Graphical abstract
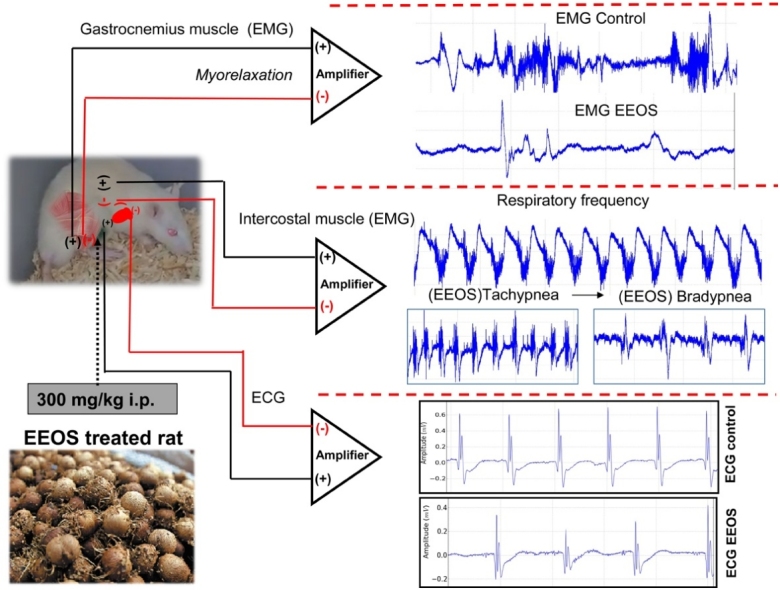
Abbreviations: EEOS, extract of E. oleracea stone; EMG, electromyographic; GABAA, γ-aminobutyric acid type A; CNS, Central Nervous System; mg GAE/g DE, milligrams of gallic acid equivalents per gram of dried extract; mg MRE/g DE, miligrams of myricetin-3-O-α-l-rhamnopyranoside equivalents per gram of dried extract; DMACA, p-dimethylaminocinnamaldehyde; mg CAE/g DE, milligrams of catechin equivalents per gram of dried extract; mg CE/g DE, milligrams of cyanidin equivalents per gram of dried extract; HPLC, High Performance Liquid Chromatography; ESI-IT-MS, Electrospray ionization Ion-Trap Mass spectrometry; EMGs, electromyographs; ACB, abdominal-costal breathing; DZP, diazepam; RC, Respiratory Control; RPR, Rhythmic and Profound Respiration; RD, respiratory depression
Keywords: Sedative, Myorelaxant, EMG, ECG, Euterpe oleracea
Highlights
-
•
Evaluation of EMG, ECG, and respiratory records of EEOS activity in rats.
-
•
EEOS showed sedative and muscle relaxant effects, which were similar to diazepam.
-
•
Bioactive compounds from EEOS indicate potential in pharmaceutical areas.
Abstract
The biological and pharmacological properties of natural polyphenols of the extract of Euterpe oleracea stone (EEOS) are associated with the central nervous system (CNS). To investigate the sedative and myorelaxant activity of EEOS in vivo, this study aimed to present the myorelaxant and sedative effects of EEOS in Wistar rats using spontaneous locomotor activity and motor electrophysiology. A total of 108 animals were used in the following experiments: a) behavioral tests (n = 27); b) electromyographic recordings of skeletal muscle (n = 27); c) respiratory muscle activity recordings (n = 27); d) cardiac muscle activity recordings (n = 27). The behavioral characteristics were measured according to the latency time of onset, the transient loss of posture reflex and maximum muscle relaxation. Electrodes were implanted in the gastrocnemius muscle and in the tenth intercostal space for electromyographic (EMG) signal capture to record muscle contraction, and in the D2 lead for electrocardiogram acquisition. After using the 300 mg/kg dose of EEOS intraperitoneally, a myorelaxant activity exhibited a lower frequency of contractility with an amplitude pattern of low and short duration at gastrocnemius muscle and intercostal muscle, which clearly describes a myorelaxant activity and changes in cardiac activity. The present report is so far the first study to demonstrate the myorelaxant activity of this extract, indicating an alternative route for açai stone valorization and its application in pharmaceutical fields.
1. Introduction
Different bioactive compounds have been investigated in recent decades as new therapeutic tools for myorelaxation and sedation with a focus on products that are naturally present in human food or folk medicine. The biodiversity in the Amazon region represents an important source for the development of new bioactive compounds [1].
The palm Euterpe oleracea Martius, family Arecaceae, known as açai palm, produces fruit at global production of 1.1 M tons in the Eastern Amazon basin (Brazil). The entire market is focused on the juice and derivatives prepared with pulp and water. The juice was extensively studied because of its richness in antioxidants, primarily phenolic compounds and tocopherols. Notably, the stone represents approximately 85 % of the drupe weight, and little is known about these stones. The hydroalcoholic or aqueous extract of açai stone [2] is characterized by phenolic acids, flavones, flavonols, and proanthocyanidins. The latter group consists exclusively of (epi)catechin units called procyanidins, which are the most abundant type of proanthocyanidins in plants. The oligomeric form, as tetramers, is the most abundant compound in açai stone extract, and procyanidin trimers are the most predominant. However, the bioactive compounds of extract of Euterpe oleracea stone (EEOS) in myorelaxant activity are not known.
Previous studies have demonstrated that the hydroalcoholic extract of açai stone, which is rich in polymeric forms of proanthocyanidins, exhibited important vasodilatory [3], antihypertensive [4], antioxidant [5], anti-inflammatory [6] and antinociceptive activities in experimental animals of acute and neuropathic pain [7].
Flavonoids are low molecular weight compounds that are present in all higher plants. More than 10,000 structurally distinct flavonoids have been described to date [8]. The biological and pharmacological properties of part of the phenolic compounds may occur via binding to γ-aminobutyric acid type A (GABAA) receptors in the central nervous system (CNS), which led to the hypothesis that these compounds act as benzodiazepine-like molecules. This hypothesis is supported by the behavioral effects of these compounds in animal models, such as myorelaxation, anxiety, sedation and convulsion [[9], [10], [11], [12]].
Benzodiazepines, cyclobenzaprine and thiocolchicoside are examples of myorelaxing drugs that can be used for various pathologies that involve pain due to persistent muscle tension [[13], [14], [15]], i.e., these agents induce muscle relaxation [14,16,17] which may be therapeutically desirable.
Açai stones are generally wasted as a byproduct of açai juice production. Still, they may possess an added value via the extraction of new bioactive compounds, which may be useful in food and pharmaceutical industries [2].
The present study is the first report to describe the myorelaxant and sedative effects of Euterpe oleracea stone in Wistar rats using spontaneous locomotor activity, motor and cardiac electrophysiology and provide further information on the bioactive compounds in this extract.
2. Materials and methods
2.1. Animals and ethical aspects
Male Wistar rats (160–200 g) of ten weeks were maintained in a controlled environment (25 ± 2 °C; 12 h light/dark cycle) with access to food and water ad libitum. A total of 108 animals were used, divided into four experiments: a) behavioral tests (n = 27); b) electromyographic recordings (n = 27); c) Electromyography of the intercostal musculature (n = 27) and d) Electrocardiogram (n = 27). All experimental procedures were approved by the Committee for Ethics in Experimental Research with Animals of Universidade Federal do Pará (Brazil; license n° 1089220518) and followed the guidelines suggested by the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to reduce the number of animals and minimize animal suffering.
2.2. Preparation of the extract of Euterpe oleracea stone
Euterpe oleracea fruits were obtained from a local market (Belém, Pará, Brazil). The State of Pará is the largest national producer of açai, producing 112,676 tons/year of the fruit with 1,439,249 tons in 2018 [18]. Of this total, 93,521 (83 %) tons/year is residue (stones). The residue of commercial juice was used to obtain the extract in our model. Açai stones (100 g) were washed with water and dried for 24 h at 105 °C. Stones were ground using a hammer mill shredder to a granular consistency (16 mesh). The extraction was made with 200 mL ethanol, shaken for 2 h, and stored in dark bottles at 4 °C for 10 days. After the maceration period, the ethanolic extract of pulverized açai stones was filtered through Whatman paper n°1 filter and rotoevaporated under low pressure at 40 °C. The dried extract was frozen at -20 °C until use. The average extract production yield was 300 mg dried extract from 35 mL ethanolic extract.
2.3. Determination of phenolic compounds of EEOS
The total phenolic content was determined using the Folin-Ciocalteu colorimetric method [19] and expressed as milligrams of gallic acid equivalents per gram of dried extract (mg GAE/g DE) [20].
Flavonols were quantified using aluminum chloride assay as described by [21]. The results are expressed as mg myricetin-3-O-α-l-rhamnopyranoside equivalents (mg MRE/g DE).
Flavanol content was determined using p-dimethylaminocinnamaldehyde (DMACA) according to the protocol proposed by Delcour and Varebeke [22]. The results are expressed as mg catechin equivalents (mg CAE/g DE).
Proanthocyanidins (condensed tannins) were measured at 555 nm after hydrolysis with n-butanol/HCl (2 h, 90 °C) according to [23]. The results are expressed as mg cyanidin equivalents (mg CE/g DE).
2.4. Chromatographic analysis (LC-ESI-IT-MS/MS)
The extract was analyzed using a Shimadzu Prominence liquid chromatography system with two Shimadzu LC-20AD automatic injector (SIL-20A HT) pumps. A C18 Phenomenex Gemini (250 × 4.6 mm – 5 μm) column was used in the analyses. The mobile phase was acidified ultrapure water (0.1 % HCOOH) and HPLC grade methanol, also acidified (0.1 % HCOOH), at a flow rate of 1.0 mL/min, with the methanol gradient increasing as follows: 0 % methanol in 0–5 min; 0 %–10 % methanol in 5−40 min; 10 %–14.5 % methanol in 40−45 min; 19 %–55 % methanol in 55−65 min and 55 %–80 % methanol in 65−66 min. The injection volume was 20.0 μL. The LC was coupled to a mass spectrometer (Amazon X, Bruker, Massachusetts, USA) equipped with electrospray ionization (ESI) and an ion-trap (IT) type analyzer in negative mode, under the following conditions: 5 kV capillary voltage, capillary temperature 325 °C, entrainment gas (N2) flow 12 L/min, nitrogen nebulizer pressure at 10 psi. The acquisition range was m/z 100–1500, with two or more events. The chemical composition were identified based on the molecule fragmentation pattern and compared with mass spectral data available in Mass Bank database.
2.5. Behavioral characterization of animals after administration of EEOS
The dried açai stone extract was solubilized with distilled water (100 mg/mL) and filtered through a 0.22-μm PVDF filter before injection. Three groups of animals were used for this experiment (n = 9 for each group). A previous study [6] used 300 mg/kg per animal. Therefore, we evaluated the effects of 200 (n = 9), 300(n = 9) and 400 (n = 9) mg/kg intraperitoneal doses (for each group) of EEOS according to the latency time of onset. The behavioral characteristics measured were the transient loss of posture reflex and maximum muscle relaxation.
2.6. Electromyographic recordings (EMG)
Conjugated electrodes were implanted in the gastrocnemius muscle for electromyographic (EMG) signal capture, and each electrode was 0.01 mm in diameter. The electrodes were connected to a data acquisition system, which was composed of a high impedance amplifier (Grass Technologies, P511) and monitored using an oscilloscope (Protek, 6510). The data were scanned continuously at a rate of 1 KHz by a computer equipped with a data acquisition board (National Instruments, Austin, TX). Data were stored on a hard disk and processed using specialized software (LabVIEW express). The entire experiment was performed inside a Faraday cage.
The control group (n = 9) received the volume equally at the dose of 0.9 % saline solution i.p., the EEOS group was injected at 300 mg/kg intraperitoneally, and EMG recordings were obtained for 5 min for each animal (n = 9). A positive control group of 5 mg/kg diazepam was used for comparison (n = 9). Conjugated electrodes were also positioned in the tenth intercostal space to record muscle contraction during observed respiratory movement characteristics: Control (n = 9), EEOS (n = 9) and DZP (n = 9).
2.7. Electrocardiographic recordings (ECG)
The electrodes were inserted following the vector in lead D2, as described by [24]. After administration of 300 mg/kg of EEOS i.p. a total time of 10 min according to the latency time of onset, then the records were made at lasted 5 min for each animal, following analyzes of: Amplitude (mV), Heart rate (bpm), RR interval, PQ interval, QT interval and QRS duration.
2.8. Electrophysiological data analysis
A tool was built to analyze the acquired signals using Python language version 2.7. The Numpy and Scipy libraries were used for mathematical processing, and the Matplolib library was used for graphics. The graphical interface was developed using the PyQt4 library [25].
2.9. Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 8.0). Parametric data from electromyographs (EMGs) were analyzed using ANOVA followed by Tukey’s post hoc test, and the results are expressed as the means ± standard error of the mean (S.E.M). Differences in analyses were considered significant for p values <0.05.
3. Results
3.1. Bioactive compounds of the extract of Euterpe oleracea stone
The content of total polyphenols in 1 g dried EEOS was 25.12 mg GAE/g DE as evaluated using the Folin-Ciocalteu method [23]. The contents of flavanols, flavonols and condensed tannins were 9.048 mg CAE, 0.258 mg MRE, and 9.798 mg CE per g DE, respectively.
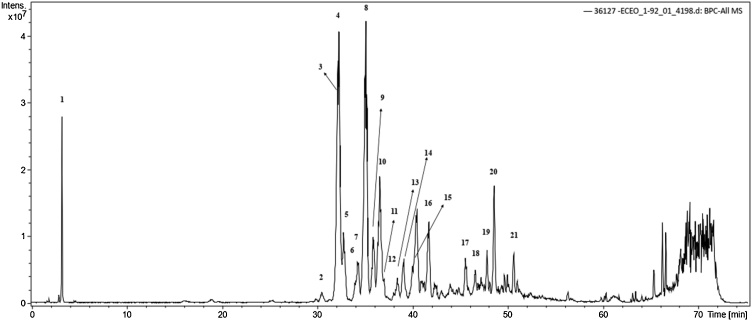
HPLC analysis showed high levels of caffeic acid, procyanidin, catechin as the major compounds, followed by polymeric proanthocyanidins, cinnamtannin and other phenolic compounds (Fig. 1 and Table 1). The chemical characterization identified by molecule fragmentation pattern compared with mass spectral data showed 21 major compounds (Supplementary Figs. S1–S21).
Fig. 1.
Negative ion HPLC/ESI-IT-MS analysis of compounds present in extract of Euterpe oleracea stone.
Table 1.
Compounds obtained after chemical characterization of extract of Euterpe oleracea stone analyzed by HPLC-ESI-IT/MS.
| ID | Retention Time (min) | [M-H]− | MSna | Compound |
|---|---|---|---|---|
| 1 | 3.3 | 683 | 341; 179 | Caffeic acid hexoxide dimer |
| 2 | 30.5 | 879 | 865; 695; 577; 407; 287 | 2-Procyanidin dimer digallate (A-type) |
| 3 | 32.0 | 577 | 425; 289 | Proanthocyanidin B2 |
| 4 | 32.0 | 1155 | 577; 425; 289 | Procyanidin tetramer |
| 5 | 32.8 | 1153 | 983; 863; 739; 575; 499; 289 | Procyanidin tetramer-B-type |
| 6 | 34.0 | 865 | 695; 577; 407; 289 | Procyanidin trimer |
| 7 | 34.3 | 579 | 289 | Cathechin dimer |
| 8 | 35.1 | 865 | 847; 695; 577; 432; 289 | Procyanidin trimer |
| 9 | 35.9 | 865 | 727; 695; 575; 287 | Procyanidin trimer |
| 10 | 36.6 | 1153 | 1027; 983; 863; 739; 576; 289 | Procyanidin tetramer-B-type |
| 11 | 36.8 | 865 | 695; 577; 289 | Procyanidin trimer |
| 12 | 38.2 | 720 | 575; 289 | Procyanidin trimer-B-type C-glycosylated |
| 13 | 38.4 | 865 | 720; 695; 577; 287 | Procyanidin trimer-B-type |
| 14 | 39.1 | 865 | 577; 287 | Procyanidin trimer-B-type C-glycosylated |
| 15 | 40.4 | 720 | 635; 576; 287 | Procyanidin trimer-B-type C-glycosylated |
| 16 | 41.8 | 727 | 642; 577; 287 | Procyanidin trimer-B-type O-gallate |
| 17 | 42.4 | 720 | 865; 635; 575; 287 | Procyanidin trimer-B-type C-glycosylated |
| 18 | 46.5 | 865 | 720; 575; 289 | Procyanidin trimer |
| 19 | 47.8 | 865 | 720; 575; 289 | Procyanidin trimer |
| 20 | 48.6 | 865 | 720; 451; 289 | Procyanidin trimer |
| 21 | 50.6 | 865 | 577; 425; 289 | Procyanidin trimer |
Fragment ions.
3.2. Intraperitoneal administration of EEOS alters spontaneous locomotor activity
IP administrations of 200, 300 and 400 mg of EEOS/kg produced abdominal writhing in all animals, which was characterized by momentary pain after the injection, followed by decreased motility, myorelaxation, animal somnolence, and deep and rhythmic breathing. An increased time of drug exposure accentuated respiratory depression as characterized by abdominal-costal breathing. All doses tested were effective based on the appearance of the behavioral characteristic of respiratory depression, as indicated in Table 2. A transient loss of posture reflex behavior was observed at 300 and 400 mg/kg, and a dose-dependent effect was observed on duration (Table 2). A dose-dependent effect on behavior was observed. Animals that received lower doses recovered normal box-field exploration more quickly than animals that received higher doses. The transient loss of posture reflex was the choice for minimal dose-effect, i.e., 300 mg/kg with a latency time of 842.10 ± 46.39 s. There was no effect of transient loss of posture reflex following oral administration in rats. In contrast, the intraperitoneal administration produced sedative effects and prolonged the elimination phase.
Table 2.
Latency in seconds in behavioral alterations after EEOS administration at different doses for the evaluation of myorelaxation.
| Doses (mg/kg) | Decreased Motility | Myorelaxation | Deep and rhythmic breathing | Somnolence | Respiratory depression (ACB*) | Transient loss of posture reflex | Return |
|---|---|---|---|---|---|---|---|
| 200 | 365.7 ± 27.59 | 413.7 ± 18.30 | 454.6 ± 11.52 | 501.6 ± 11.30 | 788.6 ± 90.58 | – | 3047 ± 306.1 |
| 300 | 218.4 ± 15.27 | 275.2 ± 10.59 | 312.1 ± 24.76 | 344.0 ± 23.93 | 645.0 ± 50.56 | 842.1 ± 46.39 | 4404 ± 276.0 |
| 400 | 133.0 ± 16.01 | 151.0 ± 15.94 | 176.9 ± 11.85 | 209.4 ± 20.20 | 253.9 ± 19.13 | 384.1 ± 34.70 | 8596 ± 600.2 |
| DZP (5 mg/kg i.p.) | 54.89 ± 11.16 | 104.9 ± 11.69 | – | 170.6 ± 11.42 | 333.3 ± 17.11 | 398.9 ± 16.30 | 10,959 ± 193.9 |
ACB: Abdominal-costal breathing.
Our behavioral evaluation and electrical recordings were useful and objective tools to verify alterations in neuronal excitability in control and EEOS-treated rats.
3.3. Intraperitoneal administration of EEOS caused myorelaxation and respiratory depression
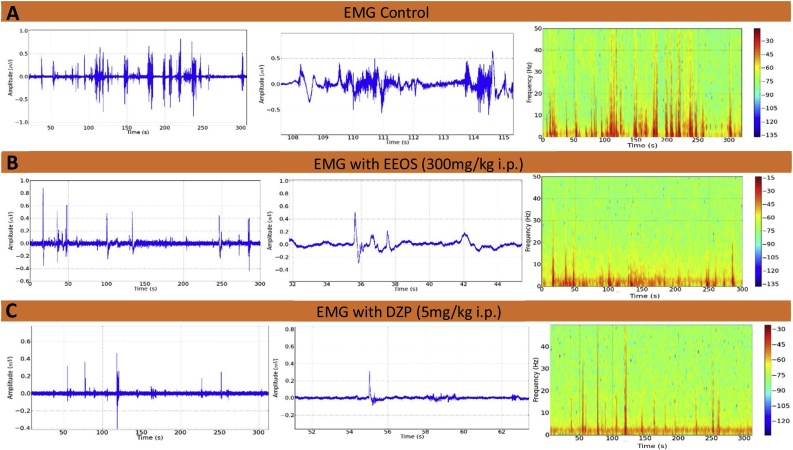
The electromyographic recordings showed contraction of the gastrocnemius muscle in control animals, with a higher frequency and duration of contractions, which can be seen in the spectrogram of Fig. 2A. Lower frequencies of contractility were observed in animals that received EEOS (Fig. 2B) and diazepam (Fig. 2C). The contractions exhibited low amplitudes and short contraction durations, which clearly indicate myorelaxant activity in the gastrocnemius muscle.
Fig. 2.
Electromyogram (EMG) of gastrocnemius muscle contraction. Records of control animals (A), after 300 mg/kg EEOS and (B), after 5 mg/kg diazepam (DZP) (C) (n = 9).
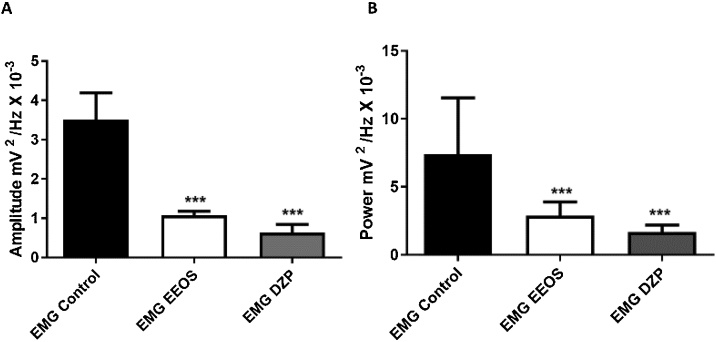
The amplitude graph accumulated up to 50 Hz in Fig. 3A shows a difference in amplitude between the contractions of the gastrocnemius muscle between the groups. EMG exhibited average amplitudes of 3.506 ± 0.6895, 1.073 ± 0.1033, and 0.6305 ± 0.2076 mV2/Hz x 10−3 for the control, EEOS and diazepam groups, respectively (Fig. 3A). A statistically significant difference between the EEOS and DZP groups was observed compared to the control group [F(2, 24) = 122.3; P < 0.0001].
Fig. 3.
Histograms comparing the accumulated amplitudes in frequencies up to 50 Hz of electromyograms (EMG) in a linear manner for the control, extract of Euterpe oleracea stone (EEOS) and diazepam (DZP) groups; averages and standard deviation of amplitudes over time (A) and power of the strongest and prolonged contraction registers (B). (***) Statistical difference for the control group; after ANOVA followed by Tukey’s test (p < 0.05, n = 9).
The longest and largest amplitude contractions recorded were 7.406 ± 4.134 mV2/Hz x 10−3 for the control electromyograms and 2.886 ± 1.003 mV2 /Hz x 10-3 for EEOS-treated rats during average contraction power, which were significantly different from the control group [F(2, 24) = 13.41; P = 0.0001]. The diazepam group (1.676 ± 0.5216 mV2/Hz x 10−3) was not significantly different from the EEOS group (Fig. 3B).
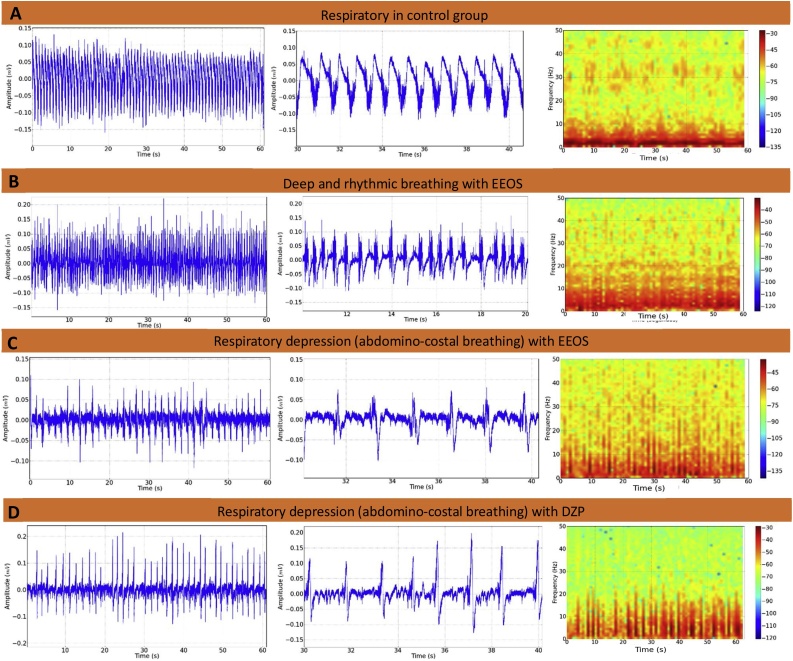
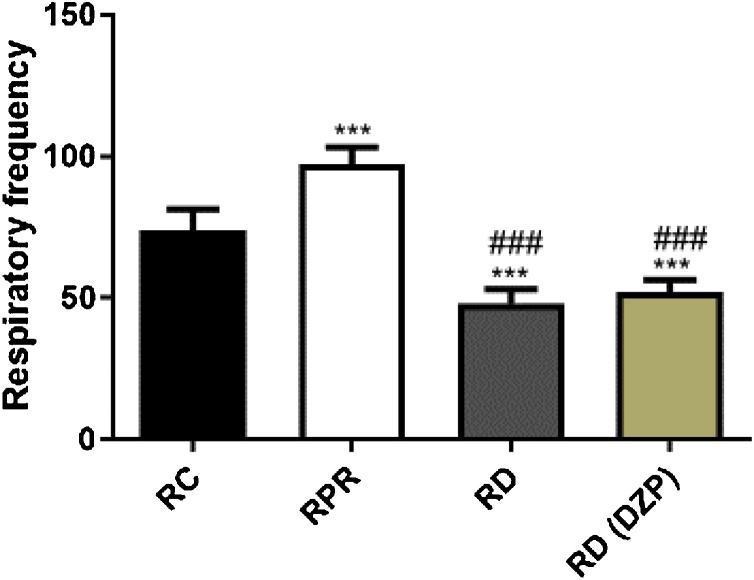
The intercostal muscle EMG recordings demonstrated that the amplitude of regular traces indicated normal respiration, and the normal respiratory rate was 73.78 ± 7.53 contractions per min (Fig. 4A). The latency for the appearance of the behavioral characteristic of deep and rhythmic respiratory rate was 312.10 ± 24.76 s. The animals exhibited a significant increase (P < 0.001) in the frequency of contraction of the intercostal muscle during this phase, with an average of 97.22 ± 5.99 contractions per min (Fig. 4B). The onset of respiratory depression occurred with a latency of 645.70 ± 50.56 s and an average of 48 ± 5.0 contractions per min, which were significantly different compared to the control group during deep and rhythmic breathing behavior (Fig. 4C and D) [F(3, 32) = 135.5; P < 0.0001].
Fig. 4.
Electromyograms of the tenth intercostal space. The intercostal muscle contraction records demonstrate the frequency of contraction in the control animals (A), after extract of Euterpe oleracea stone (EEOS) administration, which shows deep and rhythmic breathing (B), and an electromyogram of the intercostal muscle of an animal exhibiting respiratory depression (C) (B and C are sequences of observed behaviors), contraction of the intercostal muscle of the group that received DZP (D). (n = 9).
This method produces minimal damage and is sufficiently sensitive to identify differences in muscle contractions (characterized by increased frequencies and/or amplitude of waves) that cannot be quantified solely with behavioral observation.
A characteristic behavioral trait of a deep and rhythmic respiratory rate in EEOS-treated rats may present depressed activity of the CNS during intercostals muscle contraction (Fig. 5).
Fig. 5.
Respiratory rates recorded by intercostal muscle contractions in the control group (RC), animals exhibiting rhythmic and profound respiration (RPR) and respiratory depression (RD) treated by EEOS and respiratory depression treated by DZP. (***) Statistical difference for the control group; (###) Statistical difference for the RPR group, after ANOVA followed by Tukey’s test (p < .05, n = 9).
3.4. Intraperitoneal administration of EEOS caused changes in the electrocardiographic tracing
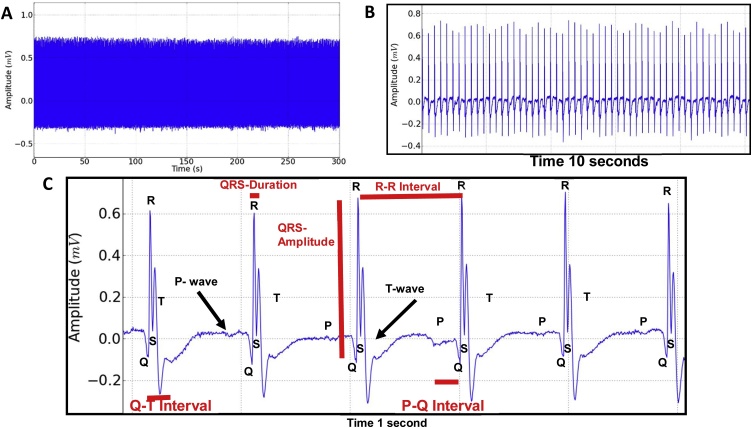
The rat ECG record (lead D2) demonstrated an intense activity where all deflagrations were observed, the P- wave, QRS complex and T-wave (Fig. 6). In the expansion of the record, the evaluation data is shown: Amplitude (mV), Heart rate (BPM), R-R interval (s), QRS duration (s), Q-T interval (s) and P-Q interval (s). These parameters demonstrated cardiac functionality for the control group (Fig. 6C) with subsequent comparison with the EEOS (Fig. 7C) and DZP (Fig. 7F) groups.
Fig. 6.
Electrocardiographic record (ECG) of the rat lasting 5 min (A), enlarged electrocardiogram with 10-second tracing (B); Magnification of 1 s demonstrating the components of the ECG: P- wave, QRS Complex and T- wave and data evaluated during the tracing: Heart rate (BPM), QRS amplitude (mV), R-R interval (s), QRS duration (s), Q-T interval (s) and P-Q interval (s) (C).
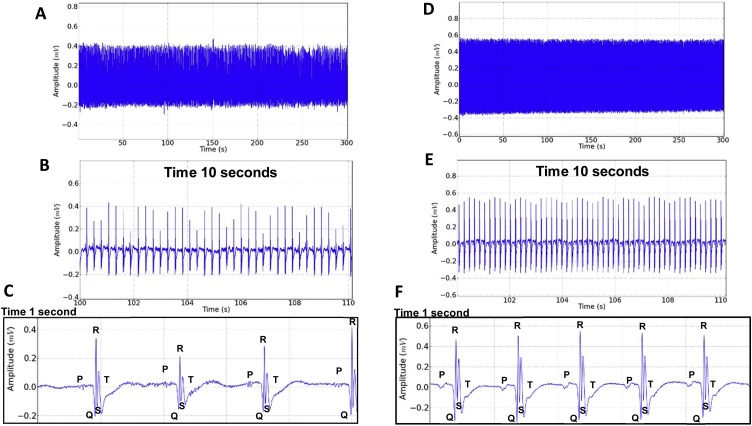
Fig. 7.
Electrocardiographic record (ECG) of the rat after administration of 300 mg/kg i.p. of EEOS, traced for 5 min (A), Expansion of the record in 10 s showing variation in the amplitude of the QRS complex after using the EEOS (B), one second magnification of the trace showing the ECG components (C). Electrocardiographic record (ECG) of the rat after administration of 5 mg/kg i.p. Diazepam, lasting 5 min (D); Expansion of the record showing 10 s (E); one second magnification of the record showing the EEG deflagrations (F).
The electrocardiographic record (lead D2) after the administration of EEOS, represented in the tracing of Fig. 7A, showed irregularities in the outbreaks that can be better observed in Fig. 7 B and C. However, the sinus rhythm with variation in the amplitude of the QRS heart rate that indicates important changes for the analysis of the drug’s action on cardiac function.
After applying the DZP, little variation occurred (lead D2), maintaining sinus rhythm and normal frequency (Fig. 7D–F).
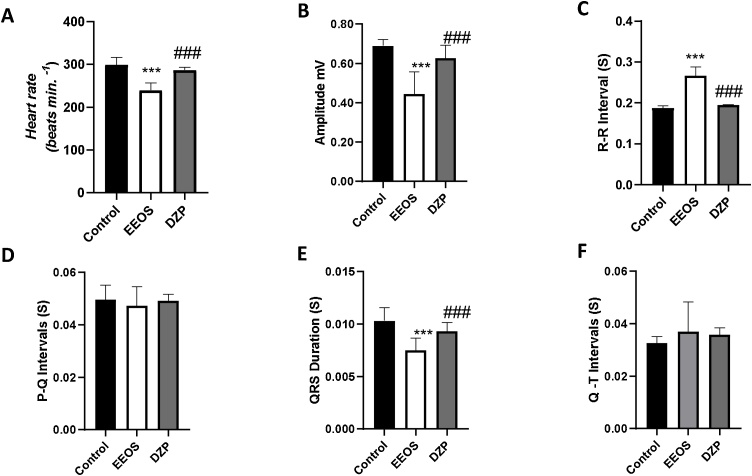
The analysis of the electrocardiographic data showed that the administration of EEOS caused a decrease in heart rate. The mean heart rate for the control group was 298.7 ± 18.28 (BPM), there was no statistical difference for the DZP group (286.7 ± 6708 BPM), these groups maintained statistical difference for the EEOS group with an average of 239.6 ± 17.60 (BPM) [F (2, 24) = 38.27 P < 0.0001] (Fig. 8A).
Fig. 8.
Comparison between the average heart rate (BPM) recorded in the control group, EEOS and DZP (A); Evaluation of the means of amplitude (mV) of the electrocardiographic records of the control, EEOS and DZP groups (B); evaluation between the means of R-R interval (s), registered in the control group, EEOS and DZP (C); evaluation of the P-Q interval means (s) of the electrocardiographic records (D); Evaluation of the mean duration of the QRS complex (s) in the electrocardiographic records (E); Evaluation of the average of Q-T intervals (s) (F). (***) Indicates statistical difference for the control; (###) indicates statistical difference for the EEOS group [ANOVA and Tukey’s test (P < 0.05, n = 9).
The amplitude of the electrocardiographic record was changed by the administration of the EEOS (0.4449 ± 0.1130 mV) and maintained a statistical difference for the control groups (0.6897 ± 0.03157 mV) and treated with DZP (0.6268 ± 0.06540 mV) [F (2, 24) = 24.18 P < 0.0001] (Fig. 8B).
The evaluation of R-R intervals showed an increase of the intervals and higher irregularity in the time of appearance of the R wave in the group treated with EEOS (0.2669 ± 0.02125s). It showed a statistical difference for the group treated with DZP (0.1947 ± 0.001349s) and the control group (0.1876 ± 0.005841 s) [F (2, 24) = 106.5 P < 0.0001] (Fig. 8C).
For the PQ interval that demonstrates atrial depolarization and the first deflagration of the QRS complex, there were no statistical differences between the groups, presenting means for the control group (0.04963 ± 0.007285 s), EEOS (0.04728 ± 0.007285 s) and DZP (0.04911 ± 0.002472s) [F (2, 24) = 0.4627, P = 0.6351] (Fig. 8D).
QRS duration indicates the speed of ventricular contraction, this parameter after EEOS administration decreased, demonstrating that the QRS complex happens more quickly. Thus, after applying the EEOS, the average was 0.007511 ± 0.001144s, showing statistical difference for the control group (0.01032 ± 0.001242s) and DZP (0.009333 ± 0.0008261 s) [F (2, 24) = 15.50, P < 0.0001] (Fig. 8E).
The cycle of ventricular activity observed at the time of depolarization represented by the QRS complex and repolarization with the T- wave, demonstrates the time it takes the heart to depolarize and repolarize. After analyzing the Q-T interval, there was no statistical difference between the groups, but there was a wide variation in the interval means for the EEOS group (0.0370 ± 0.01130s), control (0.03262 ± 0.002382s) and DZP (0.03582 ± 0.002566s) [F (2.24) = 0.9897, P = 0.3864] (Fig. 8F).
4. Discussion
Despite the importance of bioprospection of EEOS, many in vivo aspects after injection are poorly understood. The present study showed that EEOS exerted sedative and myorelaxation effects in rats, demonstrated by behavioral evaluation and electromyographic records.
Extracts of E. oleracea stone have been shown to have antioxidant and anti-inflammatory properties [3,5]. The antioxidant capacity of açai stones seems to be associated with an anticonvulsant action because of high phenolic compound contents, such as flavones, proanthocyanidins (procyanidins), which are the main bioactive compounds in açai stone extracts [6,2]. More attention must be paid to the nature and abundance of proanthocyanidins to understand their absorption, metabolism, circulating forms and bioactivity at target sites. For instance, previous studies associated neuro- and nefroprotective effect of grape seed proanthocyanidin extract against thalidomide and carboplatin (chemotherapeutic agents) through modulation of inflammation and minimization of oxidative stress [26], and gastroprotective activity in stomach ulcer induced by indomethacin with Byrsonima sericea extract [27].
Polyphenolic compounds, such as flavonoids, e.g., rutin and quercetin, produce anti-inflammatory [28,29], analgesic [7], and antioxidant [5] effects. Other flavonoids may impact animal models of sedation and myorelaxation. For example, Azevedo et al. [30] showed that rutin and quercetin prevented mechanical and thermal nociceptive-induced responses in oxaliplatin-induced neuropathic pain in rats via mediation of oxidative stress-induced damage. Other bioactive drugs that include flavonoid glycosides, such as rutin, quercitrin, isoquercitrin, goodyerin, and quercetin-3-O-(6″-feruloyl)-β-d-galactopyranoside, produced a depressant action on the CNS of rats following systemic administration [31].
An early study [32] investigated the acute intraperitoneal administration of a proanthocyanidin-rich fraction isolated from the bark of Croton celtidifolius (Euphorbiaceae). The neurobehavioral effects in rats included decreased spontaneous locomotor activity because of the putative existence of hypnosedative, anticonvulsant and anxiolytic compounds.
The myorelaxant effects of plant extracts are explained based on their anxiolytic and sedative properties, the influence on neuroreceptors, and the modulation of neurotropic factors [12]. Ligand binding to the alpha-2 agonist, glycine, GABAA receptor complex exerts numerous pharmacological actions, such as muscle relaxation, sedation [17,33,34]. Chloride ion flux conductance through this channel inhibits the firing of new action potentials, which attenuate the inhibition that reduces changes in membrane excitability, as well as the decrease in noradrenergic conduction at the locus coeruleus causes myorelaxation [11,34].
The present study showed differences in EEOS-treated rats with respect to deep and rhythmic breathing behaviors that were not described previously. Many anesthetic drugs enhance opioid effects on respiratory depression. Drugs that are considered respiratory depressants, such as propofol, sevoflurane, and midazolam, are agonists of GABA receptors and antagonists of NMDA receptors and exhibit additive or synergistic effects on respiration when combined with opioids [35].
Respiratory depression is associated with many physiological effects, including pain, stress response, appetite, and thermoregulation, which are modulated by endogenous opioid receptors of CNS. Ligand binding to opioid receptors activates inhibitory intracellular pathways that lead to the closing of voltage-sensitive calcium channels, the stimulation of potassium efflux, and a reduction in cyclic adenosine monophosphate (cAMP) production. These intracellular changes lead to reduced neuronal excitability [35].
The differential effects on breathing control rhythm are suggestive of numerous potential therapeutic targets with different neuronal functions. Our results showed strong sedative and muscle relaxant effects. The response induced by the EEOS suggests further investigation to elucidate the underlying mechanisms that trigger myorelaxation after its administration, suggesting several routes of action such as: agonist activity for GABA, Glycine, alpha-2 adrenergic receptors [17,33,34,36,37]. The molecular mechanism to the myorelaxant effects of EEOS is unknown. Further studies are needed to fully answer this question.
These results are more remarkable when we compared the new therapeutic tool with a classical drug, such as benzodiazepines and Alpha 2 agonist [38]. The sedative and myorelaxant effect of EEOS is particularly important considering the amount of stone produced in the açai chain of production. Therefore, the residues abundant in açai, which is a common palm that exhibits widespread distribution in the Amazon, may be an essential finding for further research on biotherapeutic resources that are abundantly accessible but neglected.
ECG recording can check drug effects on the heart through electrical signal changes, providing important insights into functional and structural characteristics [24,39]. In the current study, the EEOS caused changes in the rats’ heart rate, in their amplitudes and the wave intervals.
In rodents, most studies use a limb II shunt that is sufficient for the general analysis of ECG parameters [24,40]. The traits analyzed can vary due to several factors, including the use of drugs and toxic agents, being a pharmacological tool of great utility when assessing the heart’s function as a functional biomarker [24,41].
According to the electrocardiographic recording (lead D2), there were irregularities in the QRS amplitudes; this complex is responsible for the time propagation of depolarization through the ventricles [39]. These oscillations may occur during the amplitudes in supraventricular arrhythmias or left ventricular hypertrophy [39,42].
Bradycardia was observed after the administration of EEOS. This phenomenon can be caused due to the interaction of the extract components with the cardiac tissue modifying its automaticity. However, they can be linked to other levels of control, such as baroreceptors or chemoreceptors associated with the solitary nucleus tract (NTS) [43,44].
The increase in R-R intervals and their greater irregularity suggest that there was a longer expiration time [45,46], which is in accordance with the respiratory data presented, due to muscle relaxation.
The Q-T intervals represent the depolarization and repolarization time of the ventricular cardiomyocytes [39,47]. This parameter indicates disturbances in the electrical activity of the heart, therefore, it has been considered a useful indicator of drug cardiotoxicity [39]. The results of the EEOS extract showed an increase in the Q-T segment, increasing the interval of the ventricular cardiac cycle, which resulted in bradycardia, but the rhythm was sinus.
Several intervention studies have investigated the cardiovascular protective effects of citrus flavonoid [48] and various berries, including Euterpe oleracea [49,50]. The results from these studies suggest a positive impact of berry consumption. Referring the current study, the EEOS also has a protective effect and could be used in patients with hypertension because of its heart rate-lowering effect.
The potential application on the field of anticonvulsant and myorelaxant drugs or respiratory and cardiac muscle depressant drugs, relies on further studies in isolation, purification and evaluation of efficacy/toxicity of EEOS extract or part of it.
5. Conclusion
EEOS has myorelaxative, sedative, respiratory depressant and cardiac muscle depressant activity. These effects can be mediated by the CNS. However, we do not rule out the possibility of the drug’s interaction with molecular structures of the skeletal and cardiac striated muscles, or even a set of effects. Thus, the precise mechanisms of EEOS activity and the relationship between receptor subunits or other neurotransmitter systems should be further investigated in the future.
Author statement
Nilton Akio Muto: Conceptualization, Writing. Moisés Hamoy: Methodology, Software. David Cristian Rodrigues Lucas: Investigation. Bruno Brito Teixeira: Data curation. Adrielle Felicia Santos Almeida: Original draft preparation. Thamires de Castro Navegantes: Visualization. Vaniza Sheila de Sousa Ferreira de Sá, Brenda Pinto de Moraes,João Paulo do Vale Medeiros, Yasmin Amorim dos Santos: Investigation. Claúdia Quintino da Rocha, Vanessa Joia de Mello: Validation. Hervé Rogez: Reviewing and Editing. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We thank the Laboratory of Pharmacology and Toxicology of Natural Products and the Centre for Valorization of Amazonian Bioactive Compounds (CVACBA), Belém, Pará, Brazil for kindly providing the animals and the samples of extract of Euterpe oleracea for this study, respectively. This work was supported by Conselho Nacional de Ciencia e Tecnologia em Pesquisa (CNPq, Brazil) and Pro-Reitoria de Pesquisa da UFPA (PROPESP, UFPA, Brazil). A slightly different version of this paper has appeared in November 2019 in 13th Latin American Symposium of Food Science.
Edited by Dr. A.M. Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.03.024.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Almeida C.F., Ramos M.A., Silva R.R., Melo J.G., Medeiros M.F., Araujo T.A., Almeida A.L., Amorim E.L., Alves R.R., Albuquerque U.P. Intracultural variation in the knowledge of medicinal plants in an urban-rural community in the atlantic forest from northeastern Brazil. Evid. Complement. Alternat. Med. 2012;2012 doi: 10.1155/2012/679373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barros L., Calhelha R.C., Queiroz M.J.R.P., Maria João R.P., Queiroz B., Santos-Buelga C., Santos E.A., Regis W.C.B., Ferreira I.C.F.R. The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds. Ind. Crops Prod. 2015;76:318–322. [Google Scholar]
- 3.Rocha A.P., Carvalho L.C., Sousa M.A., Madeira S.V., Sousa P.J., Tano T., Schini-Kerth V.B., Resende A.C., Soares de Moura R. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart. (Acai) extracts in mesenteric vascular bed of the rat. Vascul. Pharmacol. 2007;46:97–104. doi: 10.1016/j.vph.2006.08.411. [DOI] [PubMed] [Google Scholar]
- 4.Rocha A.P.M., Resende A.C., Souza M.A.V., Carvalho L.C.R.M., Souza P.J.C., Tano T., Soares De Moura R. Antihypertensive effects and antioxidant action of a hydro-alcoholic extract obtained from fruits of Euterpe oleracea Mart.(Acai) J. Pharmacol. Toxicol. 2008;3(6):435–448. [Google Scholar]
- 5.Moura R.S., Pires K.M., Santos Ferreira T., Lopes A.A., Nesi R.T., Resende A.C., Sousa P.J., Silva A.J., Porto L.C., Valenca S.S. Addition of acai (Euterpe oleracea) to cigarettes has a protective effect against emphysema in mice. Food Chem. Toxicol. 2011;49:855–863. doi: 10.1016/j.fct.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Moura R.S., Ferreira T.S., Lopes A.A., Pires K.M., Nesi R.T., Resende A.C., Souza P.J., Silva A.J., Borges R.M., Porto L.C., Valenca S.S. Effects of Euterpe oleracea Mart. (ACAI) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine. 2012;19:262–269. doi: 10.1016/j.phymed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Sudo R.T., Neto M.L., Monteiro C.E., Amaral R.V., Resende A.C., Souza P.J., Zapata-Sudo G., Moura R.S. Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (Acai) in a rodent model of acute and neuropathic pain. BMC Complement. Altern. Med. 2015;15:208. doi: 10.1186/s12906-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos E.L., Maia B.H.L.N.S., Ferriani A.P., Teixeira S.D. Flavonoids-From Biosynthesis to Human Health. IntechOpen; London, UK: 2017. Flavonoids: classification, biosynthesis and chemical ecology; pp. 1–14. [Google Scholar]
- 9.Marder M., Paladini A.C. GABA(A)-receptor ligands of flavonoid structure. Curr. Top. Med. Chem. 2002;2:853–867. doi: 10.2174/1568026023393462. [DOI] [PubMed] [Google Scholar]
- 10.Licata S.C., Platt D.M., Cook J.M., Van Linn M.L., Rowlett J.K. Contribution of alpha1 subunit-containing gamma-aminobutyric acidA (GABAA) receptors to motor-impairing effects of benzodiazepines in squirrel monkeys. Psychopharmacology (Berl.) 2009;203:539–546. doi: 10.1007/s00213-008-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D.D., Kriegstein A.R. Defining the role of GABA in cortical development. J. Physiol. 2009;587:1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H.L., Wan J.B., Wang Y.T., Li B.C., Xiang C., He J., Li P. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55:3–16. doi: 10.1111/epi.12463. [DOI] [PubMed] [Google Scholar]
- 13.Johnston G.A. GABA(A) receptor channel pharmacology. Curr. Pharm. Des. 2005;11:1867–1885. doi: 10.2174/1381612054021024. [DOI] [PubMed] [Google Scholar]
- 14.Bourazani M., Papageorgiou E., Zarkadas G., Petrakopoulou T., Kaba E., Fasoi G., Kelesi M. The role of muscle relaxants - spasmolytic (Thiocochlicoside) in postoperative pain management after mastectomy and breast reconstruction. Asian Pac. J. Cancer Prev. 2019;20:743–749. doi: 10.31557/APJCP.2019.20.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assis M.R., Dos Santos Paiva E., Helfenstein M., Jr., Heymann R.E., Pollak D.F., Provenza J.R., Ranzolin A., Rezende M.C., Ribeiro L.S., Souza E.J.R., Martinez J.E. Treatment data from the Brazilian fibromyalgia registry (EpiFibro) Adv. Rheumatol. 2020;60:9. doi: 10.1186/s42358-019-0108-2. [DOI] [PubMed] [Google Scholar]
- 16.Milic M., Divljakovic J., Rallapalli S., van Linn M.L., Timic T., Cook J.M., Savic M.M. The role of alpha1 and alpha5 subunit-containing GABAA receptors in motor impairment induced by benzodiazepines in rats. Behav. Pharmacol. 2012;23:191–197. doi: 10.1097/FBP.0b013e3283512c85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoyama H., Doura T. Selective acetylcholinesterase inhibitors derived from muscle relaxant dantrolene. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2019.126888. [DOI] [PubMed] [Google Scholar]
- 18.SEDAP (Secretaria de Desenvolvimento Agropecuário e da Pesca). PANORAMA AGRÍCOLA DO PARÁ 2010/2018.
- 19.George S., Brat P., Alter P., Amiot M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005;53:1370–1373. doi: 10.1021/jf048396b. [DOI] [PubMed] [Google Scholar]
- 20.Ungurianu A., Şeremet O., Gagniuc E., Olaru O.T., Guţu C., Grǎdinaru D. Preclinical and clinical results regarding the effects of a plant-based antidiabetic formulation versus well established antidiabetic molecules. Pharmacol. Res. 2019;150 doi: 10.1016/j.phrs.2019.104522. [DOI] [PubMed] [Google Scholar]
- 21.Quettier-Deleu C., Gressier B., Vasseur J., Dine T., Brunet C., Luyckx M., Cazin M., Cazin J.C., Bailleul F., Trotin F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 22.Delcour J.A., Varebeke D.Jd. A new colourimetric assay for flavanoids in pilsner beers. J. Inst. Brew. 1985;91:37–40. [Google Scholar]
- 23.Giner-Chavez B.I., Van Soest P.J., Robertson J.B., Lascano C., Reed J.D., Pell A.N. A method for isolating condensed tannins from crude plant extracts with trivalent ytterbium. J. Sci. Food Agric. 1997;74(3):359–368. [Google Scholar]
- 24.Farraj A.K., Hazari M.S., Cascio W.E. The utility of the small rodent electrocardiogram in toxicology. Toxicol. Sci. 2011;121(1):11–30. doi: 10.1093/toxsci/kfr021. [DOI] [PubMed] [Google Scholar]
- 25.Souza-Monteiro J.R., Hamoy M., Santana-Coelho D., Arrifano G.P., Paraense R.S., Costa-Malaquias A., Mendonca J.R., Silva R.F., Monteiro W.S., Rogez H., Oliveira D.L., Nascimento J.L., Crespo-Lopez M.E. Anticonvulsant properties of Euterpe oleracea in mice. Neurochem. Int. 2015;90:20–27. doi: 10.1016/j.neuint.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Yousef M.I., Khalil D.K., Abdou H.M. Neuro-and nephroprotective effect of grape seed proanthocyanidin extract against carboplatin and thalidomide through modulation of inflammation, tumor suppressor protein p53, neurotransmitters, oxidative stress and histology. Toxicol. Rep. 2018;5:568–578. doi: 10.1016/j.toxrep.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araújo Rodrigues P., de Morais S.M., Aguiar L.A., Vila-Nova N.S., Benjamin S.R. Effect of Byrsonima sericea DC. leaf extracts on mice gastrointestinal tract. Toxicol. Rep. 2019;6:1182–1187. doi: 10.1016/j.toxrep.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manivannan V., Johnson M. Total phenolic, tannin, triterpenoid, flavonoid and sterol contents, anti-diabetic, anti-inflammatory and cytotoxic activities of Tectaria paradoxa (Fee.) Sledge. Toxicol. Rep. 2020;7:1465–1468. doi: 10.1016/j.toxrep.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margină D., Ungurianu A., Purdel C., Nitulescu G.M., Tsoukalas D., Sarandi E. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem. Toxicol. 2020 doi: 10.1016/j.fct.2020.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azevedo M.I., Pereira A.F., Nogueira R.B., Rolim F.E., Brito G.A., Wong D.V., Lima-Junior R.C., Albuquerque Ribeiro R., Vale M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain. 2013;9:53. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez S.P., Wasowski C., Loscalzo L.M., Granger R.E., Johnston G.A., Paladini A.C., Marder M. Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol. 2006;539:168–176. doi: 10.1016/j.ejphar.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Moreira E.L., Rial D., Duarte F.S., Carvalho C.R., Horst H., Pizzolatti M.G., Prediger R.D., Ribeiro-do-Valle R.M. Central nervous system activity of the proanthocyanidin-rich fraction obtained from Croton celtidifolius in rats. J. Pharm. Pharmacol. 2010;62:1061–1068. doi: 10.1111/j.2042-7158.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- 33.Gardner C.R., Tully W.R., Hedgecock C.J. The rapidly expanding range of neuronal benzodiazepine receptor ligands. Prog. Neurobiol. 1993;40:1–61. doi: 10.1016/0301-0082(93)90047-v. [DOI] [PubMed] [Google Scholar]
- 34.Matsukawa T., Hikasa Y. Effects of imidazoline and nonimidazoline alpha-adrenoceptor agonists and antagonists, including xylazine, medetomidine, dexmedetomidine, yohimbine, and atipamezole, on aggregation of feline platelets. Am. J. Vet. Res. 2020;81:159–171. doi: 10.2460/ajvr.81.2.159. [DOI] [PubMed] [Google Scholar]
- 35.Pattinson K.T. Opioids and the control of respiration. Br. J. Anaesth. 2008;100:747–758. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 36.Mascia M.P., Bachis E., Obili N., Maciocco E., Cocco G.A., Sechi G.P., Biggio G. Thiocolchicoside inhibits the activity of various subtypes of recombinant GABA(A) receptors expressed in Xenopus laevis oocytes. Eur. J. Pharmacol. 2007;558:37–42. doi: 10.1016/j.ejphar.2006.11.076. [DOI] [PubMed] [Google Scholar]
- 37.Nirwan N., Vyas P., Vohora D. Animal models of status epilepticus and temporal lobe epilepsy: a narrative review. Rev. Neurosci. 2018;29:757–770. doi: 10.1515/revneuro-2017-0086. [DOI] [PubMed] [Google Scholar]
- 38.Al-Abbasi F.A., Kumar V., Anwar F. Biochemical and toxicological effect of diazepam in stress-induced cardiac dysfunctions. Toxicol. Rep. 2020;7:788–794. doi: 10.1016/j.toxrep.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konopelski P., Ufnal M. Electrocardiography in rats: a comparison to human. Physiol. Res. 2016;65(5) doi: 10.33549/physiolres.933270. [DOI] [PubMed] [Google Scholar]
- 40.Buschmann G., Schumacher W., Budden R., Kühl U.G. Evaluation of the effect of dopamine and other catecholamines on the electrocardiogram and blood pressure of rats by means of on-line biosignal processing. J. Cardiovasc. Pharmacol. 1980;2(6):777–795. doi: 10.1097/00005344-198011000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Rautaharju P.M., Park L.P., Gottdiener J.S., Siscovick D., Boineau R., Smith V., Powe N.R. Race-and sex-specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African-Americans. J. Electrocardiol. 2000;33(3):205–218. doi: 10.1054/jelc.2000.7667. [DOI] [PubMed] [Google Scholar]
- 42.Merentie M., Lipponen J.A., Hedman M., Hedman A., Hartikainen J., Huusko J. Mouse ECG findings in aging, with conduction system affecting drugs and in cardiac pathologies: development and validation of ECG analysis algorithm in mice. Physiol. Rep. 2015;3(12) doi: 10.14814/phy2.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callera J.C., Bonagamba L.G., Nosjean A., Laguzzi R., Machado B.H. Activation of GABAA but not GABAB receptors in the NTSblocked bradycardia of chemoreflex in awake rats. Am. J. Physiol.-Heart Circ. Physiol. 1999;276(6):H1902–H1910. doi: 10.1152/ajpheart.1999.276.6.H1902. [DOI] [PubMed] [Google Scholar]
- 44.Sidhu S., Marine J.E. Evaluating and managing bradycardia. Trends Cardiovasc. Med. 2020;30(5):265–272. doi: 10.1016/j.tcm.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Castro R.R.T.D., Ramalho S.H.R., Nóbrega A.C.L.D. Critérios para seleção do intervalo RR no eletrocardiograma para quantificação da arritmia sinusal respiratória. Rev. Bras. Med. Do Esporte. 2000;6(1):5–8. [Google Scholar]
- 46.Pezolato V.A., Mascarin A.L., Ferreira R.B., Dias R., Silva C.A. Acompanhamento eletrocardiográfico no desenvolvimento de ratos Wistar. Arq. Bras. Med. Vet. Zootec. 2017;69(1):39–48. [Google Scholar]
- 47.Hayes E., Pugsley M.K., Penz W.P., Adaikan G., Walker M.J.A. Relationship between QaT and RR intervals in rats, guinea pigs, rabbits, and primates. J. Pharmacol. Toxicol. Methods. 1994;32(4):201–207. doi: 10.1016/1056-8719(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 48.Rezaee R., Sheidary A., Jangjoo S., Ekhtiary S., Bagheri S., Kohkan Z. Cardioprotective effects of hesperidin on carbon monoxide poisoned in rats. Drug Chem. Toxicol. 2019:1–6. doi: 10.1080/01480545.2019.1650753. [DOI] [PubMed] [Google Scholar]
- 49.Basu A., Rhone M., Lyons T.J. Berries: emerging impact on cardiovascular health. Nutr. Rev. 2010;68(3):168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ros E., Tapsell L.C., Sabaté J. Nuts and berries for heart health. Curr. Atheroscler. Rep. 2010;12(6):397–406. doi: 10.1007/s11883-010-0132-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.