Termination of Pregnancy. Available at: https://ClinicalTrials.gov/show/NCT00945997; 2000. Accessed 15 May 2020.
- 10.Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007;76:267–272. doi: 10.1016/j.contraception.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 13.Force USPST. 2015. U.S. Preventive Services Task Force procedure manual. [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane; 2019. Cochrane handbook for systematic reviews of interventions version 6.0.www.training.cochrane.org/handbook Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson WF. Contraceptive therapy following therapeutic abortion: an analysis. Obstet Gynecol. 1974;44:853–857. [PubMed] [Google Scholar]
- 17.Kurunmaki H. Contraception with levonorgestrel-releasing subdermal capsules, Norplant, after pregnancy termination. Contraception. 1983;27:473–482. doi: 10.1016/0010-7824(83)90044-6. [DOI] [PubMed] [Google Scholar]
- 18.Querido L, Ketting E, Haspels AA. IUD insertion following induced abortion. Contraception. 1985;31:603–610. doi: 10.1016/0010-7824(85)90060-5. [DOI] [PubMed] [Google Scholar]
- 19.Martin CW, Brown AH, Baird DT. A pilot study of the effect of methotrexate or combined oral contraceptive on bleeding patterns after induction of abortion with mifepristone and a prostaglandin pessary. Contraception. 1998;58:99–103. doi: 10.1016/s0010-7824(98)00072-9. [DOI] [PubMed] [Google Scholar]
- 20.Tang OS, Gao PP, Cheng L, Lee SW, Ho PC. A randomized double-blind placebo-controlled study to assess the effect of oral contraceptive pills on the outcome of medical abortion with mifepristone and misoprostol. Hum Reprod. 1999;14:722–725. doi: 10.1093/humrep/14.3.722. [DOI] [PubMed] [Google Scholar]
- 21.Ortayli N, Bulut A, Sahin T, Sivin I. Immediate postabortal contraception with the levonorgestrel intrauterine device, Norplant, and traditional methods. Contraception. 2001;63:309–314. doi: 10.1016/s0010-7824(01)00212-8. [DOI] [PubMed] [Google Scholar]
- 22.Tang OS, Xu J, Cheng L, Lee SW, Ho PC. The effect of contraceptive pills on the measured blood loss in medical termination of pregnancy by mifepristone and misoprostol: a randomized placebo controlled trial. Hum Reprod. 2002;17:99–102. doi: 10.1093/humrep/17.1.99. [DOI] [PubMed] [Google Scholar]
- 23.Steinauer JE, Sokoloff A, Roberts EM, Drey EA, Dehlendorf CE, Prager SW. Immediate versus delayed initiation of the contraceptive patch after abortion: a randomized trial. Contraception. 2014;89:42–47. doi: 10.1016/j.contraception.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Barros Pereira I, Carvalho RM, Graca LM. Intra-abortion contraception with etonogestrel subdermal implant. Eur J Obstet Gynecol Reprod Biol. 2015;185:33–35. doi: 10.1016/j.ejogrb.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Hognert H, Kopp Kallner H, Cameron S, Nyrelli C, Jawad I, Heller R. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484–2490. doi: 10.1093/humrep/dew238. [DOI] [PubMed] [Google Scholar]
- 26.Raymond EG, Weaver MA, Tan YL, Louie KS, Bousieguez M, Lugo-Hernandez EM. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;127:306–312. doi: 10.1097/AOG.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 27.Raymond EG, Weaver MA, Louie KS, Tan Y, Bousieguez M, Arangure-Peraza AG. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739–745. doi: 10.1097/AOG.0000000000001627. [DOI] [PubMed] [Google Scholar]
- 28.Douthwaite M, Candelas JA, Reichwein B, Eckhardt C, Ngo TD, Dominguez A. Efficacy of early induced medical abortion with mifepristone when beginning progestin-only contraception on the same day. Int J Gynaecol Obstet. 2016;133:329–333. doi: 10.1016/j.ijgo.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang XF, Zhong M, Liu J. Effect of short-acting combined oral contraceptives on bleeding after induced abortion. Zhonghua Yi Xue Za Zhi. 2017;97:3255–3257. doi: 10.3760/cma.j.issn.0376-2491.2017.41.012. [DOI] [PubMed] [Google Scholar]
- 30.Hou SP, Zhu WL, Li SM, Teng YC. Acceptance and continuation of contraceptive methods immediate postabortion. Gynecol Obstet Invest. 2017;82:86–95. doi: 10.1159/000445292. [DOI] [PubMed] [Google Scholar]
- 31.Lang C, Chen ZE, Johnstone A, Cameron S. Initiating intramuscular depot medroxyprogesterone acetate 24-48 hours after mifepristone administration does not affect success of early medical abortion. BMJ Sex Reprod Health. 2018;44:242–247. doi: 10.1136/bmjsrh-2017-101928. [DOI] [PubMed] [Google Scholar]
- 32.Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM. Potency of progestogens used in hormonal therapy: toward understanding differential actions. J Steroid Biochem Mol Biol. 2014;142:39–47. doi: 10.1016/j.jsbmb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber C, Barnhart K. Elsevier Saunders; Philadelphia: 2014. Contraception. [Google Scholar]
- 34.Said S, Omar K, Koetsawang S, Kiriwat O, Srisatayapan Y, Kazi A. A multicentred phase III comparative clinical trial of depot-medroxyprogesterone acetate given three-monthly at doses of 100 mg or 150 mg: 1. Contraceptive efficacy and side effects. World Health Organization Task Force on Long-Acting Systemic Agents for Fertility Regulation. Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:223–235. doi: 10.1016/0010-7824(86)90004-1. [DOI] [PubMed] [Google Scholar]
- 35.Tepper NK, Boulet SL, Whiteman MK, Monsour M, Marchbanks PA, Hooper CW. Postpartum venous thromboembolism: incidence and risk factors. Obstet Gynecol. 2014;123:987–996. doi: 10.1097/AOG.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 36.Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257–2266. doi: 10.1056/NEJMoa1111840. [DOI] [PubMed] [Google Scholar]
- 37.Center MM. Testing the efficiency of Karman Curette in the treatment of misoprostol failure in women with missed abortion. Available at: https://ClinicalTrials.gov/show/NCT02917785; 2016. Accessed 15 May 2020.
- 38.Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;8 doi: 10.1002/14651858.CD011054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diedrich JT, Zhao Q, Madden T, Secura G, Peipert JF. Three-year continuation of reversible contraception. Am J Obstet Gynecol. 2015;213 doi: 10.1016/j.ajog.2015.08.001. 662.e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grunloh DS, Casner T, Secura GM, Peipert JF, Madden T. Characteristics associated with discontinuation of long-acting reversible contraception within the first 6 months of use. Obstet Gynecol. 2013;122:1214–1221. doi: 10.1097/01.AOG.0000435452.86108.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creinin MD, Chen MJ. Medical abortion reporting of efficacy: the MARE guidelines. Contraception. 2016;94:97–103. doi: 10.1016/j.contraception.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Whitehouse KC, Kim CR, Ganatra B, Duffy JMN, Blum J, Brahmi D. Standardizing abortion research outcomes (STAR): a protocol for developing, disseminating and implementing a core outcome set for medical and surgical abortion. Contraception. 2017;95:437–441. doi: 10.1016/j.contraception.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor D, Upadhyay UD, Fjerstad M, Battistelli MF, Weitz TA, Paul ME. Standardizing the classification of abortion incidents: the Procedural Abortion Incident Reporting and Surveillance (PAIRS) Framework. Contraception. 2017;96:1–13. doi: 10.1016/j.contraception.2017.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.