Abstract
Autologous chondrocyte implantation (ACI) is a regenerative procedure used to treat focal articular cartilage defects in knee joints. However, age has been considered as a limiting factor, and ACI is not recommended for patients older than 40–50 years of age. One reason for this may be due to the reduced capacity of aged chondrocytes in generating new cartilage. Currently, the underlying mechanism contributing to aging-associated functional decline in chondrocytes is not clear, and no proven approach exists to reverse chondrocyte aging. Given that chondrocytes in healthy hyaline cartilage typically display a spherical shape, believed to be essential for chondrocyte phenotype stability, we hypothesize that maintaining aged chondrocytes in a suspension culture that forces the cells to adopt a round morphology may help to “rejuvenate” them to a younger state, thus leading to enhanced cartilage regeneration. Chondrocytes isolated from aged donors displayed reduced proliferation potential and impaired capacity in generating hyaline cartilage, compared to cells isolated from young donors, indicated by increased hypertrophy and cellular senescence. To test our hypothesis, the “old” chondrocytes were seeded as a suspension onto an agarose-based substratum, where they maintained a round morphology. After the 3-day suspension culture, aged chondrocytes displayed enhanced replicative capacity, compared to those grown adherent to tissue culture plastic. Moreover, chondrocytes subjected to suspension culture formed new cartilage in vitro with higher quality and quantity, with enhanced cartilage matrix deposition, concomitant with lower levels of hypertrophy and cellular senescence markers. Mechanistic analysis suggested the involvement of the RhoA and ERK1/2 signaling pathways in the “rejuvenation” process. In summary, our study presents a robust and straightforward method to enhance the function of aged human chondrocytes, which can be conveniently used to generate a large number of high-quality chondrocytes for ACI application in the elderly.
Keywords: chondrocytes, suspension cell culture, cell shape, RhoA, ERK1/2, cellular rejuvenation
1. INTRODUCTION
Due to limited intrinsic self-repair capacity, injury of articular cartilage often leads to tissue degeneration and may result in joint pain and impaired function and pain (1). Currently, several preservative and surgical treatments are clinically available. In particular, autologous chondrocyte implantation (ACI) has shown efficacy in reducing pain and restoring join function (2). In vitro expansion of primary chondrocytes is required to collect a sufficient number of cells for ACI (3). However, ACI or matrix-assisted ACI procedures in the clinic often exclude old adults (45 years old and older) (4), despite some reports exploring the feasibility of ACI for aged patients (5, 6). Several considerations have led to age-restricted patient selection in ACI. In addition to the general situations related to health status of the elderly, such as increased risk of surgery and extended recovery time, the reduced quantity and quality of chondrocytes from old donors account for another significant reason. In the aged patients, less cartilage tissue is available to ACI, which also contains lower abundance of chondrocytes. In addition, aged chondrocytes have reduced proliferation capacity, and thus potentially unable to yield sufficient cell number for successful implantation (7–10). Moreover, the quality of neocartilage generated by chondrocytes declined with donor age (8, 11). Hence, enhancing the functionality of chondrocytes from the old donors is critical to adopt ACI for hyaline cartilage tissue repair in the elderly.
Currently, very few studies have been conducted to “rejuvenate” old chondrocytes. With cartilage aging or degeneration, the shape of chondrocytes changes from a rounded/elliptical morphology to an abnormal morphology, associating with cytoskeleton alteration and aberrant matrix metabolism (12, 13). It is noteworthy that, in monolayer culture, chondrocytes also lose the spherical shape that they possess in vivo and assume a flattened fibroblastic structure. The process is termed as “dedifferentiation”, which is accompanied by reduced gene expression of the major cartilage matrix components, collagen type II (COL2) and aggrecan (ACAN) (14), along with the loss of proliferative capacity (10, 13). Changes in chondrocyte shape also regulates gene expression of catabolic factors that degrade the extracellular matrix (15). Importantly, maintenance of a round cell shape as a result of cell-cell aggregation or condensation has long been shown as a prerequisite for mesenchymal chondrogenesis during embryonic development (16–19). Taken together, these results strongly suggest that modulation of cell shape may be a promising strategy to reverse the inferior properties of old chondrocytes.
Currently, suspension culture or encapsulating chondrocytes within a biomaterial scaffold has been shown to improve the chondrogenic phenotype and partially reverse the cell dedifferentiation due to in vitro expansion (20–23). In particular, agarose is the most tested scaffold for such applications (20, 24–26). After being encapsulated in the agarose-based culture system, the morphology of the chondrocytes changed from a flat to a spherical shape, and cartilage-specific marker gene expression levels were elevated, and proteoglycan and collage secretion increased. The cytoskeletal structure of the agarose cultured chondrocytes was similar to those observed in situ (26).
In light of the demonstrated association between cell morphology and phenotype, we hypothesize that a suspension culture that compels old chondrocytes to adopt a round morphology can “rejuvenate” them to a younger state, thus leading to enhanced cartilage regeneration. To test this hypothesis, we first assessed the aging-relevant alterations in human chondrocytes, with a special focus on the capacity of generating new cartilage. Next, we cultured the old chondrocytes as a suspension on an agarose-based substrate, on which chondrocytes were unable to adhere, thus maintaining a round morphology. After 72 hours of suspension culture, the treated chondrocytes were harvested, and their proliferation and chondrogenic potential were examined, using untreated old chondrocytes as the control. In particular, the degree of senescence in the neocartilage formed by the cultured chondrocytes was determined, as a critical evaluation of cartilage quality. Lastly, changes in subcellular signaling pathways were probed to assess the mechanistic aspects of the “rejuvenation” process.
2. MATERIALS AND METHODS
2.1. Materials
Fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), Dulbecco’s modified Eagle medium (DMEM, Invitrogen), insulin-transferrin-selenium supplement (ITS, Invitrogen), antibiotic-antimycotic (anti-anti, Invitrogen), trypsin-EDTA (0.25%, Invitrogen), Quant-iT PicoGreen dsDNA reagent and kits (Invitrogen), TRIZOL reagent (Invitrogen), SuperScript VILO cDNA synthesis kit (Invitrogen), Applied Biosystems Power SYBR Green PCR Master Mix (Invitrogen), Halt protease inhibitors, Halt phosphatase inhibitors, Pierce™ BCA protein assay kit (ThermoFisher Scientific, Waltham, MA), and SuperSignal West Dura Extended Duration Substrate (ThermoFisher Scientific) were purchased from the indicated suppliers. Collagenase type II, CellTiter 96 AQueous One Solution cell proliferation assay (MTS), and Accumax cell dissociation solution were purchased from Worthington Biochemical (Lakewood, NJ), Promega (Madison, WI), and Innovative Cell Technologies (San Diego, CA), respectively. All other chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless stated otherwise.
2.2. Isolation and culture of chondrocytes
Primary chondrocytes were isolated from deidentified human knee articular cartilage without signs of osteoarthritis or injury obtain through the National Disease Research Interchange (Philadelphia, PA), with approval from the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents (CORID). All experiments were performed in compliance with institutional guidelines and with approval from the University of Pittsburgh Institutional Review Board (IRB). Briefly, fresh articular cartilage tissues were rinsed with DMEM containing 2× anti-anti and then cut into ~1-mm3 pieces. Afterwards, the tissue pieces were digested with collagenase type II (0.2% w/v) in a 37°C shaker overnight. The debris was removed with 40 μm cell strainer (BD Falcon, Bedford, MA) and the cell suspension was centrifuged at 300 × g for 5 minutes. The cell pellet was resuspended in the growth medium (DMEM containing 10% FBS and 1× anti-anti) and seeded in a cell culture flask at a density of 1 × 104 cells/cm2. After chondrocytes reached 70% confluency, the cells were dissociated with trypsin-EDTA and passaged. Passage 2 (P2) chondrocytes were used for all experiments. The young and old chondrocytes used in this study were pooled by equally mixing cells isolated from three young (15, 42 and 43 years old) and aged (>70, 72, 79 years old) donors. For cell area measurement, 100 cells were reandomly selected from phase-contrast micrographs, and cell area was determined using ImageJ software.
2.3. Proliferation assay
Young or old chondrocytes were seeded in a 24-well culture plate at 4,000 cells /well. At days 0, 1, 3, and 7, the metabolic activity and DNA content of cell culture were measured by the MTS and PicoGreen assays, respectively.
2.4. Pellet culture
To examine cartilage-generating capacity, conventional chondrocyte pellet culture was used. Briefly, 250,000 chondrocytes were resuspended in chondrogenic medium (high-glucose DMEM, 1% antibiotic-antimycotic, 1% ITS, 100 nM dexamethasone, 50 μM ascorbic acid, 23 μM L-proline, and 10 ng/mL transforming growth factor-β3 (TGF-β3; PeproTech, Rocky Hill, NJ), and pellets were formed in 96-well conical bottom plate via centrifugation for 10 minutes at 300 × g. The medium was changed every 2–3 days. The pellet cultures were harvested on day 14 for different analyses.
2.5. Real-time RT-PCR for marker gene expression analysis
The phenotype of newly formed cartilage from pellet culture was characterized using real-time reverse transcription polymerase chain reaction (qRT-PCR).The pellets were washed with HBSS and crushed in TRIZOL reagent. Total RNA was then extracted and purified using RNeasy Mini Kit (QIAGEN, Cat. No. 74104, Germany Hilden), followed by reverse transcription into cDNA, as described in our previous study (27). SYBR green-based method was used for qRT-PCR. Specifically, the expression levels of the following genes were analyzed: chondrogenic genes - SOX-9, aggrecan (AGCN), collagen type II (COL2); chondrocyte hypertrophy markers – collagen type X (COL10), alkaline phosphatase (ALP), matrix metalloproteinase 13 (MMP13); and senescence-associated markers - p16Ink4a (P16), P21 and P53. Ribosomal protein L13a (RPL13a) was used as an endogenous housekeeping gene. The gene primers used are shown in Supplementary Table 1.
2.6. Histology (Alcian Blue and Safranin-O/Fast Green staining)
Pellets were fixed overnight at 4 °C in 10 % buffered formalin (Fisher Chemical, Hampton, NH), dehydrated in ethanol, cleared in xylene, and then embedded in paraffin. The blocks were sectioned at 6 μm thickness using a Leica microtome (Model RM 2255). For Alcian Blue staining, sections were deparaffinized using Histo-Clear II (National Diagnostics, Atlanta, GA, USA), rehydrated, and stained with Alcian-Blue staining solution (Sigma). For Safranin O/Fast Green staining, slides were stained with hematoxylin (Mayer, Sigma) for 8 min, 0.05% Fast Green (Fisher Scientific, Pittsburgh, PA, USA) for 3 min, rinsed with 1% acetic acid, and stained with 0.5% Safranin O (Millipore, Billerica, MA) for 20 min. Histological staining was imaged using a microscope equipped with a color digital camera (Nikon Eclipse E800).
2.7. Immunohistochemistry
Immunohistochemistry was performed using the Vectastain Elite ABC-HRP Kit (Vector Labs, Burlingame, CA). Sectioned samples were prepared for histology as described above. Sections were blocked with 10% horse serum in phosphate-buffered saline (PBS) for 1 hour and then incubated overnight at 4 °C with primary antibodies against MMP-13 (Abcam, Cambridge, MA; Cat. No. ab39012), COL10 (Abcam; ab49945), COL2 (Abcam; ab34712), p16 (Abcam; ab108349), and p21 (Abcam; ab218311), respectively. Afterwards, the sections were sequentially incubated with biotinylated secondary antibodies for 1 h, and prepared Vectastain Elite ABC reagent for 30 minutes. Lastly, peroxidase substrate was added followed by incubation for various time periods appropriate for the different antigenic targets. After counterstaining with hematoxylin, slides were dehydrated, mounted, and coverslipped.
2.8. “Rejuvenating” old chondrocytes with on-agarose suspension culture
As modified from our previously published protocol (28), a sterile agarose solution (3% w/v in PBS, type VII) was cast in 24-well culture plate, followed by equilibration in growth medium for 24 hours. Old chondrocytes (1 million cells in 1 mL growth medium supplemented with 2% FBS) were then seeded on the agarose and maintained for up to 72 hours. At different time points, the cultured cells were collected for Western blot assay. After 72 hours, the cell spheroids that were formed were dissociated using Accutase® cell detachment solution (Innovative Cell Technologies, San Diego, CA) at room temperature for 1 hour, and filtered through 40 μm strainer to generate single cells. The old chondrocytes were named as Old-SUS or Old-CON group for cells with or without being subjected to suspension culture, respectively. The proliferation capacity and cartilage formation potential of chondrocytes from Old-SUS and Old-CON groups were compared using the methods described above.
2.9. Western blotting
Chondrocytes collected at different time points during the “rejuvenation” process were homogenized and extracted in RIPA buffer (Sigma; Cat. No. R0278) supplemented with Protease and Phosphatase Inhibitor Single-Use Cocktail (100×) (Thermofisher; Cat. No. 78442) and then centrifuged at 10,000 g for 10 minutes to obtain the supernatant. Protein concentration in the supernatant was determined by BCA protein assay. The samples were diluted in RIPA, mixed with Laemmli buffer (Bio-Rad, Hercules, CA), and then denatured at 95 °C for 5 min. Proteins were fractionated electrophoretically on NuPAGETM 4–12% Bis-Tris Polyacrylamide Gel (Invitrogen) and then transferred to a polyvinylidene fluoride (PVDF) membrane using the iBlot Dry Blotting System (Invitrogen). The membrane was blocked with 5% bovine serum albumin (BSA) at room temperature for 1 hour, washed, and incubated with primary antibodies at 4 °C overnight on a rotating shaker. Next, the membrane was washed three times with TBST buffer and incubated with horseradish peroxidase (HRP)-linked secondary antibodies (GE Healthcare Life Sciences, Malborough, MA) for 1 hour at room temperature and washed three times, followed by incubation with the chemiluminescence substrate. Primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cat. No. 5174), total ERK1/2 (4695), phosphorylated ERK1/2 (9101), RhoA (2117), and SOX9 (82630) were purchased from Cell Signaling Technology (Danvers, MA), and antibodies against p53 (Cat. No. ab26), p21 (ab218311), p16 (ab108349), and MMP13 (ab39012) were purchased from Abcam. Images were acquired using the ChemiDoc™ Touch Imaging System (BIO-RAD, Hercules, CA).
2.10. Statistics analysis
Quantitative data were expressed as mean ± standard deviations (S.D.). Statistics were analyzed by Student’s t-test and p values <0.05 are considered statistically significant.
3. RESULTS
3.1. Old chondrocytes displayed lower proliferation capacity than young chondrocytes
The morphology of chondrocytes isolated from young (15–43 years old) and aged (>70 years old) donors are shown in Figure 1A. Old chondrocytes were generally larger than young chondrocytes when initiated seeded on tissue culture plastic. The ratio of old chondrocytes with large cell surface area (>2, 000 μm2) was ~11.1%, about 4.3 times higher than that in young chondrocytes (Figure 1B).
Figure 1.
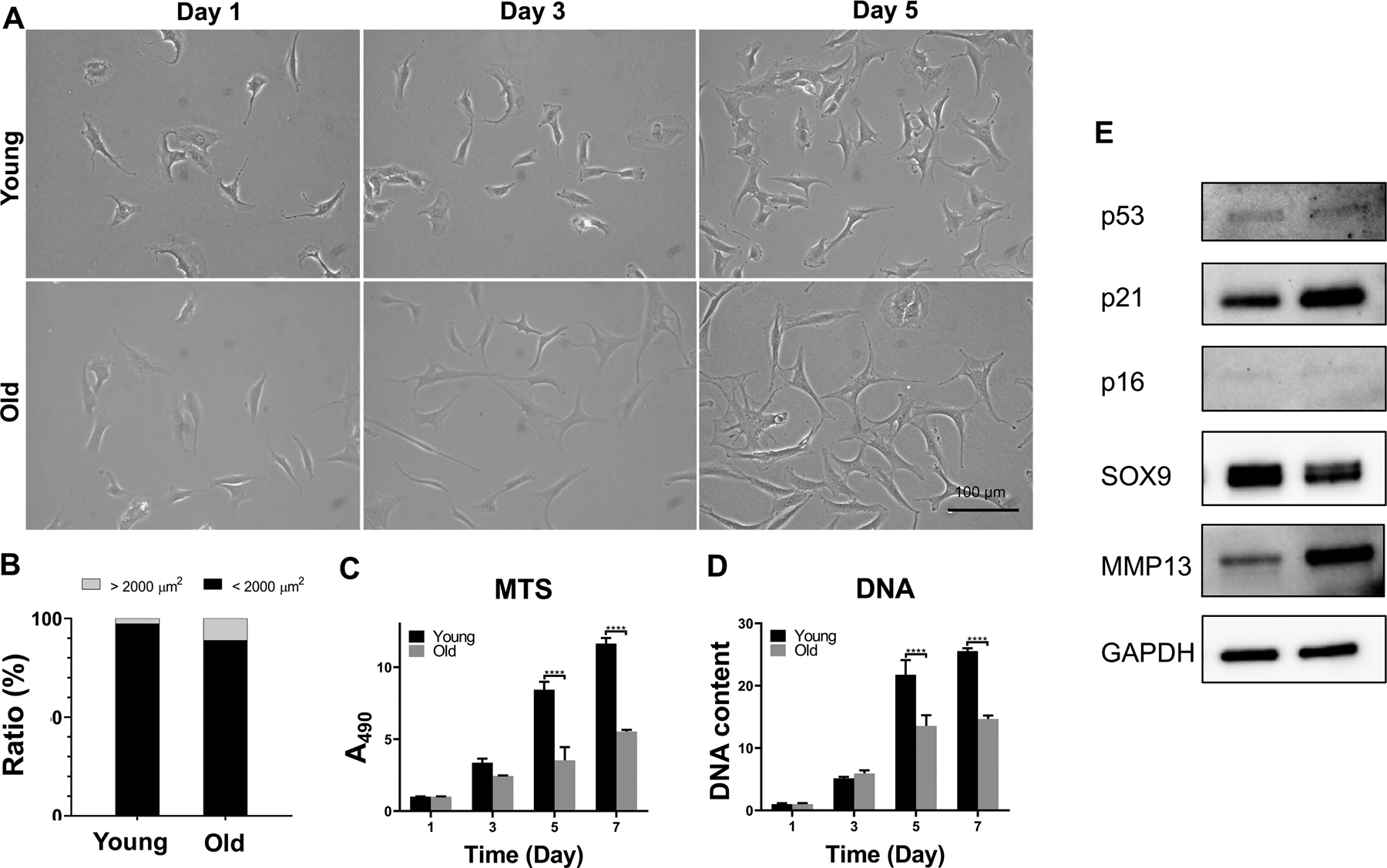
Characterization of human chondrocytes isolated from young and old donors. (A) Morphology of chondrocytes in monolayer culture on tissue culture plate after 1, 3, and 5 days of culture. Scale bar: 100 μm. (B) Cell size distribution in day 5 monolayer cultures of young and old chondrocytes. Cell size was morphometrically calculated and the number of cells with area less than or greater than 2,000 μm2 were determined and calculated as percentages of total cell number. One hundred cells were randomaly selected for cell size measurement. (C and D) Proliferation potential of young and aged chondrocytes in monolayer culture, measured with (C) MTS assay and (D) DNA quantitation. ****, p<0.0001; N= 4. (E) Western analysis of protein expression levels of representative chondrogenic, hypertrophic, and senescence markers after 3 days of monolayer culture.
Chondrocytes isolated from old donors also displayed reduced proliferation capacity when compared to young chondrocytes (Figure 1C and D). After 7 days, in cultures initially seeded at the same density, both MTS assay and total DNA quantitation showed that the number of old chondrocytes was 50% less than that of the young chondrocytes.
3.2. Old chondrocytes showed reduced SOX9 expression with enhanced p21 level
The phenotype of chondrocytes derived from donors of different ages was further examined with Western blot (Figure 1E). When compared to young chondrocytes, old chondrocytes displayed lower protein levels of the chondrogenic marker gene, SRY-type high-mobility group box-9 (SOX9), and higher expression of the catabolic enzyme marker, matrix metalloproteinase-13 (MMP-13). Protein levels of the senescence-associated markers, p16, p21, and p53, were also assessed, and the results showed a higher level of p21 in old chondrocytes. The cartilage formation capacity of the chondrocytes was examined by culturing cell pellets in chondrogenic medium. The results did not show significant difference in chondrogenic potential between young and old chondrocytes, as shown by qRT-PCR analysis of chondrogenesis marker gene expression. However, the neocartilage generated by old chondrocytes displayed significantly higher expression levels of hypertrophy marker genes (Figure S1) than the young counterparts.
In summary, compared to chondrocytes isolated from young donors, old chondrocytes displayed enlarged morphology, reduced proliferation capacity, enhanced expression of p21, as well as inferior cartilage formation potential.
3.3. On-agarose suspension culture increased the chondrogenic potential of old chondrocytes
The potential of on-agarose suspension culture on “rejuvenating” old chondrocytes was then examined (Figure 2A). During the suspension culture, cells aggregated with time and eventually formed large cell clumps (Figure 2B). The progress of the formation of the neocartilage was assessed by Western blot analysis of several markers and signaling pathways known to be relevant in chondrogenesis (Figure 2C). Ras homolog gene family member A (RhoA) protein level in chondrocytes was found to decline with time during suspension culture. Interestingly, protein levels of SOX9 rapidly increased in chondrocytes after being subjected to suspension culture, reaching a peak level after 4 hours of culture which was maintained up to 24 hours. Moreover, the level of phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2) was down-regulated while the levels of total ERK 1/2 was elevated in an incubation time-dependent manner.
Figure 2.

Suspension culture to “rejuvenate” old chondrocytes. (A) Schematic illustration of the “rejuvenation” process. (B) Microscopy imaging of chondrocyte suspension culture. (C) Protein levels of SOX9, RhoA, p-ERK 1/2 and ERK 1/2 in old chondrocytes examined after 0, 0.5, 1, 4, 8, 19, 24, 30, 48 and 72 hours of suspension culture.
3.4. On-agarose suspension culture enhanced the proliferation rate of old chondrocytes
Old chondrocytes that had been subjected to on-agarose suspension culture (Old-SUS) were then seeded in 24 well plates to examine their proliferation capacity. Interestingly, chondrocytes from the Old-SUS group displayed a smaller cell size than untreated control (Old-CON) (Figure 3A). The ratio of cells with a large surface area (>2,000 μm2) decreased from 11% (Old-CON) to 5.77% (Old-SUS) (Figure 3B). In addition, the Old-SUS chondrocytes exhibited higher proliferation capacity than the untreated control (Figure 3C and D), as shown by MTS and Picogreen assays.
Figure 3.

Characterization of old chondrocytes after the “rejuvenation” treatment. (A) Morphology of old chondrocytes with (Old-SUS) or without (Old-CON) being subjected to suspension culture, after 1, 3, and 5 days of subsequent monolayer culture on tissue culture plastic. Scale bar: 100 μm. (B) Cell size distribution in day 5 monolayer cultures of old chondrocytes with or without prior on-agarose suspension culture. Cell size was morphometrically calculated and the number of cells with area less than or greater than 2,000 μm2 were determined and calculated as percentages of total cell number. One hundred cells were randomaly selected for cell size measurement. (C and D) Proliferation activity of chondrocytes on TCP assessed with (C) MTS assay and on the basis of (D) DNA quantitation. *, p<0.05; ***, p<0.001; ****, p<0.0001; N= 4.
3.5. On-agarose culture augmented chondrogenic potential of old chondrocytes
The chondrocytes from the Old-SUS and Old-CON groups were then placed as cell pellets and cultured in chondrogenic medium. After 21 days of culture, the cartilage pellets generated by chondrocytes from Old-SUS groups displayed higher expression of COL2 and ACAN (Figure 4A; no significant difference in SOX9 expression was observed) as well as more histologically evident deposition of sGAG (Figure 4B and C) and COL2 protein (Figure 4D) than those from the Old-CON group. The extent of chondrocyte hypertrophy was also examined by qRT-PCR analysis and immunohistochemistry. The results showed reduced hypertrophy in the Old-SUS group, on the basis of lower gene expression levels of collagen type X (COL10) and MMP-13 (Figure 5A), as well as decreased matrix deposition of COL10 and MMP-13 protein in the constructs (Figure 5B).
Figure 4.

Enhanced chondrogenic potential of old chondrocytes after suspension culture. (A) Relative gene expression levels of COL2 and ACAN in cartilage generated by pellet cultures of chondrocytes from Old-CON and Old-SUS groups. ***, p<0.001; N= 4. (B) Safranin O/Fast green staining, (C) Alcian blue staining, and (D) COL2 immunohistochemistry (IHC) to assess the deposition of cartilage matrix. Scale bar: 100 μm. A more pronounced cartilaginous matrix was produced by the rejuvenated old chondrocytes (Old-SUS), when compare to the cotrol (Old-CON).
Figure 5.
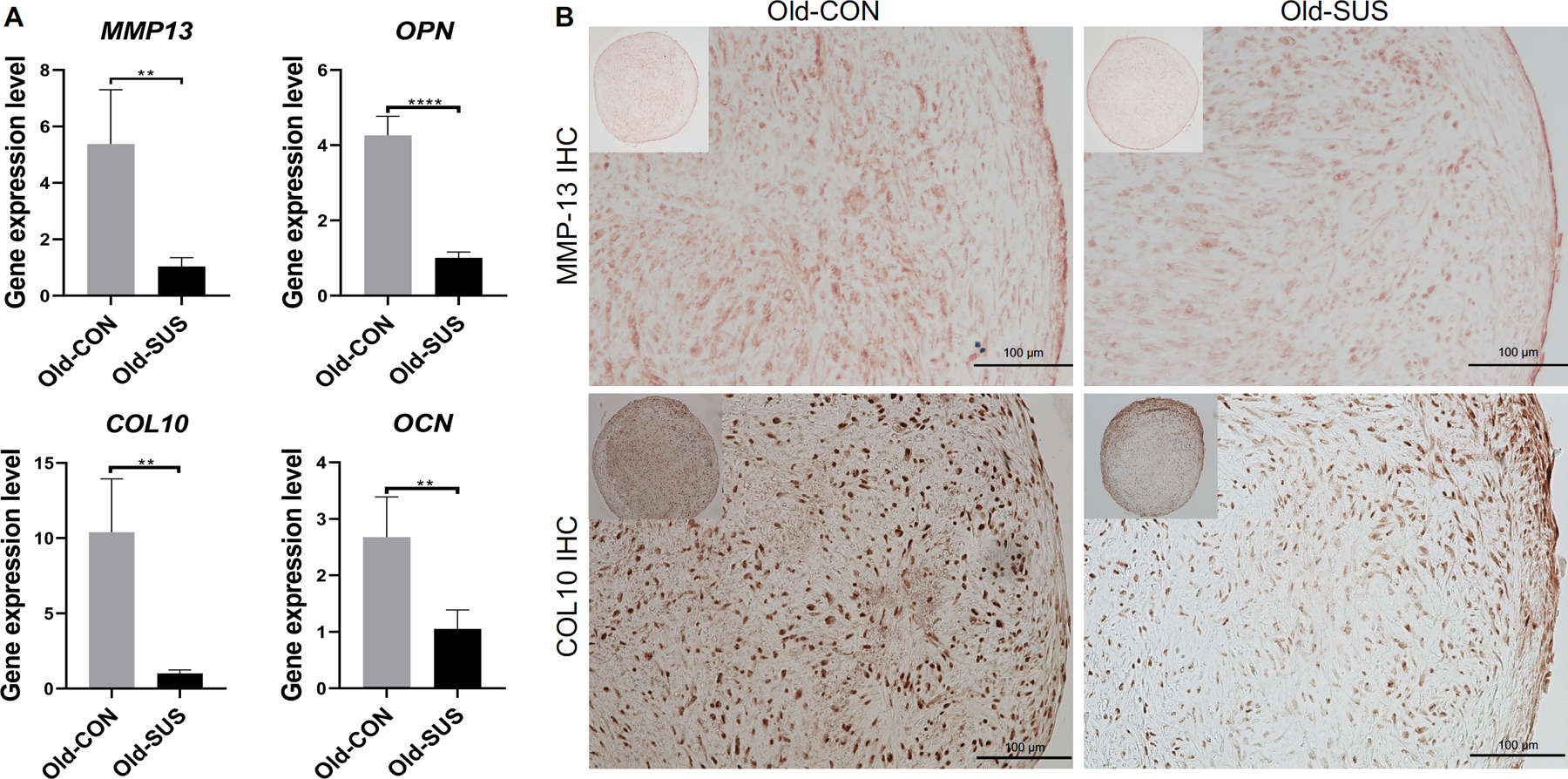
Reduced chondrocyte hypertrophy exhibited by old chondrocytes after suspension culture. (A) Relative gene expression levels of MMP-13, OPN, COL10 and OCN. *, p<0.05; **, p<0.01; ***, p<0.001; N= 4; and (B) immunohistochemical detection of MMP-13 and COL10 in cartilage generated by pellet cultures of chondrocytes from Old-CON and Old-SUS groups. Scale bar: 100 μm.
Lastly, the extent of senescence in the neo-cartilage formed by the old chondrocytes was examined. The expression level of p16 was significantly lower in the cartilage derived from the Old-SUS group (Figure 6A) than that from the Old-CON group. Moreover, immunostaining showed significantly lower levels of p16 and p21 in chondrocytes in the cartilage pellets from the Old-SUS group (Figure 6B). Taken together, these results suggest a lower senescence phenotype in the newly formed cartilage.
Figure 6.

Decreased cellular senescence level exhibited by old chondrocytes after suspension culture. (A) Relative gene expression levels of P16, P21 and P53. *, p<0.05; **, p<0.01; N=4; and (B) immunohistochemical assessment of the protein levels of p16 and p21 in cartilage generated by pellet cultures of chondrocytes from Old-CON and Old-SUS groups. Scale bar: 100 μm.
4. DISCUSSION
Given the compromised clinical outcomes of ACI in elderly patients, likely related to reduced cell quality and quantity of aged chondrocytes (10, 29), the aim of this study was to “rejuvenate” aging chondrocytes through modulating cell morphology to achieve improvement of cell quality, thus enabling potential future application of ACI in aged patients who suffer from cartilage degeneration.
During standard monolayer cell culture, some of the aged chondrocytes displayed abnormal, enlarged cell body compared with chondrocytes derived from young donors (Figure 1A & B). Chondrocyte volume and morphology has been shown to strongly impact cell phenotype. For example, Karim et al. previously used confocal laser scanning microscopy to observe the morphology of human chondrocytes in situ (12). They observed that in non-degraded cartilage the majority of chondrocytes were morphologically normal, i.e., elliptical/rounded. In contrast, in degenerated cartilage, the number of chondrocytes with abnormal morphology, such as short or long cytoplasmic processes, swollen and clustering, increased (12, 30). In addition, the cell morphology of in vitro expanded chondrocytes also changed from polygonal or round to a flattened, amoeboid-like shape, with a massive increase and prolonged extension in well-developed actin stress fibers and microtubules (31). We examined the gene expression of young and chondrocytes with or without the presence of the chondroinductive factor, TGF-β3. The native young chondrocytes displayed higher chondrogenic potential than old chondrocytes, as indicated by higher expression of SOX9. However, upon culturing in a strongly chondroinductive environment (10 ng/mL TGF-β3), levels of SOX9 were significantly upregulated in both cell types, as previously reported (32, 33). Consequently, the original difference in SOX9 expression between them was masked. Upon chondrogenic culture, we also observed significantly higher expression levels of hypertrophy marker genes (MMP-13, COL10 and OCN) in old chondrocytes, which may be associated with the increased cell size and volume observed in Figure 1A (34).
Analysis of the phenotype of the aged chondrocytes showed reduced proliferation rate, attenuated SOX9 expression, and enhanced MMP-13 and p21 production, compared to young cells (Figures 1 C–E and S1), in agreement with previous reports (13, 31, 35). Interestingly, we did not observe any difference in p16 expression between young and old chondrocytes (Figure 1E), which however was previously shown to significantly increase in OA chondrocytes compared to those in age-matched normal cartilage tissue (36). Therefore, natural aging may not necessarily lead to enhanced cellular senescence. Thus, we selected p21 as a more appropriate marker to assess natural aging.
3D culture systems typically exhibit better capabilities of maintaining the chondrocyte phenotype by providing a more favorable environment similar to that in native cartilage (37). Dedifferentiation phenotype of in vitro expanded chondrocytes could be reversed by restoring the cell morphology to spheroidal shape through cytoskeletal re-organization by cytoskeletal dissolution/disruption agent treatment or 3D culture (17, 20, 38). In this study, in order to “rejuvenate” old chondrocytes, an on-agarose suspension 3D culture strategy was adopted (Figure 2A) (28), by exploiting the inability of chondrocytes to adhere to the agarose substratum and thereby undergoing natural cell-cell aggregation. During the “rejuvenation” process, the cells were forced to maintain a round shape (Figure 2B), as reported in a previous study (20). The advantage of the on-agarose culture versus the more conventional in-agarose culture is that the chondrocytes achieve a round morphology as a result of natural cell aggregation and extracellular matrix formation, instead of being compacted by a non-native, non-interactive hydrogel scaffold. We previously showed that such a culture system was able to enhance the chondrogenic activity of embryonic mesenchymal cells (28). Notably, the proliferation potential of old chondrocytes was also enhanced after treatment, which was not reported before. Upon chondrogenic stimulation, the expression levels of chondrogenic marker genes (COL2 and ACAN) of old chondrocytes significantly increased, and the expression levels of hypertrophic marker genes (COL10, MMP-13, OCN) decreased. Similar results were reported in the study of reversing the dedifferentiation of extensively expanded chondrocytes (39).
As shown in Figure 6, we observed that the new cartilage generated from old chondrocytes contained a significant number of senescent cells. It has been reported that compared with non-senescent cell transplantation, transplanting senescent cells into the knee region caused pain, decreased rotarod performance, and induced an osteoarthritis-like microenvironment in mice (40). Furthermore, selective clearance of senescent chondrocytes decreased expression of senescence and inflammatory markers (p16, MMP-13, IL6, etc.) and increased expression of cartilage tissue extracellular matrix proteins in in vitro cultures. The removal of senescent cells through transgenic mouse models or pharmaceutical intervention after anterior cruciate ligament transection reduced post-traumatic osteoarthritis and improved cartilage repair in aged mice (41). Taken together, these observations suggest that the high senescence level in old chondrocyte-derived cartilage may not only impair the reparative outcome but also potentially cause a detrimental effect on the resident healthy native cartilage. Interestingly, as shown in Figure 6, old chondrocytes that were undergoing suspension culture formed new cartilage with significantly reduced senescent phenotype, compared to untreated control. Therefore, the “rejuvenation” process enhances both the quantity and quality of old chondrocytes-derived carriage.
Next, we investigated the potential mechanisms underlying the “rejuvenation” process. Haudenschild et al. found that the cytoskeletal organization in chondrocytes cultured within agarose scaffold was remarkably different from that in monolayer culture (26). The formation of actin stress fibers and cell spreading was also inhibited (42). Thus, the positive effects of 3D culture on chondrogenic phenotype improvement are probably not due to cell shape per se, but more likely associated with changes in cytoskeleton-related signaling pathways (30). Several studies have found that cytoskeletal modulation through the RhoA signaling pathway promoted chondrocyte redifferentiation and chondrogenesis (43, 44). RhoA signaling represses SOX9 expression in the chondrogenesis of dedifferentiated chondrocytes maintained on plastic (45). Overexpression of RhoA led to an inhibitory effect on SOX9-mediated chondrogenic differentiation (46), while the loss of RhoA protein and actin polymerization induced expression and transcriptional activity of SOX-9 (45). In our study, the level of RhoA declined rapidly in the first 4 hours, coincident with the robust increase of SOX9 expression, signifying the role of RhoA in regulating SOX9. After 8 hours, the level of RhoA was seen to be maintained at a low level (Figure 2C). We thus expected the maintenance of high-level expression of SOX9. However, SOX9 expression declined, which suggested the involvement of a different regulatory mechanism. As shown in Figure 2B, upon seeding on agarose, chondrocytes transitioned from monolayer to 3D aggregates. Interestingly, the level of SOX9 was reduced with the increased formation of cell aggregates. It has been reported that the cells in pellets expressed significantly lower levels of N-cadherin than monolayer culture (47), and N-cadherin has been shown to be a critical regulator of chondrogenesis (48–50). In particular, our previous study showed that immunoblocking of N-cadherin suppressed SOX9 expression (51). Therefore, we speculate that the decrease in SOX9 expression after 8 hours may be associated with a decrease in N-cadherin resulting from the formation of cell aggregates, a topic that will be further explored in the future.
ERK1/2, a member of the mitogen-activated protein kinase (MAPK) family, has been reported to play a crucial role in regulating the terminal differentiation of growth plate chondrocytes during endochondral ossification (52, 53). It has been found that ERK1/2 activity increased 15-fold during chondrocyte dedifferentiation, and thus inhibiting ERK1/2 activation was shown to enhance COL2 and proteoglycan production and prevented dedifferentiation during monolayer cultures (54). Additionally, inhibiting MEK/ERK activation rescued chondrogenesis of transforming growth factor-α induced articular cartilage degradation (55). ERK1/2 activation induced MMP secretion in response to proinflammatory cytokines involved in the pathogenesis of osteoarthritis and rheumatoid arthritis, and inhibition of ERK1/2 reversed the deleterious effects caused by IL1-β and TNF-α on the engineered cartilage (56). In our study, we also found that phosphorylation of ERK1/2 in chondrocyte suspension culture decreased with time (Figure 2C), which likely contributed to the restoration of chondrocytic phenotype of old cells.
It should be noted that the “rejuvenation” process will need to be further characterized on the basis of additional parameters, including proliferation markers, telomere length, effects of extrinsic or intrinsic oxidative stress, proinflammatory markers, etc. (57). In addition, the in vivo cartilage formation capacity of the ‘rejuvenated’ aged chondrocytes should be further evaluated in the context of aged animal models. Moreover, modulating RhoA and ERK1/2 by inhibitors or agonists will be conducted in the future to further understand their roles in the “rejuvenation” process.
In this study, we found that aged chondrocytes contained more cells with enlarged cell morphology, and displayed reduced proliferation rate and loss of chondrocytic phenotype, in comparison with young cells. After suspension culture for 72 hours, the “rejuvenated” chondrocytes displayed enhanced replicative capacity and chondrogenic potential. Upon chondrogenic stimulation, “rejuvenated” chondrocytes generated higher-quality hyaline cartilage, indicated by enhanced chondrogenic gene expression and cartilage matrix deposition, as well as reduced hypertrophy and senescence levels. Our findings demonstrated that the exposure to a relatively short 3D suspension culture modulated the phenotype of aged chondrocytes cell morphology, likely via suppression of RhoA activation. This reversal of chondrocyte aging resulted in “rejuvenated” chondrocytes with improved regenerative potential, which may be applied to ACI for cartilage repair in the aged population.
Supplementary Material
Acknowledgment
This work was partially supported by the National Institutes of Health (R21AG056819, P30AG024827, and UL1TR001857).
Nonstandard abbreviations
- ACI
autologous chondrocyte implantation
- ACAN
aggrecan
- ALP
alkaline phosphatase
- COL2
collagen type II
- DMEM
Dulbecco’s modified Eagle medium
- ERK1/2
extracellular signal-regulated protein kinase 1/2
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ITS
insulin-transferrin-selenium supplement
- MMP13
matrix metalloproteinase-13
- MTS
CellTiter 96 AQueous One Solution cell proliferation assay
- P-ERK1/2
phosphorylated ERK1/2
- RhoA
ras homolog gene family member A
- RPL13a
ribosomal protein L13a
- sGAG
soluble glycosaminoglycan
- SOX9
SRY-type high-mobility group box-9
- TGF-β3
transforming growth factor-β3
Footnotes
Conflict of Interest statement
The authors declare that they have no competing interests.
Availability of data and materials
The data sets supporting the conclusions of this article are included within the article and its supplementary files.
Ethics approval and consent to participate
All experiments were approved by the University of Pittsburgh Institutional Review Board.
References
- 1.Sessa A, Perdisa F, Di Martino A, Zaffagnini S, and Filardo G (2019) Cell-free biomimetic osteochondral scaffold: implantation technique. JBJS Essent. Surg. Tech 9, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vavken P, and Samartzis D (2010) Effectiveness of autologous chondrocyte implantation in cartilage repair of the knee: a systematic review of controlled trials. Osteoarthr. Cartil 18, 857–863 [DOI] [PubMed] [Google Scholar]
- 3.Makris EA, Gomoll AH, Malizos KN, Hu JC, and Athanasiou KA (2015) Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol 11, 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones DG, and Peterson L (2007) Autologous chondrocyte implantation. Instr. Course. Lect 56, 429–445 [PubMed] [Google Scholar]
- 5.Rosenberger RE, Gomoll AH, Bryant T, and Minas T (2008) Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am. J. Sports. Med 36, 2336–2344 [DOI] [PubMed] [Google Scholar]
- 6.Kon E, Filardo G, Condello V, Collarile M, Di Martino A, Zorzi C, and Marcacci M (2011) Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am. J. Sports. Med 39, 1668–1675 [DOI] [PubMed] [Google Scholar]
- 7.Giannoni P, Pagano A, Maggi E, Arbicò R, Randazzo N, Grandizio M, Cancedda R, and Dozin B (2005) Autologous chondrocyte implantation (ACI) for aged patients: development of the proper cell expansion conditions for possible therapeutic applications. Osteoarthr. Cartil 13, 589–600 [DOI] [PubMed] [Google Scholar]
- 8.Bobacz K, Erlacher L, Smolen J, Soleiman A, and Graninger WB (2004) Chondrocyte number and proteoglycan synthesis in the aging and osteoarthritic human articular cartilage. Ann. Rheum. Dis 63, 1618–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaney Davidson EN, Scharstuhl A, Vitters EL, van der Kraan PM, and van den Berg WB (2005) Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res. Ther 7, R1338–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Kraan PM, and Van den Berg WB (2008) Osteoarthritis in the context of ageing and evolution. Loss of chondrocyte differentiation block during ageing. Ageing Res. Rev 7, 106–113 [DOI] [PubMed] [Google Scholar]
- 11.Verbruggen G, Cornelissen M, Almqvist KF, Wang L, Elewaut D, Broddelez C, de Ridder L, and Veys EM (2000) Influence of aging on the synthesis and morphology of the aggrecans synthesized by differentiated human articular chondrocytes. Osteoarthr. Cartil 8, 170–179 [DOI] [PubMed] [Google Scholar]
- 12.Karim A, Amin AK, and Hall AC (2018) The clustering and morphology of chondrocytes in normal and mildly degenerate human femoral head cartilage studied by confocal laser scanning microscopy. J. Anat 232, 686–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominice J, Levasseur C, Larno S, Ronot X, and Adolphe M (1986) Age-related changes in rabbit articular chondrocytes. Mech. Ageing Dev 37, 231–240 [DOI] [PubMed] [Google Scholar]
- 14.Stokes DG, Liu G, Coimbra IB, Piera-Velazquez S, Crowl RM, and Jiménez SA (2002) Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 46, 404–419 [DOI] [PubMed] [Google Scholar]
- 15.Page-McCaw A, Ewald AJ, and Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol 8, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archer CW, Rooney P, and Wolpert L (1982) Cell shape and cartilage differentiation of early chick limb bud cells in culture. Cell Differ. 11, 245–251 [DOI] [PubMed] [Google Scholar]
- 17.Zanetti NC, and Solursh M (1984) Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J. Cell Biol 99, 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods A, Wang G, and Beier F (2007) Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J. Cell. Physiol 213, 1–8 [DOI] [PubMed] [Google Scholar]
- 19.Solursh M (1984) Cell-cell interactions and chondrogenesis. In: “Cartilage: Development, Differentiation, and Growth.” Hall BK (ed). Orlando, FL: Academic Press. 2, 121–141 [Google Scholar]
- 20.Benya PD, and Shaffer JD (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30, 215–224 [DOI] [PubMed] [Google Scholar]
- 21.Häuselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, Kuettner KE, and Thonar EJ (1994) Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J. Cell Sci 107 (Pt 1), 17–27 [DOI] [PubMed] [Google Scholar]
- 22.Binette F, McQuaid DP, Haudenschild DR, Yaeger PC, McPherson JM, and Tubo R (1998) Expression of a stable articular cartilage phenotype without evidence of hypertrophy by adult human articular chondrocytes in vitro. J. Orthop. Res 16, 207–216 [DOI] [PubMed] [Google Scholar]
- 23.Shortkroff S, Barone L, Hsu HP, Wrenn C, Gagne T, Chi T, Breinan H, Minas T, Sledge CB, Tubo R, and Spector M (1996) Healing of chondral and osteochondral defects in a canine model: the role of cultured chondrocytes in regeneration of articular cartilage. Biomaterials 17, 147–154 [DOI] [PubMed] [Google Scholar]
- 24.Parreno J, Bianchi VJ, Sermer C, Regmi SC, Backstein D, Schmidt TA, and Kandel RA (2018) Adherent agarose mold cultures: An in vitro platform for multi-factorial assessment of passaged chondrocyte redifferentiation. J. Orthop. Res 36, 2392–2405 [DOI] [PubMed] [Google Scholar]
- 25.Tran-Khanh N, Chevrier A, Lascau-Coman V, Hoemann CD, and Buschmann MD (2010) Young adult chondrocytes proliferate rapidly and produce a cartilaginous tissue at the gel-media interface in agarose cultures. Connect. Tissue Res 51, 216–223 [DOI] [PubMed] [Google Scholar]
- 26.Sasazaki Y, Seedhom BB, and Shore R (2008) Morphology of the bovine chondrocyte and of its cytoskeleton in isolation and in situ: are chondrocytes ubiquitously paired through the entire layer of articular cartilage? Rheumatology (Oxford) 47, 1641–1646 [DOI] [PubMed] [Google Scholar]
- 27.Shen H, Lin H, Sun AX, Song S, Zhang Z, Dai J, and Tuan RS (2018) Chondroinductive factor-free chondrogenic differentiation of human mesenchymal stem cells in graphene oxide-incorporated hydrogels. J Mater. Chem. B 6, 908–917 [DOI] [PubMed] [Google Scholar]
- 28.Jacenko O, San Antonio JD, and Tuan RS (1995) Chondrogenic potential of chick embryonic calvaria: II. Matrix calcium may repress cartilage differentiation. Dev. Dyn 202, 27–41 [DOI] [PubMed] [Google Scholar]
- 29.Martin JA, and Buckwalter JA (2003) The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J. Bone Joint Surg. Am 85-A Suppl 2, 106–110 [DOI] [PubMed] [Google Scholar]
- 30.Hall AC (2019) The role of chondrocyte morphology and volume in controlling phenotype-implications for osteoarthritis, cartilage repair, and cartilage engineering. Curr. Rheumatol. Rep 21, 38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashraf S, Cha BH, Kim JS, Ahn J, Han I, Park H, and Lee SH (2016) Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil 24, 196–205 [DOI] [PubMed] [Google Scholar]
- 32.Coricor G, and Serra R (2016) TGF-β regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci. Rep 6, 38616–38627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jha SK, Jha NK, Kumar D, Ambasta RK, and Kumar P (2017) Linking mitochondrial dysfunction, metabolic syndrome and stress signaling in neurodegeneration. Biochim. Biophys. Acta Mol. Basis. Dis 1863, 1132–1146 [DOI] [PubMed] [Google Scholar]
- 34.Singh P, Marcu KB, Goldring MB, and Otero M (2019) Phenotypic instability of chondrocytes in osteoarthritis: on a path to hypertrophy. Ann. N. Y. Acad. Sci 1442, 17–34 [DOI] [PubMed] [Google Scholar]
- 35.Loeser RF (2009) Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil 17, 971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou HW, Lou SQ, and Zhang K (2004) Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology (Oxford) 43, 555–568 [DOI] [PubMed] [Google Scholar]
- 37.Smeriglio P, Lai JH, Yang F, and Bhutani N (2015) 3D hydrogel scaffolds for articular chondrocyte culture and cartilage generation. J. Vis. Exp, e53085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blain EJ (2009) Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int. J. Exp. Pathol 90, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, van Rhijn LW, and Welting TJ (2012) Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr. Cartil 20, 1170–1178 [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, and Kirkland JL (2017) Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci 72, 780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, and Elisseeff JH (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med 23, 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haudenschild DR, Chen J, Steklov N, Lotz MK, and D’Lima DD (2009) Characterization of the chondrocyte actin cytoskeleton in living three-dimensional culture: response to anabolic and catabolic stimuli. MCB Mol. Cell. Biomech 6, 135–144 [PMC free article] [PubMed] [Google Scholar]
- 43.Kim MJ, Kim S, Kim Y, Jin EJ, and Sonn JK (2012) Inhibition of RhoA but not ROCK induces chondrogenesis of chick limb mesenchymal cells. Biochem. Biophys. Res. Commun 418, 500–505 [DOI] [PubMed] [Google Scholar]
- 44.Rottmar M, Mhanna R, Guimond-Lischer S, Vogel V, Zenobi-Wong M, and Maniura-Weber K (2014) Interference with the contractile machinery of the fibroblastic chondrocyte cytoskeleton induces re-expression of the cartilage phenotype through involvement of PI3K, PKC and MAPKs. Exp. Cell Res 320, 175–187 [DOI] [PubMed] [Google Scholar]
- 45.Kumar D, and Lassar AB (2009) The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol. Cell. Biol 29, 4262–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods A, Wang G, and Beier F (2005) RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem 280, 11626–11634 [DOI] [PubMed] [Google Scholar]
- 47.Gao L, McBeath R, and Chen CS (2010) Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells Cloning 28, 564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gegg C, and Yang F (2020) The effects of ROCK inhibition on mesenchymal stem cell chondrogenesis are culture model dependent. Tissue Eng. Part A 26, 130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, and Tuan RS (2003) Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem 278, 41227–41236 [DOI] [PubMed] [Google Scholar]
- 50.Bian L, Guvendiren M, Mauck RL, and Burdick JA (2013) Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. U. S. A 110, 10117–10122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Kuang B, Rothrauff BB, Tuan RS, and Lin H (2019) Robust bone regeneration through endochondral ossification of human mesenchymal stem cells within their own extracellular matrix. Biomaterials 218, 119336–119348 [DOI] [PubMed] [Google Scholar]
- 52.Chen Z, Yue SX, Zhou G, Greenfield EM, and Murakami S (2015) ERK1 and ERK2 regulate chondrocyte terminal differentiation during endochondral bone formation. J. Bone Miner. Res 30, 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bobick BE, Matsche AI, Chen FH, and Tuan RS (2010) The ERK5 and ERK1/2 signaling pathways play opposing regulatory roles during chondrogenesis of adult human bone marrow-derived multipotent progenitor cells. J. Cell. Physiol 224, 178–186 [DOI] [PubMed] [Google Scholar]
- 54.Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, and Chun JS (2002) Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J. Biol. Chem 277, 8412–8420 [DOI] [PubMed] [Google Scholar]
- 55.Appleton CT, Usmani SE, Mort JS, and Beier F (2010) Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab. Invest 90, 20–30 [DOI] [PubMed] [Google Scholar]
- 56.Djouad F, Rackwitz L, Song Y, Janjanin S, and Tuan RS (2009) ERK1/2 activation induced by inflammatory cytokines compromises effective host tissue integration of engineered cartilage. Tissue Eng. 15, 2825–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeon OH, David N, Campisi J, and Elisseeff JH (2018) Senescent cells and osteoarthritis: a painful connection. J. Clin. Invest 128, 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


