Abstract
Natural killer (NK) cells, the primary effector cells of the innate immune system, utilize multiple strategies to recognize tumor cells by (1) detecting the presence of activating receptor ligands, which are often upregulated in cancer; (2) targeting cells that have a loss of major histocompatibility complex (MHC); and (3) binding to antibodies that bind to tumor-specific antigens on the tumor cell surface. All these strategies have been successfully harnessed in adoptive NK cell immunotherapies targeting cancer. In this review, we review the applications of NK cell therapies across different tumor types. Similar to other forms of immunotherapy, tumor-induced immune escape and immune suppression can limit NK cell therapies’ efficacy. Therefore, we also discuss how these limitations can be overcome by conferring NK cells with the ability to redirect their tumor-targeting capabilities and survive the immune-suppressive tumor microenvironment. Finally, we also discuss how future iterations can benefit from combination therapies with other immunotherapeutic agents.
Keywords: NK cell, Tumor immunotherapy, NK activating receptors, NK inhibitory receptors, ADCC
A lymphocyte of the innate immune response, natural killer (NK) cells are phenotypically defined by the absence of CD3 and the presence of CD56 on their surface [1,2]. Functionally, they resemble CD8+ cytotoxic T cells [3]. NK cells derive their name from their ability to spontaneously kill their targets without the need for a prior encounter of the antigen, as they have readily available lytic granules that can activate within minutes [4], unlike their T cell counterparts. NK cell targets include stressed, virally infected, and transformed cells [5].
The ability of NK cells to target tumor cells makes them attractive effector cells for cancer immunotherapy approaches. When encountering their targets, NK cells mediate lysis through several mechanisms as follows:
Fas ligand on the surface of NK cells binds to its target death receptor on the malignant cell, leading to programmed cell death.
Preformed granules within their cytoplasm (containing the cytotoxic proteins perforin and granzyme B) [6,7] form pores on the surface of the malignant cell upon their release [7-12].
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), induced by IFN-γ, binds to tumor receptors (eg, DR4, DR5, TRID, TRAIL-R4, and OPG) [13], resulting in apoptosis of the cancer cell [14].
In addition, NK cells are the main effector cell participating in antigen-dependent cell-mediated cytotoxicity (ADCC) by recognizing IgG antibodies bound on the tumor cell surface [15], and they release proinflammatory cytokines, such as IFN-γ.
Although multiple cytolytic mechanisms suggest redundancy, a preference for distinct NK cell killing strategies appears to predominate [16]. Transformed NK cells, like the cell line NK92, favor lytic granule-mediated cytotoxicity. In contrast, primary NK cells mainly rely on Fas ligand (FasL)-mediated apoptosis [17] since lytic granules become depleted in primary cells, which necessitates that they rely on upregulated Fas ligand to mediate killing [18].
NK cells are also polyfunctional and capable of secreting multiple cytokines that help orchestrate immune responses. Besides the expression of FasL and TRAIL and secretion of perforin and granzyme B, NK cells produce IFN-γ during activation. This cytokine helps shape a subsequent antitumor immune response, exerts antiproliferative effects on malignant cells, and activates macrophage killing of phagocytosed tumor cells [10,19,20]. NK cells also secrete TNF-α upon binding of multiple receptors [21] and are known to cooperate with IL-12 to increase the secretion of IFN-γ [22]. Both IFN-γ and TNF-α act to stimulate dendritic cell (DC) maturation upon NKp30 receptor binding [23]. Hence, IFN-γ, TNF-α FasL, and perforin/granzyme B all play a part in NK cell tumor surveillance [9,24,25].
NK cells express a complex array of receptors, including the cytokine receptors (IL-2R, IL-12R, IL-15R, IL-18R, IL-21R) [26], which allow them to respond to cytokines secreted by cells they typically interact with including T cells, dendritic cells, macrophages, and bone marrow stromal cells. NK cells also express chemokine receptors, including:
CXCR1 allowing colocalization with DCs, T cells, and neutrophils
CXCR2 allowing colocalization with neutrophils
CXCR3 allowing colocalization with T cells
CXCR4, CCR5, allowing colocalization with immature dendritic cells and proinflammatory monocytes, Th1 T cells, and cytotoxic T cells [27]
Notably, however, various groups have reported different patterns of chemokine receptor expression on NK cells [28,29]. In addition, NK cells express the activating (KIR2DL4, KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, KIR3DS1, NKG2C, NKG2E, NKG2D, NCRs, NKp30, NKp44, NKp46, NKp80, DNBAM-1, 2B4) and inhibitory (KIR2DL1, KIR2DL2/3, KIR2DL5, KIR3DL1, KIR3DL2, NKG2A, LILR, KLRG1) receptors [4,30] discussed in the sections below.
The NK-mediated killing of tumor targets is the result of the net signal from the ligation of activating and inhibitory receptors within the NK cell synapse [31]. NK cells express several inhibitory and activating receptors [32]. The study of NK cell receptors was key to the understanding of these innate effectors; the basic biology underlying this immune cell was only understood once their various activating and inhibitory receptors (and their properties) became known [22]. Understanding the biology of these receptors is also critical to harnessing the potential of these cells for cancer immunotherapy.
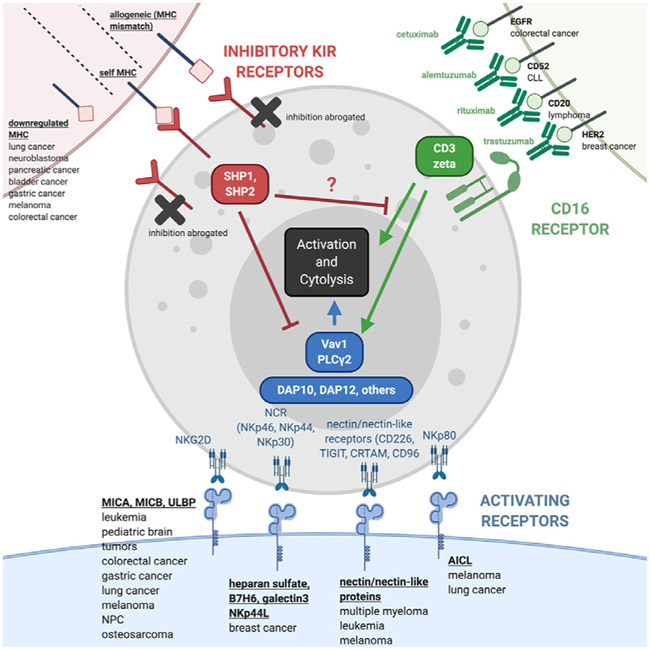
NK cells are predominantly controlled by inhibitory receptors that prevent activation (typically by activating receptor signaling)—a “fail-safe” to protect healthy cells from unwanted killing [4]. Dominance of inhibitory receptors occurs because they cluster more rapidly than activating receptors, and their blockade of activating receptors occurs early in the signal cascade [33]. There is evidence that this inhibition may be localized: only preventing activation by co-clustered receptors [34]. Activation of NK cells relies on an absence of inhibitory receptor engagement and concomitant engagement of multiple activating receptors [22]. Except for CD16, no other receptor can sufficiently activate NK cells by itself [34]. Consequently, activation and subsequent killing by NK cells is a coordinated effort, resulting in more signaling from activating receptors and less signaling from inhibitory receptors (see Figure 1) [34].
Figure 1.
Top left, in red: NK cells recognize tumor targets that lack MHC, as this prevents the inhibitory response mediated by KIR. Several tumors down-regulate MHC in response to T cell immune pressure. The same pathway does not become activated in the setting of allogeneic NK cells and is only engaged when NK cell effectors recognize self MHC. Bottom, in blue: NK cell activating receptor ligands are expressed by numerous malignancies [45-60,62,242-247], and these tumors engage NK-activating receptors, some of which associate with ITAM-containing DAP10 and DAP12 to mediate NK cell activation via proteins such as Vav1 and PLCγ2. Top right, in green: NK cells are the principal effectors of ADCC, mediating tumor lysis in settings when antibodies targeting overexpressed surface targets are used. Various antibodies have been developed to recruit ADCC against tumor cells bearing targets such as Her2, CD20, EGFR, and/or CD52. These antibodies bind to the CD16 receptor, which, in turn, is associated with ITAM-containing proteins such as the TCRζ chain–leading to NK cell activation.
TARGETING THE MALIGNANT CELL: AUTOLOGOUS NK CELLS AND ACTIVATING LIGANDS ON TUMOR CELLS
NK cell-activating receptors include activating killer immunoglobulin-like receptors (KIRs) [35]; NKG2D [36]; the natural cytotoxicity receptors (NCRs) NKp46, NKp44, and NKp30 [37]; the nectin and nectin-like receptors CD226, TIGIT, CRTAM, and CD96 [38]; and NKp80 [39]. These receptors recognize MHC, NKG2D ligands (MICA, MICB, ULBP), NCR ligands (heparan sulfate glycosa-minoglycans, B7-H6, galectin-3, NKp44L), nectin and nectin-like proteins, and activation-induced C-type lectin, respectively.
In contrast to inhibitory receptors (discussed in the section on non-self-recognition below), where the receptors themselves have immunoreceptor tyrosine-based inhibitory motifs, most activating receptors need to associate with immunoreceptor tyrosine-based activation motif (ITAM)-containing proteins [34]. Two of the most understood ITAMs are DAP10 (which associate with NKG2D) and DAP12 (which associate with activating KIRs). Phosphorylation of these ITAMs leads to signaling through molecules such as PLCγ2, Vav1, PI3K, Syk, and Ras [34,40].
Ligands for these receptors are upregulated in cancer cells (see Figure 1) [41]. Among activating receptors, NKG2D ligands are perhaps the best understood: they are upregulated during cellular stress (characteristic of malignant transformations) [42] and are otherwise absent in healthy tissue. MICA/MICB expression has been observed in several malignancies—including myeloma, leukemia, cervical cancer, and glioma [43]. UL16-binding proteins (ULBPs), initially identified for their ability to bind the UL16 protein expressed by cytomegalovirus-infected cells, bind strongly to NKG2D and DAP10, triggering multiple cascades that lead to NK cell activation and cytotoxicity [44]. ULBP1 to ULBP6 are expressed in several cancers, including multiple leukemias [45-54], colorectal cancer [55], Ewing sarcoma [56], gastric cancer [57], non-small-cell lung cancer [58], melanoma [59], nasopharyngeal cancer [60], cervical cancer [61], pediatric brain tumors [61], and osteosarcoma [62].
Mechanisms involved in upregulating ligand expression in malignancy vary, but events associated with tumorigenesis play a prominent role. Cellular senescence signals parallel expression of NKG2D ligands—following initiation of replicative senescence and DNA damage, MICA and ULBP2 are upregulated [63]. Genomic damage is thought to increase the presence of DNA in the cytoplasm, activating cyclic GMP-AMP synthase, which consequently induces expression of NKG2D ligands [64].
NK cell ligand expression can also indirectly result from other responses to malignancy. For example, increased protein production in cancer can lead to impaired folding, causing endoplasmic reticulum stress [65]. Endoplasmic reticulum stress has been shown to upregulate B7H6 mRNA and surface expression via proteins involved in the unfolded protein response [66].
Of interest, these ligands are expressed by some cancer stem cell populations. There is increasing evidence that NK cells preferentially target putative tumor-initiating populations, which seem to preferentially upregulate MICA/B [67]. NK cells lyse cancer stem cells of squamous cell carcinoma and glioblastoma in co-culture [68,69]. Stem cell differentiation is associated with decreased susceptibility to NK cell attack [70]. This mechanism is not the same across tumors, however; a recent study reports that leukemia stem cells can evade NK cells because they lack NKG2D ligand expression [71].
Their prognostic value highlights the importance of NKG2D ligands in immune-mediated rejection: a recent meta-analysis of 19 studies featuring more than 2500 patients and 10 different tumor types shows that patients with high MICA/B expression had significantly longer overall survival than their low-expressing counterparts [72]. Conversely, NK cell dysfunction appears to be a contributing factor for the development of malignancy since several functional deficiencies in NK cells have been observed in hematologic malignancies and solid tumors [73-75]. Harnessing autologous/patient-derived NK cells for immunotherapy, therefore, requires stimulation and/or ex vivo manufacture in non-immune-suppressed environments. Moreover, since NK cells comprise only 10% of circulating lymphocytes, obtaining sufficient autologous NK cells for clinical use is another reason why in vitro expansion is required [76]. Initial efforts focused on IL-2 for the expansion of “lymphokine-activated killer cells,” which were shown to be capable of killing tumors otherwise resistant to isolated NK cells [77]. However, no clear benefit was observed in a subsequent randomized phase III clinical trial [78].
Various manufacturing protocols for expanding autologous NK cells have since been developed [76], and these products have shown success in preclinical studies [79,80]. For example, patient-derived NK cells can be expanded 1000-fold in 3 weeks and can lyse breast cancer cell lines as well as allogeneic and autologous patient-derived breast cancer cells. These expanded NK cells also prevented tumor establishment in xenograft murine models [79].
Transplanting these preclinical results to human clinical trials has revealed that over the past 5 years, clinical studies have shown that adoptively transferred NK cells are safe. However, such studies have also reported very limited antitumor responses in patients with cancer, although some anecdotal responses have been reported. In 1 such study, a patient with inoperable nonsmall-cell lung cancer achieved tumor control after receiving multiple infusions of ex vivo activated NK cells. However, it was difficult to determine the direct therapeutic effect of the NK cells since the patient also received radiochemotherapy and nivolumab in combination with the NK cells [81]. In contrast, in another phase I clinical trial, ex vivo expanded autologous NK cells were administered intraventricularly to children with recurrent medulloblastoma and ependymoma [82]. In this trial, no toxicities were observed, but all except 1 patient still had progressive disease [82]. In another phase I trial, autologous NK cells expanded with OK432, IL-2, and FN-CH296-induced T cells were administered to patients with advanced or metastatic gastrointestinal cancer, and the results reflected the previously published experience using LAK cells [83]. In this trial, NK cells were successfully expanded between 500- and 4000-fold from patients who had previously failed standard therapies and were infused without notable adverse events. While the infused NK cell products expressed the appropriate markers and demonstrated tumor lytic abilities in vitro, no clinical responses were observed [83]. In summary, over the past 2 decades, most clinical trials published using autologous NK cells to treat various malignancies have failed to demonstrate sustained antitumor effects in vivo [84-87].
Examples of active trials using autologous NK cells are listed in Table 1.
Table 1.
Sample List of Active Trials Involving Autologous NK Cells
| NCT Number | Institution | Biologic/Drugs | Conditions |
|---|---|---|---|
| NCT00720785 | National Heart, Lung, and Blood Institute, National Institutes of Health | NK cells Bortezomib |
Chronic myeloid leukemia; pancreatic cancer; colorectal carcinoma; multiple myeloma; non-small-cell lung cancer |
| NCT02185781 | Gruppo Italiano Malattie EMatologiche dell'Adulto | NK cells | Acute lymphoblastic leukemia |
| NCT02271711 | M.D. Anderson Cancer Center; National Cancer Institute | NK cells | Recurrent childhood medulloblastoma; recurrent ependymoma |
| NCT02507154 | National University Hospital, Singapore | NK cells Cetuximab |
Nasopharyngeal carcinoma and head and neck squamous cell carcinoma |
| NCT02661685 | National Defense Medical Center, Taiwan∣ Ministry of Science and Technology, Taiwan ∣Academia Sinica, Taiwan | Interferon-producing killer dendritic cells (subset of NK cells) | Metastatic cancer |
| NCT03003728 | University of Arkansas; Bristol-Myers Squibb; Altor BioScience | NK cells Elotuzumab Melphalan Autologous stem cell transplant ALT-803 |
Multiple myeloma |
| NCT03410368 | First Hospital of Jilin University | NK cells | Small cell lung cancer |
| NCT03415100 | The Third Affiliated Hospital of Guangzhou Medical University | Autologous or allogeneic NK cells transfected with mRNA expressing NKG2D-directed CAR | Metastatic solid tumors |
| NCT03499834 | Ivy Life Sciences, Co., Ltd, Taipei Veterans General Hospital, Taiwan, Tri-Service General Hospital | Immune killer cells | Non-small-cell lung cancer stage IV |
| NCT03592706 | Ivy Life Sciences, Co., Ltd, Tri-Service General Hospital | Immune killer cells | Hepatocellular carcinoma |
| NCT03662477 | Shenzhen Fifth People's Hospital | NK cells | Advanced lung adenocarcinoma |
| NCT03941262 | NKMax America, Inc. | NK cells | Any histologically confirmed malignancy where disease confirmed to be metastatic and/or unresectable |
| NCT03958097 | First Hospital of Jilin University | NK cells Sintilimab |
Non-small-cell lung cancer |
TARGETING NON-SELF: ALLOGENEIC NK CELLS AND ALTERNATIVE CELL SOURCES
As previously discussed, the activation of NK cells relies on the balance between activating and inhibitory receptors, where the engagement of NK cells through activating receptor ligands dominates over any negative signals from self MHC. In cancer cells, MHC expression is downregulated as tumors attempt to escape the cytotoxic T cell response, thereby rendering them susceptible to NK cell-mediated lysis (ie, stimulated by the “missing self”) [88]. Selective pressure from tumor-specific T cells drives defects in MHC presentation by the tumor cells [89], so they are able to avoid recognition by T cells [90]. In tumor-bearing animals, MHC class I-negative tumor cells are often detected in immune-competent mice. In contrast, in T cell immune-deficient mice, MHC class I-positive tumor cells predominate [91]. Multiple mechanisms are responsible for MHC downregulation, including loss of heterozygosity associated with tumor progression in solid tumors like head and neck carcinomas (see Figure 1) [92].
It is difficult to ascertain whether MHC always remains downregulated in tumor cells since cancer cells can also potentially upregulate MHC expression [93]. NK cells express inhibitory receptors that recognize MHC class I on autologous cells [94]. These KIRs bind to MHC and send negative signals to the NK cell that effectively abrogate activating signals. This consequently inhibits NK cell lysis of the self MHC class I-expressing target [32,40,94]. KIRs function through the recruitment of the SHP-1 phosphatase after intracellular immunoreceptor tyrosine-based inhibitory motifs becomes phosphorylated [95,96]. The phosphatase reverses phosphorylation of activation motifs, neutralizing signaling from the activating receptors before propagating [34].
Allogeneic, HLA-mismatched NK cells circumvent the dominant inhibition from self MHC and are an alternative approach to the use of patient-derived NK cells. The advantage of using an allogeneic product is the off-the-shelf potential. In addition, the mismatch between the effector NK cell and the tumor target does not lead to inhibitory receptor engagement, and additional expression of activating receptor ligands on tumor cells engages activating receptors in this setting. Further, any potential for inducing GVHD can be mitigated by removing T cells from the final allogeneic NK cell product. In contrast to T cells, however, NK cells are more intolerant of cryopreservation methods—they often require culture following thaw—limiting the sites where these cells can be used [97]. Ongoing efforts are therefore under way to identify processes that can overcome this limitation [98].
Studies in the HLA-mismatched hematopoietic stem cell transplant setting provide the most compelling evidence for the antitumor potency of allogeneic NK cells. The first of these trials demonstrated alloreactivity from graft-derived NK cells against acute myelogenous leukemia (AML) with no acute graft-versus-host disease (GVHD) and no effects on engraftment. KIR-mismatched NK cells (in the graft-versus-host direction) improved the probability of survival at 5 years in patients with AML (60% in patients who were infused with alloreactive KIR-mismatched NK cells versus 5% in patients who did not receive allogeneic NK cells) [99]. Since these seminal studies, KIR-ligand mismatch in hematopoietic stem cell transplants is now considered predictive of a strong graft-versus-leukemia effect in AML [100]. NK cell ability to reject cells with aberrant MHC class I expression seems to correlate with the magnitude of the inhibitory response; this, in turn, can be determined from the strength and number of inhibitory receptor to MHC class I interactions [101,102].
Expanding this approach to the nontransplant setting (ie, administering haploidentical related donor-derived NK cells after high-intensity cyclophosphamide and fludarabine conditioning and subcutaneous IL-2) resulted in complete remissions in 5 out of 19 poor-prognosis patients with AML [103]. In this study, 75% of those with a KIR/KIR-ligand mismatch achieved remission, whereas only 13% of those without such a mismatch achieved a remission [103]. Surprisingly, despite their potent effects against non-self MHC-expressing tumor cells, these NK cells appeared to spare healthy tissue [104], and it is believed that NK cell elimination of host antigen-presenting cells contributes to the lack of GVHD [99].
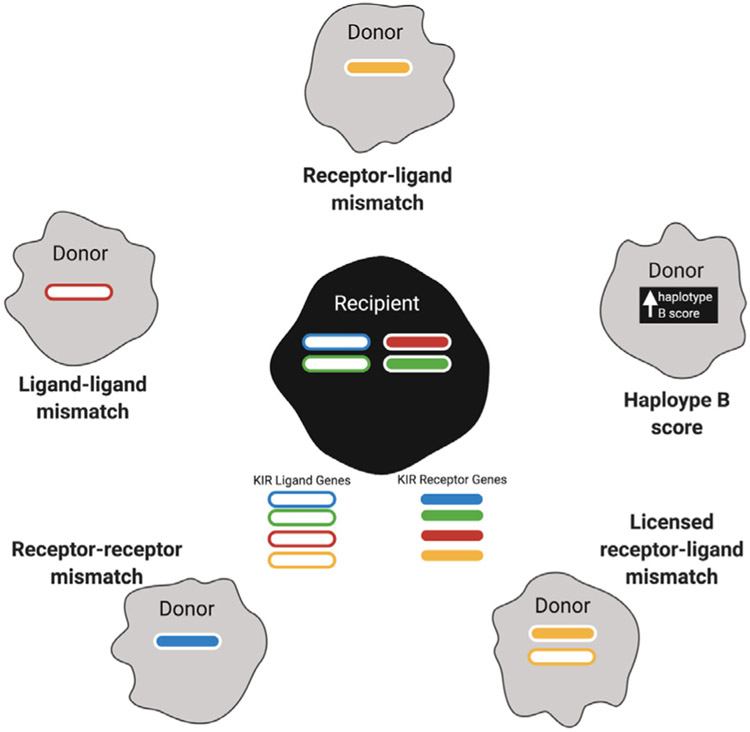
Allogeneic NK cells can be activated ex vivo in several ways (reviewed in Fang et al. [76)]. Because healthy donor-derived NK cells are not subject to immune dysfunction from tumors or drugs, they would theoretically be better mediators of cytotoxicity than patient-derived NK cells. Allogeneic NK cells have been variously selected according to donors and recipients, as seen in Figure 2. Strategies for selection of optimal NK cell donors relied on experience seen in haploidentical stem cell transplant and were enumerated by Wang et al. [105], and they have been classified by donor and recipient ligand-ligand mismatch [105,106] (selected donors express a KIR ligand gene that is missing in the recipient), receptor-ligand mismatch [105,107] (selected donors express a KIR receptor gene that is missing in the recipient and whose corresponding ligand is missing in the recipient), receptor-receptor mismatch [105,108] (selected donors express a KIR receptor gene that is missing in the recipient), and licensed receptor-ligand mismatch [105,109] (selected donors express a KIR receptor gene and the corresponding ligand, and this corresponding ligand is absent in the recipient—based on the idea that for a KIR receptor to be active, it needs to be licensed by interaction with its ligand first) or by haplotype B score [105,110] (selected donors have the best score—calculated by the number of their KIR B alleles; the KIR B haplotype has a variable amount of inhibitory receptors and more activating receptors than KIR A haplotypes—the more closely the donors are to the haplotype, the more activating they likely are [22]).
Figure 2.
Donor selection relies on gene expression of KIR-related genes in both donor and recipient. KIR ligand genes are shown as outlined rectangles, and KIR receptor genes are shown as filled rectangles. The presence of a gene in the donor or recipient is shown, and ligand-receptors pairs are shown in the same color. Selection is based on the presence of genes in the donor and absence in the recipient (receptor-receptor and ligand-ligand), a haplotype B score (see text for explanation), presence of matched receptor-gene pairs in the donor and absence in the recipient (licensed receptor-ligand), and presence of receptor genes in the donor and absence of ligand genes in the recipient (receptor-ligand).
While most studies use healthy donor peripheral blood as a source of allogeneic NK cells, exploring alternative donors and alternative sources of starting material has been the subject of numerous studies [111-114]. These alternatives include umbilical cord blood-derived NK cells [115], cell lines [116,117], and stem cell-derived NK cells [118-120].
Umbilical Cord Blood.
Cord blood (CB) appears to contain more naive NK cells than peripheral blood (PB) since approximately 30% of all lymphocytes in CB are NK cells compared to 10% in PB [121]. Moreover, given recent purification and expansion techniques, the absence of CD3+ T cell contamination in cord blood reduces the likelihood of GVHD [122]. While CB NK cells have lower expression of perforin/granzyme B and higher expression of inhibitory NKG2A [123,124], which translates to a lower spontaneous killing of targets [125], these can be overcome by ex vivo activation and expansion [126,127]. Cord blood NK cells have been found to express the same number of activating receptors as their more mature peripheral blood counterparts and produce comparable quantities of IFN-γ and TNF-α [123,124]. Their broad availability from numerous cord blood banks around the world makes them attractive donor cell sources for off-the-shelf NK cell approaches.
Stem Cells.
Progenitor cells can also be utilized to expand mature cytolytic NK cells. These progenitors can be derived from (1) CD34+ hematopoietic stem cells (HSCs) harvested from cord blood, bone marrow, or mobilized peripheral blood [128] or (2) induced pluripotent stem cells (iPSCs, which involve the use of mature cells with reactivated stemness via overexpression of pluripotency transcription factors [118]). NK cells derived from HSCs have demonstrated activity against myeloma, pancreatic cancer, and ovarian cancer cells in vivo [129-132]. On the other hand, iPSC-derived NK cells are derived from peripheral blood cells [133] whose pluripotency has been reactivated following overexpression of transcription factors such as Oct4, Sox2, cMyc, and KLF4 [118]. iPSC-derived cells show similar features to their HSC-derived counterparts but are more accessible, homogeneous, and renewable [132].
Established NK Cell Lines.
The use of NK cell lines avoids the labor-intensive expansion protocols used to generate sufficient NK cell numbers from autologous and allogeneic sources. Available NK cell lines include NK-92 and NKG [117,134]. These lines are derived from patients with leukemia or lymphoma [117] and provide an unlimited supply of homogeneous natural killer cell populations that can be maintained at a lower cost than allogeneic and autologous counterparts [135]. In particular, NK92 cell lines have been widely explored in multiple preclinical and clinical studies [136-138]. NK92 cells lack inhibitory KIRs other than KIR2DL4 and thus cannot recognize, and can in fact be inhibited by, HLA [139,140]. Unlike PB- or CB-derived NK cells, NK92 cells do not express CD16 [117] and are unable to elicit antibody-dependent cell cytotoxicity ADCC (see section below) unless modified to express this Fc receptor. Because these are transformed cells, these cell lines must also be irradiated before clinical use to prevent proliferation and persistence in their new host. Nevertheless, preclinical studies of NK92 cells have demonstrated their use against different malignancies and their combination of other immunotherapy modalities [136-138].
Adoptive transfer of ex vivo expanded allogeneic NK cells has been safely used as immunotherapy for several hematologic malignancies and solid tumors. In the past 5 years, several clinical trials have been reported as follows:
Haploidentical donor-derived NK cells were administered to children with intermediate or high-risk acute myeloid leukemia (although showed no clinical benefit) [141].
NK cell line lysate-activated haploidentical donor NK cells were infused to patients with high-risk AML during their first remission and mediated durable complete remissions in 3 patients [142].
Umbilical cord blood stem cell-derived NK cells were used to treat metastatic colorectal cancer and showed antitumor efficacy irrespective of EGFR or RAS status [143].
Allogeneic NK cells were administered to patients with unresectable liver cancer combined with irreversible electroporation (use of electric fields to form pores in cells, to induce cell death) [144] with observed effects seen, including decreased circulating tumor cells and increased Karnofsky performance status [145].
Allogeneic NK cells administered to patients with refractory acute myeloid leukemia induced increased leukemia control in patients who had an apparent increase in NK cell density detected in bone marrow biopsy specimens [146].
CB-derived NK cells administered to patients with multiple myeloma undergoing autologous stem cell transplants showed a very good partial response [147].
Overall, while these diverse trials have demonstrated a sound safety profile, the clinical efficacy reported has been relatively limited, necessitating ongoing trials. Hence, additional information detailing active clinical trials is shown in Table 2, and an overview of older clinical trials is summarized in Cheng et al. [148,149].
Table 2.
Sample List of Active Trials Involving Allogeneic NK Cells
| NCT Number | Institution | Biologic/Drugs | Conditions |
|---|---|---|---|
| NCT03056339 | M.D. Anderson Cancer Center | iC9/CAR.19/IL15-transduced CB-NK cells AP1903 |
B-lymphoid malignancies: acute lymphocytic leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma |
| NCT00900809 | NantKwest, Inc. | NK92 cell line | Refractory or relapsed acute myeloid leukemia |
| NCT00990717 | University Health Network, Toronto | NK92 cell line | Leukemia; lymphoma; myeloma; Hodgkin disease |
| NCT01040026 | University Hospital, Basel, Switzerland | Haploidentical NK cells | Multiple myeloma |
| NCT01700946 | St. Jude Children's Research Hospital; Cookies for Kids' Cancer; Assisi Foundation | Haploidentical NK cells Rituximab Chemotherapy |
Relapsed precursor B cell acute lymphoblastic leukemia and lymphoblastic lymphoma |
| NCT01729091 | M.D. Anderson Cancer Center; National Cancer Institute | CB-derived NK cells Autologous hematopoietic stem cell transplantation Elotuzumab Lenalidomide Melphalan |
Plasma cell leukemia; plasma cell myeloma |
| NCT01807468 | Samsung Medical Center | Haploidentical NK cells Haploidentical stem cell transplantation |
Neuroblastoma; Ewing sarcoma; rhabdomyosarcoma; osteosarcoma; soft tissue sarcoma |
| NCT01823198 | M.D. Anderson Cancer Center/National Cancer Institute | Donor NK cells Allogeneic hematopoietic stem cell transplantation Busulfan Fludarabine |
High-risk myeloid malignancies |
| NCT02100891 | Medical College of Wisconsin | Haploidentical NK cells Haploidentical stem cell transplantation |
Ewing sarcoma; neuroblastoma; rhabdomyosarcoma; osteosarcoma; CNS tumors |
| NCT02229266 | Technische Universität Dresden; German Research Foundation | Haploidentical NK cells Cytarabine |
Acute myeloid leukemia |
| NCT02409576 | National University Hospital, Singapore | Haploidentical NK cells | Ewing sarcoma; rhabdomyosarcoma |
| NCT02452697 | Agilent Technologies, Inc.; Duke University | NK cell-enriched donor lymphocyte infusions | Myeloid malignancies; lymphoid malignancies |
| NCT02465957 | NantKwest, Inc. | NK92 cell line | Stage IIIB and IV merkel cell carcinoma |
| NCT02727803 | M.D. Anderson Cancer Center; National Cancer Institute | NK92 cell line modified to express IL-2 and CD16 (haNK) | Myelodysplastic syndrome, leukemia, lymphoma, multiple myeloma |
| NCT02742727 | PersonGen BioTherapeutics (Suzhou) Co., Ltd.; The First People's Hospital of Hefei; Hefei Binhu Hospital | NK92 cell line modified to express anti-CD7 CAR with CD28, 41BB, and TCREζ | CD7-positive leukemia and lymphoma |
| NCT02763475 | Instituto de Investigatión Hospital Universitario La Paz | Cyclophosphamide Fludarabine Haploidentical NK cells IL-2 |
Acute myeloid leukemia |
| NCT02809092 | Hospital de Clinicas de Porto Alegre | Haploidentical NK cells Fludarabine Cytarabine granulocyte-colony stimulating factor |
Acute myeloid leukemia |
| NCT02892695 | PersonGen BioTherapeutics (Suzhou) Co., Ltd.; The First People's Hospital of Hefei; Hefei Binhu Hospital | NK92 cell line modified to express anti-CD19 CAR with CD28, 41BB, and TCREζ | CD19-positive leukemia and lymphoma |
| NCT02944162 | PersonGen BioTherapeutics (Suzhou) Co., Ltd.; The First People's Hospital of Hefei; Hefei Binhu Hospital | NK92 cell line modified to express anti-CD33 CAR | Relapsed/refractory AML |
| NCT02955550 | Celularity Incorporated | NK cells IL-2 |
Multiple myeloma |
| NCT03019640 | M.D. Anderson Cancer Center; National Cancer Institute | CB-derived NK cells Rituximab Stem cell transplant |
Recurrent or refractory B cell non-Hodgkin lymphoma |
| NCT03027128 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) | Metastatic of locally advanced solid tumors |
| NCT03068819 | Washington University School of Medicine | Cytokine-induced memory-like NK cells CD3+ T cells donor lymphocyte infusion |
Relapsed AML |
| NCT03136406 | NantKwest, Inc. | NK92 cell line ALT-803 Other immune modulators |
Pancreatic cancer |
| NCT03167164 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Merkel cell carcinoma |
| NCT03167177 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Melanoma |
| NCT03169738 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Non-small-cell lung cancer |
| NCT03169764 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Head and neck squamous cell carcinoma |
| NCT03169777 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Colorectal cancer |
| NCT03169790 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Non-Hodgkin lymphoma |
| NCT03175666 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Triple-negative breast cancer |
| NCT03197571 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Urothelial carcinoma |
| NCT03197584 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Ovarian cancer |
| NCT03300492 | University Hospital, Basel, Switzerland | Haploidentical NK cells | Acute myeloid leukemia; myelodysplastic syndromes |
| NCT03329248 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Pancreatic cancer |
| NCT03383978 | Johann Wolfgang Goethe University Hospital | NK92 cell line gene modified to express anti-Her2 CAR-expressing CD28 and ζ | Glioblastoma |
| NCT03387085 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Triple-negative breast cancer |
| NCT03387098 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Pancreatic cancer |
| NCT03387111 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Squamous cell carcinoma |
| NCT03415100 | The Third Affiliated Hospital of Guangzhou Medical University | Autologous or allogeneic NK cells transfected with mRNA expressing NKG2D-directed CAR | Metastatic solid tumors |
| NCT03420963 | M.D. Anderson Cancer Center/National Cancer Institute | CB-derived NK cells Cyclophosphamide Etoposide |
Relapsed or refractory solid tumors |
| NCT03539406 | Radboud University; Dutch Cancer Society | CB-derived NK cells | Recurrent ovarian, fallopian tube, or primary peritoneal cancer |
| NCT03554109 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Triple-negative breast cancer |
| NCT03563144 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Metastatic pancreatic cancer |
| NCT03563157 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Colorectal cancer |
| NCT03563170 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Hepatocellular carcinoma |
| NCT03574649 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Non-small-cell lung cancer |
| NCT03586869 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Pancreatic cancer |
| NCT03647423 | NantKwest, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Other immune modulators |
Chordoma |
| NCT03656705 | Xinxiang Medical University | CCCR-modified NK92 cell line | Non-small-cell lung cancer |
| NCT03669172 | Hospital General Universitario Gregorio Marañón | Donor IL-15-stimulated NK cells | Acute myeloid leukemia |
| NCT03778619 | Green Cross LabCell Corporation | Allogeneic NK cells Rituximab Fludarabine Cyclophosphamide IL-2 |
Relapsed and refractory non-Hodgkin lymphoma |
| NCT03821519 | A.O. Ospedale Papa Giovanni XXIII | Haploidentical cytokine-induced killer cells | Relapsed hematologic malignancy after transplant |
| NCT03853317 | NantKwest, Inc.; NantCell, Inc. | NK92 cell line modified to express IL-2 and CD16 (haNK) Avelumab N-803 |
Merkel cell carcinoma |
| NCT03937895 | SMT bio Co., Ltd. | NK cells Pembrolizumab |
Advanced biliary tract cancer |
| NCT03940833 | Asclepius Technology Company Group (Suzhou) Co., Ltd. | NK92 cell line modified to express B cell maturation antigen CAR | Multiple myeloma |
| NCT04162158 | Beijing 302 Hospital; Shenzhen Third People's Hospital; The First People's Hospital of Zhengzhou | NK cells | Advanced hepatocellular carcinoma |
| NCT04309084 | Celularity Incorporated | Human placental CD34+-derived NK cells | Multiple myeloma |
| NCT04310592 | Celularity Incorporated | Human placental CD34+-derived NK cells | Acute myeloid leukemia |
Regardless of the source, it is increasingly evident that allogeneic NK cells are potentially advantageous for adoptive immunotherapy of malignancy, because
they may be used in an off-the-shelf setting,
their low association with GVHD, and
they do not harbor the same inherent dysfunction as autologous patient-derived NK.
COUPLING WITH ANTIBODIES: ANTIBODY-DEPENDENT CELL CYTOTOXICITY
In recent years, several tumor-targeting antibodies have been developed the mediating antitumor functions not just by direct induction of tumor death, but also through the recruitment of NK cells that by antibody-dependent cell-mediated cytotoxicity (ADCC) [150]. Studies have shown that ADCC plays an important role in tumor clearance. Enhancing ADCC could, therefore, prove beneficial in a tumor setting [151-154].
ADCC specificity is dependent on the binding of the antibody to the tumor-associated antigen on the target cell through the antigen-binding portion (Fab) of the antibody [155,156]. Effector function is mediated by the constant region (Fc) of the antibody, which binds to Fc receptors on cytotoxic effector cells [152]. Several factors influence ADCC engagement, including the subclass/isotype, the glycoform, the density of target epitopes on the tumor cell, and Fc receptor polymorphisms [157]. Antibody class strongly determines ADCC, with IgG being the principal antibodies binding to Fc receptors that engage cell-mediated cytotoxicity [158]. Among the IgG antibodies, it is also clear that certain subclasses are better than others. For example, comparisons of 2 anti-EGFR antibodies with different subtypes show that IgG1-isotype antibodies (cetuximab) are more potent at activating NK cells than their IgG2-isotype counterpart (panitumumab) [159]. There are several classes of Fc receptors (Ia, IIa-b-c, IIIa-b) that differ in their ability to initiate an activating cytotoxic response [160], with some mediating strong inhibitory signals like FcgRIIb [160]. The critical role of NK cells in mediating ADCC stems from their lack of inhibitory Fc receptor expression, while all other FcgR-expressing cells express both activating and inhibitory Fc receptors [160]. NK cells mostly express FcgRIIIa (CD16a) and FcgRIIc (CD32c) at low levels. Both of these are activating receptors [160,161]. Certain CD16 genotypes have differential abilities to elicit ADCC. Polymorphisms in the 158 aa position alter affinity to the Fc portion of IgG—valine (V/V) at this position has a higher affinity (and better ADCC) compared with phenylalanine (V/F and F/F) [162]. The value of these polymorphisms in response to ADCC-eliciting antibodies remains under investigation, although 1 trial in Thailand could not identify a correlation between these polymorphisms and responses to rituximab in patients with large B cell lymphoma [163].
The ADCC activity of NK cells depends on CD16 (FcγRIIIA) binding to antibodies bound to the antigen on target cell surfaces. Activation through CD16 is sufficient to provide NK cell ADCC [164]. Following binding of target cell-bound antibody to CD16, the receptor associates with FcεRIγ, resulting in activation of its component ITAMs, in conjunction with phosphorylation of SLP-76 (a target of other activating receptors) [165]. In contrast to activating receptors, CD16 binding is sufficient to activate resting NK cells [165]. The metalloprotease ADAM17 also regulates CD16 expression. Following activation, the ectodomain of CD16 is cleaved by ADAM17, an enzyme involved in ectodomain shedding of neutrophils and other leukocytes [166].
Rituximab was the first monoclonal antibody (mAb) with ADCC activity approved for non-Hodgkin lymphoma treatment in 1997; many other mAbs with ADCC have been subsequently developed as therapy of solid tumors and hematologic malignancies [154]. Other mAbs mediating ADCC include trastuzumab (targeting Her2) against breast cancer [167], cetuximab (targeting EGFR) against metastatic colorectal cancer or squamous cell carcinoma [168], enoblituzumab (targeting B7-H3) against a wide range of solid tumors [169], and hu14.18K322A (targeting GD2) against neuroblastoma [170].
More recently, attempts to improve ADCC have been explored, including combination therapies (eg, antibody combinations and/or combining NK cells with antibodies) to prevent immune escape and antibody engineering to improve binding. For example, a phase III clinical trial comparing the efficacy of combining pertuzumab and trastuzumab (with docetaxel versus placebo/trastuzumab/docetaxel) showed improvements in overall survival, maintained after more than a median of 8 years [171]. Combining trastuzumab and pertuzumab to target breast cancer delayed therapy resistance, presumably maximizing ADCC and improving inhibition of tumor growth. The 2 antibodies together enhanced the recruitment of NK cells in xenograft models, demonstrating additive ADCC at subsaturation doses [172]. In addition, Fc modifications have been used to improve the effector functions of antibodies as well as to prolong their serum half-lives [173]. These chimeric antibodies (eg, margetuximab) have been safely administered in patients with HER2-expressing solid tumors eliciting clinical responses, including partial remissions in 12% and stable disease in 50% with tumor reductions occurring in 78% of evaluable responders [174]. Studies evaluating the Fc-modified CD20 antibody have been particularly instructive. Obinutuzumab is a glycoengineered antibody with reduced fucosylation on its Fc region, to increase its affinity to CD16 [175]. It has demonstrated improved ADCC compared to rituximab in vivo [176]. This modification also enabled this antibody to effectively bypass inhibitory KIRs; thus, ADCC is not impaired by KIR/HLA interaction [177]. Moreover, combining allogeneic NK cells and obinutuzumab demonstrated robust antitumor responses in vitro and in preclinical in vivo ADCC models [178]. Several genotypes have shown enhanced ADCC responses, which may be particularly helpful when deciding the type of allogeneic cell to use in combination approaches with antibodies. For example, patients with follicular lymphoma with KIR2DL2 and KIR3DL1 genotypes showed improved outcomes after receiving ADCC-mediating antibodies, suggesting that combining antibody therapies with adoptively transferred NK cells expressing these KIRs may be a potentially beneficial therapeutic approach [179]. Currently, there are several trials exploring combinations of NK cells and ADCC-mediating antibodies, as shown in Table 3.
Table 3.
Sample List of Active Trials Involving Infusion of ADCC-Mediating Antibody and NK Cells
| NCT Number | Sponsor | Intervention | Disease |
|---|---|---|---|
| NCT00625729 | Masonic Cancer Center, University of Minnesota | Rituximab (anti-CD20 antibody) Allogeneic NK cells |
Relapsed non-Hodgkin lymphoma; chronic lymphocytic leukemia |
| NCT00877110 | Memorial Sloan Kettering Cancer Center | Hu3F8 (anti-GD2 antibody) Allogeneic NK cells Cyclophosphamide Vincristine Topotecan |
Neuroblastoma |
| NCT01576692 | St. Jude Children's Research Hospital | Hu14.18K322A (anti-GD2 antibody) Allogeneic NK cells |
Neuroblastoma |
| NCT01729091 | M.D. Anderson Cancer Center | Elotuzumab (anti-SLAMF7 antibody) CB-derived NK cells |
Multiple myeloma |
| NCT02573896 | New Approaches to Neuroblastoma Therapy Consortium; Nationwide Children's Hospital; United Therapeutics | Ch14.18 (anti-GD2 antibody) Autologous NK cells |
Neuroblastoma |
| NCT02650648 | Memorial Sloan Kettering Cancer Center; Y-mAbs Therapeutics | Hu3F8 (anti-GD2 antibody) Allogeneic NK cells |
High-risk neuroblastoma |
| NCT02845999 | Centre Hospitalier Universitaire de Besancon | Cetuximab (anti-EGFR antibody) Allogeneic NK cells |
Metastatic gastrointestinal cancer |
| NCT03019640 | M.D. Anderson Cancer Center | Rituximab (anti-CD20 antibody) CB-derived NK cells |
Recurrent or refractory B cell non-Hodgkin lymphoma |
| NCT03242603 | National University Hospital, Singapore | Ch14.18 (anti-GD2 antibody) Autologous NK cells |
Recurrent neuroblastoma |
| NCT03554889 | Hangzhou Cancer Hospital | Nimotuzumab (anti-EGFR antibody) Autologous NK cells |
Recurrent or metastatic cancer |
| NCT04074746 | M.D. Anderson Cancer Center | AFM13 (bispecific antibody–anti-CD30 and anti-CD16A) CB-derived NK cells |
Refractory/relapsed CD30-positive lymphoid malignancies |
| NCT04211675 | Nationwide Children's Hospital | Dinutuximab (anti-GD2 antibody) Autologous NK cells |
Relapsed and refractory neuroblastoma |
ENHANCING NK CELLS I: REDIRECTING ACTIVITY THROUGH CHIMERIC ANTIGEN RECEPTORS
The apparent lack of robust antitumor activity reported in clinical trials using NK cells has prompted multiple investigators to explore strategies to enhance NK cell function for improved activity in vivo. Tumors can escape NK cell activity by downregulating activating receptor ligands, and several pathways that result in downregulation of ligand expression have been identified, including exosome release, proteolytic cleavage, internalization and degradation, reduced stability of transcripts, decreased translation, alternative glycosylation and lipidation, increased intracellular retention, misfolding, and alternative splicing [180]. Tumors can also escape NK cell activity by upregulating MHC, thereby engaging their inhibitory receptors [93].
To overcome some of these limitations, genetic modification of NK cells with chimeric antigen receptors (CARs) has been explored to enhance NK cell function by conferring more potent signaling and targeting. Chimeric antigen receptors are artificial receptors that feature an extracellular domain derived from the variable regions of an antibody, to confer targeting capabilities, coupled to intracellular domains that signal like activating receptors. Currently, CAR-modified NK cell therapeutics under investigation include (1) CAR-transduced primary NK or NK-92 cell targeting CD3 and CD5 for the treatment of T cell lymphoblastic leukemia [181,182], (2) CS-1 and CD138 CAR NK cells for myeloma [183], CD19-CAR-transduced NK cells for the treatment of CD19+ B cell lymphoid malignancies (NCT030563390 and NCT04245722), (4) EGFR-CAR NK92 cells targeting brain metastasis in breast cancer [184], (5) HER2 and EGFR CAR-transduced NK cells for brain metastases [184] or HER2-positive glioblastoma (NCT03383978), (6) GPA7-CAR NK cells for melanoma [185], (7) HER2 CAR-transduced NK cells for breast cancer [186], (8) WT-1-CAR NK cells for the treatment of Wilms tumor [150], and finally, (9) ROR-1 [187] and/or GD2 CAR-transduced NK cells for the treatment of neuroblastoma or neuroectodermal tumors [188]. See Tables 1 and 2 for examples of other trials.
The first larger-scale (n = 11) phase I/II clinical trial using CAR-NKs was published in early 2020, in which Liu et al. [189] evaluated a novel CD19-CAR construct that expressed IL-15 and transduced CB-derived NK cells as an off-the-shelf therapy for the treatment of patients with either refractory or relapsed chronic lymphocytic leukemiaor non-Hodgkin lymphoma. Allogeneic off-the-shelf cord blood donors were originally chosen using a partial (4/6) HLA match, but the lack of GVHD prompted the investigators to transition to KIR-ligand mismatched products for the last 2 patients. Patients received lymphodepleting chemotherapy with fludarabine and cyclophosphamide, followed by CB-derived CD19-CAR-IL15 NK cells. Seven of the 11 patients were in complete remission at a median follow-up of 13.8 months (range, 2.8 to 20.0). The absence of CRS, neurotoxicity, and GVHD, combined with encouraging clinical responses, therefore, demonstrated the potential safety and efficacy of CAR-NK cell therapy [189].
Despite these promising clinical results, CAR design improvements are continually being evaluated to enhance NK cell function. Signaling domains of adaptor molecules associated with activating NK receptors have been used to mimic physiologic NK signaling better. For example, the DAP12 intracellular domain exhibited enhanced cytotoxicity against prostate cancer stem cells compared with a CAR-NK relying on a CD3ζ domain in a preclinical model [190]. DAP12 is heavily involved in the signaling of activating receptors such as NKp44 and NKG2C. Further, DAP10 is another signaling molecule that activates NK cells associating with NKG2D [191], and evaluating a novel NKG2D-DAP10-CD3ζ construct showed increased specificity and activation against solid tumor-derived cell lines, including osteosarcoma, prostate carcinoma, and rhabdomyosarcoma [192]. Finally, another strategy to enhance costimulatory activation and NK signaling is via CD244 (2B4), a signaling lymphocyte activation molecule-related receptor. Studies have shown that CD244 has robust costimulatory roles in NK effector cells targeting CD19 or GD2, indicating that antigen-specific CD244-ζ-expressing NK cells may have great potential to boost signaling in NK cells retargeted to tumor cells [193]. In summary, multiple gene engineering approaches utilizing CAR constructs are being evaluated on the NK cell platform and have been comprehensively reviewed (see Patel et al. [194] and Rezvani [195]).
ENHANCING NK CELLS II: PROTECTION FROM TUMOR-INDUCED IMMUNE SUPPRESSION
Establishing effective NK therapeutics also requires overcoming the negative effects of the tumor microenvironment (TME). While TGF-β secretion is widely employed as a potent tumor immune evasion mechanism, multiple other factors within the TME can directly or indirectly affect NK cell function, proliferation, and/or maturation in vivo.
However, as discussed above, TGF-β plays a prominent role in tumorigenesis. Increased TGF-β expression by tumor cells is associated with metastasis in several cancers, including breast, prostate, and colorectal cancer [196-199], and there is a strong link between TGF-β production and metastatic spread. TGF-β is also secreted by the immune, endothelial, and smooth muscle cells in the surrounding tumor stroma [200,201]. TGF-β is also a prominent and critical component of the immune-suppressive environment maintained by the tumor. Elevated TGF-β levels in the tumor microenvironment have an adverse effect on antitumor immunity and inhibit host immune surveillance [200]. This suppressive cytokine [200,201] abrogates the secretion of critical Th1 cytokines, such as IFN-γ, and impairs NK cell cytolytic activity and proliferation in vitro and in vivo [202,203]. TGF-β also inhibits activating receptors such as NKG2D and is shown to impact ADCC [204-206]. Enhancing resistance to TGF-β is, therefore, of interest to several groups and takes many forms. One approach involves the genetic modification of NK cells with artificial TGF-β receptors to confer TGF-β resistance to NK cells and T cells. Such approaches can enhance antitumor immune responses against malignancies that rely on TGF-β-induced immune suppression as a potent immune evasion strategy [207-209]. In several studies, a mutant receptor lacking the kinase domain of the TGF-βRII receptor acts as a dominant-negative receptor since ligand (TGF-β) binding is unable to elicit downstream receptor signaling [207]. Gene engineering NK cells to express this dominant-negative receptor have demonstrated superior antitumor efficacy in multiple preclinical models, including neuroblastoma [210], glioblastoma [211], leukemia [212], and medulloblastoma [213].
Other ways to circumvent TGF-β-mediated NK cell immune suppression include SMAD3 suppression during expansion [214] and TGF-β signaling blockade with antagonistic drugs such as galunisertib, which reverses downregulation of NKp30, NKp46, NKG2D, and DNAM1 on NK cells [215]. Generation of TGF-β receptor 2-negative NK cells using CRISPR-Cas9 RNP complexes enhanced in vivo activity of NK cells [215] and knockdown of SMAD3, which increased cytotoxicity and IFN-γ production in vitro and improved antitumor effects of NK92 cells in mice [216]. TGF-β-specific antibodies have also been used to enhance treatment of xenograft models of head and neck squamous cell carcinoma with the anti-EGFR antibody cetuximab [217].
Outside of TGF-β, several additional TME mechanisms affect NK cell function, including changes in metabolism and production of adenosine, hypoxia, lactate, and checkpoint molecules. The majority of data to date focused on cellular metabolism, and immune cell functioning is focused on T cells and macrophages in the TME [218,219], but some studies are evaluating the role of the TME and metabolic effects on NK cell function. The TME lacks glucose and glutamine, both of which are critical for optimal NK function [220]. Because glycolysis is increased in activated NK cells, changes in the expression of metabolic enzymes such as fructose bisphosphatase 1 (eg, in lung cancer models [221]) can limit NK viability in vivo. NK cell function is also affected by metabolism in models of obesity, including murine and human models, where cytotoxicity, perforin, granzyme B, and INF-γ levels are lower in obese models [222]. Conversely, production of prostaglandin E2, produced in the TME by multiple tumors, also can reduce NK function [223]. Further, blocking of prostaglandin E2 has been shown to improve NK function in both metastatic breast cancer [224] and gastric cancer preclinical models [225].
Adenosine is another factor known to decrease NK cell function directly but can also increase the effects of immune-suppressive Tregs and myeloid-derived suppressor cells in vivo [223]. Reductions in tumor growth and increased NK infiltration of tumors are observed when adenosine effects are reduced by blocking CD39 and the A2A receptor [226]. Hypoxia also causes dysfunction of NK cells infiltrating tumors by downregulation of NKG2D, NKp44, NKp30, NKp46, granzyme B, and perforin [227-230]. Lactate is known to cause acidosis and immunosuppression in the TME [231] with a direct effect on the cytolytic activity of NK cells and downregulation of NKp46 expression [232,233]. Finally, immune checkpoint pathways can also be utilized to improve NK cell function by targeting NK cell-specific receptors (KIR, CD94/NKG2A) and targeting T cell and NK cell markers of exhaustion (TIM-3, TIGIT, CD96, and LAG-3). However, there is still some controversy regarding the exact mechanisms that checkpoint inhibitors employ to enhance NK cell function in vitro and in vivo [231,234].
CONCLUSIONS
The ability of NK cells to target cells without a priming event while showing little toxicity against healthy cells makes them promising cells for tumor immunotherapy [235,236]. Therefore, these cells are being actively explored as off-the-shelf platforms. Numerous malignancies have heterogeneous antigen expression with variable MHC expression and have heterogeneous and often unknown targets, which makes identifying and targeting the appropriate tumor antigens difficult. As we describe here, many avenues seek to harness NK cells’ ability to target activating receptor-ligand expression on tumor cells, exploiting non-self mismatch, and ADCC. Genetic modification of NK cells further allows investigators to redirect their activity, increase their potency, and abrogate immune-suppressive environments mediated by TGF-β to increase NK cell persistence and efficacy [237]. One active area of research involves NK cell homing and tumor infiltration, and several strategies to ensure effective NK cell homing to the tumor or potential sites for spread are under study. For example, induction of chemokines such as chemerin and CXCR3 ligands has been shown to improve NK cell infiltration and tumor killing in mice [238].
In summary, while NK cell therapies have elicited modest clinical benefits in the majority of malignant settings, there is clearly promise with combination approaches [239] since these cells interface with so many different components of the antitumor immune response, including antibodies. Currently, combination therapies are under active investigation, for example, combinations with CD47 blockade [240] or oncolytic viruses [241].
ACKNOWLEDGMENTS
Financial disclosure: This work is partially funded by a Cancer Moonshot grant, 1U01CA239258-01, Enhancing Cell Therapy for Brain Tumors (contact principal investigator (PI) Bollard/co-PI Cruz/co-PI Savoldo/co-PI Jones), and by the Alex’s Lemonade Stand Foundation.
Footnotes
Conflict of interest statement: C.R.Y.C. and C.M.B. are cofounders of a biotechnology company, Mana Therapeutics, developing T cell therapies for cancer. C.R.Y.C. and C.M.B. have filed a patent involving TGF-β-based constructs for gene-engineered NK cell therapies. C.M.B. is a cofounder and SAB member of Catamaran Bio, an NK cell therapy company. C.R.Y.C. is a consultant for the same company. C.R.Y.C. and C.M.B. are performing research sponsored by Catamaran Bio, a company developing NK cell therapies.
REFERENCES
- 1.Lanier LL, Testi R, Bindl J, Phillips JH Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritz J, Schmidt RE, Michon J, Hercend T, Schlossman SF Characterization of functional surface structures on human natural killer cells. Adv Immunol. 1988;42:181–211. [DOI] [PubMed] [Google Scholar]
- 3.Cortez VS, Robinette ML, Colonna M Innate lymphoid cells: new insights into function and development. Curr Opin Immunol. 2015;32:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanier LL NK cell recognition. Annu Rev Immunol. 2005;23:225–274. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S Functions of natural killer cells. Nat Immunol. 2002;9:503–510. [DOI] [PubMed] [Google Scholar]
- 6.Lettau M, Schmidt H, Kabelitz D, Janssen O Secretory lysosomes and their cargo in T and NK cells. Immunol Lett. 2007;108:10–19. [DOI] [PubMed] [Google Scholar]
- 7.Orange JS Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocikat R, Braumuller H, Gumy A, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. [DOI] [PubMed] [Google Scholar]
- 11.Smyth MJ, Cretney E, Kelly JM, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–510. [DOI] [PubMed] [Google Scholar]
- 12.Tripp CS, Wolf SF, Unanue ER Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993;90:3725–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, El-Deiry WS TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. [DOI] [PubMed] [Google Scholar]
- 14.Smyth MJ, Cretney E, Takeda K, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backes CS, Friedmann KS, Mang S, Knorck A, Hoth M, Kummerow C Natural killer cells induce distinct modes of cancer cell death: discrimination, quantification, and modulation of apoptosis, necrosis, and mixed forms. J Biol Chem. 2012;293:16348–16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, Huang B, Shi J Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target. Oncotarget. 2016;7:47163–47172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prager I, Liesche C, van Ooijen H, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. 2019;216:2113–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipe-Santos O, Bustamante J, Chapgier A, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–361. [DOI] [PubMed] [Google Scholar]
- 20.Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM Interferon: cellular executioner or white knight. Curr Med Chem. 2007;14:1279–1289. [DOI] [PubMed] [Google Scholar]
- 21.Fauriat C, Long EO, Ljunggren HG, Bryceson YT Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul WE Fundamental Immunology. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 23.Vitale M, Della Chiesa M, Carlomagno S, et al. NK-dependent DC maturation is mediated by TNF alpha and IFN gamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. [DOI] [PubMed] [Google Scholar]
- 24.Dunn GP, Old LJ, Schreiber RD The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. [DOI] [PubMed] [Google Scholar]
- 25.Wallace ME, Smyth MJ The role of natural killer cells in tumor control–effectors and regulators of adaptive immunity. Springer Semin Immuno-pathol. 2005;27:49–64. [DOI] [PubMed] [Google Scholar]
- 26.Romee R, Leong JW, Fehniger TA Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. 2014;2014:205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima M, Leander M, Santos M, et al. Chemokine receptor expression on normal blood CD56(+) NK-cells elucidates cell partners that comigrate during the innate and adaptive immune responses and identifies a transitional NK-cell population. J Immunol Res. 2015;2015: 839684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell JJ, Qin SX, Unutmaz D, et al. Unique subpopulations of CD56(+) NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. [DOI] [PubMed] [Google Scholar]
- 29.Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ Evidence for NK cell subsets based on chemokine receptor expression. J Immunol. 2006;177:7833–7840. [DOI] [PubMed] [Google Scholar]
- 30.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–224. [DOI] [PubMed] [Google Scholar]
- 31.Caligiuri MA Human natural killer cells. Blood. 2002;112:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanier LL Face off–the interplay between activating and inhibitory immune receptors. Curr Opin Immunol. 2001;13:326–331. [DOI] [PubMed] [Google Scholar]
- 33.Abeyweera TP, Merino E, Huse M Inhibitory signaling blocks activating receptor clustering and induces cytoskeletal retraction in natural killer cells. J Cell Biol. 2011;192:675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart CA, Laugier-Anfossi F, Vely F, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci U S A. 2005;102:13224–13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [DOI] [PubMed] [Google Scholar]
- 37.Bottino C, Moretta L, Moretta A NK cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol. 2006;298:175–182. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs A, Colonna M The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16:359–366. [DOI] [PubMed] [Google Scholar]
- 39.Welte S, Kuttruff S, Waldhauer I, Steinle A Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. [DOI] [PubMed] [Google Scholar]
- 40.Vivier E, Nunes JA, Vely F Natural killer cell signaling pathways. Science. 2004;306:1517–1519. [DOI] [PubMed] [Google Scholar]
- 41.Dhar P, Wu JD NKG2D and its ligands in cancer. Curr Opin Immunol. 2018;51:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez S, Groh V, Spies T Immunobiology of human NKG2D and its ligands. Curr Top Microbiol Immunol. 2006;298:121–138. [DOI] [PubMed] [Google Scholar]
- 43.Suresh PK Membrane-bound versus soluble major histocompatibility complex class I-related chain A and major histocompatibility complex class I-related chain B differential expression: mechanisms of tumor eradication versus evasion and current drug development strategies. J Cancer Res Ther. 2016;12:1224–1233. [DOI] [PubMed] [Google Scholar]
- 44.Cao W, He W UL16 binding proteins. Immunobiology. 2004;209:283–290. [DOI] [PubMed] [Google Scholar]
- 45.Romanski A, Bug G, Becker S, et al. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Exp Hematol. 2005;33:344–352. [DOI] [PubMed] [Google Scholar]
- 46.Pende D, Spaggiari GM, Marcenaro S, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the poliovirus receptor (CD155) and nectin-2(CD112). Blood. 2005;105:2066–2073. [DOI] [PubMed] [Google Scholar]
- 47.Verheyden S, Demanet C NK cell receptors and their ligands in leukemia. Leukemia. 2008;22:249–257. [DOI] [PubMed] [Google Scholar]
- 48.Epling-Burnette PK, Bai F, Painter JS, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109:4816–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salih HR, Antropius H, Gieseke F, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. [DOI] [PubMed] [Google Scholar]
- 50.Nowbakht P, Ionescu MCS, Rohner A, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal mye-lomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–3622. [DOI] [PubMed] [Google Scholar]
- 51.Sconocchia G, Lau M, Provenzano M, et al. The antileukemia effect of HLA-matched NK and NK-T cells in chronic myelogenous leukemia involves NKG2D-target-cell interactions. Blood. 2005;106:3666–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poggi A, Venturino C, Catellani S, et al. V delta 1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64:9172–9179. [DOI] [PubMed] [Google Scholar]
- 53.Nowbakht P, Ionescu MC, Rohner A, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal mye-lomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–3622. [DOI] [PubMed] [Google Scholar]
- 54.Diermayr S, Himmelreich H, Durovic B, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111:1428–1436. [DOI] [PubMed] [Google Scholar]
- 55.McGilvray RW, Eagle RA, Watson NF, et al. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res. 2009;15:6993–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verhoeven DH, de Hooge AS, Mooiman EC, et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol. 2008;45:3917–3925. [DOI] [PubMed] [Google Scholar]
- 57.Mimura K, Kamiya T, Shiraishi K, et al. Therapeutic potential of highly cytotoxic natural killer cells for gastric cancer. Int J Cancer. 2014;135:1390–1398. [DOI] [PubMed] [Google Scholar]
- 58.Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M Clinicopathological relevance of tumor expression of NK group 2 member D ligands in resected non-small cell lung cancer. Oncotarget. 2019;10:6805–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgado S, Sanchez-Correa B, Casado JG, et al. NK cell recognition and killing of melanoma cells is controlled by multiple activating receptor-ligand interactions. J Innate Immun. 2011;3:365–373. [DOI] [PubMed] [Google Scholar]
- 60.Mei JZ, Guo KY, Wei HM, Song CY Expression of NKG2D ligands in multi-drug-resistant nasopharyngeal carcinoma cell line CNE2/DDP and their effects on cytotoxicity of natural killer cells [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao.2007;27:887–889. [PubMed] [Google Scholar]
- 61.Cho H, Chung JY, Kim S, et al. MICA/B and ULBP1 NKG2D ligands are independent predictors of good prognosis in cervical cancer. BMC Cancer. 2014;14:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandez L, Valentin J, Zalacain M, Leung W, Patino-Garcia A, Perez-Martinez A Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett. 2015;368:54–63. [DOI] [PubMed] [Google Scholar]
- 63.Sagiv A, Burton DG, Moshayev Z, et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging. 2016;8:328–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raulet DH, Marcus A Coscoy L Dysregulated cellular functions and cell stress pathways provide critical cues for activating and targeting natural killer cells to transformed and infected cells. Immunol Rev. 2017;280:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oakes SA Endoplasmic reticulum stress signaling in cancer cells. Am J Pathol. 2020;190:934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Obiedat A, Charpak-Amikam Y, Tai-Schmiedel J, et al. The integrated stress response promotes B7H6 expression. J Mol Med (Berl). 2020;98:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ames E, Canter RJ, Grossenbacher SK, et al. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J Immunol. 2015;195:4010–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tseng H-C, Arasteh A, Paranjpe A, et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS ONE. 2010;5:e11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castriconi R, Daga A, Dondero A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–3539. [DOI] [PubMed] [Google Scholar]
- 70.Jewett A, Man Y-g, Cacalano N, Kos J, Tseng H-C Natural killer cells as effectors of selection and differentiation of stem cells: role in resolution of inflammation. J Immunotoxicol. 2014;11:297–307. [DOI] [PubMed] [Google Scholar]
- 71.Paczulla AM, Rothfelder K, Raffel S, et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature. 2019;572:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao Y, Chen N, Yu Y, et al. Prognostic value of MICA/B in cancers: a systematic review and meta-analysis. Oncotarget. 2017;8:96384–96395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlsten M, Jaras M Natural killer cells in myeloid malignancies: immune surveillance, NK cell dysfunction, and pharmacological opportunities to bolster the endogenous NK cells. Front Immunol. 2019;10:2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chambers AM, Matosevic S Immunometabolic dysfunction of natural killer cells mediated by the hypoxia-CD73 axis in solid tumors. Front Mol Biosci. 2019;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiu J, Ernst DM, Keating A Acquired natural killer cell dysfunction in the tumor microenvironment of classic Hodgkin lymphoma. Front Immunol. 2018;9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang F, Xiao W, Tian Z NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54. [DOI] [PubMed] [Google Scholar]
- 77.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA Lymphokine-activated killer cell phenomenon: lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Law TM, Motzer RJ, Mazumdar M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824–832. [DOI] [PubMed] [Google Scholar]
- 79.Shenouda MM, Gillgrass A, Nham T, et al. Ex vivo expanded natural killer cells from breast cancer patients and healthy donors are highly cytotoxic against breast cancer cell lines and patient-derived tumours. Breast Cancer Res. 2017;19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siegler U, Kalberer CP, Nowbakht P, Sendelov S, Meyer-Monard S, Wodnar-Filipowicz A Activated natural killer cells from patients with acute myeloid leukemia are cytotoxic against autologous leukemic blasts in NOD/SCID mice. Leukemia. 2005;19:2215–2222. [DOI] [PubMed] [Google Scholar]
- 81.Kokowski K, Stangl S, Seier S, Hildebrandt M, Vaupel P, Multhoff G Radiochemotherapy combined with NK cell transfer followed by second-line PD-1 inhibition in a patient with NSCLC stage IIIb inducing long-term tumor control: a case study. Strahlenther Onkol. 2019;195:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khatua S, Cooper LJN, Sandberg DI, et al. Phase I study of intraventricular infusions of autologous ex-vivo-expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro Oncol. 2020;22:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sakamoto N, Ishikawa T, Kokura S, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishikawa E, Tsuboi K, Saijo K, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–1871. [PubMed] [Google Scholar]
- 86.Krause SW, Gastpar R, Andreesen R, et al. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase I trial. Clin Cancer Res. 2004;10:3699–3707. [DOI] [PubMed] [Google Scholar]
- 87.Lister J, Rybka WB, Donnenberg AD, et al. Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with activated natural killer cells in the immediate posttransplant period. Clin Cancer Res. 1995;1:607–614. [PubMed] [Google Scholar]
- 88.Bubenik J MHC class I down-regulation: tumour escape from immune surveillance? (review). Int J Oncol. 2004;25:487–491. [PubMed] [Google Scholar]
- 89.Chang CC, Ferrone S Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seliger B, Harders C, Wollscheid U, Staege MS, Reske-Kunz AB, Huber C Suppression of MHC class I antigens in oncogenic transformants: association with decreased recognition by cytotoxic T lymphocytes. Exp Hematol. 1996;24:1275–1279. [PubMed] [Google Scholar]
- 91.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maleno I, Lopez-Nevot MA, Cabrera T, Salinero J, Garrido F Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol Immunother. 2002;51:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Classen CF, Falk CS, Friesen C, Fulda S, Herr I, Debatin KM Natural killer resistance of a drug-resistant leukemia cell line, mediated by up-regulation of HLA class I expression. Haematologica. 2003;88:509–521. [PubMed] [Google Scholar]
- 94.Bryceson YT, March ME, Ljunggren HG, Long EO Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Binstadt BA, Brumbaugh KM, Dick CJ, et al. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. [DOI] [PubMed] [Google Scholar]
- 96.Burshtyn DN, Scharenberg AM, Wagtmann N, et al. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klingemann H Challenges of cancer therapy with natural killer cells. Cytotherapy. 2015;17:245–249. [DOI] [PubMed] [Google Scholar]
- 98.Damodharan SN, Walker KL, Forsberg MH, et al. Analysis of ex vivo expanded and activated clinical-grade human NK cells after cryopreservation. Cytotherapy. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (New York, N.Y.). 2002;295:2097–2100. [DOI] [PubMed] [Google Scholar]
- 100.Van Elssen CHMJ, Ciurea SO NK cell therapy after hematopoietic stem cell transplantation: can we improve anti-tumor effect? Int J Hematol. 2018;107:151–156. [DOI] [PubMed] [Google Scholar]
- 101.Johansson S, Johansson M, Rosmaraki E, et al. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J Exp Med. 2005;201:1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. [DOI] [PubMed] [Google Scholar]
- 103.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. [DOI] [PubMed] [Google Scholar]
- 104.Joncker NT, Shifrin N, Delebecque F, Raulet DH Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment.J Exp Med. 2010;207:2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang W, Erbe AK, DeSantes KB, Sondel PM Donor selection for ex vivo-expanded natural killer cells as adoptive cancer immunotherapy. Future Oncol. 2017;13:1043–1047. [DOI] [PubMed] [Google Scholar]
- 106.Curti A, Ruggeri L, D'Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. [DOI] [PubMed] [Google Scholar]
- 107.Geller MA, Cooley S, Judson PL, et al. A phase II study ofallogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gagne K, Brizard G, Gueglio B, et al. Relevance of KIR gene polymorphisms in bone marrow transplantation outcome. Hum Immunol. 2002;63:271–280. [DOI] [PubMed] [Google Scholar]
- 109.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kloess S, Oberschmidt O, Dahlke J, et al. Preclinical assessment of suitable natural killer cell sources for chimeric antigen receptor natural killer-based "off-the-shelf" acute myeloid leukemia immunotherapies. Hum Gene Ther. 2019;30:381–401. [DOI] [PubMed] [Google Scholar]
- 112.Alnabhan R, Madrigal A, Saudemont A Differential activation of cord blood and peripheral blood natural killer cells by cytokines. Cytotherapy. 2015;17:73–85. [DOI] [PubMed] [Google Scholar]
- 113.Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xing D, Ramsay AG, Gribben JG, et al. Cord blood natural killer cells exhibit impaired lytic immunological synapse formation that is reversed with IL-2 exvivo expansion. J Immunother (Hagerstown, Md.: 1997). 2010;33:684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarvaria A, Jawdat D, Madrigal JA, Saudemont A Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klingemann H, Boissel L, Toneguzzo F Natural killer cells for immunotherapy–advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suck G, Odendahl M, Nowakowska P, et al. NK-92: an 'off-the-shelf therapeutic' for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eguizabal C, Zenarruzabeitia O, Monge J, et al. Natural killer cells for cancer immunotherapy: pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front Immunol. 2014;5:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knorr DA, Kaufman DS Pluripotent stem cell-derived natural killer cells for cancer therapy. Transl Res. 2010;156:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang K, Han Y, Cho WC, Zhu H The rise of human stem cell-derived natural killer cells for cancer immunotherapy. Expert Opin Biol Ther. 2019;19:141–148. [DOI] [PubMed] [Google Scholar]
- 121.Kotylo PK, Baenzinger JC, Yoder MC, Engle WA, Bolinger CD Rapid analysis of lymphocyte subsets in cord blood. Am J Clin Pathol. 1990;93:263–266. [DOI] [PubMed] [Google Scholar]
- 122.Nomura A, Takada H, Jin CH, Tanaka T, Ohga S, Hara T Functional analyses of cord blood natural killer cells and T cells: a distinctive interleukin-18 response. Exp Hematol. 2001;29:1169–1176. [DOI] [PubMed] [Google Scholar]
- 123.Luevano M, Daryouzeh M, Alnabhan R, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol. 2012;73:248–257. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, Xu H, Zheng X, Wei H, Sun R, Tian Z High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell Mol Immunol. 2007;4:377–382. [PubMed] [Google Scholar]
- 125.Dalle JH, Menezes J, Wagner E, et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatr Res. 2005;57:649–655. [DOI] [PubMed] [Google Scholar]
- 126.Gaddy J, Broxmeyer HE Cord blood CD16+56− cells with low lytic activity are possible precursors of mature natural killer cells. Cell Immunol. 1997;180:132–142. [DOI] [PubMed] [Google Scholar]
- 127.Herrera L, Santos S, Vesga MA, et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci Rep. 2019;9:18729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Luevano M, Madrigal A, Saudemont A Generation of natural killer cells from hematopoietic stem cells in vitro for immunotherapy. Cell Mol Immunol. 2012;9:310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Woll PS, Grzywacz B, Tian X, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Woll PS, Martin CH, Miller JS, Kaufman DS Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–5103. [DOI] [PubMed] [Google Scholar]
- 131.Hermanson DL, Bendzick L, Pribyl L, et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells. 2016;34:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nianias A, Themeli M Induced pluripotent stem cell (iPSC)-derived lymphocytes for adoptive cell immunotherapy: recent advances and challenges. Curr Hematol Malig Rep. 2019;14:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng M, Ma J, Chen Y, et al. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transplant. 2011;20:1731–1746. [DOI] [PubMed] [Google Scholar]
- 135.Hermanson DL, Kaufman DS Utilizing chimeric antigen receptors to direct natural killer cell activity. Front Immunol. 2015;6:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Williams BA, Wang X-H, Leyton JV, et al. CD16+NK-92 and anti-CD123 monoclonal antibody prolongs survival in primary human acute myeloid leukemia xenografted mice. Haematologica. 2018;103:1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang J, Zheng H, Diao Y Natural killer cells and current applications of chimeric antigen receptor-modified NK-92 cells in tumor immunotherapy. Int J Mol Sci. 2019;20:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sivori S, Meazza R, Quintarelli C, et al. NK cell-based immunotherapy for hematological malignancies. J Clin Med. 2019;8:1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–1570. [DOI] [PubMed] [Google Scholar]
- 140.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009;21:525–530. [DOI] [PubMed] [Google Scholar]
- 141.Nguyen R, Wu H, Pounds S, et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J Immunother Cancer. 2019;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fehniger TA, Miller JS, Stuart RK, et al. A phase 1 trial of CNDO-109-activated natural killer cells in patients with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant. 2018;24:1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Veluchamy JP, Lopez-Lastra S, Spanholtz J, et al. In vivo efficacy of umbilical cord blood stem cell-derived NK cells in the treatment of metastatic colorectal cancer. Front Immunol. 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Narayanan G Irreversible electroporation. Semin Intervent Radiol. 2015;32:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Y, Qin Z, Du D, et al. Safety and short-term efficacy of irreversible electroporation and allogenic natural killer cell immunotherapy combination in the treatment of patients with unresectable primary liver cancer. Cardiovasc Intervent Radiol. 2019;42:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grzywacz B, Moench L, McKenna D Jr, et al. Natural killer cell homing and persistence in the bone marrow after adoptive immunotherapy correlates with better leukemia control. J Immunother (Hagerstown, Md.: 1997). 2019;42:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shah N, Li L, McCarty J, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cheng M, Chen Y, Xiao W, Sun R, Tian Z NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bachanova V, Sarhan D, DeFor TE, et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother. 2018;67:483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rajasekaran N, Chester C, Yonezawa A, Zhao X, Kohrt HE Enhancement of antibody-dependent cell mediated cytotoxicity: a new era in cancer treatment. Immunotargets Ther. 2015;4:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism. Br J Cancer. 2006;94:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Clynes RA, Towers TL, Presta LG, Ravetch JV Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. [DOI] [PubMed] [Google Scholar]
- 153.Manches O, Lui G, Chaperot L, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–954. [DOI] [PubMed] [Google Scholar]
- 154.Weiner LM, Surana R, Wang S Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alderson KL, Sondel PM Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ravetch JV, Bolland S IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. [DOI] [PubMed] [Google Scholar]
- 157.Jefferis R Antibody therapeutics: isotype and glycoform selection. Expert Opin Biol Ther. 2007;7:1401–1413. [DOI] [PubMed] [Google Scholar]
- 158.Bruggemann M, Williams GT, Bindon CI, et al. Comparison of the effector functions of human-immunoglobulins using a matched set of chimeric antibodies. J Exp Med.1987;166:1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trivedi S, Srivastava RM, Concha-Benavente F, et al. Anti-EGFR targeted monoclonal antibody isotype influences antitumor cellular immunity in head and neck cancer patients. Clin Cancer Res. 2016;22:5229–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lo Nigro C, Macagno M, Sangiolo D, Bertolaccini L, Aglietta M, Merlano MC NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med. 2019;7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nimmerjahn F, Ravetch JV Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. [DOI] [PubMed] [Google Scholar]
- 162.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180:6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Angsirisak N, Wittayalertpanya S, Limpanasithikul W, Bunworasate U, Owattanapanich D Correlation of FcgammaRIIIa polymorphisms to the response of rituximab in thai patients with diffuse large B-cell lymphoma. J Med Assoc Thai. 2015;98:1215–1221. [PubMed] [Google Scholar]
- 164.Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci U S A. 1999;96:5640–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Watzl C How to trigger a killer: modulation of natural killer cell reactivity on many levels. Adv Immunol. 2014;124:137–170. [DOI] [PubMed] [Google Scholar]
- 166.Romee R, Foley B, Lenvik T, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood. 2013;121:3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pham D-H, Kim J-S, Kim S-K, et al. Effects of ADAM10 and ADAM17 inhibitors on natural killer cell expansion and antibody-dependent cellular cytotoxicity against breast cancer cells in vitro. Anticancer Res. 2017;37:5507–5513. [DOI] [PubMed] [Google Scholar]
- 168.Monteverde M, Milano G, Strola G, et al. The relevance of ADCC for EGFR targeting: a review of the literature and a clinically-applicable method of assessment in patients. Crit Rev Oncol Hematol. 2015;95:179–190. [DOI] [PubMed] [Google Scholar]
- 169.Loo D, Alderson RF, Chen FZ, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–3845. [DOI] [PubMed] [Google Scholar]
- 170.Federico SM, McCarville MB, Shulkin BL, et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. [DOI] [PubMed] [Google Scholar]
- 172.Toth G, Szoor A, Simon L, Yarden Y, Szollosi J, Vereb G The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity. Mabs. 2016;8:1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saxena A, Wu DH Advances in therapeutic Fc engineering–modulation of igG-associated effector functions and serum half-life. Front Immunol. 2016;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12:2031–2042. [DOI] [PubMed] [Google Scholar]
- 177.Terszowski G, Klein C, Stern M KIR/HLA interactions negatively affect rituximab- but not GA101 (obinutuzumab)-induced antibody-dependent cellular cytotoxicity. J Immunol. 2014;192:5618–5624. [DOI] [PubMed] [Google Scholar]
- 178.Sanchez-Martinez D, Allende-Vega N, Orecchioni S, et al. Expansion of allogeneic NK cells with efficient antibody-dependent cell cytotoxicity against multiple tumors. Theranostics. 2018;8:3856–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Erbe AK, Wang W, Carmichael L, et al. Follicular lymphoma patients with KIR2DL2 and KIR3DL1 and their ligands (HLA-C1 and HLA-Bw4) show improved outcome when receiving rituximab. J Immunother Cancer. 2019;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schmiedel D, Mandelboim O NKG2D ligands–critical targets for cancer immune escape and therapy. Front Immunol. 2018;9:2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen KH, Wada M, Firor AE, et al. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget. 2016;7:56219–56232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen KH, Wada M, Pinz KG, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia. 2017;31:2151–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jiang H, Zhang W, Shang P, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen X, Han J, Chu J, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7:27764–27777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang G, Liu R, Zhu X, et al. Retargeting NK-92 for anti-melanoma activity by a TCR-like single-domain antibody. Immunol Cell Biol. 2013;91:615–624. [DOI] [PubMed] [Google Scholar]
- 186.Daldrup-Link HE, Meier R, Rudelius M, et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15:4–13. [DOI] [PubMed] [Google Scholar]
- 187.Park H, Awasthi A, Ayello J, et al. ROR1-specific chimeric antigen receptor (CAR) NK cell immunotherapy for high risk neuroblastomas and sarcomas. Biol Blood Marrow Transplant. 2017;23:S136–S137. [Google Scholar]
- 188.Esser R, Müller T, Stefes D, et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Töpfer K, Cartellieri M, Michen S, et al. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol. 2015;194:3201–3212. [DOI] [PubMed] [Google Scholar]
- 191.Park YP, Choi SC, Kiesler P, et al. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the gammac cytokines and TGF-beta1.Blood.2011;118:3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chang Y-H, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–1786. [DOI] [PubMed] [Google Scholar]
- 193.Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15:4857–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Patel S, Burga RA, Powell AB, et al. Beyond CAR T cells: other cell-based immunotherapeutic strategies against cancer. Front Oncol. 2019;9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rezvani K Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transplant. 2019;54:785–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen XL, Chen ZQ, Zhu SL, et al. Prognostic value of transforming growth factor-beta in patients with colorectal cancer who undergo surgery: a meta-analysis. BMC Cancer. 2017;17:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen Y, Ma L, He Q, Zhang S, Zhang C, Jia W TGF-beta1 expression is associated with invasion and metastasis of intrahepatic cholangiocarcinoma. Biol Res. 2015;48:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Biswas S, Guix M, Rinehart C, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lebrun JJ The dual role of TGFbeta in human cancer: from tumor suppression to cancer metastasis. ISRN Mol Biol. 2012;2012: 381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Massague J TGFbeta in cancer. Cell. 2008;134:215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17:4296–4308. [DOI] [PubMed] [Google Scholar]
- 203.Dix AR, Brooks WH, Roszman TL, Morford LA Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–232. [DOI] [PubMed] [Google Scholar]
- 204.Letterio JJ, Geiser AG, Kulkarni AB, et al. Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Invest. 1996;98:2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Trotta R, Dal Col J, Yu J, et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181:3784–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pickup M, Novitskiy S, Moses HL The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. [DOI] [PubMed] [Google Scholar]
- 208.Foster AE, Dotti G, Lu A, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother (Hagerstown, Md.: 1997). 2008;31:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lacuesta K, Buza E, Hauser H, et al. Assessing the safety of cytotoxic T lymphocytes transduced with a dominant negative transforming growth factor-beta receptor. J Immunother (Hagerstown, Md.: 1997). 2006;29:250–260. [DOI] [PubMed] [Google Scholar]
- 210.Burga RA, Williams E, Yvon E, Fernandes R, Cruz CRY, Bollard CM Generating suppression-resistant natural killer cells as an enhanced immunotherapeutic for neuroblastoma. J Immunol. 2018;200. 179.6. [Google Scholar]
- 211.Yvon ES, Burga R, Powell A, et al. Cord blood natural killer cells expressing a dominant negative TGF-beta receptor: implications for adoptive immunotherapy for glioblastoma. Cytotherapy. 2017;19:408–418. [DOI] [PubMed] [Google Scholar]
- 212.Rouce RH, Shaim H, Sekine T, et al. The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia. 2016;30:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Powell AB, Yadavilli S, Saunders D, et al. Medulloblastoma rendered susceptible to NK-cell attack by TGFbeta neutralization. J Transl Med. 2019;17:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Foltz JA, Moseman JE, Thakkar A, Chakravarti N, Lee DA TGFbeta imprinting during activation promotes natural killer cell cytokine hypersecretion. Cancers (Basel). 2018;10:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Naeimi Kararoudi M, Dolatshad H, Trikha P, et al. Generation of knockout primary and expanded human NK cells using Cas9 ribonucleoproteins.J Vis Exp. 2018;136:58237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang Q-M, Tang PM-K, Lian G-Y, et al. Enhanced cancer immunotherapy with Smad3-silenced NK-92 cells. Cancer Immunol Res. 2018;6:965–977. [DOI] [PubMed] [Google Scholar]
- 217.Bedi A, Chang X, Noonan K, et al. Inhibition of TGF-β enhances the in vivo antitumor efficacy of EGF receptor-targeted therapy. Mol Cancer Ther. 2012;11:2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wenes M, Shang M, Di Matteo M, et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. 2016;24:701–715. [DOI] [PubMed] [Google Scholar]
- 219.Kishton RJ, Sukumar M, Restifo NP Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26:94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chang CH, Qiu J, O'Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cong J, Wang X, Zheng X, et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. 2018;28.243–255.e245. [DOI] [PubMed] [Google Scholar]
- 222.Michelet X, Dyck L, Hogan A, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. 2018;19:1330–1340. [DOI] [PubMed] [Google Scholar]
- 223.Melaiu O, Lucarini V, Cifaldi L, Fruci D Influence of the tumor microenvironment on NK cell function in solid tumors. Front Immunol. 2019;10:3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ma X, Holt D, Kundu N, et al. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013;2:e22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li T, Zhang Q, Jiang Y, et al. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology. 2016;5:e1069936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Young A, Ngiow SF, Gao Y, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78:1003–1016. [DOI] [PubMed] [Google Scholar]
- 227.Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Balsamo M, Manzini C, Pietra G, et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol. 2013;43:2756–2764. [DOI] [PubMed] [Google Scholar]
- 229.Sceneay J, Chow MT, Chen A, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. [DOI] [PubMed] [Google Scholar]
- 230.Fink T, Ebbesen P, Koppelhus U, Zachar V Natural killer cell-mediated basal and interferon-enhanced cytotoxicity against liver cancer cells is significantly impaired under in vivo oxygen conditions. Scand J Immunol. 2003;58:607–612. [DOI] [PubMed] [Google Scholar]
- 231.Kim N, Kim HS Targeting checkpoint receptors and molecules for therapeutic modulation of natural killer cells. Front Immunol. 2018;9:2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Husain Z, Huang Y, Seth P, Sukhatme VP Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–1495. [DOI] [PubMed] [Google Scholar]
- 233.Husain Z, Seth P, Sukhatme VP Tumor-derived lactate and myeloid-derived suppressor cells: linking metabolism to cancer immunology. Oncoimmunology. 2013;2:e26383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Beldi-Ferchiou A, Caillat-Zucman S Control of NK cell activation by immune checkpoint molecules. Int J Mol Sci. 2017;18:2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Herberman RB, Nunn ME, Holden HT, Lavrin DH Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors, II: characterization of effector cells. Int J Cancer. 1975;16:230–239. [DOI] [PubMed] [Google Scholar]
- 236.Kiessling R, Klein E, Wigzell H "Natural" killer cells in the mouse, I: cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. [DOI] [PubMed] [Google Scholar]
- 237.Nayyar G, Chu Y, Cairo MS Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front Oncol. 2019;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pachynski RK, Zabel BA, Kohrt HE, et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J Exp Med. 2012;209:1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Qian X, Wang X, Jin H Cell transfer therapy for cancer: past, present, and future. J Immunol Res. 2014;2014:525913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Valipour B, Abedelahi A, Naderali E, et al. Cord blood stem cell derived CD16(+) NK cells eradicated acute lymphoblastic leukemia cells using with anti-CD47 antibody. Life Sci. 2020;242:117223. [DOI] [PubMed] [Google Scholar]
- 241.Gao J, Zhang W, Mese K, Bunz O, Lu F, Ehrhardt A Transient chimeric Ad5/37 fiber enhances NK-92 carrier cell-mediated delivery of oncolytic adenovirus type 5 to tumor cells. Mol Ther Methods Clin Dev. 2020;18:376–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Haberthur K, Brennan K, Hoglund V, et al. NKG2D ligand expression in pediatric brain tumors. Cancer Biol Ther. 2016;17:1253–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Triki H, Charfi S, Bouzidi L, et al. CD155 expression in human breast cancer: clinical significance and relevance to natural killer cell infiltration. Life Sci. 2019;231:116543. [DOI] [PubMed] [Google Scholar]
- 244.Sun J, Tao H, Li X, et al. Clinical significance of novel costimulatory molecule B7-H6 in human breast cancer. Oncol Lett. 2017;14:2405–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Koh J, Lee SB, Park H, Lee HJ, Cho NH, Kim J Susceptibility of CD24(+) ovarian cancer cells to anti-cancer drugs and natural killer cells. Biochem Biophys Res Commun. 2012;427:373–378. [DOI] [PubMed] [Google Scholar]
- 246.El-Sherbiny YM, Meade JL, Holmes TD, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–8449. [DOI] [PubMed] [Google Scholar]
- 247.Fionda C, Soriani A, Zingoni A, Santoni A, Cippitelli M NKG2D and DNAM-1 ligands: molecular targets for NK cell-mediated immunotherapeutic intervention in multiple myeloma. Biomed Res Int. 2015;2015:178698. [DOI] [PMC free article] [PubMed] [Google Scholar]