Abstract
Background
There is concern about the risk of yellow fever (YF) establishment in Asia, owing to rising numbers of urban outbreaks in endemic countries and globalisation. Following an outbreak in Angola in 2016, YF cases were introduced into China. Prior to this, YF had never been recorded in Asia, despite climatic suitability and the presence of mosquitoes. An outbreak in Asia could result in widespread fatalities and huge economic impact. Therefore, quantifying the potential risk of YF outbreaks in Asia is a public health priority.
Methods
Using international flight data and YF incidence estimates from 2016, we quantified the risk of YF introduction via air travel into Asia. In locations with evidence of a competent mosquito population, the potential for autochthonous YF transmission was estimated using a temperature-dependent model of the reproduction number and a branching process model assuming a negative binomial distribution.
Results
In total, 25 cities across Asia were estimated to be at risk of receiving at least one YF viraemic traveller during 2016. At their average temperatures, we estimated the probability of autochthonous transmission to be <50% in all cities, which was primarily due to the limited number of estimated introductions that year.
Conclusion
Despite the rise in air travel, we found low support for travel patterns between YF endemic countries and Asia resulting in autochthonous transmission during 2016. This supports the historic absence of YF in Asia and suggests it could be due to a limited number of introductions in previous years. Future increases in travel volumes or YF incidence can increase the number of introductions and the risk of autochthonous transmission. Given the high proportion of asymptomatic or mild infections and the challenges of YF surveillance, our model can be used to estimate the introduction and outbreak risk and can provide useful information to surveillance systems.
Keywords: Arbovirus, Travel, Flavivirus, Aedes, Modelling, Surveillance, Outbreak
Introduction
Yellow fever (YF) is a zoonotic disease affecting individuals living in and travelling to the tropical and subtropical regions of Africa and South America.1 It is an arbovirus of the Flavivirus genus, maintained through up to three different transmission cycles.2 Of concern is the urban cycle, which occurs through sustained human-to-human transmission by the vector Aedes aegypti and can result in explosive outbreaks.3 These outbreaks can lead to large loss of life due to the severity of YF, with a case fatality rate of 67% reported amongst hospitalised cases.4 Diagnosis of YF is difficult as the clinical spectrum ranges from asymptomatic infection to fatal disease.1 Although an efficacious vaccine exists, there are global shortages due to difficulties in scaling-up vaccine production and the increasing number of YF outbreaks requiring response vaccination, which limits the extent to which routine immunisation can be conducted in several parts of Africa and South America.5,6
Critically, a 2016 outbreak in Angola resulted in the first recorded introduction of YF into Asia, after infected workers returned home to China.7 There was no onward transmission in China; however, >2 billion individuals in Asia live in areas infested with A. aegypti and Aedes albopictus, another competent and highly adaptable vector.8–12 Additionally, large parts of Asia are hyperendemic or have recorded outbreaks of dengue, chikungunya and Zika, which are transmitted by Aedes mosquitoes, suggesting conditions are also supportive for YF virus transmission. Whilst the circulation of dengue virus, chikungunya virus and Zika virus in Asia has been recorded since the mid-20th century, all three have become significant public health concerns in the last 50 years. Dengue incidence has increased 30-fold, with over half the global burden in South and South East Asia alone.13,14 Chikungunya has expanded globally since 2005, with widespread outbreaks across Asia.15 Finally, many Asian countries recorded Zika virus introduction and autochthonous transmission following the 2016 epidemic in the Americas.16,17
Factors driving the global spread of these arboviruses include urbanisation, climate change and unsustainable vector control, all of which facilitate the expansion of Aedes mosquito habitats.18 Globalisation also means that distance is no longer a limiting factor in the spread of disease and should an individual travel whilst infectious or incubating a virus there is a risk of them seeding an outbreak in a novel location.19,20 Critically, these demographic, entomological and epidemiological factors are also increasing the potential risk of YF transmission, suggesting YF could be next to spread globally.21 As vaccination coverage is near non-existent outside of endemic countries, an outbreak in Asia could require hundreds of millions of doses to mitigate an epidemic.22 In recognition of the major public health threat that the international spread of YF poses, many countries require arriving travellers to have a certificate of vaccination.6 However, the introduction of cases into China from Angola, both countries which required certificate of vaccination, demonstrates that the system can be circumvented.23,24
Given the presence of dengue, Zika and chikungunya in Asia, the absence of YF is the subject of much debate. Phylogenetic data indicate that YF originated in Africa and was introduced to the Americas via the slave trade out of West Africa.25 It has been suggested that YF had fewer historic opportunities for introduction into Asia, as Asian–African trade routes were primarily with East Africa, where YF incidence was lower.26,27 However, as YF spread to both the Americas and Europe, it is unlikely that the opportunity for YF introduction into Asia never occurred.25 Successful control of YF across Africa and the South America, through preventive vaccination campaigns or outbreak response vaccination, may have limited YF introduction into Asia during the 20th century.1 Conversely, dengue, chikungunya and Zika do not have licenced or effective vaccines, which has likely contributed to their global expansion.
Cross-protective immunity, due to high population exposure to dengue and other flaviviruses widespread across Asia, has also been suggested to limit YF introduction.28 Studies have demonstrated that although previous exposure to flaviviruses does not inhibit infection with YF, it does reduce viral load that could impede onward transmission to mosquitoes.29–31 Cross-immunity of flaviviruses would also explain why Zika outbreaks in Asia have been far more limited than those in the Americas.17,32 On the other hand, dengue and YF coexist in both Africa and South America, suggesting cross-immunity is not the only barrier. Other hypotheses include dengue virus outcompeting YF virus within the vector; competition between A. aegypti, the primary YF vector, and A. albopictus and geographical differences in vector competence or YF viral genotypes.8,33–35
Given the uncertainty surrounding the barriers to YF in Asia, predicting YF introductions remains a significant concern. Lack of historical introduction cannot be relied upon given the rise of urban YF, the expansion of global air travel and the rapid ecological and demographic changes that have occurred in the last 50 years, all of which increase the risk of YF introductions into Asia. Based on their interconnectivity with endemic countries, previous studies have suggested China, India, the United Arab Emirates and Saudi Arabia are at the greatest risk of YF introduction; however, the risk of autochthonous transmission is unknown.36,37
To address this, we used detailed origin–destination flight data from 2016 and YF incidence estimates, to predict the number of viraemic travellers capable of seeding local transmission in Asia. Next, in locations at risk of YF introduction, we used temperature-dependent reproduction number (R0) estimates and a branching process model to predict the probability of autochthonous transmission. Estimating the number of introductions capable of seeding onward transmission contributes to our understanding of the absence of YF in Asia.
Methods
YF introductions into Asia
To estimate the number of viraemic travellers who could potentially introduce YF into Asia, the model developed by Dorigatti et al.38 was implemented (see Supplementary Materials). The model links the flow of travellers between Asia and endemic countries, with the risk of contracting YF in the endemic country. Viraemic travellers include those that travel during their incubation or infectious periods. Data used to parameterise the model are presented in Table 1. We assumed air travel was the most likely route of international spread. We therefore quantified passenger volumes between endemic countries and Asian airports using 2016 travel data from the International Air Transport Association (IATA). The journey origin was assumed to be the passengers’ residency, and this analysis considered only the final destination (i.e. no transits or stopovers were included). All airports from countries within the United Nations Asia-Pacific regional group, along with those in Hong Kong, Macau and Taiwan, were included in this analysis.39 Estimates of the 2016 incidence of severe YF for endemic countries were obtained using the model developed by Garske et al.,40 which has been recently updated and extended to include South America and Africa.41 Reflecting estimates that 1 in 10 YF cases are severe,42 the incidence of severe YF was scaled to obtain an estimate of the total YF incidence in each endemic country.
Table 1.
Parameters for the model developed by Dorigatti et al.,38 used to estimate the number of YF cases introduced into Asia.
| Endemic countries | Population size | 2016 incidence estimates of YF | Duration of stay of international tourists (days) |
|---|---|---|---|
| Africa | |||
| Angola | 28 813 000 | 12 760 | 3.40 |
| Benin | 10 872 000 | 4360 | 5.51 |
| Burkina Faso | 18 646 000 | 12 200 | 6.60 |
| Burundi | 10 524 000 | 2490 | 15.0 |
| Cameroon | 23 439 000 | 60 320 | 4.85 |
| Central African Republic | 4 595 000 | 8350 | 2.70 |
| Chad | 14 453 000 | 13 970 | 7.50 |
| Congo | 5 126 000 | 9110 | 2.70 |
| Ivory Coast (Cote d’Ivoire) | 23 696 000 | 32 040 | 3.00 |
| Democratic Republic of the Congo | 78 736 000 | 429 080 | 6.26 |
| Equatorial Guinea | 1 221 000 | 4640 | 3.78 |
| Eritrea | 4 955 000 | 750 | 8.40 |
| Ethiopia | 102 403 000 | 44 860 | 8.40 |
| Gabon | 1 980 000 | 10 660 | 2.70 |
| Gambia | 2 039 000 | 1780 | 3.50 |
| Ghana | 28 207 000 | 52 170 | 10.5 |
| Guinea | 12 396 000 | 16 250 | 8.88 |
| Guinea Bissau | 1 816 000 | 6620 | 22.0 |
| Kenya | 48 462 000 | 28 240 | 10.4 |
| Liberia | 4 614 000 | 5370 | 6.29 |
| Mali | 17 995 000 | 6700 | 6.00 |
| Mauritania | 4 301 000 | 2820 | 3.50 |
| Niger | 20 673 000 | 6500 | 8.00 |
| Nigeria | 185 990 000 | 82 480 | 7.00 |
| Rwanda | 11 918 000 | 2910 | 3.20 |
| Sao Tome and Principe | 200 000 | 40 | 2.70 |
| Senegal | 15 412 000 | 9960 | 3.50 |
| Sierra Leone | 7 396 000 | 9100 | 7.00 |
| Somalia | 14 318 000 | 6430 | 9.40 |
| South Sudan | 12 231 000 | 25 230 | 6.27 |
| Sudan | 39 579 000 | 7530 | 7.17 |
| Tanzania | 55 572 000 | 23 850 | 10.0 |
| Togo | 7 606 000 | 2580 | 2.00 |
| Uganda | 41 488 000 | 31 630 | 5.50 |
| Zambia | 16 591 000 | 4350 | 4.00 |
| South America | |||
| Argentina | 43 847 000 | 1400 | 10.0 |
| Bolivia | 10 888 000 | 1360 | 19.0 |
| Brazil | 207 653 000 | 51 980 | 23.4 |
| Colombia | 48 653 000 | 2540 | 19.0 |
| Ecuador | 16 385 000 | 1790 | 8.50 |
| French Guiana | 244 000 | 230 | 2.60 |
| Guyana | 773 000 | 370 | 26.6 |
| Panama | 4 034 000 | 1110 | 8.00 |
| Paraguay | 6 725 000 | 660 | 4.30 |
| Peru | 31 774 000 | 3980 | 1.80 |
| Suriname | 558 000 | 390 | 15.0 |
| Trinidad and Tobago | 1 365 000 | 440 | 14.0 |
| Venezuela | 31 568 000 | 3280 | 11.7 |
The population size of each endemic country in 2016 was obtained from the World Health Organisation (WHO).43 The average length of stay of international tourists to each endemic country was obtained from either the World Tourism Organisation, the World Bank or the country’s national tourist website (Supplementary Table 1). We performed sensitivity analysis by increasing and decreasing the average length of stay of international tourists by 20%.
Finally, the incubation period for YF was assumed to follow a log-normal distribution with mean 4.6 days and variance 2.7 days.44 The infectious period was assumed to follow a normal distribution with mean 4.5 days and variance 0.6 days.1 Uncertainty was accounted for by sampling from these two distributions 10 000 times, giving full distributions for the number of YF introductions. Results are reported as mean and 95% confidence interval (CI). Only airports with an upper 95% CI exceeding one introduction are presented in the results section.
Probability of autochthonous transmission
In cities served by an airport where the upper 95% CI exceeded one introduction and with evidence of an A. aegypti or A. albopictus mosquito population presence (Supplementary Table 2), we estimated the probability of autochthonous transmission. The transmission intensity in each city was quantified using R0, the number of secondary cases produced on average by each infectious case in an entirely susceptible population. For YF, R0 is the product of the average number of infectious mosquitoes produced per infectious human (R0HM) and the average number of infectious humans produced per infectious mosquito (R0MH).19 R0HM and R0MH values were estimated for both A. aegypti and A. albopictus, separately. As temperature is a key determinant of YF transmission,44–48, we estimated R0MH and R0HM using a temperature-dependent model, following Gaythorpe et al.49 and Mordecai et al.50 (see Supplementary Materials). The mosquito biting rate and the extrinsic incubation period (EIP) were modelled using thermal response estimates (Supplementary Table 3). Temperature-dependent estimates for the EIP of A. albopictus were unavailable, so we scaled those of A. aegypti by the ratio of the point estimate recorded for A. albopictus (9 days at 26.7°C),51 divided by an estimate predicted by the temperature-dependent model recorded for A. aegypti at the same temperature (7.3 days at 26.7°C). Vector longevity was parameterised using estimates on A. albopictus and A. aegypti mortality from field observations, over a range of different temperatures.45 The temperature-dependent variables were estimated at each city’s average, minimum and maximum temperature52 (Table 2).
Table 2.
Location-specific data used to estimate R0 in Asian cities predicted to be at risk of YF introduction with A. aegypti and/or A. albopictus populations.
| Country | City | Average temperature (minimum—maximum) (°C) | EIP (days) a | Mosquito biting rate per day a | Average mosquito lifespan (days) a | Proportion of mosquitoes surviving EIP a | Female mosquitoes per person |
|---|---|---|---|---|---|---|---|
| Aedes aegypti | |||||||
| India | Ahmedabad | 25.19 (11.34–39.04) | 9.01 | 0.61 | 16.13 | 0.57 | 0.88 |
| Thailand | Bangkok | 27.47 (20.14–34.81) | 6.89 | 0.67 | 15.90 | 0.65 | 0.85 |
| Hong Kong | Hong Kong | 21.02 (11.67–30.38) | 20.61 | 0.47 | 16.13 | 0.28 | 0.85c |
| Saudi Arabia | Jeddah | 23.20 (7.87–38.52) | 7.29 | 0.66 | 15.98 | 0.63 | 0.85d |
| Malaysia | Kuala Lumpur | 26.58 (21.18–31.99) | 7.57 | 0.65 | 16.02 | 0.62 | 0.82 |
| Philippines | Manila | 27.48 (21.27–33.69) | 6.88 | 0.67 | 15.90 | 0.65 | 0.85c |
| Saudi Arabia | Medina | 23.20 (7.87–38.52) | 7.98 | 0.64 | 16.06 | 0.61 | 0.85d |
| India | Mumbai | 26.55 (13.78–39.33) | 7.60 | 0.65 | 16.02 | 0.62 | 0.88 |
| Oman | Muscat | 25.33 (14.44–36.22) | 8.85 | 0.61 | 16.10 | 0.58 | 0.85d |
| India | New Delhi | 23.89 (7.22–40.55) | 10.99 | 0.57 | 16.17 | 0.51 | 0.88 |
| Singapore | Singapore | 27.08 (22.62–31.54) | 7.17 | 0.66 | 15.96 | 0.64 | 0.82c |
| Aedes albopictus | |||||||
| Thailand | Bangkok | 27.47 (20.14–34.81) | 8.26 | 0.35 | 43.69 | 0.83 | 2.70c |
| China | Beijing | 8.50 (−12.52–29.53) | *b | 0.00 | 22.00 | 0.00 | 1.97 |
| Lebanon | Beirut | 21.09 (10.44–31.74) | 24.26 | 0.16 | 43.25 | 0.57 | 2.36d |
| China | Guangzhou | 20.09 (8.33–31.84) | 33.97 | 0.13 | 42.10 | 0.45 | 2.80 |
| Hong Kong | Hong Kong | 21.02 (11.67–30.38) | 24.73 | 0.16 | 43.15 | 0.56 | 2.80c |
| Malaysia | Kuala Lumpur | 26.58 (21.18–31.99) | 9.09 | 0.33 | 44.27 | 0.81 | 2.70 |
| Philippines | Manila | 27.48 (21.27–33.69) | 8.25 | 0.35 | 43.69 | 0.83 | 2.70c |
| India | Mumbai | 26.55 (13.78–39.33) | 9.12 | 0.33 | 44.27 | 0.81 | 2.70c |
| India | New Delhi | 23.89 (7.22–40.55) | 13.18 | 0.25 | 45.00 | 0.75 | 2.70c |
| South Korea | Seoul | 10.43 (−8.22 to 29.09) | *b | 0.00 | 26.52 | 0.00 | 1.97c |
| China | Shanghai | 16.00 (0.77–31.23) | *b | 0.03 | 36.85 | 0.00 | 1.97 |
| Singapore | Singapore | 27.08 (22.62–31.54) | 8.60 | 0.34 | 43.96 | 0.82 | 2.70c |
| Japan | Tokyo | 13.62 (−2.48 to 29.71) | *b | 0.00 | 33.23 | 0.00 | 1.97c |
| Data Sources | 52 | 49 , 51 | 49 | 45 | 45 , 49–51 | 53–57 | |
aTemperature-dependent variable.
bAverage temperature at this location is outside of the temperature range for the EIP. Therefore, parasite development within the mosquito will not occur and this location is unsuitable for transmission at the average temperature.
cEstimate from nearby country.
dNo local estimate available. Average of all the studies of either A. aegypti or A. albopictus density that we identified across Asia.
Where possible, we obtained local estimates for the number of female A. aegypti and A. albopictus per person.53–57 Where these data were unavailable, an alternative estimate of Aedes mosquito density or an estimate from the nearest location were used (Table 2). All other parameters were assumed to be constant across spatial units. The effective transmission rates between vector and host were taken from a study, which assessed the YF infection and transmission rates of Asian A. aegypti and A. albopictus.9 Thus, the effective transmission rate from human to A. aegypti was set at 0.8, and the effective transmission rate from A. aegypti to human was set at 0.24. For A. albopictus these values were 0.26 and 0.13, respectively. We performed sensitivity analyses on the assumed number of female Aedes mosquitoes per person and their competency (see Supplementary Materials).
We assumed independent introductions of infectious humans and quantified the outbreak probability utilising the methodology developed by Johansson et al.19 and adapted by Luo et al.58 This model uses a branching process and draws from negative binomial offspring distributions with means R0MH and R0HM and dispersion parameter 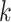 (see Supplementary Materials). The dispersion parameter controls individual heterogeneity in infectiousness and was assumed to be high (
(see Supplementary Materials). The dispersion parameter controls individual heterogeneity in infectiousness and was assumed to be high (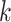 = 0.1), as estimated for other vector-borne diseases.59–61 We performed sensitivity analyses to evaluate the role of individual-level heterogeneity in infectiousness (
= 0.1), as estimated for other vector-borne diseases.59–61 We performed sensitivity analyses to evaluate the role of individual-level heterogeneity in infectiousness (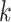 ) and population immunity (varied from 0 to 100%) on the probability of autochthonous transmission.
) and population immunity (varied from 0 to 100%) on the probability of autochthonous transmission.
Analyses were performed in R version 3.6.1 and using the Epiflows package.62
Results
We estimated that 25 airports in Asia were at risk of YF introductions during 2016, defined as having an upper 95% CI exceeding one introduction (Figure 1). These estimates refer to the overall number of introductions aggregated from all endemic countries. China, India, Saudi Arabia and the United Arab Emirates all had multiple airports predicted to be at risk of at least one YF introduction. Sensitivity analyis on the duration of stay did not change the risk of YF introduction predicted into each city (not shown).
Figure 1.
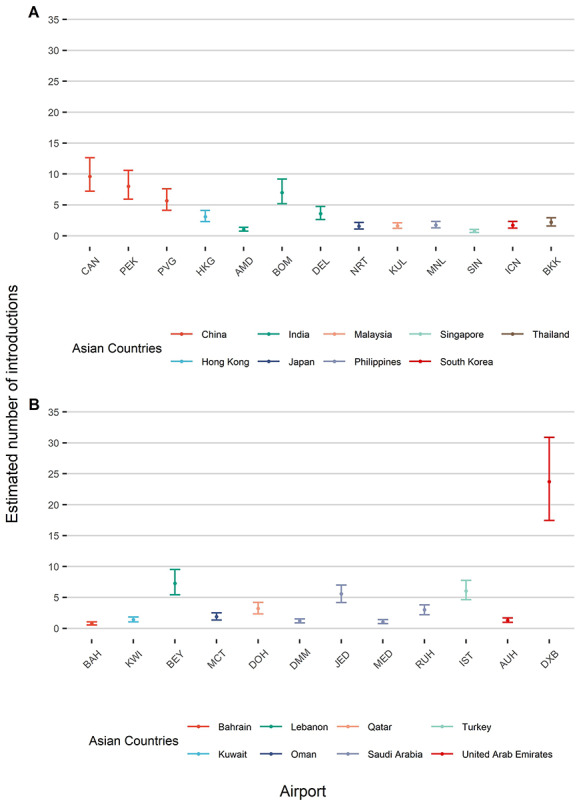
Mean (point) and 95% CIs (bars) of the total predicted number of introductions of YF into (A) airports in South and East Asia and (B) airports in West Asia and the Middle East. Introductions presented are aggregated from all endemic countries. Only airports with an upper 95% confidence limit greater than one introduction are shown. CAN: Guangzhou Baiyun International Airport, PEK: Beijing Capital International Airport, PVG: Shanghai Pudong International Airport, HKG: Hong Kong International Airport, AMD: Sardar Vallabhbhai Patel International Airport; BOM: Chhatrapati Shivaji International Airport, DEL: Indira Gandhi International Airport, NRT: Narita International Airport, KUL: Kuala Lumpur International Airport, MNL: Ninoy Aquino International Airport, SIN: Singapore Changi Airport, ICN: Incheon International Airport, BKK: Suvarnabhumi Airport, BAH: Bahrain International Airport, KWI: Kuwait International Airport, BEY: Beirut–Rafic Hariri International Airport, MCT: Muscat International Airport, DOH: Hamad International Airport, DMM: King Fahd International Airport, JED: King Abdulaziz International Airport, MED: Prince Mohammad bin Abdulaziz International Airport, RUH: King Khalid International Airport, IST: Istanbul Airport, AUH: Abu Dhabi International Airport, DXB: Dubai International Airport
Next, we estimated R0 values for cities with evidence of a competent mosquito population (Supplementary Figure 1). In cities where A. albopictus and A. aegypti are both present, we estimated two independent R0 values. The R0 estimate and number of introductions for each city were then used to predict the outbreak probability. Figure 2A shows that the risk of autochthonous transmission is <50% across all temperatures for cities in Asia with an A. aegypti population. Similarly, Figure 2B shows the risk of autochthonous transmission is <50% for Asian cities with A. albopictus populations, apart from Guangzhou at its maximum temperature, where the upper bound of the 95% CI exceeds 50%.
Figure 2.
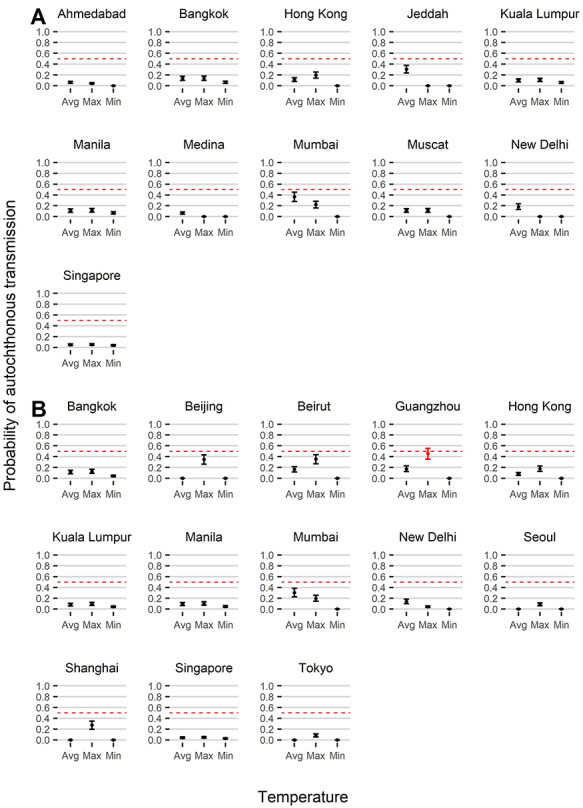
Mean (point) and 95% CIs (bars) of the predicted probabilities of autochthonous transmission in Asian cities, assuming transmission by (A) A. aegypti and (B) A. albopictus, given the independent introduction of at least one infectious individual. Probabilities are estimated at the average, minimum and maximum temperature for each location. Probabilities in red indicate an upper 95% probability of autochthonous transmission exceeding 0.5, denoted by the dashed red line
Increasing individual heterogeneity in infectiousness (which is obtained by decreasing the dispersion parameter 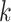 to 0.01) reduced the risk of onward transmission to near 0 in all cities (Figure 3). Conversely, only high levels of population immunity had a large reduction on the risk of transmission (Figure 3). For instance, in Mumbai, assuming achievement of the WHO recommended 80% vaccination coverage is estimated to reduce the probability of transmission by 36%, whereas a vaccination coverage of 95% would reduce transmission by 68%. Sensitivity analysis found that the effective transmission rate from mosquitoes to humans had a minimal effect on the risk of transmission (Supplementary Figure 2). Finally, in cities where the probability of autochthonous transmission was very low, the number of female mosquitoes per person had a limited effect on this risk. Conversely, cities predicted to have a higher probability of transmission were more sensitive to assumptions about the number of female mosquitoes per person.
to 0.01) reduced the risk of onward transmission to near 0 in all cities (Figure 3). Conversely, only high levels of population immunity had a large reduction on the risk of transmission (Figure 3). For instance, in Mumbai, assuming achievement of the WHO recommended 80% vaccination coverage is estimated to reduce the probability of transmission by 36%, whereas a vaccination coverage of 95% would reduce transmission by 68%. Sensitivity analysis found that the effective transmission rate from mosquitoes to humans had a minimal effect on the risk of transmission (Supplementary Figure 2). Finally, in cities where the probability of autochthonous transmission was very low, the number of female mosquitoes per person had a limited effect on this risk. Conversely, cities predicted to have a higher probability of transmission were more sensitive to assumptions about the number of female mosquitoes per person.
Figure 3.
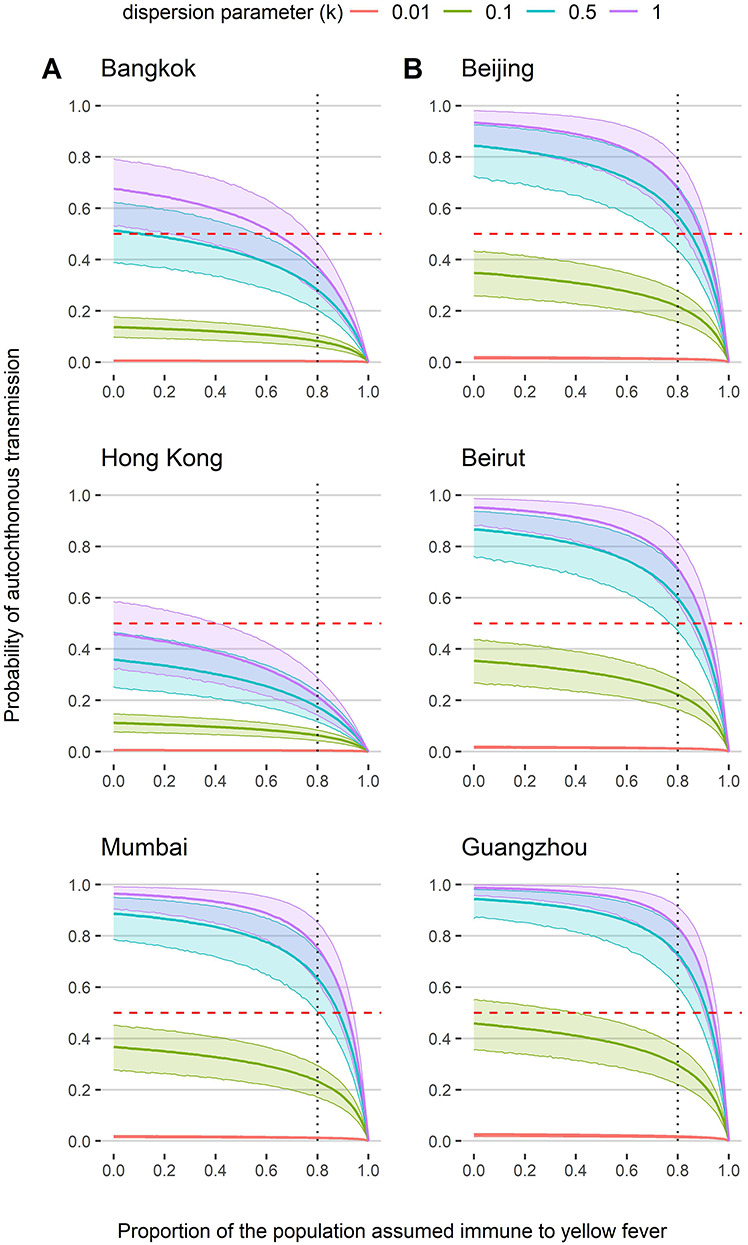
Probability of autochthonous transmission across increasing levels of population immunity at (A) the average temperature in Bangkok, Hong Kong and Mumbai assuming transmission by A. aegypti and (B) the maximum temperature in Beijing, Beirut and Guangzhou assuming transmission by A. albopictus. Also presented are probabilities at different values of the dispersion parameter k, where purple = 1, blue = 0.5, green = 0.1(baseline value) and red = 0.01. Thick lines are the mean and shaded areas are the 95% CIs. Horizontal dashed red line represents the point at which the probability of autochthonous transmission is <0.5. Vertical dashed line denotes 80% population immunity recommended by the WHO to stop local transmission in endemic countries
Discussion
Quantifying the risk of YF introduction by viraemic travellers who have the potential to propagate local transmission is critical for assessing the risk of global YF spread and can provide useful information when additional control measures are considered.63 We identified 25 cities in Asia at risk of YF introduction from endemic countries during 2016. However, except for Guangzhou at its maximum temperature, the outbreak probability across Asia was low (i.e. the 95% CI of the outbreak probability did not exceed 50%).
We predicted introductions into multiple airports in China, in accordance with the observed case introductions in 2016.7 Additionally, case introductions were predicted into multiple airports in India, the United Arab Emirates and Saudi Arabia, not observed in 2016, but in agreement with a study analysing travel patterns between YF endemic and at-risk countries.36 Brent et al.36 reported the total number of passengers flying between endemic countries and countries at risk for YF (defined by their suitability for dengue), as a proxy for the risk of YF introduction. In contrast, we use estimates of the number of travellers provided by IATA, to predict the expected number of viraemic travellers introduced, which was then used to also quantify the probability of autochthonous transmission. As we predicted a low probability of autochthonous transmission, introductions are likely to go undetected, given the high proportion of cases that have no or non-specific symptoms.1 This may explain the absence of recorded case introductions into Asia, outside of China.
Other assessments of YF in Asia highlight South and Southeast Asia as suitable for transmission.64,65 In agreement with this, we predicted transmission intensity to be highest in India, Malaysia, the Philippines, Singapore, Hong Kong and Thailand, with temperature-dependent R0 estimates close to R0 values seen during urban outbreaks in endemic countries.66,67 Despite this, we found low support for autochthonous transmission due to an estimated low number of case introductions. Although flight data were unavailable beyond 2016, tourist arrivals in Asia are estimated to increase on average by 5% annually,68 so the risk of introduction can also be expected to have increased in recent years. Equally, introductions are likely to increase following rises in YF incidence in endemic countries, as seen with the Angola outbreak.7
Higher probabilities of autochthonous transmission in China and Lebanon at warmer temperatures suggest that the potential for YF transmission by A. albopictus is seasonal. At the maximum temperature we predicted a risk of autochthonous transmission in Guangzhou, which regularly experiences dengue outbreaks in the summer, supporting the continued use of seasonal vector control measures.69 The YF case introductions into China in 2016 occurred in March and April, when A. albopictus populations were reduced, and could explain the absence of onward transmission.70 However, climate change is expanding the habitat of Aedes mosquitoes and extending periods of transmission suitability, indicating the risk will increase.71 Cities predicted to be at risk of future establishment of Aedes mosquitoes should also be monitored.72 For instance, in Dubai a high number of introductions were predicted, and dengue outbreaks occur in neighbouring Saudi Arabia and Oman.73,74
Alongside insufficient introductions and seasonality, we found heterogeneity in individual-level infectiousness to be a limiting factor to YF in Asia, which may arise through several mechanisms including asymptomatic infection and human, virus or mosquito genetics.61,75 Although cross-immunity with related flaviviruses could limit YF transmission in Asia, a high proportion of the population would need to develop immunity to significantly reduce the probability of autochthonous transmission.
We estimate that reduced vector competency is unlikely to be a substantial barrier to YF transmission in Asia, as the effective transmission rate from mosquito to humans had a limited effect on the probability of autochthonous transmission. Furthermore, the R0 estimates and corresponding probabilities of autochthonous transmission for A. albopictus were similar to A. aegypti, despite much lower effective transmission rates. This may be explained by A. albopictus’ longer lifespan and higher probability of surviving the EIP, which increases its competency as a YF vector, as has previously been suggested for dengue.76 It is also thought that adaptation of chikungunya virus to A. albopictus may have facilitated global spread and the recent chikungunya outbreaks in Asia.15 Thus, A. albopictus has the potential to be an active YF vector and its widespread distribution is unlikely to be a major barrier to YF establishment in Asia.35
Our results suggest that a limited number of case introductions in 2016 was the primary reason for the low predicted probability of autochthonous transmission in Asia. However, we also find that a number of other factors can reduce the probability of autochthonous transmission, and these could have an additive effect in preventing the establishment of YF in Asia. This is in agreement with the current literature, which suggests that multiple societal, biological and environmental factors likely contribute to the absence of YF in Asia.8,27,77
This study is not without limitations. When estimating case introductions, we assumed that the origin of each journey was the passengers’ residency and that the final travel destinations in the data set were the actual final destinations, rather than stopovers. Thus, the risk of introduction and the probability of autochthonous transmission may be overestimated for hubs, which act as transit even though appear as final destinations in the data set. Only air travel was accounted for, whereas introductions could occur via sea and land, especially between the Middle East and northeast Africa.78 Our estimates do not account for medium- or long-term migration, for instance due to work.37,79 Including migrants, along with tourists, in the model would increase the estimated number of importations but in the absence of detailed data on the migrant population between endemic countries and Asia and on their length of stay, it is hard to quantify the impact of migration on the risk of YF introduction.
We assumed force of infection was homogeneous within each endemic country, overlooking geographic variation, especially in holoendemic countries. Infection risk is also generally higher for residents, although some tourists may be high risk owing to lower immunity (through lack of natural exposure or vaccination) or due to behavioural risks, like ecotourism, increasing exposure.23,25,77 Force of infection was assumed to be constant over time and does not capture the time-varying intensity of transmission during an outbreak, nor the seasonality in travel patterns. Finally, the socioeconomic characteristics of at-risk countries were not accounted for, like those with health infrastructures weakened by conflict.80
Cities with higher probabilities of transmission were sensitive to assumptions made about mosquito densities, indicating the risk could have been either under- or overestimated. There are limited data available on mosquito densities and those that are available are heterogeneous due to variability in the density indicator, season, timing (i.e. in response to an outbreak), trap location and trap type.53–57,73,81–83 Data on vector density at a higher spatial resolution using standardised methodology would provide a more comprehensive analysis. Alternatively, Massad et al.84,85 previously used the incidence of dengue to estimate A. aegypti density and the probability of urban YF outbreaks, following the theoretical introduction of an infected traveller into Brazil. In future analyses, it would be interesting to apply this method to assess the risk of YF in Asia, considering our vector density data limitations. However, if dengue is a barrier to YF in Asia, either through cross-immunity or viral competition, data on dengue incidence may overestimate the risk in dengue hyperendemic areas and underestimate the risk in areas with competent mosquito populations but less dengue transmission, such as Southern China.8
We also assumed exclusively urban transmission and parameterised the model using A. aegypti and A. albopictus data. However, the exact contribution of each species to YF transmission is unknown, even in countries endemic for YF, so for cities infested by both mosquitoes we have reported two independent estimates of the probability of autochthonous transmission. Finally, we did not attempt to distinguish between different YF viral genotypes and vector competence was assessed solely using a West African genotype of the YF virus.9
The study has several strengths. The methods applied in this study have previously been used to predict Zika introductions into the USA and agreed with the observed number of introductions.58 Equally, temperature-dependent R0 estimates have previously been shown to be important predictors of the probability of autochthonous transmission of vector-borne diseases.50 Moreover, the risk of autochthonous transmission quantified here is dependent on both the number of introductions and on the local intensity of transmission, which provides critical information to public health officials beyond YF suitability and travel patterns.
We show that despite the rise of air travel, limited YF introductions capable of seeding onwards transmission continue to act as a barrier to YF in Asia. This supports ongoing surveillance, in order to monitor the future number of introductions, as increases in travel volumes or in the incidence of YF in endemic countries may increase the outbreak probability in Asia. Surveillance of YF presents challenges, owing to YF’s broad clinical spectrum and cross-reactivity with other flaviviruses. Therefore, the modelling framework in this study is a useful tool to monitor the risk of YF in Asia, by estimating future YF case introductions. Our results also suggest that ensuring travellers from and to YF endemic countries are vaccinated would be the most effective control measure to minimise the outbreak risk in Asia.
Should YF introduction occur, the large volumes of intraregional travel within Asia would facilitate the spread of YF further, as seen with dengue transmission.86,87 This analysis also suggests that vaccination coverages >80% would be required to significantly reduce the probability of autochthonous transmission, which may pose challenges given the current shortage of vaccine doses available.
This study quantifies the risk of YF in Asia using the limited data available and tests whether the expected number of YF introductions would have supported local transmission. We found a limited number of YF introductions producing a low probability of autochthonous transmission in 2016, suggesting that a limited number of introductions over past years may have historically prevented YF establishment in Asia. Although the model simplifies the complexity of YF transmission, it allows us to produce data-driven estimates of the expected number of introductions and of the probability of YF establishment in Asia, which can serve as a useful tool to monitor the risk of YF introduction. Given the global challenges in YF surveillance, the methods presented in this study can help identify the areas at risk of YF introduction and inform decisions to minimise the outbreak potential to reduce the global burden of YF in the future.
Supplementary Material
Contributor Information
Bethan Cracknell Daniels, MRC Centre for Global Infectious Disease Analysis; and the Abdul Latif Jameel Institute for Disease and Emergency Analytics (J-IDEA), School of Public Health, Imperial College London.
Katy Gaythorpe, MRC Centre for Global Infectious Disease Analysis; and the Abdul Latif Jameel Institute for Disease and Emergency Analytics (J-IDEA), School of Public Health, Imperial College London.
Natsuko Imai, MRC Centre for Global Infectious Disease Analysis; and the Abdul Latif Jameel Institute for Disease and Emergency Analytics (J-IDEA), School of Public Health, Imperial College London.
Ilaria Dorigatti, MRC Centre for Global Infectious Disease Analysis; and the Abdul Latif Jameel Institute for Disease and Emergency Analytics (J-IDEA), School of Public Health, Imperial College London.
Author contributions
B.C.D.: Software, Formal analysis, Writing—original draft and editing; N.I.: Conceptualization, Methodology, Writing—review and editing, Supervision; K.G.: Conceptualization, Methodology, Writing—review and editing, Supervision; I.D.: Conceptualization, Methodology, Writing—review and editing, Supervision.
Funding
MRC Centre for Global Infectious Disease Analysis [MR/R015600/1]; the UK Medical Research Council (MRC); the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union; the Wellcome Trust and the Royal Society, Sir Henry Dale fellowship [213 494/Z/18/Z to I.D.] and by the Medical Research Council UK as part of a PhD [to BCD].
Code availability
The code and data used to produce all results are available at: https://github.com/bnc19/YF_in_Asia
Conflict of interest: None declared.
References
- 1. Monath TP. Yellow fever: an update. Lancet Infect Dis 2001; 1:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Chen LH, Wilson ME. Yellow fever control: current epidemiology and vaccination strategies. Trop Dis Travel Med Vaccines 2020; 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monath TP. Facing up to re-emergence of urban yellow fever. Lancet 1999; 353:1541. [DOI] [PubMed] [Google Scholar]
- 4. Ho YL, Joelsons D, Leite GFC et al. Severe yellow fever in Brazil: clinical characteristics and management. J Travel Med 2019; 26:taz040. [DOI] [PubMed] [Google Scholar]
- 5. Monath TP, Woodall JP, Gubler DJ et al. Yellow fever vaccine supply: a possible solution. Lancet 2016; 387:1599–600. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organisation . Eliminate Yellow fever Epidemics (EYE): a global strategy, 2017-2026. In: Geneva, 2017.
- 7. World Health Organisation . Emergencies preparedness, response: yellow fever–China. 2016. https://www.who.int/csr/don/22-april-2016-yellow-fever-china/en/ (accessed 6 January 2021).
- 8. Wasserman S, Tambyah PA, Lim PL. Yellow fever cases in Asia: primed for an epidemic. Int J Infect Dis 2016; 48:98–103. [DOI] [PubMed] [Google Scholar]
- 9. Lataillade LG, Vazeille M, Obadia T et al. Risk of yellow fever virus transmission in the Asia-Pacific region. Nat Commun 2020; 11:5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amraoui F, Pain A, Piorkowski G et al. Experimental adaptation of the yellow fever virus to the mosquito Aedes albopictus and potential risk of urban epidemics in Brazil. South America Sci Rep 2018; 8:14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amraoui F, Vazeille M, Failloux AB. French Aedes albopictus are able to transmit yellow fever virus. Euro Surveill 2016; 21:30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamgang B, Vazeille M, Yougang AP et al. Potential of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to transmit yellow fever virus in urban areas in Central Africa. Emerg Microbes Infect 2019; 8:1636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM. Mapping global variation in dengue transmission intensity. Sci Transl Med 2020; 12:1–11. [DOI] [PubMed] [Google Scholar]
- 14. Guo C, Zhou Z, Wen Z et al. Global epidemiology of dengue outbreaks in 1990-2015: a systematic review and meta-analysis. Front Cell Infect Microbiol 2017; 7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wimalasiri-Yapa B, Stassen L, Huang X et al. Chikungunya virus in Asia-Pacific: a systematic review. Emerg Microbes Infect 2019; 8:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duong V, Dussart P, Buchy P. Zika virus in Asia. Int J Infect Dis 2017; 54:121–8. [DOI] [PubMed] [Google Scholar]
- 17. Lim SK, Lim JK, Yoon IK. An update on Zika virus in Asia. Infect Chemother 2017; 49:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kraemer MUG, Reiner RC, Brady OJ et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol 2019; 4:854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Gallagher N, Marano N, Staples JE. Assessing the risk of international spread of yellow fever virus: a mathematical analysis of an urban outbreak in Asuncion, 2008. Am J Trop Med Hyg 2012; 86:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol 2006; 62:293–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butler D. Fears rise over yellow fever's next move. Nature 2016; 532:155–6. [DOI] [PubMed] [Google Scholar]
- 22. Gubler DJ. Pandemic yellow fever: a potential threat to global health via travelers. J Travel Med 2018; 25. [DOI] [PubMed] [Google Scholar]
- 23. Song R, Guan S, Lee SS et al. Late or lack of vaccination linked to importation of yellow fever from Angola to China. Emerg Infect Dis 2018; 24:1383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilder-Smith A, Massad E. Estimating the number of unvaccinated Chinese workers against yellow fever in Angola. BMC Infect Dis 2018; 18:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol 2007; 52:209–29. [DOI] [PubMed] [Google Scholar]
- 26. Cathey JT, Marr JS. Yellow fever, Asia and the east African slave trade. Trans R Soc Trop Med Hyg 2014; 108:252–7. [DOI] [PubMed] [Google Scholar]
- 27. Kuno G. The absence of yellow fever in Asia: history, hypotheses, vector dispersal, possibility of YF in Asia, and other enigmas. Viruses 2020; 12:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rathore APS, St John AL. Cross-reactive immunity among Flaviviruses. Front Immunol 2020; 11:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao SY, Guzman H, da Rosa AP, Zhu HB, Tesh RB. Alteration of clinical outcome and histopathology of yellow fever virus infection in a hamster model by previous infection with heterologous flaviviruses. Am J Trop Med Hyg 2003; 68: 695–703. [PubMed] [Google Scholar]
- 30. Izurieta RO, Macaluso M, Watts DM et al. Anamnestic immune response to dengue and decreased severity of yellow fever. J Glob Infect 2009; 1:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Theiler M, Anderson CR. The relative resistance of dengue-immune monkeys to yellow fever virus. Am J Trop Med Hyg 1975; 24:115–7. [DOI] [PubMed] [Google Scholar]
- 32. Katzelnick LC, Bos S, Harris E. Protective and enhancing interactions among dengue viruses 1-4 and Zika virus. Curr Opin Virol 2020; 43:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abrao EP, da Fonseca BA. Infection of mosquito cells (C6/36) by Dengue-2 virus interferes with subsequent infection by yellow fever virus. Vector Borne Zoonotic Dis 2016; 16:124–30. [DOI] [PubMed] [Google Scholar]
- 34. Massad E, Coutinho FA, Burattini MN, Lopez LF. The risk of yellow fever in a dengue-infested area. Trans R Soc Trop Med Hyg 2001; 95:370–4. [DOI] [PubMed] [Google Scholar]
- 35. Amaku M, Coutinho FA, Massad E. Why dengue and yellow fever coexist in some areas of the world and not in others? Biosystems 2011; 106:111–20. [DOI] [PubMed] [Google Scholar]
- 36. Brent SE, Watts A, Cetron M et al. International travel between global urban centres vulnerable to yellow fever transmission. Bull World Health Organ 2018; 96:343–54B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilder-Smith A, Leong WY. Importation of yellow fever into China: assessing travel patterns. J Travel Med 2017; 24. [DOI] [PubMed] [Google Scholar]
- 38. Dorigatti I, Hamlet A, Aguas R et al. International risk of yellow fever spread from the ongoing outbreak in Brazil, December 2016 to may 2017. Euro Surveill 2017; 22:30572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. United Nations . United Nations Regional Groups of Member States. https://www.un.org/dgacm/en/content/regional-groups (accessed 4 January 2021).
- 40. Garske T, Van Kerkhove MD, Yactayo S et al. Yellow fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med 2014; 11:e1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gaythorpe K, Hamlet A, Cibrelus L, Garske T, Ferguson N. The global burden of yellow fever. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johansson MA, Vasconcelos PF, Staples JE. The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans R Soc Trop Med Hyg 2014; 108:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organisation . Global Health Observatory data repository. Population (in thousands), 2016. https://apps.who.int/gho/data/view.main.POP2020.
- 44. Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Staples JE. Incubation periods of yellow fever virus. Am J Trop Med Hyg 2010; 83:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brady OJ, Johansson MA, Guerra CA et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors 2013; 6:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamlet A, Jean K, Perea W et al. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Negl Trop Dis 2018; 12:e0006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kraemer MU, Sinka ME, Duda KA et al. The global distribution of the arbovirus vectors Aedes aegypti and A. albopictus. Elife 2015; 4:e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mordecai EA, Caldwell JM, Grossman MK et al. Thermal biology of mosquito-borne disease. Ecol Lett 2019; 22:1690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gaythorpe KA, Hamlet A, Cibrelus L, Garske T, Ferguson NM. The effect of climate change on yellow fever disease burden in Africa. Elife 2020; 9:e55619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mordecai EA, Cohen JM, Evans MV et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis 2017; 11:e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miller BR, Mitchell CJ, Ballinger ME. Replication, tissue tropisms and transmission of yellow fever virus in Aedes albopictus. Trans R Soc Trop Med Hyg 1989; 83:252–5. [DOI] [PubMed] [Google Scholar]
- 52. Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land Areas. Int J Climatol 2017; 37:4302–15. [Google Scholar]
- 53. Almeida AP, Baptista SS, Sousa CA et al. Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. J Med Entomol 2005; 42:419–28. [DOI] [PubMed] [Google Scholar]
- 54. Singh G, Tilak R, Kaushik SK. Bio-eco-social determinants of Aedes breeding in field practice area of a medical college in Pune, Maharashtra. Indian J Public Health 2019; 63:324–9. [DOI] [PubMed] [Google Scholar]
- 55. Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg 2000; 62:11–8. [PubMed] [Google Scholar]
- 56. Ahmad Zaki Z, Che Dom N, Ahmed Alhothily I. Efficacy of bacillus thuringiensis treatment on Aedes population using different applications at high-rise buildings. Trop Med Infect Dis 2020; 5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu H, Liu L, Cheng P et al. Bionomics and insecticide resistance of Aedes albopictus in Shandong, a high latitude and high-risk dengue transmission area in China. Parasit Vectors 2020; 13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luo XS, Imai N, Dorigatti I. Quantifying the risk of Zika virus spread in Asia during the 2015-16 epidemic in Latin America and the Caribbean: a modeling study. Travel Med Infect Dis 2020; 33:101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anibal Munoz LEAB, Carlos A, Abello M. Numerical analysis of a model for dengue incidence with spreading factors in an endemic region. Appl Math Sci 2018; 12:1453–63. [Google Scholar]
- 60. Grubaugh ND, Ladner JT, Kraemer MUG et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature 2017; 546:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature 2005; 438:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moraga P, Dorigatti I, Kamvar ZN et al. Epiflows: an R package for risk assessment of travel-related spread of disease. F1000Res 2018; 7:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dorigatti I, Morrison S, Donnelly CA, Garske T, Bowden S, Grills A. Risk of yellow fever virus importation into the United States from Brazil, outbreak years 2016-2017 and 2017-2018. Sci Rep 2019; 9:20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shearer FM, Longbottom J, Browne AJ et al. Existing and potential infection risk zones of yellow fever worldwide: a modelling analysis. Lancet Glob Health 2018; 6:e270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rogers DJ, Wilson AJ, Hay SI, Graham AJ. The global distribution of yellow fever and dengue. Adv Parasitol 2006; 62:181–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kraemer MUG, Faria NR, Reiner RC et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: a modelling study. Lancet Infect Dis 2017; 17:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ndeffo-Mbah ML, Pandey A. Global risk and elimination of yellow fever epidemics. J Infect Dis 2020; 221:2026–34. [DOI] [PubMed] [Google Scholar]
- 68. Glaesser D, Kester J, Paulose H, Alizadeh A, Valentin B. Global travel patterns: an overview. J Travel Med 2017; 24. [DOI] [PubMed] [Google Scholar]
- 69. Zhang Z, Jing Q, Chen Z et al. The increasing menace of dengue in Guangzhou, 2001-2016: the most important epicenter in mainland China. BMC Infect Dis 2019; 19:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen J, Lu H. Yellow fever in China is still an imported disease. Biosci Trends 2016; 10:158–62. [DOI] [PubMed] [Google Scholar]
- 71. Iwamura T, Guzman-Holst A, Murray KA. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat Commun 2020; 11:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ducheyne E, Tran Minh NN, Haddad N et al. Current and future distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in WHO Eastern Mediterranean Region. Int J Health Geogr 2018; 17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Al-Abri SS, Kurup PJ, Al Manji A et al. Control of the 2018-2019 dengue fever outbreak in Oman: a country previously without local transmission. Int J Infect Dis 2020; 90:97–103. [DOI] [PubMed] [Google Scholar]
- 74. Humphrey JM, Cleton NB, Reusken CB, Glesby MJ, Koopmans MP, Abu-Raddad LJ. Dengue in the Middle East and North Africa: a systematic review. PLoS Negl Trop Dis 2016; 10:e0005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Woolhouse ME, Dye C, Etard JF et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci U S A 1997; 94:338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brady OJ, Golding N, Pigott DM et al. Global temperature constraints on Aedes aegypti and A. albopictus persistence and competence for dengue virus transmission. Parasit Vectors 2014; 7:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comp Immunol Microbiol Infect Dis 2004; 27:319–30. [DOI] [PubMed] [Google Scholar]
- 78. Phillips. Refugees and Migrants at Sea: Another View from the Middle East and North Africa Region. Proceedings of the Annual Meeting, Vol. 2016. American Society of International Law, 2016, pp. 169–73. [Google Scholar]
- 79. Botchwey G, Crawford G, Loubere N, Lu J. South-South Labour Migration And The Impact Of The Informal China-Ghana Gold Rush 2008-13. WIDER Working Paper. 2018. Helsinki: UNU-WIDER. [Google Scholar]
- 80. Mowafi H. Conflict, displacement and health in the Middle East. Glob Public Health 2011; 6:472–87. [DOI] [PubMed] [Google Scholar]
- 81. Furuya H. Estimation of reproduction number and probable vector density of the first autochthonous dengue outbreak in Japan in the last 70 years. Environ Health Prev Med 2015; 20:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang JN, Hou J, Zhong JY et al. Relationships between traditional larval indices and meteorological factors with the adult density of Aedes albopictus captured by BG-mosquito trap. PLoS One 2020; 15:e0234555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aziz AT, Dieng H, Ahmad AH et al. Household survey of container-breeding mosquitoes and climatic factors influencing the prevalence of Aedes aegypti (Diptera: Culicidae) in Makkah City, Saudi Arabia. Asian Pac J Trop Biomed 2012; 2:849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Massad E, Amaku M, Coutinho FAB et al. The risk of urban yellow fever resurgence in Aedes-infested American cities. Epidemiol Infect 2018; 146:1219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Massad E, Amaku M, Coutinho FAB et al. Estimating the size of Aedes aegypti populations from dengue incidence data: implications for the risk of yellow fever outbreaks. Infect Dis Model 2017; 2:441–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tian H, Sun Z, Faria NR et al. Increasing airline travel may facilitate co-circulation of multiple dengue virus serotypes in Asia. PLoS Negl Trop Dis 2017; 11:e0005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lai S, Johansson MA, Yin W et al. Seasonal and interannual risks of dengue introduction from South-East Asia into China, 2005-2015. PLoS Negl Trop Dis 2018; 12:e0006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


