Abstract
Nonalcoholic fatty liver disease comprises a wide spectrum of liver injuries from simple steatosis to steatohepatitis and cirrhosis. Nonalcoholic steatohepatitis (NASH) is defined when liver steatosis is associated with inflammation, hepatocyte damage, and fibrosis. A genetic predisposition and environmental insults (ie, dietary habits, obesity) are putatively responsible for NASH progression. Here, we present the impact of the lipid-sensing nuclear receptors in the pathogenesis and treatment of NASH. In detail, we discuss the pros and cons of the putative transcriptional action of the fatty acid sensors (peroxisome proliferator-activated receptors), the bile acid sensor (farnesoid X receptor), and the oxysterol sensor (liver X receptors) in the pathogenesis and bona fide treatment of NASH.
Keywords: Nonalcoholic Steatohepatitis (NASH), Nuclear Receptors, Peroxisome Proliferator Activated Receptors (PPARs), Farnesoid X Receptor (FXR), Liver X Receptor (LXR)
Abbreviations used in this paper: ALT, alanine aminotransferase; APO-E2, apolipoprotein-E2; AST, aspartate aminotransferase; ATP, adenosine triphosphate; BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; CCl4, carbon tetrachloride; CoA, Coenzyme A; CYP7A1, cytochrome P450 7A1; FGF, fibroblast growth factor; FLINT, FXR ligand obeticholic acid for noncirrhotic, nonalcoholic steatohepatitis trial; FXR, farnesoid X receptor; HDL, high-density lipoprotein; HFD, high-fat diet; HSC, hepatic stellate cell; LPS, lipopolysaccharide; LXR, liver X receptor; MCDD, methionine- and choline-deficient diet; MUFA, monounsaturated fatty acid; NAFL, nonalcoholic fatty liver; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NOS, nitric oxide synthase; NR, nuclear receptor; OCA, obeticholic acid; PNPLA3, polymorphisms in patatin-like phospholipase 3; PPAR, peroxisome proliferator activated receptor; REGENERATE, Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment; SCD1, stearoyl-CoA desaturase 1; SHP, small heterodimer partner; SREBP1c, sterol regulatory element-binding protein 1c; TLR, Toll-like receptor; TNF, tumor necrosis factor; VLDLR, very-low-density lipoprotein receptor; WAT, white adipose tissue
Summary.
This review analyzes the impact of the lipid-sensing nuclear receptors peroxisome proliferator activated receptors, farnesoid X receptor, and liver X receptors in the pathogenesis and treatment of nonalcoholic steatohepatitis. Agonists of peroxisome proliferator-activated receptors and farnesoid X receptor have been studied extensively in mouse models, and phase II and III clinical trials currently are ongoing to test the safety and efficacy of these nuclear-receptor–based drugs for treating nonalcoholic steatohepatitis.
In the world, with approximately 2 billion adults being overweight or obese, obesity currently is considered a pandemic of the 21st century.1 In particular, obesity represents a risk factor for different clinical conditions, including cardiovascular and gastrointestinal diseases. Among the latter, nonalcoholic fatty liver disease (NAFLD) has become one of the most studied hepatic dysfunctions in the past years, also because of its continuous increasing prevalence worldwide (∼25%).2 NAFLD encompasses a wide spectrum of liver injuries, ranging from simple steatosis to steatohepatitis, and eventually fibrosis and cirrhosis. NAFLD-related cirrhosis represents one of the major known causes for the onset of hepatocellular carcinoma, and obese individuals have a 2-fold increased risk of hepatocellular carcinoma–related mortality.3, 4, 5
On the basis of the degree of disease severity, NAFLD can be subdivided into NAFL and nonalcoholic steatohepatitis (NASH). Usually, the term NAFL refers to the accumulation of lipids within hepatocytes, without evident markers of inflammatory activity or cell damage, whereas NASH is associated with liver steatosis, inflammation, and hepatocyte damage (ie, hepatocyte ballooning), with or without fibrosis.6
The diagnosis of NAFLD occurs in the presence of hepatic steatosis and the concomitant absence of other types of liver pathologies.7 Nowadays, the diagnosis is based mainly on liver biopsies, an invasive procedure with several degrees of potential complications. However, other types of noninvasive biomarkers currently are available or under investigation.8
Different theories have been postulated to explain the onset and progression of NAFLD. If previously the two-hits hypothesis was considered the principal model for NAFLD pathogenesis, at present the multiple-hits hypothesis is the one that best recapitulates the process at the basis of the disease. Briefly, in the two-hits hypothesis, an imbalance between hepatic lipid input and output was observed, which eventually resulted in triglyceride accumulation within the hepatocytes (first hit). This renders the liver more exposed to other forms of injuries, which ultimately lead to the activation of inflammatory processes and hepatic stellate cells (HSCs), with consequent extracellular matrix deposition and onset of NASH and fibrosis (second hit).9 Although initially considered as the most reliable model to explain NAFLD development, scientific advances made clear that the two-hits hypothesis is too simplistic to explain the vast complexity of this disease. Contrarily, the multiple-hits hypothesis considers NAFLD as the resultant combination of parallel insults acting on genetically predisposed individuals.10
Notably, differently from the previous hypothesis, the multiple-hits hypothesis also takes into consideration the contribution of extrahepatic tissue to liver inflammation, including the gut and adipose tissue. Gut-derived molecules such as endotoxin, a key component of many bacteria present in the microbiota, might contribute to the exacerbation of both hepatic lipid accumulation and inflammation.11 Moreover, adipokines secreted by the adipose tissue may impact NAFLD perpetration by regulating hepatic fat accumulation, insulin resistance, and fibrosis.12
Genome-wide association studies have identified several genes involved in NAFLD pathogenesis. Nonsynonymous polymorphisms in patatin-like phospholipase 3 (PNPLA3), a multifunctional enzyme involved mainly in triacylglycerol hydrolysis, have been associated with the severity of NAFLD in both pediatric and adult individuals (rs738409 C/G). In subjects carrying 2 minor G alleles (rs738409 G/G), fatty liver progresses directly to NASH.13,14 Intriguingly, the absence of PNPLA3 in mouse liver or in cultured hepatocytes determines a decreased accumulation of triglycerides, finally conferring protection against NAFLD.15,16 Further studies surely are needed to clarify the role of PNPLA3 in liver steatosis and its sequelae. In addition to PNPLA3, other genes have been correlated with NAFLD susceptibility, including the transmembrane 6 superfamily 2 and the glucokinase regulator, whose genetic variants have been associated with histologic hepatic lipid accumulation.17, 18, 19
The genetic predisposition alone is not sufficient to promote NAFLD development. Environmental insults (ie, dietary habits, obesity, and so forth) are involved in the disease progression as well, driving toward a progressive inflammatory phenotype, particular to NASH.10
To fully elucidate NAFLD and NASH peculiarities and the mechanisms involved in their progression toward severe forms of disease, several animal models have been used. Each model showed advantages and disadvantages, but none reliably reflected all features of human disease. Two major groups can be distinguished: mice that acquire the disease after dietary or pharmacologic manipulation and the genetically modified ones. Among dietary models, the methionine- and choline-deficient diet (MCDD), high-fat diet (HFD) of diverse compositions, high-cholesterol diet, and fructose-based diets are the most representative. However, different criticisms have emerged in regard to administration of these diets because they do not recapitulate the principal physiological characteristic of NAFLD. For instance, although mice fed with MCDD utterly reproduce steatohepatitis, they display weight loss and decreased concentration of circulating cholesterol and triglycerides, thus not completely resembling human disease. On the contrary, a HFD can induce insulin resistance and obesity, but fails to induce severe liver injury.20,21 Furthermore, the administration of carbon tetrachloride (CCl4), a hepatotoxin, has been widely used to induce oxidative stress to the liver, followed by accumulation of toxic lipid species and tissue necrosis. However, fibrosis phenotype promptly regresses after discontinuing drug administration.22 Finally, genetic models including ob/ob and db/db mice (both characterized by disrupted leptin signaling), as well as genetically engineered rodents, have been used to improve the understanding of the molecular processes involved in NAFLD onset and progression. Despite providing the opportunity to control both genetic and environmental factors, most of these models required concomitant dietary or drug administration to fully develop the disease and often recap only one aspect of NAFLD.23 In any case, the use of mouse models allowed a deep comprehension of the molecular determinants of NAFLD and facilitated the identification of the potential pharmacologic intervention for NAFLD therapy.
Intriguingly, although several drugs have been tested or currently are under evaluation in clinical trials, no effective therapy has been approved for the treatment of NAFLD.24 Major changes in lifestyle, such as dietary improvements and increased physical exercise, beneficially impact the management of NAFLD and may delay the progression of the disease, however, for many individuals this is not achievable, with consequent frequent relapse.25
Here, we report the impact of the principal nuclear receptors (NRs) in the pathogenesis and treatment of NASH. NRs act as ligand-activated transcription factors, and constitute a superfamily of 48 members divided into 7 subfamilies designated as NR0–NR6.26 They mediate a wide range of physiological processes, including development, metabolism, and reproduction,27 in particular, the NR1 subfamily is implicated in energy/nutrient control. These NRs form a heterodimer with retinoid X receptor and are activated by binding with ligands. When the ligand is unbound, NRs are inactivated and linked to co-repressors. The binding of NRs with ligand and retinoid X receptor allows the release of co-repressors and the recruitment of co-activators starting the transcription of target genes.28 Peroxisome proliferator-activated receptor (PPAR)α, β/δ, and γ (NR1C1-3); farnesoid X receptor (FXR, NR1H4); and liver X receptor α and β (LXR, NR1H2-3) belong to this subfamily, which overall exert a key role in the pathogenesis of NAFLD and NASH.
PPARs in Hepatic Physiology and NASH
The PPAR subfamily members play a crucial role in the regulation of lipid metabolism in different tissues. Although they usually are considered as master regulators of fatty acids, PPARs now also have been recognized for playing an important role in lipid and carbohydrate metabolism, as well as in inflammation and cellular proliferation.29 There are 3 PPAR isotypes, termed PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3), which display a different tissue distribution. PPARα is found mainly in tissue with high fatty acid catabolism, such as the liver. PPARβ/δ is distributed ubiquitously, with a hepatic expression that varies from low to moderate in human beings and rats, and from moderate to high in mice. On the contrary, PPARγ is present at high levels in white adipose tissue. Intriguingly, although PPARγ is expressed weakly in healthy liver, its expression is correlated positively with liver steatosis in obese patients.30, 31, 32, 33
PPARα
In the liver, PPARα coordinates different pathways involved in fatty acid metabolism as well as inflammation.31,34,35 Upon binding to fatty acid derivatives formed during lipolysis or to synthetic ligands (ie, fibrates), PPARα mediates the transcriptional regulation of several genes that are widely implicated in the adaptive or protective response exerted by this nuclear receptor in the liver.36, 37, 38, 39 PPARα directly regulates the expression of fatty acid transporters involved in cellular fatty acid uptake, and controls the expression of the principal enzymes of peroxisomal β-oxidation, the catabolic process by which fatty acids are broken down to produce energy.40, 41, 42, 43, 44 Moreover, activated PPARα up-regulates the mitochondrial hydroxymethylglutaryl-Coenzyme A (CoA) synthase, the rate-limiting enzyme of ketogenesis. PPARα fasting knock-out mice show impaired fatty acid β-oxidation, hypoglycemia, and an inability to produce ketone bodies.45,46 Notably, PPARα also is able to regulate the hepatic lipogenic program. Indeed, in addition to the direct induction of sterol regulatory element-binding protein 1c (SREBP1c), PPARα also indirectly can coordinate SREBP1c expression through cross-regulation with the LXR signaling pathway.47,48 Although these functions may appear conflicting, it is plausible that in a fed state PPARα controls de novo lipogenesis to provide lipids for storage. On the contrary, during fasting PPARα activity shifts to fatty acid uptake and fatty acid β-oxidation. In this way, PPARα is able to supply energy to peripheral tissues via ketogenesis. Finally, PPARα shows an anti-inflammatory activity in a murine model of systemic inflammation. Indeed, lipopolysaccharide (LPS)-induced acute-phase response is inhibited by fenofibrate treatment in hepatic-specific PPARα mice, but not in PPARα-deficient mice.49
Early evidence regarding the hepatoprotective role of PPARα in NAFLD comes from preclinical studies. PPARα-null mice subjected to HFD show massive hepatic lipid accumulation owing to inhibition of fatty acid uptake and β-oxidation.45 Moreover, both HFD-fed mice and obese Zucker rats treated with selective PPARα agonists show improved insulin sensitivity, suggesting that PPARα is active in the early pathologic stages to guarantee a healthy liver.50 Interestingly, mice with a hepatocyte-specific deletion of PPARα fed with a standard diet develop steatosis in aging, without becoming overweight, thus indicating that hepatic PPARα regulates liver as well as whole-body fatty acid homeostasis.51
In addition to steatosis, PPARα also can ameliorate NASH pathology. Indeed, in mice, MCDD-induced steatohepatitis and fibrosis can be reversed by treatment with the PPARα agonist Wy-14,643. The activation of PPARα prevents intrahepatic lipid accumulation and inflammation by lowering the number of activated macrophages and HSCs, finally promoting the normalization of the histologic changes typical of NASH.52,53 Moreover, mice lacking adipose triglyceride lipase, which fail to generate endogenous PPARα agonists, are more prone to develop hepatic inflammation when challenged with LPS and MCDD compared with wild-type mice.54
The contribution of PPARα to early stages of NASH have been studied in apolipoprotein-E2 (APO-E2) knock-in mice, which mimic human type III hyperlipoproteinemia.55 The whole-body deletion of PPARα in APO-E2 knock-in mice fed a Western diet exacerbates hepatic steatosis and inflammation. On the contrary, APO-E2 knock-in mice treated with fibrates show induction of PPARα activity. This results in the down-regulation of proinflammatory genes and in the up-regulation of genes involved in lipid catabolism. Overall, these changes inhibit NASH progression.56,57
The hepatoprotective effects of PPARα activity are partially mediated by Vanin 1, a pantetheinase expressed in liver and secreted in serum that regulates tissue adaptation to stress. The concentration of serum Vanin 1 reflects PPARα activation in the liver. Vanin 1 ablation in mice as well as inhibition of Vanin 1 activity in rats results in hepatic steatosis in response to fasting associated with a change in the expression of inflammatory and oxidative genes.58,59
Finally, the healthy benefits of PPARα also are attributable to fibroblast growth factor 21 (FGF21), a hepatokine secreted from the liver directly into the bloodstream, which upon binding to a specific receptor complex in target tissues improves systemic insulin sensitivity and lipid turnover. In the liver, activation of PPARα results in a significant increase of both hepatic and serum levels of FGF21, and PPARα null mice are FGF21-deficient. Notably, mice lacking both PPARα and FGF21 or FGF21 alone are more prone to develop hepatic steatosis when fed a MCDD.
Intriguingly, in human beings the expression of PPARα negatively correlates with the presence of NASH and the severity of steatosis.60 The administration of PPARα agonists (discussed in more detail later) in pilot studies and clinical trials has further assessed the clinical relevance of the contribution of PPARα to NAFLD/NASH.
Generally, PPARα controls energy and nutrient homeostasis, both directly, via activation of genes encoding enzymes involved in fatty acid metabolism, and, indirectly, by means of FGF21. This capacity, coupled with its anti-inflammatory actions, results in the overall protection against hepatic fatty acid accumulation and progression toward NASH.
PPARβ/δ
PPARβ/δ is the least studied in this family of nuclear receptor, although its expression is fundamental for embryo development. Indeed, disruption of the PPARβ/δ gene lead to embryonic death in the first days of development owing to impaired placenta development and giant cell differentiation.61 Despite its role in embryogenesis, PPARβ/δ plays a role in the regulation of energy metabolism in several organs, including the liver.62 In the liver, PPARβ/δ is highly expressed in hepatocytes, HSCs, and Kupffer cells, thus indicating a potential role of this nuclear receptor in inflammation and fibrosis.63 Intriguingly, the hepatic action of PPARβ/δ and PPARα in fatty acid β-oxidation and transportation appears moderately redundant. However, PPARβ/δ fails to compensate for the absence of PPARα in PPARα-null mice fed with a HFD.45,62,64
Several monounsaturated fatty acids (MUFAs) can bind to and activate PPARβ/δ, inducing balanced control of both hepatic fatty acids and glucose metabolism.65 PPARβ/δ mediates the activation of the principal enzyme designated to endogenous MUFAs synthesis, the stearoyl-CoA desaturase 1 (SCD1), finally resulting in a positive loop of regulation that culminates in a liver safeguard.65,66 Indeed, animals with liver-specific adenovirus-mediated PPARβ/δ activation fed with a HFD show less hepatic damage, despite increased lipid accumulation. This is mostly owing to the induced expression of SCD1, which avoids lipotoxicity by converting saturated fatty acids into MUFA.65 The saturated fatty acid:MUFA ratio is fundamental to preserve cellular homeostasis; indeed, a shift toward saturated fatty acid has been related to several pathologic conditions.67 Furthermore, hepatic PPARβ/δ overexpression or activation in db/db mice inhibits the expression of SREBP1c, the master regulator of lipid biogenesis, finally leading to improved hepatic steatosis.68 Interestingly, the expression of SCD1 can be induced by SREBP1c.69 Therefore, it is plausible that both PPARβ/δ and SREBP1c contribute to the fine-tuning of this enzyme in the liver to limit the accumulation of toxic lipid species with consequential detrimental effects.
Mice with hepatocyte PPARβ/δ activation show high circulating levels of phosphatidylcholine (18:0/18:1), which promotes muscle fatty acid uptake and catabolism via PPARα. On the contrary, hepatic PPARβ/δ ablation shows the opposite effect. Notably, administering phosphatidylcholine (18:0/18:1) to db/db mice improves metabolic homeostasis, thus corroborating the protective role for PPARβ/δ in liver steatosis.70 Another mechanism through which PPARβ/δ elicits amelioration of NAFLD resides in its capacity to regulate hepatic very-low-density lipoprotein receptor (VLDLR). Indeed, the expression of VLDLR correlates negatively with the abundance of PPARβ/δ in steatotic liver biopsy specimens, and the absence of the nuclear receptor in mice and primary cultured hepatocytes resulted in increased VLDLR levels.71 However, several studies have shown that VLDLR expression is up-regulated by several PPAR agonists, including PPARβ/δ ones.72, 73, 74 The administration of the PPARβ/δ agonist GW501516 increases VLDLR levels and triglycerides accumulation in the liver of wild-type mice, but in PPARβ/δ knockout animals this effect was blunted.74 In macrophages, VLDL particles bind to PPARβ/δ and lead to the activation of a downstream pathway, eventually inducing triglyceride accumulation. Notably, in this context the expression of VLDLR also increases when PPARβ/δ expression is null.75 Overall, this suggests that PPARβ/δ is essential for orchestrating the transcriptional response of VLDL particles and finely modulates the level of VLDLR, probably on the basis of the available ligands. However, it also is possible that VLDLR is required to guarantee the action of exogenous PPARβ/δ ligands.
In addition to its function in hepatic metabolism, PPARβ/δ also has a major impact on inflammation.29,76,77 However, the exact role of PPARβ/δ activation in liver inflammation is not well established, given the conflicting results obtained until now. On one hand, the activation of PPARβ/δ has been correlated with the induction of anti-inflammatory signals. Indeed, CCl4-treated PPARβ/δ-null mice show higher levels of liver fibrosis than wild-type mice, owing to induced HSC proliferation. Moreover, the administration of GW0742 as well as KD3010, 2 PPARβ/δ agonists, to the wild-type mice resulted in amelioration of a fibrosis condition both in the CCl4-fibrotic model and in the cholestasis-induced fibrosis model.78,79 On the other hand, activating PPARβ/δ using the synthetic ligand GW501516 or L165041 in CCl4-treated mice enhanced the fibrotic response owing to increased expression of proinflammatory and profibrotic genes, as well as HSC stimulation.80,81 Further studies are needed to clarify the contribution of PPARβ/δ to inflammation-driven hepatic injuries.
PPARγ
In mammals, PPARγ exists as 2 protein isoforms, both deriving from a single gene, which differ in length and tissue expression.82,83 Although PPARγ2 (G2 isoform) is expressed mainly in adipose tissue, where it governs lipid storage and adipocytes differentiation, PPARγ1 (G1 isoform) also can be found ubiquitously at low levels in non–white adipose tissue (WAT) such as liver, spleen, and heart.83,84 Moreover, PPARγ1 is expressed abundantly in macrophages, where it regulates cholesterol homeostasis, macrophage activation, and repression of inflammation.85, 86, 87, 88 Notably, the high abundance of PPARγ messenger RNA in the liver is a manifest feature of the steatotic liver in both human beings and experimental animal models.33,89,90 Mice treated with HFD show up-regulation of PPARγ with concomitant induction of liver steatosis.91 Accordingly, hepatocyte PPARγ selective ablation exerts a protective effect against hepatic steatosis in HFD-fed mice as well as ob/ob mice.92,93 Indeed, evidence in liver-specific PPARγ knockout mice indicates that PPARγ induces hepatic lipid accumulation by promoting the synthesis of new fatty acids together with their increased uptake.93 Furthermore, the treatment with PPARγ ligand rosiglitazone results in an increased steatogenic effect in the liver of KK-Ay mice, which recapitulates the features of human NAFLD, including altered adipokine expression, obesity, dyslipidemia, and insulin resistance.94 However, rosiglitazone administration to NASH patients ameliorates insulin sensitivity and histologic markers of steatosis.95,96 In a murine model of MCDD-induced fibrosis, rosiglitazone treatment prevents NASH development.97 Moreover, adenovirus-mediated PPARγ overexpression in mice fed with MCDD for 2 months causes the resolution of liver fibrosis via decreased HSC proliferation and cell-cycle arrest and apoptosis.98 Indeed, the activated phenotype of HSCs may be reversed to quiescent ones upon PPARγ ligands, thus pointing at PPARγ capacity to modulate proinflammatory and profibrogenic gene expression.99,100 Although activation of PPARγ elicits a harmful outcome in hepatocytes with the promotion of NAFLD progression, in HSCs its activity exerts beneficial effects that result in the resolution of NASH. Indeed, the disruption of PPARγ expression in macrophages and HSCs aggravates the fibrogenic response to CCl4-induced liver injury.101 Finally, PPARγ expression in liver macrophages, both Kupffer cells and infiltrating monocytes, is necessary for an alternative macrophage activation (M2) pathway, which is associated with decreased release of inflammatory cytokines and growth factors, therefore resulting in attenuated fibrosis.102 Indeed, macrophage PPARγ deletion predisposes animals to develop diet-induced obesity and insulin resistance, as well as worsens CCl4-induced liver fibrosis.101,103 If overall PPARγ activation is driving or diminishing hepatic damage is still not completely clear. Further studies aimed at identifying a proper therapy to selectively balance desirable and detrimental effects is of primary importance.
PPARs and NASH Treatments
The first line in the management of NASH patients is represented by lifestyle modifications, which includes weight loss through a proper dietary regimen and concomitant increased physical exercise.7,104 Indeed, by losing up to 10% of body weight, NASH patients showed diminished inflammation and regression of fibrosis.105,106 However, because lifestyle modifications are not easily achieved and kept over time, the treatment of NASH patients also required a combination with pharmacologic intervention.
Since now, several drugs have been tested and most of them have been designed to specifically target NRs in the liver. However, NR-based therapies usually showed poor efficacy in human beings. Today, new combined drugs targeting NRs, including dual-/triple-agonists and NR modulators, are emerging as promising pharmacologic interventions in NASH patients, with minimal negative metabolic effects107 (Figure 1, Table 1).
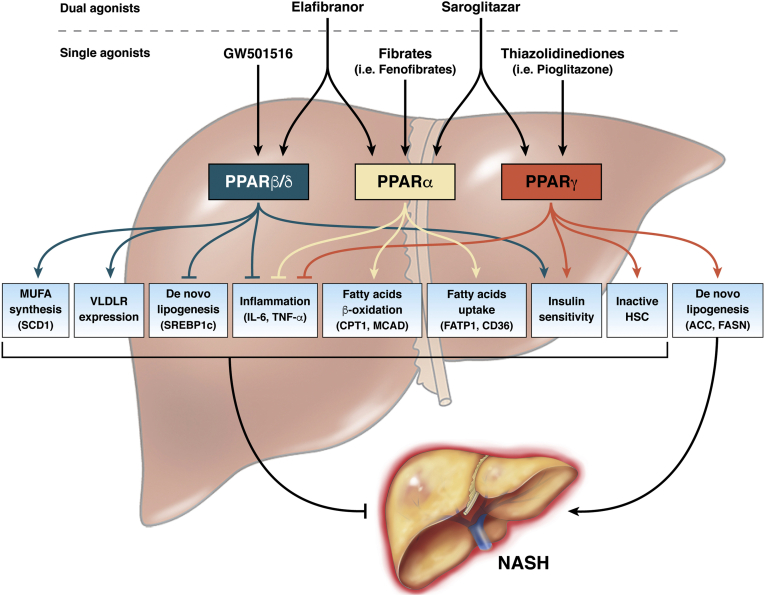
Figure 1.
Role of selective agonists of PPARs in NASH. In the liver, the 3 existing isoforms of PPAR can be activated by selective agonists. Although initially developed as a single agonist, able to selectively activate 1 single isoform, recently, dual agonists simultaneously targeting 2 PPAR isoforms represented the better therapeutic strategy to limit the detrimental effects of NASH. The beneficial effect of PPAR activation in the liver is the result of a complex cross-talk between different cellular and molecular pathways, which overall down-regulate lipid accumulation and contrast inflammation, thus contributing to improved liver health. Despite the role of PPARα and PPARβ/δ in NASH pathology being unambiguously clear, some concerns remain for PPARγ. Indeed, when activated in hepatocytes, PPARγ acts to promote fatty acid accumulation, steatosis, and progression toward NASH. On the contrary, the activation of PPARγ in HSCs exerts beneficial effects that result in the resolution of NASH. Red lines indicate the pathways down-regulated by PPAR activation, whereas green lines specify the pathways induced by PPAR agonism. ACC, acetyl CoA carboxylase; CD36, fatty acid translocase CD36; CPT1, carnitine palmitoyltransferase 1; FASN, fatty acid synthase; FATP1, Fatty acid transport protein 1; IL6, interleukin 6; MCAD, medium-chain acyl-coenzyme A dehydrogenase.
Table 1.
PPARs, FXR–FGF19, and NAFLD/NASH Clinical Trials
| Trial identifier | Trial phase (status) | Disease | Intervention |
|---|---|---|---|
| PPAR | |||
| NCT03008070 | Active, not recruiting | NASH | Drug: lanifibranor Drug: placebo |
| NCT02285205 | Completed | Type 2 diabetes NAFLD |
Drug: oral administration of lobeglitazone |
| NCT00252499 | Terminated, has results | Fatty liver insulin resistance | Drug: rosiglitazone Drug: fenofibrate Drug: placebo for rosiglitazone Drug: placebo for fenofibrate |
| NCT00633282 | Completed | NAFLD | Behavioral: lifestyle intervention Drug: pioglitazone Drug: berberine |
| NCT01694849 | Completed | NASH | Drug: elafibranor 80 mg Drug: elafibranor 120 mg Drug: placebo |
| NCT03639623 | Recruiting | Liver transplant complications NAFLD |
Drug: saroglitazar |
| NCT00062764 | Completed, has results | NASH | Drug: Actos (pioglitazone) |
| NCT00013598 | Completed | Fatty liver NASH |
Drug: pioglitazone |
| NCT03883607 | Recruiting | NASH | Drug: elafibranor 80 mg Drug: elafibranor 120 mg |
| NCT03953456 | Recruiting | NAFLD | Drug: elafibranor 120 mg Drug: placebo |
| NCT02704403 | Recruiting | NASH with fibrosis | Drug: elafibranor Drug: placebo |
| NCT03617263 | Recruiting | NAFLD in women with PCOS | Drug: saroglitazar magnesium 4-mg tablet Drug: placebo |
| NCT03061721 | Active, not recruiting | NASH NAFLD |
Drug: saroglitazar magnesium 1 mg Drug: saroglitazar magnesium 2 mg Drug: saroglitazar magnesium 4 mg Drug: placebo |
| NCT03863574 | Recruiting | NASH | Drug: saroglitazar magnesium 2 mg Drug: saroglitazar magnesium 4 mg Drug: placebo |
| NCT02265276 | Unknown | Fatty liver | Drug: saroglitazar Drug: pioglitazone |
| NCT04193982 | Not yet recruiting | NAFLD | Drug: saroglitazar Drug: vitamin E Drug: combination drug Behavioral: lifestyle changes |
| NCT02891408 | Completed | NASH | Drug: firsocostat Drug: fenofibrate |
| NCT01289639 | Terminated, has results | Fatty liver | Drug: fenofibrate Drug: pioglitazone Drug: placebo |
| NCT00262964 | Completed, has results | NAFLD | Drug: niacin Drug: fenofibrate Drug: placebo |
| NCT02781584 | Recruiting | NASH NAFLD |
Drug: SEL Drug: firsocostat Drug: cilofexor Drug: fenofibrate Drug: vascepa |
| NCT03646292 | Not yet recruiting | NAFLD Type 2 diabetes |
Drug: pioglitazone Drug: empagliflozin Drug: combination of pioglitazone and empagliflozin |
| NCT00994682 | Completed, has results | Type 2 diabetes mellitus NASH NAFLD |
Drug: pioglitazone study drug Drug: placebo Drug: pioglitazone open label |
| NCT02365233 | Terminated, has results | Type 2 diabetes mellitus NAFLD |
Drug: DPP4 inhibitor Drug: pioglitazone Drug: Lantus insulin |
| FXR–FGF19 | |||
| NCT01265498 | Completed, has results | NAFLD NASH |
Drug: obeticholic acid Drug: placebo |
| NCT02855164 | Active, not recruiting | NASH | Drug: tropifexor (LJN452) Drug: placebo |
| NCT03976687 | Recruiting | NASH Healthy |
Drug: EYP001a |
| NCT01999101 | Completed | NAFLD | Drug: Px-104 |
| NCT04065841 | Recruiting | NASH | Drug: tropifexor Drug: licogliflozin |
| NCT03836937 | Recruiting | NAFLD | Drug: obeticholic acid |
| NCT00501592 | Completed, has results | Diabetes mellitus, type II Fatty liver |
Drug: INT-747 Drug: placebo |
| NCT04328077 | Not yet recruiting | NASH | Drug: TERN-101 Other: placebo |
| NCT02808312 | Completed | NASH | Drug: GS-9674 (30 mg) Drug: GS-9674 (10 mg) |
| NCT02654002 | Completed | NASH | Drug: GS-9674 Drug: placebo |
| NCT02854605 | Completed | NASH | Drug: GS-9674 Drug: placebo to match GS-9674 |
| NCT02918929 | Completed | Presumptive NAFLD | Drug: EDP 305 Drug: placebo |
| NCT02633956 | Completed, has results | NASH | Drug: obeticholic acid Drug: atorvastatin Drug: placebo |
| NCT02548351 | Active, not recruiting | NASH | Drug: obeticholic acid Drug: placebo |
| NCT03439254 | Active, not recruiting | Compensated cirrhosis NASH |
Drug: obeticholic acid (10 mg) Drug: obeticholic acid (10–25 mg) Drug: placebo |
| NCT03912532 | Recruiting | NASH | Biological: NGM282 Other: placebo |
| NCT02443116 | Active, not recruiting | NASH | Biological: NGM282 Other: placebo |
| NCT04210245 | Recruiting | Compensated cirrhosis NASH |
Biological: aldafermin Other: placebo |
Actos (pioglitazone) (Takeda Global R&D Centre Ltd, Tokyo, Japan); DPP4, Dipeptidyl peptidase-4; PCOS polycystic ovarian syndrome; SEL, Selonsertib.
Given the hepatoprotective role of PPARα, therapies aimed at restoring its expression or activity are widely considered beneficial for the treatment of NASH patients. Fibrates represent the first class of PPARα ligands able to improve lipid accumulation and inflammation. However, despite their extensive use in the treatment of hypertriglyceridemia, fibrates do not show any benefit in the treatment of NAFLD and its sequelae. Indeed, fibrates are weak PPARα agonists, and their administration has been limited owing to dose-related adverse events.35,108,109
Although few data still exist concerning the role of PPARβ/δ in NASH, it is widely recognized that active PPARβ/δ is able to attenuate insulin resistance and the inflammatory process. A PPARβ/δ agonist (GW501516) originally was designed, however, despite the promising results in the initial trial, the drug has been withdrawn because of safety concerns.110 Seladelpar (also known as MBX-8025) is another selective PPARβ/δ agonist and its administration in preclinical and clinical studies ameliorated the serum lipid profile, lowered liver enzyme levels, and contrasted the accumulation of lipotoxic lipid species, thus indicating beneficial properties for NAFLD. However, it has been suspended from a phase II trial because of unexpected histologic findings.111, 112, 113, 114 In any case, the development of new pharmacologic drugs targeting PPARβ/δ has to take into consideration a preponderant side effect: the activation of this nuclear receptor triggers inflammatory pathways in the epidermis and enhances keratinocyte proliferation, thus inducing psoriasis.115,116
A dual-agonist targeting PPARα and PPARβ/δ has been developed, namely Elafibranor (GFT505, Genfit, France). Treatment with this drug improves NASH conditions in human beings, with a regression in fibrosis stage.117 However, Elafibranor recently failed to pass the phase III clinical trial that investigates the efficacy against NASH and the safety of long-term administration.
PPARγ activators thiazolidinediones have been broadly used in the management of diabetes as insulin sensitizers, and their effectiveness in NASH was shown recently. However, side effects such as weight gain and risk of heart failure associated with thiazolidinediones (ie, pioglitazone) may limit their use.118, 119, 120 A dual PPARα/γ agonist, saroglitazar, has been approved in India for the treatment of diabetic patients. However, this drug decreases biomarkers of hepatic health in both NAFLD and NASH patients.121
The protective effects of PPARs against fibrosis and inflammation lead to the generation of PPAR pan agonist, with the belief that, differently from targeting a single isotype, orchestrating all the PPARs together will induce an optimal metabolic response able to contrast and reduce NASH, given the combined effect of single PPAR agonists. Bezafibrate was one of the earlier PPAR pan agonists developed. Its administration in mice fed with MCDD improved hepatic steatosis and inflammation when used in combination with the PPARβ/δ agonist GW501516.122 Lanifibranor (IVA337), another PPAR pan agonist targeting all 3 isotypes, decreased liver steatosis, hepatocyte ballooning, and fibrosis in different mouse models of NASH.123,124 It was proved to be effective against skin and lung fibrosis.125,126 Lanifibranor currently is being tested in a phase 2b clinical trial.
FXR–FGF15/19 in the Gut–Liver Axis
FXR was described in 1995127 and it is the master regulator of bile acid (BA) homeostasis.128 It has a specific tissue distribution in the gastrointestinal tract with a peak in the liver and ileum, as well as in the kidney and adrenal glands.127,129,130 BA homeostasis is the result of the cross-talk between the liver and the intestine orchestrated by tissue-specific FXR activities. This NR reduces BA de novo synthesis in the liver, promotes BA secretion in bile, increases BA intestinal re-absorption, and decreases hepatic basolateral BA re-uptake.
BAs are amphipathic detergents synthesized in the liver via a multistep reaction that converts cholesterol into BAs.131 In the classic pathway, cholesterol is oxidized by the rate-limiting enzyme cytochrome P450 7A1 (CYP7A1) to produce cholic acid (CA). Alternative or acidic pathways lead to the formation of chenodeoxycholic acid (CDCA) through 27-hydroxylase. CA and CDCA are conjugated with taurine or glycine to form less-toxic, more hydrophilic bile salts. BAs are stored in the gallbladder and then secreted into the small intestine, where they contribute to the digestion of lipids, cholesterol, and fat-soluble vitamins.132 In the intestine, gut microbiota via bile salt hydrolase mediates the deconjugation of CA and CDCA to secondary BAs: deoxycholic acid and lithocholic acid or ursodeoxycholic acid, respectively.133 Finally, BAs are reabsorbed back through the portal circulation into the liver,132 recycling 95% of BAs and reducing their de novo synthesis.
At the distal ileum, BAs are reabsorbed by the apical sodium-dependent bile acid transporter.134 In enterocytes, BAs are able to activate FXR, inducing the fibroblast growth factor FGF15/19 (mouse and human, respectively) expression. FGF19 is an enterokine that travels through the portal circulation, reaches the liver, and binds to the FGF receptor 4/β-Klotho complex. The binding leads to the activation of the c-jun N-terminal kinase-dependent pathway, which ultimately down-regulates CYP7A1 expression and reduces BA synthesis.135 In enterocytes, FXR increases BA intestinal re-absorption and secretion in the portal vein, up-regulating the intestinal BA binding protein (which shuttles BAs from the apical to the basolateral membrane),136,137 and the heterodimeric organic solute transporter α/β.138 Furthermore, FXR reduces hepatic basolateral BA re-uptake, negatively regulating the sodium–taurocholate cotransporter protein and organic anion transporting polypeptide expression.
In hepatocytes, FXR activation induces the small heterodimer partner (SHP), reducing CYP7A1 expression and BA synthesis.129 Notably, in the liver, FXR activation promotes BA excretion but does not reduce BA synthesis with the same intensity of the FGF15/19 pathway.139
FXR and NASH: Preclinical Studies
The role of FXR activation on the development and protection against NASH has been evaluated in several studies using different mouse models and FXR agonists. Systemic activation of FXR prevents hepatic steatosis, inflammation, and fibrosis. In mice fed a HFD, the administration of FXR agonists (GW4064 and obeticholic acid [OCA]) improved glucose tolerance, and reduced body weight, fat mass, and hepatic triglyceride accumulation, ameliorating steatosis severity.140,141 In mice fed a HFD and in LDLR-/- mice, a model of Western diet, the administration of GW4064 and WAY-362450, 2 synthetic FXR agonists, reduced triglyceride and cholesterol levels, and WAY-362450 treatment also decreased high-density lipoprotein (HDL) levels.141,142 C57BL/6 mice fed a MCDD, a well-established NASH model, and treated with WAY-362450 for 4 weeks, showed a reduction of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, inflammatory cell infiltration, and hepatic steatosis.143 These positive effects of WAY-362450 were abolished in FXR-/- mice fed a MCDD. FXR-/- mice showed worse liver damage than wild-type mice.144 Interestingly, FXR-/- mice fed a MCDD developed hepatic cholestasis owing to the high concentration of BAs in the liver and the inhibition of genes involved in fatty acid uptake and triglyceride accumulation.144
It has been shown that hepatic FXR activation via SHP induction decreases lipogenesis and increases fatty acid oxidation through PPARα, leading to protection against NASH development.145,146 Interestingly, FXR induces PPARα expression and fatty acid oxidation only in human beings because the murine PPARα promoter does not present a functional FXR responsive element. In addition, FXR regulates hepatic glucose metabolism and immune response. In db/db mice the administration of GW4064 for 5 days activated glycogenesis and ameliorated insulin sensitivity.147 Animal model studies showed that FXR activation is able to induce the expression of genes involved in the acute-phase response and the activation of Natural Killer T (NKT) cells.148,149 In mice fed a MCDD and treated with WAY-362450, the activation of FXR reduced inflammatory infiltrates and Monocyte Chemoattractant Protein-1 (MCP1) levels.143 In line with this, monocytes isolated from FXR-/- mice showed high levels of interleukin 1β, tumor necrosis factor α (TNFα), and interferon γ, and were less responsive to anti-inflammatory drugs.150 Activation of FXR in HSCs induced SHP expression, reducing hepatic fibrosis.151,152 Furthermore, in rat HSCs, OCA administration induced PPARγ expression, reducing collagen gene induction.153
Mice fed a HFD and treated with fexaramine, an intestinal-specific FXR agonist, showed a reduction of body weight and lipogenesis expression genes and an induction of browning adipose tissue and energy expenditure ameliorating triglyceride levels and steatosis.154,155 In addition, fexaramine treatment improved insulin sensitivity, reducing fasting serum insulin and increasing serum glucagon-like peptide 1 levels.155 This FXR agonist is able to change BA composition, up-regulating taurolithocholic acid (TLCA) and lithocholic acid levels, strong agonists of FXR-Takeda G-protein receptor 5, which up-regulates serum glucagon-like peptide 1 levels.156,157 Furthermore, intestinal FXR activation leads to FGF15/19 induction, which prevents steatosis, inflammation, fibrosis, and the metabolic syndrome, major causes of NASH. Fgf15-/- mice fed a HFD showed severe steatosis.158,159 FGF15/19 administration down-regulates the expression of genes involved in lipid synthesis such as fatty acid synthase, Scd1, and diacylglycerol O-acyltransferase 2, and modifies BA composition, increasing tauro-β-muricholic acid (TβMCA) levels.159 In mice fed a high-fat, high-fructose, and high-cholesterol diet, treatment with FGF19 analog (M70) reduced hepatic inflammation and fibrosis.159 Furthermore, FGF19 acts on the metabolic syndrome, decreasing total weight and body fat mass, dyslipidemia, and ameliorating glucose homeostasis.159
Very recently, it has been shown that high-fructose and high-fat–fed pigs developed NASH, cholestasis, and impaired FXR–FGF19 signaling in the gut–liver axis. In this animal model, the severity of NASH was correlated with the reduction of FGF19 levels that lead to gut dysbiosis and increased colonic levels of choline metabolites and secondary BAs (FXR, NASH, and microbiota).
Several preclinical studies focused on the role of OCA, a selective FXR agonist with 100-fold activity higher than CDCA,160 in the prevention of NASH development, highlighting the ability of OCA to modulate glucose and lipid homeostasis and to promote hepatic anti-inflammatory and antifibrotic effects.161 In Zucker fa/fa obese rats, the administration of OCA (10 mg/kg) reduced insulin resistance and hepatic steatosis as well as body weight gain and liver fat deposition.162 Similar effects were observed in APO-E2-/- mice treated with OCA for 12 weeks.163 Furthermore, in these mice, drug administration prevented aortic plaque formation, reducing hepatic triglycerides and cholesterol content, although the development of atherosclerosis was not inhibited.164 OCA administration in a rabbit model of metabolic syndrome reduced visceral fat and improved glucose tolerance.165 OCA also shows immunomodulatory and anti-inflammatory effects. In vascular smooth muscle cells OCA down-regulated nuclear factor-κB–dependent expression of inducible nitric oxide synthetase (NOS) and cyclooxygenase-2.166 In a mouse model of hepatitis, OCA treatment reduced serum AST, interferon γ, and TNF-α levels.148 Furthermore, OCA prevents hepatic fibrosis from acting on HSC activation.152 In a thioacetamide rat model of liver fibrosis, OCA treatment decreased fibrosis and cirrhosis, reducing portal hypertension.167 Taken together, these data indicate that OCA ameliorates glucose levels and insulin sensitivity, and reduces hepatic lipid synthesis and inflammation, preventing liver damage.
FXR and NASH: Clinical Studies
FXR agonists represent an attractive class of drugs for patients with chronic liver disease. Currently, several human clinical trials are testing the safety and effects of these compounds (Table 1). In particular, OCA, a 6-ethyl-CDCA, has been approved for the treatment of primary biliary cholangitis. Clinical trials tested OCA in patients with NAFLD with type II diabetes and NASH.168,169 In a phase II clinical trial, 64 patients with NAFLD and type II diabetes were randomized to placebo, 25 mg OCA, and 50 mg OCA. The drug improved insulin sensitivity, body weight, serum levels of ALT, serum levels of γ-glutamyltransferase, serum levels of triglycerides, and fibrosis markers. OCA increased serum levels of alkaline phosphatase and LDL, and reduced HDL concentration. As expected, the drug increased FGF19 levels and reduced BA concentration, confirming FXR activation.168
In the second trial, a multicenter, randomized, phase III study, the FXR ligand obeticholic acid for noncirrhotic, nonalcoholic steatohepatitis trial (FLINT), 283 patients were treated for 72 weeks and randomized to placebo or 25 mg OCA. FLINT showed that OCA administration improved liver histology (measured as NAFLD Activity Score (NAS) score), steatosis, inflammation, and fibrosis. OCA also reduced body weight and serum ALT and γ-glutamyltransferase levels. In line with previous studies, the drug increased alkaline phosphatase and LDL levels and reduced HDL concentration. On the contrary, the FXR agonist increased fasting insulin and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), and 23% of patients had intense/severe pruritus. A phase II randomized trial in Japan (FLINT-J) showed that high OCA doses (40 mg/d) significantly resolved NASH in patients with mild fibrosis.169 Trials suggested that high doses of OCA increased the frequency and severity of pruritus. Furthermore, in 2017, the use of OCA (5 mg/d, quantity was lower compared with the dose tested in the FLINT study) was associated with major side effects including liver transplantation and deaths in cirrhotic patients with advanced liver disease (F4 fibrosis), causing a warning by the Food and Drug Administration and European Medicines Agency (EMA) (FDA adds Boxes Warning to highlight correct dosing of Ocaliva February 1, 2018; https//www.fda.gov/Drugs/Drugsafety/ucm594941.htm). To evaluate the side effects and safety of OCA clinical trials are ongoing. In a phase II, double-blind, randomized study, OCA and statin therapy were administered to NASH patients with fibrosis stages 1–4 (clinical trial: NCT02633956).
A phase III, randomized, double-blind, placebo-controlled trial (Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment [REGENERATE] study; clinical trial: NCT02548351) evaluated OCA safety and efficacy in 2400 patients with NASH with liver fibrosis at stages 2 or 3. Participants received placebo or OCA 10 mg/d or 25 mg/d for 18 months. The REGENERATE trial analyzed the improvement of liver fibrosis and the resolution of NASH.
A phase III trial (Randomized Phase 3 Study Evaluating the Efficacy and Safety of Obeticholic Acid (OCA) in Subjects with Compensated Cirrhosis due to NASH (REVERSE) study; clinical trial: NCT03439254) investigated the OCA effects in 540 compensated cirrhotic NASH patients, evaluating fibrosis improvement using the NASH Clinical Research Network scoring system. Conclusive data from the REVERSE and REGENERATE studies are expected in 2020 and 2022, respectively.
Several nonsteroidal FXR agonists (tropifexor, nidufexor, and turofexorate) have been tested in phase I trials and currently are in phase II. Very recently, a phase II trial on GS-9674 (cilofexor), an FXR agonist close to GW4064, showed that the administration of cilofexor for 24 weeks was well tolerated and ameliorated hepatic steatosis, liver biochemistry, and serum bile acids in patients with NASH (clinical trial: NCT02854605). Severe pruritus was the common side effect, especially in patients receiving high doses of the drug (100 mg).170 Unlike OCA administration, no changes in lipid profile were observed after cilofexor treatment, highlighting the differences in the molecular structure and properties of these compounds.
Given the role of BA homeostasis in NASH development, clinical trials also have been conducted on the FGF19 analogue.171 In particular, NGM282 is currently in clinical trials to evaluate the safety, tolerability, and efficacy in NASH patients. NGM282 reduced body weight, body mass index, insulin levels, and HOMA-IR, as well as lipid content in the liver, serum ALT and AST levels, and fibrosis biomarkers. A total of 84% and 42% of patients showed an improvement in their NAS score and fibrosis stage, respectively. Common side effects were diarrhea, abdominal pain, and nausea.172 Taken together, these data highlight the importance of the FXR pathway as a promising target for NASH therapy (Figure 2).
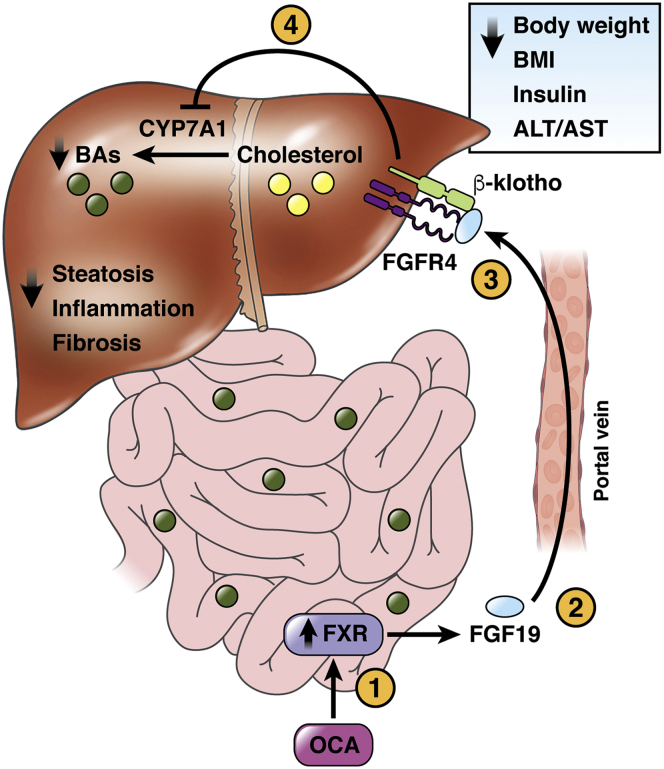
Figure 2.
Role of FXR and FGF19 in NASH. OCA-dependent FXR activation induces secretion in the portal circulation of FGF19, which reaches the liver through the portal circulation and binds the receptor FGFR4 with the co-receptor β klotho, repressing CYP7A1 expression and thus reducing BA synthesis. The effects of FXR activation improve liver steatosis, inflammation, and fibrosis. In addition, FGF19 is able to repress CYP7A1 expression, ameliorating body weight, BMI, insulin concentration, and serum ALT/AST levels. BMI, body mass index; FGFR4, fibroblast growth factor receptor 4.
LXRs in Hepatic Metabolism
LXRs are the cholesterol sensors and play a central role in the regulation of fatty acids, cholesterol, and glucose metabolism, as well as in the control of inflammation.173 LXRs exist as 2 isotypes: LXRα is expressed mainly in the liver, adipose tissue, kidney, and macrophages, whereas LXRβ is expressed ubiquitously.174, 175, 176 LXRs are activated by oxysterols (cholesterol derivatives) such as 24(S),25-epoxycholesterol, 25-hydroxycholesterol, and 22(R)-hydroxycholesterol,174, 175, 176 leading to the excretion of cholesterol as bile acids. At the same time, LXR activation reduces cholesterol synthesis and its uptake, improving cholesterol removal from the body and lipoprotein profile.174 LXRs regulate reverse cholesterol transport, in which the excess cholesterol reaches the liver and it is eliminated via feces. In rodents, LXRs induce CYP7A1 gene expression, the rate-limiting enzyme that converts cholesterol into bile acids. LXR activation also induces gene expression of the adenosine triphosphate (ATP)-binding cassette transporters ABCA1 and ABCG1, which promote cholesterol efflux from macrophages to HDL and apolipoproteins, leading to cholesterol transport to the liver.177,178
LXRs play a central role in fatty acid liver metabolism, inducing the expression of SREBP1c, a master regulator of triglycerides and fatty acid synthesis.
SREBP1c induces several enzymes used in fatty acid biosynthesis such as ATP citrate lyase, acetyl-CoA carboxylase and fatty acid synthase, SCD1, and glycerol-3-phosphate acyltransferase, a key enzyme in triglyceride and phospholipid synthesis.179 Overall, LXR-mediated induction of SREBP1c promotes lipid synthesis and regulates fatty acids and sterol homeostasis.
Furthermore, LXRs are involved in carbohydrate regulatory element-binding protein activation, a transcription factor implicated in the glycolysis and de novo lipogenesis in the liver.180, 181, 182 Carbohydrate regulatory element-binding protein up-regulates liver-pyruvate kinase gene expression, the rate-limiting enzyme of glycolysis. This transcription factor also is involved in the conversion of excess dietary carbohydrates into triglycerides. It works in synergy with LXR and SREBP1c, inducing the expression of genes such as fatty acid synthase (FAS), acetyl-CoA carboxylase, and SCD1.
LXRs exert anti-inflammatory functions via direct and indirect mechanisms as shown by the suppression of proinflammatory genes such as cyclooxygenase-2 and inducible NOS after LXR agonist treatment.183, 184, 185 LXR activation also inhibits Toll-like receptor (TLR) ligand-dependent inflammatory pathway through ABCA1 induction.186,187 In macrophages of atherosclerotic mice, cholesterol induces desmosterol production, an LXR ligand, modulating inflammation and lipid metabolism in a LXR-dependent fashion.188 Furthermore, in dendritic cells, LXR modulates cell migration via CCL19 and CCL21, which induce the expression of CD38, involved in leukocyte trafficking.189 Treatment with LXR agonists induces regulatory T cells (Treg) differentiation and inhibits T-helper (Th)1 and Th17 polarization.190
LXR and NASH
The role of LXR in the prevention and development of NASH is debated. NASH is characterized by hepatic inflammation resulting from adipose tissue and intestine dysfunction. As described previously, LXR activation is able to modulate inflammation.191 It has been shown that Small Ubiquitin-like MOdifier (SUMO)ylated forms of LXR down-regulate gene expression of inflammatory genes such as interleukin 1β and NOS, and inhibits nuclear factor-κB activity.183,185 In a NAFLD mouse model, LXR activation inhibited the phosphoinositide-3-kinase cascade, reducing TNF-α gene expression and liver injury.192 These data also were confirmed in a rat model of LPS-induced liver damage, in which treatment with the LXR agonist GW3965 reduced TNF-α and prostraglandin E2 gene expression.193 In APO-E2 knock-in mice, treatment with LXR agonist reduced cholesterol levels and inflammation but increased liver triglyceride levels.194 In bone marrow–derived macrophages, LXR activation inhibited TLR2, TLR4, and TLR9, as well as mitogen-activated protein kinase signaling,186 reducing the recruitment of these cells. In LXRα/β-/- mice, LPS administration induced proinflammatory cytokine expression and bone marrow–derived macrophage recruitment,195 showing the role of LXR in the regulation of the inflammatory response in acute liver injury.
On the other hand, hepatic LXR expression is correlated with the severity of NAFLD.196, 197, 198 In patients with NAFLD, LXR expression is up-regulated in liver and monocytes, whereas it is down-regulated in the ileum.198 In a mouse model of NASH, high levels of 24(S)-hydroxycholesterol and 7β- hydroxycholesterol have been observed.199 LXR agonists activate hepatic de novo lipogenesis and promote steatosis, inducing the expression of SREBP1c, FAS, and SCD1.181,200 Notably, in Kupffer cells without SREBP1c expression, 27-hydroxycholesterol reduces HFD-induced steatosis, inhibiting leukocyte recruitment and proinflammatory gene expression.201 In high-cholesterol diet–fed mice, LXRα deletion promoted cholesterol accumulation and increased serum ALT and AST levels as well as macrophage recruitment and Kupffer cell activation supporting inflammation,202,203 highlighting the protective role of LXRα in NASH. Furthermore, LXRα/β-/- mice show hepatic fibrosis, as shown by hepatic lipid droplet accumulation and by the induction of profibrotic genes such as Acta2 and Col1a1.204
LXRs play a key role in the maintenance of cholesterol homeostasis and they represent a promising therapeutic target in the management of atherosclerosis and cholesterol-related disorders. Several selective LXR agonists such as desmosterol, GW6340, and the LXRβ agonist LXR-623 are well tolerated but less used in the treatment of NAFLD.205, 206, 207 Further studies are needed to evaluate the safety and efficacy of LXR agonists in NASH treatment.
Conclusions
The physiological role of NRs in the gut–liver–adipose axis was explained by Evans and Mangelsdorf26 in the energy vector of nutrient homeostasis concept. In the fed state, PPARs, FXR, and LXR are involved in nutrient absorption from the gut and distribution from the gut/liver to peripheral tissues (white adipose tissue and muscles). BAs activate intestinal FXR, abetting nutrient acquisition and gut microbiota homeostasis. Absorbed dietary lipids are exported from the liver to peripheral tissue and excess cholesterol is removed from the body via reverse cholesterol transport controlled by the enterokine FGF19/15 (FXR target gene) and/or the activation of LXR by oxysterols. FGF19 stimulates transintestinal cholesterol excretion208 and reduces postprandial hyperglycemia, promoting hepatic glycogenesis. In the periphery, nutrients are consumed by muscle or stored in WAT thanks to PPARβ/δ and γ. In the fasting state, the retained energy in adipose tissue is metabolized. Through lipolysis, triacylglycerols stored in WAT are converted into fatty acids and released in the circulation to be used as an energy source by the organs. In the liver, fatty acids activate PPARα, promoting fatty acid catabolism and the production of ATP, ketone bodies, and FGF21. Ketone bodies are used as an energy source in the brain and FGF21 represents a stress signal to prepare other organs for energy deprivation.
Considering that the gut–liver–adipose axis dysfunction and abnormal energy homeostasis are the principal causes of NAFLD/NASH, the dysfunction of energy vectors could be considered as a mechanism by which NRs contributes to NAFLD/NASH development.
Several drugs that act on key pathogenic mechanisms are under development for the treatment of NASH. Agonists of PPARs and FXR have been studied extensively in mouse models, and phase II and III clinical trials currently are ongoing to test the safety and efficacy of these NR-based drugs for treating NASH.
Footnotes
Conflicts of interest A.M. has received scientific grants from Intercept Pharmaceuticals (San Diego, CA, USA) and NGM Biopharmaceuticals (San Diego, CA, USA), for the treatment of intestinal and hepatic diseases during the years 2014–2019. No money has been paid by Intercept Pharmaceuticals and NGM Biopharmaceuticals or any other agency to write this article.The remaining authors disclose no conflicts.
Funding This work was supported by Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) Progetti di Rilevante Interesse Nazionale (PRIN) 2017J3E2W2_002, Joint Programming Initiative - European Union (JPI-EU) FATMAL, European Regional Development Fund (Interreg V-A) Greece-Italy 2014-2020 Monitoring Information System (MIS) 5003627, Nuclear Receptors Network (NR-NET) Seventh Framework Programme (FP7) Marie Curie Initial Training Networks (ITN), Associazione Italiana Ricerca sul Cancro (AIRC) IG23239, and Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) Programma Operativo Nazionale "Ricerca e Innovazione (PON “R&I”) 2014-2020 n. ARS01_01220.
References
- 1.Swinburn B.A., Sacks G., Hall K.D., McPherson K., Finegood D.T., Moodie M.L., Gortmaker S.L. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Lindenmeyer C.C., McCullough A.J. The natural history of nonalcoholic fatty liver disease-an evolving view. Clin Liver Dis. 2018;22:11–21. doi: 10.1016/j.cld.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baffy G., Brunt E.M., Caldwell S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A., Das A., Majumder K., Arora N., Mayo H.G., Singh P.P., Beg M.S., Singh S. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. Am J Clin Oncol. 2018;41:874–881. doi: 10.1097/COC.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654 e1-9. doi: 10.1016/j.cgh.2014.04.014. quiz e39–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 8.Wong V.W., Adams L.A., de Ledinghen V., Wong G.L., Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 9.Day C.P., James O.F. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 10.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L., Baker R.D., Baker S.S. Gut microbiome and nonalcoholic fatty liver diseases. Pediatr Res. 2015;77:245–251. doi: 10.1038/pr.2014.157. [DOI] [PubMed] [Google Scholar]
- 12.Adolph T.E., Grander C., Grabherr F., Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017;18:1649. doi: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valenti L., Alisi A., Galmozzi E., Bartuli A., Del Menico B., Alterio A., Dongiovanni P., Fargion S., Nobili V. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1274–1280. doi: 10.1002/hep.23823. [DOI] [PubMed] [Google Scholar]
- 14.Sookoian S., Castano G.O., Burgueno A.L., Gianotti T.F., Rosselli M.S., Pirola C.J. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009;50:2111–2116. doi: 10.1194/jlr.P900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W., Chang B., Li L., Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–1142. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao L., Ito K., Huang K.H., Sae-tan S., Lambert J.D., Ross A.C. Shifts in dietary carbohydrate-lipid exposure regulate expression of the non-alcoholic fatty liver disease-associated gene PNPLA3/adiponutrin in mouse liver and HepG2 human liver cells. Metabolism. 2014;63:1352–1362. doi: 10.1016/j.metabol.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahali B., Halligan B., Speliotes E.K. Insights from genome-wide association analyses of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:375–391. doi: 10.1055/s-0035-1567870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjaerg-Hansen A., Vogt T.F., Hobbs H.H., Cohen J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zain S.M., Mohamed Z., Mohamed R. Common variant in the glucokinase regulatory gene rs780094 and risk of nonalcoholic fatty liver disease: a meta-analysis. J Gastroenterol Hepatol. 2015;30:21–27. doi: 10.1111/jgh.12714. [DOI] [PubMed] [Google Scholar]
- 20.Hebbard L., George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 21.Van Herck M.A., Vonghia L., Francque S.M. Animal models of nonalcoholic fatty liver disease-a starter's guide. Nutrients. 2017;9:1072. doi: 10.3390/nu9101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen H.H., Feigh M., Veidal S.S., Rigbolt K.T., Vrang N., Fosgerau K. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov Today. 2017;22:1707–1718. doi: 10.1016/j.drudis.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Nagarajan P., Mahesh Kumar M.J., Venkatesan R., Majundar S.S., Juyal R.C. Genetically modified mouse models for the study of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:1141–1153. doi: 10.3748/wjg.v18.i11.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oseini A.M., Sanyal A.J. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37(Suppl 1):97–103. doi: 10.1111/liv.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison S.A., Day C.P. Benefits of lifestyle modification in NAFLD. Gut. 2007;56:1760–1769. doi: 10.1136/gut.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans R.M., Mangelsdorf D.J. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangelsdorf D.J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R.M. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson S.P., Dunn C., Laughter A., Yoon L., Swanson C., Stulnig T.M., Steffensen K.R., Chandraratna R.A., Gustafsson J.A., Corton J.C. Overlapping transcriptional programs regulated by the nuclear receptors peroxisome proliferator-activated receptor alpha, retinoid X receptor, and liver X receptor in mouse liver. Mol Pharmacol. 2004;66:1440–1452. doi: 10.1124/mol.104.005496. [DOI] [PubMed] [Google Scholar]
- 29.Wahli W., Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Braissant O., Foufelle F., Scotto C., Dauca M., Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 31.Mandard S., Muller M., Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girroir E.E., Hollingshead H.E., He P., Zhu B., Perdew G.H., Peters J.M. Quantitative expression patterns of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) protein in mice. Biochem Biophys Res Commun. 2008;371:456–461. doi: 10.1016/j.bbrc.2008.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettinelli P., Videla L.A. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011;96:1424–1430. doi: 10.1210/jc.2010-2129. [DOI] [PubMed] [Google Scholar]
- 34.Xu J., Xiao G., Trujillo C., Chang V., Blanco L., Joseph S.B., Bassilian S., Saad M.F., Tontonoz P., Lee W.N., Kurland I.J. Peroxisome proliferator-activated receptor alpha (PPARalpha) influences substrate utilization for hepatic glucose production. J Biol Chem. 2002;277:50237–50244. doi: 10.1074/jbc.M201208200. [DOI] [PubMed] [Google Scholar]
- 35.Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Yu K., Bayona W., Kallen C.B., Harding H.P., Ravera C.P., McMahon G., Brown M., Lazar M.A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 37.Chakravarthy M.V., Pan Z., Zhu Y., Tordjman K., Schneider J.G., Coleman T., Turk J., Semenkovich C.F. "New" hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Sapiro J.M., Mashek M.T., Greenberg A.S., Mashek D.G. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50:1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fruchart J.C. Selective peroxisome proliferator-activated receptor alpha modulators (SPPARMalpha): the next generation of peroxisome proliferator-activated receptor alpha-agonists. Cardiovasc Diabetol. 2013;12:82. doi: 10.1186/1475-2840-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin G., Schoonjans K., Lefebvre A.M., Staels B., Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- 41.Frohnert B.I., Hui T.Y., Bernlohr D.A. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem. 1999;274:3970–3977. doi: 10.1074/jbc.274.7.3970. [DOI] [PubMed] [Google Scholar]
- 42.Hostetler H.A., McIntosh A.L., Atshaves B.P., Storey S.M., Payne H.R., Kier A.B., Schroeder F. L-FABP directly interacts with PPARalpha in cultured primary hepatocytes. J Lipid Res. 2009;50:1663–1675. doi: 10.1194/jlr.M900058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulick T., Cresci S., Caira T., Moore D.D., Kelly D.P. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci U S A. 1994;91:11012–11016. doi: 10.1073/pnas.91.23.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoyama T., Peters J.M., Iritani N., Nakajima T., Furihata K., Hashimoto T., Gonzalez F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 45.Kersten S., Seydoux J., Peters J.M., Gonzalez F.J., Desvergne B., Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Djouadi F., Weinheimer C.J., Saffitz J.E., Pitchford C., Bastin J., Gonzalez F.J., Kelly D.P. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator- activated receptor alpha- deficient mice. J Clin Invest. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Alvarez A., Alvarez M.S., Gonzalez R., Cucarella C., Muntane J., Casado M. Human SREBP1c expression in liver is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 2011;286:21466–21477. doi: 10.1074/jbc.M110.209973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hebbachi A.M., Knight B.L., Wiggins D., Patel D.D., Gibbons G.F. Peroxisome proliferator-activated receptor alpha deficiency abolishes the response of lipogenic gene expression to re-feeding: restoration of the normal response by activation of liver X receptor alpha. J Biol Chem. 2008;283:4866–4876. doi: 10.1074/jbc.M709471200. [DOI] [PubMed] [Google Scholar]
- 49.Mansouri R.M., Bauge E., Staels B., Gervois P. Systemic and distal repercussions of liver-specific peroxisome proliferator-activated receptor-alpha control of the acute-phase response. Endocrinology. 2008;149:3215–3223. doi: 10.1210/en.2007-1339. [DOI] [PubMed] [Google Scholar]
- 50.Guerre-Millo M., Gervois P., Raspe E., Madsen L., Poulain P., Derudas B., Herbert J.M., Winegar D.A., Willson T.M., Fruchart J.C., Berge R.K., Staels B. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- 51.Montagner A., Polizzi A., Fouche E., Ducheix S., Lippi Y., Lasserre F., Barquissau V., Regnier M., Lukowicz C., Benhamed F., Iroz A., Bertrand-Michel J., Al Saati T., Cano P., Mselli-Lakhal L., Mithieux G., Rajas F., Lagarrigue S., Pineau T., Loiseau N., Postic C., Langin D., Wahli W., Guillou H. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202–1214. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ip E., Farrell G.C., Robertson G., Hall P., Kirsch R., Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–132. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- 53.Ip E., Farrell G., Hall P., Robertson G., Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 54.Jha P., Claudel T., Baghdasaryan A., Mueller M., Halilbasic E., Das S.K., Lass A., Zimmermann R., Zechner R., Hoefler G., Trauner M. Role of adipose triglyceride lipase (PNPLA2) in protection from hepatic inflammation in mouse models of steatohepatitis and endotoxemia. Hepatology. 2014;59:858–869. doi: 10.1002/hep.26732. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan P.M., Mezdour H., Quarfordt S.H., Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe∗2. J Clin Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiri-Sverdlov R., Wouters K., van Gorp P.J., Gijbels M.J., Noel B., Buffat L., Staels B., Maeda N., van Bilsen M., Hofker M.H. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J Hepatol. 2006;44:732–741. doi: 10.1016/j.jhep.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 57.Lalloyer F., Wouters K., Baron M., Caron S., Vallez E., Vanhoutte J., Bauge E., Shiri-Sverdlov R., Hofker M., Staels B., Tailleux A. Peroxisome proliferator-activated receptor-alpha gene level differently affects lipid metabolism and inflammation in apolipoprotein E2 knock-in mice. Arterioscler Thromb Vasc Biol. 2011;31:1573–1579. doi: 10.1161/ATVBAHA.110.220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rommelaere S., Millet V., Gensollen T., Bourges C., Eeckhoute J., Hennuyer N., Bauge E., Chasson L., Cacciatore I., Staels B., Pitari G., Galland F., Naquet P. PPARalpha regulates the production of serum Vanin-1 by liver. FEBS Lett. 2013;587:3742–3748. doi: 10.1016/j.febslet.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 59.van Diepen J.A., Jansen P.A., Ballak D.B., Hijmans A., Hooiveld G.J., Rommelaere S., Galland F., Naquet P., Rutjes F.P., Mensink R.P., Schrauwen P., Tack C.J., Netea M.G., Kersten S., Schalkwijk J., Stienstra R. PPAR-alpha dependent regulation of vanin-1 mediates hepatic lipid metabolism. J Hepatol. 2014;61:366–372. doi: 10.1016/j.jhep.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 60.Francque S., Verrijken A., Caron S., Prawitt J., Paumelle R., Derudas B., Lefebvre P., Taskinen M.R., Van Hul W., Mertens I., Hubens G., Van Marck E., Michielsen P., Van Gaal L., Staels B. PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol. 2015;63:164–173. doi: 10.1016/j.jhep.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Nadra K., Anghel S.I., Joye E., Tan N.S., Basu-Modak S., Trono D., Wahli W., Desvergne B. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol Cell Biol. 2006;26:3266–3281. doi: 10.1128/MCB.26.8.3266-3281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka T., Yamamoto J., Iwasaki S., Asaba H., Hamura H., Ikeda Y., Watanabe M., Magoori K., Ioka R.X., Tachibana K., Watanabe Y., Uchiyama Y., Sumi K., Iguchi H., Ito S., Doi T., Hamakubo T., Naito M., Auwerx J., Yanagisawa M., Kodama T., Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoekstra M., Kruijt J.K., Van Eck M., Van Berkel T.J. Specific gene expression of ATP-binding cassette transporters and nuclear hormone receptors in rat liver parenchymal, endothelial, and Kupffer cells. J Biol Chem. 2003;278:25448–25453. doi: 10.1074/jbc.M301189200. [DOI] [PubMed] [Google Scholar]
- 64.Chen J., Montagner A., Tan N.S., Wahli W. Insights into the role of PPARbeta/delta in NAFLD. Int J Mol Sci. 2018;19:1893. doi: 10.3390/ijms19071893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu S., Hatano B., Zhao M., Yen C.C., Kang K., Reilly S.M., Gangl M.R., Gorgun C., Balschi J.A., Ntambi J.M., Lee C.H. Role of peroxisome proliferator-activated receptor {delta}/{beta} in hepatic metabolic regulation. J Biol Chem. 2011;286:1237–1247. doi: 10.1074/jbc.M110.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogowski M.P., Flowers M.T., Stamatikos A.D., Ntambi J.M., Paton C.M. SCD1 activity in muscle increases triglyceride PUFA content, exercise capacity, and PPARdelta expression in mice. J Lipid Res. 2013;54:2636–2646. doi: 10.1194/jlr.M035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piccinin E., Cariello M., De Santis S., Ducheix S., Sabba C., Ntambi J.M., Moschetta A. Role of oleic acid in the gut-liver axis: from diet to the regulation of its synthesis via stearoyl-CoA desaturase 1 (SCD1) Nutrients. 2019;11:2283. doi: 10.3390/nu11102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin X., Xie X., Fan Y., Tian J., Guan Y., Wang X., Zhu Y., Wang N. Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology. 2008;48:432–441. doi: 10.1002/hep.22334. [DOI] [PubMed] [Google Scholar]
- 69.Flowers M.T., Ntambi J.M. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu S., Brown J.D., Stanya K.J., Homan E., Leidl M., Inouye K., Bhargava P., Gangl M.R., Dai L., Hatano B., Hotamisligil G.S., Saghatelian A., Plutzky J., Lee C.H. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502:550–554. doi: 10.1038/nature12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarei M., Barroso E., Palomer X., Dai J., Rada P., Quesada-Lopez T., Escola-Gil J.C., Cedo L., Zali M.R., Molaei M., Dabiri R., Vazquez S., Pujol E., Valverde A.M., Villarroya F., Liu Y., Wahli W., Vazquez-Carrera M. Hepatic regulation of VLDL receptor by PPARbeta/delta and FGF21 modulates non-alcoholic fatty liver disease. Mol Metab. 2018;8:117–131. doi: 10.1016/j.molmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tao H., Hajri T. Very low density lipoprotein receptor promotes adipocyte differentiation and mediates the proadipogenic effect of peroxisome proliferator-activated receptor gamma agonists. Biochem Pharmacol. 2011;82:1950–1962. doi: 10.1016/j.bcp.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Gao Y., Shen W., Lu B., Zhang Q., Hu Y., Chen Y. Upregulation of hepatic VLDLR via PPARalpha is required for the triglyceride-lowering effect of fenofibrate. J Lipid Res. 2014;55:1622–1633. doi: 10.1194/jlr.M041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarei M., Barroso E., Palomer X., Escola-Gil J.C., Cedo L., Wahli W., Vazquez-Carrera M. Pharmacological PPARbeta/delta activation upregulates VLDLR in hepatocytes. Clin Investig Arterioscler. 2019;31:111–118. doi: 10.1016/j.arteri.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 75.Chawla A., Lee C.H., Barak Y., He W., Rosenfeld J., Liao D., Han J., Kang H., Evans R.M. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo C.H., Massett M.P., Shishido T., Itoh S., Ding B., McClain C., Che W., Vulapalli S.R., Yan C., Abe J. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor delta (PPARdelta) stimulation. J Biol Chem. 2006;281:32164–32174. doi: 10.1074/jbc.M602369200. [DOI] [PubMed] [Google Scholar]
- 77.Rival Y., Beneteau N., Taillandier T., Pezet M., Dupont-Passelaigue E., Patoiseau J.F., Junquero D., Colpaert F.C., Delhon A. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol. 2002;435:143–151. doi: 10.1016/s0014-2999(01)01589-8. [DOI] [PubMed] [Google Scholar]
- 78.Shan W., Palkar P.S., Murray I.A., McDevitt E.I., Kennett M.J., Kang B.H., Isom H.C., Perdew G.H., Gonzalez F.J., Peters J.M. Ligand activation of peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol Sci. 2008;105:418–428. doi: 10.1093/toxsci/kfn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iwaisako K., Haimerl M., Paik Y.H., Taura K., Kodama Y., Sirlin C., Yu E., Yu R.T., Downes M., Evans R.M., Brenner D.A., Schnabl B. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proc Natl Acad Sci U S A. 2012;109:E1369–E1376. doi: 10.1073/pnas.1202464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hellemans K., Michalik L., Dittie A., Knorr A., Rombouts K., De Jong J., Heirman C., Quartier E., Schuit F., Wahli W., Geerts A. Peroxisome proliferator-activated receptor-beta signaling contributes to enhanced proliferation of hepatic stellate cells. Gastroenterology. 2003;124:184–201. doi: 10.1053/gast.2003.50015. [DOI] [PubMed] [Google Scholar]
- 81.Kostadinova R., Montagner A., Gouranton E., Fleury S., Guillou H., Dombrowicz D., Desreumaux P., Wahli W. GW501516-activated PPARbeta/delta promotes liver fibrosis via p38-JNK MAPK-induced hepatic stellate cell proliferation. Cell Biosci. 2012;2:34. doi: 10.1186/2045-3701-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu Y., Qi C., Korenberg J.R., Chen X.N., Noya D., Rao M.S., Reddy J.K. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 84.Saladin R., Fajas L., Dana S., Halvorsen Y.D., Auwerx J., Briggs M. Differential regulation of peroxisome proliferator activated receptor gamma1 (PPARgamma1) and PPARgamma2 messenger RNA expression in the early stages of adipogenesis. Cell Growth Differ. 1999;10:43–48. [PubMed] [Google Scholar]
- 85.Ricote M., Huang J., Fajas L., Li A., Welch J., Najib J., Witztum J.L., Auwerx J., Palinski W., Glass C.K. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tontonoz P., Nagy L., Alvarez J.G., Thomazy V.A., Evans R.M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 87.Jiang C., Ting A.T., Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 88.Majdalawieh A., Ro H.S. PPARgamma1 and LXRalpha face a new regulator of macrophage cholesterol homeostasis and inflammatory responsiveness. AEBP1. Nucl Recept Signal. 2010;8 doi: 10.1621/nrs.08004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gavrilova O., Haluzik M., Matsusue K., Cutson J.J., Johnson L., Dietz K.R., Nicol C.J., Vinson C., Gonzalez F.J., Reitman M.L. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 90.Yu S., Matsusue K., Kashireddy P., Cao W.Q., Yeldandi V., Yeldandi A.V., Rao M.S., Gonzalez F.J., Reddy J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 91.Inoue M., Ohtake T., Motomura W., Takahashi N., Hosoki Y., Miyoshi S., Suzuki Y., Saito H., Kohgo Y., Okumura T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 92.Moran-Salvador E., Lopez-Parra M., Garcia-Alonso V., Titos E., Martinez-Clemente M., Gonzalez-Periz A., Lopez-Vicario C., Barak Y., Arroyo V., Claria J. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- 93.Matsusue K., Haluzik M., Lambert G., Yim S.H., Gavrilova O., Ward J.M., Brewer B., Jr., Reitman M.L., Gonzalez F.J. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bedoucha M., Atzpodien E., Boelsterli U.A. Diabetic KKAy mice exhibit increased hepatic PPARgamma1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J Hepatol. 2001;35:17–23. doi: 10.1016/s0168-8278(01)00066-6. [DOI] [PubMed] [Google Scholar]
- 95.Neuschwander-Tetri B.A., Brunt E.M., Wehmeier K.R., Oliver D., Bacon B.R. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 96.Ratziu V., Giral P., Jacqueminet S., Charlotte F., Hartemann-Heurtier A., Serfaty L., Podevin P., Lacorte J.M., Bernhardt C., Bruckert E., Grimaldi A., Poynard T., Group L.S. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) trial. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 97.Nan Y.M., Fu N., Wu W.J., Liang B.L., Wang R.Q., Zhao S.X., Zhao J.M., Yu J. Rosiglitazone prevents nutritional fibrosis and steatohepatitis in mice. Scand J Gastroenterol. 2009;44:358–365. doi: 10.1080/00365520802530861. [DOI] [PubMed] [Google Scholar]
- 98.Yu J., Zhang S., Chu E.S., Go M.Y., Lau R.H., Zhao J., Wu C.W., Tong L., Zhao J., Poon T.C., Sung J.J. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol. 2010;42:948–957. doi: 10.1016/j.biocel.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Marra F., Efsen E., Romanelli R.G., Caligiuri A., Pastacaldi S., Batignani G., Bonacchi A., Caporale R., Laffi G., Pinzani M., Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 100.Galli A., Crabb D.W., Ceni E., Salzano R., Mello T., Svegliati-Baroni G., Ridolfi F., Trozzi L., Surrenti C., Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 101.Moran-Salvador E., Titos E., Rius B., Gonzalez-Periz A., Garcia-Alonso V., Lopez-Vicario C., Miquel R., Barak Y., Arroyo V., Claria J. Cell-specific PPARgamma deficiency establishes anti-inflammatory and anti-fibrogenic properties for this nuclear receptor in non-parenchymal liver cells. J Hepatol. 2013;59:1045–1053. doi: 10.1016/j.jhep.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 102.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Odegaard J.I., Ricardo-Gonzalez R.R., Goforth M.H., Morel C.R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A.W., Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang M.A., Greenson J.K., Chao C., Anderson L., Peterman D., Jacobson J., Emick D., Lok A.S., Conjeevaram H.S. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 105.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R., Fava J.L., Wing R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., Friedman S.L., Diago M., Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378 e5. doi: 10.1053/j.gastro.2015.04.005. quiz e14–e15. [DOI] [PubMed] [Google Scholar]
- 107.Tran M., Liu Y., Huang W., Wang L. Nuclear receptors and liver disease: Summary of the 2017 basic research symposium. Hepatol Commun. 2018;2:765–777. doi: 10.1002/hep4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Musso G., Gambino R., Cassader M., Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 109.Fernandez-Miranda C., Perez-Carreras M., Colina F., Lopez-Alonso G., Vargas C., Solis-Herruzo J.A. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Riserus U., Sprecher D., Johnson T., Olson E., Hirschberg S., Liu A., Fang Z., Hegde P., Richards D., Sarov-Blat L., Strum J.C., Basu S., Cheeseman J., Fielding B.A., Humphreys S.M., Danoff T., Moore N.R., Murgatroyd P., O'Rahilly S., Sutton P., Willson T., Hassall D., Frayn K.N., Karpe F. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 111.Jones D., Boudes P.F., Swain M.G., Bowlus C.L., Galambos M.R., Bacon B.R., Doerffel Y., Gitlin N., Gordon S.C., Odin J.A., Sheridan D., Worns M.A., Clark V., Corless L., Hartmann H., Jonas M.E., Kremer A.E., Mells G.F., Buggisch P., Freilich B.L., Levy C., Vierling J.M., Bernstein D.E., Hartleb M., Janczewska E., Rochling F., Shah H., Shiffman M.L., Smith J.H., Choi Y.J., Steinberg A., Varga M., Chera H., Martin R., McWherter C.A., Hirschfield G.M. Seladelpar (MBX-8025), a selective PPAR-delta agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 112.Bays H.E., Schwartz S., Littlejohn T., 3rd, Kerzner B., Krauss R.M., Karpf D.B., Choi Y.J., Wang X., Naim S., Roberts B.K. MBX-8025, a novel peroxisome proliferator receptor-delta agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. J Clin Endocrinol Metab. 2011;96:2889–2897. doi: 10.1210/jc.2011-1061. [DOI] [PubMed] [Google Scholar]
- 113.Choi Y.J., Roberts B.K., Wang X., Geaney J.C., Naim S., Wojnoonski K., Karpf D.B., Krauss R.M. Effects of the PPAR-delta agonist MBX-8025 on atherogenic dyslipidemia. Atherosclerosis. 2012;220:470–476. doi: 10.1016/j.atherosclerosis.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 114.Haczeyni F., Wang H., Barn V., Mridha A.R., Yeh M.M., Haigh W.G., Ioannou G.N., Choi Y.J., McWherter C.A., Teoh N.C., Farrell G.C. The selective peroxisome proliferator-activated receptor-delta agonist seladelpar reverses nonalcoholic steatohepatitis pathology by abrogating lipotoxicity in diabetic obese mice. Hepatol Commun. 2017;1:663–674. doi: 10.1002/hep4.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romanowska M., al Yacoub N., Seidel H., Donandt S., Gerken H., Phillip S., Haritonova N., Artuc M., Schweiger S., Sterry W., Foerster J. PPARdelta enhances keratinocyte proliferation in psoriasis and induces heparin-binding EGF-like growth factor. J Invest Dermatol. 2008;128:110–124. doi: 10.1038/sj.jid.5700943. [DOI] [PubMed] [Google Scholar]
- 116.Romanowska M., Reilly L., Palmer C.N., Gustafsson M.C., Foerster J. Activation of PPARbeta/delta causes a psoriasis-like skin disease in vivo. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L., Romero-Gomez M., Boursier J., Abdelmalek M., Caldwell S., Drenth J., Anstee Q.M., Hum D., Hanf R., Roudot A., Megnien S., Staels B., Sanyal A., Group G.-I.S. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159 e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 118.Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J., Balas B., Gastaldelli A., Tio F., Pulcini J., Berria R., Ma J.Z., Dwivedi S., Havranek R., Fincke C., DeFronzo R., Bannayan G.A., Schenker S., Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 119.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., Van Natta M., Clark J., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R., Nash C.R.N. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lincoff A.M., Wolski K., Nicholls S.J., Nissen S.E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 121.Pai V., Paneerselvam A., Mukhopadhyay S., Bhansali A., Kamath D., Shankar V., Gambhire D., Jani R.H., Joshi S., Patel P. A Multicenter, Prospective, Randomized, Double-blind Study to Evaluate the Safety and Efficacy of Saroglitazar 2 and 4 mg Compared to Pioglitazone 45 mg in Diabetic Dyslipidemia (PRESS V) J Diabetes Sci Technol. 2014;8:132–141. doi: 10.1177/1932296813518680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagasawa T., Inada Y., Nakano S., Tamura T., Takahashi T., Maruyama K., Yamazaki Y., Kuroda J., Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 123.Wettstein G., Luccarini J.M., Poekes L., Faye P., Kupkowski F., Adarbes V., Defrene E., Estivalet C., Gawronski X., Jantzen I., Philippot A., Tessier J., Tuyaa-Boustugue P., Oakley F., Mann D.A., Leclercq I., Francque S., Konstantinova I., Broqua P., Junien J.L. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol Commun. 2017;1:524–537. doi: 10.1002/hep4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lefere S., Puengel T., Hundertmark J., Penners C., Frank A.K., Guillot A., de Muynck K., Heymann F., Adarbes V., Defrene E., Estivalet C., Geerts A., Devisscher L., Wettstein G., Tacke F. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J Hepatol. 2020;73:757–770. doi: 10.1016/j.jhep.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 125.Ruzehaji N., Frantz C., Ponsoye M., Avouac J., Pezet S., Guilbert T., Luccarini J.M., Broqua P., Junien J.L., Allanore Y. Pan PPAR agonist IVA337 is effective in prevention and treatment of experimental skin fibrosis. Ann Rheum Dis. 2016;75:2175–2183. doi: 10.1136/annrheumdis-2015-208029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Avouac J., Konstantinova I., Guignabert C., Pezet S., Sadoine J., Guilbert T., Cauvet A., Tu L., Luccarini J.M., Junien J.L., Broqua P., Allanore Y. Pan-PPAR agonist IVA337 is effective in experimental lung fibrosis and pulmonary hypertension. Ann Rheum Dis. 2017;76:1931–1940. doi: 10.1136/annrheumdis-2016-210821. [DOI] [PubMed] [Google Scholar]
- 127.Forman B.M., Goode E., Chen J., Oro A.E., Bradley D.J., Perlmann T., Noonan D.J., Burka L.T., McMorris T., Lamph W.W., Evans R.M., Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 128.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 129.Lu T.T., Repa J.J., Mangelsdorf D.J. Orphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism. J Biol Chem. 2001;276:37735–37738. doi: 10.1074/jbc.R100035200. [DOI] [PubMed] [Google Scholar]
- 130.Wang H., Chen J., Hollister K., Sowers L.C., Forman B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 131.Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 132.Li T., Chiang J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Selwyn F.P., Csanaky I.L., Zhang Y., Klaassen C.D. Importance of large intestine in regulating bile acids and glucagon-like peptide-1 in germ-free mice. Drug Metab Dispos. 2015;43:1544–1556. doi: 10.1124/dmd.115.065276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wong M.H., Oelkers P., Dawson P.A. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270:27228–27234. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]
- 135.Inagaki T., Moschetta A., Lee Y.K., Peng L., Zhao G., Downes M., Yu R.T., Shelton J.M., Richardson J.A., Repa J.J., Mangelsdorf D.J., Kliewer S.A. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tochtrop G.P., DeKoster G.T., Covey D.F., Cistola D.P. A single hydroxyl group governs ligand site selectivity in human ileal bile acid binding protein. J Am Chem Soc. 2004;126:11024–11029. doi: 10.1021/ja047589c. [DOI] [PubMed] [Google Scholar]
- 137.Toke O., Monsey J.D., DeKoster G.T., Tochtrop G.P., Tang C., Cistola D.P. Determinants of cooperativity and site selectivity in human ileal bile acid binding protein. Biochemistry. 2006;45:727–737. doi: 10.1021/bi051781p. [DOI] [PubMed] [Google Scholar]
- 138.Dawson P.A., Hubbert M., Haywood J., Craddock A.L., Zerangue N., Christian W.V., Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim I., Ahn S.H., Inagaki T., Choi M., Ito S., Guo G.L., Kliewer S.A., Gonzalez F.J. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 140.Gai Z., Visentin M., Gui T., Zhao L., Thasler W.E., Hausler S., Hartling I., Cremonesi A., Hiller C., Kullak-Ublick G.A. Effects of farnesoid X receptor activation on arachidonic acid metabolism, NF-κB signaling, and hepatic inflammation. Mol Pharmacol. 2018;94:802–811. doi: 10.1124/mol.117.111047. [DOI] [PubMed] [Google Scholar]
- 141.Ma Y., Huang Y., Yan L., Gao M., Liu D. Synthetic FXR agonist GW4064 prevents diet-induced hepatic steatosis and insulin resistance. Pharm Res. 2013;30:1447–1457. doi: 10.1007/s11095-013-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evans M.J., Mahaney P.E., Borges-Marcucci L., Lai K., Wang S., Krueger J.A., Gardell S.J., Huard C., Martinez R., Vlasuk G.P., Harnish D.C. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G543–G552. doi: 10.1152/ajpgi.90585.2008. [DOI] [PubMed] [Google Scholar]
- 143.Zhang S., Wang J., Liu Q., Harnish D.C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 144.Wu W., Liu X., Peng X., Xue R., Ji L., Shen X., Chen S., Gu J., Zhang S. Bile acids override steatosis in farnesoid X receptor deficient mice in a model of non-alcoholic steatohepatitis. Biochem Biophys Res Commun. 2014;448:50–55. doi: 10.1016/j.bbrc.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 145.Pineda Torra I., Claudel T., Duval C., Kosykh V., Fruchart J.C., Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 146.Watanabe M., Houten S.M., Wang L., Moschetta A., Mangelsdorf D.J., Heyman R.A., Moore D.D., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kong B., Luyendyk J.P., Tawfik O., Guo G.L. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–122. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mencarelli A., Renga B., Migliorati M., Cipriani S., Distrutti E., Santucci L., Fiorucci S. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J Immunol. 2009;183:6657–6666. doi: 10.4049/jimmunol.0901347. [DOI] [PubMed] [Google Scholar]
- 149.Porez G., Gross B., Prawitt J., Gheeraert C., Berrabah W., Alexandre J., Staels B., Lefebvre P. The hepatic orosomucoid/alpha1-acid glycoprotein gene cluster is regulated by the nuclear bile acid receptor FXR. Endocrinology. 2013;154:3690–3701. doi: 10.1210/en.2013-1263. [DOI] [PubMed] [Google Scholar]
- 150.Renga B., D'Amore C., Cipriani S., Mencarelli A., Carino A., Sepe V., Zampella A., Distrutti E., Fiorucci S. FXR mediates a chromatin looping in the GR promoter thus promoting the resolution of colitis in rodents. Pharmacol Res. 2013;77:1–10. doi: 10.1016/j.phrs.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 151.Carino A., Biagioli M., Marchiano S., Scarpelli P., Zampella A., Limongelli V., Fiorucci S. Disruption of TFGbeta-SMAD3 pathway by the nuclear receptor SHP mediates the antifibrotic activities of BAR704, a novel highly selective FXR ligand. Pharmacol Res. 2018;131:17–31. doi: 10.1016/j.phrs.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 152.Fiorucci S., Antonelli E., Rizzo G., Renga B., Mencarelli A., Riccardi L., Orlandi S., Pellicciari R., Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 153.Fiorucci S., Rizzo G., Antonelli E., Renga B., Mencarelli A., Riccardi L., Morelli A., Pruzanski M., Pellicciari R. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther. 2005;315:58–68. doi: 10.1124/jpet.105.085597. [DOI] [PubMed] [Google Scholar]
- 154.Fang S., Suh J.M., Reilly S.M., Yu E., Osborn O., Lackey D., Yoshihara E., Perino A., Jacinto S., Lukasheva Y., Atkins A.R., Khvat A., Schnabl B., Yu R.T., Brenner D.A., Coulter S., Liddle C., Schoonjans K., Olefsky J.M., Saltiel A.R., Downes M., Evans R.M. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pathak P., Xie C., Nichols R.G., Ferrell J.M., Boehme S., Krausz K.W., Patterson A.D., Gonzalez F.J., Chiang J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68:1574–1588. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., Hinuma S., Fujisawa Y., Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 157.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Nakamura T., Itadani H., Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 158.Alvarez-Sola G., Uriarte I., Latasa M.U., Fernandez-Barrena M.G., Urtasun R., Elizalde M., Barcena-Varela M., Jimenez M., Chang H.C., Barbero R., Catalan V., Rodriguez A., Fruhbeck G., Gallego-Escuredo J.M., Gavalda-Navarro A., Villarroya F., Rodriguez-Ortigosa C.M., Corrales F.J., Prieto J., Berraondo P., Berasain C., Avila M.A. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut. 2017;66:1818–1828. doi: 10.1136/gutjnl-2016-312975. [DOI] [PubMed] [Google Scholar]
- 159.Zhou M., Learned R.M., Rossi S.J., DePaoli A.M., Tian H., Ling L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun. 2017;1:1024–1042. doi: 10.1002/hep4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Edwards P.A., Kast H.R., Anisfeld A.M. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res. 2002;43:2–12. [PubMed] [Google Scholar]
- 161.Adorini L., Pruzanski M., Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988–997. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 162.Cipriani S., Mencarelli A., Palladino G., Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mencarelli A., Renga B., Distrutti E., Fiorucci S. Antiatherosclerotic effect of farnesoid X receptor. Am J Physiol Heart Circ Physiol. 2009;296:H272–H281. doi: 10.1152/ajpheart.01075.2008. [DOI] [PubMed] [Google Scholar]
- 164.Miyazaki-Anzai S., Levi M., Kratzer A., Ting T.C., Lewis L.B., Miyazaki M. Farnesoid X receptor activation prevents the development of vascular calcification in ApoE-/- mice with chronic kidney disease. Circ Res. 2010;106:1807–1817. doi: 10.1161/CIRCRESAHA.109.212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vignozzi L., Morelli A., Filippi S., Comeglio P., Chavalmane A.K., Marchetta M., Toce M., Yehiely-Cohen R., Vannelli G.B., Adorini L., Maggi M. Farnesoid X receptor activation improves erectile function in animal models of metabolic syndrome and diabetes. J Sex Med. 2011;8:57–77. doi: 10.1111/j.1743-6109.2010.02073.x. [DOI] [PubMed] [Google Scholar]
- 166.Li Y.T., Swales K.E., Thomas G.J., Warner T.D., Bishop-Bailey D. Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 167.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X., Burgess S.C., Li C., Ruddy M., Chakravarthy M., Previs S., Milstein S., Fitzgerald K., Kelley D.E., Horton J.D. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 2017;26:576. doi: 10.1016/j.cmet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 168.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H.U., Kipnes M., Adorini L., Sciacca C.I., Clopton P., Castelloe E., Dillon P., Pruzanski M., Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582 e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 169.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F., Chalasani N., Dasarathy S., Diehl A.M., Hameed B., Kowdley K.V., McCullough A., Terrault N., Clark J.M., Tonascia J., Brunt E.M., Kleiner D.E., Doo E., Network N.C.R. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Patel K., Harrison S.A., Elkashab M., Trotter J.F., Herring R., Rojter S., Kayali Z., Wong V.W., Greenbloom S., Jayakumar S., Shiffman M.L., Freilich B., Lawitz E.J., Gane E., Harting E., Xu J., Billin A.N., Chung C., Djedjos C.S., Subramanian G.M., Myers R.P., Middleton M.S., Rinella M., Noureddin M. Cilofexor, a nonsteroidal FXR agonist, in non-cirrhotic patients with nonalcoholic steatohepatitis: a phase 2 randomized controlled trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 171.Schumacher J.D., Guo G.L. Pharmacologic modulation of bile acid-FXR-FGF15/FGF19 pathway for the treatment of nonalcoholic steatohepatitis. Handb Exp Pharmacol. 2019;256:325–357. doi: 10.1007/164_2019_228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L., Kugelmas M., Bashir M.R., Jaros M.J., Ling L., Rossi S.J., DePaoli A.M., Loomba R. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 173.Zelcer N., Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen W., Chen G., Head D.L., Mangelsdorf D.J., Russell D.W. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Janowski B.A., Grogan M.J., Jones S.A., Wisely G.B., Kliewer S.A., Corey E.J., Mangelsdorf D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Janowski B.A., Willy P.J., Devi T.R., Falck J.R., Mangelsdorf D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 177.Costet P., Luo Y., Wang N., Tall A.R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 178.Repa J.J., Turley S.D., Lobaccaro J.A., Medina J., Li L., Lustig K., Shan B., Heyman R.A., Dietschy J.M., Mangelsdorf D.J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 179.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cha J.Y., Repa J.J. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 181.Repa J.J., Liang G., Ou J., Bashmakov Y., Lobaccaro J.M., Shimomura I., Shan B., Brown M.S., Goldstein J.L., Mangelsdorf D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang B., Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol. 2018;14:452–463. doi: 10.1038/s41574-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ghisletti S., Huang W., Ogawa S., Pascual G., Lin M.E., Willson T.M., Rosenfeld M.G., Glass C.K. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Joseph S.B., Castrillo A., Laffitte B.A., Mangelsdorf D.J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 185.Venteclef N., Jakobsson T., Ehrlund A., Damdimopoulos A., Mikkonen L., Ellis E., Nilsson L.M., Parini P., Janne O.A., Gustafsson J.A., Steffensen K.R., Treuter E. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 2010;24:381–395. doi: 10.1101/gad.545110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ito A., Hong C., Rong X., Zhu X., Tarling E.J., Hedde P.N., Gratton E., Parks J., Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife. 2015;4 doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thomas D.G., Doran A.C., Fotakis P., Westerterp M., Antonson P., Jiang H., Jiang X.C., Gustafsson J.A., Tabas I., Tall A.R. LXR suppresses inflammatory gene expression and neutrophil migration through cis-repression and cholesterol efflux. Cell Rep. 2018;25:3774–3785 e4. doi: 10.1016/j.celrep.2018.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Spann N.J., Garmire L.X., McDonald J.G., Myers D.S., Milne S.B., Shibata N., Reichart D., Fox J.N., Shaked I., Heudobler D., Raetz C.R., Wang E.W., Kelly S.L., Sullards M.C., Murphy R.C., Merrill A.H., Jr., Brown H.A., Dennis E.A., Li A.C., Ley K., Tsimikas S., Fahy E., Subramaniam S., Quehenberger O., Russell D.W., Glass C.K. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Villablanca E.J., Raccosta L., Zhou D., Fontana R., Maggioni D., Negro A., Sanvito F., Ponzoni M., Valentinis B., Bregni M., Prinetti A., Steffensen K.R., Sonnino S., Gustafsson J.A., Doglioni C., Bordignon C., Traversari C., Russo V. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 190.Herold M., Breuer J., Hucke S., Knolle P., Schwab N., Wiendl H., Klotz L. Liver X receptor activation promotes differentiation of regulatory T cells. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Endo-Umeda K., Makishima M. Liver X receptors regulate cholesterol metabolism and immunity in hepatic nonparenchymal cells. Int J Mol Sci. 2019;20:5045. doi: 10.3390/ijms20205045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu Y., Han X., Bian Z., Peng Y., You Z., Wang Q., Chen X., Qiu D., Ma X. Activation of liver X receptors attenuates endotoxin-induced liver injury in mice with nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:390–398. doi: 10.1007/s10620-011-1902-9. [DOI] [PubMed] [Google Scholar]
- 193.Wang Y.Y., Dahle M.K., Agren J., Myhre A.E., Reinholt F.P., Foster S.J., Collins J.L., Thiemermann C., Aasen A.O., Wang J.E. Activation of the liver X receptor protects against hepatic injury in endotoxemia by suppressing Kupffer cell activation. Shock. 2006;25:141–146. doi: 10.1097/01.shk.0000191377.78144.d9. [DOI] [PubMed] [Google Scholar]
- 194.Wouters K., van Bilsen M., van Gorp P.J., Bieghs V., Lutjohann D., Kerksiek A., Staels B., Hofker M.H., Shiri-Sverdlov R. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS Lett. 2010;584:1001–1005. doi: 10.1016/j.febslet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 195.Endo-Umeda K., Nakashima H., Komine-Aizawa S., Umeda N., Seki S., Makishima M. Liver X receptors regulate hepatic F4/80 (+) CD11b(+) Kupffer cells/macrophages and innate immune responses in mice. Sci Rep. 2018;8:9281. doi: 10.1038/s41598-018-27615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ni M., Zhang B., Zhao J., Feng Q., Peng J., Hu Y., Zhao Y. Biological mechanisms and related natural modulators of liver X receptor in nonalcoholic fatty liver disease. Biomed Pharmacother. 2019;113:108778. doi: 10.1016/j.biopha.2019.108778. [DOI] [PubMed] [Google Scholar]
- 197.Tanaka N., Aoyama T., Kimura S., Gonzalez F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–157. doi: 10.1016/j.pharmthera.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ahn S.B., Jang K., Jun D.W., Lee B.H., Shin K.J. Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2014;59:2975–2982. doi: 10.1007/s10620-014-3289-x. [DOI] [PubMed] [Google Scholar]
- 199.Raselli T., Hearn T., Wyss A., Atrott K., Peter A., Frey-Wagner I., Spalinger M.R., Maggio E.M., Sailer A.W., Schmitt J., Schreiner P., Moncsek A., Mertens J., Scharl M., Griffiths W.J., Bueter M., Geier A., Rogler G., Wang Y., Misselwitz B. Elevated oxysterol levels in human and mouse livers reflect nonalcoholic steatohepatitis. J Lipid Res. 2019;60:1270–1283. doi: 10.1194/jlr.M093229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schultz J.R., Tu H., Luk A., Repa J.J., Medina J.C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D.J., Lustig K.D., Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bieghs V., Hendrikx T., van Gorp P.J., Verheyen F., Guichot Y.D., Walenbergh S.M., Jeurissen M.L., Gijbels M., Rensen S.S., Bast A., Plat J., Kalhan S.C., Koek G.H., Leitersdorf E., Hofker M.H., Lutjohann D., Shiri-Sverdlov R. The cholesterol derivative 27-hydroxycholesterol reduces steatohepatitis in mice. Gastroenterology. 2013;144:167–178 e1. doi: 10.1053/j.gastro.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 202.Endo-Umeda K., Nakashima H., Umeda N., Seki S., Makishima M. Dysregulation of Kupffer cells/macrophages and natural killer T cells in steatohepatitis in LXRalpha knockout male mice. Endocrinology. 2018;159:1419–1432. doi: 10.1210/en.2017-03141. [DOI] [PubMed] [Google Scholar]
- 203.Kremer M., Thomas E., Milton R.J., Perry A.W., van Rooijen N., Wheeler M.D., Zacks S., Fried M., Rippe R.A., Hines I.N. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51:130–141. doi: 10.1002/hep.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Beaven S.W., Wroblewski K., Wang J., Hong C., Bensinger S., Tsukamoto H., Tontonoz P. Liver X receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology. 2011;140:1052–1062. doi: 10.1053/j.gastro.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hong C., Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 206.Muse E.D., Yu S., Edillor C.R., Tao J., Spann N.J., Troutman T.D., Seidman J.S., Henke A., Roland J.T., Ozeki K.A., Thompson B.M., McDonald J.G., Bahadorani J., Tsimikas S., Grossman T.R., Tremblay M.S., Glass C.K. Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages. Proc Natl Acad Sci U S A. 2018;115:E4680–E4689. doi: 10.1073/pnas.1714518115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tice C.M., Noto P.B., Fan K.Y., Zhuang L., Lala D.S., Singh S.B. The medicinal chemistry of liver X receptor (LXR) modulators. J Med Chem. 2014;57:7182–7205. doi: 10.1021/jm500442z. [DOI] [PubMed] [Google Scholar]
- 208.de Boer J.F., Schonewille M., Boesjes M., Wolters H., Bloks V.W., Bos T., van Dijk T.H., Jurdzinski A., Boverhof R., Wolters J.C., Kuivenhoven J.A., van Deursen J.M., Oude Elferink RPJ. Moschetta A., Kremoser C., Verkade H.J., Kuipers F., Groen A.K. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology. 2017;152:1126–1138 e6. doi: 10.1053/j.gastro.2016.12.037. [DOI] [PubMed] [Google Scholar]