Abstract
Objective
To assess associations between head injury (HI) with loss of consciousness (LOC), ageing and markers of later‐life cerebral pathology; and to explore whether those effects may help explain subtle cognitive deficits in dementia‐free individuals.
Methods
Participants (n = 502, age = 69–71) from the 1946 British Birth Cohort underwent cognitive testing (subtests of Preclinical Alzheimer Cognitive Composite), 18F‐florbetapir Aβ‐PET and MR imaging. Measures include Aβ‐PET status, brain, hippocampal and white matter hyperintensity (WMH) volumes, normal appearing white matter (NAWM) microstructure, Alzheimer’s disease (AD)‐related cortical thickness, and serum neurofilament light chain (NFL). LOC HI metrics include HI occurring: (i) >15 years prior to the scan (ii) anytime up to age 71.
Results
Compared to those with no evidence of an LOC HI, only those reporting an LOC HI>15 years prior (16%, n = 80) performed worse on cognitive tests at age 69–71, taking into account premorbid cognition, particularly on the digit‐symbol substitution test (DSST). Smaller brain volume (BV) and adverse NAWM microstructural integrity explained 30% and 16% of the relationship between HI and DSST, respectively. We found no evidence that LOC HI was associated with Aβ load, hippocampal volume, WMH volume, AD‐related cortical thickness or NFL (all p > 0.01).
Interpretation
Having a LOC HI aged 50’s and younger was linked with lower later‐life cognitive function at age ~70 than expected. This may reflect a damaging but small impact of HI; explained in part by smaller BV and different microstructure pathways but not via pathology related to AD (amyloid, hippocampal volume, AD cortical thickness) or ongoing neurodegeneration (serum NFL).
Introduction
Head injury (HI) is implicated as a risk factor for all‐cause dementia and Alzheimer’s disease (AD). 1 , 2 HI has been associated with pathological measures of increased β‐amyloid (Aβ), 3 cerebral atrophy, 4 reduced hippocampal volume, 5 white matter damage and reduced connectivity, 6 , 7 tau pathology 8 and shorter term axonal damage indexed by increased neurofilament light protein in blood (NFL), 9 , 10 which may confer risk of cognitive impairment.
Studies of the effects of HI have largely been focused on populations at risk of severe or repetitive head injury at younger ages, such as military personnel and athletes, and often have relatively short follow‐up periods. Longer term cognitive and pathological effects of minor HI that may occur in the general population have been less well‐studied, particularly in older people. This has limited our understanding of the long‐term cognitive and pathological effects of an injury and the interplay between injury and ageing processes; whereby, as part of normal ageing, cognitive decline and brain atrophy changes occur. Given that studies of HI in older populations are at risk of reverse causality, where cognitive impairment or ageing processes such as frailty may cause HI, 14 the timing of HI needs to be taken into account to help establish long‐term effects from HI incidents, or effects from HI coupled with ageing processes. Establishing long‐term associations between HI and pathological markers observed in dementia‐free individuals may further help explain subtle cognitive deficits observed or indicate potential future decline. 11 , 12 , 13
The MRC National Survey of Health and Development (NSHD, 1946 British Birth cohort) has followed individuals since their birth. Using this population‐based study we previously demonstrated that HI with LOC at least 15 years prior was associated with lower word learning memory by age 69, but we did not show evidence of faster cognitive decline. 15 Recently, 502 members aged 69–71 underwent extensive cognitive examination and imaging as part of the neuroimaging sub‐study (Insight 46), as well as updating a HI‐related questionnaire. We now assess associations between HI with LOC with cognitive function at age 69–71, and the extent to which any associations are explained by pathology measures at this age (cerebral Aβ‐PET, whole brain and hippocampal volume, AD‐related cortical thickness signature, white matter hyperintensity (WMH) volume, measures of white matter microstructural integrity in normal appearing white matter (NAWM), and greater axonal injury indexed by serum NFL). We use two indicators of HI to differentiate potential effects of LOC HI (i) occurring >15 years prior to the scan, which limits the possibility of reverse causality; and (ii) occurring at any time across the life course, which may be coupled with ageing‐related processes.
Methods
Study participants were from Insight 46, a sub‐study of the NSHD which initially comprised 5362 individuals born throughout mainland Britain in one week of March 1946. Eligibility criteria 16 and an overview of recruitment for Insight 46 17 are outlined in detail elsewhere. Briefly 502 participants aged 69–71 were assessed with detailed and consistent clinical, cognitive, and brain imaging protocols (https://doi.org/10.5522/NSHD/Q103). Ethical approval for the neuroscience sub‐study was granted by the National Research Ethics Service (NRES) Committee London (14/LO/1173). All participants gave written informed consent.
Head injury
In this analysis we used two indicators of HI to differentiate potential effects of LOC HI occurring (i) >15 years prior to the scan and (ii) occurring at any time across the life course:
“LOC HI >15 years prior to the scan”; At age 53, history of HI was ascertained by the response to the following question “Have you ever been knocked unconscious?”. The responses at age 53 were coded into a single HI variable with two levels: 0) no evidence of a HI with loss of consciousness (LOC) up to age 53; 1) evidence of at least one LOC HI in the life course up to age 53.
“LOC HI any time in life course”; At age 53 and 60–64, history of HI was ascertained by the response to the following question “Have you ever been knocked unconscious?”. At age 69–71, history of HI was ascertained by the response to the following question “Have you ever had a loss of consciousness”, participants positive responses were followed up with: “Have you ever had a loss of consciousness following a head injury?”, “Have you ever had an admission to hospital following a head injury?” and “Have you ever had a skull fracture?”. We used the response to “Have you ever had a loss of consciousness following a head injury?” as the main variable at age 69–71. The responses at each age were combined into a single HI variable with two levels: (0) no evidence of a LOC HI up to age 71; (1) evidence of at least one LOC HI in the life course at any age.
Cognitive function
As previously described, 18 we used cognitive performance on the Preclinical Alzheimer Cognitive Composite (PACC) as the main outcome. This composite consists of the Mini Mental State Examination (MMSE) assessing cognitive state; logical memory delayed score from the Wechsler Memory Scale‐Revised (WMS‐R) – a test of verbal episodic recall; digit‐symbol substitution test (DSST) from the Wechsler Adult Intelligence Scale‐Revised (WAIS‐R) – a test of executive function and psychomotor speed; and the 12‐item Face‐Name test (FNAME‐12) – a measure of free memory recall.
Pathological measures
Imaging was performed on a single Biograph mMR 3T PET/MRI scanner (Siemens Healthcare, Erlangen), with simultaneous acquisition of dynamic PET/MR data; the full imaging protocol has been described previously 16 and published papers have used these derived variables accordingly.
PET data were acquired following injection of 370 MBq florbetapir F18 (Amyvid). Aβ burden was assessed over a 10‐min period, ~50 min after injection. Global standardized uptake value ratios (SUVRs) were calculated from cortical regions of interest, normalized to eroded subcortical white matter. Aβ status (+/−) was determined by taking the 99th percentile of the lower (Aβ‐) Gaussian as the cut‐point (0.6104), whereby Aβ+ indicates greater Aβ load.
Volumetric T1‐weighted and FLAIR images underwent visual QC, before processing using automated pipelines 16 : whole‐brain segmentation using Multi‐Atlas Propagation and Segmentation 19 and hippocampal volume using Similarity and Truth Estimation for Propagated Segmentations 20 with appropriate manual editing. Models including brain volume were all adjusted for total intracranial volume (TIV), as calculated using Statistical Parametric Mapping 12.
Cortical thickness estimation from T1‐weighted images was performed using Freesurfer version 6.0 as previously described. 21 Cortical thickness regions of interest (ROIs) included a proposed composite signatures of AD cortical thinning: “ADsig Mayo” 22 which is comprised of select temporal cortex regions (entorhinal, fusiform, inferior and middle temporal).
A validated, unsupervised automated algorithm, BaMoS (Bayesian Model Selection) 23 was used to segment WMH from T1/FLAIR images, followed by visual QC, generating a global WMH volume including subcortical grey matter but excluding infratentorial regions. Higher WMHV indicate worse small vessel disease.
Diffusion MRI enables sensitive estimation of tissue microstructure environments and typically includes parameters of fractional anisotropy (FA) and mean diffusivity (MD). NODDI (neurite orientation dispersion and density imaging) is a multicompartmental modelling technique that further estimates microstructural complexity parameters including neurite density index (NDI) and orientation dispersion index (ODI). 24 To generate these parameters, diffusion‐weighted images underwent automated processing pipelines, 16 followed by visual QC. NAWM masks were generated from GIF‐generated by the GIF pipeline eroded by one voxel to avoid CSF and grey matter cerebrospinal fluid contamination, followed by subtraction of corresponding BaMoS‐derived lesion map 25 using FSL Maths. 26 Mean values of NAWM microstructural integrity metrics (fractional anisotropy (FA), mean diffusivity (MD), neurite density index (NDI), orientation dispersion index (ODI)) were extracted from T1‐registered diffusion maps using FSL 26 and NODDI toolbox. 24 Higher values for FA and NDI represent high directionality whereas lower values for MD and ODI indicate more constrained diffusion.
Serum NFL is a marker of axonal injury whereby higher values reflect greater neuronal damage, 27 and has previously been implicated in head injury. 9 , 10 A single 500 μL aliquot of serum for each individual was thawed at room temperature over 1 h and vortexed. 130 μL of the supernatant was pipetted onto a 96‐well plate for analysis in duplicate, using commercially available Simoa immunoassay kits of the same batch (Quanterix, Billerica, MA). Serum NFL value was quantified with an intra‐assay CV <15%, and inter‐assay CV were <30%.
Confounders
Based on previous work examining predictors of later‐life cognitive function 28 the following variables were treated as potential confounders: gender, age, childhood cognitive ability, educational attainment, childhood socioeconomic position (SEP); high affective (depression and anxiety) symptoms near the time of testing; and APOE‐ε4 status.
Childhood cognitive ability was measured at age 8 and standardized. The highest educational attainment achieved by 26 years was grouped as: no qualifications; education up to age 16; education from age 17 onwards. Childhood SEP was derived from paternal occupational class and coded according to the UK Registrar General’s Standard Occupational Classification. Affective case‐level problems were generated using a validated threshold for the 28‐item General Health Questionnaire (GHQ‐28) at age 69. 29 Genotyping of the two single nucleotide polymorphisms, rs439358 and rs7412, were used to determine APOE‐ɛ4 genotype and categorised as no ε4; heterozygous ε4 and homozygous ε4.
Statistical analysis
Sample
To be included in this analysis participants had to: be dementia‐free at the time of scanning based on expert consensus, informed by clinical history, informant history, MMSE (score ≥26) and cognitive performance (WMS‐R Logical Memory test and WAIS‐R digit‐symbol substitution test); have complete HI data; and have acceptable quality for the imaging modality of interest (Fig. 1). Differences in characteristics between those with and without HI were investigated using t‐tests and χ 2 tests for continuous and categorical characteristics, respectively.
Figure 1.
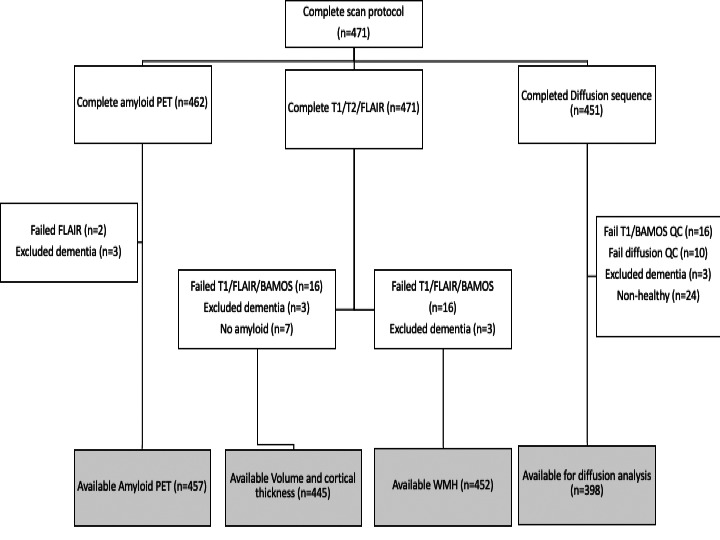
Flow chart of available imaging data.
Analysis
Multivariable linear regression models were conducted to examine the association between both LOC HI indexes (used as a binary variable) and cognitive function measures, whole brain volume, hippocampal volume, cortical thickness measures and NAWM microstructural integrity measures derived at age 69–71. Due to the non‐normal distribution of WMH and NFL, generalized linear models using the gamma distribution and log link were used to investigate relationships between history of HI and WMH volume, and NFL. Multivariable logistic regression models were conducted to examine the association between both LOC HI indexes and Aβ status (as a dichotomous variable) at age 69–71. A differential influence of HI by APOE‐ɛ4 status on Aβ was additionally tested.
We tested the extent to which specific pathological measures explained the effect between LOC HI on later‐life cognition. This was tested by re‐fitting regression models between LOC HI and later‐life cognition, adjusting for brain pathology measures of interest and estimating the effect difference between models. To reduce multiple testing, only the cognitive and cerebral pathology variables that showed prior associations with an HI index were used for further testing.
Adjustments
For all analyses, Model 1 was adjusted for gender and age at scan; Model 2 further adjusted for childhood cognitive ability, educational attainment and childhood SEP. All models which included brain, hippocampal or WMH volume as outcomes were additionally adjusted for TIV. Results are shown as mean difference in cognitive scores with 95% confidence intervals; adjustments were not made for multiple comparisons in line with previous studies. 30
As sensitivity analyses, all models including pathological measures as outcomes were additionally re‐run, adjusting for affective problems; APOE‐ɛ4 status; additionally adjusting for other concurrent pathology; and excluding those who met criteria for (MCI) mild cognitive impairment at the time of the scan (n = 13). All analyses were conducted using Stata version 15.1.
Data availability
Anonymized data will be shared by request from qualified investigators (skylark.ucl.ac.uk/NSHD/doku.php).
Results
Demographics
Of 502 individuals assessed, 499 were dementia‐free and 471 completed the imaging protocol; 49% were female; age at scan ranged from 69.3 to 71.9 years. 80 individuals (16%) reported LOC HI >15 years prior; 104 (21%) participants reported having suffered at least one LOC HI at any time in the life course (Table 1). Compared to those with no evidence of LOC HI, those who reported LOC HI at any time were more likely to be male (68% of cases). There were no statistical differences between LOC HI groups for age at testing, childhood cognitive ability, education, childhood SEP, affective problems or APOE‐ɛ4 status (Table 1).
Table 1.
Characteristics of participants reporting a head injury with loss of consciousness at any point before age 71.
| All (n = 499) | Scanned only (n = 471) | |||
|---|---|---|---|---|
| LOC HI occurring >15 years prior to scan | 80 (16%) | 77 (17%) | ||
| LOC HI occurring at any age up to age 71 | 104 (21%) | 99 (21%) | ||
| All | No HI up to 71 | HI up to 71 | p | |
|---|---|---|---|---|
| Max n | 499 (100%) | 395 (79%) | 104 (21%) | |
| Characteristics | ||||
| Childhood cognition (mean, SD) 1 | 0.39 (0.7) | 0.38 (0.7) | 0.46 (0.7) | 0.37 |
| Male | 255 (51%) | 173 (47%) | 67 (68%) | |
| Female | 244 (49%) | 196 (53%) | 32 (32%) | <0.01 |
| Educational attainment | ||||
| None | 76 (15%) | 60 (16%) | 12 (12%) | |
| Up to GCE | 151 (30%) | 119 (32%) | 23 (23%) | |
| A‐level and above | 272 (55%) | 190 (52%) | 64 (65%) | 0.14 |
| Childhood SEP | ||||
| Manual | 214 (44%) | 160 (44%) | 36 (36%) | |
| Non‐manual | 280 (56%) | 205 (56%) | 63 (64%) | 0.18 |
| APOE‐ɛ4 | ||||
| no ɛ4 | 351 (72%) | 258 (72%) | 69 (73%) | |
| ɛ4 Heterozygous | 122 (25%) | 94 (26%) | 22 (23%) | |
| ɛ4 Homozygous | 13 (3%) | 9 (2%) | 3 (3%) | 0.56 |
| Affective problems, 69 | 38 (8%) | 23 (6%) | 9 (9%) | 0.38 |
| Age at testing (mean, SD) | 70.7 (0.6) | 70.7 (0.4) | 70.6 (0.6) | 0.20 |
Abbreviations: HI, head injury; LOC, loss of consciousness; SD, standard deviation; SEP, socioeconomic position.
Childhood cognition Z‐scores were based on the full National Survey of Health and Development cohort of N = 5362, so the mean for Insight 46 participants indicates that they had higher childhood cognitive ability on average than their peers not recruited to this study.
Cognitive function
Compared to those without evidence of LOC HI, those who reported a LOC HI>15 years prior had lower digit‐symbol substitution scores (DSST) and trend‐level lower MMSE and PACC scores at age 69–71, even when adjusting for known common predictors of later‐life cognition including childhood cognition and education (Table 2, Fig. 2). This pattern remained in sensitivity analyses (Table S1). However, for those who reported LOC HI at any time in the life course up to age 71, associations with cognitive scores at age 69–71 were attenuated with adjustments for known common predictors of later‐life cognition (Table 2, Fig. 2).
Table 2.
Association between reported head injury (HI) and later‐life cognitive function at age 69–71.
| Model 1 (gender and age) | Model 2 (gender, age, childhood cognition, education, childhood SEP) | |||||
|---|---|---|---|---|---|---|
| Standardized mean difference† | p | 95% CI | Standardized mean difference† | p | 95% CI | |
| LOC HI occurring >15 years prior | ||||||
| Digit‐symbol substitution test | −0.23 | 0.04 | [−0.48, −0.01] | −0.28 | 0.02 | [−0.52, −0.05] |
| Logical memory | 0.01 | 0.93 | [−0.23, 0.25] | −0.02 | 0.85 | [−0.25, 0.21] |
| FNAME | −0.05 | 0.71 | [−0.29, 0.20] | −0.09 | 0.41 | [−0.32, 0.13] |
| MMSE | −0.17 | 0.18 | [−0.42, 0.08] | −0.22 | 0.07 | [−0.45, 0.02] |
| PACC | −0.13 | 0.28 | [−0.38, 0.11] | −0.20 | 0.07 | [−0.42, 0.01] |
| LOC HI occurring at anytime up to age 71 | ||||||
| Digit‐symbol Substitution test | −0.03 | 0.78 | [−0.27, 0.20] | −0.12 | 0.27 | [−0.34, 0.10] |
| Logical memory | 0.09 | 0.43 | [−0.14, 0.32] | 0.03 | 0.77 | [−0.18, 0.25] |
| FNAME | 0.04 | 0.73 | [−0.19, 0.27] | −0.04 | 0.71 | [−0.25, 0.17] |
| MMSE | −0.06 | 0.63 | [−0.29, 0.17] | −0.13 | 0.26 | [−0.35, 0.10] |
| PACC | 0.02 | 0.84 | [−0.20, 0.25] | −0.08 | 0.44 | [−0.28, 0.12] |
HI, head injury; FNAME, Face‐Name test; SEP, socioeconomic position; MMSE, mini mental state examination; PACC, Preclinical Alzheimer’s Cognitive Composite;
Model 1 adjusts for gender and age; Model 2 adjusts for gender, age, childhood cognition, education and childhood SEP. All outcome measures are standardized so estimates reflect differences in mean of the standardized cognitive outcome between HI groups, whereby those without evidence of head injury with loss of consciousness are the reference group. Bold reflects p < 0.05.
†estimates reflect differences in mean of the standardized cognitive outcome between HI groups.
Figure 2.
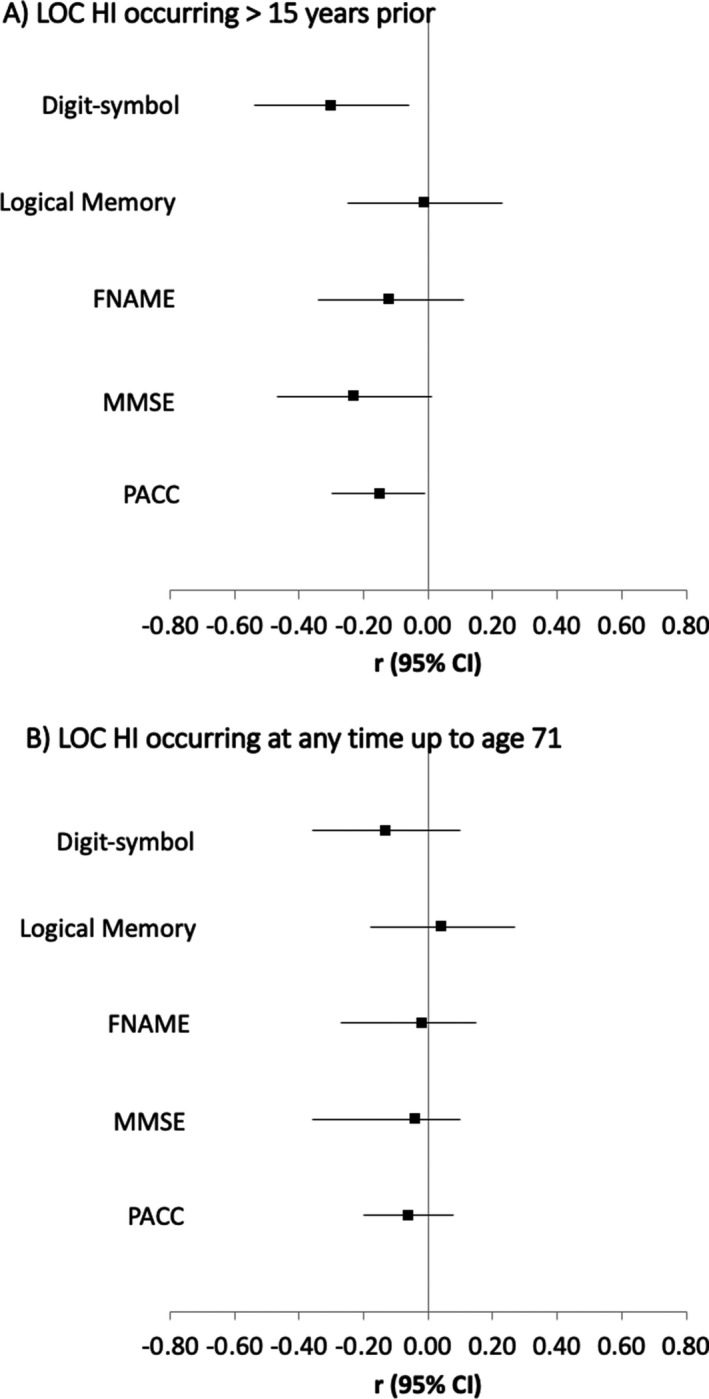
Forest plot of estimates from a linear regression model showing the mean difference in cognitive outcomes at age 69–71 by (A) LOC HI occurring >15 years prior, and (B) LOC HI occurring at anytime up to age 71 using no LOC HI as the reference group. Estimates are standardized and adjusted for gender, childhood cognition, education, childhood socioeconomic position, age at testing, APOE‐ɛ4 status. FNAME, face name test; MMSE, mini mental state examination; PACC, Preclinical Alzheimer Cognitive Composite.
Pathological markers
There was no evidence that LOC HI was associated with Aβ status on PET imaging, smaller AD‐related cortical thickness, increased WMH or increased NFL (Table 3, Fig. 3). A differential influence of any LOC HI by APOE‐ɛ4 status on Aβ was tested but interactions were not significant; Aβ status p = 0.41.
Table 3.
Association between reported head injury (HI) and pathological measures at age 69–71.
| Model 1 (gender and age) | Model 2 (gender, age, childhood cognition, education, child SEP) | |||||
|---|---|---|---|---|---|---|
| Standardized mean difference 1 | p | 95% CI | Standardized mean difference 1 | p | 95% CI | |
| LOC HI occurring >15 years prior | ||||||
| Whole brain volume | −0.31 | 0.02 | [−0.56, −0.06] | −0.31 | 0.02 | [−0.56, −0.06] |
| Hippocampal volume | −0.08 | 0.55 | [−0.33, 0.18] | −0.07 | 0.59 | [−0.33, 0.19] |
| AD CT signature | −0.07 | 0.57 | [−0.32, 0.18] | −0.06 | 0.63 | [−0.32, 0.19] |
| NAWM FA | −0.34 | 0.02 | [−0.62, −0.06] | −0.36 | 0.01 | [−0.64, −0.07] |
| NAWM NDI | −0.33 | 0.02 | [−0.62, −0.05] | −0.34 | 0.02 | [−0.62, −0.05] |
| NAWM MD | 0.28 | 0.06 | [−0.01, 0.56] | 0.28 | 0.05 | [−0.00, 0.57] |
| NAWM ODI | 0.16 | 0.26 | [−0.12, 0.45] | 0.19 | 0.18 | [−0.09, 0.48] |
| Aβ status 2 | 0.98 | 0.95 | [0.51, 1.88] | 1.00 | 0.99 | [0.52, 1.91] |
| WMHV 3 | 1.02 | 0.88 | [0.77, 1.35] | 1.01 | 0.94 | [0.76, 1.34] |
| NFL 3 | 0.92 | 0.22 | [0.79, 1.05] | 0.92 | 0.24 | [0.80, 1.06] |
| LOC HI occurring at anytime up to age 71 | ||||||
| Whole brain volume | −0.26 | 0.03 | [−0.49, −0.02] | −0.27 | 0.03 | [−0.50, −0.03] |
| Hippocampal volume | −0.13 | 0.28 | [−0.37, 0.11] | −0.13 | 0.29 | [−0.37, 0.11] |
| AD CT signature | −0.13 | 0.27 | [−0.37, 0.10] | −0.13 | 0.30 | [−0.36, 0.11] |
| NAWM FA | −0.28 | 0.04 | [−0.54, −0.01] | −0.29 | 0.04 | [−0.55, −0.02] |
| NAWM NDI | −0.26 | 0.05 | [−0.53, 0.00] | −0.28 | 0.04 | [−0.55, −0.01] |
| NAWM MD | 0.23 | 0.09 | [−0.04, 0.49] | 0.23 | 0.09 | [−0.04, 0.50] |
| NAWM ODI | 0.08 | 0.57 | [−0.19, 0.34] | 0.11 | 0.40 | [−0.15, 0.38] |
| Aβ status 2 | 0.91 | 0.77 | [0.49, 1.68] | 0.92 | 0.79 | [0.49, 1.71] |
| WMHV 3 | 1.00 | 0.99 | [0.77, 1.29] | 0.99 | 0.92 | [0.76, 1.28] |
| NFL 3 | 0.92 | 0.20 | [0.80, 1.05] | 0.92 | 0.23 | [0.81, 1.05] |
Model 1 adjusts for gender and age; Model 2 adjusts for gender, age, childhood cognition, education and childhood SEP.
Abbreviations: AD, Alzheimer’s disease; CT, cortical thickness; FA, fractional anisotropy; HI, head injury; MCI, mild cognitive impairment; MD, mean diffusivity; NAWM, normal appearing white matter; NDI, neurite density index; NFL, serum neurofilament light chain; ODI, orientation dispersion index; WMHV, white matter hyperintensity volume.
Linear regression models were used with whole brain, hippocampal volume, CT signature, NAWM measures whereby all these outcomes measures were standardized so estimates reflect differences in mean of the standardized outcome between HI groups.
Logistic regressions were used with Aβ status as an outcome (Aβ‐ as the reference) so estimates reflect odds ratio and 95% CI.
Generalized linear models using the gamma distribution and log link were used to investigate relationships between prior HI and WMH load and NFL so estimates reflect relative increases and 95% CI. In all cases, those without evidence of a head injury with loss of consciousness are the reference group. Bold reflects p < 0.05.
Figure 3.
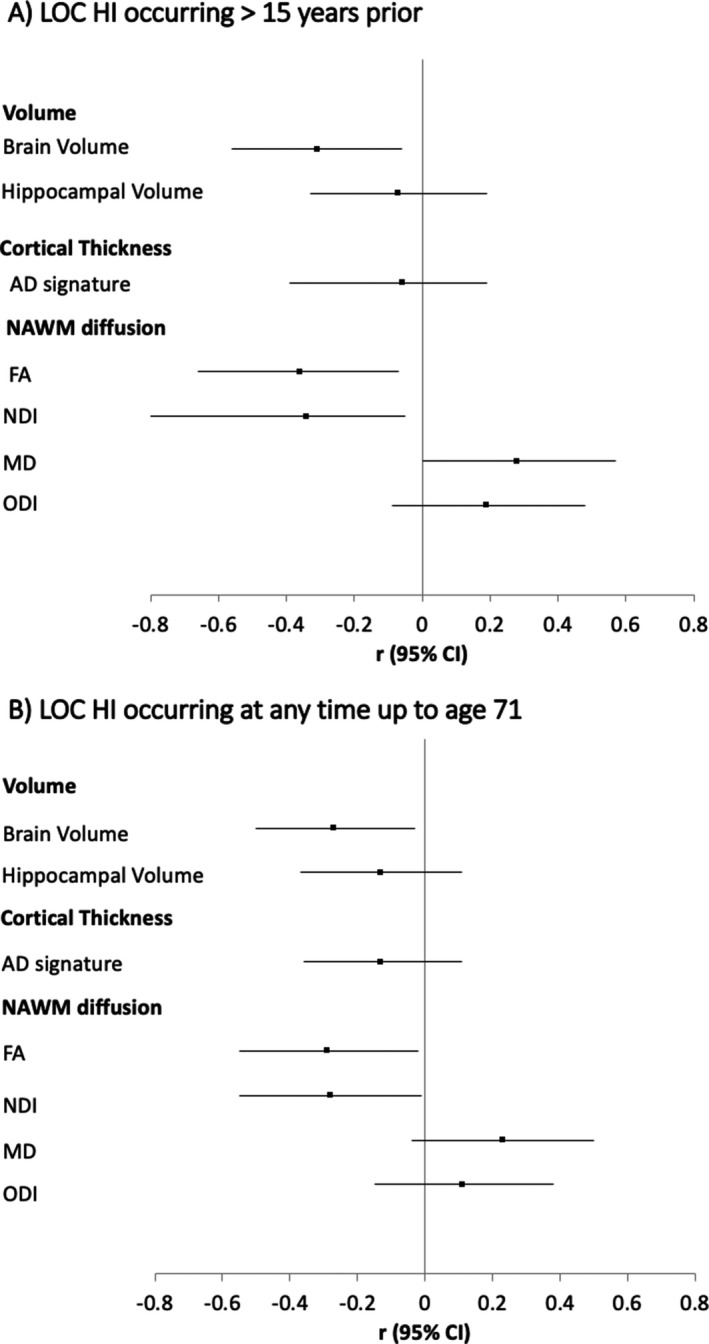
Forest plot of estimates from a linear regression model showing the mean difference in continuous neuroimaging outcomes at age 69–71 by (A) LOC HI occurring >15 years prior, and (B) LOC HI occurring at anytime up to age 71. Estimates are standardized and adjusted for gender, age at testing, childhood cognition, education and childhood socioeconomic position. Volumetric measures additionally adjusted for total intracranial volume. NAWM, normal appearing white matter; FA, fractional anisotropy; MD, mean diffusivity; NDI, neurite density index; ODI, orientation dispersion index.
Compared to those without evidence of LOC HI, those with LOC HI>15 years prior and LOC HI anytime in the life course, was associated with smaller whole brain volume relating to an observed decrease of 12 mL (1%) and 14 mL (1%), respectively; and associated with altered NAWM microstructure measures at age 69–71 (decreased mean FA and decreased NDI and higher MD and higher ODI) (Table 3, Fig. 3). The pattern of results remained with adjustments sensitivity analyses (Table S1). There were trend‐level associations between LOC HI at any time in the life course and smaller hippocampal volume at age 69–71. However, the associations were largely attenuated when adjusting for whole brain volume (Table S1), implying hippocampal reduction was not disproportionate to brain volume reduction.
As the strongest associations with LOC HI>15 years prior were DSST scores (cognition); and whole brain volume and lower FA (pathology markers), we tested to what extent the association observed between LOC HI occurring >15 years before and DSST at age 69–71 was explained by brain volume or FA‐related pathways. As previously noted, the estimate of the total effect of LOC HI>15 years prior on DSST in later‐life in the fully adjusted model was negative (n = 445 with available brain volume data; −0.29 [95% CI −0.51, −0.05], p < 0.01, R 2 = 0.17). This effect was attenuated and became non‐significant when additionally adjusting for TIV‐adjusted brain volume (−0.18 [95% CI −0.42, 0.04], p = 0.09, R 2 = 0.24). This demonstrates that brain volume explained approximately 38% of the relationship between LOC HI<15 years prior and DSST. In contrast, the effect between LOC HI and DSST was only slightly attenuated when FA was additionally adjusted (changing from −0.25 [95% CI −0.50, −0.02], p < 0.03, R 2 = 0.18 with n = 398 available FA data to −0.21 [95% CI −0.47, 0.05], p = 0.11, R 2 = 0.20 with adjustment of FA), demonstrating that FA explains 16% of the relationship between LOC HI<15 years prior and DSST.
Discussion
We found that reporting a HI with LOC is fairly common in a population‐based birth study (21%) and is most likely to occur in men (68% of cases). Compared to those who had never reported a LOC HI, those with a LOC HI at least 15 years prior, when participants were aged 53 or younger, performed worse on a digit‐symbol substitution test (DSST) at age 69–71 than expected based on known predictors of later‐life cognition such as their childhood cognitive performance. The relationship between LOC HI and DSST was partly explained by smaller brain volume (38%) and worse microstructural integrity (16%). We found no strong evidence that those with a LOC HI at any time was associated with pathology linked to AD (amyloid burden, cortical thickness in AD‐related signature region), axonal injury (NFL) or WMH at age 69–71 years. These findings may suggest that LOC HI that occur aged 50’s and earlier is associated with subtly worse cognition at age ~70 years, partly via diffuse brain volume‐related pathways, but not via pathology related to AD (amyloid, hippocampal volume, AD signature regions cortical thickness) or ongoing neurodegeneration (NFL). Yet, given that brain volume and NAWM differences occur in normal ageing trajectories it is unclear whether HI relates to an acute but stable loss of volume, interacts with normal ageing processes or reflects ongoing neurodegeneration, to infer susceptibility for dementia.
Our findings show that LOC HI is associated with lower cognitive function on a DSST at least 15 years later. This test measures a range of cognitive domains including speed, motor skills and attention and is sensitive to cognitive impairment 31 and brain damage. 32 Notably, DSST scores were only lower in those with LOC HI occurring at least 15 years prior, not in those with a HI occurring at anytime in the life course. Whilst the relationship between HI and ageing processes are still unclear, given that our participants are born in the same week limiting age‐effects between groups, and we take into account participants’ own prior cognition limiting reverse causation, finding subtle associations only with HI 15 years prior is a potentially elucidating finding. It could be a spurious association or suggests the importance of: (i) a longer duration of life following exposure; (ii) a temporal effect amplification of early exposure or (iii) age of HI. Growing research provides evidence that greater time since injury is associated with greater cognitive decline 33 and older brain ageing, 34 suggesting that HI accelerates brain atrophy and cognitive decline ageing trajectories. It is also known that the likely causes of HI are different across age groups, for example occupational, road accidents and sports‐related injuries in younger populations and falls in older populations, which may infer different risk and interact with ageing processes differently. 33 , 35 It is plausible that later‐life injuries occurring closer to the scan may be due to ageing processes linked to susceptibility to falls, comorbidities and related medication or as a consequence of prodromal cognitive impairment 36 , 37 and therefore the measure of HI across the life course may have potential cases of reserve causation. Further work with better characterization of HI will help to elucidate the relationship between features of HI (i.e. duration, frequency, timing) and later‐life cognitive function and brain health.
Our finding that HI is associated with lower whole brain volume is in line with well‐documented evidence of traumatic brain injury‐associated brain volume loss, 4 estimated around 4% after 1 year. 38 We also demonstrated that the smaller brain volume is partly explained lower DSST scores in HI participants, suggesting that HI‐associated cerebral volume loss is likely on the mechanistic pathway from HI to lower cognitive performance.
Our evidence that HI is associated with lower NAWM FA and NDI is in accordance with the main findings of recent meta‐analyses, demonstrating impaired structural integrity in all severities of TBI 39 and that FA, a metric of directionality, is a stronger correlate of LOC HI than mean diffusivity. 40 It is not possible to directly infer the underlying microstructural changes related to diffusion metrics without supporting histology, however post‐mortem work in cerebral amyloid angiopathy has shown that decreased FA can be caused by tissue rarefaction and reduction in neuronal density, while increasing MD can reflect decreasing myelination. 41 Notably, FA did not explain much of the variance between HI and lower DSST score, indicating that it is not a big explanatory influence, or it is a noisier measure; regional measures may closely relate to cognitive performance. 42
Acute disruptions to biochemical and cytoskeletal functions are thought to happen in all HI events, even in the mild HI that is likely expected here, and could explain how HI‐related changes affect whole brain volume and NAWM microstructural indexes. 2 Given the existing wider evidence, it is feasible that acute HI‐induced brain volume loss and different microstructure occurs as a direct effect of the initial pathological response and may reduce brain reserve and neuroplasticity. HI‐induced atrophy and microstructural changes could also be the basis of a long‐latency or temporal amplification effects, whereby it makes the brain more vulnerable to ageing, inflammation, and neurodegenerative effects many years after the initial injury, and consequently at greater risk of cognitive impairment.
Despite studies reporting links between HI and Alzheimer’s disease, and a few studies providing evidence that HI is associated with AD‐related pathology markers including increased β‐amyloid (Aβ) 3 and reduced hippocampal volume, 5 we find a lack of strong associations between LOC HI and a range of AD‐biomarkers (Aβ‐PET, disproportional hippocampal volume loss, AD‐signature region cortical thickness 43 ). Our findings suggest that HI does not increase cognitive impairment risk via earlier development of pathology typically associated with the AD, at least not at this relatively early stage of the pathophysiological continuum of AD. 44 Instead our findings suggest that HI is linked to diffuse brain volume‐related pathways at this age, and may interact with normal ageing processes, or reflect ongoing neurodegeneration, which may infer susceptibility for dementia.
We did not observe greater cerebral global WMH indicative of small vessel disease or find differences in serum NFL, a marker of axonal injury. NFL may be a more suitable biomarker as an acute index of neuronal damage instead of chronic damage 45 ; and worse in those with pre‐existing neurological conditions. 46 In addition, as the severity of head injury in this cohort is expected to be mild it is possible that the neuronal injury is too low to be detected by NFL in blood samples. 47 Long‐term effects of mild and moderate head injury on NFL are not yet established and would be key to elucidate the stability and dynamics of these markers.
A key strength is the use of a population‐based birth cohort with data spanning over 70 years, enabling prospectively ascertained reports of HI and adjustment for key predictors of later‐life cognition not usually available, such as premorbid childhood cognition. Notably, the inclusion of covariates including earlier cognition, education and social class had almost no effect on the association between head injury and brain health outcomes, suggesting these had limited confounding effects. Participants were born in the same week which reduces the risk of confounding by age on otherwise age‐sensitive measures. The age of participants at the time of the scan (69–71) reflects a life course stage whereby pathology is expected to accumulate, but clinical manifestations of dementia are still limited. However, in this context, some of the null findings reported here may reflect the relatively early stage of pathophysiological continuum of AD that we expect some participants to be in, potentially many years before onset of AD‐related neurodegeneration. One caveat is only one time point of imaging, so inferences concerning the temporal order and progressive nature of neuropathology are limited; this is particularly important to understand whether HI is associated with an acute but stable loss of brain volume or an ongoing neurodegenerative process. In addition, while we were able to explore differences between prior HI at least 15 years prior to testing and HI occurring at any time across the life course, a main limitation of this study is that more detailed information about timing, frequency and severity of HI was not available. Ongoing assessments in our sample are planned to gain more detailed information about HI which will be informative in due course. There are a high volume of tests but, in line with previous studies, 30 adjustments have not made for multiple comparisons and results are shown as mean difference with 95% confidence intervals at every stage to enable the reader to judge the biological importance of the results.
We demonstrated that even in the general population (i.e., not just professional athletes or veterans) a head injury sufficient to cause a loss of consciousness in people in their 50’s or younger is associated with poorer psychomotor speed and executive function at age ~70, even after adjusting for prior education and childhood cognitive ability. The effect was partly explained by the presence of smaller global brain volumes and microstructural changes, although other pathological underpinnings may be present. Continued follow‐up will provide greater insight into whether the negative effects of HI on pathological markers and cognitive function are static or dynamic. If replicated, these findings suggest experiencing a LOC HI earlier in life may have implications for cognitive and brain health lasting decades later, into later life.
Author Contributions
MR, NCF and JMS conceived and obtained funding for the study. CAL, TP, AK, SB, SK, HMS, MB, AW, KM, JC and SNJ had a major role in the acquisition of data. CAL, DC, IM, CS, WC, LP performed imaging processing and QC. JMN provided statistical support. SNJ performed the analysis and drafted the initial manuscript. All authors contributed to revision, interpretation and editing of the manuscript.
| Name | Location | Role | Contribution |
|---|---|---|---|
| Sarah‐Naomi James | University College London | Author | Major role in the acquisition of data. Drafting/revising the manuscript for content. |
| Jennifer M. Nicholas | London School of Hygiene and Tropical Medicine | Author | Provided statistical support. Interpreted the data; revised the manuscript for intellectual content. |
| Christopher A. Lane | University College London | Author | Major role in the acquisition of data and QC. Interpreted the data; revised the manuscript for intellectual content. |
| Thomas D. Parker | University College London | Author | Major role in the acquisition of data. Interpreted the data; revised the manuscript for intellectual content. |
| Kirsty Lu | University College London | Author | Major role in the acquisition of data. Interpreted the data; revised the manuscript for intellectual content. |
| Ashvini Keshavan | University College London | Author | Major role in the acquisition of data. Interpreted the data; revised the manuscript for intellectual content. |
| Sarah M. Buchanan | University College London | Author | Major role in the acquisition of data. Interpreted the data; revised the manuscript for intellectual content. |
| Sarah E. Keuss | University College London | Author | Major role in the acquisition of data. Interpreted the data; revised the manuscript for intellectual content. |
| Heidi Murray‐Smith | University College London | Author | Major role in the acquisition of data. Interpreted the data; revised the manuscript for intellectual content. |
| Andrew Wong | University College London | Author | Major role in the acquisition of data. Interpreted the data; revised the manuscript for intellectual content. |
| David M. Cash | University College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Ian B. Malone | University College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Josephine Barnes | University College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Carole H. Sudre | King’s College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Will Coath | University College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Lloyd Prosser | University College London | Author | Major role in the derivation of data. Revised the manuscript for intellectual content. |
| Sebastien Ourselin | King’s College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Marc Modat | King’s College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| David Thomas | University College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Jorge Cardoso | King’s College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Amanda Heslegrave | University College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Henrik Zetterberg | University College London, University of Gothenburg | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Sebastian J. Crutch | University College London | Author | Major role in the derivation of data. Interpreted the data; revised the manuscript for intellectual content. |
| Jonathan M. Schott | University College London | Author | Conceived and obtained funding for the study. Interpreted the data; revised the manuscript for intellectual content. |
| Marcus Richards | University College London | Author | Conceived and obtained funding for the study. Interpreted the data; revised the manuscript for intellectual content. |
| Nick C. Fox | University College London | Author | Conceived and obtained funding for the study. Interpreted the data; revised the manuscript for intellectual content. |
Conflicts of Interest
HZ is a co‐founder of Brain Biomarker Solutions, a GU Ventures‐based platform company at the University of Gothenburg, has served on advisory boards of Roche Diagnostics, Wave, Samumed and CogRx and has given lectures in symposia sponsored by Biogen and Alzecure. NCF has received research funding from Eisai, Elan, Eli Lilly, GE, IXICO, Janssen, Lundbeck, Pfizer, Roche Sanofi‐Aventis and Wyeth. NCF has served on a Data Safety Monitoring Committee for Biogen. JS has received research funding from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly), has consulted for Roche Pharmaceuticals, Biogen, and Eli Lilly, given educational lectures sponsored by GE, Eli Lilly and Biogen, and serves on a Data Safety Monitoring Committee for Axon Neuroscience SE. All other authors have no conflicts of interest to declare.
Supporting information
Table S1. Means of cognitive and neuroimaging data available.
Table S2. Association between reported head injury (HI) and later‐life cognitive function at age 69–71 with additional sensitivity analyses.
Table S3. Association between reported head injury (HI) and pathology at age 69–71 with additional sensitivity analyses.
Acknowledgements
We are very grateful to those study members who helped in the design of the study through focus groups, and to the participants both for their contributions to Insight 46 and for their commitments to research over the last seven decades.
Funding information
This study is principally funded by grants from Alzheimer’s Research UK (ARUK‐PG2014‐1946, ARUK‐PG2017‐1946), the Medical Research Council Dementias Platform UK (CSUB19166), the Wolfson Foundation (PR/ylr/18575) and The Drake Foundation. The biomarker analyses are funded by the Brain Research Trust (UCC14191) and Weston Brain Institute and Selfridges Group Foundation award (176724). Florbetapir amyloid tracer is kindly provided by AVID Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly) who had no part in the design of the study. The MRC National Survey of Health and Development is funded by the Medical Research Council (MC_UU_12019/1, MC_UU_12019/3). The NSHD, MR and AW are funded by the Medical Research Council (MC_UU_12019/1, MC_UU_12019/3). Some researchers are supported by the NIHR Queen Square Dementia BRU (JMS, NCF), UCL Hospitals Biomedical Research Centre (JMS), Leonard Wolfson Experimental Neurology Centre (JMS, NCF, MM, DT). JB is supported by an Alzheimer’s Research UK Senior Fellowship. TP is supported by a Wellcome Trust Clinical Research Fellowship (200109/Z/15/Z). AK was supported by a Wolfson Foundation Clinical Research Fellowship. CS is supported by an Alzheimer’s Society Junior Fellowship (AS‐JF‐17‐011). MM is supported by an Alzheimer’s Society Project Grant (AS‐PG‐15‐025). SC is supported by an Alzheimer’s Research UK Senior Research Fellowship (ARUK‐SRF2013‐8). SO receives funding from the EPSRC (EP/H046410/1, EP/J020990/1, EP/K005278), the MRC (MR/J01107X/1), the EU‐FP7 project VPH‐DARE@IT (FP7‐ICT‐2011‐9‐601055), and NIHR BRC UCLH/UCL High Impact Initiative. HZ acknowledges support from the UCL/UCLH NIHR Biomedical Research Centre, the UK Dementia Research Institute at UCL and a Wellcome Trust Multi‐User Equipment Grant. JMS acknowledges the EPSRC (EP/J020990/1) and European Union’s Horizon 2020 research and innovation programme (Grant 666992) and a Weston Brain Institute and Selfridges Group Foundation award (176724). NCF acknowledges support from the UCL/UCLH NIHR Biomedical Research Centre, an NIHR Senior Investigator award and the UK Dementia Research Institute at UCL.
Funding Statement
This work was funded by Alzheimer's Society UK grants ARUK‐SRF2013‐8, AS‐JF‐17‐011, AS‐PG‐15‐025, and AS‐JF‐17‐011; Wellcome Trust grant 200109/Z/15/Z; Alzheimer's Research UK grants ARUK‐PG2014‐1946 and ARUK‐PG2017‐1946; Medical Research Council Dementias Platform UK grant CSUB19166; The Wolfson Foundation grant PR/ylr/18575; Brain Research Trust grant UCC14191; Engineering and Physical Sciences Research Council grants EP/H046410/1, EP/J020990/1, and EP/K005278; Weston Brain Institute and Selfridges Group Foundation award grant 176724; Medical Research Council grants MC_UU_12019/1 and MC_UU_12019/3.
References
- 1. Li Y, Li Y, Li X, et al. Head injury as a risk factor for dementia and Alzheimer’s disease: a systematic review and meta‐analysis of 32 observational studies. PLoS One 2017;12:e0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. LoBue C, Munro C, Schaffert J, et al. Traumatic brain injury and risk of long‐term brain changes, accumulation of pathological markers, and developing dementia: a review. J Alzheimer’s Dis 2019;70:629–654. [DOI] [PubMed] [Google Scholar]
- 3. Roberts GW, Gentleman SM, Lynch A, et al. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1994;57:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross DE. Review of longitudinal studies of MRI brain volumetry in patients with traumatic brain injury. Brain Injury 2011;25:1271–1278. [DOI] [PubMed] [Google Scholar]
- 5. Monti JM, Voss MW, Pence A, et al. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci 2013;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Narayana PA. White matter changes in patients with mild traumatic brain injury: MRI perspective. Concussion 2017;2:CNC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aoki Y, Inokuchi R, Gunshin M, et al. Diffusion tensor imaging studies of mild traumatic brain injury: a meta‐analysis. J Neurol Neurosurg. Psychiatry 2012;83:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stern RA, Adler CH, Chen K, et al. Tau positron‐emission tomography in former national football league players. N Engl J Med 2019;380:1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016;6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shahim P, Tegner Y, Marklund N, et al. Neurofilament light and tau as blood biomarkers for sports‐related concussion. Neurology 2018;90:e1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Keeffe E, Kelly E, Liu Y, et al. Dynamic blood brain barrier regulation in mild head trauma. J. Neurotrauma 2019;37(2):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham J, Broglio SP, O’Grady M, Wilson F. History of sport‐related concussion and long‐term clinical cognitive health outcomes in retired athletes: a systematic review. J Athl Train 2020;55(2),132–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gallo V, Motley K, Kemp SPT, et al. Concussion and long‐term cognitive impairment among professional or elite sport‐persons: a systematic review. J.Neurol Neurosurg Psychiatry 2020;jnnp‐2019‐321170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fann JR, Ribe AR, Pedersen HS, et al. Long‐term risk of dementia among people with traumatic brain injury in Denmark: a population‐based observational cohort study. The Lancet Psychiatry 2018;5(5),424–431. [DOI] [PubMed] [Google Scholar]
- 15. James S‐N, Davis D, Rawle M, et al. Head injury with loss of consciousness and subsequent cognitive decline: follow‐up in the 1946 British Birth Cohort Study. Alzheimer’s Dement 2018;14:P278–P279. [Google Scholar]
- 16. Lane CA, Parker TD, Cash DM, et al. Study protocol: insight 46 – a neuroscience sub‐study of the MRC National Survey of Health and Development. BMC Neurol 2017;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. James S‐N, Lane CA, Parker TD, et al. Using a birth cohort to study brain health and preclinical dementia: recruitment and participation rates in Insight 46. BMC Res Notes 2018;11:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu K, Nicholas JM, Collins JD, James SN, Cognition at age 70. Neurology 2019;93(23):e2144–e2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leung KK, Barnes J, Modat M, et al. Brain MAPS: an automated, accurate and robust brain extraction technique using a template library. NeuroImage 2011;55:1091–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jorge Cardoso M, Leung K, Modat M, et al. STEPS: Similarity and Truth Estimation for Propagated Segmentations and its application to hippocampal segmentation and brain parcelation. Med Image Anal 2013;17:671–684. [DOI] [PubMed] [Google Scholar]
- 21. Parker TD, Cash DM, Lane CA, et al. Amyloid β influences the relationship between cortical thickness and vascular load. Alzheimer’s Dement Diagn Assess Dis Monit 2020;12(1):e12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jack CR, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain 2015;138(Pt 12):3747–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sudre CH, Cardoso MJ, Bouvy WH, et al. Bayesian model selection for pathological neuroimaging data applied to white matter lesion segmentation. IEEE Trans Med Imaging 2015;34:2079–2102. [DOI] [PubMed] [Google Scholar]
- 24. Zhang H, Schneider T, Wheeler‐Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 2012;61:1000–1016. [DOI] [PubMed] [Google Scholar]
- 25. Iverson GL, Hakulinen U, Wäljas M, et al. To exclude or not to exclude: white matter hyperintensities in diffusion tensor imaging research. Brain Injury 2011;25:1325–1332. [DOI] [PubMed] [Google Scholar]
- 26. Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 27. Gaetani L, Blennow K, Calabresi P, et al. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 2019;90(8):870–881. [DOI] [PubMed] [Google Scholar]
- 28. Richards M, Barnett JH, Xu MK, et al. Lifetime affect and midlife cognitive function: prospective birth cohort study. Br J Psychiatry 2014;204:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med 1979;9:139–145. [DOI] [PubMed] [Google Scholar]
- 30. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed] [Google Scholar]
- 31. Jaeger J. Digit symbol substitution test. J Clin Psychopharmacol 2018;38:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russell EW. WAIS factor analysis with brain‐damaged subjects using criterion measures. J Consult Clin Psychol 1972;39:133–139. [DOI] [PubMed] [Google Scholar]
- 33. Sendroy‐Terrill M, Whiteneck GG, Brooks CA. Aging with traumatic brain injury: cross‐sectional follow‐up of people receiving inpatient rehabilitation over more than 3 decades. Arch Phys Med Rehabil 2010;91:489–497. [DOI] [PubMed] [Google Scholar]
- 34. Cole JH, Leech R, Sharp DJ; Alzheimer’s Disease Neuroimaging Initiative . Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol 2015;77:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc 2006;54:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pierce MB, Silverwood RJ, Nitsch D, et al. Clinical disorders in a post war British cohort reaching retirement: evidence from the first National Birth Cohort Study. PLoS One 2012;7:e44857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters ME, Gardner RC. Traumatic brain injury in older adults: do we need a different approach? Concussion 2018;3:CNC56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sidaros A, Skimminge A, Liptrot M, et al. Long‐term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. NeuroImage 2009;44:1–8. [DOI] [PubMed] [Google Scholar]
- 39. Wallace EJ, Mathias JL, Ward L. Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: a meta‐analysis. Brain Imaging Behav 2018;12:1607–1621. [DOI] [PubMed] [Google Scholar]
- 40. Zhang J, Wei R‐L, Peng G‐P, et al. Correlations between diffusion tensor imaging and levels of consciousness in patients with traumatic brain injury: a systematic review and meta‐analysis. Sci Rep 2017;7:2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Veluw SJ, Reijmer YD, van der Kouwe AJ, et al. Histopathology of diffusion imaging abnormalities in cerebral amyloid angiopathy. Neurology 2019;92:e933–e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wallace EJ, Mathias JL, Ward L. The relationship between diffusion tensor imaging findings and cognitive outcomes following adult traumatic brain injury: a meta‐analysis. Neurosci Biobehav Rev 2018;92:93–103. [DOI] [PubMed] [Google Scholar]
- 43. Ridha BH, Barnes J, Bartlett JW, et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol 2006;5:828–834. [DOI] [PubMed] [Google Scholar]
- 44. Jack CR, Wiste HJ, Weigand SD, et al. Age‐specific and sex‐specific prevalence of cerebral β‐amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross‐sectional study. Lancet Neurol 2017;16:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iverson GL, Reddi PJ, Posti JP, et al. Serum neurofilament light is elevated differentially in older adults with uncomplicated mild traumatic brain injuries. J Neurotrauma 2019;36:2400–2406. [DOI] [PubMed] [Google Scholar]
- 47. Wallace C, Zetterberg H, Blennow K, van Donkelaar P. No change in plasma tau and serum neurofilament light concentrations in adolescent athletes following sport‐related concussion. PLoS One 2018;13:e0206466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Means of cognitive and neuroimaging data available.
Table S2. Association between reported head injury (HI) and later‐life cognitive function at age 69–71 with additional sensitivity analyses.
Table S3. Association between reported head injury (HI) and pathology at age 69–71 with additional sensitivity analyses.
Data Availability Statement
Anonymized data will be shared by request from qualified investigators (skylark.ucl.ac.uk/NSHD/doku.php).


