Key Points
Question
Is spinal manipulative therapy associated with changes in the immune system?
Findings
In this systematic review of 13 studies comprising 795 participants, no clinical studies investigated the efficacy or effectiveness of spinal manipulative therapy in preventing or improving disease-specific outcomes among patients with infectious disease. Preliminary laboratory experiments indicated that spinal manipulative therapy may, in the short term, be associated with levels of change in immunological biomarkers among asymptomatic participants.
Meaning
These findings suggest that, given the limitations of the evidence, claims that spinal manipulative therapy is associated with changes in the immune system are premature and further clinical studies should be completed.
Abstract
Importance
Claims that spinal manipulative therapy (SMT) can improve immune function have increased substantially during the COVID-19 pandemic and may have contributed to the rapid spread of both accurate and inaccurate information (referred to as an infodemic by the World Health Organization).
Objective
To identify, appraise, and synthesize the scientific literature on the efficacy and effectiveness of SMT in preventing the development of infectious disease or improving disease-specific outcomes in patients with infectious disease and to examine the association between SMT and selected immunological, endocrine, and other physiological biomarkers.
Evidence Review
A literature search of MEDLINE, the Cumulative Index to Nursing and Allied Health Literature, the Index to Chiropractic Literature, the Cochrane Central Register of Controlled Trials, and Embase was conducted from inception to April 15, 2020. Randomized clinical trials and cohort studies were included. Eligible studies were critically appraised, and evidence with high and acceptable quality was synthesized using the Synthesis Without Meta-Analysis guideline.
Findings
A total of 2593 records were retrieved; after exclusions, 50 full-text articles were screened, and 16 articles reporting the findings of 13 studies comprising 795 participants were critically appraised. The literature search found no clinical studies that investigated the efficacy or effectiveness of SMT in preventing the development of infectious disease or improving disease-specific outcomes among patients with infectious disease. Eight articles reporting the results of 6 high- and acceptable-quality RCTs comprising 529 participants investigated the effect of SMT on biomarkers. Spinal manipulative therapy was not associated with changes in lymphocyte levels or physiological markers among patients with low back pain or participants who were asymptomatic compared with sham manipulation, a lecture series, and venipuncture control groups. Spinal manipulative therapy was associated with short-term changes in selected immunological biomarkers among asymptomatic participants compared with sham manipulation, a lecture series, and venipuncture control groups.
Conclusions and Relevance
In this systematic review of 13 studies, no clinical evidence was found to support or refute claims that SMT was efficacious or effective in changing immune system outcomes. Although there were limited preliminary data from basic scientific studies suggesting that SMT may be associated with short-term changes in immunological and endocrine biomarkers, the clinical relevance of these findings is unknown. Given the lack of evidence that SMT is associated with the prevention of infectious diseases or improvements in immune function, further studies should be completed before claims of efficacy or effectiveness are made.
This systematic review investigates the association of spinal manipulative therapy with the prevention of infectious disease, improvement of disease-specific outcomes, and changes in immune system function.
Introduction
At a time when the rapid spread of both accurate and inaccurate information (ie, infodemics, as referred to by the World Health Organization) has produced substantial concern for public health,1,2 claims that spinal manipulative therapy (SMT) can improve immune function have substantially increased with the onset of the COVID-19 pandemic, especially in Canada and the US.3,4,5 These claims were highlighted in a March 28, 2020, online report of the US-based International Chiropractors Association, which stated, “The observation that those who use chiropractic regularly and do not become ill with colds, flu, and other community shared illnesses is frequent within the profession and should not be ignored.”6 Such claims have a long history within the chiropractic profession.7,8 Proponents of these claims state that their position is informed by scientific evidence.6 However, the validity of these claims has been questioned.9
State chiropractic licensing boards in the US have provided limited and heterogenous guidance regarding chiropractic practice during the COVID-19 pandemic.10 In Canada, the College of Chiropractors of British Columbia, whose mission is to protect the public by regulating chiropractors to ensure safe, qualified, and ethical delivery of care,11 requested that we conduct an independent rapid review of the scientific literature to investigate the association of SMT with immunity. Therefore, we performed a systematic review of the literature to examine whether SMT was associated with efficacy and effectiveness in (1) preventing the development of infectious disease and (2) improving disease-specific health outcomes among patients with infectious disease. We also aimed to synthesize data from laboratory experiments to investigate the association between SMT and immunological, endocrine, and other physiological biomarkers.
Methods
We conducted our review using the methodology recommended by the World Health Organization12,13,14,15 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for systematic reviews.16 Ethics approval was not required, as this study was a systematic review. The review protocol was published in the Open Science Framework Registry on April 21, 202017 (eMethods 1 in the Supplement).
We systematically searched MEDLINE, the Cumulative Index to Nursing and Allied Health Literature, the Index to Chiropractic Literature, the Cochrane Central Register of Controlled Trials, and Embase from inception to April 15, 2020. Search terms consisted of subject headings specific to each database (eg, medical subject headings in MEDLINE, such as musculoskeletal manipulations, immunity, and communicable disease) and free-text words relevant to our study objectives and design (search strategy and specific search terms are available in eMethods 2 in the Supplement). Randomized clinical trials (RCTs) and cohort studies were included if they were published in English, included participants who were healthy or symptomatic, examined SMT that was provided by any health care professional, compared SMT with no intervention or other interventions, and measured clinical outcomes or changes in the levels of immunological, endocrine, and other physiological biomarkers.
Data including study characteristics, participant demographic characteristics, intervention characteristics, and outcome data were extracted. The lead author (N.C.) critically appraised the internal validity of relevant articles using the Scottish Intercollegiate Guidelines Network criteria for RCTs and cohort studies.18 These appraisals were verified by the senior authors, which included epidemiologists (C.C., J.D.C., and P.C.), a biostatistician (S.H.J.), and general scientists (S.M., J.T., and S.I.). Disagreements regarding study quality were resolved through discussion. We categorized RCTs by study phases, as described by Campbell et al,19 and we synthesized the evidence from high- and acceptable-quality studies based on the Synthesis Without Meta-analysis guideline.20 We restricted the synthesis to studies with high and acceptable quality because those with low and unacceptable quality were more likely to yield biased estimates of effect sizes.21,22,23,24,25
Results
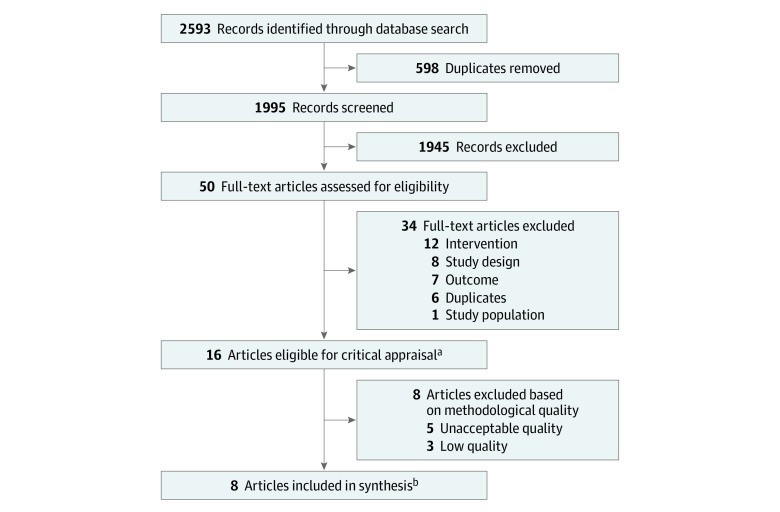
The initial database search retrieved 2593 records. After excluding 598 duplicates, 1995 titles and abstracts were screened (Figure). The eligibility of a random sample of 200 titles and abstracts (10.0%) was then evaluated by 2 independent reviewers (N.C. and S.M.) to determine the interreviewer agreement of the screening process, which was found to be 98.5%. The full text of 50 articles was assessed to confirm eligibility.26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69 Of those, 16 articles26,27,29,32,34,35,38,40,43,44,46,47,48,49,50,68 reporting the results of 13 studies comprising 795 participants met the inclusion criteria and were critically appraised.
Figure. Flow Diagram of Study Selection.
aThe 16 eligible articles reported findings from 13 studies.
bArticles included in the synthesis reported findings from 6 randomized clinical trials, with 3 of those articles reporting the results of 1 randomized clinical trial.26
Risk of Bias Within Studies
Of the 16 eligible articles, 1 article50 was rated as having high-quality evidence, and 7 articles26,27,29,44,46,49,68 were rated as having acceptable-quality evidence. Eight articles were rated as having low-quality38,43 or unacceptable-quality32,34,35,40,47,48 evidence; these articles were excluded from the evidence synthesis because of the high risk of bias. Studies with a high risk of bias had the following methodological limitations: (1) inadequate or unclear randomization (n = 5)34,38,43,47,48 and (2) inadequate or unclear allocation concealment (n = 3)32,35,40 (Table). We contacted the authors of 10 articles32,34,35,38,40,43,47,48,49,50 to inquire about their study methodology. Only 1 author responded and clarified that adequate methods were used for randomization, allocation concealment, and blinding.49,50 Therefore, 8 articles26,27,29,44,46,49,50,68 reporting the results of 6 high- or acceptable-quality RCTs comprising 529 participants were included in the evidence synthesis. These studies had various methodological limitations, including inadequate allocation concealment or blinding, dissimilar groups at baseline, and the use of outcomes with inadequate validity and reliability (Table).
Table. Risk of Bias.
| Source | Yes, no, or cannot be determined | Loss to follow-up | Quality of evidence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appropriate and clear research question | Adequate randomization | Adequate allocation concealment | Blinding of participants and investigators | Groups similar at start of study | Treatment is only difference between groups | Valid and reliable outcomes | Used intention-to-treat analysis | Results comparable between sites | Statistical analysis | Specimen collection | |||
| Brennan et al,50 1994 | Y | Y | Y | Y | Y | Y | Y | N | NA | Y | Y | 26.0% overall (unclear from which groups the participants withdrew) | High |
| Brennan et al,49 1991 | Y | Y | Y | Y | Y | Y | N | N | NA | Y | Y | 9.5% SMT; 21.0% soft tissue; 7.9% sham |
Acceptable |
| Mackawan et al,27 2007 | Y | Y | CBD | N | Y | Y | CBD | Y | NA | Y | Y | 0% TTM; 0% joint mobilization |
Acceptable |
| Puhl et al,68 2012 | Y | Y | N | N | CBD | Y | Y | NA | NA | Y | Y | 5.3% SMT; 14.3% sham |
Acceptable |
| Sampath et al,44 2017 | Y | Y | Y | CBD | N | Y | Y | Y | NA | Y | Y | 0% TS SMT; 0% sham |
Acceptable |
| Teodorczyk-Injeyan et al,26 2006 | Y | Y | N | Y | CBD | Y | Y | N | NA | CBD | Y | 0% SMT; 0% sham; 0% VC |
Acceptable |
| Teodorczyk-Injeyan et al,29 2008 | Y | Y | N | Y | CBD | Y | Y | N | NA | Y | Y | 3.3% SMT; 8.0% sham; 11.0% VC |
Acceptable |
| Teodorczyk-Injeyan et al,46 2010 | Y | Y | N | Y | CBD | Y | Y | N | NA | Y | Y | 10.0% SMT; 0% sham; 18.5% VC |
Acceptable |
| Molina-Ortega et al,38 2014 | Y | CBD | Y | CBD | CBD | Y | Y | Y | NA | Y | CBD | 0% CS SMT; 0% TS SMT; 0% control |
Low |
| Plaza-Manzano et al,43 2014 | Y | CBD | Y | N | N | CBD | Y | Y | NA | Y | Y | 0% CS SMT; 0% TS SMT; 0% control |
Low |
| Davison et al,48 2003 | Y | CBD | N | CBD | CBD | Y | CBD | Y | NA | N | Y | 0% SMT; 0% sham | Unacceptable |
| Degenhardt et al,35 2017 | Y | Y | N | CBD | CBD | Y | Y | N | NA | Y | CBD | 7.1% MT; 7.1% sham US; 0% no treatment |
Unacceptable |
| Licciardone et al,40 2012 | Y | Y | CBD | CBD | N | CBD | Y | CBD | NA | Y | N | Not reported | Unacceptable |
| Licciardone et al,32 2013 | Y | Y | CBD | CBD | CBD | CBD | Y | CBD | NA | Y | N | Not reported | Unacceptable |
| Selano et al,34 1994 | N | CBD | N | CBD | CBD | CBD | Y | N | NA | N | N | 55.0% overall (unclear from which groups the participants withdrew) | Unacceptable |
| Whelan et al,47 2002 | Y | CBD | N | CBD | CBD | CBD | Y | CBD | NA | N | N | Not reported | Unacceptable |
Abbreviations: CS, cervical spine; CBD, cannot be determined; MT, manual treatment; NA, not applicable; US, ultrasound; SMT, spinal manipulative therapy; TS, thoracic spine; TTM, traditional Thai massage; VC, venipuncture control group.
Study Characteristics
Among the 8 articles (6 RCTs)26,27,29,44,46,49,50,68 included in the evidence synthesis, 6 articles26,29,46,49,50,68 included SMT that was provided by chiropractors, and 2 articles27,44 included SMT that was provided by physiotherapists. Three RCTs26,29,46,49,50 evaluated the association between SMT and levels of immunological biomarkers (eTable 1 in the Supplement), and 3 RCTs27,44,68 examined the association between SMT and levels of endocrine and other physiological biomarkers (eTable 2 in the Supplement). Four RCTs26,29,44,46,49,68 included healthy and/or asymptomatic adults, while 2 RCTs27,50 included adult patients with low back pain. No studies reported on the incidence of infections, and no studies included children or patients with an infectious disease.
Evidence Synthesis
We did not identify any eligible RCTs or cohort studies that investigated the association between SMT and efficacy, effectiveness, or surveillance in the prevention of infectious disease. We also did not find any eligible RCTs or cohort studies that investigated the association between SMT and efficacy, effectiveness, or surveillance in the improvement of disease-specific outcomes among patients with infectious disease.
Spinal manipulative therapy was associated with immediate changes in the levels of selected immunological biomarkers (polymorphonuclear neutrophils,49 monocytes,49 tumor necrosis factor α,26 interleuken 1β,26 interleuken 2,29 immunoglobulin G,46 and immunoglobulin M46) in asymptomatic participants compared with sham manipulation and a lecture series49 and with venipuncture control groups.26,29,46 The duration of these changes and their physiological or clinical significance is unknown. However, SMT was not associated with changes in lymphocyte levels46,50 among patients with low back pain50 or participants who were asymptomatic46 compared with sham manipulation and a lecture series50 and with sham manipulation and venipuncture control groups (eTable 1 in the Supplement).46 With the exception of 1 study, which was designed solely to investigate associations between SMT and changes in lymphocyte subpopulations,50 the levels of biomarkers of interest were assessed in vitro26,29,46,49 (eTable 1 in the Supplement).
In all studies with the exception of one,44 SMT was not associated with levels of selected physiological markers (substance P,26,27,49 testosterone,44 testosterone to cortisol ratio,44 oxyhemoglobin,44 heart rate variability,44 norepinephrine,68 or epinephrine68), in the immediate term among patients with chronic low back pain27 or participants who were asymptomatic44 (eTable 1 and eTable 2 in the Supplement). However, 1 study44 reported that SMT was associated with changes in the level of salivary cortisol in the immediate term among asymptomatic participants compared with sham SMT (eTable 2 in the Supplement). The physiological or clinical significance of these changes and their duration is unknown.
Discussion
In this systematic review, no evidence from acceptable- or high-quality RCTs was found to support or refute the efficacy or effectiveness of SMT to prevent the development of infectious disease or to improve disease-specific outcomes among patients with infectious disease through its consequences for the immune system. Although 8 high- or acceptable-quality articles were identified that suggested SMT may be associated with immediate changes in immunological and endocrine biomarkers, these findings were preliminary and were mostly based on in vitro observations that appeared to be dependent on study methodology. Furthermore, the studies were conducted among asymptomatic participants or patients with low back pain, and their clinical relevance is unknown. It is important to note that all of the studies included in this systematic review were phase 0 (exploratory) studies, including those with a high risk of bias that were not included in the synthesis and therefore could not be used to assess the efficacy and effectiveness of SMT in preventing the development of infectious disease or improving disease-specific outcomes in patients with infectious disease.
Implications and Future Research
The findings from this review were based on phase 0 (exploratory) studies that included healthy participants or patients with low back pain but did not include patients with an infectious disease. None of the studies included in the review were phase 2 (biologic activity) studies, which would have established proof of concept that an intervention had any biologic activity. Although some of the studies in the present review appeared to indicate an association between SMT and selected immunological parameters, the clinical implications of SMT for the immune system are unknown.
Any intervention must be properly evaluated in clinical trials, including phase 2 (biologic activity) and phase 3 (efficacy or effectiveness) studies, before widespread use. The findings from several phase 0 (exploratory) studies suggested that there was a possible association between SMT and selected biomarkers. However, no clear and consistent associations across studies were identified. Moreover, none of the included studies considered the implications of SMT for immunity but instead explored the association between SMT and selected biomarkers associated with the human inflammatory response. If this area of research is to be prioritized, then the exploratory nature of our findings and the current available evidence-based interventions for the treatment of infectious diseases should be considered, and appropriately designed clinical trials should be conducted. However, given the current lack of clear and consistent data regarding the association between SMT and immune markers, it would be premature to conduct RCTs without high-quality evidence from future phase 0 clinical trials.
Strengths and Limitations
This study has several strengths. These strengths include adherence to the PRISMA checklist,16 establishment of a protocol before review completion and registration with the Open Science Framework Registry, formulation of a clear research question, use of a robust literature search strategy of 5 databases that was reviewed by 2 librarians, screening of interrater reliability comparison, critical appraisal of eligible studies, and inclusion of a review process that was conducted by senior scientists at each step of the rapid review. We also provided a full electronic search strategy, including limits used, for at least 1 database so that the search can be replicated.
This study also has limitations. First, we only included studies published in English and in peer-reviewed journals, which has the potential to introduce publication bias; however, most studies are published in English,70 and the exclusion of articles written in a language other than English would not be likely to produce bias, given that most clinical trials are published in the English-language literature.71,72,73,74,75 Furthermore, we consulted content experts (S.I., J.T.I., and J.D.C.) regarding their knowledge of other studies in the field to minimize publication bias. Second, screening, critical appraisal, and data extraction were conducted by 1 investigator rather than 2. However, we implemented a structured quality assurance methodology to minimize errors in the screening and selection of articles and data extraction.
Conclusions
No clinical evidence from high- or acceptable-quality RCTs was found to support or refute claims that SMT is efficacious or effective in preventing or improving infectious diseases. We found limited exploratory evidence from primarily in vitro studies that SMT may be associated with immediate changes in immunological and endocrine biomarkers among healthy participants or patients with low back pain. However, the clinical relevance of these findings is unknown, particularly among patients with infectious disease. Given the lack of evidence that SMT prevents infectious diseases or improves immune function, further studies are warranted before claims of efficacy or effectiveness can be made.
eMethods 1. Protocol and Registration, Eligibility Criteria, Information Sources, Study Selection, Risk of Bias in Individual Studies, Data Extraction, Data Items, Statistical Analysis, and Evidence Synthesis
eMethods 2. Search Strategies
eTable 1. Evidence Table for Immune Markers
eTable 2. Evidence Table for Endocrine and Other Physiological Markers
eReferences
References
- 1.World Health Organization . Managing the COVID-19 infodemic: promoting healthy behaviours and mitigating the harm from misinformation and disinformation. Updated September 23, 2020. Accessed November 19, 2020. https://www.who.int/news/item/23-09-2020-managing-the-covid-19-infodemic-promoting-healthy-behaviours-and-mitigating-the-harm-from-misinformation-and-disinformation
- 2.Naeem SB, Bhatti R. The COVID-19 ‘infodemic’: a new front for information professionals. Health Info Libr J. 2020;37(3):233-239. doi: 10.1111/hir.12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawchuk G, Hartvigsen J, Harsted S, Nim CG, Nyiro L. Misinformation about spinal manipulation and boosting immunity: an analysis of Twitter activity during the COVID-19 crisis. Chiropr Man Therap. 2020;28(1):34. doi: 10.1186/s12998-020-00319-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawchuk G, Hartvigsen J, Innes S, Simpson JK, Gushaty B. The use of internet analytics by a Canadian provincial chiropractic regulator to monitor, evaluate and remediate misleading claims regarding specific health conditions, pregnancy, and COVID-19. Chiropr Man Therap. 2020;28(1):24. doi: 10.1186/s12998-020-00314-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axen I, Bergstrom C, Bronson M, et al. Misinformation, chiropractic, and the COVID-19 pandemic. Chiropr Man Therap. 2020;28(1):65. doi: 10.1186/s12998-020-00353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Chiropractors Association . Immune function and chiropractic: what does the evidence provide? Revised March 28, 2020. Accessed November 19, 2020. https://www.chiropractic.org/immune-function-and-chiropractic-what-does-the-evidence-provide-revised/
- 7.Pero R. Medical researcher excited by CBSRF project results. Chiropr J. 1989;32. [Google Scholar]
- 8.Smith RK. One hundred thousand cases of influenza with a death rate of one-fortieth of that officially reported under conventional medical treatment. 1919. J Am Osteopath Assoc. 2000;100(5):320-323. [PubMed] [Google Scholar]
- 9.Côté P, Bussieres A, Cassidy JD, et al. A united statement of the global chiropractic research community against the pseudoscientific claim that chiropractic care boosts immunity. Chiropr Man Therap. 2020;28(1):21. doi: 10.1186/s12998-020-00312-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neff SM, Roecker CB, Okamoto CS, et al. Guidance concerning chiropractic practice in response to COVID-19 in the U.S.: a summary of state regulators’ web-based information. Chiropr Man Therap. 2020;28(1):44. doi: 10.1186/s12998-020-00333-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.College of Chiropractors of British Columbia . About the College of Chiropractors of BC. Accessed August 23, 2020. https://www.chirobc.com/about-ccbc/
- 12.Tricco AC, Antony J, Zarin W, et al. A scoping review of rapid review methods. BMC Med. 2015;13:224. doi: 10.1186/s12916-015-0465-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricco AC, Langlois EV, Straus SE, eds. Rapid reviews to strengthen health policy and systems: a practice guide. World Health Organization; 2017. Accessed April 6, 2020. https://apps.who.int/iris/bitstream/handle/10665/258698/9789241512763-eng.pdf?sequence=1 [Google Scholar]
- 14.Varker T, Forbes D, Dell L, et al. Rapid evidence assessment: increasing the transparency of an emerging methodology. J Eval Clin Pract. 2015;21(6):1199-1204. doi: 10.1111/jep.12405 [DOI] [PubMed] [Google Scholar]
- 15.Featherstone RM, Dryden DM, Foisy M, et al. Advancing knowledge of rapid reviews: an analysis of results, conclusions and recommendations from published review articles examining rapid reviews. Syst Rev. 2015;4:50. doi: 10.1186/s13643-015-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow N, Côté P, Cancelliere C, et al. Does spinal manipulative therapy impact the immune system? a rapid review of the literature. OSF Registries; 2020. Accessed April 21, 2020. https://osf.io/ux7wc
- 18.Scottish Intercollegiate Guidelines Network . Checklists. 2013. Accessed August 23, 2020. https://www.sign.ac.uk/what-we-do/methodology/checklists/
- 19.Campbell CM, Gilron I, Doshi T, Raja S. Designing and conducting proof-of-concept chronic pain analgesic clinical trials. Pain Rep. 2019;4(3):e697. doi: 10.1097/PR9.0000000000000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell M, McKenzie JE, Sowden A, et al. Synthesis Without Meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hewitt CE, Kumaravel B, Dumville JC, Torgerson DJ; Trial Attrition Study Group . Assessing the impact of attrition in randomized controlled trials. J Clin Epidemiol. 2010;63(11):1264-1270. doi: 10.1016/j.jclinepi.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 22.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135(11):982-989. doi: 10.7326/0003-4819-135-11-200112040-00010 [DOI] [PubMed] [Google Scholar]
- 23.Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, Gotzsche PC. Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol. 2007;36(4):847-857. doi: 10.1093/ije/dym087 [DOI] [PubMed] [Google Scholar]
- 24.Trowman R, Dumville JC, Torgerson DJ, Cranny G. The impact of trial baseline imbalances should be considered in systematic reviews: a methodological case study. J Clin Epidemiol. 2007;60(12):1229-1233. doi: 10.1016/j.jclinepi.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 25.Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601-605. doi: 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther. 2006;29(1):14-21. doi: 10.1016/j.jmpt.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 27.Mackawan S, Eungpinichpong W, Pantumethakul R, Chatchawan U, Hunsawong T, Arayawichanon P. Effects of traditional Thai massage versus joint mobilization on substance P and pain perception in patients with non-specific low back pain. J Bodyw Mov Ther. 2007;11(1):9-16. doi: 10.1016/j.jbmt.2005.11.001 [DOI] [Google Scholar]
- 28.Teodorczyk-Injeyan JA, McGregor M, Triano JJ, Injeyan SH. Elevated production of nociceptive CC chemokines and sE-selectin in patients with low back pain and the effects of spinal manipulation: a nonrandomized clinical trial. Clin J Pain. 2018;34(1):68-75. doi: 10.1097/AJP.0000000000000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teodorczyk-Injeyan JA, Injeyan HS, McGregor M, Harris GM, Ruegg R. Enhancement of in vitro interleukin-2 production in normal subjects following a single spinal manipulative treatment. Chiropr Osteopat. 2008;16:5. doi: 10.1186/1746-1340-16-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saggio G, Docimo S, Pilc J, Norton J, Gilliar W. Impact of osteopathic manipulative treatment on secretory immunoglobulin A levels in a stressed population. J Am Osteopath Assoc. 2011;111(3):143-147. [PubMed] [Google Scholar]
- 31.Roy RA, Boucher JP, Comtois AS. Inflammatory response following a short-term course of chiropractic treatment in subjects with and without chronic low back pain. J Chiropr Med. 2010;9(3):107-114. doi: 10.1016/j.jcm.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licciardone JC, Kearns CM, Hodge LM, Minotti DE. Osteopathic manual treatment in patients with diabetes mellitus and comorbid chronic low back pain: subgroup results from the OSTEOPATHIC trial. J Am Osteopath Assoc. 2013;113(6):468-478. [PubMed] [Google Scholar]
- 33.Noll DR, Degenhardt BF, Stuart MK, Werden S, McGovern RJ, Johnson JC. The effect of osteopathic manipulative treatment on immune response to the influenza vaccine in nursing homes residents: a pilot study. Altern Ther Health Med. 2004;10(4):74-76. [PubMed] [Google Scholar]
- 34.Selano JL, Hightower BC, Pfleger B, Collins KF, Grostic JD. The effects of specific upper cervical adjustments on the CD4 counts of HIV positive patients. Chiropr Res J. 1994;3(1):32-39. [Google Scholar]
- 35.Degenhardt BF, Johnson JC, Fossum C, Andicochea CT, Stuart MK. Changes in cytokines, sensory tests, and self-reported pain levels after manual treatment of low back pain. Clin Spine Surg. 2017;30(6):E690-E701. doi: 10.1097/BSD.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 36.Noll DR, Degenhardt BF, Johnson JC. Multicenter osteopathic pneumonia study in the elderly: subgroup analysis on hospital length of stay, ventilator-dependent respiratory failure rate, and in-hospital mortality rate. J Am Osteopath Assoc. 2016;116(9):574-587. doi: 10.7556/jaoa.2016.117 [DOI] [PubMed] [Google Scholar]
- 37.Achalandabaso A, Plaza-Manzano G, Lomas-Vega R, et al. Tissue damage markers after a spinal manipulation in healthy subjects: a preliminary report of a randomized controlled trial. Dis Markers. 2014;2014:815379. doi: 10.1155/2014/815379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molina-Ortega F, Lomas-Vega R, Hita-Contreras F, et al. Immediate effects of spinal manipulation on nitric oxide, substance P and pain perception. Man Ther. 2014;19(5):411-417. doi: 10.1016/j.math.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 39.Walkowski S, Singh M, Puertas J, Pate M, Goodrum K, Benencia F. Osteopathic manipulative therapy induces early plasma cytokine release and mobilization of a population of blood dendritic cells. PLoS One. 2014;9(3):e90132. doi: 10.1371/journal.pone.0090132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Licciardone JC, Kearns CM, Hodge LM, Bergamini MVW. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: results from the OSTEOPATHIC trial. J Am Osteopath Assoc. 2012;112(9):596-605. doi: 10.7556/jaoa.2012.112.9.596 [DOI] [PubMed] [Google Scholar]
- 41.Noll DR, Shores JH, Gamber RG, Herron KM, Swift J Jr. Benefits of osteopathic manipulative treatment for hospitalized elderly patients with pneumonia. J Am Osteopath Assoc. 2000;100(12):776-782. [PubMed] [Google Scholar]
- 42.Steele KM, Carreiro JE, Viola JH, Conte JA, Ridpath LC. Effect of osteopathic manipulative treatment on middle ear effusion following acute otitis media in young children: a pilot study. J Am Osteopath Assoc. 2014;114(6):436-447. doi: 10.7556/jaoa.2014.094 [DOI] [PubMed] [Google Scholar]
- 43.Plaza-Manzano G, Molina-Ortega F, Lomas-Vega R, Martínez-Amat A, Achalandabaso A, Hita-Contreras F. Changes in biochemical markers of pain perception and stress response after spinal manipulation. J Orthop Sports Phys Ther. 2014;44(4):231-239. doi: 10.2519/jospt.2014.4996 [DOI] [PubMed] [Google Scholar]
- 44.Sampath KK, Botnmark E, Mani R, et al. Neuroendocrine response following a thoracic spinal manipulation in healthy men. J Orthop Sports Phys Ther. 2017;47(9):617-627. doi: 10.2519/jospt.2017.7348 [DOI] [PubMed] [Google Scholar]
- 45.Newberry MA, Bowen GS, Trubey K, Fernandez MI. Can laypersons be trained to effectively deliver osteopathic manual therapy to patients with HIV? a pilot study. J Am Osteopath Assoc. 2011;111(5):325-330. [PubMed] [Google Scholar]
- 46.Teodorczyk-Injeyan JA, McGregor M, Ruegg R, Injeyan HS. Interleukin 2–regulated in vitro antibody production following a single spinal manipulative treatment in normal subjects. Chiropr Osteopat. 2010;18:26. doi: 10.1186/1746-1340-18-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whelan TL, Dishman JD, Burke J, Levine S, Sciotti V. The effect of chiropractic manipulation on salivary cortisol levels. J Manipulative Physiol Ther. 2002;25(3):149-153. doi: 10.1067/mmt.2002.122328 [DOI] [PubMed] [Google Scholar]
- 48.Davison S, Parkin-Smith GF. The possible effect of upper cervical chiropractic manipulation on short-term lymphocytic response: a pilot study. Eur J Chiropr. 2003;51:19-28. [Google Scholar]
- 49.Brennan PC, Kokjohn K, Kaltinger CJ, et al. Enhanced phagocytic cell respiratory burst induced by spinal manipulation: potential role of substance P. J Manipulative Physiol Ther. 1991;14(7):399-408. [PubMed] [Google Scholar]
- 50.Brennan PC, Graham MA, Triano JJ, Hondras MA, Anderson RJ. Lymphocyte profiles in patients with chronic low back pain enrolled in a clinical trial. J Manipulative Physiol Ther. 1994;17(4):219-227. [PubMed] [Google Scholar]
- 51.Belcastro MR, Backes CR, Chila AG. Bronchiolitis: a pilot study of osteopathic manipulative treatment, bronchodilators, and other therapy. J Am Osteopath Assoc. 1984;83(9):672-676. [PubMed] [Google Scholar]
- 52.Brennan PC, Triano JJ, McGregor M, Kokjohn K, Hondras MA, Brennan DC. Enhanced neutrophil respiratory burst as a biological marker for manipulation forces: duration of the effect and association with substance P and tumor necrosis factor. J Manipulative Physiol Ther. 1992;15(2):83-89. [PubMed] [Google Scholar]
- 53.Kline CA. Osteopathic manipulative therapy, antibiotics, and supportive therapy in respiratory infections in children: comparative study. J Am Osteopath Assoc. 1965;65(3):278-281. [PubMed] [Google Scholar]
- 54.Fernandez-Perez AM, Peralta-Ramirez MI, Pilat A, Moreno-Lorenzo C, Villaverde-Gutierrez C, Arroyo-Morales M. Can myofascial techniques modify immunological parameters? J Altern Complement Med. 2013;19(1):24-28. doi: 10.1089/acm.2011.0589 [DOI] [PubMed] [Google Scholar]
- 55.Noll DR. The short-term effect of a lymphatic pump protocol on blood cell counts in nursing home residents with limited mobility: a pilot study. J Am Osteopath Assoc. 2013;113(7):520-528. doi: 10.7556/jaoa.2013.003 [DOI] [PubMed] [Google Scholar]
- 56.Vander Have KL, Karmazyn B, Verma M, et al. Community-associated methicillin-resistant Staphylococcus aureus in acute musculoskeletal infection in children: a game changer. J Pediatr Orthop. 2009;29(8):927-931. doi: 10.1097/BPO.0b013e3181bd1e0c [DOI] [PubMed] [Google Scholar]
- 57.Ho AK, Shrader MW, Falk MN, Segal LS. Diagnosis and initial management of musculoskeletal coccidioidomycosis in children. J Pediatr Orthop. 2014;34(5):571-577. doi: 10.1097/BPO.0000000000000147 [DOI] [PubMed] [Google Scholar]
- 58.Noll DR, Degenhardt BF, Stuart M, McGovern R, Matteson M. Effectiveness of a sham protocol and adverse effects in a clinical trial of osteopathic manipulative treatment in nursing home patients. J Am Osteopath Assoc. 2004;104(3):107-113. [PubMed] [Google Scholar]
- 59.Goldstein M. Osteopathic manipulative treatment for pneumonia. Osteopath Med Prim Care. 2010;4(1):3. doi: 10.1186/1750-4732-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boone W, Oswald P, Holt K, Beck R, Singh K, Ashton A. Physical, physiological, and immune status changes, coupled with self-perceptions of health and quality of life, in subjects receiving chiropractic care: a pilot study. J Vertebral Subluxation Res. 2006:1-6. [Google Scholar]
- 61.Tuchin PJ. The effect of chiropractic spinal manipulative therapy on salivary cortisol levels. Australas Chiropr Osteopathy. 1998;7(2):86-92. [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell C, Kent C, Banne AF, Amiri A, Pero R. Surrogate indication of DNA repair in serum after long term chiropractic intervention—a retrospective study. J Vertebral Subluxation Res. 2005:1-5. [Google Scholar]
- 63.Henderson AT, Fisher JF, Blair J, Shea C, Li TS, Bridges KG. Effects of rib raising on the autonomic nervous system: a pilot study using noninvasive biomarkers. J Am Osteopath Assoc. 2010;110(6):324-330. [PubMed] [Google Scholar]
- 64.Lohman EB, Pacheco GR, Gharibvand L, et al. The immediate effects of cervical spine manipulation on pain and biochemical markers in females with acute non-specific mechanical neck pain: a randomized clinical trial. J Man Manip Ther. 2019;27(4):186-196. doi: 10.1080/10669817.2018.1553696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perrin RN, Edwards J, Hartley P. An evaluation of the effectiveness of osteopathic treatment on symptoms associated with myalgic encephalomyelitis. a preliminary report. J Med Eng Technol. 1998;22(1):1-13. doi: 10.3109/03091909809009993 [DOI] [PubMed] [Google Scholar]
- 66.Degenhardt BF, Darmani NA, Johnson JC, et al. Role of osteopathic manipulative treatment in altering pain biomarkers: a pilot study. J Am Osteopath Assoc. 2007;107(9):387-400. [PubMed] [Google Scholar]
- 67.Jackson KM, Steele TF, Dugan EP, Kukulka G, Blue W, Roberts A. Effect of lymphatic and splenic pump techniques on the antibody response to hepatitis B vaccine: a pilot study. J Am Osteopath Assoc. 1998;98(3):155-160. [PubMed] [Google Scholar]
- 68.Puhl AA, Injeyan HS. Short-term effects of manipulation to the upper thoracic spine of asymptomatic subjects on plasma concentrations of epinephrine and norepinephrine—a randomized and controlled observational study. J Manipulative Physiol Ther. 2012;35(3):209-215. doi: 10.1016/j.jmpt.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 69.Pacheco GR, Lohman EB. The effect of high-velocity low-amplitude thrust manipulation of the cervical spine on biomarkers related to stress and pain perception in females with mechanical neck pain. J Womens Health Phys Ther. 2018;42(1):38. doi: 10.1097/JWH.0000000000000091 [DOI] [Google Scholar]
- 70.Johnson AP, Sikich NJ, Evans G, et al. Health technology assessment: a comprehensive framework for evidence-based recommendations in Ontario. Int J Technol Assess Health Care. 2009;25(2):141-150. doi: 10.1017/S0266462309090199 [DOI] [PubMed] [Google Scholar]
- 71.Moher D, Fortin P, Jadad AR, et al. Completeness of reporting of trials published in languages other than English: implications for conduct and reporting of systematic reviews. Lancet. 1996;347(8998):363-366. doi: 10.1016/S0140-6736(96)90538-3 [DOI] [PubMed] [Google Scholar]
- 72.Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7(41):1-90. doi: 10.3310/hta7410 [DOI] [PubMed] [Google Scholar]
- 73.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320(7249):1574-1577. doi: 10.1136/bmj.320.7249.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31(1):115-123. doi: 10.1093/ije/31.1.115 [DOI] [PubMed] [Google Scholar]
- 75.Nussbaumer-Streit B, Klerings I, Dobrescu AI, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42-54. doi: 10.1016/j.jclinepi.2019.10.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Protocol and Registration, Eligibility Criteria, Information Sources, Study Selection, Risk of Bias in Individual Studies, Data Extraction, Data Items, Statistical Analysis, and Evidence Synthesis
eMethods 2. Search Strategies
eTable 1. Evidence Table for Immune Markers
eTable 2. Evidence Table for Endocrine and Other Physiological Markers
eReferences