Abstract
Introduction
Obesity and higher BMI is one of the leading comorbidities to increase the risk of COVID-19 severity. This paper presents a systematic review and meta-analysis estimating the effects of overweight and obesity on COVID-19 disease severity.
Method
Two electronic databases (Medline and Cochrane library) and one grey literature database (Grey Literature Report) were searched. The risks of bias of the selected studies were assessed by using the Navigation Guide method for human data. Both random and fixed effect meta-analyses were determined using Review Manager (RevMan) software version 5.4.
Results
After initial screening, 12 studies were fulfilled the eligibility criteria, comprising a total of 405359 patients, and included in the systematic review. The pooled risk of COVID-19 severity was 1.31 times higher based on both fixed and random effect model among those overweight patients, I2 0% and 2.09 and 2.41 times higher based on fixed and random effect respectively among obese patients, I2 42% compared to healthy individuals.
Conclusion
Overweight and obesity are found to be risk factors for disease severity of COVID-19 patients. However, further assessment of metabolic parameters is required to estimate the risk factors of COVID-19 patients and understanding the mechanism between COVID-19 and body mass index.
Keywords: COVID-19, Overweight, Obesity, BMI
1. Introduction
Coronavirus disease 2019 (COVID-2019)—caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus—was declared a pandemic by the World Health Organization on March 11, 2020 (Sohrabi et al., 2020). As of February 11, 2021, COVID-19 has infected almost 107 million people worldwide, with a death toll of over 2.3 million (WHO, 2021). Previously, two highly pathogenic Coronaviruses resulted in outbreaks of a severe acute respiratory syndrome (SARS) in 2003 in Guangdong province, China, and the Middle East respiratory syndrome (MERS) in Middle Eastern countries in 2012 (Assiri et al., 2013; Drosten et al., 2003; Zaki et al., 2012; Zhong et al., 2003). Multiple risk factors are associated with incidence and mortality in COVID-19 patients (Alam et al., 2021). An increasing body of data suggests that individuals with diabetes mellitus (Morgan et al., 2010), hypertension, and severe obesity (BMI ≥ 40 kg/m2) are more likely to be infected and are at a higher risk for complications and death from COVID-19 (Centers for Disease Control and Prevention (CDC), 2020; Bhatraju et al., 2020; Guan et al., 2020; Kassir, 2020; Onder et al., 2020; Yang et al., 2020a; Zhou et al., 2020). Many countries mentioned body mass index (BMI) as a clinical risk factor of COVID-19, such as China (Li et al., 2020), Italy (Grasselli et al., 2020), United States (Bhatraju et al., 2020), as the immunity system plays a vital role in obesity-induced adipose tissue inflammation (Kassir, 2020). Emerging literature suggested that adults with obesity under the age of 60 are more likely to be hospitalized (Zhang et al., 2020). The prevalence of obesity among adults is increasing day by day due to insufficient physical activities. A previous study showed a strong correlation between obesity and complications of viral infections (influenza virus, SARS, and MERS) (Muniyappa and Gubbi, 2020). Many studies found that excessive weight gain ≥18 kg may increase the risk of developing community-acquired pneumonia (Morgan et al., 2010; Louie et al., 2011). Severe obesity might increase the duration of hospital stay and the case fatality rate (Zhou et al., 2020; Deng et al., 2020a). However, two earlier reports suggested no difference in body mass index (BMI) between severe and non-severe groups (Zhang et al., 2020; EL‐Arabey and Abdalla, 2020). Although several studies addressed the impact of the body mass index (BMI) on COVID-19, a definite conclusion has not been drawn yet. Hence, this meta-analysis was conducted to elucidate the relationship between obesity and COVID-19 by searching existing literature.
2. Methods
2.1. Literature search
We searched two electronic databases: MEDLINE (on October 20, 2020), Cochrane library (on October 21, 2020), and one grey literature database: Grey Literature Report (on October 21, 2020). Searches were carried out following an appropriate search strategy in English. We searched the literature using the following keywords: overweight, obesity, body mass index, respiratory disease, coronavirus, COVID-19. Manual searching was also performed to identify potentially eligible studies.
2.2. Study selection
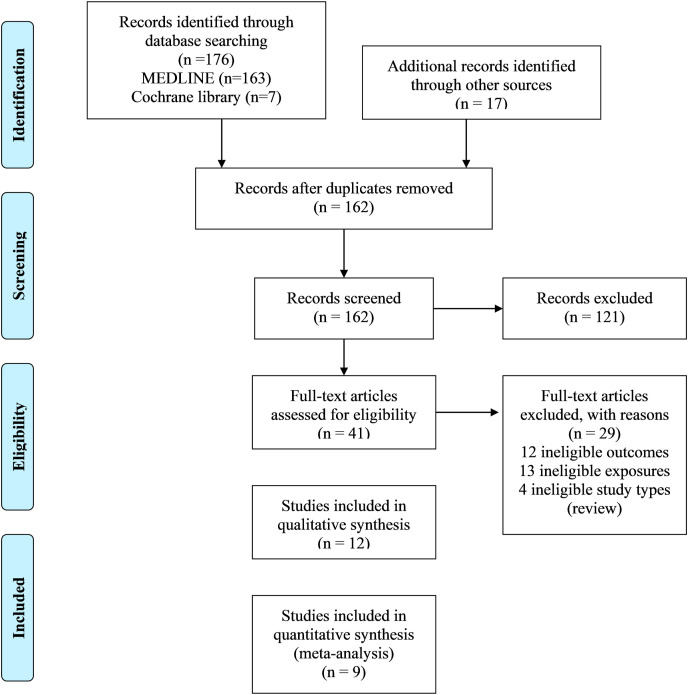
All articles found in the searches were downloaded, and duplicate articles were identified and excluded. Two independent authors screened the titles and abstracts for finding duplicates and then screened the full texts to select the eligible articles. If there were any disagreements between the review authors, a third author's option was considered to reach a decision. Following the PRISMA guideline, the study selection process is presented in a flow chart (Fig. 1 ).
Fig. 1.
The flow chart of searching and selecting studies based on selected criteria for systematic review and meta-analysis.figure.
2.3. Eligibility criteria
PECO definitions are described below:
-
•
Population: We included all studies of people aged (≥15 years) and reported positive for the presence of coronavirus in their bodies by the RT-PCR technique. Studies that measured the body mass index (BMI) were included with standard procedure.
-
•
Exposures: Studies that defined overweight and obesity with standard definition were included.
-
•
Comparators: Healthy participants with optimum BMI were used as a comparator. All other comparators were excluded.
-
•
Outcomes: Severity of disease was used as an outcome in this systematic review. Here, the term “Severity” is defined as the impact of COVID-19 on fatality, utilization of health care resources such as increase of hospital stay, ventilation, other services, and comorbidities (Prochaska et al., 2013).
2.4. Types of study
We included studies that measured the effect of overweight and obesity on COVID-19 disease severity. Eligible studies were randomized control trials, cohort studies (both prospective and retrospective), case-control studies. Records published only in the English language were considered. We have included both published and unpublished studies. Studies conducted using unethical practices were excluded.
2.5. Types of effect measures
We included measures of the relative effect of overweight and obesity on the severity of disease (prevalence and incidence), compared with the patient with optimum BMI. We included relative effect measures such as RRs, ORs, and Hazard ratios. Some of our studies were retrospective case-control studies for which RR could not be calculated. Hence, we had to recalculate the RR and HR of other studies into OR (Supplementary Table 1). If a study presented estimates for effect from two or more alternative models that had been adjusted for different variables, then we systematically prioritized the estimate from the model that provided information on the relevant confounders or mediators, at least the core variables: age, sex, and socioeconomic position. We prioritized estimates from models adjusted for more potential confounders over those from models adjusted for fewer. For example, if a study presents estimates from a crude, unadjusted model (Model A), a model adjusted for one potential confounder (e.g., age; Model B) and a model adjusted for two potential confounders (e.g., age and sex; Model C), then we prioritized the estimate from Model C.
2.6. Data extraction
Two independent reviewers extracted the data on study characters (study authors, study country, population size, study year, exposure, and outcome), study design, and risk of bias (including source population representation, blinding, exposure assessment, outcome assessment, confounding, incomplete outcome data, selective outcome reporting, conflict of interest and other sources of bias).
2.7. Risk of bias assessment
There is no standard method of assessing the risk of bias of selected studies for the systematic review. The risk of bias of this review was assessed by nine risk factors of bias included in the Navigation Guide method for human data. These were: (i) source population representation; (ii) blinding; (iii) exposure assessment; (iv) outcome assessment; (v) confounding; (vi) incomplete outcome data; (vii) selective outcome reporting; (viii) conflict of interest; and other sources of bias. The ratings for all domains were: “low,”; “unclear,” and “high.” Two independent reviewers assessed the risk of bias of selected studies. When there is a disagreement between the two reviewers, a third reviewer's option was considered. Funnel plots were generated to assess the potential concerns on publication bias (Supplementary figure 1-4).
2.8. Statistical analysis
We assessed heterogeneity by reporting the I 2 (% residual variation due to heterogeneity) and tau2 (method of moments estimates of between-study variance) of the pooled estimate. As to account for cross-study heterogeneity and check for robustness and potential outliers, both random effect and fixed-effect models were used to measure the relationship between obesity and COVID-19 disease severity. The 95% confidence interval has been reported in a pooled analysis. All analysis was done by using Review Manager (RevMan) software version 5.4.
3. Result
3.1. Study selection
A total of 193 individual studies were identified in our searches. Twelve studies fulfilled our eligibility criteria and were included in the systematic review (Fig. 1). Of the 12 included studies (Cai et al., 2020; Deng et al., 2020b; Hamer et al., 2020; Hu et al., 2020; Kalligeros et al., 2020; Klang et al., 2020; Lighter et al., 2020; McMichael et al., 2020; Petrilli et al., 2020; Richardson et al., 2020; Simonnet et al., 2020; Zheng et al., 2020), nine studies were included in the meta-analysis (Cai et al., 2020; Hamer et al., 2020; Hu et al., 2020; Kalligeros et al., 2020; Klang et al., 2020; Lighter et al., 2020; Petrilli et al., 2020; Simonnet et al., 2020; Zheng et al., 2020). The initial excluded 121 articles had little to no relevance to our present study. Some of the excluded articles were review articles, meta-analysis, and in some cases, complete literature was unavailable.
3.2. Characteristics of included studies
Most of the studies were cohort studies (7 studies), followed by case-control studies (4 studies) and one cross-sectional analysis. The total population of the included studies was 405359. The most commonly studied countries were the United States (6 studies) and China (4 studies). The comparator of most studied was BMI ≤25 kg/m2 (Table 1 ).
Table 1.
Result of systematic review (published and grey article).
| Source | Study design | Country | Population (n) | Median Age (IQR) | Sex | Used WHO interim guidance | Method of COVID-19 testing | Defined obesity | Other comorbidities measured | Findings | Definition of comparator |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Klang et al. | Retrospective cohort study | USA | 572 were young, and 2834 were old | NR | M/F | NR | Nasopharyngeal swab PCR test |
BMI | Coronary artery disease (CAD), Congestive heart failure (CHF), Hypertension (HTN), Diabetes mellitus, cancer, hyperlipidemia |
For both younger and the aged population who had a BMI above 40 kg/m2, was independently associated with mortality (p < 0.001) | BMI<30 |
| Hamer et al. | Cohort study | UK | 387,109 | NR | M/F | NR | RT-PCR | BMI | Diabetes, hypertension, cardiovascular disease | The relative risk ratio was higher among obese people with COVID-19 compared with a healthy weight. | BMI<25 |
| Simonnet et al. | Retrospective study | France | 124 | 60 (51–70) | M/F | Yes | Real-time reverse transcriptase–PCR | BMI | Diabetes, hypertension, dyslipidemia | Overweight and obesity were significantly more frequent among SARS-CoV-2 participants, and the requirement of IMV was significantly higher among obese and overweight participant | BMI<25 |
| Hu et al. | Retrospective study | China | 323 | 61 | M/F | Yes | RT-PCR, CT | BMI | Smoking, diabetes, critical disease designation, hypertension, WBC count, neutrophil count | BMI showed no significant effects on patients outcome (p > 0.05) | BMI<25 |
| Kalligeros et al. | Retrospective cohort study | USA | 103 | 60 (50–72) | M/F | NR | Reverse transcriptase–PCR assay | BMI | Hypertension, diabetes, heart disease | Admission to ICU and requirement of IMV were significantly associated with obesity and severe obesity | BMI<25 |
| McMichael et al. | Case report | USA | 167 | 72 | M/F | NR | rRT-PCR | NR | Hypertension, cardiac disease, renal disease, diabetes mellitus, cancer, liver disease, pulmonary disease | Most of the facility residents had chronic health conditions with obesity | NR |
| Richardson et al. | Case series | USA | 5700 | 63 (52–75) | M/F | NR | Nasopharyngeal swab PCR test |
BMI | Hypertension, diabetes, cancer, cardiovascular disease, liver disease, kidney disease, asthma | Obesity was identified as a common comorbidities | NR |
| Cai et al. | Case series | China | 383 | NR | M/F | Yes | Real-time reverse transcription PCR method | BMI | Diabetes, hypertension, cardiovascular disease, liver disease, cancer | The risk of developing severe COVID-19 was 1.84 times and 3.40 times higher among overweight and obese patients, respectively, especially in men. | BMI: 18.5–23.9 |
| Zheng et al. | NR | China | 214 | NR | M/F | NR | Real-time reverse transcription PCR method | BMI | T2D, hypertension, dyslipidemia | The presence of obesity with metabolic associated fatty liver disease was significantly associated with the increased risk of severe COVID-19 disease | BMI<25 |
| Deng et al. | Retrospective study | China | 112 | 65 (49–78) | M/F | Yes | RT-PCR test | BMI | Hypertension, diabetes, coronary heart disease, atrial fibrillation | Body mass index was not significantly associated with the disease severity of COVID-19 patients | NR |
| Petrilli et al. (unpublished) | Cross-sectional study | USA | 4103 | 52 (36–65) | M/F | NR | Real-time RT-PCR | BMI | Diabetes, cancer, coronary kidney disease, coronary artery disease | BMI of the patients was significantly associated with hospitalization. | BMI:<30 |
| Lighter et al. | Retrospective study | USA | 3615 | NR | M/F | NR | PCR | BMI | None | Higher BMI(≥30) and age<60 patients had high risk of admission in acute and critical care than lower BMI patients. | BMI<30 |
Nine studies reported the relation between COVID-19 disease severity with overweight and obesity (Table 3 ).
Table 3.
Odds ratio of selective studies for meta-analysis.
| Klang et al. | Simonnet et al. | Kalligeros et al. | Zheng et al. | Hu et al. | Cai et al. | Hamer et al. | Lighter et al. | Petrilli et al. | |
|---|---|---|---|---|---|---|---|---|---|
| Overweight | 1.1 (0.5–2.3) | 1.69 (0.52–5.48) | 2.27 (0.59–8.83) | – | 0.65 (0.19–2.23) | 1.74 (1.03–2.93) | 1.32 (1.09–1.60) | 1.1 (0.8–1.7) | 1.38 (1.03–1.85) |
| Obese | 5.1 (2.3–11.1) | 7.36 (1.63–33.14) | 5.39 (1.13–25.64) | 6.32 (1.16–34.54) | 2.86 (0.79–10.31) | 2.69 (1.31–5.52) | 1.97 (1.61–2.42) | 1.5 (0.9–2.3) | 1.73 (1.03–2.90) |
*Reference: Person with a normal BMI (18.5–24.9 wt/m.2.
3.3. Risk of bias at individual study level
The risks of bias rating for each domain for all 12 studies for this outcome are presented in Table 2. Our symmetrical funnel plots depict that our study is not prone to publication or reporting bias (Supplementary figure 1-4).
Table 2.
Risk of bias assessment.
| Klang et al. | Hamer et al. | Simonnet et al. | Hu et al. | Kalligeros et al. | McMichael et al. | Richardson et al. | Cai et al. | Zheng et al. | Deng et al. | Petrilli et al. | Lighter et al. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Are the study group at risk of not representing their source populations in a manner that might introduce selection bias? | Unclear | High | Unclear | Low | Low | Unclear | Low | Low | Unclear | Low | Low | Low |
| Was knowledge of the group assignments adequately prevented (i.e., blinded or masked) during the study, potentially leading to the subjective measurement of either exposure or outcome? | Unclear | Unclear | Low | Low | Low | Low | Low | Low | Low | Low | Unclear | Low |
| Were exposure assessment methods lacking accuracy? | Low | Low | Low | Low | Low | Low | Low | Unclear | Low | Low | Low | Low |
| Were outcome assessment methods lacking accuracy? | Low | Low | Low | Low | Low | Low | Low | Unclear | Low | Unclear | Low | Low |
| Was potential confounding inadequately incorporated? | Unclear | Low | Low | Unclear | Low | High | Unclear | Low | Low | Unclear | Unclear | Unclear |
| Were incomplete outcome data inadequately addressed? | Low | Low | Low | Unclear | Low | Low | Low | Low | Low | Unclear | Low | Low |
| Does the study report appear to have selective outcome reporting | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Did the study receive any support from a company, study author, or other entity having a financial interest in any of the exposures studied? | Low | Low | Low | Low | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Did the study appear to have problems that could put it at risk of bias? | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Total score (Extra 2 points for peer-reviewed article) | 17 | 17 | 19 | 18 | 20 | 16 | 19 | 18 | 19 | 17 | 16 | 17 |
3.4. Measured outcome
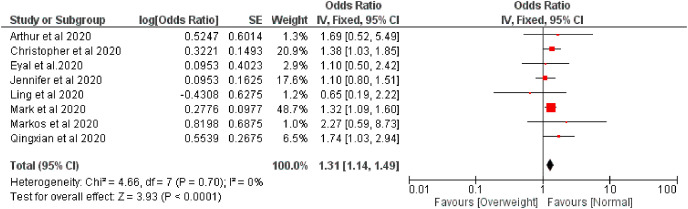
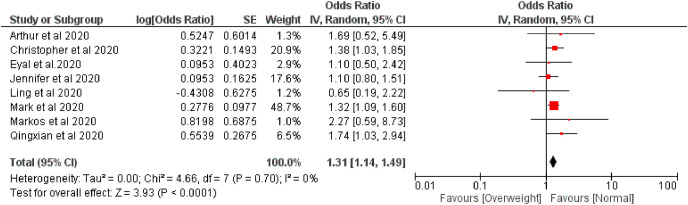
The effect of overweight on disease severity of COVID-19 patients was measured comparing with normal body weight. The meta-analysis of selected nine studies showed that the pooled risk of disease severity was 1.31 times higher based on both fixed and random effect model among those patients who were overweight, I 2 0% (Fig. 2, Fig. 3 ).
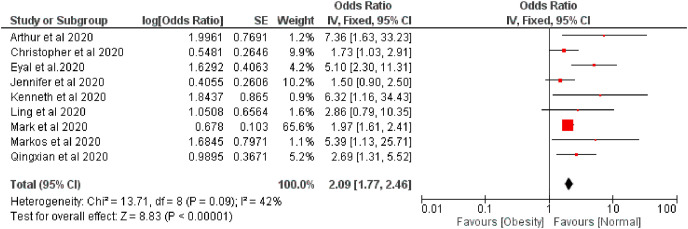
Fig. 2.
Forest plot illustrating the Fixed effect model of the association between overweight and COVID-19 severity.
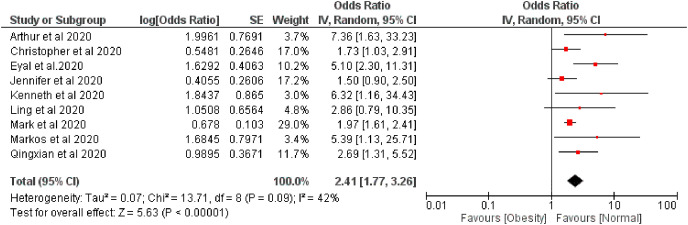
Fig. 3.
Forest plot illustrating the Random effect model of the association between overweight and COVID-19 severity.
The pooled risk of disease severity was 2.09 and 2.41 higher based on fixed and random effect respectively among obese patients compared with regular bodyweight patients, I 2 42% (Fig. 4, Fig. 5 ).
Fig. 4.
Forest plot illustrating the Fixed effect model of the association between obesity and COVID-19 severity.
Fig. 5.
Forest plot illustrating the Random effect model of the association between obesity and COVID-19 severity.
4. Discussion
Of the 41 studies examined, some studies failed to find any association between BMI and COVID-19 (Deng et al., 2020b; Hu et al., 2020), and some studies did not measure BMI as a risk factor of COVID-19. So finally, we included 12 studies that covered all the selection criteria. The present study has accumulated all the findings related to COVID-19 and BMI. Interestingly, it was found that BMI is a risk factor for COVID-19 patients. Overweight patients were 1.31 times higher at the risk of disease severity of COVID-19, and obese patients were 2.09 and 2.41 times higher susceptible to the severity of COVID-19 according to fixed and random effect model, respectively. Our study result is consistent with what has been found in previous reports (Du et al., 2020; Földi et al., 2020; Malik et al., 2020; Pranata et al., 2020; Yang et al., 2020b). A meta-analysis showed that obese people are severely affected by COVID-19 than non-obese people (OR: 2.31, 95% CI: 1.3–4.12) (Yang et al., 2021), which is similar to our present study. Another meta-analysis showed that obesity increased the severity of COVID-19 patients, and obesity is considered a significant risk factor (Földi et al., 2020). Various studies previously documented for different viral pathogens, including influenza, that obesity was a substantial risk factor for disease severity (Kwong et al., 2011; Moser et al., 2019; Maccioni et al., 2018). During the 2009 H1N1 pandemic, it was found that the rates of hospitalization and deaths were higher among overweight and obese patients (Morgan et al., 2010).
Several parameters with overweight and obesity play a role in the disease severity of COVID-19. However, there is no exact mechanism that explains the contribution of overweight and obesity to severe COVID-19 outcomes. Nevertheless, obesity has adverse effects on lung function, diminishing forced expiratory volume and forced vital capacity (Sattar et al., 2020). It is also reported that respiratory physiology is changed by obesity with the decreased functional ability of the respiratory system (Parameswaran et al., 2006). Another study found that obesity impaired immune system surveillance and response (Huttunen and Syrjänen, 2013). Obesity was also found to weaken the respiratory function, gas exchange, lung volume, increase comorbidities (CVD, T2D, kidney disease), and metabolic risk (hypertension, insulin resistance, and dyslipidemia), which contributed to the disease severity of COVID-19 patients (Stefan et al., 2020). Some studies explained why obese people presented a worse clinical outcome than a typical patient. These studies concluded that overweight and obese people have a different innate and adaptive immune response and have higher leptin and lower adiponectin concentrations, which leads to dysregulation of immune response and contributes to worsening pathogenesis conditions (Andersen et al., 2016; Richard et al., 2017; Ouchi et al., 2011). Another study found that obesity reduced the activity of macrophages when an antigen is presented (Ahn et al., 2015). Obesity was also directly associated with basal inflammatory status characterized by higher circulating Interleukin 6 and C-reactive protein levels (Sattar et al., 2020). Obesity also impaired the adaptive immune system responses to the influenza virus (Green and Beck, 2017). It is crucial to understand and determine the relationship between obesity and COVID-19 to reduce the risk of developing severe COVID-19 illness. The lifestyle of people should be improved to lessen risk both in the current and subsequent waves of COVID-19.
The present study has some limitations. We only used articles that were published in the English language and had full-text availability. In few instances, we could not find full articles that were excluded from our study. Some of our studies were retrospective case-control studies; therefore, we could not calculate the RR for those studies. We had to recalculate the RR and HR of other studies into OR, which has a likelihood of overestimation. Since our study population is predominantly from China, the USA, UK, and France, it limits the opportunity to assess the universal scenario.
5. Conclusion
The study found that overweight and obesity to be potential risk factors for increased disease severity of COVID-19 patients. Nevertheless, further assessment of metabolic biomarkers is required to estimate the risk factors of COVID-19 patients and understanding the mechanism between COVID-19 and body mass index. Therefore, we recommend that additional attention be given to obese patients and other patients during this epidemic.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval was not required for this study.
Availability of data and material
The datasets generated during this study are available from the corresponding author on a reasonable request.
CRediT authorship contribution statement
Akibul Islam Chowdhury: Conceptualization, Formal analysis, Data curation, Writing – original draft. Mohammad Rahanur Alam: Conceptualization, Writing – original draft, Formal analysis, Data curation. Md. Fazley Rabbi: Funding acquisition, Writing – original draft. Tanjina Rahman: Funding acquisition, Writing – original draft. Sompa Reza: Funding acquisition, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to express our gratitude to the authors of the studies included in our study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obmed.2021.100340.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ahn S.Y., Sohn S.H., Lee S.Y., et al. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ. Toxicol. Pharmacol. 2015;40:924–930. doi: 10.1016/j.etap.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Alam M.R., Kabir M.R., Reza S. Comorbidities might be a risk factor for the incidence of COVID-19: evidence from a web-based survey. Prev. Med. Rep. 2021;21:101319. doi: 10.1016/j.pmedr.2021.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., McGeer A., Perl T.M., et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region—case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Chen F., Wang T., et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) People with certain medical conditions. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html
- Deng G., Yin M., Chen X., Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 2020;24:1–3. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Hu B., Zhang Y., et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Du Y., Lv Y., Zha W., Zhou N., Hong X. Association of Body mass index (BMI) with Critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. Metabolism. 2020;117:154373. doi: 10.1016/j.metabol.2020.154373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL‐Arabey A.A., Abdalla M. Metformin and COVID‐19: a novel deal of an old drug. J. Med. Virol. 2020;92:2293–2294. doi: 10.1002/jmv.25958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Földi M., Farkas N., Kiss S., et al. Obesity is a risk factor for developing critical condition in COVID‐19 patients: a systematic review and meta‐analysis. Obes. Rev. 2020;21 doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W.D., Beck M.A. Obesity impairs the adaptive immune response to influenza virus. Ann. Am. Thorac. Soc. 2017;14:S406–S409. doi: 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Kivimäki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav. Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Chen S., Fu Y., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin. Infect. Dis. 2020;71:2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen R., Syrjänen J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- Kalligeros M., Shehadeh F., Mylona E.K., et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassir R. Risk of COVID‐19 for patients with obesity. Obes. Rev. 2020;21 doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Severe obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity. 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong J.C., Campitelli M.A., Rosella L.C. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin. Infect. Dis. 2011;53:413–421. doi: 10.1093/cid/cir442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighter J., Phillips M., Hochman S., et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie J.K., Acosta M., Samuel M.C., et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin. Infect. Dis. 2011;52:301–312. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- Maccioni L., Weber S., Elgizouli M., et al. Obesity and risk of respiratory tract infections: results of an infection-diary based cohort study. BMC Publ. Health. 2018;18:271. doi: 10.1186/s12889-018-5172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik V.S., Ravindra K., Attri S.V., Bhadada S.K., Singh M. Higher body mass index is an important risk factor in COVID-19 patients: a systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2020;27:42115–42123. doi: 10.1007/s11356-020-10132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael T.M., Currie D.W., Clark S., et al. Epidemiology of covid-19 in a long-term care facility in king county, Washington. N. Engl. J. Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan O.W., Bramley A., Fowlkes A., et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A (H1N1) disease. PloS One. 2010;5 doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J.A.S., Galindo-Fraga A., Ortiz-Hernández A.A., et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir. Virus. 2019;13:3–9. doi: 10.1111/irv.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J. Am. Med. Assoc. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran K., Todd D.C., Soth M. Altered respiratory physiology in obesity. Canc. Res. J. 2006;13:203–210. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. MedRxiv. 2020 doi: 10.1101/2020.04.08.20057794. [DOI] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., et al. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2020:101178. doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J., Gellman M., Turner J. Springer; New York: 2013. Encyclopedia of Behavioral Medicine. [Google Scholar]
- Richard C., Wadowski M., Goruk S., Cameron L., Sharma A.M., Field C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care. 2017;5 doi: 10.1136/bmjdrc-2016-000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. J. Am. Med. Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., McInnes I.B., McMurray J.J. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi C., Alsafi Z., O'Neill N., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO Coronavirus disease (COVID-19) dashboard. 2021. https://covid19.who.int/
- Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:P91–P95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Tian C., Chen Y., Zhu C., Chi H., Li J. Obesity aggravates COVID‐19: an updated systematic review and meta‐analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hu J., Zhu C. Obesity aggravates COVID‐19: a systematic review and meta‐analysis. J. Med. Virol. 2021;93:257–261. doi: 10.1002/jmv.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A., van B.S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y., et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zheng K.I., Gao F., Wang X.B., et al. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N., Zheng B.J., Li Y.M., et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during this study are available from the corresponding author on a reasonable request.