Abstract
Background and aims:
Diffuse idiopathic skeletal hyperostosis (DISH) is a common incidental finding on medical imaging and often thought to be benign. Our objective was to investigate whether DISH is associated with coronary artery disease as measured with the coronary artery calcification (CAC) score in a large cohort of current and former smokers.
Methods:
In a subset of subjects from the COPDGene study, DISH was scored by a minimum of two independent readers if there were four adjacent levels of flowing osteophytes and a third reader adjudicated discrepancies. CAC was calculated using a modified Agatston method. Associations of DISH with the presence and extent of CAC were analyzed with and without adjustment for COPD and known atherosclerotic risk factors, including age, sex, race, diabetes, hypertension, high cholesterol, body mass index and smoking.
Results:
DISH was present in 361 subjects (13.2%) from a total group of 2728. Median (interquartile range) Agatston was 81 (0–329) in DISH subjects compared to 0 (0–94 in subjects without DISH (p < 0.001). DISH prevalence was 8.8% in CAC = 0, 12.8% in CAC1–100, 20.0% in CAC100–400 and 24.7% in CAC.400. Subjects with DISH had a significantly higher risk of having coronary artery calcifications; OR [CI95%] 1.37[1.05–1.78] (p=0.019) after correction for age, gender, race, COPD and atherosclerotic risk factors.
Conclusions:
Subjects with DISH, a common musculoskeletal disorder involving bone formation anterior to the spine, have an increased burden of coronary artery disease, and therefore DISH may be a more relevant incidental finding than commonly thought.
Keywords: Calciumscore, CT, DISH, Coronary artery disease, Atherosclerosis, Agatson score
1. Introduction
As far as we know, in 1950, Forestier and Querol were the first to report on diffuse idiopathic skeletal hyperostosis (DISH) [1]. They described it as a peculiar type of ankylosing hyperostosis of the spine characterized by ossification of the anterior and right lateral aspects of the vertebral column, particularly in the thoracic region. The clinical, pathologic, and radiological features of the disorder allowed its differentiation from other spinal diseases including ankylosing spondylitis and osteoarthrosis [1]. The etiology of DISH is still unclear, but genetic, metabolic and probably inflammatory pathways are involved [2,3]. The hallmark of the condition is new bone formation, partly in entheses [3]. Although the exact prevalence and incidence remain undetermined, it is well known that DISH is more frequent in men, and that the incidence increases with age, predominantly affecting white patients over the age of 40 years [4].
DISH is commonly identified as an incidental finding on medical imaging and it is often regarded as a benign characteristic outside the setting of spine trauma. However, since the early descriptions of DISH, there have been associations reported to obesity, large waist circumference, hypertension, cardiomegaly, diabetes mellitus, hyper-insulinaemia, dyslipidaemia and hyperuricaemia [5–8]. In addition, both coronary heart disease by history, and cerebrovascular accidents were found to be associated with DISH and/or spondylosis deformans [9,10]. However DISH as an independent risk factor for cardiovascular disease has never been investigated.
Coronary artery calcifications (CAC) reflects the burden of coronary atherosclerosis and it is a strong risk factor for adverse cardiovascular outcomes [11]. Since the early ‘90s, the Agatston score has been used for an accurate estimation of the 10-year coronary heart disease risk and it is related to classic risk factors such as age, gender, race/ethnicity, diabetes, current smoker, cholesterol levels and others [12]. There is a continuous debate as to whether the calcification formation itself is either harmful or protective. The dominant opinion is that in the early stages, coronary calcifications make atherosclerotic plaques unstable, but in the later stages, the plaque is stabilized with a very low chance of plaque rupture although the stenosis associated with the calcified plaque can be hemodynamically significant [13].
It is not known whether subjects with DISH have more atherosclerosis, but it is our hypothesis that subjects with DISH would have more atherosclerosis and it may even be that DISH and CAC share common underlying (bone formation) pathways. We aimed in this cross-sectional study to investigate whether subjects with DISH have more CAC as a reflection of coronary atherosclerosis.
2. Patients and methods
2.1. Subjects
The Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) Study is a longitudinal, observational study designed to identify subtypes of disease and genetic risk factors for COPD in a biracial population of non-Hispanic white (NHW) (approximately two-thirds) and African American (approximately one-third) current and former smokers with at least a 10–packyears history of cigarette use and an age-range at inclusion between 45 and 80 years. COPDGene participants were enrolled between 2007 and 2011 from 21 study clinical centers in the United Stated of America. The cohort includes smokers with and without spirometric COPD. This ancillary study presents results from the baseline visit that was completed by 10,129 smokers. We investigated a subset of 2817 subjects who had their CT scan done with a calcium calibration phantom [INTable™ CT scanner pads (Image Analysis Inc., Columbia, Kentucky)], where scored for the presence of DISH and who had a successful CAC measurement. In 89 subjects not all four vessels could be scored and these subjects were excluded from the analyses, resulting in 2728 subjects included.
2.2. Risk factor assessment
COPDGene conducted an extensive study visit for each participant. For the present study we used demographic data, medical history, measures of pulmonary function after inhaled bronchodilator (albuterol) and chest CT. Medical history included self-reported physician diagnosis of diabetes, high blood pressure and a high cholesterol.
2.3. CT scanning and assessment of DISH
The scans were acquired using multi-detector CT scanners and standardized protocols as described before in detail [14]. Visual scoring for DISH was performed by trained imaging analysts using TeraRecon software and 3D sharp reconstructions from the stored image files. There were a minimum of two readers per scan who scored DISH if there were three adjacent levels of flowing osteophytes. A third reader adjudicated scans with divergent reads. Extensive disc narrowing associated with the flowing new bone formation was not scored as DISH.
2.4. Coronary artery calcification (CAC) quantification
We quantified CAC following Agatston’s algorithm [15,16] on ungated CT scans, later on referred to as CAC or modified Agatston. Briefly, coronary calcium was classified using a CT threshold of 130 Hounsfield units (HU) involving 3 contiguous voxels for identification of a calcific lesion resulting in a minimum lesion area of 1.02 mm2. The lesion score was calculated using the area density method, by multiplying the lesion area by a density factor [17] of 1 for lesions whose maximal density was 130–199 HU, 2 for lesions 200–299 HU, 3 for lesions 300–399 HU, and 4 for lesions > 400 HU. A total coronary calcium score was determined by summing individual lesion scores from each of four anatomic sites (left main, left anterior descending, circumflex, and right coronary arteries [18]. The calcium score was stratified into categories of 0, 1–100, 101–400 and > 400 modified Agatston Units, which reflects no, mild/minimal, moderate and extensive plaque burden, respectively [19]. In 89 subjects, not all four vessels could be scored and these were excluded from the analyses. The accuracy and reliability of CAC scoring using un-gated CT has been validated in comparisons with ECG-gated studies in COPDGene participants [18].
2.5. Statistical analysis
Normally distributed variables were stated as mean (standard deviation (SD)) and non-normal data as median (interquartile range (Q1–Q3)). Differences between DISH and no DISH subjects were compared using a Student’s t-test (normal continuous variables) and a Mann-Whitney U test (non-normal continuous variables) and a Chi square test (categorical variables). Prevalence of DISH was compared between those with and without CAC and among the CAC severity categories (0, 1–100, > 100–400 and > 400 modified Agatston).
Univariate logistic regression analysis with presence of CAC as independent factor and DISH status (present/absent) as dependent factor was performed. Second, multivariable logistical regression analysis was done first adjusting for age, gender, and race and subsequently additionally for BMI, height, packyears, smoking status, diabetes, cholesterol, blood pressure and COPD. This analysis was done in a stepwise backward elimination based on a p-value < 0.10.
Subsequently given that the logarithmically transformed calcium score was not normally distributed multinomial logistic regression analysis was done with CAC categories (1–100, > 100–400 and > 400) as independent factor and CAC of 0 as reference category and DISH status (present/absent) as dependent factor. First a univariate multinomial logistic regression was performed, subsequently a gender, age and race adjusted analysis and finally a full multivariate multinomial logistic regression analysis. The same correction factors were included and elimination strategy was done as for the logistic regression analysis. We compared the prevalence of DISH and CAC according to the number of risk factors (hypertension, high cholesterol, diabetes, BMI > 30) present. One-way ANOVA was performed with least significance difference testing for multiple comparisons. Subjects with no risk factors were used as reference. A significance level of < 0.05 was set. Statistical analyses were performed with SPSS version 22 (IBM Statistics, Chicago, Illinois, USA).
3. Results
3.1. Baseline characteristics
Of the 2728 subjects included, 13.2% (n = 361) had DISH. Table 1 lists the differences in demographics and cardiovascular risk factors between subjects with and without DISH. Subjects with DISH were significantly older (63.8 vs. 58.7 years), more often male (74.0% vs 47.6%) and former smoker (62.0% vs. 47.2%), compared to subjects without DISH. In addition, subjects with DISH had a significantly higher BMI (32.2 vs. 28.3) and more diabetes (23.8% vs. 10.1%), high blood pressure (58.7% vs. 40.0%) and high cholesterol (50.1% vs. 36.0%).
Table 1.
Baseline characteristics.
| Variable | NO DISH [N = 2367] | DISH [N = 361] | p - value |
|---|---|---|---|
| Age [years], mean [SD] | 58.7 [8.9] | 63.8 [8.8] | < 0.001 |
| Gender [F/M], N | 1240/1127 | 94/267 | < 0.001 |
| Race (White/Black) | 1694/673 | 290/71 | < 0.001 |
| Weight [kg], mean [SD] | 81.7 [19.0] | 96.5 [19.9] | < 0.001 |
| Height [cm], mean [SD] | 169.7 [9.5] | 173.1 [9.0] | < 0.001 |
| BMI [kg/m2], mean [SD] | 28.3 [6.0] | 32.2 [6.2] | < 0.001 |
| Pack years, mean [SD] | 42.9 [24.5] | 47.7 [25.6] | 0.001 |
| Current smoker*, N [%] | 1249 [52.8%] | 137 [38.0%] | < 0.001 |
| Diabetes*, N [%] | 239 [10.1%] | 86 [23.8%] | < 0.001 |
| High blood pressure*, N [%] | 946[40.0%] | 212 [58.7%] | < 0.001 |
| COPD status yes, N [%] | 1036 [43.8%] | 163 [45.2%] | 0.956 |
| High cholesterol*, N [%] | 853 [36.0%] | 181 [50.1%] | < 0.001 |
| CAC present, N [%] | 1110 [46.9%] | 241 [66.8%] | < 0.001 |
| Agatston, median [IQR] | 0 [0–94] | 81 [0–329] | < 0.001 |
| Total CAC volume, median [IQR] | 0 [0–90] | 75 [0–284] | < 0.001 |
N = number; SD= Standard deviation; BMI=Body Mass Index.
Questionnaire derived.
3.2. Coronary artery calcifications in relation to the presence of DISH
Coronary artery calcifications were significantly more prevalent in DISH subjects, 66.8% had coronary artery calcifications compared to 46.9% in those without DISH, p < 0.001. The median (interquartile range) modified Agatston score was 81 (0–329) in DISH subjects compared to 0 (0–94) in subjects without DISH (p < 0.001). An example of a patient with DISH and extensive CAC is shown in Fig. 1. Table 2 lists the results of the logistic regression analysis with the presence of CAC as outcome. Subjects with DISH had a significantly higher odds of having coronary artery calcifications; 1.27 [1.051.78] (p=0.019), after extensive correction for known age, gender, race, cardiovascular risk factors and COPD. DISH prevalence also increased with the modified Agatston strata (Table 3). In those with a modified Agatston > 400, DISH was present in 24.7% while in those with a modified Agatston 1–100 DISH was present in 12.8%. Table 4 shows the results from the multinomial logistic regression analysis with the different CAC categories (1–100; > 100–400 and > 400) as outcome compared to those without CAC as a reference category. In the crude analysis, there was a clear and statistically significant association between DISH presence and increasing CAC strata, which attenuated after adjustment for age, gender and race. After full adjustment in the strata CAC > 100–400 and > 400, DISH significantly associated with the presence of CAC, OR [95%CI] 1.56 [1.12–2.17], p=0.008 and 1.47 [1.02–2.12], p=0.041.
Fig. 1.
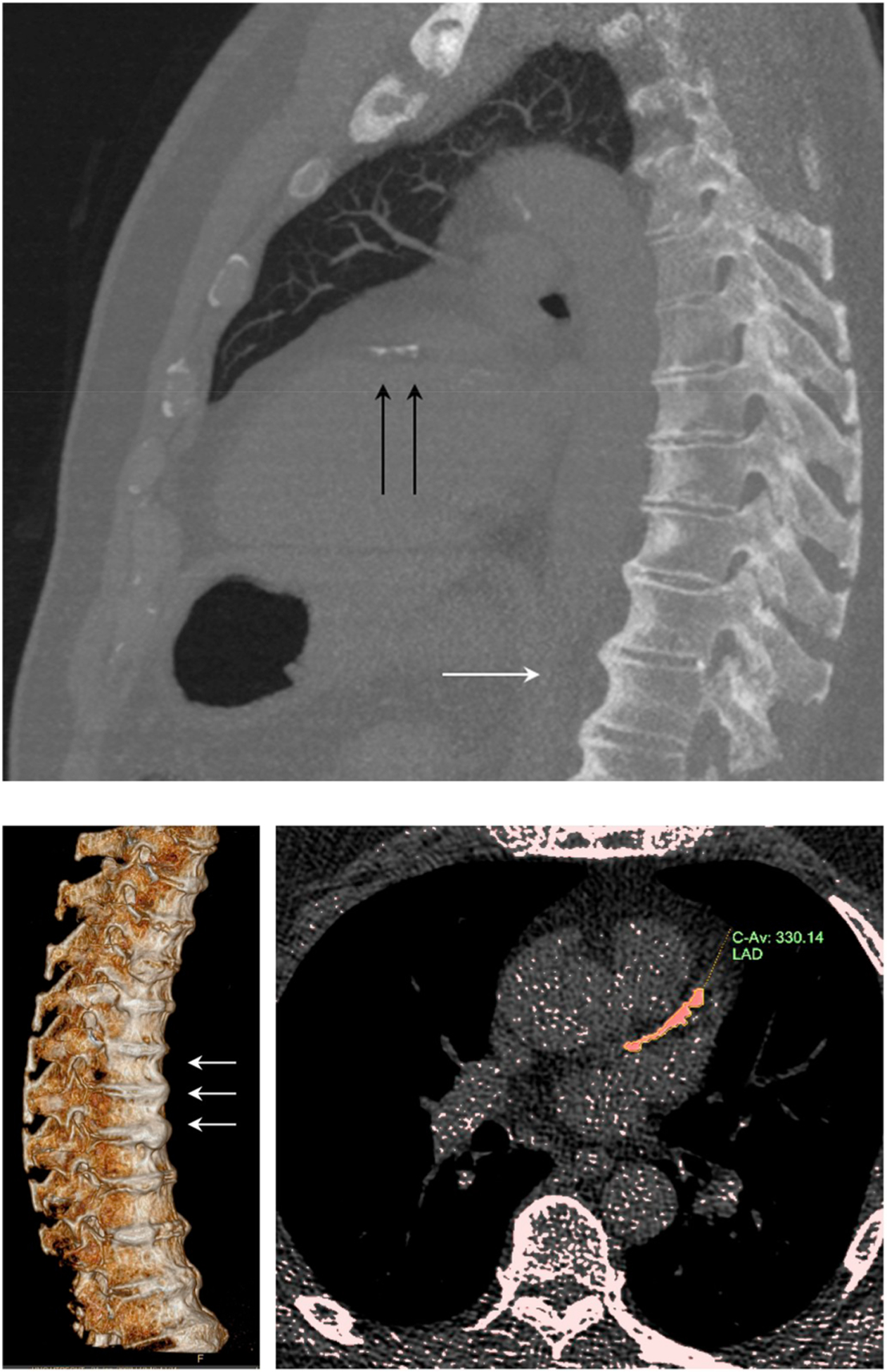
Illustration of a 68 year old male with DISH and CAC score category > 100–400.
(Upper panel) MIP 7 mm sagittal/oblique. White arrows: thoracic DISH; black arrows: calcifications in the left anterior descending coronary artery (LAD). (Lower left panel) 3D reconstruction of the spine showing DISH. (Lower right panel) LAD calcification quantification.
Table 2.
Logistic regression analysis on the association of DISH and the presence of coronary artery calcifications.
| Variable | Units | Univariate model | Age, gender and race adjusted | Full multivariate model | |||
|---|---|---|---|---|---|---|---|
| OR [95%CI] | p-value | OR [95%CI] | p-value | OR [95%CI] | p-value | ||
| DISH | Present vs. absent | 1.32 [1.01–1.72] | < 0.041 | 1.41 [1.09–1.82] | 0.009 | 1.37[1.05–1.78] | 0.019 |
| Age | +1 year | 1.08 [1.07–1.011] | < 0.001 | – | – | 1.08 [1.07–1.10] | < 0.001 |
| Gender | Male vs. female | 1.67 [1.41–1.99] | < 0.001 | – | – | 1.69 [1.43–2.00] | < 0.001 |
| Race | White vs. Black | 1.24 [1.01–1.53] | 0.37 | – | – | 1.26 [1.03–1.55] | 0.028 |
| Packyears | + 10 pack years | 1.01 [1.00–1.01] | 0.135 | 1.01 [1.00–1.01] | 0.01 | ||
| Diabetes | Present vs. absent | 1.05 [0.80–1.38] | 0.721 | 1.36 [1.05–1.7] | 0.019 | – | – |
| BMI | + 1 kg/m2 | 1.01 [0.99–1.02] | 0.364 | 1.02 [1.00–1.03] | 0.030 | – | – |
| Smoking status | Current vs. former | 1.37 [1.13–1.68] | 0.002 | 1.32 [1.09–1.60] | 0.004 | 1.39 [1.15–1.69] | 0.001 |
| Hypertension | Present vs. absent | 1.53 [1.27–1.83] | < 0.001 | 1.71[1.45–2.02] | < 0.001 | 1.56 [1.31–1.87] | < 0.001 |
| High cholesterol | Present vs. absent | 1.25 [1.04–1.50] | 0.019 | 1.40 [1.18–1.66] | < 0.001 | 1.27 [1.06–1.53] | 0.010 |
| COPD | Present vs. absent | 1.61 [1.35–1.93] | < 0.001 | 1.64 [1.38–1.95] | < 0.001 | 1.64 [1.38–1.95] | < 0.001 |
DISH = Diffuse idiopathic skeletal hyperostosis. BMI = Body Mass Index. COPD = Chronic obstructive pulmonary disease. OR = odds ratio. CI = confidence interval. Multivariate model was designed stepwise based on backward elimination at p < 0.10.
Table 3.
Prevalence of DISH in the CAC severity categories.
| CAC category | ||||
|---|---|---|---|---|
| 1 (CAC = 0) n = 1404 | 2 (CAC = 1–100) n = 586 | 3 (CAC > 100–400) n = 430 | 4 (CAC > 400) n = 308 | |
| NO DISH | 1280 (91.2%) | 511 (87.2%) | 344 (80.0%) | 232 (75.3%) |
| DISH | 124 (8.8%) | 75 (12.8%) | 86 (20.0%) | 76 (24.7%) |
CAC is presented as the modified Agatston score as quantified on ungated CT scans. DISH = diffuse idiopathic skeletal hyperostosis.
Table 4.
–Multinomial logistic regression analysis on the association of DISH and CAC severity.
| (CAC = 1–100) | (CAC > 100–400) | (CAC > 400) | ||||
|---|---|---|---|---|---|---|
| OR [95%CI] | p | OR [95%CI] | p | OR [95%CI] | p | |
| DISH crude | 1.52 [1.17–2.05] | 0.007 | 2.58 [1.91–3.48] | <0.001 | 3.38 [2.46–4.65] | <0.001 |
| DISH age, gender, race adjusted | 1.15 [0.84–1.58] | 0.385 | 1.56 [1.13–2.16] | 0.007 | 1.59 [1.11–2.28] | 0.011 |
| DISH fully adjusted* | 1.13 [0.82–1.56 | 0.453 | 1.56[1.12–2.17] | 0.008 | 1.47 [1.02–2.12] | 0.041 |
CAC = coronary artery calcium. OR = odds ratio. CI = confidence interval.
CAC category (1–100; > 100–400 and > 400) is the outcome compared to those without CAC as reference category.
Fully adjusted for age, sex, race, packyears, diabetes, BMI, smoking status, hypertension, high cholesterol and COPD. The fully adjusted model was designed stepwise based on backward elimination at p < 0.10.
The number of CVD risk factors (0–4) in relation with the presence of CAC and DISH is shown in Table 5.
Table 5.
Number of risk factors in relation with the presence of DISH and CAC.
| Number of risk factorsa | |||||
|---|---|---|---|---|---|
| 0 (n=834) | 1 (n=821) | 2 (n=620) | 3 (n=350) | 4 (n=103) | |
| CAC present; n (%) | 324 (38.8%) | 375 (45.7%) * | 381 (61.5%) # | 203 (58.0%) # | 68 (66.0%)# |
| DISH present; n (%) | 58 (7.0%) | 74 (9.0%)NS | 106 (17.1%) # | 86 (24.6%)# | 37 (35.9%)# |
p-value:
< 0.001;
< 0.05; NS > 0.05. Compared with no risk factors (one-way ANOVA with least significance difference testing for multiple comparisons).
Risk factors: hypertension, high cholesterol, diabetes, BMI > 30.
4. Discussion
Disorders involving ectopic mineralisation or bone formation outside the skeleton are common and often regarded as innocent when identified as an incidental imaging finding. In our study population the prevalence of DISH was 13.6%. We showed that these subjects with DISH have a substantial higher burden of coronary artery disease compared to subjects without DISH, independent of age, gender and race. Although DISH was known to be associated with worse outcome in the setting of trauma, there is now growing evidence that DISH is associated with restrictive respiratory physiology [20] and with these findings is associated with coronary artery disease. This challenges the notion that the incidental finding of DISH on radiography or cross-sectional imaging is a benign characteristic or event. Investigations into the interactions between DISH, respiratory disease and coronary artery disease may lead to better understanding of the relationships between musculoskeletal conditions and other systemic diseases.
We hypothesized that subjects with DISH would have an increased burden of atherosclerosis given previous reports on the association between DISH and common cardiovascular risk factors and previous reports of associations with a history of cardiovascular disease [5–10]. The independent associations between these two entities have not been investigated and we were able to directly measure coronary artery disease burden on the same scans on which DISH was diagnosed. Previously DISH was largely diagnosed on radiographs using the criteria proposed by Resnick et al. [21], but we have recently described CT criteria [22]. Since chest radiographs are inadequate to reliably identify coronary calcifications it was previously difficult to investigate the relationship between DISH and coronary artery disease. In our large cohort with CT scans available, we now confirm an association of DISH with coronary atherosclerosis. Even without known CVD risk factors there are subjects with DISH and CAC; so the presence of DISH (as a biomarker) is of importance as part of CVD risk burden. Coronary artery disease remains underdiagnosed in the face of potential primary and secondary preventative strategies. DISH can potentially serve as an early warning of the vulnerable cardiovascular patient especially since chest radiographs are probably the most commonly ordered radiological test worldwide. This association adds to growing evidence on the relevance of DISH, which has also been shown to be related to back pain and to reduced respiratory function.
Can our study shed some light on the etiology of DISH? In medicine, diseases are more and more seen as ‘problems with organs’. DISH is traditionally seen as a musculoskeletal condition as coronary calcification is seen as a cardiovascular problem. It, however, remains important to better understand (and treat) disease to have a more holistic approach based on embryonic origins of tissue or systemic derangements such as low grade inflammation which appears to play a role in many linked diseases in the skeleton, vascular system and beyond.
We realise that much caution is needed in a cross-sectional cohort study. In the crude multinomial regression analyses, we observed a strong and significant association between DISH and the CAC strata, but in the fully adjusted model these associations weakened and only remained significant in the CAC > 100–400 and > 400 strata. One of the explanations could be that some factors such as obesity (high BMI) and diabetes are in the causal pathway of the common findings of CAC and DISH [23]. In diabetes patients, insulin can promote bone formation, which could lead to excess bone formation when insulin levels may be high in the presence of insulin resistance [24]. Adipokines like leptin are associated with obesity and also have an association with an increase in bone metabolism [25] and obese rats with increased leptin receptor genes develop ossifications of the spinal ligaments [26]. Another hypothesis is that ectopic bone formation (as in DISH and CAC) is a general mechanism of tissue repair. At the moment, this is largely speculation, but as therapeutic options are increasing, we think that a better understanding of the etiology of bone forming disorders is important. For example, currently, bisphosphonates are being investigated in vascular calcification disorders, osteoarthritis and brain calcifications [27–29]. Genetic syndromes may help further understand etiology. With respect to DISH, the loss of equilibrative nucleoside Transporter 1 (ENT1) leads to progressive ectopic mineralization resembling DISH in mice, but the vascular status is unknown [30]. In humans, arteries calcify in Generalised Arterial Calcification of infancy (GACI) [31], pseudoxantoma elasticum (PXE), Fahr’s disease or idiopathic basal ganglia calcifications and ACDC [32] but whether these patients develop DISH is unknown. Although care is needed with etiological implications of our study, we think our findings do stimulate further research into a possible shared etiology of DISH and CAC.
4.1. Limitations
Strengths of this study are the large well-defined cohort with CT derived quantitative CAC, a thorough DISH diagnosis and information on important confounding factors such as smoking status, BMI, diabetes, hypertension, high cholesterol was available. However, this study has also limitations. Information on diabetes, high cholesterol and/or high blood pressure were self-reported and the cholesterol was not subdivided into LDL, HDL and triglycerides. This could induce misclassification bias in our adjusted associations. Furthermore, our study was cross-sectional and therefore caution is needed with etiological conclusions. Another limitation is that we used the established diagnostic criteria to diagnose DISH, but did not quantify the severity of DISH. Inflammatory blood markers were not investigated, which would have been interesting given the etiological discussion. Nevertheless, we did show that subjects with DISH have a higher burden of coronary atherosclerosis, which may have medical consequences.
4.2. Conclusion
This the first study that shows that subjects with DISH have more CAC as a reflection of coronary atherosclerosis. Better understanding of underlying, similar pathways of these entities may contribute to our knowledge and eventually treatment of diseases involving ectopic mineralisation. DISH is usually observed as an incidental finding on imaging and may be an important finding to stimulate assessment of occult cardiovascular disease.
HIGHLIGHTS.
Diffuse idiopathic skeletal hyperostosis (DISH) is a common incidental finding on medical imaging and often thought to be benign.
Subjects with DISH have more CAC as a reflection of coronary atherosclerosis.
DISH is usually observed as an incidental finding on imaging and may be an important finding to stimulate assessment of occult cardiovascular disease.
Financial support
The project described was supported by Award Number U01 HL089897 and Award Number U01 HL089856 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
COPD foundation funding
The COPDGene® project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion.
Footnotes
Conflicts of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- [1].Forestier J, Rotes Querol J, Senile ankylosing hyperostosis of the spine, Ann. Rheum. Dis 9 (December 1950) 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vezyroglou G, Mitropoulos A, Kyriazis N, et al. , A metabolic syndrome in diffuse idiopathic skeletal hyperostosis: a controlled study, J. Rheumatol 23 (1996) 672–676. [PubMed] [Google Scholar]
- [3].Mader R, Verlaan JJ, et al. , Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms, Nat. Rev. Rheumatol 9 (12) (2013. December) 741–750. [DOI] [PubMed] [Google Scholar]
- [4].Nascimento FA, Gatto LA, Lages RO, et al. , Diffuse idiopathic skeletal hyperostosis: a review, Surg. Neurol. Int 5 (Suppl 3) (2014. April 16) S122–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Littlejohn GO, Smythe HA, Marked hyperinsulinemia after glucose challenge in patients with diffuse idiopathic skeletal hyperostosis, J. Rheumatol 8 (1981) 965–968. [PubMed] [Google Scholar]
- [6].Littlejohn GO, Insulin and new bone formation in diffuse idiopathic skeletal hyperostosis, Clin. Rheumatol 4 (1985) 294–300. [DOI] [PubMed] [Google Scholar]
- [7].Denko CW, Boja B, Moskowitz RW, Growth promoting peptides in osteoarthritis and diffuse idiopathic skeletal hyperostosis- insulin, insulin-like growth factor-I, growth hormone, J. Rheumatol 21 (1994) 1725–1730. [PubMed] [Google Scholar]
- [8].Kiss C, Szilagyi M, Paksy A, Poor G, Risk factors for diffuse idiopathic skeletal hyperostosis: a case control study, Rheumatology 41 (2002) 27–30. [DOI] [PubMed] [Google Scholar]
- [9].Julkunen, et al. , Diffuse idiopathic skeletal hyperostosis (DISH) and spondylosis deformans as predictors of cardiovascular diseases and cancer, Scand. J. Rheumatol 10 (3) (1981) 241–248. [DOI] [PubMed] [Google Scholar]
- [10].Wilson PW, et al. , Prediction of coronary heart disease using risk factor categories, Circulation 97 (18) (1998. May 12) 1837–1847. [DOI] [PubMed] [Google Scholar]
- [11].Madhavan MV, et al. , Coronary artery calcification: pathogenesis and prognostic implications, J. Am. Coll. Cardiol 63 (17) (2014. May 6) 1703–1714. [DOI] [PubMed] [Google Scholar]
- [12].Pletcher MJ, et al. , Interpretation of the coronary artery calcium score in combination with conventional cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA), Circulation 128 (10) (2013. September 3) 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takata K, Imaizumi S, Zhang B, et al. , Stabilization of high-risk plaques, Cardiovasc. Diagn. Ther 6 (4) (2016. August) 304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Regan Elizabeth A., et al. , Genetic Epidemiology of COPD (COPDGene) study design, COPD 7 (1) (2010. February) 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE, American heart association committee on cardiovascular I, intervention, American heart association council on cardiovascular R, intervention, American heart association committee on cardiac imaging CoCC. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American heart association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology, Circulation 114 (2006) 1761–1791. [DOI] [PubMed] [Google Scholar]
- [16].Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., R. Detrano, Quantification of coronary artery calcium using ultrafast computed tomography, J. Am. Coll. Cardiol 15 (1990) 827–832. [DOI] [PubMed] [Google Scholar]
- [17].Shemesh J, et al. , Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease, Radiology 257 (2010) 541. [DOI] [PubMed] [Google Scholar]
- [18].Budoff MJ, et al. Coronary Artery and Thoracic Calcium on Noncontrast Thoracic CT Scans: Comparison of Ungated and Gated Examinations in Patients from the COPD Gene Cohort. [DOI] [PMC free article] [PubMed]
- [19].Budoff Matthew J., Nasir Khurram, McClelland Robyn L., Detrano Robert, Wong Nathan, Blumenthal Roger S., et al. , Coronary calcium predicts events better with absolute calcium scores than age-gender-race percentiles – the Multi-Ethnic Study of Atherosclerosis (MESA), J. Am. Coll. Cardiol 53 (4) (2009. January 27) 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oudkerk SF, Buckens CF, Mali WP, De Koning HJ, et al. , Diffuse idiopathic skeletal hyperostosis is associated with lower Lung volumes in current and former smokers, Am. J. Respir. Crit. Care Med 194 (2016. July 15) 241–242. [DOI] [PubMed] [Google Scholar]
- [21].Resnick D, Niwayama G, Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH), Radiology 119 (1976) 559–568. [DOI] [PubMed] [Google Scholar]
- [22].Oudkerk SF, de Jong PA, Attrach M, et al. , Diagnosis of diffuse idiopathic skeletal hyperostosis with chest computed tomography: inter-observer agreement, Eur. Radiol 27 (1) (2017. January) 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pillai S, Littlejohn G, Metabolic factors in diffuse idiopathic skeletal hyperostosis - a review of clinical data, Open Rheumatol. J 8 (2014) 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sencan D, Elden H, Nacitarhan V, Sencan M, Kaptanoglu E, The prevalence of diffuse idiopathic skeletal hyperostosis in patients with diabetes mellitus, Rheumatol. Int 25 (7) (2005. September) 518–521. [DOI] [PubMed] [Google Scholar]
- [25].Li H, Jiang LS, Dai LY, Hormones and growth factors in the pathogenesis of spinal ligament ossification, Eur. Spine J 16 (2007) 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shirakura Y, Sugiyama T, Tanaka H, Taguchi T, Kawai S, Hyperleptinemia in female patients with ossification of spinal ligaments, Biochem. Biophys. Res. Commun 267 (2000) 752–755. [DOI] [PubMed] [Google Scholar]
- [27].Kranenburg G, et al. , The effect of etidronate on ectopic mineralization in pseudoxanthoma elasticum - a randomized, double-blind, placebo-controlled trial, J. Am. Coll. Cardiol 71 (10) (2018. March 13) 1117–1126.29519353 [Google Scholar]
- [28].Neogi T, Li S, Peloquin C, et al. , Effect of bisphosphonates on knee replacement surgery, Ann. Rheum. Dis 77 (1) (2018. January) 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oliveira JR, Oliveira MF, Primary brain calcification in patients undergoing treatment with the biphosphanate alendronate, Sci. Rep 6 (2016. March 15) 22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Warraich S, Bone DB, et al. , Loss of equilibrative nucleoside transporter 1 in mice leads to progressive ectopic mineralization of spinal tissues resembling diffuse idiopathic skeletal hyperostosis in humans, J. Bone Miner. Res 28 (2013) 1135–1149. [DOI] [PubMed] [Google Scholar]
- [31].Edouard T, Chabot G, Miro J, Buhas DC, et al. , Efficacy and safety of 2-year etidronate treatment in a child with generalized arterial calcification of infancy, Eur. J. Pediatr 170 (12) (2011. December) 1585–1590. [DOI] [PubMed] [Google Scholar]
- [32].Jin H, St Hilaire C, Huang Y, Yang D, et al. , Increased activity of TNAP compensates for reduced adenosine production and promotes ectopic calcification in the genetic disease ACDC, Sci. Signal 9 (458) (2016. December 13) ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]


