Abstract
Background
Vietnam has emerged as one of the world's leading success stories in responding to COVID-19. After a prolonged period of little to no transmission, there was an outbreak of unknown source in July, 2020, in the Da Nang region, but the outbreak was quickly suppressed. We aimed to use epidemiological, behavioural, demographic, and policy data from the COVID-19 outbreak in Da Nang to calibrate an agent-based model of COVID-19 transmission for Vietnam, and to estimate the risk of future outbreaks associated with reopening of international borders in the country.
Methods
For this modelling study, we used comprehensive data from June 15 to Oct 15, 2020, on testing, COVID-19 cases, and quarantine breaches within an agent-based model of SARS-CoV-2 transmission to model a COVID-19 outbreak in Da Nang in July, 2020. We applied this model to quantify the risk of future outbreaks in Vietnam in the 3 months after the reopening of international borders, under different behavioural scenarios, policy responses (ie, closure of workplaces and schools), and ongoing testing.
Findings
We estimated that the outbreak in Da Nang between July and August, 2020, resulted in substantial community transmission, and that higher levels of symptomatic testing could have mitigated this transmission. We estimated that the outbreak peaked on Aug 2, 2020, with an estimated 1060 active infections (95% projection interval 890–1280). If the population of Vietnam remains highly compliant with mask-wearing policies, our projections indicate that the epidemic would remain under control even if a small but steady flow of imported infections escaped quarantine into the community. However, if complacency increases and testing rates are relatively low (10% of symptomatic individuals are tested), the epidemic could rebound again, resulting in an estimated 2100 infections (95% projected interval 1050–3610) in 3 months. These outcomes could be mitigated if the behaviour of the general population responds dynamically to increases in locally acquired cases that exceed specific thresholds, but only if testing of symptomatic individuals is also increased.
Interpretation
The successful response to COVID-19 in Vietnam could be improved even further with higher levels of symptomatic testing. If the previous approaches are used in response to new COVID-19 outbreaks, epidemic control is possible even in the presence of low levels of imported cases.
Funding
Ministry of Science and Technology (Vietnam).
Translation
For the Vietnamese translation of the abstract see Supplementary Materials section.
Introduction
Vietnam was one of the first ten countries to be affected by the COVID-19 pandemic, with the first confirmed case reported on Jan 23, 2020.1 By Feb 6, 2020, 150 cases had been reported outside of mainland China, of which ten (7%) were identified in Vietnam, putting it in the top ten most affected countries.2 However, by the end of 2020, only 1465 cumulative COVID-19 cases and 0·4 deaths per million inhabitants had been reported in Vietnam, ranking the country among the five countries with the lowest COVID-19 disease burden, and among the three countries with lowest overall mortality.3 Understanding how a low level of transmission was maintained and whether this can be sustained will be crucial when international borders are reopened. At the time of writing, entry to Vietnam has only been granted for diplomatic, official, repatriation, and employment purposes; all individuals must remain in quarantine for 14 days and test negative for SARS-CoV-2 twice before entering the community.
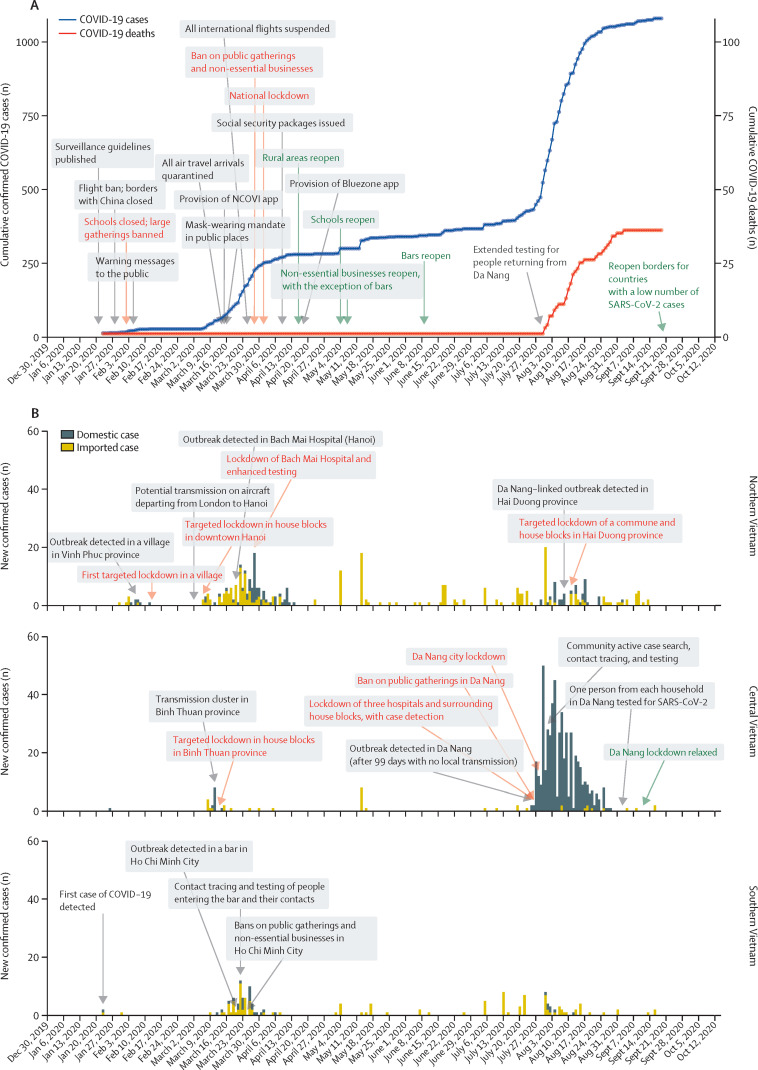
The successful suppression of COVID-19 transmission in Vietnam has been widely praised.4, 5 The initial response to COVID-19 was characterised by a series of measures to prevent onward transmission following importation (figure 1 ). The Vietnamese Government closed borders with China on Jan 28, 2020. The travel restriction policy extended to other countries affected by COVID-19 in mid-February, and to all international travel on March 25, 2020. Additional border control measures included screening and quarantine of all travellers entering Vietnam. The country closed all land border crossings with the three neighbouring countries of China, Laos, and Cambodia, and denied cruise ships permission to dock. Schools and universities closed initially for the 1-week Tet holidays (the Vietnamese Lunar New Year), and the closure was extended in response to the COVID-19 epidemic. In March, 2020, the Vietnamese Government issued a mask-wearing mandate, a ban on public gatherings, and a 2 m physical distancing recommendation.
Figure 1.
Effect of national pandemic measures on COVID-19 cases and deaths nationally, and by region in Vietnam (January to October, 2020)
(A) Cumulative confirmed COVID-19 cases and deaths in Vietnam; red text indicates lockdown measures, and green text indicates relaxation of lockdown measures. NCOVI=Novel COrona VIrus. (B) Number of new confirmed COVID-19 cases by region and case source (domestic or imported); red text indicates lockdown measures and green text indicates relaxation of lockdown measures.
Research in context.
Evidence before this study
Mathematical modelling has been an essential component of the global response to the COVID-19 pandemic. As a result of the successful COVID-19 response in Vietnam, fewer modelling studies have been required in the country in comparison to countries with a higher pandemic burden. We searched PubMed from database inception to Jan 18, 2021, for modelling studies using the search terms (“SARS-CoV-2” OR “COVID-19”) AND (“modelling” OR “model”) AND (“Vietnam”). Our search yielded two modelling studies based on the Vietnamese population, but neither model included epidemiological data.
Added value of this study
We used epidemiological, behavioural, demographic, and policy data to model the response to COVID-19 in Vietnam to understand why the approach was successful, and to investigate potential areas for further improvements in the response considering the risks associated with reopening borders for international travel. We used this approach to quantify the potential scale of future outbreaks, which indicated that future outbreaks are possible, especially if behavioural complacency increases. We also quantified the level of testing required to avoid such outbreaks.
Implications of all the available evidence
Evidence from a diverse range of epidemiological contexts indicates that maintaining high levels of symptomatic testing is essential. Our study suggests that testing of symptomatic individuals will remain crucial even when case counts are low and community transmission has ostensibly been eliminated. Additionally, we provided an important quantitative evaluation of the COVID-19 response in Vietnam, emphasising that ongoing success will depend on the continued willingness of the population and policy to respond and adapt to new epidemiological data as they become available.
Together with enhancement of communication to the public, surveillance, isolation and quarantine, testing, and contact tracing, Vietnam adopted targeted lockdowns early in the pandemic. After several separate epidemics were detected in different locations within a short period of time, the government imposed a national lockdown from April 1 to April 15, 2020. These measures resulted in the prevention of community transmission of COVID-19 for 99 days. On July 25, 2020, a cluster of domestic cases was identified at a provincial hospital in Da Nang in central Vietnam. A lockdown was implemented in the Da Nang region on July 28, 2020, but the outbreak spread rapidly, with cases identified in 14 other provinces. The Da Nang outbreak resulted in more than 500 confirmed COVID-19 cases and the first 35 COVID-19 deaths reported in Vietnam, but was suppressed by late August, 2020.
Since the start of the COVID-19 pandemic, mathematical modelling has been an important component of the global response.6 Reopening international borders will present a challenge and, due to the absence of local transmission and robust pandemic response systems in the country, few modelling studies have used data from Vietnam. The available literature on the risks associated with international travel has shown that border closures were an important component for restricting the spread of the virus,7, 8 but only a small number of studies have assessed the risks associated with reopening borders.9, 10
Until July 25, 2020, no locally transmitted cases of COVID-19 had been reported in Vietnam for 99 days. According to a VnExpress survey of around 95 000 people done on July 26, 2020, 35% of people reported frequently using a face mask in the previous 2 weeks, and 29% of people reported wearing a face mask sometimes in the past 2 weeks. However, in response to the detection of the local cluster of cases, schools and workplaces in Da Nang were closed from July 28 (3 days after cases were detected) until mid-September, 2020, and a stay-at-home order was issued (appendix 2 p 1). According to a VnExpress survey done on Aug 2, 2020, the proportions of people who reported using a face mask in the previous 2 weeks had changed to 90% frequently and 5% sometimes. Such feedback mechanisms between the number of reported cases and the behaviour of individuals have rarely been incorporated directly into modelling frameworks, although some studies have included dynamic interventions based on trigger thresholds.11, 12, 13 We hypothesised that the inclusion of feedback mechanisms in our model would be essential to capture the evolution of the outbreak in Da Nang province, and to make reasonable estimates of the probability of future outbreaks in Vietnam and countries with similar COVID-19 profiles (eg, Taiwan and New Zealand).
In this study, we aimed to use epidemiological, behavioural, demographic, and policy data from the COVID-19 outbreak in the Da Nang region in July, 2020, to calibrate an agent-based model of COVID-19 transmission for Vietnam, model adaptive behavioural changes in response to new information about the epidemic, and to estimate the risk of future outbreaks in Vietnam in response to the reopening of international borders.
Methods
Data sources
For this modelling study, we obtained testing data (daily and cumulative numbers of tests and diagnosed cases by geographical region) from the National Institute of Hygiene and Epidemiology (Hanoi, Vietnam) and patient data from the General Department of Preventive Medicine (GDPM; Ministry of Health, Hanoi, Vietnam). For each COVID-19 case, we extracted data on age, sex, nationality, geographical origin, case classification (imported or domestic), date of diagnosis, symptoms, date of illness onset, date of isolation, date of hospitalisation, the number of close contacts infected with SARS-CoV-2, any complications developed during hospital administration, and date of death, if applicable. Between Jan 23 and Aug 22, 2020, 1014 de-duplicated laboratory-confirmed COVID-19 cases and 35 deaths were recorded in Vietnam.
Data on arrivals to airports in Vietnam between April and November, 2020, were collected from the GDPM and the Pasteur Institute of Ho Chi Minh City (Ho Chi Minh City, Vietnam). All data were de-identified, thus the requirement for written informed consent and ethical approval was waived.
Da Nang outbreak transmission model
We modelled the spread of COVID-19 in central Vietnam using Covasim, an open-source agent-based model of SARS-CoV-2 transmission and disease progression. Further details of the mathematical approach used for Covasim have been published previously, as a preprint.14 Briefly, data on age and sex composition of the population were used to create a model population of individuals with similar characteristics. We used Covasim's inbuilt methods to construct four distinct contact networks that assign these individuals to households, schools, workplaces, and communities on the basis of their age (appendix 2 p 1). The disease transmission model in Covasim was used to propagate these individuals over time. Full details of this model are available online. Briefly, each individual is characterised as susceptible, exposed, recovered, or dead, with exposed individuals additionally categorised according to whether or not viral shedding has started, and according to their symptoms (asymptomatic, mild, severe, or critical). The modelling parameters within Covasim determine the ways in which individuals progress through these states, including the probabilities associated with onward transmission and disease progression, duration of disease by acuity, and the effects of interventions.
Following the release of the initial lockdowns in April, 2020, a campaign was launched in Da Nang to attract domestic tourists, which led to a sharp increase in domestic tourist arrivals: around 250 000 individuals visited in May, 450 000 in June, and around 1·4 million in July, equating to approximately 33 000 individuals per day between June 15 and July 25, 2020. Despite the aggressive response of the Vietnamese Government to the early waves of COVID-19 infections, tests of 895 blood donors in Ho Chi Minh City between Aug 27 and Nov 7, 2020, showed low prevalence (0·2%) of neutralising antibodies against SARS-CoV-2. We therefore initialised the model on June 15, 2020, and, in the subsequent 40 days until July 25, an average of one new infected individual per day (0·003% of incoming tourists; drawn from a negative binomial distribution with dispersion of 0·25) was introduced into the population. This approach approximated the influx of domestic tourists to Da Nang between June 15 and July 25, 2020, with a large number of infected people travelling to a single municipality.
To simulate the policy environment, we include parameters that captured Vietnam's testing, tracing, isolation, quarantine, and lockdown strategies (appendix 2 p 1). The model was calibrated to data from central Vietnam on tests, diagnoses, and deaths obtained for the period June 15 to Oct 15, 2020. The model was calibrated by drawing 20 samples from a distribution of values for the per-contact transmission risk, running 500 simulations for each sample to produce 10 000 trial simulations, and retaining 1% of the simulations with the minimum absolute differences between the model projections and the data. Core parameter values and their sources are presented in appendix 2 (p 1).
An important factor in modelling the Da Nang outbreak was the speed with which both official policy and behaviour adapted to the detection of new COVID-19 cases. In addition to survey data indicating that the proportion of people who reported frequently wearing a mask increased following the reported increase in locally transmitted cases, increased vigilance with regard to hygiene and distancing protocols was also likely. A case-control study on the use of masks and other personal protective measures in Thailand found that individuals who wore masks all the time were more likely to report that their closest contacts were more than 1 m away, contact durations were limited to 15 min or less, and they washed their hands often.15 The study found a negative association between the individual-level risk of COVID-19 transmission and high mask usage (adjusted odds ratio 0·23, 95% CI 0·09–0·60). Using these estimates, we obtained an estimate for the overall individual-level impact of the reported increase in mask usage, corresponding to a reduction in the per-contact probability of transmission of 58% (95% CI 26–73; appendix 2 p 1). In the model, we assume that this estimate applies to the community, workplace, and school networks, but not to households, where people are less likely to wear masks.
Reopening of international borders scenario
To estimate the risk of an imported infection entering Vietnam and escaping quarantine, we analysed data on incoming arrivals to airports in Vietnam and calculated the number of onward transmissions per infected arrival. These calculations considered the quarantine protocols in place in Vietnam (appendix 2 p 3).
To model the risk of cases who evade quarantine causing an outbreak in Vietnam, we established a national model using the parameter values obtained via the calibration process for the Da Nang outbreak. Beginning from a point with no active cases in the community (Nov 30, 2020), we initialised 100 simulations and projected forward by 3 months. The number of new imported cases on each day was drawn from a negative binomial distribution, with parameters based on the observed distribution of imported cases between Feb 1 and Aug 22, 2020. For all future projections, we assumed that schools and workplaces would be closed if more than five cases were detected. We also assumed that all identified contacts of confirmed cases would be tested regardless of symptoms; for individuals with COVID-19-like symptoms but no known history of contact with a case, we assumed 10% of individuals would seek a test during periods of low transmission (based on an analysis of testing data between Feb 1 and Aug 22, 2020), but once more than five cases are detected, aggressive testing campaigns would increase this proportion to 90% of individuals.
Modelled scenarios
The estimation of how policy and behaviour will respond to increases in reported cases is highly relevant for analysing the future trajectory of the epidemic in Vietnam. Therefore, we simulated three scenarios: constant high compliance, increased complacency, and self-regulating behaviour. In the constant high compliance scenario, we assumed that the population would remain highly compliant with measures to stop transmission. We modelled this scenario by assuming the reduction in transmission risk in response to the detection of new locally transmitted cases in late July, 2020, represented a permanent shift in behaviour. In the increased complacency scenario, we included another behavioural change in the model, whereby the relative per-contact probability of transmission increased to the pre-outbreak value after the 14-day average of new locally transmitted cases decreased below two. Following the detection of new cases, we assumed that the probability of someone with symptoms getting tested would increase as testing capacity is scaled up and that policy actions would be implemented comparable with those implemented in the past (ie, localised school and workplace closures following the detection of more than five locally transmitted cases), but we assumed that compliance with mask wearing and non-pharmaceutical interventions would not increase again. In the self-regulating behaviour scenario, the relative per-contact probability of transmission was fully dynamic. The per-contact probability of transmission increased to the pre-outbreak value whenever the 14-day average of new locally transmitted cases decreased below two, but decreased again if the daily number of new locally transmitted cases increased above five. The self-regulating scenario was intended to capture behavioural change in response to the information conveyed by COVID-19 case counts.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
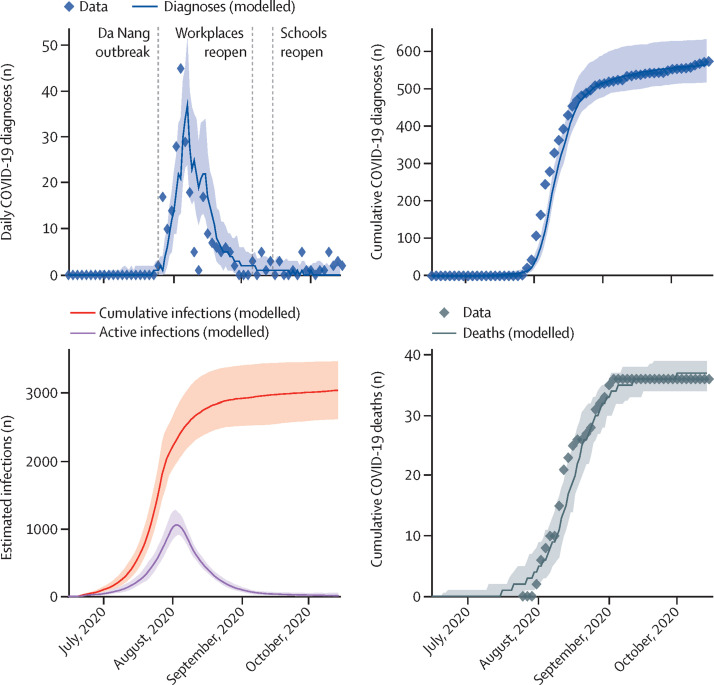
We estimated that the outbreak in central Vietnam is likely to have started before the first cases were detected, with an estimated 1480 infections (95% projection interval 1170–1870) occurring between June 15 and July 25, 2020 (figure 2 ). The level of testing was relatively low during this period, averaging around 380 tests per day, but testing was scaled up rapidly after cases were detected on July 25, 2020, to a peak of around 17 000 tests per day by Aug 13, 2020. We estimate that the outbreak peaked on Aug 2, 2020, with an estimated 1060 active infections (890–1280).
Figure 2.
Modelled projections of COVID-19 cases and deaths in central Vietnam (July to October, 2020)
Solid lines indicate the median model projections over 100 simulations, shaded areas indicate 95% projection intervals, and diamonds indicate data. The daily diagnoses data before Aug 22, 2020, include local transmissions only; diagnosis datapoints after Aug 22, 2020, could not be disaggregated by origin and thus should be interpreted as overestimates of local transmission.
Between June 15 and Oct 15, 2020, we estimated that 3020 people (95% projected interval 2600–3460) were infected, of whom only 574 (20%) were diagnosed. This relatively low case detection rate can largely be explained by the lower testing rates before July 25, 2020. Thereafter, we estimated that the majority of undiagnosed infections can be accounted for by asymptomatic transmission chains: 47% (42–51) of undiagnosed infections that occurred after July 25 were asymptomatic and, of these, 72% (59–82) of individuals acquired COVID-19 from another asymptomatic person, thus making tracing difficult. We estimated the overall infection-fatality rate was 1·2% (1·0–1·3) and case-fatality rate was 6·3% during the Da Nang outbreak.
Available data indicate that the risk of a case entering the country is low, but not zero. Approximately 337 (0·6%) of 55 079 international arrivals to airports in Vietnam tested positive for COVID-19 between April and November, 2020. Although 96% of infected arrivals who arrived during this period had no known onward transmission, this equates to a 4% risk of an infection being released into the community despite a 14-day quarantine period. This risk includes the probability that an infected person develops symptoms only after a 14-day period (around 1%),16, 17, 18 in addition to the probability of a failure in quarantine procedures.
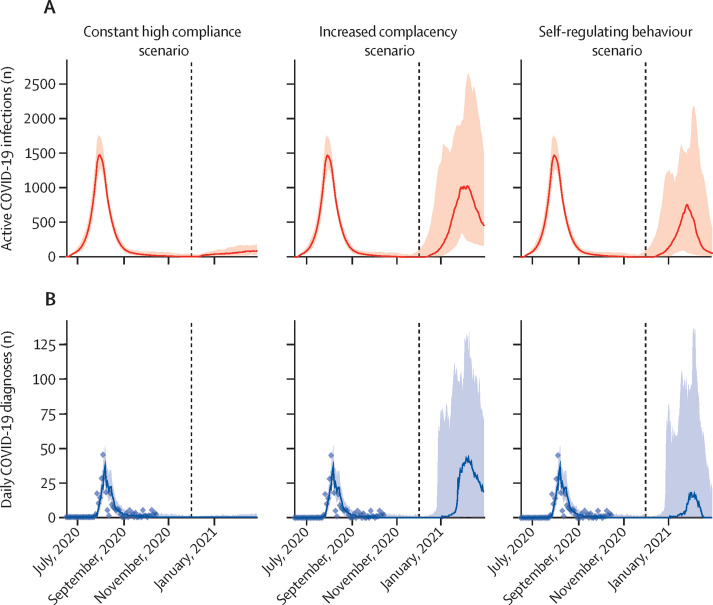
If the population of Vietnam remains highly compliant with mask wearing and other non-pharmaceutical interventions, our projections indicate that the epidemic will remain under control, even if a small but steady flow of imported infections escaped quarantine into the community (figure 3 ). In the increased complacency scenario, if mask usage declines as complacency increases, our estimates showed that the epidemic could rebound, with worst-case scenario estimates projecting a peak of 2500 active cases within 2 months of borders reopening (figure 3). The worst-case scenario could be partly mitigated if policy and behaviour respond dynamically to data showing that the daily number of locally acquired cases has exceeded a specified threshold, which in this scenario we assumed to be five cases, but delays between when infections begin to increase and when the first cases are detected could result in a substantial proportion of transmissions being missed because they occur before policy can respond (figure 3).
Figure 3.
Modelled estimates of active infections (A) and daily diagnoses (B) according to three behavioural scenarios
In the constant high compliance scenario, individuals are assumed to remain fully compliant with masks and other non-pharmaceutical interventions even after prolonged periods of low transmission. In the increased complacency scenario, increasing complacency in the population is assumed to lead to permanently decreased compliance with mask wearing, although government policies mandate the closure of schools and workplaces in response to the detection of more than five locally transmitted cases. In the self-regulating behaviour scenario, compliance with mask wearing and other behavioural interventions decreases and increases as a function of reported cases. Shaded areas show 95% projection intervals and dashed vertical lines show dates of border reopening.
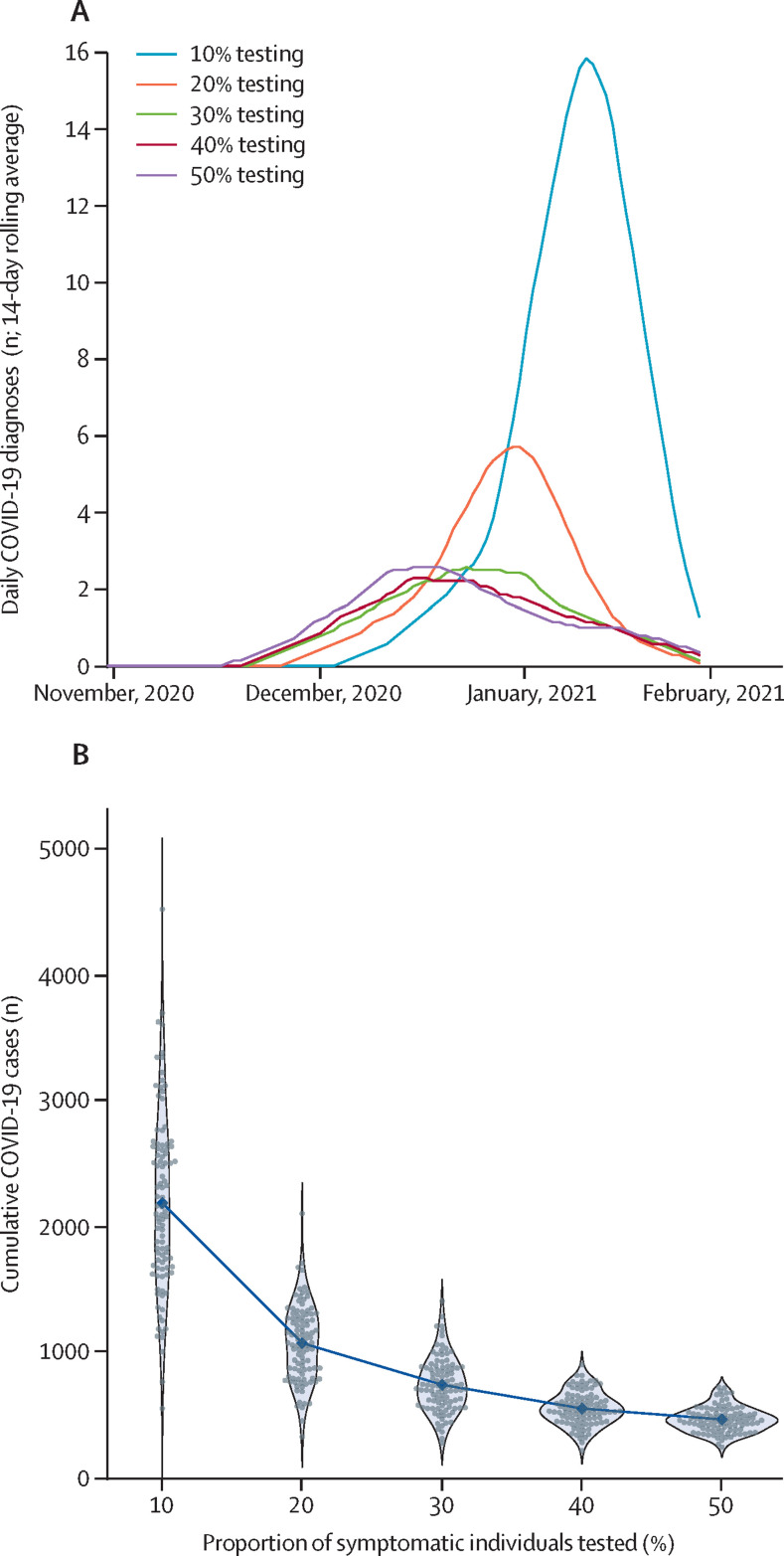
Achieving infection control during future outbreaks depends on how quickly cases are detected, which relies on ongoing testing of individuals with COVID-19 symptoms. In the three behavioural scenarios, we assumed that during periods when no cases had been reported, demand for symptomatic testing would be low, with around 10% of individuals with symptoms seeking a test. Across all simulations, lower testing rates would result in larger and more prolonged increases in transmission before the epidemic could be brought back under control (figure 4A ). The difference in number of COVID-19 cases is particularly notable when the testing rate increases from 10% to 20%, which leads to a halving of the median cumulative number of infections in the subsequent 3 months from 2110 (95% projection interval 1050–3630) to 1100 (570–1670; figure 4B).
Figure 4.
The effect of routine testing of symptomatic individuals on the potential size of an outbreak in Vietnam after border reopening
(A) Median estimated trajectories of the 14-day rolling average of daily COVID-19 diagnoses across 100 simulations. (B) Cumulative number of COVID-19 infections between Dec 1, 2020, and March 1, 2021, in each simulation (grey dots) with medians (blue dots). Blue shaded areas show the density of the projected cumulative infections across 100 simulations.
Discussion
The effectiveness of the COVID-19 response in Vietnam has been well documented.4, 5, 19 During the outbreak in Da Nang in July, 2020, closure of schools and workplaces within 3 days of cases being detected in affected areas, the immediate adoption of masks, widespread testing and quarantine of potentially exposed individuals, and rapid contact tracing enabled the epidemic curve to be flattened within a week of cases being detected. However, our results suggest that response could be further improved if individuals with COVID-19 symptoms were encouraged to seek testing even if they had no known contact with a known case. We estimated that by the time the first cases in Da Nang were detected, 1480 infections (95% projection interval 1170–1870) had occurred, which was likely to be a result of a rapid influx of domestic tourists and low testing rates in Da Nang. Since no quarantine or testing protocols applied to domestic travellers, these COVID-19 cases were not detected.
The response to the COVID-19 outbreak in Da Nang provides insight regarding approaches to implement when borders are reopened. Although reopening borders will require incoming arrivals to follow rigorous testing and quarantine protocols, our analysis of incoming arrivals to Vietnam during a 5-month period indicated that 4% of infected arrivals transmitted to one or more people despite these protocols. Consequently, permitting more international travellers presents a risk. Clear evidence of this risk emerged in late January, 2021, when a case was detected in an airport in Quang Ninh in northern Vietnam after 56 days of no community transmission. Between Jan 28 and Feb 16, 2021, 719 domestic cases were detected and targeted lockdowns were initiated.
Assuming that Vietnam continues to pursue a policy of COVID-19 elimination, our results indicate that containment of future outbreaks is likely to be achieved, assuming that features of earlier responses are repeated. However, if testing rates are low, potential exists for substantial community transmission before detection and consequent containment of new cases. We estimate that if the level of testing remains similar to that observed before the Da Nang outbreak, 1000–4000 cumulative infections could occur in the 3 months after borders are reopened, but that doubling of the testing rate from 10% of people with symptoms to 20% would result in half the number of infections. However, our modelling study was done before variants of concern had been identified, which have subsequently emerged in most countries around the world, including Vietnam. Viral sequencing indicates that four of the cases detected between Jan 28, and Feb 16, 2021 in Vietnam, involved the SARS-CoV-2 B.1.1.7. variant. The emergence of more transmissible strains of SARS-CoV-2 would increase both the probability and expected size of future outbreaks in Vietnam.
Our results highlight the importance of ongoing symptomatic testing in regions with low or no transmission, which has been supported by other studies in the literature. Two studies by our group assessed the effect of testing in settings with low or no transmission. The first study found that ongoing low levels of transmission could be largely controlled by test-and-trace strategies, but that the total number of infections in a 3-month period would be more than 30 times higher with a 50% testing rate than a 90% testing rate.20 The second study estimated the probability of a single introduced case resulting in more than five cases per day within 60 days to be around 50% with no restrictions in place and a testing rate of 25%, compared with 45% with a testing rate of 50%, or 35% with a testing rate of 75%.21 Another study found that mass random testing of 5% of the population per week combined with self-isolation, household quarantine, and tracing of all contacts would lead to a mean transmission reduction of 64%.22 Hellewell and colleagues found that with 20 initial cases and 60% of contacts being traced, less than 50% of outbreaks would be controlled, assuming that all symptomatic cases are eventually detected.23
Our study has a number of limitations. Since we used an agent-based model, our results are based on underlying assumptions about the ways in which these agents interact. We modelled individual interactions over four networks (households, schools, workplaces, and community), but did not explicitly model large gatherings that could potentially become superspreading events. Such events are known to have potential for causing outbreaks.24, 25 Our estimates of the potential scale of an outbreak in Vietnam might therefore be conservative, especially considering events such as the 1-week Vietnamese Lunar New Year in early February, 2021, and the National Congress of the Communist Party of Vietnam in late January, 2021. Superspreading is also partly driven by overdispersion of viral load among individuals, a factor which is included in the model (eg, in Seattle [WA, USA], we estimated that 50% of transmissions were caused by around 10% of infected people26).
Another limitation is that we assumed that the population is homogeneous in terms of behaviour and quarantine compliance. Generally, the omission of variability in model inputs also results in the omission of variability in the outputs. For example, when models assume that mask wearing reduces transmission risk for all individuals by a certain percentage, this actually incorporates a range of individual behavioural changes that might adjust individual-level transmission risk by varying amounts. The possibility of variation in a number of factors (eg, a single individual with a high viral load, a high number of contacts, who does not wear a mask) affects the risk of outbreaks.
Our model did not consider testing or contact tracing supply constraints, which are possible during a rapidly growing epidemic, especially for contact tracing programmes, and which might thus prevent tracing-based containment beyond a certain point. Additionally, we did not consider cost-effectiveness or economic consequences in this model.
Our estimates for parameters such as the age-dependent probability of developing symptoms or dying were derived from studies not specific to Vietnam, and are subject to revision as new information becomes available. Similarly, the population network underlying our model is based on a relatively simple network structure that might omit some aspects of mixing patterns within the Vietnamese population. Our model fitting methodology does not allow us to reliably quantify uncertainty in the transmission probability.
The success of the COVID-19 response in Vietnam is remarkable for several reasons. Compared with other countries aiming for total elimination of COVID-19 (eg, South Korea, Australia, and New Zealand), Vietnam not only has a much larger population and lower per-capita income, but has the additional challenge of monitoring land borders. To maintain this control after reopening borders to international travellers will require a continued commitment to fast and stringent policy adaptations in response to new cases and, importantly, sufficient levels of testing among symptomatic individuals, even among those with no known history of contact with a confirmed case. Rapid containment is only possible if real-time data on the progress of the epidemic is available. As countries such as Vietnam consider how to re-introduce international travel, routine testing as a surveillance measure will be crucial.
Data sharing
The model code for Covasim is available online. The code used to run all simulations contained in this Article is available from a publicly available online repository (https://github.com/optimamodel/covid_vietnam).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the Ministry of Science and Technology (Vietnam; grant: 32/20-ĐTĐL.CN-CNN). We thank additional members of the Institute for Disease Modelling team who contributed to the Covasim model. We also thank front-line colleagues from Vietnam who have devoted their lives to taking care of patients with COVID-19 and for their tremendous efforts to trace, quarantine, and test close contacts of cases and at-risk individuals during the ongoing COVID-19 pandemic. We thank Tu N Le and Thinh V Nguyen at the Pasteur Institute of Ho Chi Minh City for management of model data inputs.
Contributors
QDP, RMS, and CCK conceptualised the study. QDP, QCL, QDT, LTP, TQP, TQD, DNT, and TVN collated and curated the data. RMS and QDP verified the data. APO, QDP, CCK, and RMS visualised the data. RMS, RGA, DJK, DM, and CCK contributed to model development. RMS did data analyses. QDP, RMS, and CCK wrote the manuscript. All authors reviewed the manuscript. All author had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Pollack T, Thwaites G, Rabaa M, et al. Emerging COVID-19 success story: Vietnam's commitment to containment. Our World in Data. March 5, 2021. https://ourworldindata.org/covid-exemplar-vietnam
- 2.Ravelo JL, Jerving S. COVID-19—a timeline of the coronavirus outbreak. Devex. https://www.devex.com/news/sponsored/covid-19-a-timeline-of-the-coronavirus-outbreak-96396
- 3.Johns Hopkins University of Medicine Coronavirus Resource Center Mortality analyses. https://coronavirus.jhu.edu/data/mortality
- 4.Pham QT, Rabaa MA, Duong HL, et al. The first 100 days of SARS-CoV-2 control in Vietnam. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1130. published online Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinh L, Dinh P, Nguyen PDM, Nguyen DHN, Hoang T. Vietnam's response to COVID-19: prompt and proactive actions. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adam D. Special report: The simulations driving the world's response to COVID-19. Nature. 2020;580:316–318. doi: 10.1038/d41586-020-01003-6. [DOI] [PubMed] [Google Scholar]
- 7.Chinazzi M, Davis JT, Ajelli M, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368:395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert M, Pullano G, Pinotti F, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395:871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnet Institute traQ Study. Transparent risk assessment of quarantine. https://www.burnet.edu.au/projects/466_traq_study_transparent_risk_assessment_of_quarantine
- 10.Clifford S, Quilty BJ, Russell TW, et al. Strategies to reduce the risk of SARS-CoV-2 re-introduction from international travellers. medRxiv. 2020 doi: 10.1101/2020.07.24.20161281. published online July 25. (preprint). [DOI] [Google Scholar]
- 11.Chowdhury R, Heng K, Shawon MSR, et al. Dynamic interventions to control COVID-19 pandemic: a multivariate prediction modelling study comparing 16 worldwide countries. Eur J Epidemiol. 2020;35:389–399. doi: 10.1007/s10654-020-00649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker PGT, Whittaker C, Watson OJ, et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;369:413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malani A, Soman S, Asher S, et al. National Bureau of Economic Research; 2020. Adaptive control of COVID-19 outbreaks in India: local, gradual, and trigger-based exit paths from lockdown. [DOI] [Google Scholar]
- 14.Kerr CC, Stuart RM, Mistry D, et al. Covasim: an agent-based model of COVID-19 dynamics and interventions. medRxiv. 2020 doi: 10.1101/2020.05.10.20097469. published online May 15, 2020. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doung-ngern P, Suphanchaimat R, Panjangampatthana A, et al. Case-control study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis. 2020 doi: 10.3201/eid2611.203003. published online Sept 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linton NM, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9:E538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z, Xu X, Wu Y, Wang L, Cowling BJ, Meyers LA. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis. 2020;26:1341–1343. doi: 10.3201/eid2606.200357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nong VM, Le Thi Nguyen Q, Doan TT, et al. The second wave of COVID-19 in a tourist hotspot in Vietnam. J Travel Med. 2020 doi: 10.1093/jtm/taaa4. published online Sept 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuart RM, Abeysuriya RG, Kerr CC, et al. The role of masks, testing and contact tracing in preventing COVID-19 resurgences: a case study from New South Wales, Australia. BMJ Open (in press). [DOI] [PMC free article] [PubMed]
- 21.Abeysuriya RG, Delport D, Stuart RM, et al. Preventing a cluster from becoming a new wave in settings with zero community COVID-19 cases. medRxiv. 2020 doi: 10.1101/2020.12.21.20248595. published Dec 22. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucharski AJ, Klepac P, Conlan AJK, et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellewell J, Abbott S, Gimma A, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dave DM, Friedson AI, McNichols D, Sabia JJ. Institute of Labor Economics; September, 2020. The contagion externality of a superspreading event: the sturgis motorcycle rally and COVID-19.http://ftp.iza.org/dp13670.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemieux JE, Siddle KJ, Shaw BM, et al. Phylogenetic analysis of SARS-CoV-2 in Boston highlights the impact of superspreading events. Science. 2020;371 doi: 10.1126/science.abe3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr CC, Mistry D, Stuart RM, et al. Controlling COVID-19 via test-trace-quarantine. medRxiv. 2020 doi: 10.1101/2020.07.15.20154765. published online Oct 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The model code for Covasim is available online. The code used to run all simulations contained in this Article is available from a publicly available online repository (https://github.com/optimamodel/covid_vietnam).