ABSTRACT
Melioidosis, caused by Burkholderia pseudomallei, is increasingly recognized in several regions of the globe. The present study was performed to identify and determine the frequency of B. pseudomallei infection in localized pyogenic lesions in eastern India and describe their clinico-microbiological profile. Pus samples were subjected to standard microbiological techniques for isolation and identification of various bacteria, including B. pseudomallei, which were confirmed by PCR. The clinical and demographic details of patients with melioidosis and antimicrobial susceptibility pattern of B. pseudomallei isolates were analyzed. Of 245 samples, 126 (51.4%) were culture positive, yielding 137 isolates. Staphylococcus aureus was the predominant pathogen accounting for 54 (39.4%) isolates, followed by B. pseudomallei accounting for 34 (24.8%) isolates. The mean age of the patients with melioidosis was 39.1 years, with males (24/34; 70.6%) being affected more than females (10/34; 29.4%). A majority of the patients were laborers (12/34; 35.3), followed by homemakers (8/34; 23.5%). Head and neck abscesses (35.3%) were the most common presentation followed by pyogenic lesions of the musculoskeletal system (32.3%) and deep organ abscesses (23.5%). Clinical resolution of infection was observed in 31 (91.2%) patients, relapse in two (5.9%) patients, and death in one (2.9%) patient, respectively. Susceptibility testing revealed all B. pseudomallei isolates to be completely susceptible to the following antimicrobials: ceftazidime, trimethoprim–sulfamethoxazole, imipenem, and doxycycline, with one (2.9%) resistant to amoxicillin–clavulanic acid. Burkholderia pseudomallei is an emerging etiological agent of localized pyogenic infections in eastern India, affecting a mainly adult male population. An increased vigilance along with appropriate diagnostic techniques helps in accurate diagnosis facilitating appropriate therapy.
INTRODUCTION
Burkholderia pseudomallei, a saprophytic Gram-negative soil and water bacterium, is the causative agent of melioidosis, a disease of immense public health importance in subtropical and tropical areas such as Northern Australia and Southeast Asia.1,2 Classified as a tier 1 select agent by the CDC, the organism can produce a cluster of overlapping and protean disease manifestations ranging from localized infections and subclinical infection with late manifestations to acute fulminant septicemia.3,4 Lately, the disease, with an estimated global incidence of ∼165,000 cases and case fatality of 89,000/year, has been emerging and evolving in other countries such as India, Sri Lanka, China, and Bangladesh, with recent modeling studies predicting a surge of cases of melioidosis in India.5–7 The entity has been known to affect mainly people with regular occupational or recreational exposure to moist soil or surface water such as rice farmers of Southeast Asia, indigenous communities of Australia, other agricultural workers, construction workers, adventure travelers, soldiers, and immigrants.1,2 Known risk factors include diabetes mellitus (DM), excessive alcohol intake, thalassemia, chronic renal and lung disease, and various immunosuppressive conditions such as glucocorticoid therapy and cancer.2,4,8 The three main routes contributing to disease transmission include percutaneous inoculation, inhalation, and ingestion apart from occasional cases of transmission from mother to infants due to ingestion of breast milk, and sexual and vertical transmission.1,2,4 Some other typical epidemiological features include the increased occurrence of the entity during the rainy season (75–81%), increased prevalence in males compared with females, and the high potential of the disease to undergo latency only to be reactivated years later when the host immune status is compromised.2,8,9 The true burden of melioidosis in developing tropical countries is unknown and masked because of the lack of laboratory and clinical expertise of this disease, underdeveloped microbiological facilities, and poor reporting systems. There is also a lack of awareness about the disease, resulting in misdiagnosis and inappropriate or inadequate treatment. Pneumonia and sepsis are the major clinical manifestations of the disease, accounting for approximately 60% of infections, and can lead to a mortality rate as high as 96% in the absence of effective intervention, although crude case fatality rate ranges from 10 to 50%.3,4,9
Approximately 25–40% patients have localized infections, such as cutaneous and soft tissue abscesses, suppurative lymphadenitis, salivary gland abscesses, joint manifestations, pyomyositis, prostatic abscesses, and visceral organ abscesses such as liver and spleen abscesses, which may, however, rapidly progress to more widespread infection.4,7–11 Early and accurate detection of melioidotic lesions when the disease is still localized in conjunction with appropriate and adequate treatment can arrest the disease progression and eliminate the chances of the disease from becoming fatal or undergoing latency, and cause complications later on. A rigorous literature search showed that most of the reported cases of localized melioidosis in India have been described either as single reports or small series or as a part of the spectrum of the total culture-positive cases of melioidosis in retrospective studies.7,12–15 We found only a single prospective study from India which attempted to detect B. pseudomallei infection in various non-blood specimens, including pyogenic lesions, in the community.11
Hence, we undertook this study to identify and determine the frequency of B. pseudomallei infection in patients presenting with localized pyogenic lesions to our hospital using conventional and molecular methods, and describe their clinico-microbiological profile. In addition, the profile and antimicrobial susceptibility pattern of other pathogens recovered from the lesions were also studied.
MATERIALS AND METHODS
Study setting.
The study, approved by the Institutional Ethical Committee, was conducted over a 2-year period from August 2018 to July 2020 at a tertiary healthcare center in eastern India. Based on the available literature, patients presenting to the hospital with localized infections of the skin and soft tissue such as cutaneous and subcutaneous abscesses, suppurative lymphadenopathy, joint effusions, empyemas, deep organ abscesses, osteomyelitis, pyomyositis, or any other localized abscesses or suppurative infections of the cervical, parotid, salivary gland, and musculoskeletal system were included. Written informed consent was obtained from adults and assent with consent from children younger than 18 years. Patients with pyogenic lesions with known or contributory precipitating factors such as decubitus ulcers, chronic venous ulcers, postoperative wound infections, pyoderma, pemphigus with secondary infections, and pressure sores were excluded.
Sample collection and processing.
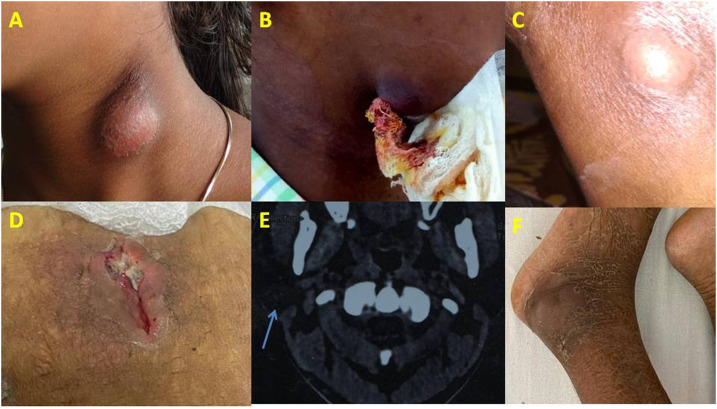
Taking due aseptic precautions, pus samples were aspirated from surface lesions during incision-drainage procedures or collected from deep/internal abscesses as radio imaging–guided aspirates (Figure 1A–F) and promptly transported to the microbiology laboratory within 2 hours of collection. Demographic details were collected as per a preset questionnaire. In the laboratory, the samples were subjected to standard microbiological culture techniques for the isolation and identification of various aerobic and facultative anaerobic bacteria, including B. pseudomallei.16–18 In brief, after noting the nature and consistency followed by a Gram stain and modified acid-fast stain using 1% sulfuric acid as decolorizer, the specimens were inoculated on 5% sheep blood agar, MacConkey agar, and Ashdown agar (as a special culture medium for B. pseudomallei). The inoculated culture plates were incubated at 37°C aerobically (MacConkey agar and Ashdown agar), and at 5–10% CO2 (blood agar) for 5 days before being declared as culture negative. Organisms were identified by their growth characteristics on different media, colony morphology, Gram stain reaction, and other standard biochemical tests.16–18 Specifically, B. pseudomallei was identified by its characteristic wrinkled appearance of colonies, oxidase activity, Gram-negative non–lactose-fermenting motile bacillus with characteristic bipolar or irregular staining with a “safety pin” appearance, resistance to polymyxins (colistin 10 µg and polymyxin B 300U disks, HiMedia, Mumbai, India), and subjecting to the Vitek-2 automated identification system (bioMérieux, Marcy l’Etoile, France).
Figure 1.
Pyogenic lesions of (A). Neck abscess. (B) Abscess in the chin. (C) Knee abscess. (D) Anterior abdominal wall abscess. (E) Parotid abscess. (F) Lower limb abscess. This figure appears in color at www.ajtmh.org.
Molecular confirmation.
The phenotypically identified isolates of B. pseudomallei were subjected to molecular confirmation by conventional PCR targeting the groEL and type III secretion system (TTS1) gene cluster as genus- and species-specific targets using oligonucleotide primer pairs (Sigma-Aldrich Ltd., St. Louis, MO) with their expected amplicon sizes as shown in Table 1.11,19 Genomic DNA was extracted by the HiPurA Bacterial Genomic DNA Purification Kit (HiMedia, Mumbai, India) as per the manufacturer’s instructions, and PCR (in 25 µL volume) was performed using DreamTaq™ Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA) which contains Taq polymerase, dNTPs, MgCl2, and the appropriate buffer. Amplification was carried out in Veriti™ Dx 96-well Thermal Cycler (Thermo Fisher Scientific) with the following cycling conditions: 1) initial denaturation at 94°C for 5 minutes, 35 cycles of denaturation (94°C, 30 seconds), annealing (52°C, 30 seconds), and extension (72°C, 45 seconds), followed by a final extension for 2 minutes at 72°C for groEL; and 2) initial denaturation at 95°C for 1 minute, 35 cycles of denaturation (95°C, 30 seconds), annealing (60°C, 30 seconds), and extension (72°C, 30 seconds), followed by a final extension for 2 minutes at 72°C for TTS1. The amplicons were analyzed by electrophoresis in 1% agarose gel stained with ethidium bromide and visualized in an automated UV transillumination system (Syngene G:BOX, Synoptics, Cambridge, United Kingdom). Burkholderia cepacia ATCC 25416 was used as a positive control for identification of genus Burkholderia. Molecular grade water was used as a negative control (Supplemental Figure).
Table 1.
Oligonucleotide primers used for molecular confirmation of B. pseudomallei
| Name | Target gene | Oligonucleotide sequence (5′–3′) | Amplicon size (bp) | Purpose | Ref |
|---|---|---|---|---|---|
| Gro1 -F | groEL | CTG GAA GAC ATC GCG ATC | 139 | Burkholderia genus–specific | 19 |
| Gro2-R | CGT CGA TGA TCG TCG TGT T | ||||
| BPTTS-F | Type III secretion system | CTTCAATCTGCTCTTTCCGTT | 548 | B. pseudomallei species–specific | 11 |
| BPTTS-R | CAGGACGGTTTCGGACGAA |
B. pseudomallei = Burkholderia pseudomallei.
Antimicrobial susceptibility testing.
Susceptibility testing of the isolates was carried out on the Vitek-2 system as well as on Mueller–Hinton agar (HiMedia) by the Kirby-Bauer disc diffusion method whenever required (except for B. pseudomallei) and interpreted as per Clinical and Laboratory Standards Institute (CLSI) M100 guidelines.20 Susceptibility testing for B. pseudomallei was performed by EzyMIC (HiMedia) to determine the minimum inhibitory concentration (MIC) and interpreted as per CLSI M45 guidelines.21 Tests to detect methicillin-resistant Staphylococcus aureus (MRSA), high-level gentamicin resistance (HLGR), and carbapenemase-producing Gram-negative bacteria were performed as per CLSI M100 guidelines.20 Reference strains of Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and S. aureus ATCC 25923 were used as quality control strains. Organisms with intermediate levels of resistance were included in the percentage of resistant organisms for final analysis.
RESULTS
A total of 245 samples from an equal number of patients were included in the study, of which 126 (51.4%) were culture positive for various pathogens, whereas 114 (46.5%) were culture negative. Coryneform bacteria, environmental bacillus species, and micrococci were grown in five (2.0%) samples which were considered contaminants, and not processed further. Among the 126 culture-positive samples, the number of isolates obtained was 137 (115 had a single pathogen and 11 had two types of bacteria), with S. aureus as the predominant pathogen, accounting for 54 (39.4%) isolates. Burkholderia pseudomallei was the second most common pathogen accounting for 34 (24.8%) isolates (Table 2). Thus, the culture positivity rate of B. pseudomallei among patients suspected with localized forms of melioidosis was 13.9% (34/245).
Table 2.
Organisms isolated from pyogenic lesions (n = 137)
| Organism | No. of isolates (%) |
|---|---|
| Staphylococcus aureus | 54 (39.4) |
| Burkholderia pseudomallei | 34 (24.8) |
| Escherichia coli | 11 (8.0) |
| Pseudomonas aeruginosa | 10 (7.3) |
| Streptococcus species* | 8 (5.8) |
| Enterococcus spp. | 7 (5.1) |
| Klebsiella pneumoniae | 7 (5.1) |
| Nocardia species | 3 (2.2) |
| Proteus mirabilis | 2 (1.4) |
| Streptococcus pneumoniae | 1 (0.7) |
| Total | 137 |
Includes four each of β-hemolytic group and viridians group.
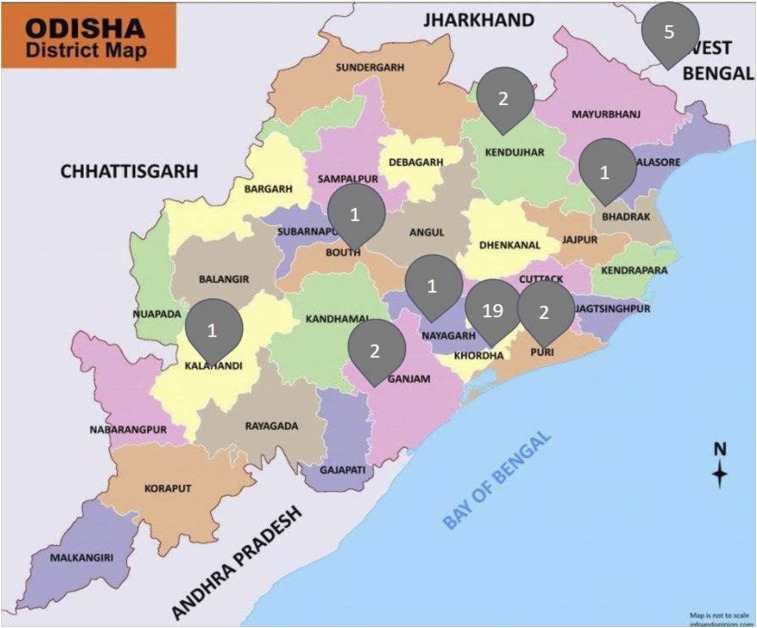
Of the 34 patients culture positive for B. pseudomallei infection, 29 were from the state of Odisha and five from the adjoining state of West Bengal, with a majority from Khordha district (19/34; 55.9%) of Odisha (Figure 2). The demographic details and clinical characteristics of these culture-positive cases are shown in Table 3. The mean age of the patients was 39.1 years. The lowest and highest age from which B. pseudomallei was isolated was a 7-year-old male child with neck abscess and a 71-year-old male elderly patient with mediastinal lymph node swelling, respectively. Most cases were isolated from the working adult age-group of 18–60 years (26/34; 76.5%), and males (24/34; 70.6%) were affected more than females (10/34; 29.4%). The majority of the patients were laborers (12/34; 35.3), followed by homemakers (8/34; 23.5%). Exposure by presumptive outdoor occupational activity was seen in a total of 18 (52.9%) and that by indoor occupational activity was seen in 16 (47.1%) patients. Head and neck abscesses (12, 35.3%) were the most common clinical presentation, followed by pyogenic lesions of the musculoskeletal system (11, 32.3%) and deep organ abscesses (8, 23.5%). The musculoskeletal lesions consisted of septic arthritis (4, 11.8%), psoas abscess (3, 8.8%), lower limb abscess (3, 8.8%), and osteomyelitis (1, 2.9%). Among deep organ abscesses, there were four (11.8%) cases of spleen abscess, three (8.8%) cases of liver abscess, and one case (2.9%) with both spleen and liver abscesses. Apart from these, one case (2.9%) each of abdominal wall abscess, pleural empyema, and mediastinal lymphadenitis were also present. Twenty-two (64.7%) cases had one or more known risk factors for this infection, with DM as the most common condition present in 15 (15/34, 44.1%) cases. A history of alcohol intake was present in nine (26.4%) patients. Diabetes was found to be statistically significantly associated with melioidosis (15/34 versus 21/211, P < 0.05), whereas alcohol intake was not found to have a statistically significant association (9/34 versus 47/211, P = 0.08). Individuals with melioidosis were seven times more likely to have diabetes and two times more likely to have a history of alcohol intake than those without melioidosis (odds ratio, 7.14 and 2.09, respectively). Six (17.6%) patients had an associated bacteremia. Thirty-three patients were discharged after the completion of initial intensive phase of therapy, with relapse seen in two (5.9%) patients at 3 months follow-up. Death occurred in one patient (2.9%) with bacteremia just before the initiation of therapy. Susceptibility testing revealed all the B. pseudomallei isolates to be completely susceptible to the antimicrobials, ceftazidime, trimethoprim–sulfamethoxazole, imipenem, and doxycycline, with only one (2.9%) isolate resistant to amoxicillin–clavulanic acid. The MIC characteristics of these 34 isolates to various antimicrobial agents have been shown in Table 4. Imipenem showed excellent activity with low MIC50 and MIC90 values (0.38 and 1.5 µg/mL, respectively), and maximum isolates (12, 35.3%) demonstrated an MIC of 0.25 µg/mL to imipenem. The lone isolate resistant to amoxicillin–clavulanic acid had an MIC of 16 µg/mL. For ceftazidime, 13 (38.2%) isolates had an MIC of 3 µg/mL, followed by nine (26.5%) and eight (23.5%) having MICs of 2 µg/mL and 4 µg/mL, respectively.
Figure 2.
District-wise distribution of Burkholderia pseudomallei cases in Odisha, eastern India. This figure appears in color at www.ajtmh.org.
Table 3.
Demographic details and clinical characteristics of culture-positive Burkholderia pseudomallei cases from pyogenic lesions (n = 34)
| Demographic profile | No. of patients (%) (n = 34) |
|---|---|
| Age-group (years) | |
| ≤ 18 | 5 (14.7) |
| > 18–60 | 26 (76.5) |
| > 60 | 3 (8.8) |
| Mean ± SD | |
| 39.1 ± 16.8 | – |
| Median | |
| 39.5 | – |
| Range | |
| 7–71 | – |
| Gender | |
| Male | 24 (70.6) |
| Female | 10 (29.4) |
| Occupation | |
| Outdoor | |
| Laborer | 12 (35.3) |
| Military personnel | 2 (5.9) |
| Driver | 2 (5.9) |
| Farmers | 2 (5.9) |
| Indoor | |
| Homemaker | 8 (23.5) |
| Student | 5 (14.7) |
| Office worker | 2 (5.9) |
| Teacher | 1 (2.9) |
| Seasonal distribution | |
| Monsoon (June–September) | 20 (58.8) |
| Autumn (October–November) | 7 (20.6) |
| Winter (December–February) | 4 (11.8) |
| Summer (March–May) | 3 (8.8) |
| Clinical presentation | |
| Head and neck | 12 (35.3) |
| Musculoskeletal lesions | 11 (32.3) |
| Deep organ abscess | 8 (23.5) |
| Abdominal wall abscess | 1 (2.9) |
| Others* | 2 (6) |
| Risk factors/comorbid conditions† | |
| DM | 10 (29.4) |
| DM and alcoholism | 5 (14.7) |
| Alcoholism | 4 (11.7) |
| Hematological disorders‡ | 3 (8.8) |
| No apparent risk factor | 12 (35.3) |
| Treatment undertaken | 33 (97.1)§ |
| Initial therapy | |
| Ceftazidime | 28 (82.4) |
| Meropenem | 4 (11.7) |
| Imipenem | 1 (2.9) |
| Eradication therapy | |
| Co-trimoxazole | 30 (88.2) |
| Doxycycline | 2 (5.9) |
| Amoxicillin–clavulanate | 1 (2.9) |
| Outcome | |
| Treated | 31 (91.2) |
| Relapse | 2 (5.9) |
| Death | 1 (2.9) |
DM = diabetes mellitus.
Includes one case each of mediastinal lymph node aspirate and pleural empyema.
P < 0.05 (significant) for occurrence of diabetes between melioidosis and non-melioidosis patients by Chi-square test.
Includes anemia, thalassemia, and sickle cell disease.
One patient died before initiation of treatment.
Table 4.
Minimum inhibitory concentration characteristics of Burkholderia pseudomallei (n = 34) to various antimicrobials
| Antibiotic | No. of isolates with MIC (µg/mL) | MIC (µg/mL) | % S | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.19 | 0.25 | 0.38 | 0.5 | 0.75 | 1 | 1.5 | 2 | 3 | 4 | 6 | 16 | Range | MIC50 | MIC90 | ||
| AMC | – | – | – | – | – | 1 | 4 | 4 | 17 | 2 | 5 | 1 | 1–16 | 3 | 6 | 97.1 |
| CAZ | – | – | – | – | – | – | 3 | 9 | 13 | 8 | 1 | – | 1.5–6 | 3 | 4 | 100 |
| DO | – | – | – | 3 | 8 | 10 | 6 | 5 | 1 | 1 | – | – | 0.5–4 | 1 | 2 | 100 |
| IPM | 4 | 12 | 7 | 6 | 1 | – | 1 | 1 | 2 | – | – | – | 0.19–3 | 0.38 | 1.5 | 100 |
| TMS | 2 | 5 | 2 | 2 | 7 | 10 | 4 | 2 | – | – | – | – | 0.19–2 | 0.75 | 1.5 | 100 |
AMC = amoxicillin–clavulanic acid; CAZ = ceftazidime; DO = doxycycline; IPM = imipenem; MIC = minimum inhibitory concentration; TMS = trimethoprim–sulfamethoxazole; S = susceptible.
Antimicrobial susceptibility pattern of other pathogens isolated from the pyogenic lesions showed that of 54 S. aureus isolates, 10 (18.5%) were MRSA, and among seven Enterococcus species, one (14.3%) demonstrated HLGR. As regards Gram-negative bacilli, E. coli was the most commonly isolated pathogen, with five (45.4%) and two (18.2%) isolates showing resistance to third-generation cephalosporins and carbapenems, respectively (Supplemental Table). Both the carbapenem-resistant E. coli isolates were metallo-beta-lactamase producers.
DISCUSSION
Our study showed a high prevalence (13.9%) of B. pseudomallei infection among localized pyogenic infections in the eastern state of Odisha. In fact, B. pseudomallei accounted for about a quarter (24.8%) of the isolated pathogens and was the second in rank order of bacteria isolated from various cases of localized pyogenic infections. Staphylococcus aureus was the most common pathogen accounting for 39.4% of the isolates. In a recently published study conducted from January 2014 to December 2015 at a teaching hospital in South India, a similar finding was noted with B. pseudomallei as the second most common pathogen among 271 patients suspected with localized forms of melioidosis, with S. aureus constituting the most common isolate.11 The culture positivity rate for B. pseudomallei (5.9%, 16/271) was however lower than that found in the current study.11 In a previous study conducted in 2002 in North India, although the findings of culture positivity rate of pus samples (48.4%, 2,437 of 5,039) as well as S. aureus being the most common pathogen were similar as in the current study, no B. pseudomallei was isolated.22 Fifteen years on, a report from the same center again has no mention of B. pseudomallei.23 A study in South India between January 2012 and 2017 shows B. pseudomallei as a rare pathogen in only three pus specimens.24 Thus, the current study shows the emergence of B. pseudomallei as a significant pathogen in localized pyogenic infections in eastern India, distributed in various districts of Odisha and West Bengal. The apparent concentration of maximum number of cases in Khordha district may be because our hospital is situated in Khordha district of Odisha, and hence the likelihood of concentration of more cases in Khordha district. Furthermore, with ours being a large tertiary-care institute, people from faraway places also visit the hospital for treatment. So, a scattered distribution of melioidosis cases in other districts of Odisha as well as in the neighboring state of West Bengal can be observed. Thus, our study shows the widespread presence of melioidosis in the eastern region of India. Active case searching might uncover more hidden reservoirs of the disease.
The age of patients in the current study (mean 39.1 years, range: 7–71 years) is in accordance with the previous report from our institute for non-bacteremic cases (mean 38.2 years, range: 1–70 years).25 Other studies from India report a slightly higher mean age of 41–48.5 years, taking all manifestations into account.7,15 Congruent with reports from other endemic regions, males (70.6%) were affected more than females (29.4%) and is likely explained due to a higher potential for activities among males facilitating exposure.8,9 Males accounted for 74.5%, 92.1%, and 83.6% of patients in studies carried out in Odisha,25 South India,12 and Malaysia,26 respectively. Although early studies indicated that environmental exposure to contaminated soil or water, like in people involved in farming and military, was an important risk factor for acquiring melioidosis, studies carried out by Vidyalaksmi et al.14 and Kingsley et al.26 and more recently by Tipre et al.7 show environmental exposure in only 36.8%, 39%, and 28% cases, respectively. In the current study, presumptive high-risk exposure by outdoor occupational history was observed in only half of the patients, and there was not much difference in frequency between people engaged in outdoor (18, 52.9%) and indoor (16, 47.1%) occupations. Thus, outdoor occupational exposure as a predominant risk factor for melioidosis seems to be blurring in recent times, and people across occupations seem to be vulnerable for melioidosis. The current study showed maximum (79.4%) patients presenting during the monsoon or in the post-monsoon season (Table 3), in agreement to previous well-documented reports from endemic regions (71.6–81% during the rainy season).14,27 During rainfall, bacteria move to surface soil, and during heavy wind, aerosolization of bacteria from surface water and soil cause infection due to inoculation through skin abrasions or by inhalation.1–3
There is very limited mention of B. pseudomallei in studies on the microbiological spectrum of skin and soft tissue infections or pyogenic lesions from India, and published literature have mainly consisted of case reports and case series. To the best of our knowledge, the earliest mention of B. pseudomallei in skin and soft tissue infections in India is by Mathew et al.,28 who described four cases of surgical presentation of melioidosis, of which three presented with splenic abscesses and one with a soft tissue abscess in the neck. Subsequently, only a few case reports of isolated visceral abscesses including brain and liver abscesses have been described in the literature.29–31 A little later, Vidyalakshmi et al.32 reported the isolation of B. pseudomallei from six pus samples of a series of 25 patients with culture-proven melioidosis consisting of supraclavicular mass (two), psoas abscess (two), scalp abscess (one), and gluteal abscess (one). Furthermore, of 32 culture-proven cases between 2005 and 2010, localized disease was observed in 43.8%, usually as septic arthritis or abscesses.33 A recent compilation of case reports from India has shown soft tissue abscess (37%) as the most common clinical presentation, followed by pneumonia (24%) and osteomyelitis/septic arthritis (18%).7 However, all these are retrospective studies describing the clinico-epidemiological trends of melioidosis incidentally diagnosed at their centers. By contrast, our study has been conducted in a prospective manner to actively search for melioidosis in patients presenting with skin and soft tissue infections and other localized manifestations.
In the current study, neck abscesses accounted for more than one-third (11/34, 32.4%) of our cases, which corroborates nicely with a recently published review, where cervical lymphadenopathy was found to account for 40% of cases of localized melioidosis cases worldwide.9 Melioidosis mimicking as well as coinfecting tubercular cold abscesses have been reported from India on many instances.34–37 Hence, melioidosis should be considered as a differential diagnosis of suppurative or granulomatous regional lymphadenitis in the neck region apart from tuberculosis, especially in a tuberculosis-endemic country like India. Parotid abscess due to melioidosis has been described predominantly in children from Thailand.8,9 In the current study, the single case of parotid abscess was found in a 33-year-old woman. As regards musculoskeletal involvement, of 26 patients from South India with musculoskeletal melioidosis, 15 (58%) had osteomyelitis, 10 (38%) had septic arthritis, and one (4%) had soft tissue abscess.38 From outside India, localized melioidosis as the predominant manifestation was seen in a study from Thailand in which, of 134 patients from January 2002 to June 2011, the majority cases (62.7%) were localized unifocal or multifocal infection without bacteremia.10 A study from Bangladesh over a prolonged period from 1961 to 2017 noted musculoskeletal (21, 43%), organ abscess/deep-seated abscess (13, 27%), and cutaneous (13, 27%) lesions to be the predominant manifestations.39 A study in Cambodia on pediatric melioidosis found that the infection was predominantly localized in 27 cases (69%) and disseminated in 12 (31%).40 A point of note is that it is vital to consider melioidosis as one of the differential diagnosis in abscesses in unusual sites such as the spleen, parotid, and prostate, especially with chronic presentation. Liver abscess is usually due to Entamoeba histolytica or enteric bacteria, but in the spleen, abscess due to protozoa and enteric bacteria is less common, and therefore, one should think about the possibility of melioidosis. In the current study, liver and spleen abscesses were found in eight (23.5%) patients.
Diabetes mellitus, with associated neutrophil dysfunction and deranged innate immunity, is the most important risk factor for melioidosis with estimates of > 50% of all worldwide cases of melioidosis to have diabetes.2–4 However, a significant proportion of patients (∼20%) have no recognized risk factors as in the current study.3,9,27 Overall, an excellent outcome was observed similar to other studies of localized cutaneous or musculoskeletal infections, where a high rate of resolution has been seen.38,41,42
Susceptibility studies from India and elsewhere reveal that antimicrobial resistance in B. pseudomallei has been extremely low to most of the antibiotics, except amoxicillin–clavulanic acid and trimethoprim–sulfamethoxazole.32,43 A study of more than 4,000 B. pseudomallei isolates in Thailand and more than 600 isolates in Singapore reported ceftazidime resistance at 0.5%, whereas multiple smaller studies demonstrated 100% susceptibility.9 However, more recently, antimicrobial resistance seems to be emerging.43–45 In a study in Malaysia, ceftazidime, imipenem, amoxicillin–clavulanic acid, and doxycycline resistance were observed in one isolate (0.6%) each, but trimethoprim–sulfamethoxazole resistance was observed in 17 (10%) isolates.43 At other places, the rate of trimethoprim–sulfamethoxazole resistance ranges from 2.5% in Australia to 13–16% in Thailand.44 In India, all 31 B. pseudomallei were sensitive to ceftazidime, trimethoprim–sulfamethoxazole, imipenem, and chloramphenicol, but for amoxicillin–clavulanate, 44% were resistant and 16% were in the intermediate range.32 In another study in Malaysia, among 81 isolates, six were multidrug resistant (resistant to meropenem, imipenem, and ceftazidime) and the remaining 75 were resistant to at least one of the antimicrobials.45 Minimum inhibitory concentration 90 for the tested antibiotics in the current study ranged from 1.5 to 6 µg/mL, slightly higher than that of the study from Malaysia, where MIC90 of most of the antibiotics was within the range of 0.38–4.0 µg/mL.43
CONCLUSION
Burkholderia pseudomallei is an emerging etiological agent of localized pyogenic infections in eastern India, principally affecting the adult male population. Various ecological factors such as high rainfall and temperature, extensive agricultural activities, rampant large-scale construction as well as high incidence of illnesses such as diabetes and tuberculosis that are present in India, especially in regions like Odisha, make the population in these regions susceptible to the infection caused by B. pseudomallei. With a predicted rise in the prevalence of diabetes in the adult Indian population from 7.1% in 2010 to 8.6% in 2030 in India (an increase from approximately 51 million to 87 million diabetics), a resultant increased risk of melioidosis is envisioned in the near future.12 Because localized melioidosis has an excellent prognosis with appropriate intensive phase and eradication phase therapy, it mandates an increased vigilance and early diagnosis on the part of physicians and laboratory personnel to further reduce the morbidity and mortality. The major limitation of the present study is that it is a single-center small-scale study. Multicentric study results will have more generalizability than single-center studies. Also, other diagnostic methods such as antigen testing and direct molecular testing from suspected clinical samples were not performed, and thus, the culture-positive cases may not reflect the true extent of melioidosis present in the population. Further studies may be undertaken in the future, such as community serosurveys and molecular testing, to delineate areas of high burden and endemicity for effective control of the disease.
Supplemental table and figure
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental table and figure appear at www.ajtmh.org.
REFERENCES
- 1.Cheng AC, Currie BJ, 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18: 383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Currie BJ, Peacock SJ, 2012. Melioidosis. N Engl J Med 367: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, Limmathurotsakul D, 2018. Melioidosis. Nat Rev Dis Primers 4: 17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie BJ, 2015. Melioidosis: evolving concepts in epidemiology pathogenesis and treatment. Semin Respir Crit Care Med 36: 111–125. [DOI] [PubMed] [Google Scholar]
- 5.Cousins S, 2016. India is at high risk from surge in cases of melioidosis, warn researchers. BMJ 352: i275. [DOI] [PubMed] [Google Scholar]
- 6.Limmathurotsakul D, et al. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1: 15008. [DOI] [PubMed] [Google Scholar]
- 7.Tipre M, Kingsley PV, Smith T, Leader M, Sathiakumar N, 2018. Melioidosis in India and Bangladesh: a review of case reports. Asian Pac J Trop Med 11: 320–329. [Google Scholar]
- 8.Kingsley PV, Arunkumar G, Tipre M, Leader M, Sathiakumar N, 2016. Pitfalls and optimal approaches to diagnose melioidosis. Asian Pac J Trop Med 9: 515–524. [DOI] [PubMed] [Google Scholar]
- 9.Gassiep I, Armstrong M, Norton R, 2020. Human melioidosis. Clin Microbiol Rev 33: e00006–e00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churuangsuk C, Chusri S, Hortiwakul T, Charernmak B, Silpapojakul K, 2016. Characteristics, clinical outcomes and factors influencing mortality of patients with melioidosis in southern Thailand: a 10-year retrospective study. Asian Pac J Trop Med 9: 256–260. [DOI] [PubMed] [Google Scholar]
- 11.Tellapragada C, Shaw T, D’Souza A, Eshwara VK, Mukhopadhyay C, 2017. Improved detection of Burkholderia pseudomallei from non-blood clinical specimens using enrichment culture and PCR: narrowing diagnostic gap in resource-constrained settings. Trop Med Intl Health 22: 866–870. [DOI] [PubMed] [Google Scholar]
- 12.Koshy M, Jagannati M, Ralph R, Victor P, David T, Sathyendra S, Veeraraghavan B, Varghese GM, 2019. Clinical manifestations, antimicrobial drug susceptibility patterns, and outcomes in melioidosis cases, India. Emerg Infect Dis 25: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay C, Shaw T, Varghese GM, Dance DAB, 2018. Melioidosis in South Asia (India, Nepal, Pakistan, Bhutan and Afghanistan). Trop Med Infect Dis 3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidyalakshmi K, Lipika S, Vishal S, Damodar S, Chakrapani M, 2012. Emerging clinico-epidemiological trends in melioidosis: analysis of 95 cases from western coastal India. Int J Infect Dis 16: e491–e497. [DOI] [PubMed] [Google Scholar]
- 15.Chrispal A, Rajan SJ, Sathyendra S, 2010. The clinical profile and predictors of mortality in patients with melioidosis in South India. Trop Doct 40: 36–38. [DOI] [PubMed] [Google Scholar]
- 16.Tille PM, ed., 2017. Bailey & Scott’s Diagnostic Microbiology, 14th edition. St. Louis, MO: Elsevier Inc. [Google Scholar]
- 17.Collee JG, Miles RS, Watt B, 1996. Test for the identification of bacteria. Collee JG, Fraser AG, Marmion BP, Simmons A, eds. Mackie and McCartney. Practical Medical Microbiology. 14th edition. London, United Kingdom: Churchill Livingstone, 131–145. [Google Scholar]
- 18.Pitt TL, Dance DA, 2005. Burkholderia spp. and related genera. Borriello SP, Murray PR, Funke G, eds. Topley & Wilsons Microbiology & Microbial Infections. Bacteriology Vol. 2. 10th edition. Chichester, West Sussex, United Kingdom: Wiley, 1607–1648. [Google Scholar]
- 19.Suppiah J, Thimma JS, Cheah SH, Vadivelu J, 2010. Development and evaluation of polymerase chain reaction assay to detect Burkholderia genus and to differentiate the species in clinical specimens. FEMS Microbiol Lett 306: 9–14. [DOI] [PubMed] [Google Scholar]
- 20.CLSI , 2018. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100, 28th edition. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 21.CLSI , 2015. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. CLSI Guideline M45, 3rd edition. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 22.Mohanty S, Kapil A, Dhawan B, Das BK, 2004. Bacteriological and antimicrobial susceptibility profile of soft tissue infections from Northern India. Indian J Med Sci 58: 10–15. [PubMed] [Google Scholar]
- 23.Agrawal SK, Panigrahy A, Perumalla S, Kapil A, Dhawan B, 2018. Microbiological profile and antibiotic resistance pattern of skin and soft-tissue infections: a study from Northern India. J Lab Physicians 10: 471–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudhaharan S, Kanne P, Chavali P, Vemu L, 2018. Aerobic bacteriological profile and antimicrobial susceptibility pattern of pus isolates from tertiary care hospital in India. J Infect Dev Ctries 12: 842–848. [DOI] [PubMed] [Google Scholar]
- 25.Behera B, et al. 2019. Melioidosis in Odisha: a clinico-microbiological and epidemiological description of culture-confirmed cases over a 2-year period. Indian J Med Microbiol 37: 430–432. [DOI] [PubMed] [Google Scholar]
- 26.Kingsley PV, Leader M, Nagodawithana NS, Tipre M, Sathiakumar N, 2016. Melioidosis in Malaysia: a review of case reports. PLoS Negl Trop Dis 10: e0005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Currie BJ, Ward L, Cheng AC, 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 4: e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathew S, Perakath B, Mathew G, Sitaram V, Nair A, Lalitha MK, John TJ, 1999. Surgical presentation of melioidosis in India. Natl Med J India 12: 59–61. [PubMed] [Google Scholar]
- 29.Lath R, Rajshekhar V, George V, 1998. Brain abscess as the presenting feature of melioidosis. Br J Neurosurg 12: 170–172. [DOI] [PubMed] [Google Scholar]
- 30.Navaneethan U, Ramesh Kumar AC, Ravi G, 2006. Multiple visceral abscess in a case of melioidosis. Indian J Med Sci 60: 68–70. [PubMed] [Google Scholar]
- 31.Mukhopadhya A, Balaji V, Jesudason MV, Amte A, Jeyamani R, Kurian G, 2007. Isolated liver abscesses in melioidosis. Indian J Med Microbiol 25: 150–151. [DOI] [PubMed] [Google Scholar]
- 32.Vidyalakshmi K, Shrikala B, Bharathi B, Suchitra U, 2007. Melioidosis: an under-diagnosed entity in western coastal India: a clinico-microbiological analysis. Indian J Med Microbiol 25: 245–248. [DOI] [PubMed] [Google Scholar]
- 33.Gopalakrishnan R, Sureshkumar D, Thirunarayan MA, Ramasubramanian V, 2013. Melioidosis: an emerging infection in India. J Assoc Physicians India 61: 612–614. [PubMed] [Google Scholar]
- 34.Vishnu Prasad NR, Balasubramaniam G, Karthikeyan VS, Ramesh CK, Srinivasan K, 2012. Melioidosis of chest wall masquerading as a tubercular cold abscess. J Surg Tech Case Rep 4: 115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kundangar RS, Bhat SN, Mohanty SP, 2017. Melioidosis mimicking tubercular cold abscess. BMJ Case Rep 2017: bcr2017221787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulaiman H, Ponnampalavanar S, Mun KS, Italiano CM, 2013. Cervical abscesses due to co-infection with Burkholderia pseudomallei, Salmonella enterica serovar Stanley and Mycobacterium tuberculosis in a patient with diabetes mellitus. BMC Infect Dis 13: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shenoy V, Kamath MP, Hegde MC, D’Souza T, Mammen SS, 2009. Melioidosis and tuberculosis: dual pathogens in a neck abscess. J Laryngol Otol 123: 1285–1287. [DOI] [PubMed] [Google Scholar]
- 38.Perumal R, Livingston A, Samuel S, Govindaraju SK, 2020. Melioidosis of the musculoskeletal system. Med Princ Pract 29: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chowdhury FR, et al. 2018. Melioidosis in Bangladesh: a clinical and epidemiological analysis of culture-confirmed cases. Trop Med Infect Dis 3: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagnarith Y, Kumar V, Thaipadungpanit J, Wuthiekanun V, Amornchai P, Sin L, Day NP, Peacock SJ, 2010. Emergence of pediatric melioidosis in Siem Reap, Cambodia. Am J Trop Med Hyg 82: 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raja NS, Scarbrook C, 2016. Burkholderia pseudomallei causing bone and joint infections: a clinical update. Infect Dis Ther 5: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibney KB, Cheng AC, Currie BJ, 2008. Cutaneous melioidosis in the tropical top end of Australia: a prospective study and review of the literature. Clin Infect Dis 47: 603–609. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad N, Hashim R, Mohd Noor A, 2013. The in vitro antibiotic susceptibility of Malaysian isolates of Burkholderia pseudomallei. Int J Microbiol 2013: 121845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweizer HP, 2012. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khosravi Y, Vellasamy KM, Mariappan V, Ng SL, Vadivelu J, 2014. Antimicrobial susceptibility and genetic characterisation of Burkholderia pseudomallei isolated from Malaysian patients. ScientificWorldJournal 2014: 132971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.