Abstract
Mediator is a modular coactivator complex involved in the transcription of the majority of RNA polymerase II-regulated genes. However, the degrees to which individual core subunits of Mediator contribute to its activity have been unclear. Here, we investigate the contribution of two essential architectural subunits of Mediator to transcription in Saccharomyces cerevisiae. We show that acute depletion of the main complex scaffold Med14 or the head module nucleator Med17 is lethal and results in global transcriptional downregulation, though Med17 removal has a markedly greater negative effect. Consistent with this, Med17 depletion impairs preinitiation complex (PIC) assembly to a greater extent than Med14 removal. Co-depletion of Med14 and Med17 reduced transcription and TFIIB promoter occupancy similarly to Med17 ablation alone, indicating that the contributions of Med14 and Med17 to Mediator function are not additive. We propose that, while the structural integrity of complete Mediator and the head module are both important for PIC assembly and transcription, the head module plays a greater role in this process and is thus the key functional module of Mediator in this regard.
Keywords: mediator, transcription, RNA polymerase II, Med14, Med17
Introduction
Mediator is a conserved, essential coactivator complex that bridges transcription factors bound to distal cis-regulatory elements and promoter-bound general transcription factors (GTFs) and RNA polymerase II (RNAPII). The 25 subunits of budding yeast Mediator are classified into four modules: head, middle, tail, and kinase. Med14 acts as a scaffold, holding together the head, middle, and tail modules (Tsai et al. 2014; Wang et al. 2014; Plaschka et al. 2015; Robinson et al. 2015). The head module contacts RNAPII and GTFs, with its formation nucleated by Med17 (Imasaki et al. 2011). Together, the head and middle modules form the functional core Mediator (cMed), an assembly of 15 mostly essential subunits (Liu et al. 2001; Plaschka et al. 2015), while the nonessential tail and kinase modules in turn regulate the recruitment and function of cMed (Jeronimo et al. 2016).
Much work on the transcriptional role of Mediator has focused on the head module core subunit Med17. As Med17 is essential (Thompson et al. 1993), various inducible approaches have been used to study its function. Early studies used srb4-138, a temperature-sensitive (ts) allele of Med17 that dissociates the head module from the remainder of the complex at elevated temperature (Linder et al. 2006). Single-gene and global mRNA measurements from this strain following Med17 inactivation revealed a decrease comparable to that seen for a ts RNAPII mutant (Thompson and Young 1995; Holstege et al. 1998), though such measurements cannot distinguish the effects of altered mRNA synthesis and decay. While more recent studies have demonstrated global decreases in nascent RNA synthesis (Plaschka et al. 2015) and RNAPII occupancy (Paul et al. 2015) in srb4-138 cells at elevated temperature, analysis of Mediator function under heat shock is complicated by the general decrease in RNAPII occupancy observed at elevated temperature (Warfield et al. 2017) and the involvement of Mediator in heat shock transcription (Kremer et al. 2012; Kim and Gross 2013; Anandhakumar et al. 2016). Furthermore, dominant mutations in the MED6 and MED22 genes, both encoding head subunits, suppress srb4-138 lethality at the restrictive temperature but do not compensate for SRB4 deletion (Lee et al. 1998), suggesting that the mutant protein retains some function at elevated temperature.
Nuclear depletion of Mediator subunits with anchor away (AA) (Haruki et al. 2008) has also been used to probe their roles in transcription. As Med17 nucleates the head module (Imasaki et al. 2011), it stands to reason that its depletion would reduce or ablate head module formation. Surprisingly, Med17 AA is not lethal and results in a relatively modest global reduction in RNAPII occupancy (Petrenko et al. 2017; Bruzzone et al. 2018). Similarly, Med14 AA leads to a moderate reduction in RNAPII occupancy at ∼400 well-transcribed genes and, notably, also maintains viability (Petrenko et al. 2017). It has thus been proposed that Mediator modules contribute independently to overall complex function and can individually promote substantial transcription such that viability is maintained despite single subunit loss. Given that deletion of Med14 or Med17 is lethal (Sakai et al. 1990; Thompson et al. 1993), while AA of these subunits is not, it is likely that AA yields inducible hypomorphic alleles that are useful for revealing sets of genes heavily dependent on Mediator components, but may not allow for determination of the full effects of individual Mediator subunit loss. Indeed, auxin-inducible degron (AID)-mediated depletion of Med14 decreases RNAPII occupancy of nearly all genes (Warfield et al. 2017), a much broader and stronger effect than that resulting from Med14 AA (Petrenko et al. 2017).
Given these disparate results, an outstanding question regarding Mediator function is whether the structural roles of Med14 and Med17 are equivalent or distinct in their contribution to the overall function of the complex. That is, is dissociation of the complete head, middle, and tail modules via Med14 removal more or less deleterious to pre-initiation complex (PIC) assembly and transcription than “beheading” of Mediator by depletion of Med17? To address this question, we directly measured transcription by labeling, purification, and sequencing of newly synthesized RNA (nsRNA) following AID-mediated ablation of Med14 or Med17. Depletion of either factor was lethal and globally downregulated transcription. Removal of Med17 caused a substantially greater downregulation of transcription and PIC formation and/or stability than Med14 degradation, indicating that the head module is key for full PIC occupancy. Notably, Med17 depletion showed little preference for downregulation of genes annotated as dependent on the Spt-Ada-Gcn5 (SAGA) coactivator complex, which have previously been reported to be specifically affected by Med17 AA. Simultaneous depletion of Med14 and Med17 resulted in transcriptional downregulation equivalent to that seen with removal of Med17 alone, indicating that the roles of Med14 and Med17 in transcription are not additive. Taken together, our results indicate that the structural integrity of complete Mediator and coherence of the head module are not equivalent in terms of their impact on PIC occupancy and transcription.
Materials and methods
Yeast methods
Med14, Med17, and Sua7 were tagged with 3xV5-IAA7 using pSB2065 (pFA6a-3xV5-IAA7-kanMX6) (Miller et al. 2016) in the GZY191 (BY4705 pGPD-OsTIR1-HIS3) background (Tourigny et al. 2018). Med14 was tagged with 3xHA-IAA7 using pGZ364 (pFA6a-3xHA-IAA7-URA3). Med14, Med17, and Sua7 were tagged with 3xFLAG using pFA6a-6xGLY-3xFLAG-hphMX4 (Funakoshi and Hochstrasser 2009) (a gift from Mark Hochstrasser, Addgene plasmid #20755) or a derivative thereof in which the hphMX4 marker is replaced with a TRP1 cassette (pGZ392). The BY4741 kin28as strain was kindly provided by Aseem Ansari (Rodríguez-Molina et al. 2016). In this strain, OsTIR1 was integrated into the leu2 locus using pSB2271 (pNH605-pGPD1-OsTIR1-LEU2) (Miller et al. 2016), Med14 was tagged with 3xHA-IAA7 using pSB2229 (pFA6a-3xHA-IAA7-kanMX6), and Med17 was tagged with 3xFLAG using pGZ393 (pFA6a-6xGLY-3xFLAG-URA3). For spot assays, strains were grown overnight and diluted to an OD600 of 1.0. Tenfold serial dilutions were plated on YPD with and without 500 μM 3-IAA and plates were imaged after 48 h of growth at 30°C. Strain genotypes are provided in Supplementary Table S1.
Western blotting
A 50 ml culture was grown in YPD to an OD600 ≥ 0.7. Prior to addition of 500 μM 3-IAA, a 9-ml sample was removed and cells were pelleted at 1500 × g for 2 min. The OD600 of the culture (0 time point) was also measured during centrifugation. Following centrifugation, the pellet was resuspended in 1 ml cold PBS and transferred to a microfuge tube. Cells were pelleted with a flash spin, the supernatant was aspirated, and cells were frozen. After 3-IAA addition, the above cell collection procedure was repeated at 15, 30, 45, and 60 min. For protein extraction, pellets were thawed and resuspended in 150 μl protein extraction buffer (100 mM NaOH, 2% SDS, 50 mM EDTA). Resuspended cells were heated at 95°C for 5 min and the lysis buffer was neutralized by addition of 3.75 μl 4 M acetic acid followed by vortexing. Samples were mixed with 50 μl 4× Laemmli buffer with DTT and heated for 95°C for 3 min. Prior to loading, samples were cleared by centrifugation at maximum speed and room temperature for 2 min. Sample loading was normalized by OD600 values. The following antibodies were used for western blotting: mouse anti-V5 (ThermoFisher R960-25), mouse anti-FLAG M2 (Sigma F3165), rat anti-HA 3F10 (Roche 15645900), rabbit anti-histone H3 (Abcam ab1791). See Supplementary Figure S1 for full blot scans.
Degron western blots were quantified using BioRad Image Lab v6.0.1 for chemiluminescent blots or LiCor ImageStudio Lite v5.2.5 for scanned fluorescent blots. The intensity of bands corresponding to V5- and/or HA-tagged targets, along with the H3 loading control in each lane, was measured using pixels surrounding the quantification area as local background. V5 and HA signals were then normalized to their respective H3 levels before calculating the proportion of signal in each lane relative to the untreated (0 min) timepoint, set to 1. Values for the 30 min time point (at which all sequencing experiments reported here were performed) were then averaged and plotted in R (Supplementary Figure S1).
4tU-seq
RNA labeling and purification were performed as previously described (Baptista et al. 2017; Baptista and Devys 2018). Sequencing libraries were constructed by the GenomEast platform at the Institut de Génétique et de Biologie Moléculaire et Cellulaire using the Illumina TruSeq Stranded Total RNA LT Sample Prep Kit. Libraries were sequenced on the on the Illumina HiSeq 4000 platform with the following parameters: 50 cycles paired-end (Med14-AID and Med17-AID), 100 cycles paired-end (Sua7-AID), 50 cycles single-end (Med14/17-AID). All 4tU-seq and total RNA-seq experiments were performed in biological duplicate.
ChIP-seq
Med17
ChIP was performed as described (Bataille and Robert 2009) with minor modifications. A 100 mL culture was grown to an OD600 ≥ 0.8 and treated with 500 μM 3-IAA or an equal volume of DMSO for 15 min. After 15 min of 3-IAA or DMSO treatment, the culture was split into two flasks. One culture was treated with 6 μM 1-Napthyl PP1 (NAPP1, Cayman Chemicals) and the other was treated with an equal volume of DMSO for 15 min. Cells were crosslinked using 1% formaldehyde for 30 min at 30°C and then quenched using Tris pH 8.0 (final concentration of 0.75 M). Crosslinked cells were lysed with bead beating using a Disruptor Genie at 4°C for 8 cycles of 3 min at 2850 rpm and 1 min on ice. Then, lysate was transferred to a new tube and centrifuged as previously described. Lysate was sonicated using a Bioruptor Pico for 8 cycles of 30 s on and 30 s off. Soluble chromatin was immunoprecipitated using 30 μL of serum bovine albumin pre-blocked Sigma FLAG M2 magnetic beads (catalog number M8823) overnight at 4°C. Beads were washed as described and decrosslinked by resuspension in 98 μl of TE/1% SDS and shaking (800 RPM) at 65°C for 12–16 h. DNA was purified from the supernatant using a Qiagen PCR purification kit per the manufacturer’s protocol. All Med17 ChIP-seq experiments were performed in biological duplicate. Sequencing libraries were constructed by the Indiana University Center for Genomics and Bioinformatics (CGB) using the NEBNext Ultra II Library Prep Kit for Illumina and sequenced for 80 cycles in paired-end mode on the Illumina NextSeq 500 platform at the CGB.
Sua7
A 100mL culture of the strain of interest was grown in YPD to an OD600 ≥ 0.8 and split into two flasks. One culture was treated with 500 μM 3-IAA and the other was treated with an equal volume of DMSO for 30 min. ChIP was performed as described (Tourigny et al. 2018). Library construction and sequencing was performed as described for Med17 ChIP-seq, except that some libraries were sequenced for 38 cycles. Sua7 ChIP-seq experiments in the Med17-AID and Med14/17-AID strains following DMSO or 3-IAA treatment were performed in biological duplicate. Our two previously published biological replicates of Sua7 ChIP-seq in the Med14-AID strain with DMSO or 3-IAA treatment were reanalyzed for comparison (Tourigny et al. 2018) (GSE112721).
Data analysis
Total RNA-seq/4tU-seq
Reads were mapped to a chimeric genome composed of the S. cerevisiae (sacCer3) and S. pombe (ASM294v2) genomes using STAR (Dobin et al. 2013) v2.5.3a. Quantification was performed with uniquely aligned reads using htseq-count (Anders et al. 2015) v0.6.1p1 with annotations from Ensembl version 94 for S. cerevisiae and Ensembl fungi version 41 for S. pombe and union mode. S. cerevisiae read counts were normalized across samples with the size factors computed by the median-of-ratios method (Anders and Huber 2010) on S. pombe genes to make these counts comparable between samples. The simple error ratio estimate (SERE) method (Schulze et al. 2012) was used to assess replicate similarity. A SERE value of 1.0 indicates dispersion between replicates attributable to Poisson variation. SERE values > 1 are indicative of overdispersion observed between biological replicates, and SERE values ≫ 1 are attributable to different biological conditions. Differential expression analysis was performed with DESeq2 (Love et al. 2014) v1.16.1. Log2(fold changes), P-values, and adjusted P-values for the 5080 analyzed transcripts are given in Supplementary Table S2. Codon usage of the 5011 mRNAs analyzed here was performed with coRdon (Elek et al. 2019) and the fraction of optimal codons for each transcript was calculated using a previously published list of optimal codons (Presnyak et al. 2015). Optimal codon percentage was then correlated with the average of spike-in-normalized nsRNA counts divided by median transcript length in kb across all DMSO-treated samples. Data analysis and visualization were performed with R. Code used for figure generation is available upon request.
ChIP-seq
Paired-end ChIP-seq data were aligned to the sacCer3 genome build using Bowtie2 (Langmead and Salzberg 2012) with default settings plus “-I 10 -X 700 --no-unal --dovetail --no-discordant --no-mixed.” SAM files were converted to sorted BAMs using SAMtools (Li et al. 2009). BigWig files were generated using deepTools (Ramírez et al. 2016) v3.3.0 bamCoverage with 1 bp bins and counts per million (CPM) normalization. Signal tracks were visualized with Gviz (Hahne and Ivanek 2016). Log2(3-IAA/DMSO) and log2(Med17-FLAG/no-tag) bigWig files were generated using deepTools bamCompare with 1 bp bins and signal extraction scaling (SES) normalization (Diaz et al. 2012). Matrices of ChIP-seq signal around TSSs to be plotted as heatmaps were generated with deepTools computeMatrix using reference-point mode, 1 bp bins, and 1000 bp windows upstream and downstream of each TSS. Heatmaps were then generated with deepTools plotHeatmap. GTF ChIP-seq signal was quantified in a window spanning −150 to +50 relative to the TSS of each gene using HOMER annotatePeaks (Heinz et al. 2010). Sua7 log2(3-IAA/DMSO) FCs were averaged for biological duplicates and plotted in R. Statistical comparisons were performed with pairwise Wilcoxon rank-sum tests and Bonferroni correction in R. The 562 highly Sua7-bound regions analyzed in Figure 5D were chosen based on a threshold of an average of ≥ 50 normalized counts when all six DMSO-treated replicates were considered.
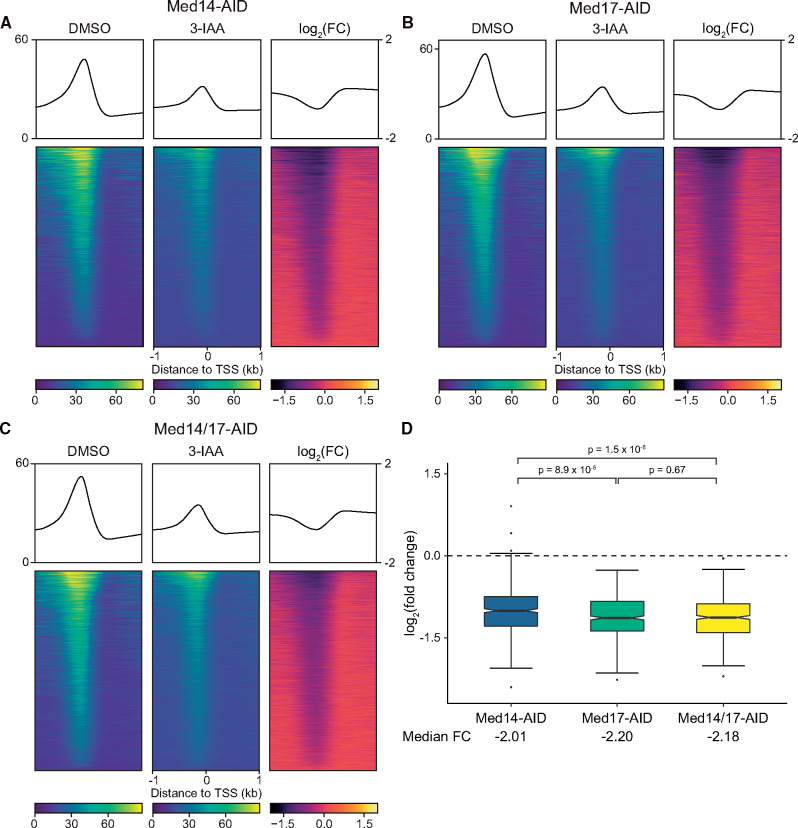
Figure 5.
Effects of Med14, Med17, and Med14/17 depletion on TFIIB promoter occupancy. (A–C) Average plots and heatmaps of Sua7 ChIP-seq signal around TSSs (n = 5,183) following DMSO or 3-IAA treatment of the indicated strain (see Supplementary Figure S6 for replicate heatmaps). The three heatmaps in each panel are sorted descending by average signal in the DMSO-treated sample. (D) Boxplots of average log2(FCs) in Sua7 promoter binding following Med14, Med17, or Med14/17 depletion at 562 promoters with an average of at least 50 normalized counts across all six DMSO-treated Sua7 ChIP-seq replicates. Whiskers represent the smallest and largest values up to IQR * 1.5. Notches represent 1.58 * IQR/sqrt(n), which equates to a ∼95% confidence interval for comparing medians. Statistical significance was assessed with pairwise Wilcoxon rank-sum tests and the Bonferroni correction for multiple comparisons.
Data availability
Supplementary Figures S1–S8 and Tables S1 and S2 have been deposited in figshare (https://doi.org/10.25386/genetics.13350521). An R Shiny app for interactive exploration of the RNA-seq data generated is available at https://zentlab.shinyapps.io/med1417. All sequencing data generated in this study have been deposited in GEO (total RNA-seq/4tU-seq: GSE146346; ChIP-seq: GSE145494).
Supplementary material is available at figshare DOI: https://doi.org/10.25386/genetics.13350521.
Results
Functional impairment of Mediator with the auxin degron system
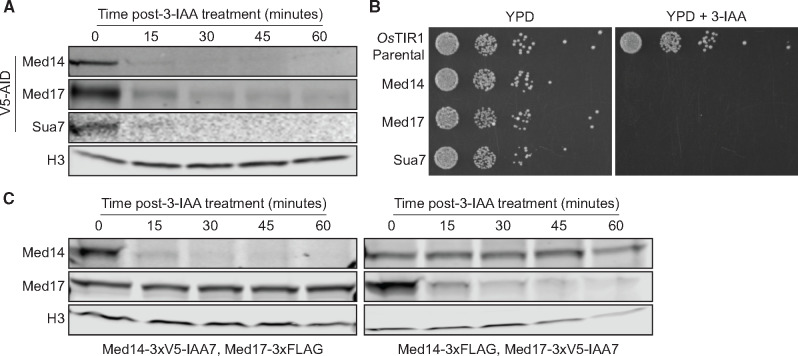
We used the AID system (Nishimura et al. 2009) to inducibly deplete the structurally essential Mediator subunits Med14 and Med17. Based on available biochemical and structural data, we reasoned that Med14 loss would dissociate Mediator into disconnected but stable head, middle, and tail modules (Tsai et al. 2014; Wang et al. 2014; Plaschka et al. 2015; Robinson et al. 2015). To ablate the head module, we targeted Med17, which acts as a scaffold for head module assembly (Imasaki et al. 2011). Depletion of Med14-AID and Med17-AID was highly efficient (Figure 1A). We also generated a strain in which Sua7/TFIIB was tagged with AID to allow us to compare the transcriptional effects of Mediator subunit depletion with those of directly impaired PIC assembly and function. Sua7-AID displayed similar depletion kinetics to those seen for Med14-AID and Med17-AID (Figure 1A). The Med14-AID, Med17-AID, and Sua7-AID strains all failed to grow on YPD medium containing 3-IAA, while the parental OsTIR1-expressing strain exhibited normal growth (Figure 1B). Given that Med14 and Med17 have been shown to be essential by classical genetic approaches (Sakai et al. 1990; Thompson et al. 1993), this result demonstrates that the AID system is suitable for full functional genomic analysis of essential Mediator subunits.
Figure 1.
Destabilization of complete Mediator and the Mediator head module with the auxin degron system. (A) Western blots showing the kinetics of 3-IAA-mediated depletion of AID-tagged factors. (B) Spot assays assessing growth of AID-tagged strains on YPD plates containing DMSO or 500 μM 3-IAA. (C) Western blots showing levels of Med17 following Med14 depletion and Med14 levels following Med17 depletion.
We next sought to validate the specificity of our approach for individual Mediator subunits. To this end, we tagged Med17 with a 3xFLAG epitope in the Med14-AID background and viceversa. We observed no effect of Med14 depletion on Med17 stability even after a 1 h 3-IAA treatment (Figure 1C), suggesting that AID targeting of Med14 does not nonspecifically affect Med17. Similarly, removal of Med17 had no impact on Med14 protein levels (Figure 1C). Thus, AID appears to target specific Mediator modules, in contrast to previous studies using AA where Med14 AA removes Med17 from the nucleus (Anandhakumar et al. 2016) and vice-versa (Petrenko et al. 2017).
Interestingly, comparison of AID- and FLAG-tagged Med14 and Med17 levels on unadjusted western blot scans indicated a substantially higher abundance of Med17 (Supplementary Figure S1). This variation is not due to differences in sample processing and imaging, as the sets of Med14-AID/Med17-FLAG and Med14-FLAG/Med17-AID samples were electrophoresed on a single gel and blotted and scanned or exposed on a single membrane to ensure comparability. While we cannot rule out differences in western blot transfer efficiency as the cause of the apparent discrepancies in Med14 and Med17 abundance we observe, these results are consistent with values recently reported in an integrative analysis of nearly two dozen quantitative protein abundance datasets (Ho et al. 2018), with median values of 1989 Med14 molecules and 3342 Med17 molecules per cell (Supplementary Figure S2).
Mediator dissociation and head module ablation downregulate global transcription to different extents
To assess the acute transcriptional effects of various forms of Mediator impairment, we performed 4-thiouracil (4tU) labeling, biotinylation, purification, and high-throughput sequencing (4tU-seq) of nsRNA. To establish a transcription-null baseline, we first assessed total and nsRNA levels following Sua7 depletion. As we observed strong depletion of all AID-targeted factors by 30 min of 3-IAA treatment (Figure 1A), we chose this early time point for 4tU labeling to avoid the potentially confounding effects of increasing cell dysfunction and death. S. cerevisiae cells were labeled for 6 min following 3-IAA treatment and spiked with a defined fraction of similarly labeled S. pombe cells to enable normalization against an exogenous reference. After sequencing, we quantified the levels of 5080 genes encoding verified ORFs and RNAPII-dependent sn/snoRNAs in steady-state (total) and nsRNA fractions using highly correlated biological duplicates (Supplementary Figures S3 and S4). The defect in transcription caused by Sua7 depletion was evident even in the total RNA fraction: at a FC cutoff of 2 and an adjusted P-value threshold of 0.05, 4129 genes were significantly downregulated, with a median FC of −4.87 for all genes (Figure 2A). Analysis of nsRNA levels following Sua7 removal revealed a massive reduction in transcription, with 5007 genes significantly downregulated and a median FC of −16.61 for all genes (Figure 2B). Having established a baseline of transcription ablation via Sua7 depletion, we next determined the transcriptional consequences of Med14 and Med17 removal. Med14 loss resulted in a modest reduction of steady-state transcript levels, with 1322 genes displaying reduced abundance and a median FC of −1.64 for all genes (Figure 2C). In contrast, analysis of nsRNA after Med14 depletion revealed a robust global downregulation of transcription, with 4869 genes significantly downregulated and a median FC of −3.63 for all genes (Figure 2D). Notably, Med17 ablation resulted in a much stronger effect on steady-state transcript levels than that of Med14, with 3424 genes significantly downregulated and a median FC of −2.47 for all genes (Figure 2E). The impact of Med17 loss on nsRNA levels was also substantially more pronounced, with 4994 genes significantly downregulated at a median FC of −6.49 for all genes (Figure 2F). From these data, we conclude that the Mediator complex as a whole and the head module are both essential for global transcription at levels supporting viability, though the effect of ablating the head module is more dramatic.
Figure 2.
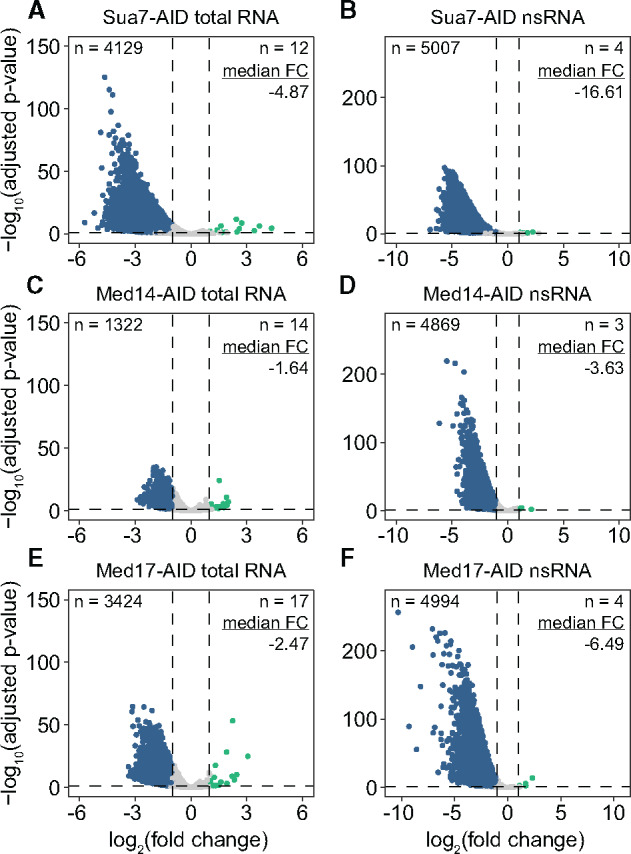
Architectural Mediator subunit depletion results in global transcriptional downregulation. (A and B) Volcano plots comparing the fold changes (FCs) and adjusted P-values of 5080 RNAPII-transcribed genes in the (A) total and (B) nsRNA fractions after Sua7 depletion. A FC cutoff of 2 with an adjusted P-value threshold of 0.05 was used to assess significance. Significantly downregulated genes are colored blue and significantly upregulated genes are colored teal. Numbers of significantly downregulated and upregulated genes as well as the median linear FC of all genes are given. (C and D) Same as (A and B) but for Med14 depletion. (E and F) Same as (A and B) but for Med17 depletion.
Simultaneous depletion of Med14 and Med17 is equivalent to loss of Med17 with respect to global transcription
Our results thus far indicate that depletion of Med17 has a greater negative effect on transcription than that of Med14 loss. We therefore speculated that the head module, which is a biochemically stable entity (Takagi et al. 2006; Imasaki et al. 2011), might remain associated with the PIC following dissociation from the middle and tail modules, and impart some residual level of transcriptional stimulation. Were this the case, we would expect that simultaneous depletion of Med14 and Med17 would result in transcriptional downregulation comparable to that observed with the destruction of Med17 alone. We therefore generated a strain bearing both Med14-AID and Med17-AID (Figure 3A). Again, we observed an apparently greater abundance of Med17 relative to Med14 (Supplementary Figure S1), consistent with our results from the AID/FLAG strains used in Figure 1. Following sequencing of total and nsRNA after 3-IAA treatment, we observed a greater decrease in total RNA levels with Med14/17 co-depletion than with either single Mediator degron (Figure 3B). However, at the level of nsRNA, Med14/17 depletion had a slightly milder negative effect than single Med17 depletion (Med14/17-AID median FC = −5.97, Med17-AID median FC = −6.49) with a similar number of genes downregulated (Med14/17-AID, 4990 genes; Med17-AID, 4994 genes) (Figure 3C). For all factors tested, we observed a substantially greater decrease in nsRNA than total RNA levels (Figure 3D), suggesting some degree of buffering of steady-state transcript levels via reduced mRNA decay (Sun et al. 2012).
Figure 3.
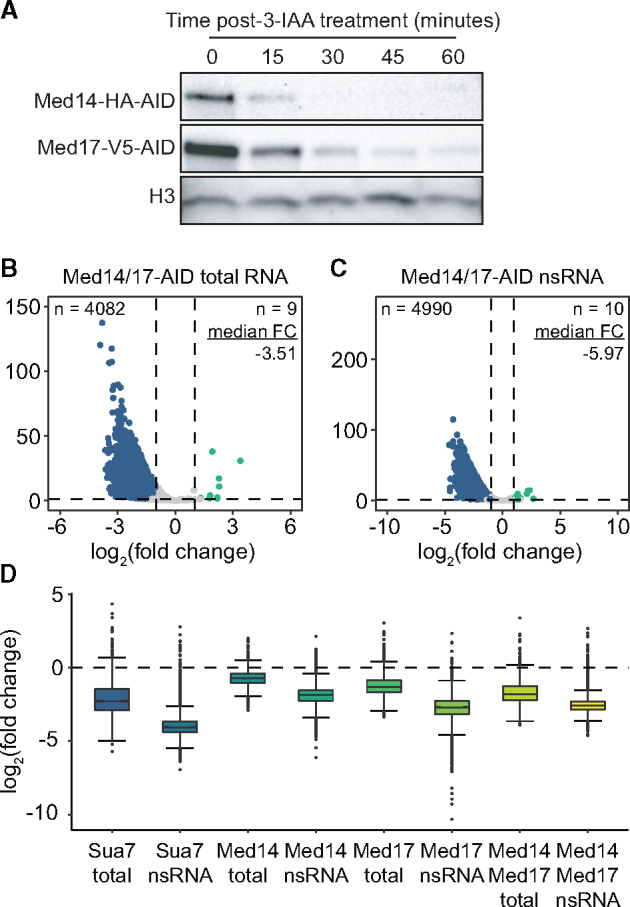
Simultaneous Med14 and Med17 depletion affects transcription to a degree comparable to loss of Med17 alone. (A) Western blots showing the kinetics of 3-IAA-mediated depletion of Med14 and Med17 in the dual degron strain. (B and C) Volcano plots comparing the FCs and adjusted P-values of 5080 RNAPII-transcribed genes in the (B) total and (C) nsRNA fractions after Med14/17 co-depletion. A FC cutoff of 2 with an adjusted P-value threshold of 0.05 was used to assess significance. Significantly downregulated genes are colored blue and significantly upregulated genes are colored teal. Numbers of significantly downregulated and upregulated genes as well as the median linear FC of all genes are given. (D) Boxplots summarizing the FCs of all 5080 analyzed genes in the total and nsRNA fractions from each degron experiment as labeled. Whiskers represent the smallest and largest values up to IQR * 1.5.
To further investigate the possibility that the head module is capable of stimulating transcription independent of the remaining complex post-Med14 depletion, we performed Med17 ChIP-seq following Med14 removal. In addition, due to the transient nature of Mediator interaction with PICs, we inhibited the TFIIH kinase Kin28 by 1-napthyl PP1 (NAPP1) inhibition of the ATP-analog sensitive kin28as allele, which traps Mediator at promoters by impairing phosphorylation of the RNAPII CTD (Jeronimo and Robert 2014; Wong et al. 2014). We performed two biological replicate ChIP-seq experiments for each of the four conditions (±NAPP1, ±3-IAA) and visualized the results as heatmaps. As expected, NAPP1 treatment of cells not depleted of Med14 resulted in a substantial accumulation of Med17 immediately upstream of TSSs (Supplementary Figures S5A and S6). This was also apparent at a selection of individual loci (Supplementary Figure S5B). However, when ChIP-seq was performed following 3-IAA treatment to deplete Med14, we were unable to detect Med17 at promoters (Supplementary Figures S5A and S6). While these data do not support the hypothesis that the head module remains associated with PICs at some level following Mediator dissociation via Med14 removal, we cannot rule out the possibility that a small amount of head module is still PIC-associated but below our detection threshold.
Gene-specific effects of Mediator impairment
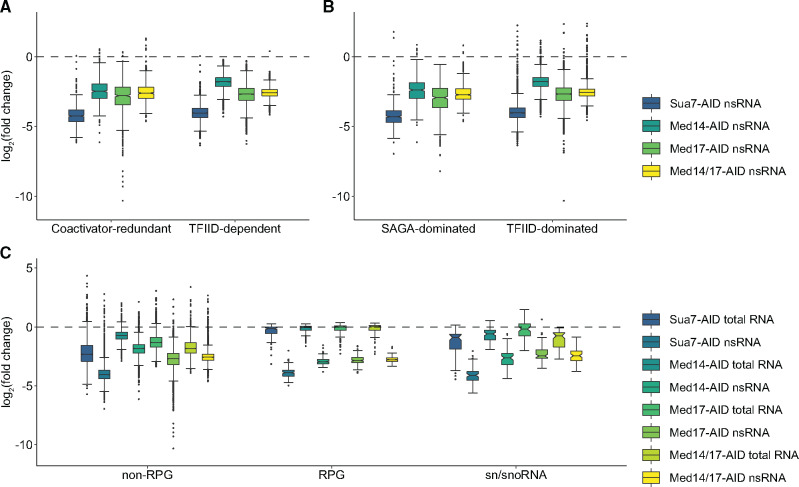
We next sought to determine the transcriptional effects of middle- and head-specific Mediator destabilization on distinct gene categories. Recent work analyzing the effects of acute depletion of SAGA and TFIID subunits led to a proposed reclassification of yeast genes as coactivator redundant (CR) or TFIID dependent (Donczew et al. 2020). CR genes are modestly sensitive to acute depletion of subunits of either SAGA or TFIID but are dramatically affected by simultaneous depletion of subunits from both complexes, while TFIID-dependent genes are heavily dependent on TFIID but show little change in transcription following rapid depletion of SAGA subunits. Med17 depletion resulted in greater downregulation of both classes of genes than did depletion of Med14, though the degrees of downregulation were more comparable for CR than for TFIID-dependent genes (Figure 4A). Consistent with our analysis of all genes (Figure 3D), co-depletion of Med14 and Med17 resulted in slightly milder downregulation of CR and TFIID-dependent genes than did ablation of Med17 alone (Figure 4A). We observed a similar effect for nonribosomal protein-coding genes previously classified as SAGA or TFIID-dominated (Huisinga and Pugh 2004) (Figure 4B).
Figure 4.
Gene-specific effects of Mediator subunit depletion. (A and B) Boxplots of average log2(FCs) in nsRNA levels following Sua7, Med14, Med17, or Med14/17 depletion for genes classified as (A) coactivator redundant or TFIID-dependent or (B) SAGA or TFIID-dominated (exclusive of RPGs). (C) Boxplots of average log2(FCs) in total and nsRNA levels following Sua7, Med14, Med17, or Med14/17 depletion for all non-RPG protein coding genes, RPGs, and sn/snoRNAs. Whiskers represent the smallest and largest values up to IQR * 1.5. Notches represent 1.58 * IQR/sqrt(n), which equates to a ∼95% confidence interval for comparing medians.
Med14 and Med17 are equally important for ribosomal protein gene and sn/snoRNA transcription
We next analyzed the effects of Mediator impairment on the transcription of ribosomal protein genes (RPGs), a co-regulated set of 137 highly transcribed genes encoding the structural components of the cytoplasmic ribosome (Warner 1999), and small nuclear and nucleolar RNAs (sn/snoRNAs), which we recently found to be dependent on Med14 for full expression (Tourigny et al. 2018). In contrast to what we observed for genes classified by coactivator dependence, Med14 and Med17 depletion reduced RPG and sn/snoRNA transcription to comparable extents (Figure 4C).
We also noted that, while RPG transcription was heavily downregulated by Sua7 or Mediator subunit depletion, steady-state RPG transcript levels were essentially unchanged, in stark contrast to non-RPG protein-coding transcripts (Figure 4C). We observed a similar phenomenon for sn/snoRNAs, though to a lesser extent. These findings suggest that RPG transcripts and potentially sn/snoRNAs are robustly buffered against decreased synthesis. In the case of RPGs, this phenomenon may be attributable to their relatively long half-lives (Presnyak et al. 2015; Chan et al. 2018). It has also been proposed that the high stability of RPG mRNAs and other long-lived transcripts is due to high codon optimality (Presnyak et al. 2015). Given that RPGs are highly transcribed and their mRNAs contain a large proportion of optimal codons, we asked if transcription level might correlate more generally with codon optimality across our data set. We examined the relationship of optimal codon usage with mean nsRNA levels across all DMSO-treated samples. This analysis revealed a moderate but highly significant positive correlation (Spearman’s ρ = 0.53, P < 2.2 × 10−16) (Supplementary Figure S7), consistent with previous comparisons of RNA synthesis rates and codon optimality in budding and fission yeast (Harigaya and Parker 2016). Further exploration of a potential functional relationship between codon optimality and transcription in budding yeast, potentially similar to that reported for Neurospora crassa (Zhou et al. 2016), is warranted.
Head module destabilization affects PIC assembly to a greater extent than complete Mediator dissociation
Given that a primary function of Mediator is promotion of PIC assembly (Ranish et al. 1999; Chen et al. 2012; Jeronimo and Robert 2014; Eyboulet et al. 2015; Eychenne et al. 2016; Grünberg et al. 2016), we next assessed the effects of Med14, Med17, and Med14/17 depletion on promoter occupancy of the PIC by ChIP-seq. We first performed two biological replicate experiments for Sua7/TFIIB in the Med17-AID and Med14/17-AID strains and compared the results to our previously published Sua7 ChIP-seq data from the Med14-AID strain (Tourigny et al. 2018). We then visualized enrichment of Sua7 around TSSs, regardless of expression level, as heatmaps, which showed apparently comparable decreases in promoter occupancy across all three strains (Figure 5, A–C, Supplementary Figure S8). However, quantification of changes in Sua7 occupancy at a set of 562 highly occupied promoters revealed modest but significant differences between strains (Figure 5D). At these promoters, Med14 removal resulted in a median FC in Sua7 occupancy of −2.01, while Med17 and Med14/17 depletion yielded median FCs of −2.2 and −2.18, respectively, corresponding to significant differences in the distribution of FCs (Med17, P = 8.9 × 10−5, Med14/17, P = 1.5 × 10−8). The distribution of FCs was not significantly different between Med17-AID and Med14/17-AID (P = 0.67). This phenomenon was also not limited to this set of highly Sua7-bound loci: when all genes were considered, both Med17 and Med14/17 displayed significantly greater reductions than Med14 (Med17, P = 1.3 × 10−3, Med14/17, P = 1.2 × 10−6) despite many lowly bound sites being considered, while remaining insignificantly different from each other (P = 0.36).
Discussion
Our results provide clear evidence that integrity of complete Mediator and the head module are both essential for viability-supporting transcription, but indicate substantial differences in the contributions of these facets of Mediator structural integrity to global transcription in yeast. Furthermore, our data indicate that while intact Mediator is necessary for full PIC assembly and function at promoters, they also show that PICs persist at some level following acute depletion of essential Mediator components, at least within the 30 min timeframe assessed here. This observation is consistent with previous work employing AA of essential subunits (Jeronimo and Robert 2014; Petrenko et al. 2017) and our present finding that depletion of Sua7, a bona fide GTF, results in a substantially higher degree of global transcriptional downregulation than loss of either Med14 or Med17.
Based on the greater negative effects of Med17 versus Med14 depletion on transcription and PIC occupancy, we hypothesized that the head module might remain associated with PICs following dissociation of Mediator after Med14 loss, thus conferring a modest degree of continued transcriptional stimulation. Indeed, co-depletion of Med14 and Med17 resulted in transcriptional downregulation similar to that seen with Med17 removal alone. However, we were unable to detect PIC association of the head module by Med17 ChIP-seq following Kin28 inhibition. It is possible that a small amount of head module remains PIC-bound following Med14 depletion, but is below our detection threshold. Regardless, our data show that when Med14 is depleted, without a major effect on Med17 protein level (and thus, presumably, the integrity of the head module), head module recruitment to promoters is greatly reduced. This was not previously known, as AA cannot deplete Med14 without also removing the head module from the nucleus (Anandhakumar et al. 2016).
The concept that the independent head module can remain associated with the PIC to potentially support a modest degree of transcriptional stimulation is consistent with studies suggesting distinct activator-dependent and activator independent pathways for Mediator recruitment (Lacombe et al. 2013; Jean-Jacques et al. 2018; Knoll et al. 2018). Activator-dependent recruitment, considered the canonical mode of Mediator recruitment, involves interaction of activators with the tail module and recruitment to UASs, followed by transient promoter association. Consistent with this model, deletion of multiple tail subunits severely compromises Mediator-UAS association (Jeronimo et al. 2016; Petrenko et al. 2016; Knoll et al. 2018). However, these studies also presented striking evidence that Mediator still associates with promoters when UAS recruitment is abolished. Furthermore, Mediator can be recruited to a promoter via artificial tethering of TFIIB or TBP (Lacombe et al. 2013; Jean-Jacques et al. 2018). Lastly, depletion of various PIC components impairs Mediator association with promoters (Knoll et al. 2018). Taken together, these lines of evidence point to an activator independent mode of Mediator recruitment, wherein cMed interacts with the promoter-bound PIC. Indeed, the head module makes extensive contacts with PIC components (Larivière et al. 2006; Takagi et al. 2006; Cai et al. 2010), and so it may be that these protein–protein interactions are responsible for Mediator-PIC association in the absence of complete tail module. Thus, it is conceivable that the independent head module could continue to associate with PICs at some level despite a lack of activator interaction.
If a small amount of head module does remain associated with PICs in the absence of Med14, how might it or another module stimulate transcription? Recombinant head module only weakly stimulates transcription (approximately twofold) when mixed with GTFs in a standard in vitro transcription assay (Takagi et al. 2006). In contrast, when a head module-containing minimal PIC (mPIC) is assembled prior to assaying transcriptional activity, an ∼18-fold enhancement of transcription is observed (Cai et al. 2012). Thus, the head module only confers minimal stimulation of transcription when PIC assembly is required, but seems to have the capacity to robustly enhance transcription at a post-PIC assembly step. Indeed, interaction of the head module with RNAPII in vitro in the context of the mPIC appears to trigger a conformational shift in RNAPII that may enhance its interaction with promoter DNA (Cai et al. 2012). It is also possible that headless Mediator, which is generated by Med17 inactivation and remains associated with the genome (Linder et al. 2006; Paul et al. 2015), is somehow deleterious to transcription, perhaps via inhibitory interactions with the PIC. A third possibility is that the tail module promotes transcription in the absence of Med14, as it has been proposed that a triad of tail subunits (Med2, Med3, and Med15) may promote transcription when severed from cMed by removal of the tail/middle connector subunit Med16 (Zhang et al. 2004; Galdieri et al. 2012). However, this would seem to also require the head module, as co-depletion of Med14 and Med17 reduces nsRNA levels to an extent much greater than that of Med14 alone, while presumably having no effect on the tail.
We observed in several western blotting experiments, using multiple epitope tags, that the apparent abundance of Med17 was significantly greater than that of Med14. This finding is consistent with previous biochemical characterization of yeast Mediator, which indicated the existence of a full Mediator complex and a smaller form of Mediator lacking the tail and kinase modules, the middle subunits Med5 and Med19, and, importantly, Med14 (Liu et al. 2001). While both complexes were able to restore transcriptional activity to nuclear extract from Mediator-mutant yeast strains, the smaller Mediator complex stimulated less transcription and reduced the transcriptional activity of full Mediator, leading to the suggestion that it competes with full Mediator for promoter association. While the presence of Med17 in both of these distinct forms of Mediator, versus the inclusion of Med14 only in full Mediator, could explain the greater overall abundance of Med17, it is unclear how the head and middle modules of the smaller Med14-less Mediator complex would interact; biochemical evidence indicates that Med14 is essential for association of recombinant yeast head and middle modules (Plaschka et al. 2015). However, recombinant human head and middle modules can interact in the absence of Med14, though they are unable to stimulate basal transcription in vitro (Cevher et al. 2014).
Of note, our data are distinct from previous work on the extent of Med17’s role in transcription and, by extension, the head module. Two independent studies using Med17 AA reported relatively modest global transcriptional changes as measured by RNAPII ChIP-seq (Petrenko et al. 2017; Bruzzone et al. 2018), with the former study further reporting a comparably mild decrease in RNAPII occupancy following Med14 AA. Both studies also indicated a preferential impact of Med17 AA on RNAPII occupancy of SAGA-dominated genes. In contrast, our data show that Med17 depletion causes a substantially greater transcriptional decrease than does Med14 loss, and that Med17 has little specificity for specific subsets of genes classified by coactivator dependence. It may be that genes primarily dependent on SAGA for expression are more sensitive to impaired Mediator function because they tend to be more highly transcribed than those that primarily require TFIID (Paul et al. 2015; Petrenko et al. 2017; Donczew et al. 2020) and are thus more strongly affected when cMed is partially depleted via Med17 AA. The preferential impact of Med14 removal on CR and SAGA-dominated genes that we observed may be attributable to disconnection of the tail from the other Mediator modules, as outright deletion of tail subunits has been reported to preferentially affect SAGA-dominated gene transcript levels (Ansari et al. 2012; Knoll et al. 2018), RNAPII occupancy (Paul et al. 2015), and promoter PIC occupancy (Jeronimo et al. 2016), all of which presumably result from reduced activator-dependent recruitment of Mediator to UASs (Paul et al. 2015; Jeronimo et al. 2016).
Taken together, our results indicate that cohesion of complete Mediator and the head module are both essential for transcription at a level that supports viability in yeast, but the degrees to which they are necessary for PIC assembly and transcription differ. The head appears to be the key functional module of Mediator with regard to PIC formation and thus promotion of optimal transcription. We propose that Med14, acting as the backbone of the complex, exerts its effects on transcription by enabling full recruitment of complete Mediator. Furthermore, while our study does not rule out the possibility that individual Mediator modules may have functions outside of the complete complex, our data indicate that these roles are likely to be minor and insufficient for viability, as disconnection of the head, middle, and tail via Med14 depletion, in addition to loss of head coherence absent Med17, are lethal.
Acknowledgments
We thank Rafal Donczew, Sebastian Grünberg, Steven Hahn, Laszlo Tora, and members of the Zentner Lab for helpful discussions throughout the course of this work, and François Robert for advice on Mediator ChIP.
Funding
This study was supported by the Agence Nationale de la Recherche [ANR-18-CE12-0026 SAGA-Retina to D.D.] and the National Institutes of Health [R35GM128631 to G.E.Z.].
Conflicts of interest
None declared.
Literature cited
- Anandhakumar J, Moustafa YW, Chowdhary S, Kainth AS, Gross DS.. 2016. Evidence for multiple mediator complexes in yeast independently recruited by activated heat shock factor. Mol Cell Biol. 36:1943–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W.. 2010. Differential expression analysis for sequence count data. Genome Biol. 11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W.. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari SA, Ganapathi M, Benschop JJ, Holstege FCP, Wade JT, et al. 2012. Distinct role of Mediator tail module in regulation of SAGA-dependent, TATA-containing genes in yeast. EMBO J. 31:44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista T, Devys D.. 2018. Saccharomyces cerevisiae metabolic labeling with 4-thiouracil and the quantification of newly synthesized mRNA as a proxy for RNA polymerase II activity. J Vis Exp. 140:e57982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista T, Grünberg S, Minoungou N, Koster MJE, Timmers HTM, et al. 2017. SAGA is a general cofactor for RNA polymerase II transcription. Mol Cell. 68:130–143.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille AR, Robert F.. 2009. Profiling genome-wide histone modifications and variants by ChIP-chip on tiling microarrays in S. cerevisiae. In: Leblanc B, Moss T, editors. DNA-Protein Interactions: Principles and Protocols. 3rd ed. Totowa, NJ: Humana Press. p. 267–279. [DOI] [PubMed] [Google Scholar]
- Bruzzone MJ, Grünberg S, Kubik S, Zentner GE, Shore D.. 2018. Distinct patterns of histone acetyltransferase and Mediator deployment at yeast protein-coding genes. Genes Dev. 32:1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Chaban YL, Imasaki T, Kovacs JA, Calero G, et al. 2012. Interaction of the Mediator head module with RNA polymerase II. Structure. 20:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Imasaki T, Yamada K, Cardelli F, Takagi Y, et al. 2010. Mediator head module structure and functional interactions. Nat Struct Mol Biol. 17:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevher MA, Shi Y, Li D, Chait BT, Malik S, et al. 2014. Reconstitution of active human core Mediator complex reveals a critical role of the MED14 subunit. Nat Struct Mol Biol. 21:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LY, Mugler CF, Heinrich S, Vallotton P, Weis K.. 2018. Non-invasive measurement of mRNA decay reveals translation initiation as the major determinant of mRNA stability. eLife. 7:e32536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-F, Lehmann L, Lin JJ, Vashisht A, Schmidt R, et al. 2012. Mediator and SAGA have distinct roles in Pol II preinitiation complex assembly and function. Cell Rep. 2:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Park K, Lim DA, Song JS.. 2012. Normalization, bias correction, and peak calling for ChIP-seq. Stat Appl Genet Mol Biol. 11:Articl 9. doi:10.1515/1544-6115.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, et al. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donczew R, Warfield L, Pacheco D, Erijman A, Hahn S.. 2020. Two roles for the yeast transcription coactivator SAGA and a set of genes redundantly regulated by TFIID and SAGA. eLife. 9:e50109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elek A, Kuzman M, Vlahovicek K.. 2019. coRdon: codon usage analysis and prediction of gene expressivity. R package version 1.8.0, https://github.com/BioinfoHR/coRdon.
- Eyboulet F, Wydau-Dematteis S, Eychenne T, Alibert O, Neil H, et al. 2015. Mediator independently orchestrates multiple steps of preinitiation complex assembly in vivo. Nucleic Acids Res. 43:9214–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eychenne T, Novikova E, Barrault M-B, Alibert O, Boschiero C, et al. 2016. Functional interplay between Mediator and TFIIB in preinitiation complex assembly in relation to promoter architecture. Genes Dev. 30:2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi M, Hochstrasser M.. 2009. Small epitope-linker modules for PCR-based C-terminal tagging in Saccharomyces cerevisiae. Yeast. 26:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdieri L, Desai P, Vancura A.. 2012. Facilitated assembly of the preinitiation complex by separated tail and head/middle modules of the Mediator. J Mol Biol. 415:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünberg S, Henikoff S, Hahn S, Zentner GE.. 2016. Mediator binding to UASs is broadly uncoupled from transcription and cooperative with TFIID recruitment to promoters. EMBO J. 35:2435–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne F, Ivanek R.. 2016. Visualizing genomic data using Gviz and bioconductor. In: Mathé E, Davis S, editors. Statistical Genomics: Methods and Protocols. New York, NY: Springer New York. p. 335–351. [DOI] [PubMed] [Google Scholar]
- Harigaya Y, Parker R.. 2016. Analysis of the association between codon optimality and mRNA stability in Schizosaccharomyces pombe. BMC Genom. 17:895–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, Laemmli UK.. 2008. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 31:925–932. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, et al. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 38:576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B, Baryshnikova A, Brown GW.. 2018. Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell Syst. 6:192–205.e3. [DOI] [PubMed] [Google Scholar]
- Holstege FCP, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, et al. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 95:717–728. [DOI] [PubMed] [Google Scholar]
- Huisinga KL, Pugh BF.. 2004. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol Cell. 13:573–585. [DOI] [PubMed] [Google Scholar]
- Imasaki T, Calero G, Cai G, Tsai K-L, Yamada K, et al. 2011. Architecture of the Mediator head module. Nature. 475:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Jacques H, Poh SL, Kuras L.. 2018. Mediator, known as a coactivator, can act in transcription initiation in an activator-independent manner in vivo. Biochim Biophys Acta. 1861:687–696. [DOI] [PubMed] [Google Scholar]
- Jeronimo C, Langelier M-F, Bataille AR, Pascal JM, Pugh BF, et al. 2016. Tail and kinase modules differently regulate core mediator recruitment and function in vivo. Mol Cell. 64:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo C, Robert F.. 2014. Kin28 regulates the transient association of Mediator with core promoters. Nat Struct Mol Biol. 21:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Gross DS.. 2013. Mediator recruitment to heat shock genes requires dual Hsf1 activation domains and mediator tail subunits Med15 and Med16. J Biol Chem. 288:12197–12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll ER, Zhu ZI, Sarkar D, Landsman D, Morse RH.. 2018. Role of the pre-initiation complex in Mediator recruitment and dynamics. eLife. 7:e39633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer SB, Kim S, Jeon JO, Moustafa YW, Chen A, et al. 2012. Role of Mediator in regulating Pol II elongation and nucleosome displacement in Saccharomyces cerevisiae. Genetics. 191:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe T, Poh SL, Barbey R, Kuras L.. 2013. Mediator is an intrinsic component of the basal RNA polymerase II machinery in vivo. Nucleic Acids Res. 41:9651–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL.. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larivière L, Geiger S, Hoeppner S, Röther S, Sträßer K, et al. 2006. Structure and TBP binding of the Mediator head subcomplex Med8–Med18–Med20. Nat Struct Mol Biol. 13:895–901. [DOI] [PubMed] [Google Scholar]
- Lee TI, Wyrick JJ, Koh SS, Jennings EG, Gadbois EL, et al. 1998. Interplay of positive and negative regulators in transcription initiation by RNA polymerase II holoenzyme. Mol Cell Biol. 18:4455–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder T, Zhu X, Baraznenok V, Gustafsson CM.. 2006. The classical srb4-138 mutant allele causes dissociation of yeast Mediator. Biochem Biophys Res Commun. 349:948–953. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ranish JA, Aebersold R, Hahn S.. 2001. Yeast nuclear extract contains two major forms of RNA polymerase II Mediator complexes. J Biol Chem. 276:7169–7175. [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S.. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MP, Asbury CL, Biggins S.. 2016. A TOG protein confers tension sensitivity to kinetochore-microtubule attachments. Cell. 165:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M.. 2009. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods. 6:917–922. [DOI] [PubMed] [Google Scholar]
- Paul E, Zhu ZI, Landsman D, Morse RH.. 2015. Genome-wide association of mediator and RNA polymerase II in wild-type and mediator mutant yeast. Mol Cell Biol. 35:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko N, Jin Y, Wong KH, Struhl K.. 2016. Mediator undergoes a compositional change during transcriptional activation. Mol Cell. 64:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko N, Jin Y, Wong KH, Struhl K.. 2017. Evidence that Mediator is essential for Pol II transcription, but is not a required component of the preinitiation complex in vivo. eLife. 6:e28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschka C, Lariviere L, Wenzeck L, Seizl M, Hemann M, et al. 2015. Architecture of the RNA polymerase II-Mediator core initiation complex. Nature. 518:376–380. [DOI] [PubMed] [Google Scholar]
- Presnyak V, Alhusaini N, Chen Y-H, Martin S, Morris N, et al. 2015. Codon optimality is a major determinant of mRNA stability. Cell. 160:1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, et al. 2016. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44:W160–W165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranish JA, Yudkovsky N, Hahn S.. 1999. Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev. 13:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Trnka MJ, Pellarin R, Greenberg CH, Bushnell DA, et al. 2015. Molecular architecture of the yeast Mediator complex. eLife. 4: e08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Molina JB, Tseng SC, Simonett SP, Taunton J, Ansari AZ.. 2016. Engineered covalent inactivation of TFIIH-kinase reveals an elongation checkpoint and results in widespread mRNA stabilization. Mol. Cell. 63:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A, Shimizu Y, Kondou S, Chibazakura T, Hishinuma F.. 1990. Structure and molecular analysis of RGR1, a gene required for glucose repression of Saccharomyces cerevisiae. Mol Cell Biol. 10:4130–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze SK, Kanwar R, Gölzenleuchter M, Therneau TM, Beutler AS.. 2012. SERE: single-parameter quality control and sample comparison for RNA-Seq. BMC Genomics. 13:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Schwalb B, Schulz D, Pirkl N, Etzold S, et al. 2012. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res. 22:1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Calero G, Komori H, Brown JA, Ehrensberger AH, et al. 2006. Head module control of mediator interactions. Mol Cell. 23:355–364. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Koleske AJ, Chao DM, Young RA.. 1993. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell. 73:1361–1375. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Young RA.. 1995. General requirement for RNA polymerase II holoenzymes in vivo. Proc Nat Acad Sci USA. 92:4587–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourigny JP, Saleh MM, Schumacher K, Devys D, Zentner GE.. 2018. Mediator is essential for small nuclear and nucleolar RNA transcription in yeast. Mol Cell Biol. 38:e00296–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K-L, Tomomori-Sato C, Sato S, Conaway RC, Conaway JW, et al. 2014. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex. Cell. 157:1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sun Q, Ding Z, Ji J, Wang J, et al. 2014. Redefining the modular organization of the core Mediator complex. Cell Res. 24:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield L, Ramachandran S, Baptista T, Devys D, Tora L, et al. 2017. Transcription of nearly all yeast RNA polymerase II-transcribed genes is dependent on transcription factor TFIID. Mol Cell. 68:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR. 1999. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci. 24:437–440. [DOI] [PubMed] [Google Scholar]
- Wong KH, Jin Y, Struhl K.. 2014. TFIIH phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Mol Cell. 54:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Sumibcay L, Hinnebusch AG, Swanson MJ.. 2004. A triad of subunits from the Gal11/Tail domain of Srb Mediator is an in vivo target of transcriptional activator Gcn4p. Mol Cell Biol. 24:6871–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Dang Y, Zhou M, Li L, Yu C-h, et al. 2016. Codon usage is an important determinant of gene expression levels largely through its effects on transcription. Proc Natl Acad Sci USA. 113:E6117–E6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary Figures S1–S8 and Tables S1 and S2 have been deposited in figshare (https://doi.org/10.25386/genetics.13350521). An R Shiny app for interactive exploration of the RNA-seq data generated is available at https://zentlab.shinyapps.io/med1417. All sequencing data generated in this study have been deposited in GEO (total RNA-seq/4tU-seq: GSE146346; ChIP-seq: GSE145494).
Supplementary material is available at figshare DOI: https://doi.org/10.25386/genetics.13350521.