Abstract
Objective
To examine whether overall lifestyles mediate associations of socioeconomic status (SES) with mortality and incident cardiovascular disease (CVD) and the extent of interaction or joint relations of lifestyles and SES with health outcomes.
Design
Population based cohort study.
Setting
US National Health and Nutrition Examination Survey (US NHANES, 1988-94 and 1999-2014) and UK Biobank.
Participants
44 462 US adults aged 20 years or older and 399 537 UK adults aged 37-73 years.
Exposures
SES was derived by latent class analysis using family income, occupation or employment status, education level, and health insurance (US NHANES only), and three levels (low, medium, and high) were defined according to item response probabilities. A healthy lifestyle score was constructed using information on never smoking, no heavy alcohol consumption (women ≤1 drink/day; men ≤2 drinks/day; one drink contains 14 g of ethanol in the US and 8 g in the UK), top third of physical activity, and higher dietary quality.
Main outcome measures
All cause mortality was the primary outcome in both studies, and CVD mortality and morbidity in UK Biobank, which were obtained through linkage to registries.
Results
US NHANES documented 8906 deaths over a mean follow-up of 11.2 years, and UK Biobank documented 22 309 deaths and 6903 incident CVD cases over a mean follow-up of 8.8-11.0 years. Among adults of low SES, age adjusted risk of death was 22.5 (95% confidence interval 21.7 to 23.3) and 7.4 (7.3 to 7.6) per 1000 person years in US NHANES and UK Biobank, respectively, and age adjusted risk of CVD was 2.5 (2.4 to 2.6) per 1000 person years in UK Biobank. The corresponding risks among adults of high SES were 11.4 (10.6 to 12.1), 3.3 (3.1 to 3.5), and 1.4 (1.3 to 1.5) per 1000 person years. Compared with adults of high SES, those of low SES had higher risks of all cause mortality (hazard ratio 2.13, 95% confidence interval 1.90 to 2.38 in US NHANES; 1.96, 1.87 to 2.06 in UK Biobank), CVD mortality (2.25, 2.00 to 2.53), and incident CVD (1.65, 1.52 to 1.79) in UK Biobank, and the proportions mediated by lifestyle were 12.3% (10.7% to 13.9%), 4.0% (3.5% to 4.4%), 3.0% (2.5% to 3.6%), and 3.7% (3.1% to 4.5%), respectively. No significant interaction was observed between lifestyle and SES in US NHANES, whereas associations between lifestyle and outcomes were stronger among those of low SES in UK Biobank. Compared with adults of high SES and three or four healthy lifestyle factors, those with low SES and no or one healthy lifestyle factor had higher risks of all cause mortality (3.53, 3.01 to 4.14 in US NHANES; 2.65, 2.39 to 2.94 in UK Biobank), CVD mortality (2.65, 2.09 to 3.38), and incident CVD (2.09, 1.78 to 2.46) in UK Biobank.
Conclusions
Unhealthy lifestyles mediated a small proportion of the socioeconomic inequity in health in both US and UK adults; therefore, healthy lifestyle promotion alone might not substantially reduce the socioeconomic inequity in health, and other measures tackling social determinants of health are warranted. Nevertheless, healthy lifestyles were associated with lower mortality and CVD risk in different SES subgroups, supporting an important role of healthy lifestyles in reducing disease burden.
Introduction
Socioeconomic status (SES) has been associated with differences in morbidity and mortality.1 Although most countries and regions have witnessed socioeconomic progress and rising standards of living in recent decades, the US and UK are the top two countries showing an increasing rate of wealth inequity among Organization for Economic Cooperation and Development countries.2 Moreover, the gap has also become more pronounced for socioeconomic inequity in survival—longevity has increased among Americans with middle and high income, whereas in poor Americans it has remained unchanged or even decreased in certain demographic groups.3 Similar trends are also observed in the UK.2 The impacts of these differences have been made more evident during the covid-19 pandemic, which has affected socially disadvantaged groups the most.4 Immediate efforts are therefore warranted to reduce socioeconomic inequities in health and to improve the resilience of populations.
Lifestyle factors are commonly viewed as mediators between SES and health and that healthy lifestyles might alleviate the socioeconomic inequities in health.5 Multiple studies have examined the contribution of an individual lifestyle factor or several in the association between SES and mortality or morbidity.6 However, important gaps remain. First, how much an overall lifestyle mediates the association between SES and health outcomes is debatable. Previous studies tended to use single variables (eg, income, wealth, occupation, education level) to represent individual level SES,6 which only partly reflected overall SES7; thus it is essential to construct a comprehensive SES variable comprising different aspects of SES. Besides, lifestyle factors are interrelated8 and few studies have built a healthy lifestyle score to reflect overall lifestyle and to evaluate its impact on the socioeconomic inequities in health. Second, limited research has been performed on the interaction and joint associations of SES and overall lifestyles with health outcomes. Third, it remains unclear whether the findings are consistent among subpopulations of different age, sex, and racial or ethnic groups.
We used data from the US National Health and Nutrition Examination Survey (US NHANES) and UK Biobank to evaluate the complex relations of lifestyles and SES with mortality and incident cardiovascular disease (CVD).
Methods
Study population
US NHANES recruited a representative sample of civilian, community dwelling members of the US population using a complex, multistage probability design. The survey was conducted periodically before 1999 and continuously thereafter. Details of the study design and data collection have been previously described.9 Although US NHANES has released cross sectional questionnaire, examination, and laboratory data up to 2018, mortality data were updated to 31 December 2015. Accordingly, the current analysis included 61 202 participants who were aged 20 years and older and not pregnant at baseline in US NHANES III (1988-94) and continuous NHANES (1999-2014) surveys. Those with missing information on socioeconomic factors (n=6939), lifestyle factors (n=8156), other covariates (n=1619), and deaths (n=26) were excluded from the analysis. Overall, 44 462 participants from US NHANES were included (supplementary fig 1).
UK Biobank recruited more than 500 000 participants aged 37 to 73 years from 22 assessment centers across England, Scotland, and Wales between 2007 and 2010. Details of the study design and data collection have been described previously.10 Among the 502 492 participants, we excluded those with missing information on socioeconomic factors (n=77 962), lifestyle factors (n=20 029), and other covariates (n=4964). Overall, 399 537 participants were included (supplementary fig 1). For the analysis of incident CVD, we only included those without prevalent CVD (n=324 517) at baseline.
Assessment of SES
In US NHANES, self-reported family income level, occupation, education level, and health insurance were used to measure SES according to previous studies,7 11 and each factor was divided into three levels (low, medium, and high) with consideration of practical interpretation and sample size within levels. The family income level was operationalized using the family poverty to income ratio, which reflected the annual family income relative to the federal poverty level and was comparable across surveys since income thresholds were updated for inflation and family size each year.12 According to a published study and the Patient Protection and Affordable Care Act, we grouped participants according to the poverty to income ratio: low (≤1), middle (1-4), and high (≥4).12 Education was categorized into less than high school diploma, high school graduate or equivalent, and college or above.13 Occupation was classified based on the widely used socioeconomic index in the US,14 and each occupation was rated according to the employees’ earnings, education level, and prestige. The socioeconomic index ranged between 13.98 and 90.45,15 and occupation was categorized into upper (socioeconomic index ≥50), lower (socioeconomic index <50, including retirees16 and students), and unemployment. Health insurance was categorized into private health insurance (including any private health insurance, Medi-Gap, or single-service plan), public health insurance only (including Medicare, Medicaid, State Children’s Healthcare Plan, military healthcare, Indian Health Service, State Sponsored Health Plan, or other government programme), and no health insurance.17 An overall SES variable was created using latent class analysis based on family income level, occupation, education level, and health insurance (each factor had three levels).7 The latent class analysis, which uses multiple observed categorical variables to generate an unmeasured variable (ie, latent variable) with a set of mutually exclusive latent classes, was conducted using PROC LCA, a new SAS procedure.18 Three latent classes were identified, which respectively represented a high, medium, and low SES according to the item-response probabilities.19 The supplementary file describes the data collection and latent class analysis in US NHANES.
In UK Biobank, total household income before tax was obtained through questionnaires, and participants could choose an option from <₤18 000 ($25 000; €21 000), ₤18 000-£30 999, ₤31 000-£51 999, ₤52 000-£100 000, >₤100 000, do not know, or prefer not to answer. A total of 14.3% of participants chose the last two options and were excluded from the main analyses as missing values; however, we included them in sensitivity analyses when evaluating single socioeconomic factors, consistent with a previous study,20 on the basis that these participants might be more likely to have lower SES. Participants reported their education qualifications as college or university degree; A levels, AS levels, or equivalent; O levels, GCSEs, or equivalent; CSEs or equivalent; NVQ, HND, HNC, or equivalent; other professional qualifications; none of the above (equivalent to less than high school diploma); or prefer not to answer (which was excluded from our analyses as missing values). As UK Biobank only acquired employment status instead of information on specific occupation at baseline, we regrouped participants into two groups: employed (including those in paid employment or self-employed, retired, doing unpaid or voluntary work, or being full or part time students) and unemployed. Because the National Health Service, a publicly funded healthcare system aiming to provide comprehensive, universal and free services, is implemented in the UK,21 we did not consider health insurance as a component of SES in UK Biobank. An overall SES variable was created using latent class analysis based on three individual socioeconomic factors (household income, education level, and employment status). We did not regroup household income and education level into three groups as we did in US NHANES because of the larger sample size in UK Biobank and failure of model convergence owing to fewer observed groups if the two variables were regrouped. Three latent classes were identified, which respectively represented a high, medium, and low SES according to the item-response probabilities. Details are reported in the supplementary file.
In UK Biobank, Townsend deprivation index was available as an area level SES variable derived from national census data according to postcodes of residence, which considered car ownership, household overcrowding, owner occupation, and unemployment.22 A higher Townsend deprivation index denotes lower area level SES.22
Assessment of lifestyle factors and other covariates
Since multiple lifestyle factors are interrelated and are associated with mortality and morbidity, we constructed a healthy lifestyle score including cigarette smoking, alcohol consumption, physical activity, and diet according to a previous US NHANES study23 and that coincided with recommendations from the World Health Organization.24 All lifestyle factors were obtained through structured questionnaires and 24 hour dietary recalls. Never smoking was considered as a healthy level, which was defined in the questionnaire as smoking fewer than 100 cigarettes in life. Frequency and volume of current alcohol consumption were self-reported, and a healthy level was defined as daily consumption of one drink or fewer for women and two drinks or fewer for men, according to the dietary guidelines in the US and UK (one drink contains 14 g of ethanol in the US and 8 g in the UK).25 26 For physical activity, different assessment questions were used between the US and UK studies, and questionnaires also varied in different survey years in US NHANES. Nevertheless, weekly metabolic equivalent hours of leisure time physical activity were calculated in US NHANES 1999-2014 and UK Biobank, whereas monthly frequency of leisure time physical activity was calculated in US NHANES 1988-94. To harmonize the data, we further classified the participants into thirds and defined the top third as a healthy level of physical activity.
In US NHANES, dietary quality was obtained from 24 hour dietary recalls and was assessed by healthy eating index (HEI) scores. The HEI-2015 was calculated for the 1999-2014 survey cycles, which aligns with the 2015-20 Dietary Guidelines for Americans.27 However, because food codes used in the 1988-94 cycles could not match those used in the 1999-2014 cycles, we used HEI-1995 for the 1988-94 cycles and the variable was directly provided by the original dataset. HEI-1995 aligns with the food guide pyramid released by the US Department of Agriculture in 1992.27 Supplementary table 1 provides details of constructions of HEI-1995 and HEI-2015, and both scores reflected the overall dietary quality according to the contemporary dietary guidelines. A healthy diet was defined as the health eating index in the top two fifths of distribution.28 In UK Biobank, dietary information was obtained through questionnaires and did not contain energy or salt intakes, thus we could not calculate the HEI scores. Instead, according to a previous UK Biobank study,29 we evaluated dietary quality using a more recent dietary recommendation for cardiovascular health, which considered adequate consumption of fruit, vegetables, whole grains, fish, shellfish, dairy products, and vegetable oils and reduced consumption of refined grains, processed meats, unprocessed meats, and sugar sweetened beverages. We defined a healthy diet as meeting at least five items of the recommendations (see supplementary table 2).
For each lifestyle factor, we assigned 1 point for a healthy level and 0 points for an unhealthy level. Thus, the healthy lifestyle score was the sum of the points and ranged between 0 and 4, with higher scores indicating healthier lifestyles. Although this simple additive method has been used widely,30 31 32 the underlying assumption is that the associations between different lifestyle factors and the outcome were identical, which might not be true. Thus we also constructed a weighted lifestyle score, where each lifestyle factor was weighted by its association with the outcome. Body mass index (BMI) was not included in the lifestyle score given the concern that it could be an intermediate factor between behavioral factors and health outcomes. In addition, the obesity paradox is a concern,33 and overweight and obesity might not be strongly associated with mortality in older people.13 Nevertheless, we also included baseline BMI in the lifestyle score in a sensitivity analysis, and healthy bodyweight was defined as a BMI of 18.5-24.9.28
Other covariates were obtained through questionnaires, including age; sex; marital status (US NHANES only); assessment centers (UK Biobank only); self-reported race; an acculturation score based on the country of birth, length of time in the US or UK, and language spoken at home (see supplementary file);34 history of hypertension, diabetes, CVD, or cancer; and history of chronic bronchitis, emphysema, or chronic obstructive pulmonary disease (UK Biobank only). Diagnoses of CVD and cancer were also obtained through linked hospital admissions data and cancer registry in the UK Biobank. Bodyweight and height were measured at baseline, with BMI calculated as weight (kg)/(height (m)2).
Outcome ascertainment
Outcomes were classified using ICD-9 and ICD-10 (international classification of diseases, ninth and 10th revisions, respectively) codes. The primary outcomes included all cause mortality, CVD mortality, and incident CVD. In US NHANES, deaths were obtained through the National Death Index to 31 December 2015.35 In UK Biobank, deaths were obtained through death certificates held within the NHS Information Centre (England and Wales) and the NHS Central Register (Scotland) to 30 April 2020.36 CVD diagnoses, including myocardial infarction (ICD-9 codes 410-412 and 429.79; ICD-10 codes I21-I23, I24.1, and I25.2) and stroke diagnoses (ICD-9 codes 430, 431, 434, and 436; ICD-10 codes I60, I61, I63, and I64), were obtained through linked hospital admissions data including Hospital Episode Statistics-Admitted Patient Care (England), Scottish Morbidity Records-General/Acute Inpatient and Day Case Admissions (Scotland), and Patient Episode Database for Wales as well as death register data to 31 January 2018.37 38 Secondary outcomes were mortality from heart disease (ICD-10 codes I00-I09, I11, I13, and I20-I51 in US NHANES), coronary heart disease (ICD-10 codes I20-I25 in UK Biobank), and stroke (ICD-10 codes I60, I61, I63, and I64 in UK Biobank), as well as incident myocardial infarction and stroke. Mortality from cerebrovascular disease or total CVD was not considered in NHANES because the US National Death Index matched mortality dataset stopped updating data on deaths from cerebrovascular diseases after 31 December 2011.
Statistical analysis
To estimate appropriate variance and statistics representative of US adults, our analysis in US NHANES considered the oversampling, stratification, and clustering according to the NHANES statistical analysis guideline.39 Baseline characteristics were described across different levels of SES, and differences among groups were tested by analysis of variance adjusted for sampling weights for continuous variables and Rao-Scott χ2 test for categorical variables in US NHANES, and by analysis of variance and χ2 test in UK Biobank.
We used Cox proportional hazard regression models to estimate the hazard ratios and 95% confidence intervals of outcomes associated with SES and lifestyle score. The proportional hazards assumption was examined by creating a product term of follow-up time and SES, and we found no significant deviation from the assumption.40 Person years were calculated from baseline until the date of death or diagnosis (for the incident CVD analysis), or end of follow-up, whichever occurred first. Based on previous researches,20 23 model 1 included SES; age; sex; self-reported race; marital status (US NHANES only); assessment centers (UK Biobank only); acculturation; BMI; and history of hypertension, diabetes, CVD, cancer, chronic bronchitis, emphysema, or chronic obstructive pulmonary disease. Model 2 additionally included the healthy lifestyle score. We used the difference method to calculate the mediation proportion by the mediator (overall lifestyle) for the association between SES and each outcome—that is, comparing estimates from models with and without the hypothesized mediator.41 We additionally calculated the C statistics of the two models to compare the predictions with versus without the healthy lifestyle score.
We further conducted a stratified analysis by latent class of SES to investigate associations of the lifestyle score with health outcomes among adults in different socioeconomic subgroups. As only 838 (2.2%) and 3495 (9.4%) US adults had 0 and 4 points of healthy lifestyle score, and the corresponding numbers in the UK Biobank were 49 545 (12.4%) and 9841 (2.5%), we merged participants with 0 points and 1 point as well as those with 3 and 4 points to increase the statistical power. In this analysis, the reference group was set as the participants with unhealthy lifestyles (lifestyle scores of 0 or 1), and we examined whether adherence to healthy lifestyles was associated with protection against mortality and incident CVD across different SES subgroups. To quantify the additive and multiplicative interactions, we additionally included a product term of SES (low, medium, and high) and healthy lifestyle score (0 or 1; 2; and 3 or 4 points) in the model. The hazard ratio with its 95% confidence interval of the product term was the measure of interaction on the multiplicative scale. We used the relative excess risk due to interaction (RERI) and corresponding 95% confidence intervals as the measure of interaction on the additive scale, calculated using the coefficients and corresponding standard errors of the product term, SES, and lifestyle score, as well as covariance matrix.42
To assess the joint associations, we further classified participants into nine groups according to SES (low, medium, and high) and healthy lifestyle score (0 or 1; 2; and 3 or 4 points) and estimated hazard ratios of mortality and incident CVD in different groups compared with those with high SES and three or four healthy lifestyle factors.
To test the robustness and potential variations in different subgroups, we repeated all analyses stratified by sex (men and women), self-reported race (white and non-white participants), and age groups (<60, and ≥60, defined as elders by the World Health Organization43).
We conducted several sensitivity analyses. First, we repeated all analyses by substituting SES with each socioeconomic factor (ie, family income level, occupation or employment status, education level, and health insurance), and these factors were mutually adjusted in the models. Similarly, we also used the individual lifestyle factors instead of the score in the models to evaluate whether the estimated mediation proportion was similar to that of the main analysis. Second, a weighted healthy lifestyle score was constructed to account for varied magnitudes of the associations between different lifestyle factors and outcomes.44 Third, we constructed a lifestyle score including baseline BMI. Fourth, we excluded individuals with prevalent diabetes, CVD, cancer, chronic bronchitis, emphysema, or chronic obstructive pulmonary disease because both lifestyles and SES could be influenced by major chronic diseases. Fifth, we excluded events that occurred within the first three years of follow-up to reduce potential reverse causation. Sixth, we restricted the analysis to those aged 40 years or older in US NHANES to coincide with the age distribution in UK Biobank, and to reduce the concern that SES is prone to change and the risk of mortality due to lifestyles is relatively lower in younger adults. As only five participants in UK Biobank were aged less than 40 years, this sensitivity analysis was not performed in UK Biobank. Seventh, we used multiple imputation to impute all missing independent variables to test the influence of missing variables.45 Eighth, we assigned 0, 1, and 2 points to each low, medium, and high level socioeconomic factor (for employment status in UK Biobank, only 0 and 2 points were assigned for unemployed and employed status) and added the scores to get a socioeconomic score (range 0-8 in US NHANES and 0-6 in UK Biobank). As only 756 (0.9%) participants in US NHANES and 5000 (1.3%) in UK Biobank had a score of 0, we merged those with 0 or 1 point. The socioeconomic score was then used in all analyses instead of the latent class derived SES variable. Ninth, in the final model in UK Biobank we further included the Townsend deprivation index, a variable reflecting the area level SES, for two purposes: to evaluate whether the association between individual level SES and health outcomes remained robust when controlling for area level SES, and to repeat all the main analysis using Townsend deprivation index as the SES variable, instead of the individual level SES variable. Tenth, we additionally included quadratic terms of age in the models to consider the possible non-linear associations of age with health outcomes.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). We considered two sided P values <0.05 to be significant.
Patient and public involvement
The analyses were based on existing data of two cohort studies in general populations, US NHANES and UK Biobank, and we did not participate in the participant recruitment. To our knowledge, no patients were involved in the design, recruitment, or conduct of the studies. The research question and outcome measures of the present study were proposed by systematically reviewing the evidence of the associations between lifestyles and non-communicable diseases, and no patients were involved in the process. Participants from the two cohorts were deidentified, and thus we could not disseminate the results to each participant; however, the results will be disseminated to the public through broadcasts and popular science articles.
Results
Population characteristics
Table 1 shows baseline characteristics of participants from US NHANES and UK Biobank. Among 44 462 participants from US NHANES (mean age 46.5 years, 48.7% men), 10 469 (33.6%) were of high SES, 20 729 (46.4%) of medium SES, and 13 264 (20.0%) of low SES. Among 399 537 participants from UK Biobank (mean age 56.1 years, 47.5% men), 79 697 (19.9%) were of high SES, 210 935 (52.8%) of medium SES, and 108 905 (27.3%) of low SES. Adults of low SES were more likely to be women, non-white people, not married, unemployed, and less educated, and to have low income, public or no health insurance, and a higher prevalence of comorbidities. Unhealthy levels of cigarette smoking, leisure time physical activity, and BMI were more prevalent among adults of low SES. Participants excluded from the current analysis owing to missing information were older, of low SES, and more likely to be women, non-white people, not married, and less accultured (see supplementary table 3).
Table 1.
Baseline characteristics of participants from US National Health and Nutrition Examination Survey (US NHANES) and UK Biobank according to socioeconomic status (SES).* Values are numbers (percentages) unless stated otherwise
| Characteristics | US NHANES | UK Biobank | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total population (n=44 462) | High SES† (n=10 469) | Medium SES† (n=20 729) | Low SES† (n=13 264) | Total population (n=399 537) | High SES‡ (n=79 697) | Medium SES‡ (n=210 935) | Low SES‡ (n=108 905) | ||
| Mean age (95% CI) (years) | 46.5 (46.1 to 46.9) | 45.9 (45.3 to 46.4) | 47.7 (47.2 to 48.2) | 44.8 (44.2 to 45.3) | 56.1 (56.1 to 56.1) | 52.5 (52.4 to 52.5) | 55.7 (55.7 to 55.8) | 59.5 (59.5 to 59.5) | |
| Men | 21 869 (48.7) | 5335 (50.9) | 10 357 (48.4) | 6177 (45.8) | 189 813 (47.5) | 40 990 (51.4) | 101 484 (48.1) | 47 339 (43.5) | |
| White ethnicity or race | 21 392 (73.6) | 6630 (84.0) | 10 878 (76.0) | 3884 (50.8) | 382 053 (95.6) | 76 596 (96.1) | 202 429 (96.0) | 103 028 (94.6) | |
| Married | 24 223 (57.4) | 6987 (68.7) | 11 860 (58.1) | 5376 (37.0) | - | - | - | - | |
| Household income§: | |||||||||
| High | 10 840 (34.6) | 8162 (81.0) | 2653 (15.9) | 25 (0.3) | 105 098 (26.3) | 78 565 (98.6) | 25 914 (12.3) | 619 (0.6) | |
| Medium | 24 629 (52.0) | 2176 (18.0) | 17 208 (80.3) | 5245 (43.1) | 206 809 (51.8) | 1132 (1.4) | 185 021 (87.7) | 20 656 (19.0) | |
| Low | 8993 (13.4) | 131 (1.0) | 868 (3.7) | 7994 (56.6) | 87 630 (21.9) | 0 | 0 | 87 630 (80.5) | |
| Occupation: | |||||||||
| Employed, student, or retired | 35 382 (81.7) | 9787 (92.8) | 18 637 (89.1) | 6958 (46.2) | 372 167 (93.1) | 75 242 (94.4) | 207 951 (98.6) | 88 974 (81.7) | |
| Upper socioeconomic index | 7267 (22.7) | 6325 (61.2) | 697 (3.8) | 245 (2.1) | - | - | - | - | |
| Lower socioeconomic index | 28 115 (59.0) | 3462 (31.6) | 17 940 (85.3) | 6713 (44.1) | - | - | - | - | |
| Unemployed | 9080 (18.3) | 682 (7.2) | 2092 (11.0) | 6306 (53.8) | 27 370 (6.9) | 4455 (5.6) | 2984 (1.4) | 19 931 (18.3) | |
| Education: | |||||||||
| College or above | 19 747 (55.1) | 10 152 (96.6) | 7394 (39.1) | 2201 (22.4) | 188 002 (47.1) | 63 753 (80.0) | 97 653 (46.3) | 26 596 (24.4) | |
| High school or equivalent | 11 511 (26.4) | 317 (3.4) | 8750 (44.7) | 2444 (22.8) | 153 752 (38.5) | 15 944 (20.0) | 103 822 (49.2) | 33 986 (31.2) | |
| Less than high school | 13 204 (18.5) | 0 | 4585 (16.2) | 8619 (54.8) | 57 783 (14.5) | 0 | 9460 (4.5) | 48 323 (44.4) | |
| Health insurance: | |||||||||
| Private | 26 795 (68.8) | 9278 (90.4) | 16 448 (79.6) | 1069 (7.5) | - | - | - | - | |
| Public only | 8907 (14.5) | 745 (5.7) | 1896 (7.8) | 6266 (44.9) | - | - | - | - | |
| None | 8760 (16.6) | 446 (4.0) | 2385 (12.5) | 5929 (47.6) | - | - | - | - | |
| More accultured | 37 584 (91.5) | 9818 (96.1) | 18 466 (93.2) | 9300 (79.6) | 367 865 (92.1) | 71 696 (90.0) | 196 119 (93.0) | 100 050 (91.9) | |
| Never smoking | 22 835 (50.5) | 6330 (59.7) | 10 369 (47.8) | 6136 (41.6) | 218 975 (54.8) | 49 681 (62.3) | 117 780 (55.8) | 51 514 (47.3) | |
| No heavy alcohol consumption | 41 747 (92.3) | 9817 (92.5) | 19 535 (92.6) | 12 395 (91.3) | 251 900 (63.0) | 45 264 (56.8) | 131 045 (62.1) | 75 591 (69.4) | |
| Top third of LTPA | 12 956 (34.0) | 4507 (45.6) | 5824 (30.4) | 2625 (22.6) | 133 182 (33.3) | 32 993 (41.4) | 71 688 (34.0) | 28 501 (26.2) | |
| Healthy diet¶ | 19 321 (43.6) | 5534 (52.5) | 8803 (41.0) | 4984 (34.9) | 56 892 (14.2) | 10 826 (13.6) | 29 278 (13.9) | 16 788 (15.4) | |
| BMI: | |||||||||
| 18.5-24.9 | 13 827 (33.4) | 3568 (36.2) | 6443 (32.6) | 3816 (30.6) | 131 018 (32.8) | 31 920 (40.1) | 68 872 (32.7) | 30 226 (27.8) | |
| <18.5 | 755 (1.8) | 149 (1.4) | 309 (1.7) | 297 (2.7) | 1998 (0.5) | 388 (0.5) | 926 (0.4) | 684 (0.6) | |
| 25.0-29.9 | 15 294 (33.4) | 3684 (34.8) | 7163 (33.3) | 4447 (31.3) | 170 781 (42.7) | 33 504 (42.0) | 91 756 (43.5) | 45 521 (41.8) | |
| ≥30.0 | 14 586 (31.4) | 3068 (27.6) | 6814 (32.5) | 4704 (35.4) | 95 740 (24.0) | 13 885 (17.4) | 49 381 (23.4) | 32 474 (29.8) | |
| Self-reported comorbidities: | |||||||||
| Hypertension | 14 746 (29.4) | 2864 (24.9) | 7171 (31.2) | 4711 (32.8) | 112 082 (28.1) | 15 123 (19.0) | 56 087 (26.6) | 40 872 (37.5) | |
| Diabetes | 4680 (7.6) | 653 (4.7) | 2116 (8.2) | 1911 (11.2) | 19 819 (5.0) | 2178 (2.7) | 8966 (4.3) | 8675 (8.0) | |
| CVD | 4609 (8.1) | 597 (4.3) | 2232 (8.9) | 1780 (12.7) | 75 020 (18.8) | 8974 (11.3) | 35 280 (16.7) | 30 766 (28.3) | |
| Cancer | 4048 (9.2) | 1034 (9.7) | 2160 (9.7) | 854 (7.3) | 33 816 (8.5) | 5252 (6.6) | 17 121 (8.1) | 11 443 (10.5) | |
| Emphysema, chronic bronchitis, or COPD | 3238 (7.6) | 474 (4.6) | 1502 (7.7) | 1262 (12.2) | 8847 (2.2) | 813 (1.0) | 3631 (1.7) | 4403 (4.0) | |
BMI=body mass index; COPD=chronic obstructive pulmonary disease; CVD=cardiovascular disease; LTPA=leisure time physical activity.
In US NHANES, all estimates accounted for complex survey designs, and P values were calculated using analysis of variance adjusting for sampling weights and Rao-Scott χ2 test for continuous and categorical variables, respectively. In UK Biobank, P values were calculated using analysis of variance and χ2 test for continuous and categorical variables, respectively. All P values were <0.001, except for heavy alcohol consumption (P=0.058).
SES in US NHANES was generated through latent class analysis using information on family income to poverty ratio, occupation, education level, and health insurance.
SES in UK Biobank was generated through latent class analysis using the information on household income, employment status, and education level.
In US NHANES, ≥4, >1 to <4, and ≤1 of family income to poverty ratios represented the high, medium, and low family income level, respectively. In UK Biobank, <£18 000 ($25 000; €21 000), £18 000-£51 999, and ≥£52 000 of average total household income before tax represented the high, medium, and low family income level, respectively.
In US NHANES III, healthy diet denoted the top two fifths of healthy eating index-1995 score. In the continuous US NHANES, healthy diet denoted the top two fifths of healthy eating index-2015 score. In UK Biobank, healthy diet denoted ideal intakes of ≥5 dietary components for cardiovascular health.
Mediation analysis of lifestyle on associations of SES with mortality and incident CVD
In US NHANES, 8906 deaths were recorded (1889 from heart disease) during a mean follow-up of 11.2 years. In UK Biobank, 22 309 deaths (4537 from CVD; a mean follow-up of 11.0 years) and 6903 incident CVD cases (4414 myocardial infarction and 2645 stroke; a mean follow-up of 8.8 years) were recorded. After adjusting for lifestyle score and other covariates, including age, sex, self-reported race, marital status, assessment centers, acculturation, BMI, and history of comorbidities, the hazards ratios when adults of low SES were compared with adults of high SES were 2.13 (95% confidence interval 1.90 to 2.38) for all cause mortality in US NHANES, and 1.96 (1.87 to 2.06) for all cause mortality, 2.25 (2.00 to 2.53) for CVD mortality, and 1.65 (1.52 to 1.79) for incident CVD in UK Biobank (table 2). The hazard ratios without adjustment for lifestyle score were larger. Each additional healthy lifestyle factor was associated with 11% to 17% lower risks of mortality and incident CVD (supplementary table 4). When low SES was compared with high SES, the proportion mediated by the lifestyle score was 12.3% (10.7% to 13.9%) for all cause mortality in US NHANES, and 4.0% (3.5% to 4.4%) for all cause mortality, 3.0% (2.5% to 3.6%) for CVD mortality, and 3.7% (3.1% to 4.5%) for incident CVD in UK Biobank (table 2). When the socioeconomic score was used to investigate more extreme socioeconomic disparities, the hazard ratios for the lowest compared with highest socioeconomic score were 2.87 and 3.23 for all cause mortality in US NHANES and UK Biobank, respectively, and 3.37 for CVD mortality and 2.46 for incident CVD in UK Biobank. However, the mediation proportion attributed to lifestyle remained similar to that of the main analyses (supplementary table 5). Additional inclusion of the healthy lifestyle score did not improve the prediction of all outcomes (supplementary table 6).
Table 2.
Associations of socioeconomic status (SES) with incident cardiovascular disease (CVD) and mortality and mediation proportion of socioeconomic inequity in health attributed to lifestyle*
| Hazard ratio (95% CI) | Mediation proportion (%) (95% CI) | ||
|---|---|---|---|
| Unadjusted for lifestyle score | Adjusted for lifestyle score | ||
| All cause mortality | |||
| US NHANES: | |||
| High SES | 1 (Reference) | 1 (Reference) | - |
| Medium SES | 1.67 (1.51 to 1.84) | 1.57 (1.42 to 1.73) | 11.9 (9.6 to 14.2) |
| Low SES | 2.36 (2.11 to 2.65) | 2.13 (1.90 to 2.38) | 12.3 (10.7 to 13.9) |
| UK Biobank: | |||
| High SES | 1 (Reference) | 1 (Reference) | - |
| Medium SES | 1.31 (1.25 to 1.37) | 1.29 (1.23 to 1.35) | 5.1 (4.0 to 6.5) |
| Low SES | 2.02 (1.92 to 2.12) | 1.96 (1.87 to 2.06) | 4.0 (3.5 to 4.4) |
| CVD in UK Biobank | |||
| CVD mortality: | |||
| High SES | 1 (Reference) | 1 (Reference) | - |
| Medium SES | 1.33 (1.19 to 1.49) | 1.31 (1.17 to 1.47) | 4.6 (2.8 to 7.2) |
| Low SES | 2.31 (2.06 to 2.59) | 2.25 (2.00 to 2.53) | 3.0 (2.5 to 3.6) |
| Incident CVD: | |||
| High SES | 1 (Reference) | 1 (Reference) | - |
| Medium SES | 1.29 (1.20 to 1.39) | 1.28 (1.18 to 1.38) | 4.1 (2.8 to 5.9) |
| Low SES | 1.69 (1.55 to 1.83) | 1.65 (1.52 to 1.79) | 3.7 (3.1 to 4.5) |
US NHANES=US National Health and Nutrition Examination Survey.
SES was generated through latent class analysis using information on family income to poverty ratio, occupation, education, and health insurance in US NHANES, and household income, education, and employment status in UK Biobank. All models included age, sex, marital status (US NHANES only), self-reported race, acculturation, study center (UK Biobank only), body mass index, and prevalent comorbidities (including history of hypertension, diabetes, CVD, cancer, chronic bronchitis, emphysema, or chronic obstructive pulmonary disease). The healthy lifestyle score consisted of never smoking, no heavy alcohol consumption, higher physical activity level, and a higher diet quality score. Analysis in US NHANES included the US population and study design weights to account for the complex survey design. Only those free from CVD at baseline were included in the analysis for incident CVD.
When low SES levels were compared with high SES levels, each individual socioeconomic factor was associated with higher risks of all primary outcomes, and the hazard ratios ranged from 1.13 to 2.09 (supplementary table 7). The proportion of the association between individual socioeconomic factors and mortality mediated by lifestyles ranged from less than 1% for household income in UK Biobank to 22.2% for education attainment in both cohorts. When the Townsend deprivation index was simultaneously included in the final model in UK Biobank, the associations of individual level SES with primary outcomes were not materially changed. In general, the associations between Townsend deprivation index and health outcomes were weaker compared with individual level SES (supplementary fig 2). Results of all sensitivity analyses were largely consistent, except that the mediation proportion increased when the healthy lifestyle score was substituted by four individual lifestyle factors in UK Biobank (supplementary table 5).
Supplementary table 8 shows the results for the mortality and morbidity of CVD subtypes. The hazard ratios when low SES was compared with high SES ranged from 1.45 for incident stroke to 2.62 for coronary heart disease mortality, and the mediation proportion by lifestyle ranged from 2.8% to 8.2%.
Interaction and joint analysis of lifestyle and SES with mortality and incident CVD
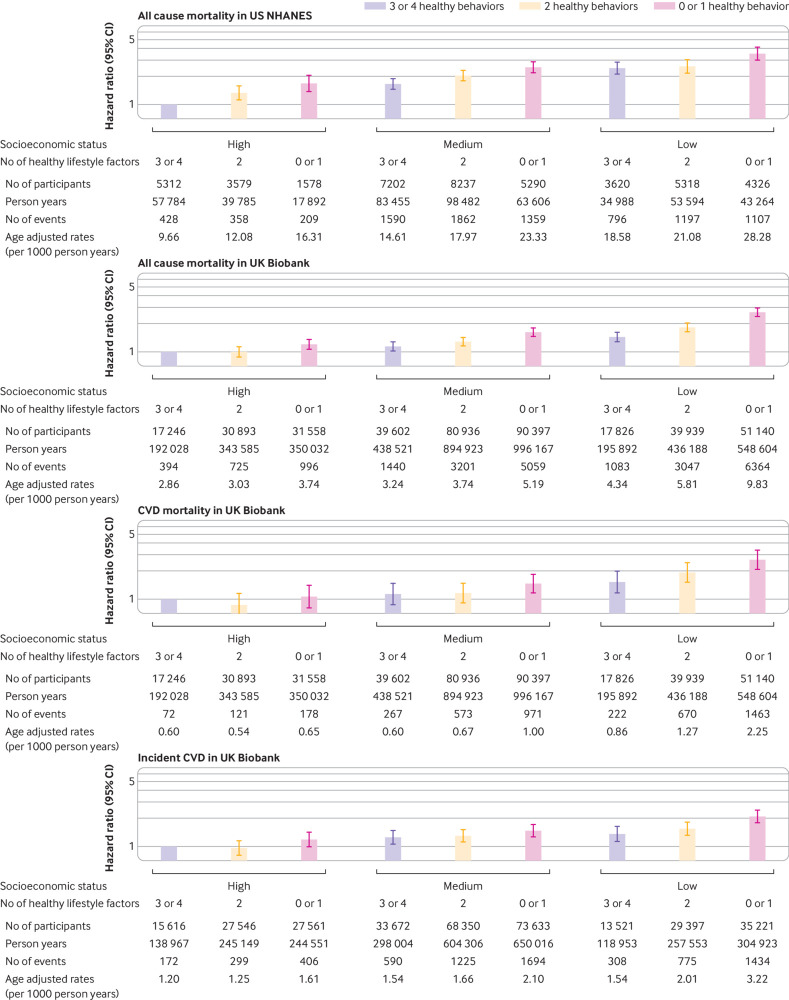
No significant interaction was found between lifestyle and SES on all cause mortality in US NHANES, whereas both multiplicative and additive interactions were observed between lifestyle and SES on all primary outcomes in UK Biobank (all P for interaction <0.02; fig 1). A healthier lifestyle score was associated with lower risks of all primary outcomes among individuals of various SES subgroups in both cohorts, whereas the associations were stronger among those from a low SES subgroup in UK Biobank (fig 1). For example, in UK Biobank, the hazard ratios for those with three or four healthy lifestyle factors compared with no or one healthy lifestyle factor for all cause mortality were 0.86 (0.76 to 0.96) among individuals of high SES, 0.70 (0.66 to 0.74) among those of medium SES, and 0.56 (0.52 to 0.59) among those of low SES. Similar patterns were found for total CVD mortality and incident CVD (fig 1), and when CVD subtypes were used as the outcomes (supplementary fig 3), as well as when the area level Townsend deprivation index was used as the SES variable (supplementary fig 2). The results remained similar in all sensitivity analyses (supplementary table 9).
Fig 1.
Associations of healthy lifestyle score with mortality and incident cardiovascular disease (CVD) by socioeconomic status (SES). In the US National Health and Nutrition Examination Survey (US NHANES), models included US population and study design weights to account for the complex survey design. Hazard ratios were adjusted for age, sex, marital status (US NHANES only), self-reported race, acculturation, study center (UK Biobank only), body mass index, and prevalent comorbidities (including history of hypertension, diabetes, CVD, cancer, chronic bronchitis, emphysema, or chronic obstructive pulmonary disease). Only those free from CVD at baseline were included in the analysis for incident CVD. Multiplicative interaction was evaluated using hazard ratios for the product term between the healthy lifestyle score (0 or 1 point v 3 or 4 points) and SES (low v high), and the multiplicative interaction was statistically significant when its confidence interval did not include 1. Additive interaction was evaluated using relative excess risk due to interaction (RERI) between the healthy lifestyle score (0 or 1 point v 3 or 4 points) and SES (low v high), and the additive interaction was statistically significant when its confidence interval did not include 0
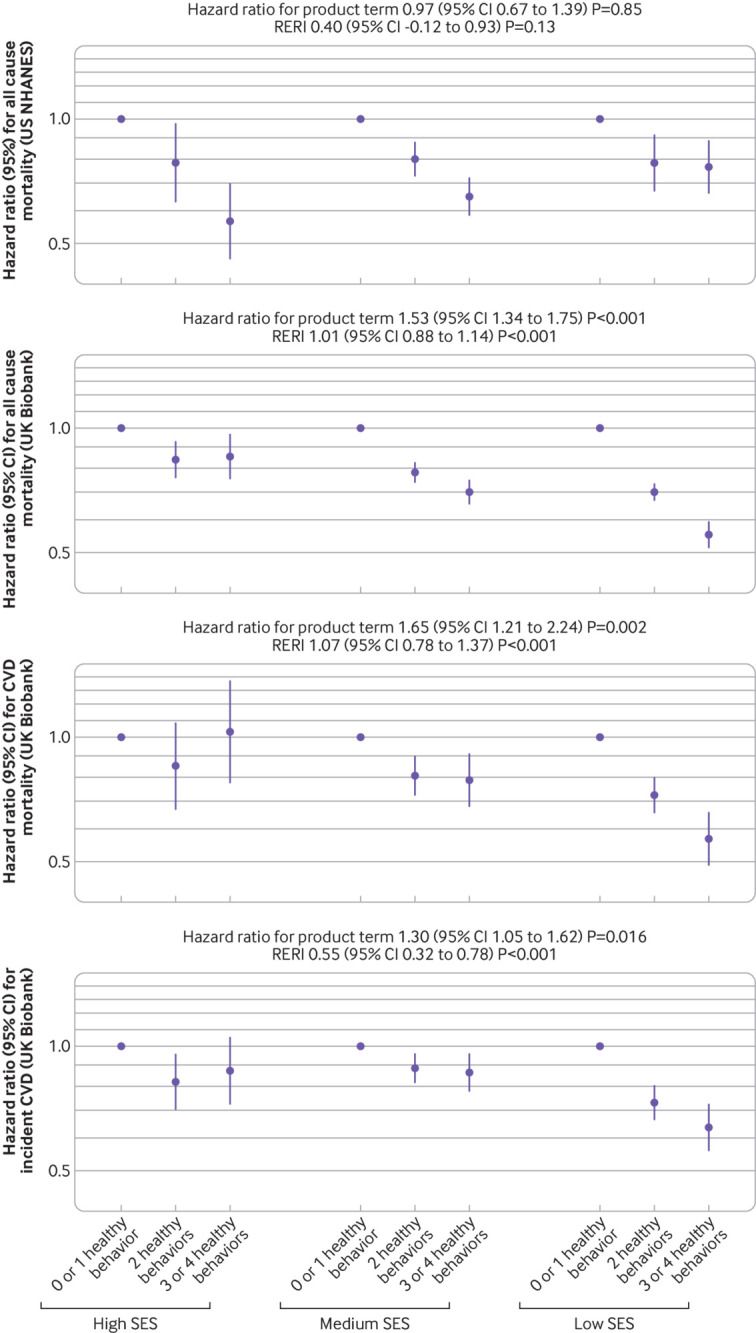
Figure 2 shows the joint association of lifestyles and SES on the primary outcomes, and hazard ratios for individuals of low SES and no or one healthy lifestyle factor compared with those with high SES and three or four healthy lifestyle factors were 3.53 (3.01 to 4.14) for all cause mortality in US NHANES, and 2.65 (2.39 to 2.94) for all cause mortality, 2.65 (2.09 to 3.38) for CVD mortality, and 2.09 (1.78 to 2.46) for incident CVD in the UK Biobank. Results were not materially changed in all sensitivity analyses (supplementary table 10), and similar patterns were found when using individual socioeconomic factors in the analysis (supplementary fig 4), as well as when using the area level Townsend deprivation index in the UK Biobank (supplementary fig 2).
Fig 2.
Joint associations of healthy lifestyle score and socioeconomic status with mortality and incident cardiovascular disease (CVD). In the US National Health and Nutrition Examination Survey (US NHANES), models included US population and study design weights to account for the complex survey design. Hazard ratios were adjusted for age, sex, marital status (US NHANES only), self-reported race, acculturation, study center (UK Biobank only), body mass index, and prevalent comorbidities (including history of hypertension, diabetes, CVD, cancer, chronic bronchitis, emphysema, or chronic obstructive pulmonary disease). Only those free from CVD at baseline were included in the analysis for incident CVD
Lifestyle and socioeconomic inequity in health among subpopulations
Supplementary tables 11 and 12 and supplementary figure 5 show results stratified by sex, self-reported race, and age group. The socioeconomic inequity in all cause mortality and the joint associations of lifestyles and SES with all cause mortality were stronger in men than in women, and in younger than older adults in both cohorts (P for interaction <0.03). The results were not substantially different between white and non-white people. The proportions of socioeconomic inequity in health mediated by lifestyles were all modest (all <20%; data not shown) and similar to those of the main analyses.
Discussion
In these two large US and UK cohorts, low SES was associated with higher risks of mortality and CVD, and 3.0% to 12.3% of the associations were mediated by lifestyle factors. In UK Biobank, significant interactions were found between lifestyle factors and SES on all primary outcomes, and the associations between lifestyle factors and health outcomes were stronger among those of low SES. The highest risks of mortality and CVD were seen in adults of low SES and with the least healthy lifestyles.
Comparison with other studies
Socioeconomic inequity in mortality has been widely discussed. A large multicohort study with 1.7 million participants from the US, Europe, and Australia found that low SES was associated with a 26% higher risk of mortality and 2.1 years of life lost between ages 40 and 85 years, and low SES might respectively contribute to 15.3% and 18.9% of deaths among women and men.1 Moreover, socioeconomic inequity in mortality has continuously widened in the US. From 2001 to 2014, longevity increased by 2.34 and 2.91 years, respectively, among the wealthiest 5% of US men and women, whereas only 0.32 and 0.04 years among the poorest 5% of US men and women.46 Similar trends were also observed in the UK, or when high education levels were compared with low education levels.2 3 Our analysis confirmed the socioeconomic disparity in mortality and extended the findings to CVD morbidity and mortality. Thus, exploring the possible methods to reduce socioeconomic inequity in health is urgently needed.
The current evidence indicates causal relations between SES and death,47 and SES could affect individuals’ access to multitudinous resources (eg, knowledge, wealth, power, prestige, and advantageous social connections) and protective factors (eg, healthy lifestyle and healthcare services). Many studies have investigated the contribution of health behaviors to socioeconomic inequity in health outcomes, including mortality and CVD. A systematic review of 31 studies6 reported that about 20% to 30% of the socioeconomic inequity in health outcomes were explained by lifestyle factors. However, substantial heterogeneity was reported, with a minimum of −59% to a maximum of 75%. Therefore, firm conclusions cannot be made, and there are several potential reasons why this is not possible. First, most studies investigated a single socioeconomic factor, and studies examining an overall individual level SES were limited. Although different socioeconomic factors might correlate with each other, they reflected different domains of SES or social class and should not be simply replaced by others. Second, most previous studies examined single or limited numbers of lifestyle factors, and only five studies considered all lifestyle factors (smoking, alcohol consumption, physical activity, and diet) in the models.48 49 50 51 52 Third, the characteristics of study populations (eg, age, sex composition, race or ethnicity, regions, SES levels, health status), study design (cross sectional or longitudinal, and follow-up duration if a cohort study), data collection methods, and statistical methods (such as adjustment for covariates) varied widely.
Our study found that in US and UK adults only up to 12.3% of the association between SES and mortality was explained by lifestyle factors. The results are consistent with several other studies in various populations.53 54 55 In the longitudinal analyses on 22 194 participants in the Moli-sani study, Italy, participants of poor SES in childhood (assessed by a score of three variables: housing tenure, access to hot water, and overcrowding in household) but an upward trajectory in both education attainment and material circumstances had lower risks of total and cause specific mortality, whereas health related behaviors explained less than 10% of the association.56 The low mediation proportion indicated that substantial reductions of the socioeconomic inequity in health could not be achieved through promoting healthy lifestyles alone, and other measures to tackle the social determinants of health are still needed.
In our study, we also confirmed that healthy lifestyles were associated with lower risks of mortality and incident CVD in the two cohorts, regardless of SES. In addition, significant interactions were observed in the UK study, and the protective associations of healthy lifestyles and health outcomes were stronger among those of low SES, which highlighted the necessity of lifestyle modification, especially among those of low SES who were more vulnerable to unhealthy lifestyles. This is consistent with a previous analysis in the UK Biobank study,20 which also found that combinations of unhealthy lifestyle factors were associated with disproportionate harm in deprived populations, as assessed by the Townsend deprivation index, an area level SES variable. However, we found no significant interaction between lifestyles and SES on total mortality in the US study, similar to an analysis of education attainment and lifestyles with CVD mortality in Japan.57 Another study in a generally low income population in the US even found a weaker association between lifestyles and mortality among men with relatively low incomes, but not among women.44 The exact reasons for the inconsistent findings were unclear, but might depend on the definition of SES and lifestyle factors as well as the population characteristics. More studies are still needed to understand the complex relations between lifestyle factors and SES on health.
We also compared the overall individual level SES variable and Townsend deprivation index in the UK Biobank and found that the associations of individual level SES with outcomes were stronger than those of area level SES, and similar patterns were observed for the joint associations of lifestyle factors and SES. Besides, effect sizes of individual level SES were not attenuated after adjusting for Townsend deprivation index. Accordingly, it is necessary to construct an overall individual level SES variable because postcode derived area level SES reflects different aspects and has several problems, such as inability to determine social causes of health, inability to distinguish individual differences, confusion with other environmental health determinants, unreliability when populations are heterogeneous or change quickly, and inapplicability to mobile communities.58
Strengths and limitations of this study
Major strengths of this study are the large sample size from two well established nationwide cohorts in the US and UK—the findings are generally consistent within the two cohorts except for the interaction between lifestyle factors and SES on health outcomes. The large sample size also allowed us to perform the joint and stratified analyses with sufficient statistical power. In addition, we constructed an overall SES variable and healthy lifestyle score to comprehensively evaluate the complex relations of lifestyle factors and SES with mortality and incident CVD. We also conducted a series of sensitivity analyses to show the robustness of the findings, and evaluated individual socioeconomic and lifestyle factors.
Nevertheless, we also acknowledge several limitations. First, information on socioeconomic level and lifestyle was mainly self-reported and was only measured once, thus measurement errors were inevitable. Besides, we could not capture the long term SES trajectories as well as lifestyle changes during adulthood. Future studies with repeated measurements are preferred. Second, the SES variable was constructed differently in the two cohorts. For example, health insurance scheme was included as a component in the US study but not in the UK study, and occupational information was not collected at baseline in the UK Biobank and thus we could only use employment status. Third, a lifestyle score derived from a sum of the number of healthy lifestyle factors assumed that all lifestyle factors had equal effects on health outcomes, which might not be true. Although we constructed a weighted lifestyle score in the sensitivity analysis and found similar results, the weighted score still cannot fully account for the complex interactions between lifestyle factors, and the weights were study specific. Fourth, the follow-up duration is relatively short (mean 8.8-11.2 years), and those who died during the study period might have had serious diseases at baseline. Both lifestyle behaviors and SES could be influenced by disease status. Although our main analysis of adjusting comorbidities at baseline and sensitivity analysis of excluding those with major chronic diseases at baseline generated robust results, the possibility of reverse causation and residual confounding (many other diseases were not measured or considered) cannot be fully eliminated. Fifth, those excluded from the analysis because of missing covariates were more likely to be of lower SES; therefore, the socioeconomic inequity in health outcomes might be underestimated in our study. Nevertheless, the results remained similar after imputing missing covariates. Sixth, owing to the nature of post hoc subgroup analyses, sample size in each subgroup was not calculated before data collection. Especially, the number of participants and events might be insufficient among the non-white subgroup in the UK Biobank, and the results should be cautiously interpreted. Finally, although we controlled for key personal characteristics and comorbidities, residual confounding was still possible and causal inference cannot be made because of the nature of observational studies.
Conclusions
Based on two large nationwide US and UK cohorts, low SES was found to be significantly associated with higher risks of mortality and incident CVD, and the associations were modestly mediated by lifestyle factors. Therefore, promoting healthy lifestyles alone might not substantially reduce the socioeconomic inequity in health without other social determinants of health being considered. The finding argues for government policies to tackle upstream social and environmental determinants of health.59 Nevertheless, individuals with disadvantaged SES and unhealthy lifestyles had the highest risks of mortality and incident CVD, which highlights the importance of lifestyle modification in reducing disease burden for all people, especially those of low SES in the UK.
What is already known on this topic
Disadvantaged socioeconomic status (SES) and unhealthy lifestyles have been associated with higher risks of mortality and incident cardiovascular disease (CVD)
Studies found that individual lifestyle factors might mediate the associations between single socioeconomic factors and health; however, the results are not consistent, and to what extent lifestyle factors mediate the associations of overall SES with mortality and incident CVD remains unclear
Little is known about the interaction and joint associations of lifestyles and SES with mortality and incident CVD
What this study adds
In two nationwide cohort studies in US and UK adults, those of low SES had higher risks of mortality and CVD, and overall lifestyle only explained 3.0% to 12.3% of the excess risks
Significant interactions were found between lifestyle factors and SES on mortality and incident CVD in UK adults, and the associations between healthy lifestyles and outcomes were stronger among those of low SES
Compared with those of high SES and the healthiest lifestyle, those of low SES and the least healthy lifestyle had 2.09-fold to 3.53-fold risks of mortality and incident CVD
Web extra.
Extra material supplied by authors
Supplementary information: Additional methods, figures, and tables
Contributors: YBZ and AP designed the study. YBZ, CC, and XFP conducted the data analysis. YBZ drafted the manuscript. JG, YL, OHF, GL, and AP critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript. AP is guarantor. The corresponding author attests that all the listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: AP was supported by the National Nature Science Foundation of China (81930124) and National Key Research and Development Program of China (2017YFC0907504). The funders had no role in the study design or implementation; data collection, management, analysis, or interpretation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Nature Science Foundation of China and National Key Research and Development Program of China (AP) for the submitted work; support from California Walnut Committee and Swiss Reinsurance Company (YL) outside the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study protocol for the US NHANES was approved by the US NHANES institutional review board and National Center for Health Statistics Research ethics review board. The North West Multi-Centre Research Ethics Committee approved the collection and use of UK Biobank data. All participants provided written informed consent. Institutional review board approval was waived for this analysis because of the publicly available and deidentified data.
Data sharing: NHANES data are available at www.cdc.gov/nchs/nhis/index.htm, and data from UK Biobank are available on application at www.ukbiobank.ac.uk/register-apply.
The lead author (AP) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results of the research will be disseminated to the public through broadcasts and popular science articles.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Stringhini S, Carmeli C, Jokela M, et al. LIFEPATH consortium . Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 2017;389:1229-37. 10.1016/S0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmot M, Allen J, Boyce T, Goldblatt P, Morrison M. Health equity in England: the Marmot review ten years on. 2020. www.health.org.uk/publications/reports/the-marmot-review-10-years-on.
- 3. Bor J, Cohen GH, Galea S. Population health in an era of rising income inequality: USA, 1980-2015. Lancet 2017;389:1475-90. 10.1016/S0140-6736(17)30571-8. [DOI] [PubMed] [Google Scholar]
- 4. Niedzwiedz CL, O’Donnell CA, Jani BD, et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. BMC Med 2020;18:160. 10.1186/s12916-020-01640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phelan JC, Link BG. Controlling disease and creating disparities: a fundamental cause perspective. J Gerontol B Psychol Sci Soc Sci 2005;60:27-33. 10.1093/geronb/60.Special_Issue_2.S27. [DOI] [PubMed] [Google Scholar]
- 6. Petrovic D, de Mestral C, Bochud M, et al. The contribution of health behaviors to socioeconomic inequalities in health: A systematic review. Prev Med 2018;113:15-31. 10.1016/j.ypmed.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 7. Quaglia A, Lillini R, Mamo C, Ivaldi E, Vercelli M, SEIH (Socio-Economic Indicators, Health) Working Group . Socio-economic inequalities: a review of methodological issues and the relationships with cancer survival. Crit Rev Oncol Hematol 2013;85:266-77. 10.1016/j.critrevonc.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 8. Shankar A, McMunn A, Steptoe A. Health-related behaviors in older adults relationships with socioeconomic status. Am J Prev Med 2010;38:39-46. 10.1016/j.amepre.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention/National Center for Health Statistics. About the National Health and Nutrition Examination Survey. 2017. www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 10. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marmot MG. Status syndrome: a challenge to medicine. JAMA 2006;295:1304-7. 10.1001/jama.295.11.1304. [DOI] [PubMed] [Google Scholar]
- 12. Odutayo A, Gill P, Shepherd S, et al. Income disparities in absolute cardiovascular risk and cardiovascular risk factors in the United States, 1999-2014. JAMA Cardiol 2017;2:782-90. 10.1001/jamacardio.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ 2019;367:l5584. 10.1136/bmj.l5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapman BP, Fiscella K, Kawachi I, Duberstein PR. Personality, socioeconomic status, and all-cause mortality in the United States. Am J Epidemiol 2010;171:83-92. 10.1093/aje/kwp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens G, Cho JH. Socioeconomic indexes and the new 1980 census occupational classification scheme. Soc Sci Res 1985;14:142-68. 10.1016/0049-089X(85)90008-0. [DOI] [Google Scholar]
- 16. Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: how the new measures measure up. Sociol Methodol 1994;24:1-72. 10.2307/270978. [DOI] [Google Scholar]
- 17. Le P, Chaitoff A, Misra-Hebert AD, Ye W, Herman WH, Rothberg MB. Use of antihyperglycemic medications in U.S. adults: an analysis of the National Health and Nutrition Examination Survey. Diabetes Care 2020;43:1227-33. 10.2337/dc19-2424. [DOI] [PubMed] [Google Scholar]
- 18. Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling 2007;14:671-94. 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins LM, Lanza ST. Latent class and latent transition analysis: with applications in the social, behavioral, and health sciences. Wiley, 2010. [Google Scholar]
- 20. Foster HME, Celis-Morales CA, Nicholl BI, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health 2018;3:e576-85. 10.1016/S2468-2667(18)30200-7. [DOI] [PubMed] [Google Scholar]
- 21.Department of Health & Social Care. Guidance: the NHS Constitution for England. 2015. www.gov.uk/government/publications/the-nhs-constitution-for-england/the-nhs-constitution-for-england.
- 22. Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the North. Croom Helm, 1988. [DOI] [PubMed] [Google Scholar]
- 23. Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality: findings from the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health 2011;101:1922-9. 10.2105/AJPH.2011.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Tackling NCDs: ‘best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases. 2017. https://apps.who.int/iris/bitstream/handle/10665/259232/WHO-NMH-NVI-17.9-eng.pdf?sequence=1&isAllowed=y.
- 25.US Department of Agriculture, US Department of Health and Human Services. Dietary guidelines for Americans, 2020-2025. 2020. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf.
- 26.National Health Service. The risks of drinking too much. 2019. www.nhs.uk/live-well/alcohol-support/the-risks-of-drinking-too-much/.
- 27.National Cancer Institute. Developing the Healthy Eating Index. 2019. https://epi.grants.cancer.gov/hei/developing.html#f1b.
- 28. Li Y, Schoufour J, Wang DD, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ 2020;368:l6669. 10.1136/bmj.l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank study. JAMA Cardiol 2018;3:693-702. 10.1001/jamacardio.2018.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Pan XF, Chen J, et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia 2020;63:21-33. 10.1007/s00125-019-04985-9. [DOI] [PubMed] [Google Scholar]
- 31. Zhang YB, Pan XF, Chen J, et al. Combined lifestyle factors, all-cause mortality and cardiovascular disease: a systematic review and meta-analysis of prospective cohort studies. J Epidemiol Community Health 2021;75:92-9. 10.1136/jech-2020-214050. [DOI] [PubMed] [Google Scholar]
- 32. Zhang YB, Pan XF, Chen J, et al. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer 2020;122:1085-93. 10.1038/s41416-020-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greenberg JA. The obesity paradox in the US population. Am J Clin Nutr 2013;97:1195-200. 10.3945/ajcn.112.045815. [DOI] [PubMed] [Google Scholar]
- 34. Yoshida Y, Broyles S, Scribner R, et al. Social support modifies the negative effects of acculturation on obesity and central obesity in Mexican men. Ethn Health 2020;25:1103-14. 10.1080/13557858.2018.1492708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics. 2015 public-use linked mortality files. 2019. www.cdc.gov/nchs/data-linkage/mortality-public.htm.
- 36.Biobank UK. Mortality data: linkage to death registries. 2020. https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/DeathLinkage.pdf.
- 37.Biobank UK. Definitions of stroke for UK Biobank phase 1 outcomes adjudication. 2017. https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_stroke.pdf.
- 38.Biobank UK. Definitions of acute myocardial infarction and main myocardial infarction pathological yypes: UK Biobank phase 1 outcomes adjudication. 2017. https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_mi.pdf.
- 39.US Centers for Disease Control and Prevention. Key concepts about NHANES survey design. 2015. www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/SampleDesign/Info1.htm.
- 40. SAS Institute . SAS/STAT® 15.1 user’s guide. SAS Institute, 2018. [Google Scholar]
- 41. Nevo D, Liao X, Spiegelman D. Estimation and inference for the mediation proportion. Int J Biostat 2017;13:/j/ijb.2017.13.issue-2/ijb-2017-0006/ijb-2017-0006.xml. 10.1515/ijb-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology 1992;3:452-6. 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. Ageing and health. 2018. www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 44. Warren Andersen S, Zheng W, Sonderman J, et al. Combined impact of health behaviors on mortality in low-income Americans. Am J Prev Med 2016;51:344-55. 10.1016/j.amepre.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan Y. Multiple imputation using SAS software. J Stat Softw 2011;45:1-25. 10.18637/jss.v045.i06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chetty R, Stepner M, Abraham S, et al. The association between income and life expectancy in the United States, 2001-2014. JAMA 2016;315:1750-66. 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kawachi I, Adler NE, Dow WH. Money, schooling, and health: Mechanisms and causal evidence. Ann N Y Acad Sci 2010;1186:56-68. 10.1111/j.1749-6632.2009.05340.x. [DOI] [PubMed] [Google Scholar]
- 48. Hastert TA, Ruterbusch JJ, Beresford SA, Sheppard L, White E. Contribution of health behaviors to the association between area-level socioeconomic status and cancer mortality. Soc Sci Med 2016;148:52-8. 10.1016/j.socscimed.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jarvandi S, Yan Y, Schootman M. Income disparity and risk of death: the importance of health behaviors and other mediating factors. PLoS One 2012;7:e49929. 10.1371/journal.pone.0049929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laaksonen M, Talala K, Martelin T, et al. Health behaviours as explanations for educational level differences in cardiovascular and all-cause mortality: a follow-up of 60 000 men and women over 23 years. Eur J Public Health 2008;18:38-43. 10.1093/eurpub/ckm051. [DOI] [PubMed] [Google Scholar]
- 51. Schrijvers CT, Stronks K, van de Mheen HD, Mackenbach JP. Explaining educational differences in mortality: the role of behavioral and material factors. Am J Public Health 1999;89:535-40. 10.2105/AJPH.89.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stringhini S, Dugravot A, Shipley M, et al. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med 2011;8:e1000419. 10.1371/journal.pmed.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feinglass J, Lin S, Thompson J, et al. Baseline health, socioeconomic status, and 10-year mortality among older middle-aged Americans: findings from the Health and Retirement Study, 1992 2002. J Gerontol B Psychol Sci Soc Sci 2007;62:S209-17. 10.1093/geronb/62.4.S209. [DOI] [PubMed] [Google Scholar]
- 54. Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA 1998;279:1703-8. 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 55. Woodward M, Oliphant J, Lowe G, Tunstall-Pedoe H. Contribution of contemporaneous risk factors to social inequality in coronary heart disease and all causes mortality. Prev Med 2003;36:561-8. 10.1016/S0091-7435(03)00010-0. [DOI] [PubMed] [Google Scholar]
- 56. Bonaccio M, Di Castelnuovo A, Costanzo S, et al. Interaction between education and income on the risk of all-cause mortality: prospective results from the MOLI-SANI study. Int J Public Health 2016;61:765-76. 10.1007/s00038-016-0822-z. [DOI] [PubMed] [Google Scholar]
- 57. Eguchi E, Iso H, Honjo K, Yatsuya H, Tamakoshi A. No modifying effect of education level on the association between lifestyle behaviors and cardiovascular mortality: the Japan Collaborative Cohort Study. Sci Rep 2017;7:39820. 10.1038/srep39820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moscrop A, Ziebland S, Bloch G, Iraola JR. If social determinants of health are so important, shouldn’t we ask patients about them? BMJ 2020;371:m4150. 10.1136/bmj.m4150. [DOI] [PubMed] [Google Scholar]
- 59. Marmot M, Friel S, Bell R, Houweling TAJ, Taylor S, Commission on Social Determinants of Health . Closing the gap in a generation: health equity through action on the social determinants of health. Lancet 2008;372:1661-9. 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional methods, figures, and tables