Abstract
In this study, an alternative extraction technique, ultrasound-assisted extraction, was used to extract the polyphenolic fraction from two different residues of the candelilla plant (Euphorbia antisyphilitica). These metabolites were further analyzed to evaluate their bioactivity as antioxidants. In addition, their functional groups were identified by Fourier transform infrared (FTIR) spectroscopy. The antioxidant assays showed statistically significant differences between the phenolic extracts, with citric acid residues (CAR) exhibiting a higher oxidant effect than sulfuric acid residues (SAR). The CAR from San Jerónimo (SJ) cultivar showed decreased IC50 values (179.441 ± 7.92 μL mL−1, DPPH•), and its polyphenolic fraction was able to inhibit lipid oxidation (70.31 ± 2.50%). FTIR analysis subsequently revealed the presence of functional groups related to polyphenolic compounds, such as hydroxy, carbonyl, carbon double bond, and amine groups. In addition, FTIR spectra showed slight differences in phenolic compounds, due to the strong acid treatment involved in the extraction of wax. The present study demonstrated that candelilla by-products from citric acid-wax extraction have a polyphenolic fraction with strong antioxidant activity, which may be useful in food and pharmaceutical products.
Keywords: E. antisyphilitica Zucc., Polyphenolic compounds, Antioxidant activity, Ultrasound-assisted extraction
E. antisyphilitica Zucc.; Polyphenolic compounds; Antioxidant activity; Ultrasound-assisted extraction.
1. Introduction
Euphorbia antisyphilitica Zucc., better known as the candelilla plant, is a non-timber resource utilized in the northern regions of Mexico. This plant is a perennial shrub with few leaves and has cylindrical stems that resemble the appearance of candles. These stems are erect and covered with wax. The wax in the plants generally fulfills the function of a waterproofing agent, and is of vital support against extreme stress factors, preventing dehydration of the plant tissue by evaporation [1]. Considering that around of 101.5 million hectares are estimated as “Drylands” in Mexico [2, 3], the northern region of Mexico is an area that favors the production of wax in the plant, which is subsequently collected by people in the communities of the region.
The production of candelilla wax is an exhaustive task, with a relevant impact on the region's economy as it represents a source of economic income for families in marginalized communities, along with other non-timber resources such as oregano, laurel, and lechuguilla. Candelilla wax is a product with numerous applications in the food and cosmetic markets, including as a fruit coating, an additive to cosmetics (e.g., lipstick, lip gloss and cleaning sticks), an insulative coating, and a component for the formation of hydrophobic surfaces, among other applications as described in patents CN109620744, CN109431877, and CN108978338 (see WIPO-Patentscope website).
Briefly, the wax extraction process includes the collection of the candelilla plant and elimination of impurities (soil and rocks), extraction using an acidic methodology involving H2SO4, which has a toxic impact on workers, and finally an impurity removal procedure [4, 5]. In light of this, De León-Zapata [6] proposed an alternative technology using citric acid, with the benefit of no toxic gas emission while obtaining similar yields compared to the traditional method. In any case, the extraction process generates a considerable amount of waste that eliminated by burning processes, or by incorrect disposal of the material in land, causing ecological problems.
Since candelilla by-products could be a rich source of bioactive compounds with possible applications in the industry, scientific projects in recent years have focused on the revaluation of residues through the integral use of residual chemistry in the cell matrix [7] or the application of additional processes that allow the generation of organic molecules of interest through solid-state fermentation processes [8, 9]. According to the literature, candelilla by-products contain polyphenolic compounds such as ellagitannins and ellagic acid [10]. Thus, the implementation of well-known emerging technologies such as ultrasound-assisted extraction (UAE) in the recovery of polyphenols is an interesting alternative with pertinent advantages compared to conventional predecessors. These advantages include a lack of necessity for high operating temperatures, a lower degree of complexity, no generation of toxic waste, high performance, and increased quality of the obtained compounds [11, 12, 13]. As a result, polyphenolic isolation can be performed by non-destructive techniques such as membrane separation, solvent extraction, and absorption [14]. Frequently, studies have applied adsorption, an easier operational process that can be carried out by non-functionalized or functionalized hydrophobic resins [15] and the addition of different polar/non-polar solvents [14]. The adsorption of polyphenolic compounds with XAD16 resins has also been successfully employed from other plant sources [16, 17].
Furthermore, there has been scarce evaluation of the antioxidant properties of candelilla by-products, and only one study relating to the DPPH• free radical-scavenging capacity of extracts from these residues has been published [10]. Therefore, the objective of the present study was to evaluate the antioxidant potential of the polyphenolic fraction from the residual material of the traditional and alternative wax extraction processes of E. antisyphilitica. Finally, to the best of our knowledge, the present study is the first to evaluate the antioxidant capacity (by different methods) and characterization of functional groups by FTIR of E. antisyphilitica by-products obtained by traditional and alternative methods from plants cultivated in northern Mexico.
2. Materials and methods
2.1. Chemicals and reagents
Ethanol, distilled water, citric acid, gallic acid, linoleic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), ABTS•+ (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)), Tween 20, Amberlite XAD-16, ferrous chloride (FeCl2), ferric chloride (FeCl3), monobasic potassium phosphate (KH2PO4), dibasic potassium phosphate (K2HPO4) potassium ferrocyanide (C6FeK4N6), sodium nitrite (NaNO2), aluminum chloride (AlCl3), and other reagents were purchased from Sigma Aldrich (Toluca, Mexico). Sodium carbonate anhydrous (Na2CO3), acetic acid (CH3COOH), sodium hydroxide (NaOH), and sodium carbonate (Na2CO3) were purchased from Sigma-Aldrich (Fair Lawn, EE. UU).
2.2. Candelilla by-products
Candelilla by-products (from the traditional process) were recovered after wax extraction in different localities distributed in the northern region of Mexico (Table 1). The samples were identified as follows: Estanque de León (León), San Jerónimo (SJ), San Miguel y Anexos (SMYA), and Lucio Blanco (LB). In the laboratory, plant material was washed with distilled water and then dried using an electric stove at 50 °C for 48 h. The dried material was subsequently crushed, and the particle size comprised 2–5 mm. Finally, the material obtained was stored in hermetic bags in the dark, at room temperature, until analysis.
Table 1.
Evaluated communities and sample IDs.
| Specie | Localities | City | State | ID |
|---|---|---|---|---|
| E. antisyphilitica | Estanque de León | Cuatrociénegas | Coah | León |
| San Jerónimo | Melchor Ocampo | Zac | SJ | |
| San Miguel y Anexos | Melchor Ocampo | Zac | SMYA | |
| Lucio Blanco | Cuatrociénegas | Coah | LB |
Coah = Coahuila de Zaragoza, Mexico.
Zac = Zacatecas, Mexico.
2.3. Wax extraction with citric acid
As the traditional procedure of candelilla wax-extraction is carried out using a solution of H2SO4, it was not possible to recollect citric acid residues on local communities. This extraction was carried out in a laboratory environment as follows. First, 200 g of small pieces (10 cm length) of candelilla plant were added to a beaker (4 L) with 1 L of a solution of boiling citric acid solution to a final concentration of 1.0%. The wax was recovered, and the plant material was dried and stored as described above.
2.4. UAE of polyphenolic compounds
The extraction of phenolic compounds was conducted using the methodology proposed by Castro-López et al. [18] with some modifications. The sample (5 g) was extracted with 60 mL of extraction solution (35:65, v/v, ethanol:water) at room temperature with a solid:liquid ratio of 1:12 (w/v). The extraction procedure was performed in dark bottles in an ultrasound bath (Model 2510, Sonics and Materials, Branson, MO, USA) to 40 kHz power (100%) for 40 min at room temperature. Subsequently, a vacuum pump and Whatman #41 filter paper were used for filtration, and the extraction solution was removed in an oven at 50 °C (48 h).
2.4.1. Purification of polyphenolic compounds
The purification was carried out using a stationary phase (Amberlite XAD-16) and a mobile phase (water and ethanol). First, the dried phenolic extract was re-suspended in 40 mL of bi-distilled water; 20 mL of extract was added to the stationary phase, and bi-distilled water was used as the eluent to discard unwanted compounds. Then, absolute ethanol was used as the eluent to obtain the polyphenolic-rich fraction. The solvent was evaporated (50 °C for 48 h) and the polyphenols were recovered as a fine powder.
2.5. Antioxidant activity assays
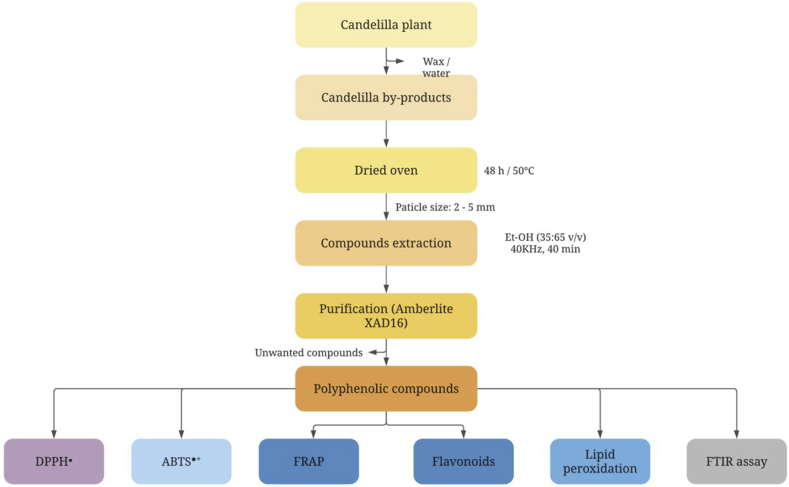
The polyphenolic compounds were resuspended in distilled water for antioxidant evaluation (Figure 1). A single concentration (250 μg mL−1) was used for lipid oxidation analysis, flavonoid assay, and ferric reducing antioxidant power (FRAP) evaluation. For anti-radical DPPH• and ABTS•+ activity, the IC50 value was determined using a concentration curve of the purified phenolic extract (1000, 750, 500, 250, and 0 μg mL−1).
Figure 1.
General process diagram of antioxidant activity evaluation of E. antisyphilitica by-products.
2.5.1. DPPH• radical scavenging assay
The free radical-scavenging capacity of the samples was evaluated from a purple color solution of radical DPPH•, using methanol as a solvent (60 mM). The assay was carried out according to the method of Brand-Williams et al. [19] with some modifications. A volume of 2,950 μL of DPPH• radical was added to each test tube for every 50 μL of sample or standard curve (gallic acid). The reaction mix was subjected to an incubation period in the dark for 30 min. Subsequently, the absorbance of the samples was recorded at a wavelength of 517 nm.
Inhibition capacity was calculated using the following Eq. (1), and the result was expressed by the IC50 value (concentration needed to reach 50% of radical inhibition):
| (1) |
where AControl represents the absorbance value of the control and ASample represents the absorbance value obtained for each specific sample.
2.5.2. ABTS•+ radical scavenging assay
Inhibition of the ABTS•+ radical was evaluated according to the methodology proposed by Van den Berg et al. [20] with slight modifications. The ABTS•+ radical cation was generated by aqueous ABTS solution (7 mM) with potassium persulfate (2.45 mM) mixing in the dark at room temperature for 12 h before use. Working ABTS•+ solution was adjusted in ethanol to 0.700 ± 0.002 nm of absorbance. A sample volume (50 μL) was mixed to react with 950 μL of the ABTS•+ solution, and after 1 min of reaction, the absorbance was measured (734 nm). The radical inhibition capacity was calculated using Eq. (2), and the result was expressed as the IC50 value (concentration needed to reach 50% of radical inhibition):
| (2) |
where AControl represents the absorbance value of the control and ASample represents the absorbance value obtained for each specific sample.
2.5.3. Ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power was determined according to the methodology proposed by Celik et al. [21] with slight modifications. Samples (500 μL) were mixed with 120 μL of phosphate buffer (pH 7), prepared by mixing dibasic potassium phosphate (61.5 mL, 1 M), monobasic potassium phosphate (38.1 mL, 1 M), and water was added to 1000 mL in a volumetric flask. Then, 220 μL of 1% potassium ferrocyanide (C6FeK4N6) was added to the reaction mix, which was homogenized and incubated at 50 °C for 20 min. Next, 10% trichloroacetic acid (12 μL), distilled H2O (45 μL), and 0.1% ferric chloride (10 μL) were added. The absorbance was recorded at a wavelength of 734 nm. Finally, the results were reported as μg equivalent gallic acid per milliliter according to a calibration curve prepared with the same standard.
2.5.4. Flavonoid content
The flavonoid content was determined according to the method of De la Rosa et al. [22] with some modifications. A sample volume (310 μL) was mixed with 93 μL of 5% sodium nitrite (w/v) and 93 μL of distilled water. The solution was manually mixed and incubated for 3 min at 40 °C. Then, 93 μL of 10% aluminum chloride (w/v) was added and incubated for 3 min. Finally, 1250 μL of 0.5 M sodium hydroxide was added and incubated for 30 min in the dark. The absorbance was recorded at 510 nm. Finally, the results were reported as μg equivalent quercetin per milliliter according to a calibration curve prepared with the same standard.
2.5.5. Lipid peroxidation inhibition (LPO) assay
The assay was performed according to Zou et al. [23] with some modifications. A linoleic acid solution was prepared by mixing 0.6 g of linoleic acid and 1.5 g of Tween 20 dissolved in 8 mL of ethanol. Next, 100 μL of a linoleic acid solution and 1500 μL of the acetate buffer (0.02 M, pH 4) were mixed with 50 μL of the sample to be analyzed (controls = 50 μL of distilled water). The samples were homogenized and incubated at 37 °C in a water bath for a min. After incubation, 750 μL of FeCl2 solution (0.01 g of FeCl2 and 0.017 g of EDTA diluted to 100 mL with distilled water) was added to induce lipid oxidation and incubated for 24 h at 37 °C. Finally, 250 μL aliquots were taken to analyze the oxidation (0 and 24 h).
For each aliquot, 1 mL of NaOH (0.1 M, in 10% ethanol, v/v) was added to stop the oxidation process, and 2.5 mL of ethanol (10%, v/v) was added to dilute the sample. Finally, the absorbance of the samples was measured at 232 nm using ethanol (10%, v/v) as a blank.
The inhibition of linoleic acid oxidation was calculated using Eq. (3):
| (3) |
where A is the difference between the absorbance of distilled water (as control) after 24 h and 0 h of incubation, and B is the difference between the absorbance of each purified phenolic extract sample after 24 h and 0 h of incubation.
2.6. Analysis by Fourier transform infrared spectroscopy
The analysis was performed using an Agilent Cary 630 FTIR coupled to a zinc selenide crystal (ZnSe) ATR. The dry purified phenolic extract was deposited on the entire surface of the reader and secured by means of the equipped press. The reading was performed using the MicroLab PC program in the spectral range of 4000 to 650 cm−1, with a cycle of 32 scans with a resolution of 2 cm−1. The analysis of the spectrum and the functional groups detected was carried out using the MicroLab Expert program, and the plot was constructed using the OriginPro 8 program.
2.7. Experimental design and statistical analysis
All experiments were conducted in triplicate and results are reported as mean ± standard deviation (SD). The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's HSD test with p = 0.05 considered statistically significant. Statistical analysis was performed using Minitab 17 Statistical Software (Minitab, Inc., State College, Pennsylvania, PA, USA).
3. Results and discussion
3.1. Flavonoid content
The global spread of COVID-19 has changed the food industry, especially in terms of food sustainability, safety, and security [24]. In addition, the novel coronavirus promotes consumer sales of supplements and nutraceutical products, with a paradigm change from a curative to a preventive model [25]. As a result, research into functional ingredients has increased, and a wide variety of bioactive compounds and their sources have been analyzed for their potential health benefits [26]. The preventive model therefore focuses on research into potential new polyphenolic sources to provide a starting material for functional products. Our results indicated that candelilla by-products can be considered a source of flavonoid-type compounds (Table 2), with a significant difference observed between the cultivars; compared to SAR, the LB cultivar had the highest value (78.95 ± 10.81 μg equivalent quercetin per milliliter), and CAR did not show any significant differences. The data suggest that the antioxidant activity of the analyzed samples does not depend only on flavonoid content, but also other polyphenolic classes (e.g., phenolic acids) could also be related to antioxidant properties. This is in line with previous findings reported by Rojas et al. [27], who observed that candelilla by-products are a source of phytochemicals such as flavonoids (catechin) and phenolic acids (gallic and ellagic acids), with potential industrial applications.
Table 2.
Flavonoid content and antioxidant activity of the purified polyphenolic compounds of E. antisyphilitica by-products from the different wax-extraction processes.
| Sample | Flavonoids |
DPPH• |
ABTS•+ |
Lipid oxidation |
FRAP |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| mE Quercetin (250 μg mL−1) |
(IC50, μg mL−1) |
(IC50, μg mL−1) |
(% Inh) 250 μg mL−1 |
mE Gallic acid (250 μg mL−1) |
||||||
| E. H2SO4 | E. C6H8O7 | E. H2SO4 | E. C6H8O7 | E. H2SO4 | E. C6H8O7 | E. H2SO4 | E. C6H8O7 | E. H2SO4 | E. C6H8O7 | |
| León | 51.80 ± 6.44BC | 50.85 ± 5.15A | 388.683 ± 7B | 211.267 ± 4.85AB | 68.16 ± 2.67A | 46.78 ± 2.04A | 45.91 ± 8.22A | 65.26 ± 6.16A | 56.45 ± 7.36A | 113.29 ± 6.83B |
| SJ | 43.71 ± 2.85C | 57.52 ± 25.16A | 319.2 ± 5.67C | 179.441 ± 7.92B | 66.96 ± 2.56A | 49.85 ± 3.05A | 45.75 ± 3.73A | 64.69 ± 1.23A | 50.172 ± 0.747A | 135.07 ± 6.01A |
| SMYA | 65.14 ± 1.42AB | 55.14A | 401.404 ± 6.45B | 189.61 ± 7.95AB | 69.01 ± 2.56A | 49.63 ± 0.54A | 43.37 ± 5.45AB | 70.31 ± 2.50A | 46.382 ± 1.635A | 123.40 ± 4.61AB |
| LB | 78.95 ± 10.81A | 64.66 ± 4.36A | 438.154 ± 11.53A | 239.43 ± 42.63A | 62.40 ± 4.65A | 48.55 ± 2.42A | 30.1 ± 4.29B | 60.71 ± 3.05A | 54.91 ± 3.09A | 124.12 ± 3.51AB |
The extractions were identified as E. H2SO4 (sulfuric acid) and E. C6H8O7 (citric acid). The letters (A, B, C) show significant differences according to the comparison of means between the same extraction method (p = 0.05). % Inh represents the percentage of inhibition of linoleic acid oxidation.
3.2. Antioxidant activity of candelilla by-products
The antioxidant analysis (Table 2) revealed significant differences between the purified polyphenolic compounds analyzed for each extraction method (p < 0.05). According to the literature, 50% of the inhibition (IC50) of the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) was used as a tool to compare the antioxidant capacity of different antioxidant extracts from plants and food products. In this regard, the evaluation of the residue obtained by wax extraction with sulfuric acid reached IC50 values that ranged from 319.2 ± 5.67 to 438.154 ± 11.53 μL mL−1. The data indicated that the samples from SJ exhibited the lowest concentration of polyphenolic compounds required to reach 50% of radical inhibition. On the other hand, the candelilla by-products obtained from the wax extraction with citric acid showed lower IC50 values (179.441 ± 7.92 to 239.43 ± 42.63 μg mL−1) than those registered for the residues obtained using the traditional method. Once again, the purified polyphenolic compounds from SJ showed the lowest IC50 values, and the samples LB from Coahuila de Zaragoza state had the highest IC50 values. However, the ABTS•+ assay did not show significant differences between the IC50 cultivars. However, as in the DPPH• assay, the data showed a lower IC50 value for CAR samples (46.78 ± 2.04 to 49.85 ± 3.05 μg mL−1) than with SAR (62.4 ± 4.65 to 69.01 ± 2.56 μg mL−1). According to Moreno-Hernández et al. [28], the high antioxidant activity could be explained by the many hydroxy (OH) groups in their chemical structure, which are responsible for conferring antioxidant power.
In comparison, other relevant studies have demonstrated the antioxidant properties (DPPH• assay) of species belonging to the family Euphorbiaceae, such as Euphorbia hirta L. cultivated in tropical countries, showed a higher IC50 value (0.803–0.989 mg mL−1) for crude material than the values reported in this study. Euphorbia retusa Forss. native from the Algerian Sahara, has a lower IC50 value (7.20 ± 0.25 μg mL−1) than the values reported in our study [29, 30]. In addition, Euphorbia gaditana extracts (cultivated in Europe) obtained IC50 values varying with applied solvent with a range between 8.28 ± 0.88 μg mL−1 and 200 μg mL−1 [31]. The antioxidant activity of Copernicia prunifera (family Arecaceae), a related plant in the wax industry that is used for carnauba wax extraction, has been evaluated in a previous study in which the registered IC50 was 50.70 μg mL−1 for ethanolic extracts [32]. The previously published data showed the potential of candelilla waste that showed a close IC50 value even after wax extraction with thermic and acid treatment. Likewise, our results show IC50 values that are comparable to those reported for other exploited resources from the northern region of Mexico such as: P. longiflora with 225 ± 9.43 μg mL−1 and 89 ± 0.63 μg mL−1 for L. graveolens [6, 33].
According to previous reports, the potential of polyphenolic compounds as functional ingredients has a beneficial impact on human health due to its antioxidant properties [34], antimicrobial/antifungal activity [7], prebiotic effect [35], and α-amylase inhibition [36]. In addition, other studies have focused on elucidating the impact of gastrointestinal digestion process on polyphenolic content, its bioactivity, and bio-accessibility [37, 38].
Furthermore, the industrial relevance of polyphenols could be extended to the cosmetic market as an auxiliary component. Relevant studies support the in vitro/in vivo potential of polyphenolic compounds against UV-Skin damage induced by free radicals generated by solar exposure, suggesting a possible photochemopreventive activity of these compounds [39, 40, 41, 42]. Additionally, other industrial applications of phytochemicals from candelilla by-products (including phenolic compounds) were reported in a recent paper published by Rojas et al. [27].
The FRAP test showed statistically significant differences between the CAR samples (Table 2) according to the following descending order: SJ > LB > SMYA > León; highlighting the polyphenolic compounds from SJ which had the highest reducing activity; SAR did not show differences for reducing power. Based on the obtained results, the samples that showed a higher value of equivalence and inhibition of the radical DPPH• also showed a higher reducing power. This is in accordance with a previous report indicating that higher content of gallic acid equivalents by means of reducing power tests (e.g., Folin-Ciocalteu, and FRAP) shows greater activity against the radical DPPH• [43, 44]. On the other hand, the low values observed could be explained by the antagonistic interactions between the main and minor components of this phenolic fraction [45, 46]. In context, the antioxidant activity is related to the amount and class of polyphenols present in purified extracts. Our data could be the result of the efficiency of mineral acid extraction in catalyzing the degradation of cell wall polysaccharides during wax extraction, and the liberation of polyphenolic extracts in wastewater during the wax extraction process. According to Hosseini et al. [47], carboxylic acids, as well as mineral acids, are unable to catalyze the degradation of the cell wall because the acid-catalyzed hydrolysis is proportional to the H+ concentration. In this sense, the residual material could have less bioactive compounds on the vegetal matrix to release.
Finally, the ability of an extract to inhibit the oxidation chain is relevant, as it is known that oxidation of fatty acids often leads to the formation and propagation of lipid radicals, uptake of oxygen, and destruction of lipids, which can affect food products. According to the lipid peroxidation assay, the phenolic fractions from candelilla by-products showed similar inhibition values with both extraction techniques, while better inhibition values were associated with a phenolic extract (70.31 ± 2.50%) from SMY with a low DPPH• IC50 value and high iron reduction capacity (FRAP). As a result, the data suggest that these extracts could be used for lipid oxidation inhibition. However, further research into potential toxicity must be undertaken before any application to a food product.
3.3. FTIR assay
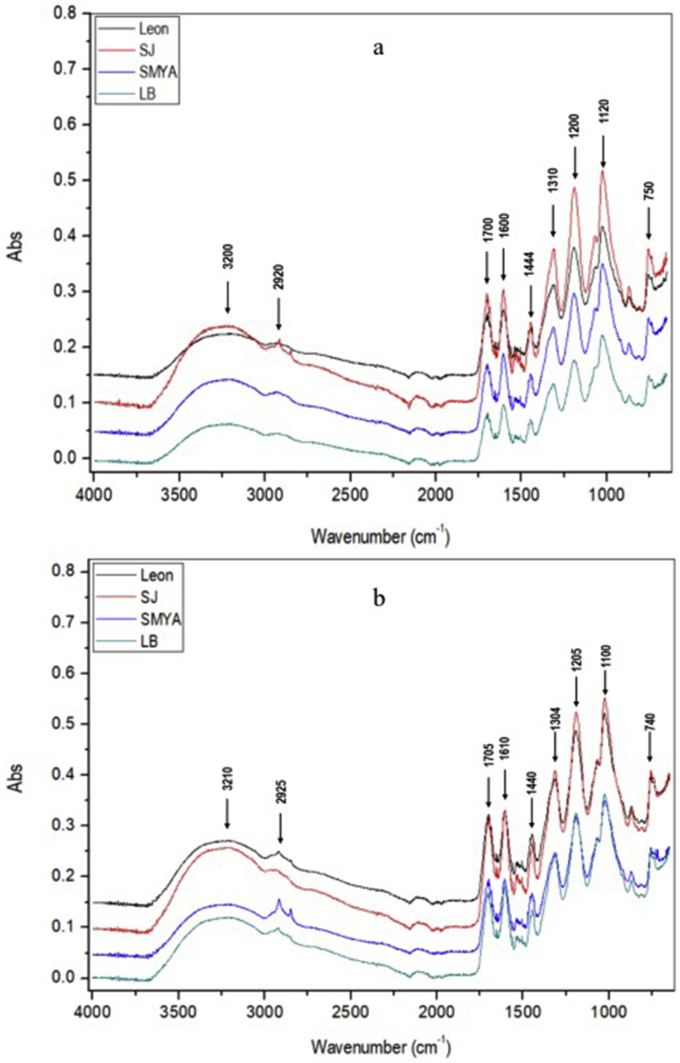
The IR spectra obtained from SAR and CAR showed that functional groups were not qualitatively affected by wax extraction techniques (Figure 2a and 2b). The signals in the range of 3300–3000 cm−1 indicate the presence of the functional group OH in hydrogenated aromatic, aliphatic, and phenolic alcoholic compounds. In the region between 2920 and 2850 cm−1, the presence of C–H is observed due to the elongation of the methyl and methylene groups present in aromatic compounds.
Figure 2.
IR spectral data of polyphenolic compounds of E. antisyphilitica by-products from different wax extraction methods. IR spectra obtained by sulfuric acid method (a); IR spectra obtained by citric acid method (b).
In the 1700 and 1600 cm−1 ranges, the presence of the N–H group is observed, which indicates the presence of compounds with an aromatic ring that may correspond to aromatic amides or amines. Signals between 1500 and 1400 cm−1 correspond to the elongation of carbonyl groups, while the peaks in the 1350 and 1300 cm−1 region are due to the deformation of the C=C bonds of the aromatic ring.
The signal at 1200 cm−1 absorption band is attributable to O–H in the deformation of polyphenols, while between 1150 and 1000 cm−1 indicates the presence of the C–O–H functional group of alcohols. Finally, in the band at 750 cm−1, the presence of the C–H group was observed, indicating the presence of aromatic carboxylic acids.
Previous polyphenolic studies reported similar IR spectra; for example, Lucarini et al. [48] carried out an analysis of phenolic compounds from grape seed varieties. Aguirre-Joya et al. [49] analyzed edible covers and films based on candelilla and polyphenols of Larrea tridentata and Oliveria et al. [50], who evaluated the phenols of five plants. However, despite the great similarity in the obtained spectra, a significant difference was one found within the region known as “fingerprint” which comprises 1500 to 800 cm−1 and whose information within this range is of great importance; additionally, more characteristic variations of each sample could be presented in this region.
In this region, Oliveria et al. [50] indicated that the band at 1645 cm−1 (C=O) is related to the presence of flavonoids. In contrast, in the results obtained, within the region between 1700 and 1600 cm−1, the presence of the N–H group, which indicates the presence of compounds with an aromatic ring that can correspond to amides or aromatic amines, coincides with the findings reported by Ay et al. [51] in samples of Punica granatum L.
3.4. Candelilla waste as source of potential functional auxiliaries
In a post-pandemic context, it is important to highlight that vaccines have been proposed as the main solution to COVID-19 by preparing the body against such viral pathogens. However, according to Han and Hoang [52], boosting cellular functions could lower the incidence and severity of a possible infection. Polyphenolic compounds could serve as functional ingredients, which play an important role in boosting cellular functions as antioxidant, antibacterial, anti-inflammatory auxiliary against some diseases [53, 54, 55]. In addition, the in silico potential of flavonoids against COVID-19 was evaluated by Adem et al. [56], who determined a possible inhibition of viral replication by molecular docking with COVID-19 main protease. Finally, the antiviral activity of polyphenolic compounds has been reported for other viruses [57].
Another relevant application of the polyphenolic fraction of candelilla by-products could be the integration of polyphenolic rich fractions to edible films and coating for food storage, using their antibacterial potential for increasing food safety. As an example, a study carried out by Vega-Menchaca [58] showed antibacterial activity of candelilla methanolic extracts against Enterobacter aerogenes 9183 and Staphylococcus aureus (30.1 and 26.8 μg mL−1, respectively). In addition, other studies have demonstrated the effectiveness of the phenolic fraction of candelilla against phytopathogens such as Fusarium oxysporum, Colletotrichum gloeosporoides, Rhizoctonia solani, and Alternaria alternate [59]. Overall, the integration of candelilla compounds into an industrial application requires a more detailed analysis.
4. Conclusion
The present study demonstrated the potential of candelilla by-products as sources of potent antioxidant agents. According to the radical DPPH• assay, the best phenolic extract was associated with citric acid wax extraction for the SJ cultivar with 179.441 ± 7.92 μg mL−1, while ABTS•+ did not indicate any differences between cultivars but evidenced lower IC50 values in CAR purified extracts. The FRAP assay supported the higher reducing power of CAR (113.29 ± 6.83 to 135.07 ± 6.01, μg equivalent gallic acid per milliliter) in contrast with SAR (42.804 ± 1.39 to 56.45 ± 7.36, μg equivalent gallic acid per milliliter). The flavonoids assay suggested that antioxidant properties could be related to other polyphenolic compounds. The lipid oxidation assay showed higher inhibition by CAR, with the best cultivar corresponding to SMYA with 70.31 ± 2.50%. The FTIR data suggested the presence of functional groups related to polyphenolic compounds, such as hydroxyl (OH), carbonyl (C=O), carbon double bound (C=C), and amine (N–H). In addition, the differences in absorbance observed in the IR spectrum could likely have resulted from the strong acid treatment in the wax extraction process. This study therefore provides novel and interesting information on the phytochemicals present in candelilla residues, and informs further applications. Despite these positive results, additional detailed studies (cytotoxic analyses) are needed to confirm the possible application of recovered antioxidant agents in a food matrix; additionally, the impacts of acid treatment must be analyzed to elucidate the possible mechanism underlying the decrease in antioxidant activity.
Declarations
Author contribution statement
Israel Bautista-Hernández: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Néstor E. Aranda-Ledesma: Performed the experiments; Wrote the paper.
Romeo Rojas: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Julio C. Tafolla-Arellano: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Guillermo C. G. Martínez-Ávila: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by CONAFOR-CONACYT (B-S-131466, B-S-65769), and PAICyT-UANL (CT1109-20).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare the following conflict of interests: Guillermo C. G. Martínez-Ávila; [is part of the Advisory board of the Food Science and Nutrition section of Heliyon].
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the Directorate of Research of UAAAN for the facilities given to the development of this study.
References
- 1.Lan Y. Plant wax. In: Melton L., Shahidi F., Varelis P., editors. Encyclopedia of Food Chemistry. Vol. 1. Elsevier; 2019. pp. 154–196. [Google Scholar]
- 2.Anda J., Shear H. Potential of vertical hydroponic agriculture in Mexico. Sustainability. 2017;9:140. [Google Scholar]
- 3.Granados R., Soria J., Cortina M. Rainfall variability, rainfed agriculture and degree of human marginality in North Guanajuato, Mexico. Singapore J. Trop. Geogr. 2017;38:153–166. [Google Scholar]
- 4.Govea M., La Candelilla . Vol. 52. Secretaría de Medio Ambiente; 2018. La última esperanza de algunos pueblos del desierto. [Google Scholar]
- 5.Rojas R., Saucedo S., De León M.A., Jasso D., Aguilar C.N. Essay past, present and future of candelilla. Rev. Mex. Cienc. Agric. 2011;2(6):7–18. ISSN: 2007-1132. [Google Scholar]
- 6.De León-Zapata M.A. Facultad de Ciencias Químicas, Universidad Autónoma de Coahuila. Saltillo, Coah; México: 2008. Mejoras tecnológicas al proceso de extracción de cera de candelilla. Profesional thesis. [Google Scholar]
- 7.Cid-Pérez T.S., Ávila-Sosa R., Ochoa-Velasco C.E., Rivera-Chavira B.E., Nevárez-Moorillón G.V. Antioxidant and antimicrobial activity of Mexican oregano (Poliomintha longiflora) essential oil, hydrosol and extracts from waste solid residues. Plants. 2019;8:22. doi: 10.3390/plants8010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh R.S., Kaur N., Kennedy J.F. Pullulan production from agro-industrial waste and its applications in food industry: a review. J. Carbohydr. Polym. 2019;217:46–57. doi: 10.1016/j.carbpol.2019.04.050. ISSN 0144-8617. [DOI] [PubMed] [Google Scholar]
- 9.Villegas-Méndez M.A., Aguilar-Machado D.E., Balagurusamy N.B., Montañez J., Morales-Oyervides L. Agro-industrial wastes for the synthesis of carotenoids by Xanthophyllomyces dendrorhous: mesquite pods-based medium design and optimization. Biochem. Eng. J. 2019;150:107260. [Google Scholar]
- 10.Burboa E.A., Ascacio-Valdés J.A., Zugasti-Cruz A., Rodríguez-Herrera R., Aguilar C.N. Antioxidant and antibacterial capacity of candelilla extracts residues. Rev. Mex. Ciencias Farm. 2014;45(1):51–56. [Google Scholar]
- 11.Mandal S., Mandal V., Kumar-Das A. In: A S., editor. Vol. 1. Academic. Press. Inc.; 2015. pp. 63–82. (Essentials of Botanical Extraction: Principles and Applications). [Google Scholar]
- 12.Hesham H.A.R., Abdurahman H.N., Rosli M.Y. Techniques for extraction of essential oils from plants: a review. Aust. J. Basic Appl. Sci. 2016;10(16):117–127. [Google Scholar]
- 13.Khalil A.A., Rahman U., Khan M., Sahar A., Mehmoodac T., Khana M. Essential oil eugenol: sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017;7:32669. [Google Scholar]
- 14.Singh N., Basu S., Balakrishnan M. Comprehensive treatment scheme for distillery wastewater targeting recovery of water, antioxidant compounds and biogas. J. Water. Proc. Eng. 2020;38:101663. [Google Scholar]
- 15.Silva M., Castellanos L., Ottens M. Capture and purification of polyphenols using functionalized hydrophobic resins. Ind. Eng. Chem. Res. 2018;57(15):5359–5369. doi: 10.1021/acs.iecr.7b05071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertin L., Ferri F., Scoma A., Marchetti L., Fava F. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem. Eng. J. 2011;171:1287–1293. [Google Scholar]
- 17.Kaushik A., Basu S., Raturi S., Batra V.S., Balakrishnan M. Recovery of antioxidants from sugarcane molasses distillery wastewater and its effect on biomethanation. J. Water. Proc. Eng. 2018;25:204–211. [Google Scholar]
- 18.Castro-López C., Bautista-Hernández I., González-Hernández M.D., Martínez-Ávila G.C.G., Rojas R., Gutiérrez-Díez A., Medina-Herrera N., Aguirre-Arzola V.E. Polyphenolic profile and antioxidant activity of leaf purified hydroalcoholic extracts from seven Mexican Persea americana cultivars. Molecules. 2019;24(173):2–18. doi: 10.3390/molecules24010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- 20.Van den Berg R., Haenen G.R., Van den Berg H., Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66:511–517. [Google Scholar]
- 21.Celik S.E., Özyürek M., Güçlü K., Apa R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta. 2010;81:4–5. doi: 10.1016/j.talanta.2010.02.025. 1300-1309. [DOI] [PubMed] [Google Scholar]
- 22.De la Rosa L.A., Vazquez-Flores A.A., Alvarez-Parrilla E., Rodrigo-García J., Medina-Campos O.N., Ávila-Nava Azalia., González-Reyes S., Pedraa-Chaverri J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J. Funct. Foods. 2014;7:1756–4646. 219-228. [Google Scholar]
- 23.Zou Y., Lu Y., Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004;52(16):5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]
- 24.Nakat Z., Bou-Mitri C. COVID-19 and the food industry: readiness assessment. Food Contr. 2020;121:107661. doi: 10.1016/j.foodcont.2020.107661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayseli Y.I., Aytekin N., Buyukkayhan D., Aslan I., Ayseli M.T. Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: advice for healthcare professionals. Trends Food Sci. Technol. 2020;105:186–199. doi: 10.1016/j.tifs.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galanakis C.M., Aldawoud T.M.S., Rizou M., Rowan N.J., Ibrahim S.A. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: a comprehensive review. Foods. 2020;9:11. doi: 10.3390/foods9111701. 1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas R., Tafolla-Arellano J.C., Martínez-Ávila G.C.G. Euphorbia antisyphilitica Zucc: a source of phytochemicals with potential applications in industry. Plants. 2021;10:8. doi: 10.3390/plants10010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno-Hernández C.L., Sáyago-Ayerdi S.G., García- Galindo H.S., Mata- Montes de Oca M., Montalvo-González E. Effect of the application of 1-methylcyclopropene and wax emulsions on proximate analysis and some antioxidants of soursop (Annona muricata L.) Sci. World J. 2014:89685. doi: 10.1155/2014/896853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basma A.A., Zakaria Z., Latha L.Y., Sasidharan S. Antioxidant activity and phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac. J. Trop. Med. 2011;4(5):386–390. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 30.Lahmadi S., Belhamra M., Karoune S., Kechebar M.S.A., Bensouici C., Kashi I., Mizab O., Ksouri R. In vitro antioxidant capacity of Euphorbia retusa Forss k from Algerian desert. J. Pharm. Pharmacogn. Res. 2019;7(5):356–366. [Google Scholar]
- 31.Badaoui M.I., Magid A.A., Voutquenne-Nazabadioko L., Benkhaled M., Harakay D., Robert A., Haba H. Antioxidant activity-guided isolation of constituents from Euphorbia gaditana Coss. and their antioxidant and tyrosinase inhibitory activities. Phytochem. Lett. 2020;39:99–104. [Google Scholar]
- 32.Da Silva Andrade L.B., Silva Juliao M.S., Vera R.C., Soares T.H., Dos Santos R.O., Coelho A.L. Antioxidant and antifungal activity of carnauba wax powder extracts. Ind. Crop. Prod. 2018;125:220–227. [Google Scholar]
- 33.García-Bores A.M., Espinosa-González A.M., Reyna-Campos A., Cruz-Toscano S., Benítez-Flores J.C., Hernández-Delgado C.T., Flores-Maya S., Urzúa-Meza M., Peñalosa-Castro I., Céspedes-Acuña C.L., Avila-Acevedo J.G. Lippia graveolens photochemopreventive effect against UVB radiation-induced skin carcinogenesiss. J. Photochem. 2016;167:72–81. doi: 10.1016/j.jphotobiol.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Tanase C., Cosarca S., Muntean Daniela-Lucia. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules. 2019;24(6):1182. doi: 10.3390/molecules24061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moorthy M., Chaiyakunapruk N., Jacob S.A., Palanisamy U.D. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: a systematic review of randomized controlled trials. Trends Food Sci. Technol. 2020;99:634–649. [Google Scholar]
- 36.Sun L., Wang Y., Miao M. Inhibition of α-amylase by polyphenolic compounds: substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020;104:190–207. [Google Scholar]
- 37.Gutiérrez-Grijalva E.P., Antunes-Ricardo M., Acosta-Estrada B.A., Gutiérrez-Uribe J.A., Heredia J.B. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Int. Food Res. J. 2019;116:676–686. doi: 10.1016/j.foodres.2018.08.096. [DOI] [PubMed] [Google Scholar]
- 38.Chait Y.A., Gunenc A., Bendali F., Hosseinian F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: bioaccessibility and bioactivity. LWT Food Sci. Technol. 2019;117:108623. [Google Scholar]
- 39.Hu S., Zhang X., Chen F., Wang M. Dietary polyphenols as photoprotective agents against UV radiation. J. Funct. Foods. 2017;30:108–118. [Google Scholar]
- 40.Liu C., Guo H., DaSilva N.A., Li D., Zhang K., Wan Y., Gao Xing-Hua, Chen Houng-Duo., Seeram N.P., Ma H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods. 2019;54:559–567. doi: 10.1016/j.jff.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perde-Schrepler M., Chereches G., Brie I., Tatomir C., Postescu I.D., Soran L., Filip A. Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J. Photochem. Photobiol. B. 2012;118:16–21. doi: 10.1016/j.jphotobiol.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Figueiredo S.A., Pinto F.M., Da Silva C.A., Cunha T.M., Dos Santos M.H., Vieira M. J. In vitro and in vivo photoprotective/photochemopreventive potential of Garcinia brasiliensis epicarp extract. J. Photochem. Photobiol., B. 2014;131:65–73. doi: 10.1016/j.jphotobiol.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Ulewicz-Magulska B., Wesolowski M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum. Nutr. 2019;74:61–67. doi: 10.1007/s11130-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma K., Guleria S. Synergistic antioxidant activity of natural products. Ann. Pharmacol. Pharm. 2017;2(8):1086. [Google Scholar]
- 45.Zardi-Bergaouia A., Jelizia S., Flaminib G., Ascrizzib R., Janneta H. Comparative study of the chemical composition and bioactivities of essential oils of fresh and dry seeds from Myoporum insulare R. Br. Ind. Crop. Prod. 2018;111:232–237. [Google Scholar]
- 46.Jomová K., Hudecova L., Lauro P., Simunkova M., Alwasel S.H., Alhazza I.M., Valko M. A switch between antioxidant and prooxidant properties of the phenolic compounds myricetin, morin, 30,40-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper(II) ions: a spectroscopic, absorption titration and DNA damage study. Molecules. 2019;24:23. doi: 10.3390/molecules24234335. 4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseini S., Gharachorloo M., Ghiassi-Tarzi B., Ghavami M. Evaluation of the organic acids ability for extraction of anthocyanins and phenolic compounds from different sources and their degradation kinetics during cold storage. J. Food Nutr. Sci. 2016;66(4):261–269. [Google Scholar]
- 48.Lucarini M., Durazzo A., Kiefer J., Santini A., Lombardi-Boccia G., Souto E.B., Bevilacqua N. Grape seeds: chromatographic profile of fatty acids and phenolic compounds and qualitative analysis by FTIR-ATR spectroscopy. Foods. 2020;9(1):10. doi: 10.3390/foods9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguirre-Joya J.A., Cerqueira M.A., Ventura-Sobrevilla J., Aguilar-Gonzalez M.A., Carbó-Argibay E., Castro L.P., Aguilar C.N. Candelilla wax-based coatings and films: functional and physicochemical characterization. Food Bioprocess Technol. 2019;12(10):1787–1797. [Google Scholar]
- 50.Oliveira R.N., Mancini M.C., Oliveira F.C.S.D., Passos T.M., Quilty B., Thiré R.M.D.S.M., McGuinness G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Materia. 2016;21(3):767–779. [Google Scholar]
- 51.Ay Ç.Ö., Özcan A.S., Erdoğan Y., Özcan A. Characterization of Punica granatum L. peels and quantitatively determination of its biosorption behavior towards lead (II) ions and Acid Blue 40. Colloids Surf., B. 2012;100:197–204. doi: 10.1016/j.colsurfb.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Han B., Hoang B.X. Opinions on the current pandemic of COVID-19: use functional food to boost our immune functions. J. Infect. Public Health. 2020;13(12):1811–1817. doi: 10.1016/j.jiph.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J., Gao L., Lian H., Chen Shao-dong. Silico screening of potential anti-COVID-19 bioactive natural constituents from food sources by molecular docking. Nutrition. 2021;82:111049. doi: 10.1016/j.nut.2020.111049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diarra M.S., Hassan Y.I., Block G.S., Drover J.C.G., Delaquis P., Oomah D. Antibacterial activities of a polyphenolic-rich extract prepared from American cranberry (Vaccinium macrocarpon) fruit pomace against Listeria spp. LWT Food Sci. Technol. 2020;123:109056. [Google Scholar]
- 55.Bhattacharya D., Sinha R., Mukherjee P., Howlader R.D., Nag D., Sarkar S., Koley H., Withey J.H., Gachhui R. Anti-virulence activity of polyphenolic fraction isolated from Kombucha against Vibrio cholerae. Microb. Pathog. 2020;140:103927. doi: 10.1016/j.micpath.2019.103927. [DOI] [PubMed] [Google Scholar]
- 56.Adem S., Eyupoglu V., Sarfraz I., Rasul A., Ali M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: an in-silico strategy unveils a hope against CORONA. Preprints. 2020:2020030333. doi: 10.1016/j.compbiomed.2022.105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khanna K., Kohli S.K., Kaur R., Bhardwaj A., Bhardwaj V., Ohri P., Sharma A., Ahmad A., Bhardwaj R., Ahmad P. Herbal immune-boosters: substantial warriors of pandemic covid-19 battle. Phytomedicine. 2020:153361. doi: 10.1016/j.phymed.2020.153361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vega-Menchaca M.C., Rivas-Morales C., Verde-Star J., Oranday-Cárdenas A., Rubio-Morales M.E., Núñez-González M.A., Serrano-Gallardo L.B. Antimicrobial activity of five plants from Northern Mexico on medically important bacteria. Afr. J. Microbiol. Res. 2013;7(43):5011–5017. [Google Scholar]
- 59.Ascacio-Valdés J., Burboa E., Aguilera-Carbo A.F., Aparicio M., Pérez-Schmidt R., Rodríguez R., Aguilar C.N. Antifungal ellagitannin isolated from Euphorbia antisyphilitica Zucc. Asian Pac. J. Trop. Biomed. 2013;3(1):41–46. doi: 10.1016/S2221-1691(13)60021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.