Abstract
Little is known about the longitudinal association between fasting glucose (FG) and the diurnal cortisol profile among those with normal fasting glucose (NFG), impaired fasting glucose (IFG) and diabetes. To assess the temporality of the relationship between cortisol and glucose, we examined the association of: A) change (Δ) in diurnal cortisol curve features with ΔFG; B) prior annual percent change in FG with diurnal cortisol curve features; and C) baseline cortisol curve features with ΔFG over 6 years among participants with NFG, IFG and diabetes in the Multi-Ethnic Study of Atherosclerosis. The main outcome measures were: A) 6-year ΔFG (n = 512); B) diurnal cortisol curve features (wake-up cortisol levels, cortisol awakening response, total area under the curve, overall decline slope and bedtime cortisol) (n = 1275); and C) 6-year ΔFG (n = 700). After full multivariable adjustment among participants with diabetes, each annual percent change increase in wake-up cortisol, total area under the curve (AUC), and overall decline slope was associated with a significant increase in FG over 6 years in all models (all p < 0.05). A 1% prior annual increase in FG was associated with a 2.8 % lower (−2.8 %; 95 % CI: −5.3 % to −0.4 %) bedtime cortisol among participants with NFG at baseline. A 1 % flatter overall decline slope was associated with a 0.19 % increase in subsequent annual % change in FG over 6 years among participants with diabetes. Among participants with diabetes there was a positive association of change in wake-up cortisol, total AUC and overall decline slope with change in FG. Baseline overall decline slope was positively associated with change in FG among the baseline diabetes group. These results suggest a detrimental role of cortisol contributing to glycemia among individuals with diabetes.
Keywords: Cortisol, Diabetes, Glucose, Glycemia, Hypothalamic-pituitary-adrenal axis
1. Introduction
The hypothalamic-pituitary-adrenal (HPA) axis constitutes the body’s major biological system that responds to imminent or perceived threats and stressors (inflammatory, traumatic or psychological) through elicitation of a response involving the release of glucocorticoids into the systemic circulation by the adrenal glands (Tsigos and Chrousos, 2002; Kyrou et al., 2006). Glucocorticoids are also important regulators of energy balance and glucose homeostasis (Tsigos and Chrousos, 2002; Kyrou et al., 2006; Joseph and Golden, 2017). There are environmental and genetic determinants that modulate inter-individual differences in cortisol exposure (Joseph and Golden, 2017). Detailed clinical studies of the HPA axis in diabetes have shown significant HPA axis dysregulation with both elevated morning, afternoon and diurnal cortisol, along with a higher prevalence of non-suppressed cortisol following the dexamethasone suppression test (Cameron, 1984; Hudson et al., 1984; Cameron et al., 1987; Roy et al., 1990). Additionally, microvascular complications in diabetes, including retinopathy and neuropathy have been linked to HPA axis dysfunction (Roy et al., 1990; Tsigos et al., 1993). Prior epidemiologic studies have also shown that metabolic disorders, obesity and type 2 diabetes are associated with HPA dysfunction (Joseph and Golden, 2017). In cross-sectional studies, individuals with type 2 diabetes display a flatter cortisol slope across the day, blunted cortisol awakening response (CAR), and higher bedtime cortisol compared to those without diabetes (Bruehl et al., 2009; Lederbogen et al., 2011; Champaneri et al., 2012; Hackett et al., 2014). Cortisol curve features, including flatter overall cortisol decline slope (awakening to bedtime) and higher bedtime cortisol and total area under the curve (AUC) cortisol were positively associated with hemoglobin A1c, a marker of chronic glycemia, in the Multi-Ethnic Study of Atherosclerosis (MESA) (Joseph et al., 2015).
Recently, the longitudinal association of diabetes status and changes in daily cortisol curve features over time were evaluated in two studies—the MESA Study and the Whitehall II Study (Spanakis et al., 2016; Hackett et al., 2016). The MESA Study showed that diabetes status did not predict change in cortisol curve features over 6 years (Spanakis et al., 2016); however, the Whitehall II Study reported higher bedtime cortisol levels and flatter diurnal cortisol slope at baseline predicted new-onset of diabetes (Hackett et al., 2016). These data suggest that HPA axis features may influence glucose metabolism and diabetes risk, but that diabetes may not influence cortisol dynamics and HPA axis function. The above studies have focused on the temporality of cortisol features with diabetes status; however, there are limited data on the longitudinal relationship of FG and cortisol features among those with NFG, IFG and diabetes. Therefore, we examined the longitudinal association between FG and cortisol curve features over 6 years in participants with NFG, IFG and diabetes utilizing prospective data from the main MESA study (MESA Exams 1–5) and the MESA Stress Ancillary Study. To examine the temporality of the relationship between cortisol and glucose, we assessed different associations: A) changes in FG with changes in diurnal cortisol features over 6 years; B) prior average annual FG change with diurnal cortisol features; and C) baseline cortisol features with changes in FG over 6 years, as presented in (Fig. 1).
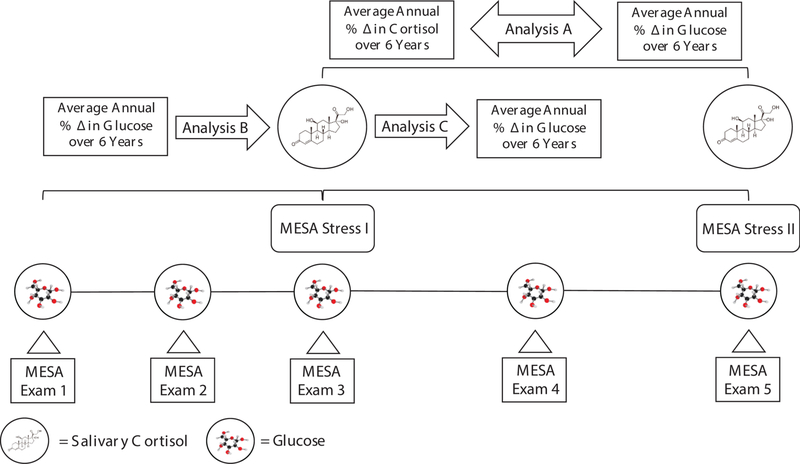
Fig. 1.
Conceptual Design of Study.
Temporality of the Relationship Between Cortisol and Glucose. The temporality of the relationship between cortisol and glucose among participants with normal fasting glucose, impaired fasting glucose and diabetes. Analysis A, change (Δ) in diurnal cortisol curve features with Δ fasting glucose among individuals who attended both MESA Stress I and MESA Stress II; Analysis B, prior annual percent change in fasting glucose with diurnal cortisol curve features among participants who attended either MESA Stress I or II; Analysis C, baseline cortisol curve features with Δ fasting glucose over 6 years among participants who attended MESA Stress I.
2. Methods
2.1. Study population
MESA is a multi-center, longitudinal cohort study of the prevalence and correlates of subclinical cardiovascular disease and the factors influencing its progression (Bild, 2002). Between July 2000 and August 2002, 6,814 men and women without clinical cardiovascular disease who identified themselves as White, Black, Hispanic or Chinese and were 45–84 years of age were recruited from six U.S. communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; St. Paul, Minnesota; and New York, New York. These individuals participated in MESA Exams 1–5; details on the sampling frames and the cohort examination procedures have been published previously (Bild, 2002). The MESA Stress I Study collected detailed measures of stress hormones, including salivary cortisol measures in 1,002 participants between 2004 and 2006 (concurrent with MESA Exams 3 and 4) at the New York and Los Angeles sites. The MESA Stress II Study collected similar data, on a subsample of 1,082 participants at the New York, Los Angeles and Baltimore sites between 2010–2012 during MESA Exam 5. There were 2,038 participant visits for MESA Stress I or MESA Stress II. Informed consent was obtained from each participant, and the institutional review boards of all the participating institutions approved the study and consent procedures.
2.2. Glycemic status
Participants fasted for 12 hours and avoided smoking and heavy physical activity for 2 hours before each examination. Fasting blood samples were drawn between 7:30 and 10:30 AM. Serum was frozen and stored at −70 °C as previously described (7). NFG was defined as FG = ≤ 5.5 mmol/l (100 mg/dl), IFG was defined as FG 5.6–6.9 mmol/L (100 mg/dL to 125 mg/dL), and diabetes status was defined according to the 2003 American Diabetes Association criteria of FG ≥ 7.0 mmol/l (126 mg/dl) or use of hypoglycemic medication (oral agents and/or insulin) as previously described (American Diabetes Association, 2009; Bild, 2002).
2.3. Salivary cortisol measures
In MESA Stress I, salivary cortisol measures were collected over 3 days with 6 time points measured per day. The first sample was taken immediately after waking (before getting out of bed), the second sample 30 min later, the third sample at around 10:00 AM, the fourth sample at around noon (or before lunch if lunch occurred before noon), the fifth sample at around 6:00 PM (or before dinner if dinner occurred before 6:00 PM), and the sixth sample right before bedtime. Participants recorded collection time on special cards. Additionally, a time-tracking device (Track Caps) automatically registered the time at which cotton swabs were extracted to collect each sample. The participants were informed of this time-tracking device. From our work in MESA Stress I, we learned that we could adequately characterize the diurnal cortisol curve features with 2 days of sample collection; thus, the third day was eliminated in MESA Stress II to reduce participant burden (Wang et al., 2014). Sample collection times in MESA Stress II corresponded to those in MESA Stress I with the following exception—two additional samples were collected at 1 hour after breakfast and 6:00 PM to better characterize the early and late decline slopes, respectively (Wang et al., 2014). Participants were instructed to record the exact time of sample collection on a special card, which was facilitated by a provided alarm clock. Participants were given similar instructions not to eat or drink or brush their teeth 15 min before collecting the salivary samples in MESA Stress I and II. They were also instructed to leave the cotton swab in their mouths for less than 2 minutes until soaked, moving it around inside their mouth.
In MESA Stress I, 97 % of participants collected samples on all 3 days and 85 % of participants collected at least 5 samples per day for all days on which they collected samples (Wang et al., 2014; Joseph et al., 2015). In MESA Stress II, 98 % of participants collected valid samples (i.e. valid cortisol sample and valid time of sample collection) on both days and 96 % of participants collected at least 5 valid samples per day for both days. Participants recorded collection time on special cards. Based on prior work in our population, the median difference between the actual collection time and recorded times was between 2 and 4 minutes depending on the sample. The 25th and 75th percentiles were between 1 and 2 and 5 and 13 minutes, respectively, with the longest times corresponding to the last sample of the day. Overall, the first sample was taken within 5 minutes of wake-up for 78 % of the days across participants and the median difference between the first and second sample was 34 minutes. While lower compliance with the collection protocol was associated with a less pronounced CAR, compliance was not associated with any other cortisol features, and adjustment for compliance did not affect the associations of cortisol features with sociodemographic characteristics (Wang et al., 2014).
For both MESA Stress I and II, saliva samples were stored at −20 °C until analysis. Before biochemical analysis, samples were thawed and centrifuged at 3000 rpm for 3 minutes to obtain clear saliva with low viscosity. Cortisol levels were determined using a commercially available chemiluminescence assay with a high sensitivity of 0.16 ng/mL (IBL, Hamburg, Germany). Intra- and inter-assay coefficients of variation were less than 8%.
2.4. Assessment of cortisol features and covariates
Healthy diurnal cortisol regulation follows a circadian pattern, with high levels upon awakening, which increase by 50–75 % between 0–30 minutes post-awakening (cortisol awakening response, CAR); and decline across the remainder of the day, reaching a nadir in the late evening some 16 to 18 hours after awakening (Joseph and Golden, 2017). We investigated five features of the daily cortisol curve: Wake-up cortisol levels, CAR, standardized total cortisol AUC, overall decline slope, and bedtime cortisol. Due to its positively skewed distribution, cortisol was log-transformed before the cortisol features were calculated (Joseph et al., 2015). Wake-up cortisol was defined as the salivary cortisol obtained at time 0. The CAR was the cortisol rise from time 0 to 30 minutes post-awakening. The overall decline slope was calculated as the rate of decline using all samples except the second sample (30 minutes post-awakening). To calculate the AUC, we used linear splines to connect the values from each of the sample times and then calculated the area under the linear spline based on the trapezoid rule (Yeh and Kwan, 1978) using all available data and restricting estimates to a 16-hour day duration for all participants (AUC was missing if less than 3 samples were collected for the exam day or the last 2 samples were missing). The AUC was then standardized by the length of duration (16-hours).
A priori we selected potential confounders in the glucose-cortisol association based on the extant literature. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2) (Joseph et al., 2017). Weight and height were measured using a balanced beam scale and a vertical ruler, respectively, with participants wearing light clothing and no shoes. Anthropometric measures were taken in duplicate and averaged. The covariates were assessed at the corresponding MESA Exam where cortisol features were ascertained. Covariates such as age, sex, race/ethnicity, and cigarette smoking were self-reported using protocols as previously described (Bild, 2002; Champaneri et al., 2012). Based on sensitivity analysis, we found similar results among individuals using medications versus not using medications such as beta blockers, hormone replacement therapy, aspirin and steroids. Therefore, we did not exclude participants based on these prescription drugs. Participants were categorized as current, former, or not current smokers (Badrick et al., 2007). Physical activity was obtained from an interviewer-administered questionnaire adapted from the Cross-Cultural Activity Participation Study (Ainsworth et al., 1999; LaMonte et al., 2001) and measured as moderate-to-vigorous physical activity in metabolic equivalent minutes per week (Bertoni et al., 2008). Depressive symptoms were assessed using the Center for Epidemiological Studies Depression (CES-D) scale (Radloff and Locke, 2008).
2.5. Statistical analysis
2.5.1. Association of annual percent change in cortisol features with annual percent change in FG over 6 years (Analysis A)
This analysis included individuals who attended both MESA Stress I and MESA Stress II (N = 580). We excluded individuals with missing data on annual percent change in cortisol features (N = 54), on important covariates including BMI, CES-D, physical activity, smoking status (n = 9) and on annual percent change in FG (N = 5). The final analytic dataset included 512 participants (Supplemental Fig. 1). We used the following formulas to calculate annual percent change FG and annual percent change cortisol features.
If MESA Stress I was collected in MESA Exam 3:
If MESA Stress I was collected in MESA Exam 4:
Multivariate linear regression models were used to examine annual percent change in cortisol feature with annual percent change in FG over 6 years. The models were adjusted as follows: Model 0 included adjustment for age, sex, and race/ethnicity; Model 1 was further adjusted for smoking status, physical activity, CES-D, and BMI.
2.5.2. Association of prior annual percent change FG and diurnal cortisol features (Analysis B)
This analysis included participants who attended either MESA Stress Exam; 969 participants were enrolled in MESA Stress I and 1,069 participants were enrolled in MESA Stress II. For individuals who attended both MESA Stress exams, we only included their MESA Stress II data, resulting in 1,458 participants (389 participants in MESA Stress I and 1,069 participants in MESA Stress II). After excluding individuals with missing data on cortisol feature values (N = 90), important covariates including BMI, CES-D, physical activity, smoking status (N = 34) and prior average annual percent change FG (N = 59), the final analytic dataset included 1,275 participants (Supplemental Fig. 1). We used the following formula for each subject to calculate fasting average annual change prior to each exam at which cortisol was assessed:
N = number of visits prior to Exam when cortisol was collected
Multivariate linear regression models were used to examine the association of prior average annual % change in FG with cortisol curve features using separate models for each cortisol curve feature. The models were adjusted as follows: Model 0 included adjustment for an exam indicator for MESA Stress Exam (I or II), age, sex, and race/ethnicity. Model 1 further adjusted for smoking status, physical activity, CES-D, and BMI. The analyses were additionally stratified by diabetes status (NFG, IFG, and diabetes).
2.5.3. Association of baseline cortisol features at MESA Stress I with subsequent annual percent change in FG (Analysis C)
The analysis included participants who attended MESA Stress I (N = 969). We excluded cortisol samples with missing cortisol feature values (N = 80), missing covariate data including BMI, CES-D, physical activity, smoking status (N = 22), and missing subsequent annual percent change FG (N = 167). The final analytic dataset included 700 participants (Supplemental Fig. 1). We used the following formulas to calculate subsequent annual percent change in FG.
If MESA Stress I cortisol features were collected during MESA Exam 3:
If MESA Stress I cortisol features were collected during MESA Exam 4:
Multivariable linear regression models were used to examine the association between baseline cortisol curve features with subsequent annual percent change in FG. The models were adjusted as follows: Model 0 included adjustment for age, sex, and race/ethnicity; Model 1 further adjusted for smoking status, physical activity, CES-D, and BMI. The analyses were additionally stratified by diabetes status (NFG, IFG, and diabetes). All analyses were conducted using the SAS system, version 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was defined as a 2-sided alpha <0.05 for main effects and <0.10 for interactions.
3. Results
For each analysis, participants with normoglycemia were younger with lower BMI and were more likely to be white compared to participants with IFG or diabetes (Table 1; all p < 0.05). The normoglycemic participants had a trend towards higher physical activity and a higher percentage of current smokers, which was significant for the population included in analysis C for physical activity. There were no differences in CES-D Score.
Table 1.
Participant baseline characteristics stratified by diabetes status.
| Parameters | Analysis Aa |
Analysis Bb |
Analysis Cc |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 512) | Normal (N = 337) | IFG (N = 101) | Diabetes (N = 74) | p-valued | Total (N = 700) | Normal (N = 443) | IFG (N = 148) | Diabetes (N = 109) | p-valued | Total (N = 1275) | Normal (N = 787) | IFG (N = 259) | Diabetes (N = 229) | p-valued | |
| Age (years) | 63.7 ± 9.1 | 62.8 ± 9.3 | 64.9 ± 9 | 66.2 ± 8 | 0.0045 | 64.4 ± 9.6 | 63.6 ± 9.8 | 64.8 ± 9.3 | 67.1 ± 8.7 | 0.0023 | 66.6 ± 9.6 | 65.9 ± 9.9 | 67.5 ± 9.4 | 67.5 ± 9.4 | 0.0035 |
| Sex, male (%) | 47.1 | 42.4 | 59.4 | 51.4 | 0.0081 | 46.7 | 43.3 | 55.4 | 48.6 | 0.0355 | 47.1 | 43.6 | 58 | 47 | 0.0003 |
| Raee/ethnicity (%) | |||||||||||||||
| White | 19.7 | 22.9 | 20.8 | 4.1 | 0.0055 | 21.1 | 25.3 | 21.6 | 3.7 | <.0001 | 26.0 | 31.3 | 25.5 | 8.7 | <.0001 |
| Black | 27.3 | 24.9 | 29.7 | 35.1 | 26.4 | 25.1 | 25 | 33.9 | 30.9 | 28.7 | 29 | 40.6 | |||
| Hispanic | 52.9 | 52.2 | 49.5 | 60.8 | 52.4 | 50.0 | 53.4 | 62.4 | 43.1 | 40.2 | 45.6 | 50.7 | |||
| Body mass index (kg/m2) | 29.0 ± 5.2 | 28.2 ± 5.1 | 30 ± 5 | 31 ± 5.3 | <.0001 | 28.9 ± 5.5 | 28.0 ± 5.4 | 29.9 ± 5.3 | 31 ± 5.1 | <.0001 | 29.2 ± 5.6 | 28.2 ± 5.2 | 30.1 ± 5.6 | 31.8 ± 5.5 | <.0001 |
| Physical activity (MET min/week) | 746.25 (210, 1702.5) | 825 (210, 1860) | 735 (263, 1575) | 638 (210, 1155) | 0.2853 | 806.25 (210, 1860) | 877.5 (150, 2100) | 735 (210, 1470) | 630 (210, 1335) | 0.1556 | 1050 (315, 2490) | 1170 (368, 2700) | 900 (165, 2025) | 930 (263, 1980) | 0.0040 |
| Smoking status (%) | |||||||||||||||
| Never | 48.8 | 50.7 | 47.5 | 41.9 | 0.2364 | 46.6 | 48.8 | 44.6 | 40.4 | 0.0745 | 44.1 | 45.5 | 41.7 | 41.9 | 0.1977 |
| Former | 42.6 | 39.5 | 45.5 | 52.7 | 45.6 | 42.0 | 49.3 | 55.1 | 47.8 | 45.4 | 52.1 | 51.1 | |||
| Current | 8.6 | 9.8 | 6.9 | 5.4 | 7.9 | 9.3 | 6.1 | 4.6 | 8.2 | 9.2 | 6.2 | 7 | |||
| CES-D-score* | 6 (2, 12) | 6 (2, 12) | 5 (2, 11) | 6 (3, 14) | 0.3358 | 6 (2.5, 12) | 6 (2, 12) | 5 (2, 11) | 5 (3, 12) | 0.2433 | 6 (3, 12) | 6 (2, 12) | 5(2, 11) | 6(3, 13) | 0.0260 |
Associations of annual percent change in cortisol feature glucose with annual percent change in glucose over 6 years stratified by diabetes status.
Associations of prior average annual changes over 6 years in fasting glucose with cortisol features stratified by diabetes status.
Associations of baseline cortisol features with subsequent annual percent change FG stratified by diabetes status.
For age and BMI, p-values were from ANOVA for physical activity and CES-D, p-values were from Kruskal-Wallis Test, for the rest of variables p-values were from chi-square test.
3.1. Association of annual percent change in cortisol features with annual percent change in FG over 6 years
We found effect modification of the association of annual percent change in cortisol features with annual percent change in FG over 6 years by glycemic status and present stratified results in Table 2. Among participants with diabetes, each annual percent increase in wake-up cortisol, total AUC, and overall decline slope was associated with a significant increase in annual percent change in FG over 6 years in all models (Table 2). For instance, a 1-unit annual percent increase in overall decline slope (flattening) was associated with a 0.015 annual percent change in FG (0.015 [95 %CI: 0.005, 0.024]). There was no association of change in CAR or bedtime cortisol with change in FG among participants with diabetes. There was no association of annual percent change in cortisol curve features with percent change in fasting glucose among those with NFG or IFG (Table 2).
Table 2.
Associations of annual percent change in cortisol feature with annual percent change in glucose over 6 years stratified by diabetes status (Normal: N = 337, Impaired fasting glucose: N = 101 and Diabetes: N = 74).
| Predictor | Outcome: Avg. annual Δglucose |
||
|---|---|---|---|
| Model 0 coefficient (95 %CI) |
Model 1 coefficient (95 %CI) |
||
| Avg. annual ΔWake-up cortisol | Normal | 0.001 (−0.006, 0.009) | 0.001 (−0.006, 0.009) |
| IFG | −0.046 (−0.189, 0.098) | −0.03 (−0.174, 0.114) | |
| Diabetes | 0.046 (0.008, 0.083) | 0.044 (0.007, 0.082) | |
| P-value for interaction | 0.0593 | 0.0790 | |
| Avg. annual ΔCAR | Normal | 0.0005 (−0.001, 0.002) | 0.0005 (−0.001, 0.002) |
| IFG | 0.00004 (−0.0002, 0.0003) | 0.00005 (−0.0002, 0.0003) | |
| Diabetes | −0.002 (−0.008, 0.004) | −0.002 (−0.008, 0.004) | |
| P-value for interaction | 0.6576 | 0.6690 | |
| Avg. annual ΔTotal AUC | Normal | −0.002 (−0.037, 0.032) | 0.001 (−0.034, 0.036) |
| IFG | −0.018 (−0.118, 0.082) | −0.008 (−0.108, 0.092) | |
| Diabetes | 0.197 (0.095, 0.299) | 0.2 (0.098, 0.302) | |
| P-value for interaction | 0.0012 | 0.0013 | |
| Avg. annual ΔOverall decline slope | Normal | 0.0004 (−0.009, 0.01) | −0.0002 (−0.01, 0.01) |
| IFG | −0.013 (−0.081, 0.056) | −0.014 (−0.083, 0.054) | |
| Diabetes | 0.015 (0.005, 0.024) | 0.015 (0.005, 0.024) | |
| P-value for interaction | 0.1039 | 0.0855 | |
| Avg. annual ΔBedtime cortisol | Normal | 0.0003 (−0.004, 0.005) | −0.00002 (−0.004, 0.004) |
| IFG | −0.0006 (−0.009, 0.008) | −0.00009 (−0.008, 0.008) | |
| Diabetes | −0.0002 (−0.0008, 0.0004) | −0.0002 (−0.0008, 0.0004) | |
| P-value for interaction | 0.9750 | 0.9968 | |
Model 0: adjust for age, sex, race/ethnicity.
Model 1: adjust for age, sex, race/ethnicity, physical activity, smoking, CES-D, BMI.
β-coefficient interpretation: One-unit change of Avg. annual ΔWake-up cortisol will result in β unit change in Avg. annual Δglucose.
Note: Bold values signifies p < 0.05.
3.2. Association of prior annual percent change in FG over 6 years with diurnal cortisol features
We found effect modification of the association of prior annual percent change in FG over 6 years with diurnal cortisol features by glycemic status and thus present stratified results. The estimated difference in cortisol curve features associated with prior annual percent change in FG is shown in Table 3. Among individuals with NFG in the fully adjusted model, greater increases in FG were associated with lower bedtime cortisol. A 1% annual increase in FG was associated with a 2.7 % lower (−2.7 %; 95 % CI: −5.2 % to −0.3 %) bedtime cortisol among participants with NFG at baseline. There was no association between annual percent change in FG and bedtime cortisol among individuals with IFG and diabetes. Prior annual percent change in FG was not associated with other cortisol curve features (wake up, CAR, total AUC, or overall cortisol slope) regardless of glycemic status.
Table 3.
Associations of prior average annual changes over 6 years in fasting glucose with cortisol features stratified by diabetes status (Normal: N = 787, Impaired fasting glucose: N = 259 and Diabetes: N = 229).
| Predictor | Outcome |
|||||
|---|---|---|---|---|---|---|
| Avg. annual Δglucose | Wake-up cortisol coefficient (95 %CI) |
CAR coefficient (95 %CI) |
Total AUC coefficient (95 %CI) |
Overall decline slope coefficient (95 %CI) |
Bedtime cortisol coefficient (95 %CI) |
|
| Model 0 | Normal | 0.00003 (−0.023, 0.023) | −0.009 (−0.03, 0.011) | −0.013 (−0.03, 0.005) | 0.00003 (−0.002, 0.002) | −0.028 (−0.052, −0.004) |
| IFG | −0.004 (−0.038, 0.037) | 0.006 (−0.028, 0.04) | −0.008 (−0.036, 0.021) | 0.0001 (−0.003, 0.003) | −0.014 (−0.054, 0.025) | |
| Diabetes | −0.007 (−0.016, 0.002) | 0.006 (−0.002, 0.014) | 0.002 (−0.004, 0.009) | 0.0002 (−0.0004, 0.0009) | 0.0007 (−0.008, 0.01) | |
| P-value for interaction | 0.8170 | 0.3918 | 0.2367 | 0.9771 | 0.0772 | |
| Model 1 | Normal | 0.002 (−0.02, 0.025) | −0.008 (−0.029, 0.012) | −0.012 (−0.03, 0.006) | −0.00005(−0.002, 0.002) | −0.027 (−0.052, −0.003) |
| IFG | −0.002(−0.04, 0.035) | 0.006 (−0.028, 0.04) | −0.01 (−0.039, 0.019) | 0.00002 (−0.003, 0.003) | −0.017 (−0.056, 0.023) | |
| Diabetes | −0.006(−0.014, 0.003) | 0.006 (−0.001, 0.014) | 0.003 (−0.004, 0.01) | 0.0002 (−0.0005, 0.0008) | 0.0008 (−0.008, 0.01) | |
| P-value for interaction | 0.7999 | 0.4207 | 0.2212 | 0.9650 | 0.0766 | |
Model 0: adjust for MESA Stress Exam 1 or 2 indicator, age, sex, race/ethnicity.
Model 1: adjust for MESA Stress Exam 1 or 2 indicator, age, sex, race/ethnicity, physical activity, smoking, CES-D, BMI.
β-coefficient interpretation: An increase of one-unit in the Avg.annual Δglucose would result in (eβ − 1)*100 percentage change in cortisol features.
Note: Bold values signifies p < 0.05.
3.3. Association of baseline cortisol feature at MESA Stress I with subsequent annual percent change in FG over 6 years
There was no association between baseline cortisol curve features and change in FG among all participants (Table 4). We found a significant interaction by glycemic status and thus stratified the analysis. Among those with diabetes, a 1% flatter overall decline slope was associated with a 0.19 % increase in subsequent annual percent change in FG over 6 years among participants with diabetes (19.21; 95 % CI: 1.519%–36.908%). No significant relationships were found between baseline cortisol curve features and annual percent change among those with IFG or NFG (Table 4).
Table 4.
Associations of baseline cortisol features with subsequent annual percent change FG stratified by diabetes status (Normal: N = 443, Impaired fasting glucose: N = 148 and Diabetes: N = 109).
| Predictor | Outcome: Avg. annual Δglucose |
||
|---|---|---|---|
| Model 0 coefficient (95 %CI) |
Model 1 coefficient (95 %CI |
||
| wake-up cortisol | Normal | 0.099(−0.71, 0.91) | 0.138 (−0.683, 0.958) |
| IFG | 0.12 (−1.305, 1.546) | 0.085 (−1.345, 1.516) | |
| Diabetes | −0.339 (−1.72, 1.042) | −0.289 (−1.674, 1.097) | |
| P-value for interaction | 0.8541 | 0.8714 | |
| CAR | Normal | −0.152 (−1.028, 0.724) | −0.127 (−1.005, 0.751) |
| IFG | 0.05 (−1.758, 1.858) | 0.151 (−1.666, 1.967) | |
| Diabetes | 0.045 (−2.257, 2.347) | 0.308(−2.029, 2.644) | |
| P-value for interaction | 0.9727 | 0.9203 | |
| Total AUC | Normal | −0.112(−1.217, 0.992) | −0.107(−1.229, 1.014) |
| IFG | 0.311 (−1.319, 1.941) | 0.188 (−1.46, 1.836) | |
| Diabetes | −0.603 (−2.499, 1.293) | −0.533 (−2.451, 1.385) | |
| P-value for interaction | 0.7712 | 0.8527 | |
| Overall decline slope | Normal | −2.226(−11.056, 6.604) | −2.607 (−11.48, 6.266) |
| IFG | 3.025 (−11.295, 17.345) | 2.52 (−11.89, 16.93) | |
| Diabetes | 19.897 (2.29, 37.5) | 19.21 (1.519, 36.908) | |
| P-value for interaction | 0.0879 | 0.0955 | |
| Bedtime cortisol | Normal | 0.017 (−0.642, 0.676) | −0.003 (−0.67, 0.66) |
| IFG | 0.247 (−0.777, 1.27) | 0.187 (−0.844, 1.218) | |
| Diabetes | 0.636 (−0.561, 1.833) | 0.651 (−0.549, 1.852) | |
| P-value for interaction | 0.6577 | 0.6365 | |
Model 0: adjust for age, sex, race/ethnicity.
Model 1: adjust for age, sex, race/ethnicity, physical activity, smoking, CES-D, BMI.
Interpretation = Betalog-cortisol feature × ln(1.01), β-coefficient interpretation: one percent increase in the cortisol features increases (or decreases) the Avg. annual Δglucose by (β/100) units.
Note: Bold values signifies p < 0.05.
4. Discussion
In this contemporary, longitudinal study of multi-ethnic adults, a greater annual percent increase in wake-up cortisol, total AUC, and flatter overall decline slope was associated with an annual percent increase in FG among participants with diabetes. Among normoglycemic individuals, a higher annualized percent change in FG was associated with lower bedtime cortisol, but not with any other cortisol curve features. Among participants with diabetes, baseline flatter cortisol decline slope was positively associated with changes in fasting glucose. All of these associations persisted following adjustment for important confounders.
4.1. Association of change in cortisol features with change in FG over 6 years
Among participants with diabetes, a higher average of annual changes in wake-up cortisol, total AUC cortisol, and flatter overall decline slope were associated with greater annual changes in FG. No significant associations between average change in cortisol curve features and average annual change in FG were observed in those with NFG and IFG.
Among all the cortisol curve features examined, the AUC is thought to reflect daily cortisol exposure as previously described in MESA (Joseph et al., 2015). Our finding may be explained by the physiology of cortisol metabolism. A major function of cortisol is to raise glucose through gluconeogenesis and decrease insulin secretion (Joseph et al., 2015). We previously reported a cross-sectional association of higher total cortisol AUC in women with diabetes compared to women without diabetes, suggesting a role of elevated cortisol in contributing to hyperglycemia (Champaneri et al., 2012). The current study used longitudinal data over 6 years to assess the association of change in cortisol curve features with change in FG; therefore, this finding suggests a temporal relationship between cortisol levels and longitudinal changes in FG. The difference between the results of the delta-delta analysis (Analysis A) and Analyses B and C may be due to Analyses B and C examining the association of annualized FG with cortisol curve features and cortisol curve features with change in glucose, in which either cortisol as an exposure or an outcome was measured at only one time point. Thus, these two analyses had cross-sectional components; whereas, in Analysis A, both FG and cortisol features were examined at two time points.
Our finding that the flattening of the overall decline slope is associated with positive changes in average FG in participants with diabetes is concordant with previous cross-sectional analyses. Joseph et al. in the MESA Stress II cohort found that a flatter overall decline slope was associated with a higher HbA1c among those with diabetes but not among normoglycemic participants (Joseph et al., 2015). Among normoglycemic African Americans in the Jackson Heart Study, a 100 % increase in morning serum cortisol was associated with 2.7 mg/dL higher FPG. Among those with diabetes in the same cohort, a 100 % increase in morning serum cortisol was associated with 23.6 mg/dL higher FPG, an 8.74-fold difference between groups (Ortiz et al., 2019). The findings are also consistent with the significant positive association of cortisol with change in annualized FG in the diabetes group in Analysis C and clinical studies of cortisol in diabetes (Cameron, 1984; Hudson et al., 1984; Cameron et al., 1987; Roy et al., 1990). There are two proposed mechanisms for the findings. First, greater cortisol burden or exposure, even within the normal range, promotes hyperglycemia which subsequently promotes HPA-axis dysfunction, leading to a vicious cycle of cortisol dysregulation and a less dynamic diurnal cortisol profile (Joseph and Golden, 2017). Second, activation of the HPA axis with greater cortisol burden or exposure due to inflammatory, traumatic, or psychological stresses and/or depression which are more prevalent in diabetes (Roy et al., 1987; Chrousos, 1995; Tsigos and Chrousos, 2002; Kyrou et al., 2006; Mezuk et al., 2008; Joseph and Golden, 2017). Diurnal cortisol slope changes can be an indicator of stress-related alterations with acute and chronic stress exposures being linked to a flatter cortisol decline (Adam and Kumari, 2009; Miller et al., 2002; Steffen et al., 2003; Suglia et al., 2010). These two mechanisms may function together or in parallel. The changes seen with cortisol curve features and glucose support an increasingly important role of cortisol dysregulation impacting glucose metabolism in diabetes.
4.2. Association of prior annual percent change in FG with diurnal cortisol features at MESA Stress I
Our data reveals an inverse association of prior annual percent change in FG with bedtime cortisol among normoglycemic participants, which remained significant following adjustment for multiple confounding variables, including BMI. To our knowledge, this association is a novel finding and has not been explored in prior studies (Joseph and Golden, 2017). In fact, most studies have focused on cross-sectional measures of glycemia or long-term incident diabetes (Joseph and Golden, 2017). Joseph et al. and others did not find a cross-sectional association of bedtime cortisol with HbA1C in non-diabetic individuals (Joseph et al., 2015). The discrepancy between this prior study versus the current study is likely due to differences in study design. The prior study was cross-sectional with a single HbA1c measure that reflected glycemia over the prior 3 months; however, in the current study, fasting glucose was evaluated over several years prior to the cortisol measure. The association suggests that increases in FG among normoglycemic participants may lead to lower bedtime cortisol. Further research is necessary to confirm this association and determine potential mechanisms.
4.3. Association of baseline cortisol features with subsequent annual percent change in FG over 6 years
We found no significant associations between baseline cortisol curve features and annual percent changes in FG among all participants. However, when individuals with NFG, IFG, or diabetes were examined separately, there was effect modification by diabetes status (p < 0.10). Among those with diabetes, a significant positive association of the overall decline slope (flatter) with subsequent annual percent change in FG existed. Previously, Joseph et al. published data showing that a one-unit increase in overall decline slope was associated with a 54.7 % higher HbA1c among those with diabetes (Joseph et al., 2015). Consistent with our study, no associations or trends were observed among normoglycemic or IFG individuals. These findings suggest that there may be a significant association of flatter overall decline slope with worsening glycemia in individuals with diabetes.
4.4. Limitation and strengths
The MESA Stress study is one of few population-based cohort studies with well-characterized, rigorous longitudinal data on diurnal cortisol curve characteristics, FG and multiple covariates from a racially diverse sample of adults in middle to older age. These strengths enabled us to perform longitudinal analyses of various cortisol curve features with FG over time. However, our study should be interpreted in light of some limitations. First, due to the observational nature of the study, causality cannot be inferred. Second, the average ages of participants in analyses 1–3 were 66.6, 64.4, and 63.7. Thus, the results may not be generalizable to younger populations. Third, we did not collect cortisol throughout the night; thus, we are unable to evaluate a full 24-hour diurnal cortisol cycle. Fourth, the study was limited by the small sample size of individuals with cortisol levels collected longitudinally. Finally, the statistical associations were interpreted without correction for multiple comparisons. Typical multiple comparison corrections assume that tests are independent and are too conservative for correlated hypotheses as we have in this study. Hence, some caution is warranted in the interpretation of our study results.
5. Conclusions
In summary, this is the first study to explore the longitudinal change in cortisol features with change in FG among individuals with NFG, IFG and diabetes. Changes in wake-up cortisol, total AUC cortisol, and overall decline slope were positively associated with changes in FG among participants with diabetes over 6 years. Second, greater changes in FG over 6 years were longitudinally associated with lower bedtime cortisol among those with NFG. Third, baseline overall decline slope was positively associated with change in FG among those with diabetes. These findings support temporality-favoring cortisol impacting changes in glucose among individuals with diabetes. These relationships were independent of BMI, suggesting that glucocorticoids impact glucose metabolism directly through effects on insulin secretion and insulin signaling (Geer et al., 2014). Future studies are needed to examine these relationships with detailed clinical and metabolic phenotyping to determine the mechanisms by which changes in cortisol secretion disrupt glucose metabolism and to develop future novel targets for treatment of type 2 diabetes.
Supplementary Material
Acknowledgements
The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions.
Funding
This research was supported by contracts HHSN268201500003I, through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from The National Center for Research Resources. MESA Stress Study was supported by RO1 HL10161-01A1 and R21 DA024273 (PI: Dr. Diez Roux). Dr. Joshua J. Joseph was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK117041) and The Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program ID#76236. Dr. Guy Brock was partially funded by National Institute of Health grant UL1TR002733from the National Center for Advancing Translational Sciences.
Footnotes
Declaration of Competing Interest
The authors have no relevant conflicts of interest to disclose.
Appendix A. Supplementary data
Supplementary material related to this article can be found in the online version at https://doi.org/10.1016/j.psyneuen.2020.104698.
References
- Adam EK, Kumari M, 2009. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34, 1423–1436. 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM, 1999. Moderate physical activity patterns of minority women: the cross-cultural activity participation study. J. Womens Health Gend. Based Med. 8, 805–813. 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association, 2009. Standards of medical care in diabetes—2010.Diabetes Care 33, S11–S61. 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M, 2007. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 92, 819–824. 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR, 2008. The association between physical activity and subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 169, 444–454. 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild DE, 2002. Multi-ethnic study of atherosclerosis: objectives and design. Am. J. Epidemiol. 156, 871–881. 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Convit A, 2009. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 34, 815–821. 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, 1984. Hypothalamic-pituitary-adrenocortical activity in patients with diabetes mellitus. Arch. Gen. Psychiatry 41, 1090. 10.1001/archpsyc.1983.01790220080013. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Thomas B, Tiongco D, Hariharan M, Greden JF, 1987. Hypercortisolism in diabetes mellitus. Diabetes Care 10, 662–664. 10.2337/diacare.10.5.662. [DOI] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Roux AD, Golden SH, 2012. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the multi-ethnic study of atherosclerosis. Metabolism 61, 986–995. 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, 1995. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 332, 1351–1362. 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Geer EB, Islam J, Buettner C, 2014. Mechanisms of glucocorticoid-induced insulin resistance. Endocrinol. Metab. Clin. North Am. 43, 75–102. 10.1016/j.ecl.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RA, Steptoe A, Kumari M, 2014. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J. Clin. Endocrinol. Metab. 99, 4625–4631. 10.1210/jc.2014-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RA, Kivimäki M, Kumari M, Steptoe A, 2016. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the Whitehall II cohort study. J. Clin. Endocrinol. Metab. 101, 619–625. 10.1210/jc.2015-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hudson MS, Rothschild AJ, Vignati L, Schatzberg AF, Melby JC, 1984. Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch. Gen. Psychiatry 41, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Joseph JJ, Golden SH, 2017. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 1391, 20–34. 10.1111/nyas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Wang X, Spanakis E, Seeman T, Wand G, Needham B, Golden SH, 2015. Diurnal salivary cortisol, glycemia and insulin resistance: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 62, 327–335. 10.1016/j.psyneuen.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Wang X, Diez Roux AV, Sanchez BN, Seeman TE, Needham BL, Golden SH, 2017. Antecedent longitudinal changes in body mass index are associated with diurnal cortisol curve features: the multi-ethnic study of atherosclerosis. Metabolism 68, 95–107. 10.1016/j.metabol.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I, Chrousos GP, Tsigos C, 2006. Stress, visceral obesity, and metabolic complications. Ann. N. Y. Acad. Sci. 1083, 77–110. 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- LaMonte MJ, Durstine JL, Addy CL, Irwin ML, Ainsworth BE, 2001. Physical activity, physical fitness, and Framingham 10-year risk score: the cross-cultural activity participation study. J. Cardpulm. Rehabil. 21, 63–70. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Hummel J, Fademrecht C, Krumm B, Kühner C, Deuschle M, Ladwig K-H, Meisinger C, Wichmann H-E, Lutz H, Breivogel B, 2011. Flattened circadian cortisol rhythm in type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 119, 573–575. 10.1055/s-0031-1275288. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, Golden SH, 2008. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 31, 2383–2390. 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK, 2002. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 21, 531–541. 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Kluwe B, Odei JB, Echouffo Tcheugui JB, Sims M, Kalyani RR, Bertoni AG, Golden SH, Joseph JJ, 2019. The association of morning serum cortisol with glucose metabolism and diabetes: the Jackson Heart Study. Psychoneuroendocrinology 103, 25–32. 10.1016/j.psyneuen.2018.12.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS, Locke BZ, 2008. Center for epidemiologic studies depression scale (CES-D). Handbook of Psychiatric Measures, 2nd ed. American Psychiatric Publishing, Inc., Washington, DC. [Google Scholar]
- Roy A, Pickar D, Paul S, Doran A, Chrousos GP, Gold PW, 1987. CSF corticotropin-releasing hormone in depressed patients and normal control subjects. Am. J. Psychiatry 144, 641–645. 10.1176/ajp.144.5.641. [DOI] [PubMed] [Google Scholar]
- Roy M, Collier B, Roy A, 1990. Hypothalamic-pituitary-adrenal axis dysregulation among diabetic outpatients. Psychiatry Res. 31, 31–37. 10.1016/0165-1781(90)90106-F. [DOI] [PubMed] [Google Scholar]
- Spanakis EK, Wang X, Sánchez BN, Diez Roux AV, Needham BL, Wand GS, Seeman T, Golden SH, 2016. Lack of significant association between type 2 diabetes mellitus with longitudinal change in diurnal salivary cortisol: the multiethnic study of atherosclerosis. Endocrine. 10.1007/s12020-016-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen PR, McNeilly M, Anderson N, Sherwood A, 2003. Effects of perceived racism and anger inhibition on ambulatory blood pressure in african americans. Psychosom. Med. 65, 746–750. 10.1097/01.PSY.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ, 2010. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol. Trauma Theory Res. Pract. Policy 2, 326–334. 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP, 2002. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 53, 865–871. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Young RJ, White A, 1993. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. J. Clin. Endocrinol. Metab. 76, 554–558. 10.1210/jcem.76.3.8383141. [DOI] [PubMed] [Google Scholar]
- Wang X, Sánchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, Seeman TE, Diez Roux AV, 2014. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology 49, 310–320. 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh KC, Kwan KC, 1978. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J. Pharmacokinet. Biopharm. 6, 79–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.