Abstract
The growing life expectancy in modern societies has raised scientific interest in identifying medical interventions to alleviate age-associated pathologies such as vascular calcification, cognitive decline, sarcopenia, osteoporosis and sexual dysfunction. Although no such single treatment has thus far been established in humans, some clinicians and patients have set their hopes on testosterone replacement therapy (TRT) as a potential “fountain of youth” for aging men. While TRT has proven effective in ameliorating distinct symptoms of late-onset hypogonadism (LOH), its safety remains to be demonstrated. Besides humans, multiple other species exhibit age-related reductions in circulating testosterone levels, raising the question whether such changes are an inherent, pathological feature of growing organismal age or rather reflect an adaptive response. In this manuscript, we apply key principles of evolutionary medicine to testosterone biology and LOH to provide a novel perspective on these two fields. Additionally, we discuss insightful data derived from the animal kingdom to illustrate the plasticity of individual testosterone trajectories across the lifespan, outline cost-benefit-considerations of TRT in LOH and highlight potential caveats of such therapies.
Keywords: testosterone, late-onset hypogonadism, evolution, TRT, aging, trade-offs, hormones, endocrinology
Introduction
Evolution refers to changes in biological characteristics over consecutive generations, which are driven by evolutionary processes such as natural selection. The latter operates when four key requirements are met: 1.) there must be variation in a trait, 2.) there must be variation in reproductive success, 3.) the correlation between the trait and reproductive success must be non-zero and 4.) the state of the trait must be heritable. In other words, genetic variation conferring enhanced reproductive fitness of an organism will be favored and ultimately selected for. At the same time, almost any given trait is involved in trade-offs, which describes the notion, that improvement in one aspect of physiology (e.g. reproduction) may negatively impact another1. Compared to other non-human primates, the human species has developed a distinct reproductive phenotype characterized by relatively late sexual maturity, low offspring number, and, in most cases, a long post-reproductive lifespan2,3. Whereas in women the beginning of the latter is sharply marked by the onset of menopause, male individuals do not exhibit such obvious changes. Rather, men experience a modest age-related decline in circulating testosterone levels (roughly 1–2 % per year starting at the age of ~40 years), which is paralleled by deterioration of sperm quality and may eventually result in the development of symptomatic hypogonadism4,5. This clinical picture was coined “late-onset hypogonadism” (LOH) almost two decades ago and has extensively been studied ever since then6. LOH is characterized by low testosterone levels in the presence of typical symptoms such as diminished libido and erectile dysfunction7. Of note, the diagnosis of LOH is complicated by the lack of a consensus threshold to define testosterone deficiency in the elderly, although levels below 10 nmol/l have been widely considered pathological7,8. In contrast to other forms of hypogonadism, LOH cannot be readily classified as either of primary (testicular defect) or secondary (hypothalamic and/or pituitary defect) origin since these patients exhibit features of both perturbations9. While much has been learned about the epidemiology and clinical presentation of LOH, very little is known concerning its evolutionary background and medical significance. Most importantly, it remains unclear whether declining testosterone levels (and specifically, LOH) in older men reflect an adaptive or maladaptive trait.
LOH: a disease after all?
The biological effects of the testes had been recognized since antiquity, long before the concept of hormones arose. Yet, it took researchers up until the 20th century to identify testosterone as the active agent facilitating the androgenic effects of testicular tissue. Subsequent scientific efforts culminated in the synthetic production of testosterone as well as the development of radioimmunoassays to adequately measure circulating levels of the hormone. Both discoveries were prerequisites to gain mechanistic insights into testosterone (patho)biology and thus of great medical significance10.
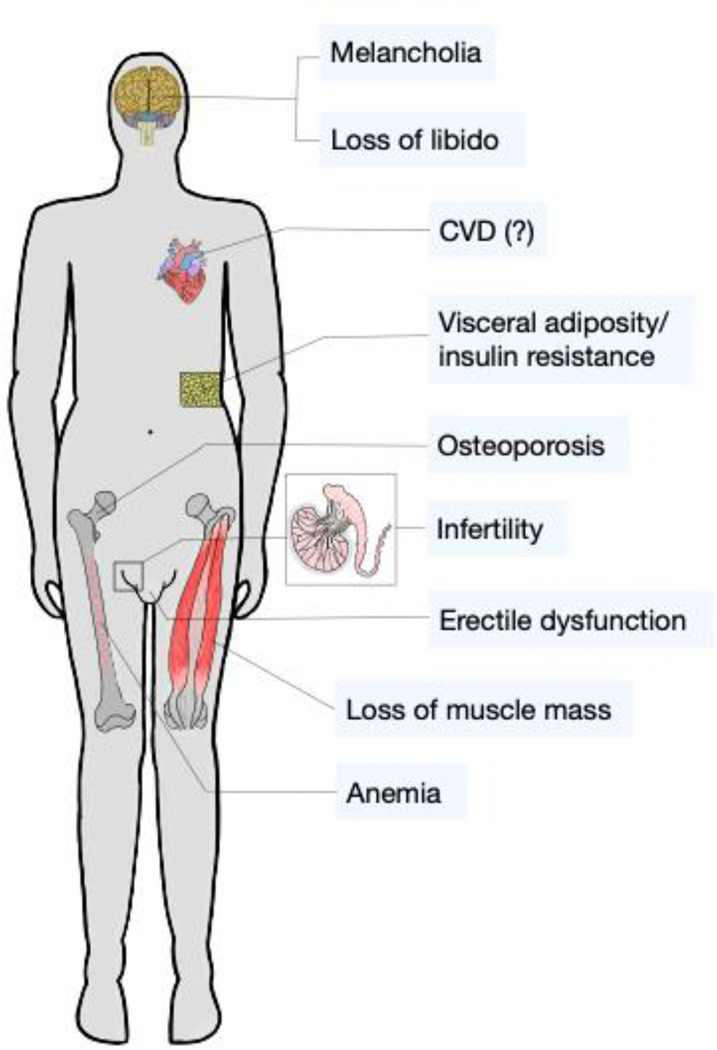
Postnatally, testosterone regulates a plethora of physiological functions in males including development and maintenance of secondary sexual characteristics, sexual desire, body composition, bone mass, mood, hematopoiesis, hemodynamics and metabolism11–13. Reciprocally, hypogonadism impairs these functions to various extents, thereby causing significant morbidity (Figure 1)14,15. Since aging is associated with perturbations in most testosterone-regulated functions and growing age is paralleled by declining testosterone levels in men, the assumption that testosterone replacement therapy (TRT) could be used to positively modulate various aspects of male health was an obvious conclusion. This hypothesis was supported by cross-sectional studies revealing inverse associations between testosterone and markers of glycemic control, BMI, waist circumference, muscle mass, bone mineral density, serum triglycerides and risk for cardiovascular disease14,16–18. In this respect, the tight interconnection between the metabolic syndrome and hypogonadism has drawn special attention, since both pathologies reciprocally deteriorate each other19.
Figure 1: Symptoms of hypogonadism.
Low circulating testosterone levels are associated with several detrimental health effects including diminished libido, erectile dysfunction, loss of muscle and bone mass, increased visceral adiposity (potentially yielding impaired glucose tolerance), melancholia (eventually culminating in depression) and anemia. Whether hypogonadism actually confers enhanced risk for cardiovascular disease is still a matter of controversy.
Subsequent prospective clinical trials demonstrated that testosterone replacement therapy could indeed positively impact distinct symptoms of hypogonadism in older men. The so-called Testosterone Trials (TTrials) were the first studies to prospectively evaluate the effects of TRT over a 12-month period on clinically meaningful endpoints in men with LOH (reviewed in20). A total of 790 participants were randomly assigned to either receive transdermal testosterone supplementation or a placebo. Inclusion criteria included age >65 years and circulating testosterone levels <275 ng/dl, whereas a high risk for (and history of) prostate cancer as well cardiovascular disease (among others) served as exclusion criteria. The TTrials revealed improvements in several aspects of LOH including sexual desire, erectile dysfunction, lean body mass, walking distance or bone mineral density21,22. However, safety concerns were raised by the observation that TRT yielded a significant increase in non-calcified coronary artery plaque volume22, which was affirmed by a meta-analysis demonstrating an elevated risk for cardiovascular events (OR=1.54, 95% CI 1.09–2.58) in patients undergoing TRT23. Conversely, other studies did not find evidence for such an association (REF24 and see below). Similarly, the effects of testosterone on prostate cancer development are still a topic of debate. While most investigations have not found a consistent increase in prostate cancer risk in patients undergoing TRT, sample sizes of these studies were generally small and follow-ups were too short to draw valid conclusions20,25. Of note, a large meta-analysis including 20 prospective studies found that low endogenous free testosterone levels (i.e. the lowest study-specific decile) were associated with a reduced risk for developing prostate cancer, whereas higher levels did not confer an elevated hazard26. These observations are in accordance with the saturation model of androgen receptor signaling27. In contrast to previously published literature28, the cited study did not find evidence for a higher frequency of more aggressive (i.e. higher Gleason grade) tumors in men with low testosterone.
In summary, TRT in (adequately diagnosed) LOH elicits several beneficial effects. However, these benefits may also come at a certain cost, i.e. potentially detrimental adverse events. From an endocrine perspective, the supplementation of a true hormonal deficit should not be associated with major adverse events if the exogenous delivery system resembles the physiological secretory pattern of the respective hormone. Indeed, parenterally administered testosterone preparations as commonly used several years ago exhibited a rather unfavorable pharmacokinetic profile, resulting in both sub- as well as supraphysiological testosterone serum levels, thus yielding adverse effects. In contrast, presently used topical formulations and subcutaneously administered short-acting esters resemble endogenous testosterone secretion more closely29–32. However, clinical data suggest that these therapies are still not entirely safe in older men22,33. Conversely, the benefits of TRT in younger men with classical primary or secondary hypogonadism are well documented and (for the most part) associated with very few adverse events34. Of note, similar observations were also made in females, where hormone replacement therapy (HRT) in younger postmenopausal individuals elicited mainly beneficial effects, whereas an increased risk for cardiovascular events was described in older women35,36
Therefore, the most relevant conceptional aspect concerning TRT in the elderly is the question whether declining testosterone levels in these individuals should be considered a pathological deficit (i.e. perturbation of homeostasis) or rather reflect a physiological, adaptive response. While the former point has already been extensively discussed in the scientific literature, little attention has been paid to the latter. In the following sections, we use common concepts of evolutionary biology to provide an alternative view on LOH.
Evolutionary considerations of testosterone biology
Life history theory reflects a theoretical framework to understand how finite organismal resources are allocated to competing and often opposing biological programs through trade-offs37. These programs include growth, reproduction and maintenance, the latter being further divided into dormancy and defense38. Depending on the environmental conditions an organism is facing at a given time, resource investment may differ significantly. While favorable environments (sufficient nutrient supply, few predators) promote resource allocation into growth and reproduction, hostile environments (characterized by nutrient scarcity, insults and other challenging conditions) favor investments into maintenance programs38. This may be exemplified by defense strategies such as the inflammatory response: The high energetic burden imposed by fighting invading pathogens necessitates a temporal suppression of competing biological responses, which themselves are metabolically costly39.
This holds especially true for reproductive functions. Indeed, testosterone levels are uniformly low in patients inflicted with inflammatory conditions such inflammatory bowel disease, arthritis or sepsis 40–42. In fact, almost any evolutionary relevant environmental challenge such as cold, starvation or stress (requiring prioritization of energy distribution) provokes reduction in circulating testosterone levels43–45. Pertinent to this, strenuous endurance exercise yielding an imbalance between energy consumption and expense (male exercising syndrome) associates with hypogonadism and subfertility46. Thus, low testosterone levels under these conditions can be considered an adaptive response to reduce the energetic burden of sex hormone-driven biological functions, thereby allowing allocation of resources into other costly programs.
It is worth noting, that not only nutrient scarcity but also excess as found in obesity is associated with hypogonadism and infertility47,48. Several lines of evidence have demonstrated that the persistent induction of pro-inflammatory cytokines in obese individuals (“low-grade inflammation”) causally contributes to low testosterone levels by impairing hypothalamic GnRH secretion49. Additionally, hypothalamic inflammation in obesity is fueled by nutrient excess per se50. Conversely, treatment of obese male individuals with the Interleukin-1 beta antagonist Anakinra increases systemic testosterone abundance51. Since the pro-inflammatory state in obesity is considered as an example for overwhelmed organismal homeostatic capacities39, low testosterone secretion in these individuals likely reflects a pathological, rather than an adaptive response.
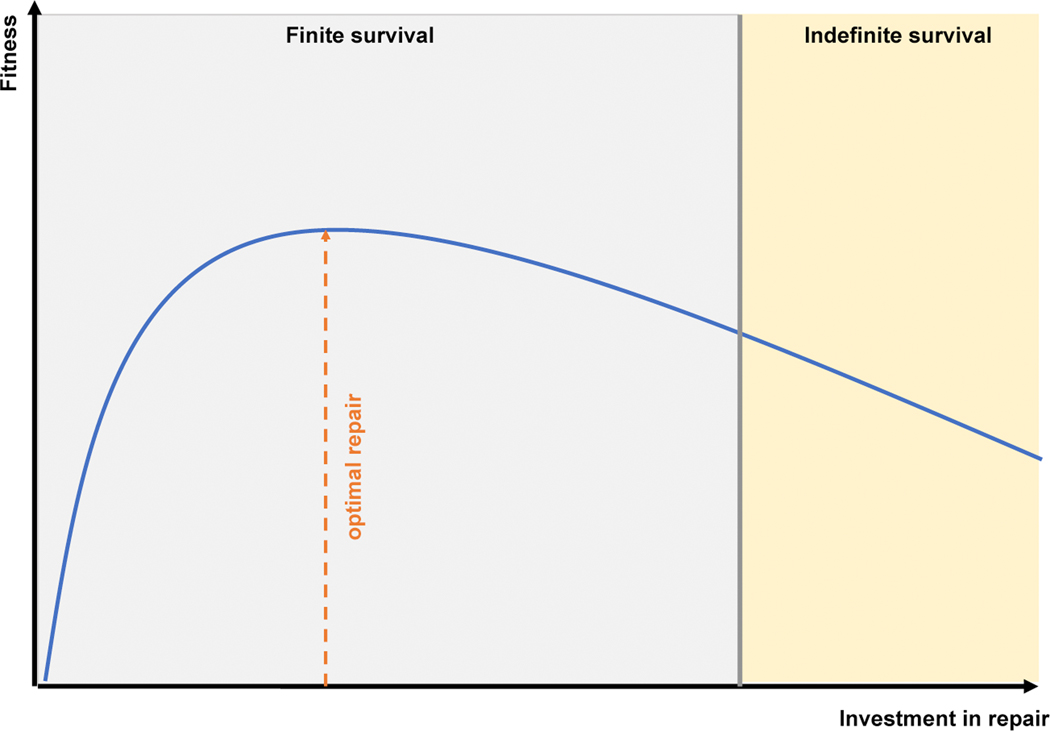
Physiologically, most (if not all) of testosterone can be considered anabolic and require high metabolic activity. Of note, these processes are not only energy-consuming, but also yield side-products, i.e. “waste”. For example, cellular protein synthesis (which is stimulated by testosterone52) is inevitably paralleled by a certain accumulation of defective and/or misfolded proteins requiring clearance. This is accomplished by maintenance programs (e.g. proteasomal degradation)1,53. With increasing age, the capacity of maintenance programs progressively declines, which is considered as one of the main drivers of highly prevalent pathologies of old age such as neurodegenerative diseases54. These disorders are thus examples for causes of intrinsic mortality. In contrast, extrinsic mortality (i.e. predators, starvation, dehydration etc.) has largely been eliminated in industrialized (“westernized”) countries. From an evolutionary perspective, the age-related impairment of maintenance programs reflects antagonistic pleiotropy (by genetic means) and a trade-off (by phenotypic means): the selection of traits ensuring sufficient reproductive success early in life opposes those favoring maintenance and survival later in life because high investment into one program (anabolism) will unavoidably reduce available resources for the other (catabolism). Thus, human aging may be considered a trade-off, where reproduction is prioritized over longevity (Figure 2)55. If one further expands this notion, then age-related reductions in anabolic hormones would represent an adaptive mechanism to alter the cost-benefit ratio of this trade-off. In other words, declining testosterone levels in aging men impair several functions regulated by the hormone (cost), while eventually allowing the fostering of others (benefit). This principle may also be exemplified by observations made among the animal kingdom.
Figure 2: Aging as a trade-off between reproduction and repair.
According to Darwin’s theory of evolution, natural selection favors traits conferring enhanced fitness. The term “fitness” is often loosely defined but essentially refers to reproductive success, i.e. higher fitness (better adaptation to a given environment) equals enhanced reproductive success. However, selection of traits conferring higher reproductive fitness will inevitably result in lower resource allocation into repair programs (maintenance) because organismal resources are finite. Consequently, prioritization of reproductive success limits lifespan (trade-off). Neither too much, nor too little investment into repair is favorable because both will result in reduced fitness: the former because of “overinvestment” of resources into repair programs, the latter due to premature death. Yet, indefinite survival would only be achieved if resources were placed sub-optimally. Since this strategy would culminate in impaired (reproductive) fitness, such traits will not be selected for. Hence, organisms do not exhibit these extensive repair programs, which may explain their finite lifespan (after Kirkwood & Cremer, 1982).
Testosterone biology: lessons from the animal kingdom
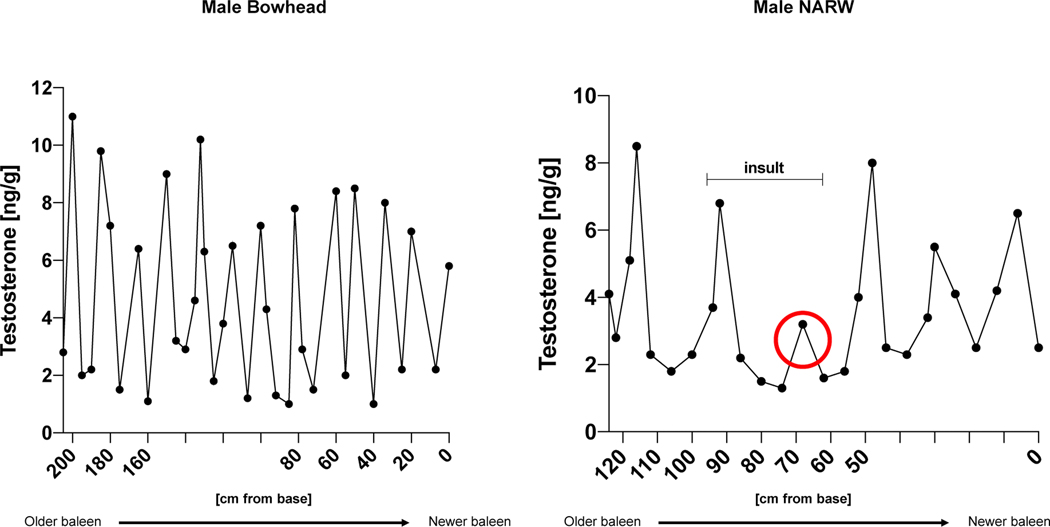
The baleen (i.e. the keratin-containing feeding-filter system inside the whale’s mouth) of baleen whales contains a multi-year record of the animal’s endocrine history because hormones are embedded into the baleen matrix in a continuous manner56. Thus, studying these materials reflects a unique opportunity to longitudinally follow testosterone trajectories of an individual. Indeed, such studies have revealed that testosterone levels in baleen whales exhibit annual cycles with peaks during the winter months (breeding season) and astonishingly low values in summer57. Moreover, the magnitude of testosterone peaks gradually declines with increasing age of the animal and exposure to stressors (entanglement, sickness) results in blunted androgen levels in the subsequent cycle (Figure 3)56.These observations have several important implications: 1.) for baleen whales, the cost of maintaining high testosterone levels throughout the year is apparently higher than its benefit, suggesting that androgen biology does not confer major advantages for the most part in these animals 2.) as in humans (and most other species), hostile environments suppress androgen secretion to allow resource allocation into other biological programs, 3.) age-associated reductions in testosterone levels are not restricted to humans but also found in other mammals and 4.) the cyclicity of testosterone levels and fertility in baleen whales (i.e. peaking in winter) mirrors human whale hunting season (summer). Whether the latter phenotype is actually shaped by human behavior (i.e. extrinsic mortality) in the sense of an adaptive response or has already been existent much longer remains elusive. Remarkably, age-associated reductions in testosterone secretion have also been described in many other species including primates, mice, or guinea pigs58–60.
Figure 3: Testosterone trajectories in baleen whales.
In baleen whales, hormones are embedded into the baleen matrix in a continuous fashion, thus providing a unique opportunity to study individual testosterone trajectories over the lifespan. The left panel shows annual testosterone cycles in a baleen bowhead whale. Material close to the base reflects new baleen corresponding to a more recent endocrine status. Note that the magnitude of the annual testosterone peak progressively declines with growing age of the animal, reminiscent of decreasing circulating testosterone levels in aging humans. The right panel illustrates similar dynamics in a male North Atlantic Right Whale (NARW). After being challenged by an insult in one year (entanglement, sickness), the animal exhibited a strongly decreased testosterone peak in the consecutive year perhaps reflecting a prioritization of resource allocation into maintenance and repair programs rather than growth/reproduction. Comparable adaptations occur in humans, where almost any persistent evolutionary relevant stressor (e.g. infection, starvation, cold) evokes suppression of testosterone levels (after Hunt et al., 2018).
The plasticity of testosterone secretion depending on the current environmental conditions may also be illustrated by data derived from lions. In these animals, the darkness and length of the animal’s mane correlate with testosterone levels, nutritional status, reproductive success and offspring survival but negatively impact the animal’s surface temperature and food intake in areas with hot climate. Consequently, both mane characteristics can vary along the lifespan of an animal because the cost of maintaining the trait may become higher than its benefit if environmental conditions change sufficiently enough (phenotypic plasticity)61.
In general, an adaptive trait is characterized by a cost that is lower than its benefit. Vice versa, a trait with a stable, unfavorable cost-benefit ratio in a given environment will undergo natural selection to either shift this ratio (mostly through lowering its cost) or eliminate it after all39. Applying this principle to the lion kingdom, persistently altered climatic conditions (i.e. high temperatures) and poor nutrient availability (secondary to climate changes) would presumably favor lions with shorter and lighter colored manes (as well as lower testosterone levels) because under these environmental circumstances, the benefits generated from such characteristics (enhanced reproductive fitness) would fall below their costs (energy consuming growth of hair despite nutrient scarcity and eventually death due to insufficient temperature regulation). Of note, it would require a considerable amount of time and millions of selective events until the most optimal mane phenotype in the new environment has been established.
Similarly, LOH could reflect one of those diseases, where genetics are still running behind rapidly changing environmental conditions because distinctly increased life expectancies are a rather recent phenomenon in human evolution (i.e. reflecting an evolutionary mismatch). On the other hand, LOH may confer adaptive benefits tailored to meet altered demands with growing age. If this holds true, TRT in the elderly would impose unfavorable consequences by disrupting energetic adaptations required for maintenance programs. There are several examples for such maintenance functions: removal of defective (pre-cancerous) cells, misfolded proteins, old organelles or membrane lipids, all of which accumulate with growing organismal age and impose metabolic costs in order to be fixed 38,62,63.If these demands cannot be met because other pathways (e.g. testosterone-driven anabolism) are overriding, maintenance capacities are overwhelmed, thus resulting in cellular and/or organ dysfunction and ultimately, culminating in disease.
Kennedy Syndrome as a paradigm for androgen-mediated toxicity
As outlined in the previous sections, testosterone secretion is highly dynamic and always tailored to meet the current environmental and/or organismal requirements. The consequences of perturbing these adaptations may be exemplified by a rare genetic disorder known as spinal and bulbar muscular atrophy (SBMA), also referred to as Kennedy Syndrome. SBMA is an X-chromosomal, recessively inherited disease caused by an abnormally extended polyglutamine stretch (encoded by a CAG repeat) within the n-terminal region of the androgen receptor (AR) that exclusively manifests in males. The length of the CAG-repeat inversely correlates with age at onset of the disease, i.e. longer repeats yield earlier manifestations64.
Typical symptoms include progressive muscle weakness, bulbar dysfunction and fasciculations. Moreover, SBMA patients exhibit endocrine abnormalities such as erectile dysfunction, testicular atrophy or gynecomastia reflecting impaired androgenic actions of poly-glutamine AR65. The molecular mechanisms evoking other alterations of the disease are incompletely understood but nuclear accumulation of toxic receptor aggregates, mitochondrial dysfunction, impaired autophagy, defective proteasome activity and altered transcriptional regulation have been proposed66. Most intriguingly, chemical or surgical castration reduces motor symptoms, neuronal degeneration and disease progression in animal models of SBMA67,68. Of note, these improvements could not be explained by decreased testosterone-mediated AR expression, but presumably included altered AR stability and reduced nuclear abundance of toxic AR aggregates68. In view of elevated circulating estradiol levels secondary to disrupted negative feedback inhibition in SBMA69, one could speculate that estrogens contribute to the pathogenesis of the disease. However, neither preclinical, nor clinical evidence has thus far established a beneficial role for interfering with estrogen biology (e.g. via aromatase inhibitors) to ameliorate SBMA symptoms or diseases trajectories.
Taken together these observations suggested that SBMA is primarily caused by abnormal proteostasis, i.e. gain-of-function mutation of AR yielding pathological accumulation of the protein.
However, additional studies revealed that activation of the AR by its cognate ligands (i.e. dihydrotestosterone and testosterone, respectively) is required to evoke toxicity of poly-glutamine AR70. Initially, this was interpreted as the necessity of ligand-dependent nuclear translocation of AR to promote local protein accumulation within this cellular compartment. Based on these assumptions, clinical trials employing the GnRH analogue leuprorelin (i.e. androgen deprivation therapy) for the treatment of SBMA were initiated, which revealed promising, although ambiguous results71–73, suggesting that effective therapeutic approaches may require a more sophisticated approach.
Subsequent investigations demonstrated that nuclear translocation of polyglutamine AR is necessary but not sufficient to evoke toxicity. Rather, DNA binding and amplification of native AR interactions were found to exert toxic effects74. As such, specific functions of the AR (excluding those promoting androgenicity) are not only preserved in SBMA but actually enhanced and function as drivers of the disease, indicating that unrestrained AR signaling (i.e. opposing catabolism) is not compatible with maintaining cellular homeostasis. Pertinent to these observations, a preclinical study in mice75 revealed improvements in several SBMA symptoms including reduced grip strength, weight loss, gait disturbances as well as testicular atrophy elicited by the selective androgen receptor modulator MEBP (1-[2-(4-methylphenoxy)ethyl]-2-[(2-phenoxyethyl)sulfanyl]1-H-benzimidazole). Mechanistically, MEBP attenuated distinct aspects of pathologically amplified AR functions through increasing corepressor recruitment to the AF2 (activation function 2) domain of the protein. Importantly, the burden of toxic AR aggregates remained unchanged by the treatment. On the other hand, testicular atrophy (i.e. a prototypical androgenic function of the AR) was attenuated upon MEBP exposure. Together, these results support the concept that a.) SBMA is not primarily driven by accumulation of toxic receptor aggregates but rather dysregulated transcriptional AR activity and b.) fine-tuning of the latter to promote abolished androgenic receptor functions, while counteracting the amplification of others is sufficient to positively modulate disease trajectories in SBMA:
Notably, the question if androgen antagonism also elicits favorable effects in other neurodegenerative diseases with less obvious links to testosterone biology (e.g. Parkinson’s disease) remains obscure. Vice versa, no convincing clinical data are available on the effects of TRT on the course of such diseases. One small study (n=15 per group) investigated the effects of TRT in patients with Parkinson’s disease. These authors did not find evidence for beneficial effects of testosterone replacement but rather reported adverse events such as worsening of dyskinesia76. While the very small sample size of this study limits its significance, preclinical data has already pointed towards unfavorable effects of androgens in promoting early (but not more advanced) Parkinson’s disease77–79.
In summary, the biological programs promoted by testosterone mainly foster anabolism, which oppose those pathways favoring catabolism. As exemplified by the impressive case of SBMA, amplification of certain AR functions is highly pathological and evokes devastating disease. With respect to ageing, the importance of maintenance programs increases and under specific circumstances, disrupting such responses is detrimental. Thus, it may be tempting to speculate that promoting anabolism through testosterone supplementation with growing age may exert negative effects on health and longevity.
Testosterone and cardiovascular disease
Cardiovascular diseases (CVD) have emerged as the leading causes of mortality in westernized countries80,81. In other words, these pathologies are currently the main drivers of limited lifespan in modern societies. Although an extensive body of literature has suggested that testosterone is linked to cardiovascular health and disease (elegantly reviewed in REF11), the precise nature of this association has still not been fully resolved. In fact, some cross-sectional studies have reported inverse associations between circulating testosterone and the risk for cardiovascular disease82, whereas several others have not83,84. Furthermore, clinical trials investigating the effects of TRT in older men have thus far not been adequately powered to assess clinically relevant cardiovascular endpoints, but retrospective studies have reported neutral, protective as well as detrimental effects33,85,86. On the other hand, supraphysiological testosterone levels (i.e. anabolic steroid abuse) are well-known to exert detrimental effects on cardiovascular health87. Along these lines, women with biochemical hyperandrogenemia (i.e. Polycystic Ovary Syndrome) display an increased risk for CVD88, although this observation might be partly driven by associated metabolic perturbations such as insulin resistance89.
Thus, the several clinical benefits associated with TRT in older men could be outweighed by unfavorable cardiovascular consequences, as already documented for HRT in women. From an evolutionary perspective, this raises two important questions: 1.) Why does testosterone modulate cardiovascular biology in the first place and 2.) why are testosterone and cardiovascular health involved in a trade-off?
The first question can be explored by the phenomenon of co-evolution in the Bornean Rock Frog (Staurois Parvus). These animals have developed a distinct hind limb movement pattern (known as the “foot flag”) to attract females for mating. This process is testosterone-driven and facilitated by high androgen receptor expression in the corresponding muscles, which is not found in other frog species devoid of the foot flag. Together, these observations suggest that the enhanced fitness conferred by specific testosterone effects required the co-evolution of other traits (i.e. high muscular AR expression) to implement such functions sufficiently90. Similarly, the fitness advantage conferred by testosterone-mediated muscle protein synthesis (that is, dominance/reproduction/survival) might have required an evolutionary linkage to the cardiovascular system in order to ensure sufficient oxygen and nutrient supply to these organs. This may explain why testosterone regulates cardiomyocyte physiology, vascular tone or hematopoiesis.
The second question, on the other hand, is more puzzling. In industrialized countries, the hemodynamic costs of maintaining physiological sex hormone concentrations with growing age may be exceptionally high (due to the risk of erythrocytosis, clotting, hypertension) because of the high prevalence of comorbidities associated with CVD91. Vice versa, men in environments without such factors would be expected to be spared from age-related reductions in circulating testosterone. Indeed, aging men from the US display a more prominent testosterone decline than those from Paraguay or Nepal92,93. Importantly, these results should be interpreted with caution since testosterone levels in the cited study92 were measured from saliva samples, which provide an acceptable, although not fully accurate estimation of systemic testosterone abundance94,95.
Nevertheless, if one expands the notions highlighted above, low sex hormone levels in the elderly in industrialized countries might confer protection from CVD (adaptation), whereas TRT would perturb this phenotype. The latter hypothesis is supported by mendelian randomization studies demonstrating an increased hazard for thromboembolism in men with genetically determined higher endogenous testosterone levels96.
Generally speaking, organismal death can be considered a trade-off between different ways to die. Thus, a “low testosterone” trait in older men might protect from cardiovascular death (currently being the most prevalent limitation to lifespan), while enhancing the risk to succumb to other diseases (e.g. fragility fracture due to osteoporosis), potentially reflecting a favorable trade-off in our current environment. Of note, both exogenous and endogenous testosterone is aromatized to estradiol by CYP19A1 and the latter fulfills several important physiological functions in males that have traditionally been ascribed solely to androgens (e.g. regulation of visceral fat mass, libido or erectile function)12,97. Reciprocally, some of the adverse effects arising from TRT could, in fact, be estradiol mediated. On the other hand, the clinical use of non-aromatizable androgens is obsolete because such treatments would render patients devoid of a variety of beneficial effects associated with TRT (e.g. increase in bone mass).
Taken together, potential cardiovascular side-effects remain the most prominent safety concern for TRT in older men, reminiscent of the debate on HRT in postmenopausal women. In 2018, the first large, prospective, randomized controlled trial (TRAVERSE) with a long-term follow-up (i.e. 5 years) investigating cardiovascular safety as the primary endpoint was initiated (NCT03518034) and this study will hopefully aid in resolving the longstanding controversy about cardiovascular consequences (and ultimately, mortality) of TRT in aging men.
Does testosterone affect lifespan?
Considering the points outlined above, it would be reasonable to assume that low testosterone levels are correlated with an increased lifespan. The most obvious example for this notion is the difference in longevity between males and females as such that on average, women live longer than men98. Moreover, a population of Korean eunuchs was found to exhibit a significantly increased lifespan compared to socio-economically matched controls99. Similar observations were made in other mammals such as primates, sheep, cats or mice100. It is worth emphasizing, that these findings are heavily influenced by multiple confounders such as hormone-driven behavioral changes (e.g. risk-taking, aggression etc.). For example, alpha male Baboons exhibit the highest testosterone but also the highest corticosterone levels, suggesting that defeating an alpha status (and high testosterone levels) comes at the cost of stress, although influences on lifespan were not explored in the cited study101.
Nevertheless, a shared mechanism of various lifespan-increasing interventions lies in a reduced activation of anabolic pathways. Genetically, this may be exemplified by Laron-Syndrome characterized by defective growth hormone-signaling yielding a short stature in the presence of longevity102. The latter effect can also be achieved by both caloric restriction as well as pharmacological mTOR blockade (via rapamycin) 103,104. Given that long-term caloric restriction lowers systemic testosterone abundance105 and mTOR is a direct molecular target of the hormone106, decreased circulating testosterone should result in reduced anabolism and thus increased lifespan. Pertinent to this, a study comparing various genetic as well as pharmacological longevity interventions found that “feminization” of gene expression was a shared characteristic of these manipulations 107. Unfortunately, testosterone levels were not reported by the authors. On the other hand, castrated rats exhibited a moderately increased lifespan compared to matched controls, supporting a protective role of low testosterone in aging108. Moreover, male C57BL/6 mice were found to exhibit the longest lifespan among a variety of different mouse strains and these animals also display exceptionally low circulating testosterone levels109,110.
In contrast to these notions, several studies in humans have reported an inverse correlation between circulating testosterone and mortality111,112. Notably, most of these findings were likely blurred by reverse causality, i.e. men with poor health develop low testosterone and have higher chances succumbing to a disease rather than the other way round20. However, the European Aging Male Study (EMAS) found an increased mortality in patients with LOH even after adjusting for potential confounders such as poor general health status and BMI113. On the other hand, mendelian randomization studies did not find evidence for such an association114. Another layer of complexity is added to the association between testosterone and mortality by sex-hormone binding globulin (SHBG). Reduced levels of the latter have been linked to an increased risk for developing type II diabetes in longitudinal studies115. Given that insulin resistance lowers hepatic SHBG production116,117, this association could arise from reverse causality. However, mendelian randomization studies have indeed supported a causal role of SHBG in glycemic dysregulation118. On the other hand, high SHBG levels have been associated with increased mortality in diabetic men, although this effect was very modest (HR=1.03, 95% CI 1.01–1.04)119. Conversely, lower SHBG levels conferred a reduced risk for ischemic heart disease mortality in the general population112.
Taken together, definite conclusions concerning the relationship between testosterone, mortality and longevity are currently difficult to draw since the interpretation of most available studies is complicated by a plethora of confounders. However, as outlined above, testosterone biology likely involves trade-offs between different ways to die.
Another unresolved aspect of testosterone physiology potentially affecting mortality are its proposed immunomodulatory functions. Given the opposing effects of testosterone and inflammatory signals on resource allocation, several authors have suggested an immunosuppressive role of the hormone45. Yet, experimental evidence supporting this hypothesis is scarce120. Interestingly, however, one study found impaired antibody responses upon influenza vaccination in men compared to women. Subsequent machine-learning approaches revealed that this inefficacy in male individuals was indeed explained by circulating testosterone abundance121. Whether similar effects also account for the higher cancer and lower autoinflammatory disease incidence in men compared to women remains elusive.
Concluding remarks
The question whether age-related changes in circulating testosterone reflect a pathological deficit or an adaptive response remains difficult to answer. The former notion is corroborated by several retrospective and some prospective clinical trials demonstrating distinct benefits of TRT in older men, whereas the latter is supported by adverse events associated with such therapies as well as data derived from animals. In fact, the observation that many other mammals besides humans experience age-related reductions in testosterone levels strongly suggests that the evolutionary cost of maintaining high androgen levels throughout lifespan are simply too high and/or the generated benefit is too low. While longevity might be compromised by TRT, most patients do not strive for an indefinite survival, but rather a high quality of life. Thus, the reasonable amount of data supporting beneficial effects of TRT in LOH currently justifies its clinical use under specific circumstances. On the other hand, the expectations of a pharmacological “fountain of youth” will most likely never be fulfilled, neither by TRT, nor by another therapy because the basic evolutionary principle of trade-offs simply cannot be overcome.
Highlights.
Testosterone secretion is highly dynamic among species and tailored to the demands of the respective environmental conditions.
Decreasing testosterone levels with age are not restricted to humans, but also found in many other mammals.
A growing demand for testosterone replacement therapy (TRT) in aging men has been noted worldwide.
The benefits of TRT need to be balanced against potential adverse events, which mainly appear to affect the cardiovascular system.
The link between testosterone, cardiovascular health and the risk for the development prostate cancer is incompletely understood.
Testosterone-driven anabolic pathways are in conflict with catabolic programs required for maintenance functions.
Acknowledgments
The authors would like to thank Tiziana Klawitter for her excellent help with the figures. This work was funded by the Deutsche Forschungsgemeinschaft (DFG) to LCH (HO 1875/24–1 and HO 1875/26–1), TDR (RA 2151/4–1) and MR (RA1923/12–1). Further funding was provided by the German Academic Scholarship Foundation and Mildred Scheel Nachwuchszentrum (MSNZ) to NJ. AW was supported by the NIH Clinical Investigator Award (K08AI128745).
Footnotes
Conflicts of interest
The authors have received honoraria, unrestricted educational grants and research funding to the individual or the institution from Alexion (LCH), Amgen (LCH, MR, TDR), Roche (TDR), Shire (LCH, TDR) and UCB (LCH, TDR). The remaining authors have no potential conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stearns S. & Medzhitov R. Evolutionary Medicine. 306 (Sinauer Associates, Oxford University Press, 2016). [Google Scholar]
- 2.Stearns SC Evolutionary medicine: its scope, interest and potential. Proc Biol Sci 279, 4305–4321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stearns SC, Allal N. & Mace R. Chapter 3: Life history theory and human development. 47–69 (Crawford CK(ed.), Lawrence Erlbaum Associates, New York, Foundations of Evolutionary Psychology, 2008). [Google Scholar]
- 4.Feldman HA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87, 589–598 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Johnson SL, Dunleavy J, Gemmell NJ & Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev 19, 22–33 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Nieschlag E. Late-onset hypogonadism: a concept comes of age. Andrology (2019). [DOI] [PubMed] [Google Scholar]
- 7.Wu FC, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 363, 123–135 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Nieschlag E, et al. Testosterone replacement therapy: current trends and future directions. Hum Reprod Update 10, 409–419 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Khera M, et al. Adult-Onset Hypogonadism. Mayo Clin Proc 91, 908–926 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Nieschlag E. & Nieschlag S. ENDOCRINE HISTORY: The history of discovery, synthesis and development of testosterone for clinical use. Eur J Endocrinol 180, R201–R212 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Gagliano-Jucá T. & Basaria S. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol 16, 555–574 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein JS, Yu EW & Burnett-Bowie SA Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 369, 2457 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Kelly DM & Jones TH Testosterone: a metabolic hormone in health and disease. J Endocrinol 217, R25–45 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Corona G, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 165, 687–701 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Saad F, Caliber M, Doros G, Haider KS & Haider A. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male 23, 81–92 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Tang YJ, et al. Serum testosterone level and related metabolic factors in men over 70 years old. J Endocrinol Invest 30, 451–458 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Lopes RF, Ferreira SA, Coeli CM & Farias ML Low body mass index and declining sex steroids explain most age-related bone loss in Brazilian men. Osteoporos Int 20, 1175–1182 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Zitzmann M, Faber S. & Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 91, 4335–4343 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 5, 673–681 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Snyder PJ, et al. Lessons From the Testosterone Trials. Endocr Rev 39, 369–386 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storer TW, et al. Effects of Testosterone Supplementation for 3 Years on Muscle Performance and Physical Function in Older Men. J Clin Endocrinol Metab 102, 583–593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder PJ, et al. Effects of Testosterone Treatment in Older Men. N Engl J Med 374, 611–624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Freeman G, Cowling BJ & Schooling CM Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 11, 108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddad RM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc 82, 29–39 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Calof OM, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60, 1451–1457 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Watts EL, et al. Low Free Testosterone and Prostate Cancer Risk: A Collaborative Analysis of 20 Prospective Studies. Eur Urol 74, 585–594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgentaler A. & Traish AM Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 55, 310–320 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 349, 215–224 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Nieschlag E. Current topics in testosterone replacement of hypogonadal men. Best Pract Res Clin Endocrinol Metab 29, 77–90 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Srinivas-Shankar U. & Wu FC Drug insight: testosterone preparations. Nat Clin Pract Urol 3, 653–665 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Kaminetsky JC, et al. A 52-Week Study of Dose Adjusted Subcutaneous Testosterone Enanthate in Oil Self-Administered via Disposable Auto-Injector. J Urol 201, 587–594 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Kaminetsky J, Jaffe JS & Swerdloff RS Pharmacokinetic Profile of Subcutaneous Testosterone Enanthate Delivered via a Novel, Prefilled Single-Use Autoinjector: A Phase II Study. Sex Med 3, 269–279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigen R, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310, 1829–1836 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Bhasin S, et al. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 103, 1715–1744 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Chester RC, Kling JM & Manson JE What the Women’s Health Initiative has taught us about menopausal hormone therapy. Clin Cardiol 41, 247–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marjoribanks J, Farquhar C, Roberts H, Lethaby A. & Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev 1, CD004143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stearns SC The Evolution of Life Histories. 249 (Oxford Univ. Press, 1992). [Google Scholar]
- 38.Wang A, Luan HH & Medzhitov R. An evolutionary perspective on immunometabolism. Science 363(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okin D. & Medzhitov R. Evolution of inflammatory diseases. Curr Biol 22, R733–740 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christeff N, Benassayag C, Carli-Vielle C, Carli A. & Nunez EA Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J Steroid Biochem 29, 435–440 (1988). [DOI] [PubMed] [Google Scholar]
- 41.Grosen A, et al. Semen Quality and Sperm DNA Integrity in Patients With Severe Active Inflammatory Bowel Disease and Effects of Tumour Necrosis Factor-alpha Inhibitors. J Crohns Colitis 13, 564–571 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Kanik KS, et al. Adrenocorticotropin, glucocorticoid, and androgen secretion in patients with new onset synovitis/rheumatoid arthritis: relations with indices of inflammation. J Clin Endocrinol Metab 85, 1461–1466 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Fanjul LF & Ruiz de Galarreta CM Effects of starvation in rats on serum levels of testosterone, dihydrotestosterone and testicular 3 beta-hydroxysteroid dehydrogenase activity. Horm Metab Res 13, 356–358 (1981). [DOI] [PubMed] [Google Scholar]
- 44.Parua S, Ghosh D, Nandi DK & Debnath J. Effect of cold exposure on testicular delta 5–3 beta and 17 beta hydroxysteroid dehydrogenase activities and plasma levels of testosterone in toad (Bufo melanostictus) in breeding and hibernating season: duration-dependent response. Andrologia 30, 105–108 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Muehlenbein MP & Bribiescas RG Testosterone-mediated immune functions and male life histories. Am J Hum Biol 17, 527–558 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Hackney AC & Aggon E. Chronic Low Testosterone Levels in Endurance Trained Men: The Exercise- Hypogonadal Male Condition. J Biochem Physiol 1(2018). [PMC free article] [PubMed] [Google Scholar]
- 47.Lotti F, et al. Metabolic syndrome and prostate abnormalities in male subjects of infertile couples. Asian J Androl 16, 295–304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lotti F, et al. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology 1, 229–239 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Morelli A, et al. Metabolic syndrome induces inflammation and impairs gonadotropin-releasing hormone neurons in the preoptic area of the hypothalamus in rabbits. Mol Cell Endocrinol 382, 107–119 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Cakir I. & Nillni EA Endoplasmic Reticulum Stress, the Hypothalamus, and Energy Balance. Trends Endocrinol Metab 30, 163–176 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Ebrahimi F, et al. IL-1 Antagonism in Men With Metabolic Syndrome and Low Testosterone: A Randomized Clinical Trial. J Clin Endocrinol Metab 103, 3466–3476 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Griggs RC, et al. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol (1985) 66, 498–503 (1989). [DOI] [PubMed] [Google Scholar]
- 53.Balchin D, Hayer-Hartl M. & Hartl FU In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Hipp MS, Kasturi P. & Hartl FU The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol 20, 421–435 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Kirkwood TB & Cremer T. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Hum Genet 60, 101–121 (1982). [DOI] [PubMed] [Google Scholar]
- 56.Hunt KE, et al. Multi-year patterns in testosterone, cortisol and corticosterone in baleen from adult males of three whale species. Conserv Physiol 6, coy049 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cates KA, et al. Testosterone trends within and across seasons in male humpback whales (Megaptera novaeangliae) from Hawaii and Alaska. Gen Comp Endocrinol 279, 164–173 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Machida T, Yonezawa Y. & Noumura T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm Behav 15, 238–245 (1981). [DOI] [PubMed] [Google Scholar]
- 59.Rigaudière N, Pelardy G, Robert A. & Delost P. Changes in the concentrations of testosterone and androstenedione in the plasma and testis of the guinea-pig from birth to death. J Reprod Fertil 48, 291–300 (1976). [DOI] [PubMed] [Google Scholar]
- 60.Beehner JC, et al. Testosterone related to age and life-history stages in male baboons and geladas. Horm Behav 56, 472–480 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.West PM & Packer C. Sexual selection, temperature, and the lion’s mane. Science 297, 1339–1343 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Eskelinen EL Autophagy: Supporting cellular and organismal homeostasis by self-eating. Int J Biochem Cell Biol 111, 1–10 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Almanza A, et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J 286, 241–278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atsuta N, et al. Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain 129, 1446–1455 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Rosenbohm A, et al. The metabolic and endocrine characteristics in spinal and bulbar muscular atrophy. J Neurol 265, 1026–1036 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Beitel LK, Alvarado C, Mokhtar S, Paliouras M. & Trifiro M. Mechanisms mediating spinal and bulbar muscular atrophy: investigations into polyglutamine-expanded androgen receptor function and dysfunction. Front Neurol 4, 53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnold FJ & Merry DE Molecular Mechanisms and Therapeutics for SBMA/Kennedy’s Disease. Neurotherapeutics 16, 928–947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katsuno M, et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35, 843–854 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Ni W, Chen S, Qiao K, Wang N. & Wu ZY Genotype-phenotype correlation in Chinese patients with spinal and bulbar muscular atrophy. PLoS One 10, e0122279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finsterer J. Perspectives of Kennedy’s disease. J Neurol Sci 298, 1–10 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Hashizume A, et al. Long-term treatment with leuprorelin for spinal and bulbar muscular atrophy: natural history-controlled study. J Neurol Neurosurg Psychiatry 88, 1026–1032 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Katsuno M, et al. Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 9, 875–884 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Banno H, et al. Phase 2 trial of leuprorelin in patients with spinal and bulbar muscular atrophy. Ann Neurol 65, 140–150 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Nedelsky NB, et al. Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron 67, 936–952 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Badders NM, et al. Selective modulation of the androgen receptor AF2 domain rescues degeneration in spinal bulbar muscular atrophy. Nat Med 24, 427–437 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okun MS, et al. Testosterone therapy in men with Parkinson disease: results of the TEST-PD Study. Arch Neurol 63, 729–735 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Gillies GE, Pienaar IS, Vohra S. & Qamhawi Z. Sex differences in Parkinson’s disease. Front Neuroendocrinol 35, 370–384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dluzen D, Jain R. & Liu B. Modulatory effects of testosterone on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurochem 62, 94–101 (1994). [DOI] [PubMed] [Google Scholar]
- 79.Yu YL & Wagner GC Influence of gonadal hormones on sexual differences in sensitivity to methamphetamine-induced neurotoxicity. J Neural Transm Park Dis Dement Sect 8, 215–221 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Finegold JA, Asaria P. & Francis DP Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol 168, 934–945 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathers CD, Boerma T. & Ma Fat D. Global and regional causes of death. Br Med Bull 92, 7–32 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Yeap BB, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab 94, 2353–2359 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Shores MM, et al. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab 99, 2061–2068 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haring R, et al. Association of sex steroids, gonadotrophins, and their trajectories with clinical cardiovascular disease and all-cause mortality in elderly men from the Framingham Heart Study. Clin Endocrinol (Oxf) 78, 629–634 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma R, et al. Association Between Testosterone Replacement Therapy and the Incidence of DVT and Pulmonary Embolism: A Retrospective Cohort Study of the Veterans Administration Database. Chest 150, 563–571 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Sharma R, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J 36, 2706–2715 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Nieschlag E. & Vorona E. Doping with anabolic androgenic steroids (AAS): Adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord 16, 199–211 (2015). [DOI] [PubMed] [Google Scholar]
- 88.de Groot PC, Dekkers OM, Romijn JA, Dieben SW & Helmerhorst FM PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update 17, 495–500 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Osibogun O, Ogunmoroti O. & Michos ED Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med 30, 399–404 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Mangiamele LA, et al. Increased androgenic sensitivity in the hind limb muscular system marks the evolution of a derived gestural display. Proc Natl Acad Sci U S A 113, 5664–5669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yusuf S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 395, 795–808 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ellison PT, et al. Population variation in age-related decline in male salivary testosterone. Hum Reprod 17, 3251–3253 (2002). [DOI] [PubMed] [Google Scholar]
- 93.Ellison PT & Panter-Brick C. Salivary testosterone levels among Tamang and Kami males of central Nepal. Hum Biol 68, 955–965 (1996). [PubMed] [Google Scholar]
- 94.Fiers T, et al. A critical evaluation of salivary testosterone as a method for the assessment of serum testosterone. Steroids 86, 5–9 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Andersson CR, Bergquist J, Theodorsson E. & Ström JO Comparisons between commercial salivary testosterone enzyme-linked immunosorbent assay kits. Scand J Clin Lab Invest 77, 582–586 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Luo S, Au Yeung SL, Zhao JV, Burgess S. & Schooling CM Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: mendelian randomisation study in UK Biobank. BMJ 364, l476 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hammes SR & Levin ER Impact of estrogens in males and androgens in females. J Clin Invest 129, 1818–1826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Austad SN & Fischer KE Sex Differences in Lifespan. Cell Metab 23, 1022–1033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Min KJ, Lee CK & Park HN The lifespan of Korean eunuchs. Curr Biol 22, R792–793 (2012). [DOI] [PubMed] [Google Scholar]
- 100.Brooks RC & Garratt MG Life history evolution, reproduction, and the origins of sex-dependent aging and longevity. Ann N Y Acad Sci 1389, 92–107 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Gesquiere LR, et al. Life at the top: rank and stress in wild male baboons. Science 333, 357–360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aguiar-Oliveira MH & Bartke A. Growth Hormone Deficiency: Health and Longevity. Endocr Rev 40, 575–601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weir HJ, et al. Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab 26, 884–896.e885 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cangemi R, Friedmann AJ, Holloszy JO & Fontana L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell 9, 236–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Basualto-Alarcón C, Jorquera G, Altamirano F, Jaimovich E. & Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc 45, 1712–1720 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Tyshkovskiy A, et al. Identification and Application of Gene Expression Signatures Associated with Lifespan Extension. Cell Metab 30, 573–593.e578 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Drori D. & Folman Y. Environmental effects on longevity in the male rat: exercise, mating, castration and restricted feeding. Exp Gerontol 11, 25–32 (1976). [DOI] [PubMed] [Google Scholar]
- 109.Yuan R, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8, 277–287 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brouillette J, Rivard K, Lizotte E. & Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res 65, 148–157 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Adelborg K, et al. Cardiovascular Outcomes and All-cause Mortality Following Measurement of Endogenous Testosterone Levels. Am J Cardiol 123, 1757–1764 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Araujo AB, et al. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med 167, 1252–1260 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Pye SR, et al. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab 99, 1357–1366 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Haring R, et al. Mendelian randomization suggests non-causal associations of testosterone with cardiometabolic risk factors and mortality. Andrology 1, 17–23 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Wallace IR, McKinley MC, Bell PM & Hunter SJ Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf) 78, 321–329 (2013). [DOI] [PubMed] [Google Scholar]
- 116.Plymate SR, Jones RE, Matej LA & Friedl KE Regulation of sex hormone binding globulin (SHBG) production in Hep G2 cells by insulin. Steroids 52, 339–340 (1988). [DOI] [PubMed] [Google Scholar]
- 117.Plymate SR, Matej LA, Jones RE & Friedl KE Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67, 460–464 (1988). [DOI] [PubMed] [Google Scholar]
- 118.Perry JR, et al. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet 19, 535–544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ramachandran S, Strange RC, Fryer AA, Saad F. & Hackett GI The association of sex hormone-binding globulin with mortality is mediated by age and testosterone in men with type 2 diabetes. Andrology 6, 846–853 (2018). [DOI] [PubMed] [Google Scholar]
- 120.O’Brien KA, Waterman JM, Anderson WG & Bennett NC Trade-offs between immunity and testosterone in male African ground squirrels. J Exp Biol 221(2018). [DOI] [PubMed] [Google Scholar]
- 121.Furman D, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A 111, 869–874 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]