Abstract
Sulfonamide (or sulphonamide) functional group chemistry (SN) forms the basis of several groups of drug. In vivo sulfonamides exhibit a range of pharmacological activities, such as anti-carbonic anhydrase and anti-t dihydropteroate synthetase allowing them to play a role in treating a diverse range of disease states such as diuresis, hypoglycemia, thyroiditis, inflammation, and glaucoma. Sulfamethazine (SMZ) is a commonly used sulphonamide drug in veterinary medicine that acts as an antibacterial compound to treat livestock diseases such as gastrointestinal and respiratory tract infections. Sulfadiazine (SDZ) is another frequently employed sulphonamide drug that is used in combination with the anti-malarial drug pyrimethamine to treat toxoplasmosis in warm-blooded animals. This study explores the research findings and the work behaviours of SN (SMZ and SDZ) drugs. The areas covered include SN drug structure, SN drug antibacterial activity, SN drug toxicity, and SN environmental toxicity.
Keywords: Sulfonamide, Sulfamethazine, Sulfadiazine, Toxicology, Environment, Bio-macromolecules
Introduction
Sulfonamides (SN) or sulfanilamides belong to an important class of synthetic antimicrobial drugs that are pharmacologically used as broad spectrum for the treatment of human and animal bacterial infections (Seydel 1968; Supuran et al. 2003). SN structures are organo-sulphur compounds containing the -SO2NH2 and/or -SO2NH- group and are characteristic of the existence of sulfanilamide group and a distinct 6- or 5-membered heterocyclic rings. SNs are not readily biodegradable and have potential to cause various unfavourable side effects including diseases of the digestive and respiratory tracts (Sultan 2015) (with some of the SN drug non-allergic reactions include diarrhoea, nausea, vomiting, dizziness, candidiasis, folate deficiency, and headaches (Mathews et al. 2015)). When used in large doses, SN drugs may cause a strong allergic reaction with two of the most serious being Stevens–Johnson syndrome and toxic epidermal necrolysis (Shah et al. 2018). The overall incidence of adverse drug reactions to sulfanamide allergy is approximately 3–8%, close to that seen for penicillin (Giles et al. 2019; Warrington et al. 2018). A key determinant feature of this allergic response involves substitution at the N4 arylamine group position such as is found in sulfamethoxazole, sulfasalazine and sulfadiazine (Dibbern and Montanaro 2008; Tilles 2001). Other SN drugs which do not contain the arylamine group tend not to induce the allergic response and may therefore be safely taken (Giles et al. 2019; Khan et al. 2019). As a result of this allergy effect, SNs are classified into two groups: (i) anti-bacterial sulfonamides (with an aromatic amine) and (ii) non-antibacterial sulphonamides (without an aromatic amine) (Igwe and Okoro 2014; Yousef et al. 2018; Zawodniak et al. 2010).
SN-derived drugs developed up till the present include sulfamethazine, sulfadiazine, sulfamethoxazole, sulfasalazine, sulfisoxazole, sulfamerazine, sulfadimethoxine, sulfafurazole, and sulphanilamide (“Antibacterial Agents, Sulfonamides” 1944; Hehui et al. 2021; Supuran 2017) (Table 1). Among these SN derivatives, the first to be developed in 1906 was sulphanilamide, although it was not used as an antimicrobial agent until the late 1930s (Ballentine 1981; Fernández-Villa et al. 2019). Sulfamethazine (SMZ) and sulfadiazine (SDZ) are among the derivatives of sulphonamides group of antibiotic drugs that contain the aromatic amine group. SMZ and SDZ are commonly used in veterinary medicine as an antibacterial compound to treat livestock diseases such as gastrointestinal and respiratory tract infections (Rama et al. 2017). SMZ has been used in animal feeds or feed additives to promote growth in animals (Awaisheh et al. 2019; Burbee et al. 1985; Chattopadhyay 2014; Dixon-Holland 1992). SDZ on the other hand is used primarily on the treatment of infection caused by the burn wounds (Banerjee et al. 2019; Dai et al. 2010; Hosseini et al. 2007). SDZ is also used in combination with the anti-malarial drug pyrimethamine to treat toxoplasmosis in mammals (Hossein Eshghia et al. 2011; Islam et al. 2013; Winters and Janney 2015). There are several reports about SN, SMZ, and SDZ that deal with its environmental effects, antibacterial effects, and its interactions with specific bio-macromolecules (Bendjeddou et al. 2016; Biošić et al. 2017; Chen et al. 2012; Genç et al. 2008a; Islam et al. 2016; Qadir et al. 2015). It is the intention of the present review article to critically assess these reports.
Table 1.
Summary of DFT study on SN, SMZ, and SDZ
| Sl no. | Molecule/Complex | Method | Basis set | Energy gap/Magnetic moment (μeff) | Year | Reference |
|---|---|---|---|---|---|---|
| 1 | Sulfonamide in gas and DMSO phase | DFT | B3LYP/6-31G (d, p) | 6.10 eV, 6.14 eV | 2014 | Boufas et al. (2014) |
| 2 | Sulfonamide moiety | Geometry optimization | M06-2X/6-311+G (d,p) |
0.27 a.u(singlet) 0.21 a.u(triplet) |
2018 | Ge et al. (2018) |
| 3 | Sulfonamide I and II | DFT | B3LYP/6-31G (d, p) | 5.38 eV, 5.33 eV | 2019 | Arshad et al. (2019) |
| 4 | Sulfadiazine | DFT | B3LYP/6-31G++(d,p) | - | 2009 | Ogruc-ildiz et al. (2009) |
| 5 | Sulfadiazine | DFT | B3LYP/6-31G (d, p) | 4.347 eV | 2019 | Dubey and Patel (2019) |
| 6 | Sulfadiazine Ru(II) and Rh(III) complexes | DFT | B3LYP/6-31G (d, p) | - | 2020 | Mansour and Radacki (2020) |
| 7 | Sulfamethazine Fe(III) complexes (binary and ternary) | TD-DFT | DFT/B3LYP |
6.05 μB 6.16 μB |
2014 | Mansour (2014) |
| 8 | Sulfamethazine Cu(II) | TD-DFT | DFT/B3LYP/LANL2DZ | 1.53 μB | 2015 | Mansour and Mohamed (2015) |
| 9 | Sulfamethazine | DFT | B3LYP/6-31+G(d,p) | 4.979 eV | 2015 | Won et al. (2015) |
| 10 | Sulfamethazine in water | DFT | B3LYP/6-31+G(d) | - | 2018 | Hazhir et al. (2018b) |
Structure and nomenclature
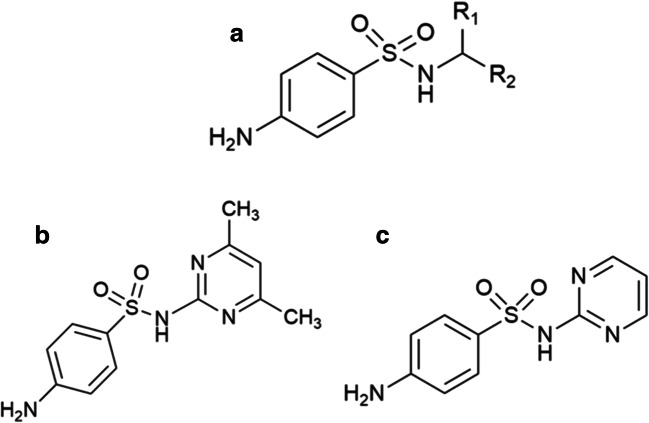
The typical structure of a tertiary SN involves a central sulfur atom, with two doubly bonded oxygens, that is also bonded to a nitrogen atom (existing as a substituted amine) and an aniline group (Fig. 1a) in which R1/R2 may also be hydrogen, alkyl, aryl, or hetero aryl groups. An alternative means of describing the prototypical SN drug structure is an organic compound consisting of aniline derivatized with a sulfonamide group (Pareek et al. 2013; Sonu et al. 2017). The difference in the derivative structure of SN (Fig. 1a) between SMZ and SDZ (Fig. 1 b and c) lies in the extra dimethyl group that is present in the 4th and 6th carbon of the pyridine ring. The IUPAC name of SN is 4-aminobenzenesulfonamide, and the two derivative drugs are 4-amino-N-(4, 6-dimethylpyrimidin-2-yl) benzene sulphonamide for SMZ and 4-amino-N-(pyrimidin-2-yl) benzene-1-sulphonamide for SDZ respectively (Robertson et al. 2020; “Sulfamethazine and Its Sodium Salt” 2001).
Fig. 1.
Chemical structures of a a generic tertiary sulfonamide (SN), b sulfamethazine (SMZ), and c sulfadiazine (SDZ)
Synthetic aspect
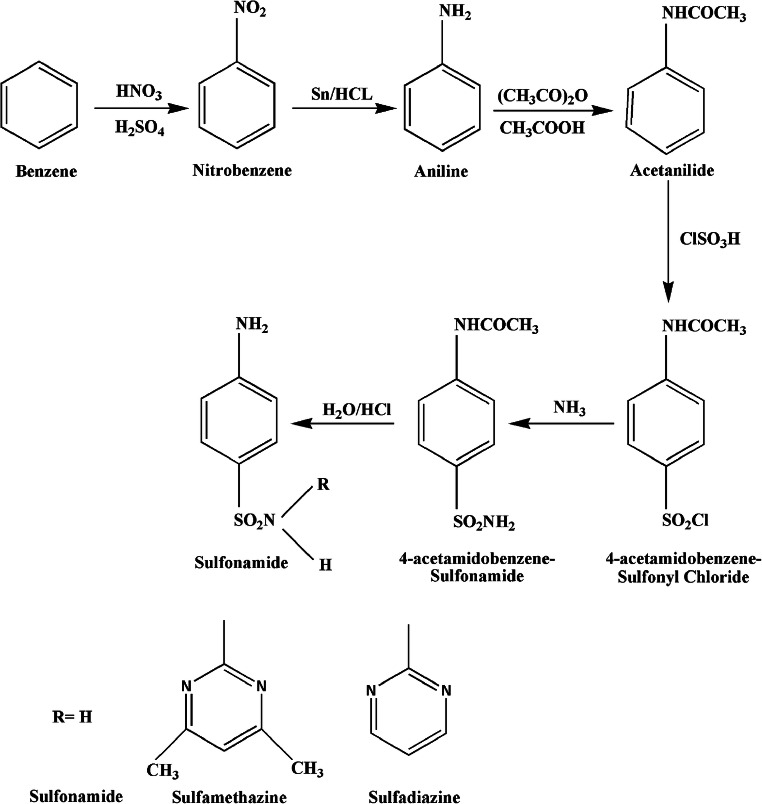
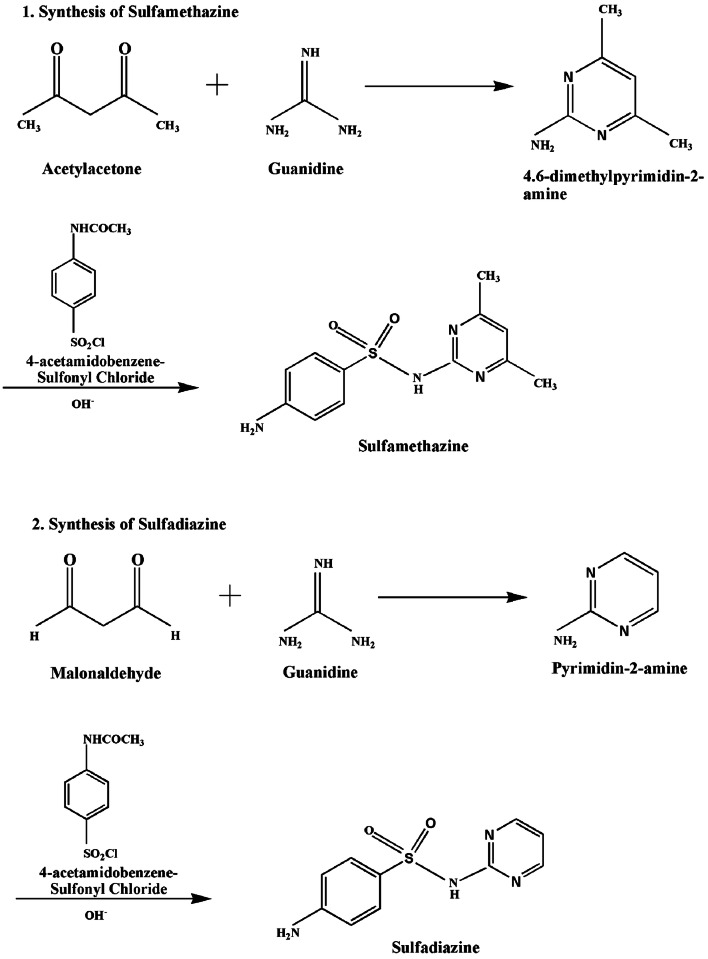
There are a number of published methods for the synthesis of sulfonamides in different research papers (Naredla and Klumpp 2013; Shah et al. 2018) yet the most frequent and common method involves a reaction of aliphatic or aromatic sulfonyl chloride with ammonia which produces a greater yield as compared with that of other methods (Bahrami et al. 2009; Dominique Guianvarc’h et al. 2004). The initial compound for the sulphonamide synthesis is benzene which follows six more steps to procure the product. Benzene undergoes nitration to give nitrobenzene which is then reduced by the reducing agent tin and hydrochloric acid to give anilinium ion and is further converted to aniline using sodium hydroxide. Acetanilide produced via acetylation in the aqueous medium then reacts with chlorosulfonic acid to give 4-acetamidobenzenesulfonyl chloride. The intermediate thus formed gives 4-acetamidobenzene sulphonamide in the presence of ammonia. The final step of the synthesis involves hydrolysis in acidic medium to form 4-aminobenzenesulfonamide (sulphanilamide). The schematic representation for the synthesis of sulfonamide drug is shown in Fig. 2 (Tacic et al. 2017). Further derivatives were synthesized using 4-acetamidobenzenesulfonyl chloride with 4,6-dimethylpyrimidin-2-amine (obtained from reacting pentane-2,4-diol with gaunidine) for SMZ (Lu and Rohani 2010; Ross and Plainfield 1968) and pyrimidine-2-amine (obtained from reacting malonaldehyde with gaunidine) for SDZ (Donizete et al. 2005; Ma et al. 2015; Shun-ichi Yamada et al. 1950) respectively, as shown in Fig. 3.
Fig. 2.
Schematic representation of the synthesis of sulphonamide in aqueous medium at room temperature (Tacic et al. 2017)
Fig. 3.
Schematic representation for the synthesis of sulfamethazine in acetone and pyridine (Banes 1974) and sulfadiazine in acetic acid solution (Abraham 1966; Shun-ichi Yamada et al. 1950)
Density functional theory study
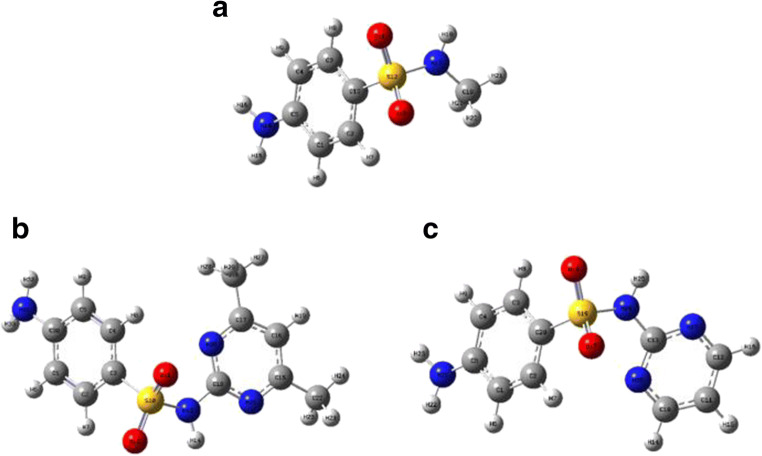
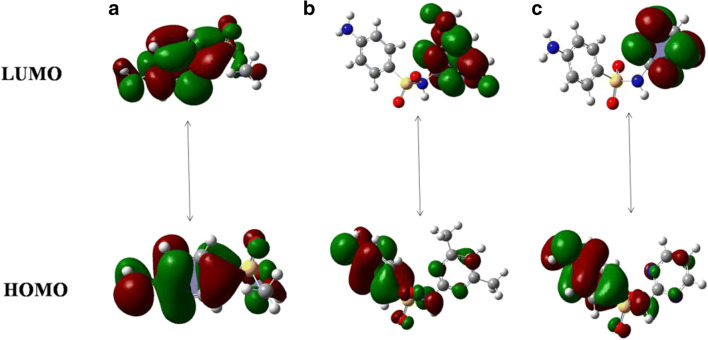
Density functional theory (DFT) is a computational method that is frequently employed for theoretical simulation of an organic compound’s electronic in structure (Karataş et al. 2017; Van Mourik et al. 2014; Verma 2018). The frontier molecular orbital (FMO) analysis is a useful property to determine molecular reactivity and electronic structure, as well as providing information on electronic transitions within molecules (Arshad et al. 2015). The chemical stability of molecules can be explained on the basis of energy gap between electronic transitions of HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) (Huang et al. 2017; Mathammal et al. 2013; Xu et al. 2020). A higher HOMO–LUMO energy gap reflects high kinetic stability and low chemical reactivity (stable towards oxidation and reduction reactions) (Farooq et al. 2019; Khan et al. 2018; Rasool et al. 2016). The calculated energy of the HOMO orbital relates to the ionization energy (I. E.), whereas the lower energy LUMO reflects the electron affinity (E. A.) (Ullah et al. 2015). Figures 4 and 5 describe the optimized molecular structure and the electronic transitions (HOMO-LUMO) of the drug SN and its derivatives SMZ and SDZ respectively. Some reports based on DFT simulation of SN, SMZ, and SDZ molecules describing the basis set used and their energy gap difference were reviewed with this information summarized in Table 1. The energy gap gives the relative idea of the stability with the molecule with SMZ being comparatively more stable than that of its parent molecule SN and its fellow derivative SDZ. The observed effective magnetic moment (μeff) values obtained for SMZ are also in the acceptable range for non-interacting magnetically diluted iron and copper complexes (Kato et al. 1964; Kohout and Krätsmár-Šmogrovič 1968).
Fig. 4.
DFT optimized structure of a sulphonamide, b sulfamethazine, and c sulfadiazine (Arshad et al. 2019; Hazhir et al. 2018a; Huschek et al. 2008).
Fig. 5.
DFT HOMO-LUMO energy levels of a sulphonamide, b sulfamethazine, and c sulfadiazine (Acar Selçuki et al. 2020; Krawczyk 2015; Mekala and Mathammal 2012).
Antibacterial activity
Sulphonamides are an important class of antibiotic drugs with a wide range of activity, being very effective against gram-positive and certain gram-negative bacteria (White and Cooper 2003). Some of the susceptible gram-negative bacteria include Klebsiella, Salmonella, Escherichia coli, and Enterobacter species; however, sulfonamides show no inhibitory activity (bacterial resistance) against Pseudomonas aeruginosa and Serratia species.(Lavanya 2017). Sulphonamides are utilized in the treatment of tonsillitis, septicemia, meningococcal meningitis, bacillary dysentery, and number of infections of urinary tract (Seneca 2015; Wiedemann et al. 2014). Sulfonamides also show inhibitory activity against some fungi (Pneumocystis carinii) and protozoa (Toxoplasma, Coccidia) (Chio et al. 1996; McFarland et al. 2016). There are many published reports showing antibacterial action by sulphonamide, sulfamethazine, and sulfadiazine drugs (Blanchard et al. 2016; Majewsky et al. 2014; Peng et al. 2015; Reddy et al. 2012; Tailor and Patel 2015; Ueda et al. 2020). SN and its derivatives showed pronounced antimicrobial activity when used against bacterial infections caused by Nocardia, Staphylococcus aureus and Escherichia coli (Genç et al. 2008b; Isik and Özdemir-Kocak 2009a). Increased antibacterial activity of the SN drug group was seen upon substitution with electron withdrawing groups such as the nitro group (Genç et al. 2008a; Isik and Özdemir-Kocak 2009b; Radha Mothilal and Thamaraichelvan 2016; Tailor and Patel 2015; Vagdevi 2018).
Mechanism and mode of action
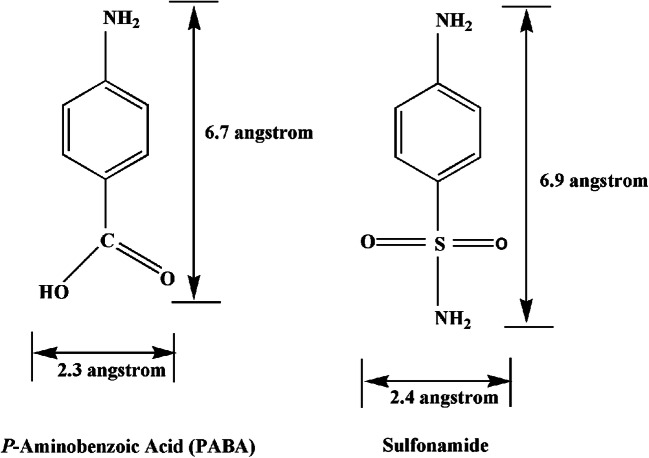
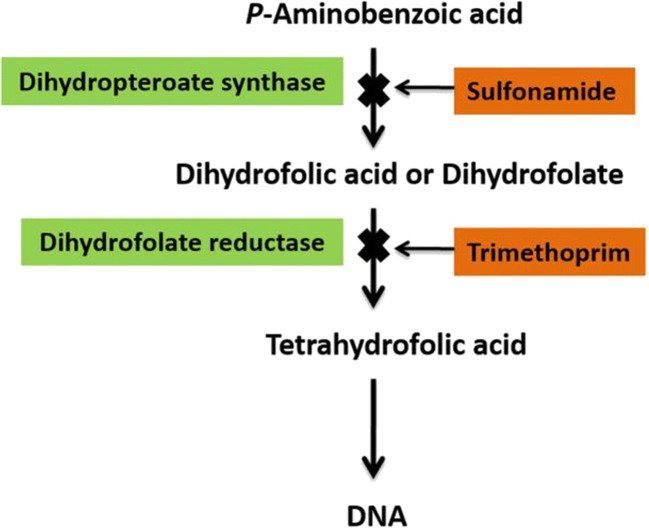
Antibiotics are chemotherapeutic agents used to inhibit or kill bacteria. Sulphonamides are competitive antagonists and structural analogues of p-aminobenzoic acid (PABA) in the synthesis of folic acid which is essential for the further production of DNA in the bacteria (Zessel et al. 2014). Similarity between the structures (Fig. 5) of SN and PABA allows SN to inhibit and replace PABA in the enzyme dihydropteroate synthetase (whose activity is important for the production of folate) and eventually inhibits the formation of dihydrofolate, tetrahydrofolate and also subsequently inhibits bacterial DNA growth and cell division or replication (Fig. 6) (Pareek et al. 2013). SN drugs along with trimethoprim are used to prevent the synthesis of tetrahydrofolate which further stops DNA replication. The effects of the drug give rise to hindrances in cell division, making the SN drugs bacteriostatic rather than bactericidal (Bohni 1976; Nemeth et al. 2015; Wood and Austrain 1941).
Fig. 6.
Structural similarity between PABA and sulphonamide (Tacic et al. 2017)
Folic acid (vitamin B9) is essential for the body cell growth and development in humans as it is required for the synthesis, repair, and methylation of DNA (Mahmood 2014). As a consequence, folic acid is critically important for women during pregnancy for a healthy foetus and also for men to improve sperm counts and motility (Dunlap et al. 2011; Gao et al. 2016). Sulfa drugs do not cause disruption in animal cells because they do not synthesize folate, but rather they consume it in the form of dietary requirement, with folic acid performing its functions only after its conversion to tetrahydrofolic acid by dihydrofolate reductase (which is believed to be slow in humans) (Bailey and Ayling 2009). Disturbances in the production of tetrahydrofolate by the SN type drugs can cause abnormality in the DNA by not providing sufficient methyl groups for methylation thereby limiting DNA synthesis which can potentially lead to carcinogenesis (Weinstein et al. 2003) (Fig. 7).
Fig. 7.
Synthesis of tetrahydrofolic acid and the mode of sulfonamide action on the synthesis of tertahydrofolic acid (Lavanya 2017; Pareek et al. 2013)
Bacterial resistance to sulphonamides
Antimicrobial resistance poses an ever increasing threat to mankind, animal, and environmental health (Prestinaci et al. 2015; Taneja and Sharma 2019). A major cause of antimicrobial resistance is the overuse of these medications; another factor is the unavailability and/or lack of new drugs to overcome the problem (Aslam et al. 2018; Ventola 2015). Bacteria can resist antibiotic medicines in two different ways—either by endogenous vertical evolution or by exogenous horizontal evolution (Courvalin 2008; Laws et al. 2019). Vertical evolution refers to the gaining of resistance from mutation occurring spontaneously within the bacterial genome that subsequently transfers to its offspring, whereas horizontal evolution describes the transfer of resistance genes between non-related bacteria (Laws et al. 2019). Bacterial resistance to SN has been frequently reported with some of the reported resistance cases being due to (i) resistance bacterial genes to trimethoprim-sulfamethoxazole (used as prophylaxis for the treatment of severe respiratory tract infections) (Pneumocystis carinii)(Pentti Huovinen 2001), (ii) resistance genes to SDZ resulted in phenotypic conversion showing a lack of sensitivity to polymyxin B for Serratia marcescen (Greenfield and Feingold 2014), (iii) resistance bacterial genes to SN spread and distributed in soils and were detected around poultry farms in China (Wang et al. 2014), (iv) resistance bacterial genes to SN discovered in environment (Razavi et al. 2017), and (v) trimethoprim-SN resistance spread among pathogenic bacteria (Huovinen et al. 1995). With increasing antibiotic resistance to SN drugs, considerable effort needs to be directed to the development of new and effective medicines.
Environmental effect
The ability of antibiotics to fight against bacterial infections plays an important role in the management of infectious diseases in humans, animals, and microorganisms. However, the overuse and misuse of antibiotics and their release into the environment can potentially pose a threat to animals and microbial communities in soil and aquatic environment (Cycoń et al. 2019; Ding and He 2010; Kraemer et al. 2019). As mentioned, SNs are frequently used antibiotics for animals (e.g. cattle and pigs) and humans for the treatment of bacterial diseases. Both after and during the use of these medicines a high fraction of the drug/medicine is excreted without metabolism through the urine or faeces, with these excretions then released into the environment as manure or sewage (Lin and Tsai 2009). Such observations provide grounds for investigation into SN drugs, covering their detection and distribution in the surrounding environment. SN drugs were also found to exist in low, but detectable levels, in the edible tissues of meat-producing animals treated with SN drugs (Bjurling et al. 2000; Hruska and Franek 2012; Poirier et al. 1999). These poorly metabolized antibiotics also accumulate within the soil which can impact soil microbial communities and functions (Thiele-Bruhn and Beck 2005; S. Wang et al. 2021). The sorption of SMZ antibiotics within the soil by black carbon has also been reported as a possible form of bioremediation (Chen et al. 2012). Trace determination of such antibiotics like SMZ and SDZ in animal feeds, human urine, and wastewater (aquatic environment) using different techniques have been variously reported (Blakemore and Thompson 1981; Ji et al. 2012; Mcardell et al. 2004). The environmental behaviour of SMZ and SDZ as reported by Biošić et al. (2017) concluded that their metabolites can bioaccumulate in the aquatic environment if they are not exposed to sunlight (Biošić et al. 2017).
Toxicological effect
Any drug used as medication can cause side effects. Antibiotics are sometime used in a manner which does not provide any benefit and can potentially cause harm (e.g. when used against certain viral infections such as common cold). As such antibiotics are not always the preferred solution as associated toxicological side effects may place the patient in unnecessary hazard. Some of the factors influencing the toxicological effect of SN drugs include duration and dosage of the drug, existence of the heterocyclic ring in N1 substituted SN, its solubility in blood and in other biological fluids, kidney state, age, and nutritional status of patient.(Shmukler et al. 2000) A research report by Boufas et al. (2014) outlined that SNs are somewhat toxic for blood cells, with sulphanilamide derivative being more toxic comparatively among the SN group of drugs while sulfadiazine was reported the least harmful. SN drugs can produce serious acute haemolytic anaemia (destruction of the red cells) causing agranulocytosis which is capable of depressing blood platelets (Kracke 1944). The potential for toxicity of SMZ in the environment is extant especially when occurring near to water (De Liguoro et al. 2009; Liu et al. 2019; Wood et al. 1957). Toxicity tests in animals of SDZ suggested that it is less toxic in comparison with other SNs while still being highly effective against common pathogens and as such has been exploited for the treatment of human bacterial infections (Finland et al. 1941; Kouroumkis et al. 1974). The measured toxicity of SDZ in water and water bodies suggested a possible increase in its toxic antimicrobial effects following its pH dependent chemical degradation, with a decrease in toxicity at higher pH values (Liu et al. 2016; Taylor et al. 2014).
Bio-macromolecule binding interaction study
SN type drugs, such as SMZ and SDZ, are widely used antibiotics with a plethora of drug targets (Islam et al. 2013; Lv et al. 2013; Sajid and Hamad 2013; Uhlemann et al. 2021). The study of which bio-macromolecules these drugs show affinity for helps us to understand their effect on humans, animals, and microorganisms. Tables 2, 3, and 4 summarize SN, SMZ, and SDZ interaction studies with a range of bio-macromolecules. The nature of the binding is principally described using the parameters of binding affinity and number of binding sites (with the target bio-macromolecule shown in the left hand column). Strong drug binding affinity to the target of > 106 M−1 can result in inhibition however the opposite can be expected for weak interaction with the target (binding affinities < 104 M−1).
Table 2.
Summary on SN-bio-macromolecule binding study
| Sl no. | Complex | Technique | Binding constant (M−1) | No. of binding sites | Year | Reference |
|---|---|---|---|---|---|---|
| 1 | Sulfonamide-bovine carbonic anhydrase | Fluorescence spectroscopy | 2.5 × 107 (dissociation constant) | - | 1967 | Chen and Kernohan (1967) |
| 2 | Sulfonamide-erythrocyte protein | Michaelis-Menten method | - | - | 1989 | Matsumoto (1989) |
| 3 | Sulfonamides-human carbonic anhydrase I enzyme | Crystallography | - | - | 1994 | Chakravarty and Kannan (1994) |
| 4 | Sulfonamide substituted 8-hydroxyquinoline-DNA | UV-Vis spectroscopy, gel electrophoresis | - | - | 2011 | Ixit et al. (2011) |
| 5 | Sulfonamide-human serum albumin | Isothermal titration calorimetry | 2.2 × 106 | 1 | 2012 | Behbehani et al. (2013) |
| 6 | Aryl bis-sulfonamides-enzyme IspF | Mass spectrometry, molecular docking | - | - | 2013 | Katharina Root et al. (2013) |
| 7 | Sulfonamide derivatives- bovine serum albumin | Fluorescence spectroscopy | 4.8 × 104 to 1.5 × 105 | 1 | 2014 | Zhang et al. (2014) |
| 8 | Sulfonamides-Ovalbumin | Fluorescence spectroscopy | 1.20 to 30.66 × 105 | 1 | 2019 | Carolina et al. (2019) |
| 9 | Cobalt complex sulfonamide-DNA | UV-Vis spectroscopy | 1.6 × 105 | 1 | 2019 | Pandya et al. (2019) |
Table 3.
Summary on SMZ-bio-macromolecule binding study
| Sl no. | Complex | Technique | Binding constant (M−1) | No. of binding sites | Year | Reference |
|---|---|---|---|---|---|---|
| 1 | Sulfamethazine-lysozyme | Fluorescence spectroscopy | - | 1 | 1984 | Atef et al. (1984) |
| 2 | Sulfamethazine-immunoglobulin G | Purification | 106 (dissociation constant) | - | 2003 | Liu et al. (2003) |
| 3 | Sulfamethazine- bovine serum albumin | Fluorescence spectroscopy | 2 × 106 | 1 | 2011 | Dawoud Bani-Yaseen (2011) |
| 4 | Sulfamethazine- human serum albumin | Fluorescence spectroscopy | 1.09 × 104 | 1.14 | 2012 | Chen et al. (2012) |
| 5 | Sulfamethazine-human immunoglobulin G | Fluorescence spectroscopy | 2 × 104 | 1 | 2015 | Wang et al. (2015) |
| 6 | Sulfamethazine- bovine serum albumin, adenine | Fluorescence spectroscopy |
1.32 × 105 2.11 × 104 |
0.98 0.92 |
2015 | Rajendiran and Thulasidhasan (2015) |
| 7 | Sulfamethazine N-acetylation-human N-acetyltrasferase-2 | HPLC | - | - | 2015 | Tahir et al. (2016) |
| 8 | Sulfamethazine-cyclodextrins | Fluorescence spectroscopy | - | - | 2019 | Ameen and Szente 2019) |
Table 4.
Summary on SDZ-bio-macromolecule binding study
| Sl no. | Complex | Technique | Binding constant (M−1) | No. of binding sites | Year | Reference |
|---|---|---|---|---|---|---|
| 1 | Silver sulfadiazine-DNA | Spectrophotometer | - | - | 1972 | Rosenkranz (1972) |
| 2 | Sulfadiazine-bovine serum albumin | Fluorescence spectroscopy | 2.48 × 104 | 1 | 2013 | Sajid and Hamad (2013) |
| 3 | Sulfadiazine sodium-ct-DNA, HSA | Fluorescence spectroscopy | 1.06 × 104, 1.63 × 104 | 1,1 | 2013 | Geng et al. (2013) |
| 4 | Sulfadiazine-ct DNA | UV-Vis spectroscopy | 2.87 × 103 | 2 | 2013 | Fotouhi et al. (2013) |
| 5 | Sulfadiazine-water-soluble proteins | Fluorescence spectroscopy | ⁓104-105 | ⁓1 | 2013 | Islam et al. (2013) |
| 6 | Sulfadiazine-human serum albumin | Fluorescence spectroscopy | 3.90 × 104 | 1 | 2014 | Ali and Al-lohedan (2014) |
| 7 | Sulfadiazine-human serum albumin | Fluorescence spectroscopy | 1.28 × 104 | 0.99 | 2014 | Huang and Liu (2014) |
| 8 | Polycation sulfadiazine-DNA | Synthesis, fluorescence spectroscopy | - | - | 2015 | Long et al. (2015) |
| 9 | Sulfadiazine-ureases | Synthesis, molecular docking | - | - | 2017 | Bean et al. (2017) |
| 10 | Sulfadiazine-lysozyme | Fluorescence spectroscopy | 1.75 × 104 | 1 | 2018 | Sonu et al. (2018) |
Conclusion
SNs are widely used synthetic antimicrobial drugs due to the fact that they that can be used for diverse purposes (such as treating bacterial infections and also promoting livestock growth). There are various forms of SN drug derivatives that have been produced of which SMZ and SDZ are the most frequently used. The purpose of this review was to examine SN drug structure, function, and toxicity in treated human and animal patients as well as the environment. This study has made clear the drug mode of action in its inhibition of bacteria through its competitive inhibition of bacterial DNA synthesis. The nomenclature and structure of the drugs (at the DFT level of approximation) have been discussed. Literature of its toxicological effect upon the environment, animals and human beings has also been reviewed.
Funding
JB received financial assistance from UGC-DAE, Mumbai Centre, BARC, Mumbai (grant no. CRS-M-266), DBT GOI (sanction no. BT/PR25026/NER/95/963/2017), and TEQIP-III Seed Grant, NIT Nagaland.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham EP. Anti-Cancer Drugs. 1966;2:1–25. doi: 10.1136/bmj.2.5525.1314. [DOI] [Google Scholar]
- Acar Selçuki N, Coşkun E, Biçer E. Combined computational and experimental studies on cysteine-sulfadiazine adduct formation. Turk J Chem. 2020;44(2):502–517. doi: 10.3906/KIM-1908-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MS, Al-lohedan HA (2014) Interaction of human serum albumin with sulfadiazine. J Mol Liq:1–7. 10.1016/j.molliq.2014.04.029
- Ameen HM, Szente L. Thermodynamic characterization of the interaction between the antimicrobial drug sulfamethazine and two selected cyclodextrins. Molecules. 2019;24:1–12. doi: 10.3390/molecules24244565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antibacterial Agents, Sulfonamides . Kirk-Othmer Encyclopedia of Chemical Technology. 1944. pp. 1–24. [Google Scholar]
- Arshad MN, Bibi A, Mahmood T, Asiri AM, Ayub K. Synthesis, crystal structures and spectroscopic properties of triazine-based hydrazone derivatives; a comparative experimental-theoretical study. Molecules. 2015;20:5851–5874. doi: 10.3390/molecules20045851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad MN, Faidallah HM, Asiri AM, Asiri AM, Mahmood T (2019) Structural, spectroscopic and nonlinear optical properties of sulfonamide derivatives; experimental and theoretical study. J Mol Struct:1–42. 10.1016/j.molstruc.2019.127393
- Aslam B, Wang W, Arshad MI, Khurshid M, Rasool MH, Nisar MA, Aslam MA, Qamar MU. Antibiotic resistance: a rundown of a global crisis. Infection and Drug Resistance. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atef EE, Hardee GE, Perrin JH. Studies on Sulfamethazine - Lysozyme Interactions by Fluorescence Quenching. Drug Dev Ind Pharm. 1984;1:57–68. doi: 10.3109/03639048409038292. [DOI] [Google Scholar]
- Awaisheh SS, Khalifeh MS, Rahahleh RJ, Al-Khaza’Leh JM, Algroom RM. Sulfamethazine contamination level and exposure assessment in domestic and imported poultry meats in Jordan. Veterinary World. 2019;12:1992–1997. doi: 10.14202/vetworld.2019.1992-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami K, Khodaei MM, Soheilizad M. Direct conversion of thiols to sulfonyl chlorides and sulfonamides. J Org Chem. 2009;74(24):9287–9291. doi: 10.1021/jo901924m. [DOI] [PubMed] [Google Scholar]
- Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. PNAS. 2009;106:15424–15429. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballentine C (1981) Sulfanilamide Disaster. U.S. Food and Drug Administration, 1–5. http://www.fda.gov/aboutfda/whatwedo/history/productregulation/sulfanilamidedisaster/default.htm
- Banerjee J, Seetharaman S, Wrice NL, Christy RJ, Natesan S. Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration. PLoS ONE. 2019;14(6):1–22. doi: 10.1371/journal.pone.0217965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banes D. Sulfamethazine. Analytical Profiles of Drug Substances. 1974;57(4):1–22. doi: 10.1093/jaoac/57.4.1010. [DOI] [Google Scholar]
- Bean J, Abbas Q, Hassan M, Raza H, Seo S. Sulfonamide-linked ciprofloxacin, sulfadiazine and amantadine derivatives as a novel class of inhibitors of jack bean urease: synthesis, kinetic mechanism and molecular docking. Molecules. 2017;22:1–20. doi: 10.3390/molecules22081352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani, G. R., Sadr, M. H., Nabipur, H., & Barzegar, L. (2013). A comparative study on the interaction of sulfonamide and nanosulfonamide with human serum albumin. J Chem 0, 1–4.
- Bendjeddou A, Abbaz T, Khacha N, Benahmed M, Gouasmia A, Villemin D. Antibacterial activity of sulfonamide derivatives against clinical strains of bacteria. Res J Pharm, Biol Chem Sci. 2016;7(2):799–804. [Google Scholar]
- Biošić M, Mitrevski M, Babić S. Environmental behavior of sulfadiazine, sulfamethazine, and their metabolites. Environ Sci Pollut Res. 2017;24(10):9802–9812. doi: 10.1007/s11356-017-8639-8. [DOI] [PubMed] [Google Scholar]
- Bjurling P, Baxter GA, Caselunghe M, Jonson C, Connor O, Elliott CT. Biosensor assay of sulfadiazine and sulfamethazine residues in pork. Analyst. 2000;125:1771–1774. doi: 10.1039/b004835f. [DOI] [PubMed] [Google Scholar]
- Blakemore WM, Thompson HC. Trace analysis of cinnamaldehyde in animal feed, human urine, and wastewater by electron capture gas chromatography. J Chromatogr Sci. 1981;19:625–633. doi: 10.1021/jf00119a032. [DOI] [PubMed] [Google Scholar]
- Blanchard C, Brooks L, Ebsworth-Mojica K, Didione L, Wucher B, Dewhurst S, Krysan D, Dunman PM, Wozniak RAF. Zinc pyrithione improves the antibacterial activity of silver sulfadiazine ointment. MSphere. 2016;1(5):1–14. doi: 10.1128/msphere.00194-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni E. Bacteriostatic and bactericidal activity of two trimethoprim-sulfonamide combinations. Chemotherapy. 1976;22:262–273. doi: 10.1159/000221933. [DOI] [PubMed] [Google Scholar]
- Boufas W, Dupont N, Berredjem M, Berrezag K, Becheker I, Berredjem H, Aouf N (2014) Synthesis and antibacterial activity of sulfonamides. SAR and DFT Studies. J Mol Struct:1–15. 10.1016/j.molstruc.2014.05.066
- Burbee CR, Green R, Matsumoto M. Antibiotics in animal feeds: risks and costs. Am J Agric Econ. 1985;67(5):966–970. doi: 10.2307/1241355. [DOI] [Google Scholar]
- Carolina A, De Lyra F, Amanda L, Silva S, Cristina E, Santos L, Maria A, López Q, Silva CS, Figueiredo IM, Carinhanha J, Santos C (2019) Molecular interaction of sulfonamides and ovalbumin, an allergenic egg protein, exploring biophysical, theoretical and biological studies. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy:1–54. 10.1016/j.saa.2019.117747 [DOI] [PubMed]
- Chakravarty S, Kannan KK. Refined Structures of Three Sulfonamide Drug Complexes of Human Carbonic Anhydrase I Enzyme. 1994. pp. 298–309. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay MK. Use of antibiotics as feed additives: a burning question. Front Microbiol. 2014;5:1–3. doi: 10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RAYMONDF, Kernohan JC. Combination with of bovine carbonic anhydrase a fluorescent. J Biol Chem. 1967;242(24):5813–5823. [PubMed] [Google Scholar]
- Chen J, Zhou X, Zhang Y, Gao H. Potential toxicity of sulfanilamide antibiotic: binding of sulfamethazine to human serum albumin. Sci Total Environ. 2012;432:269–274. doi: 10.1016/j.scitotenv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Chio LC, Bolyard LA, Nasr M, Queener SF. Identification of a class of sulfonamides highly active against dihydropteroate synthase from Toxoplasma gondii, Pneumocystis carinii, and Mycobacterium avium. Antimicrob Agents Chemother. 1996;40:727–733. doi: 10.1128/aac.40.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. Predictable and unpredictable evolution of antibiotic resistance. J Intern Med. 2008;264(1):4–16. doi: 10.1111/j.1365-2796.2008.01940.x. [DOI] [PubMed] [Google Scholar]
- Cycoń M, Mrozik A, Piotrowska-Seget Z. Antibiotics in the soil environment—degradation and their impact on microbial activity and diversity. Front Microbiol. 2019;10:1–66. doi: 10.3389/fmicb.2019.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Huang Y-Y, Sharma K, S., T. Hashmi, J., B. Kurup, D., & R. Hamblin, M. Topical antimicrobials for burn wound infections. Recent Patents on Anti-Infective Drug Discovery. 2010;5(2):124–151. doi: 10.2174/157489110791233522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawoud Bani-Yaseen A. Spectrofluorimetric study on the interaction between antimicrobial drug sulfamethazine and bovine serum albumin. J Lumin. 2011;131(5):1042–1047. doi: 10.1016/j.jlumin.2011.01.019. [DOI] [Google Scholar]
- De Liguoro M, Fioretto B, Poltronieri C, Gallina G. The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim. Chemosphere. 2009;75(11):1519–1524. doi: 10.1016/j.chemosphere.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Dibbern DA, Montanaro A. Allergies to sulfonamide antibiotics and sulfur-containing drugs. Ann Allergy Asthma Immunol. 2008;100(2):91–101. doi: 10.1016/s1081-1206(10)60415-2. [DOI] [PubMed] [Google Scholar]
- Ding C, He J. Effect of antibiotics in the environment on microbial populations. Appl Microbiol Biotechnol. 2010;87:925–941. doi: 10.1007/s00253-010-2649-5. [DOI] [PubMed] [Google Scholar]
- Dixon-Holland DE (1992) ELISA and its application for residue analysis of antibiotics and drugs in products of animal origin. Analysis of Antibiotic/Drug Residues in Food Products of Animal Origin:57–74. 10.1007/978-1-4615-3356-6_5
- Donizete Á, Borges L, Del Ponte G, Federman A, Carvalho I. Synthesis of sulfadiazine and silver sulfadiazine in semi-micro scale, as an experimental practice in drug synthesis. Quim Nova. 2005;28(4):727–731. [Google Scholar]
- Dubey RP, Patel UH (2019) Crystallography Study, Hirshfeld surface analysis and DFT studies of Cadmium complex of the triple sulfa drug constituent sulfadiazine with secondary ligand β - picoline. Journal of Chemical Crystallography:1–9. 10.1007/s10870-019-00803-7
- Dunlap B, Shelke K, Salem SA, Keith LG. Folic acid and human reproduction — ten important issues for clinicians. Journal of Experimental & Clinical Assisted Reproduction. 2011;1:13–15. [PMC free article] [PubMed] [Google Scholar]
- Eshghia H, Rahimizadeha M, Zokaeib M, Eshghic S, Eshghic S, Zinab Faghihia ET, M. K Synthesis and antimicrobial activity of some new macrocyclic bis-sulfonamide and disulfides. Eur J Chem. 2011;1(4):47–50. doi: 10.5155/eurjchem.3.3.359. [DOI] [Google Scholar]
- Farooq A, Naeem M, Hussain M, Aziz M, Ayub K, Nawaz M, Mahmood T. Synthesis , structural properties , DFT studies , antimicrobial activities and DNA binding interactions of two newly synthesized organotin ( IV ) carboxylates. J Mol Struct. 2019;1191:291–300. doi: 10.1016/j.molstruc.2019.04.066. [DOI] [Google Scholar]
- Fernández-Villa D, Aguilar MR, Rojo L. Folic acid antagonists: antimicrobial and immunomodulating mechanisms and applications. Int J Mol Sci. 2019;20:1–30. doi: 10.3390/ijms20204996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M, Strauss E, Peterson OL. Sulfadiazine therapiutic evaluation and toxic effects on four hundred and forty-six patients. J Am Med Assoc. 1941;116:2641–2648. doi: 10.1001/jama.251.11.1467. [DOI] [PubMed] [Google Scholar]
- Fotouhi L, Hashkavayi AB, Heravi MM. Interaction of sulfadiazine with DNA on a MWCNT modified glassy carbon electrode : Determination of DNA. Int J Biol Macromol. 2013;53:101–106. doi: 10.1016/j.ijbiomac.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Gao Y, Sheng C, Xie R, Sun W, Asztalos E, Moddemann D, Zwaigenbaum L, Walker M, Wen SW (2016) New perspective on impact of folic acid supplementation during pregnancy on neurodevelopment / autism in the offspring children—a systematic review. PLoS ONE:1–16. 10.1371/journal.pone.0165626 [DOI] [PMC free article] [PubMed]
- Ge P, Yu H, Chen J, Qu J, Luo Y (2018) Photolysis mechanism of sulfonamide moiety in five-membered sulfonamides: A DFT study. Chemosphere, 1–25. 10.1016/j.chemosphere.2018.01.041 [DOI] [PubMed]
- Genç Y, Özkanca R, Bekdemir Y. Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. Ann Clin Microbiol Antimicrob. 2008;7:1–6. doi: 10.1186/1476-0711-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genç Y, Özkanca R, Bekdemir Y. Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. Ann Clin Microbiol Antimicrob. 2008;7:1–6. doi: 10.1186/1476-0711-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S, Liu G, Cui F (2013) Molecular interaction of ctDNA and HSA with sulfadiazine sodium by multispectroscopic methods and molecular modeling. Luminescence:1–9. 10.1002/bio.2457 [DOI] [PubMed]
- Giles A, Foushee J, Lantz E, Gumina G. Sulfonamide allergies. Pharmacy. 2019;7(3):1–12. doi: 10.3390/pharmacy7030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S, Feingold DS. The synergistic action of the sulfonamides and the polymyxins against serratia marcescens. J Infect Dis. 2014;121:555–558. doi: 10.1093/infdis/121.5.555. [DOI] [PubMed] [Google Scholar]
- Guianvarc’h D, Duca M, Boukarim C, Kraus-Berthier L, Ste´phane Le´once. Alain Pierre´. Pfeiffer B, Renard P, Arimondo PB, Claude Monneret DD. Synthesis and biological activity of sulfonamide derivatives of epipodophyllotoxin. J Med Chem. 2004;47(2):2365–2374. doi: 10.1021/jm031117b. [DOI] [PubMed] [Google Scholar]
- Hazhir N, Kiani F, Tahermansouri H, Saraei AG-H, Koohyar F. Prediction of thermodynamic and structural properties of sulfamerazine and sulfamethazine in water using DFT and ab initio methods. J Mex Chem Soc. 2018;62:1–13. [Google Scholar]
- Hazhir N, Kiani F, Tahermansouri H, Saraei AG, Koohyar F. Prediction of thermodynamic and structural properties of sulfamerazine and sulfamethazine in water using DFT and ab Initio methods. J Mex Chem Soc. 2018;62:1–13. [Google Scholar]
- Hehui Z, Ping W, Jie L. Detection of 12 sulfonamide antibiotics in cosmetics by ultra performance liquid chromatography detection of 12 sulfonamide antibiotics in cosmetics by ultra performance liquid chromatography Determination of 12 Sulfonamides in Cosmetics by Ultra Performa. Chin J Chromatogr. 2021;53:10–11. [Google Scholar]
- Hosseini SV, Tanideh N, Kohanteb J, Ghodrati Z, Mehrabani D, Yarmohammadi H. Comparison between alpha and silver sulfadiazine ointments in treatment of Pseudomonas infections in 3rd degree burns. Int J Surg. 2007;5:23–26. doi: 10.1016/j.ijsu.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hruska K, Franek M. Sulfonamides in the environment : a review and a case report. Review Article. 2012;57(1):1–35. [Google Scholar]
- Huang F, Liu Y. Spectroscopic studies on the interaction between sulfadiazine and human serum albumin. Adv Mater Res. 2014;1044-1045(1045):181–184. doi: 10.4028/www.scientific.net/AMR.1044-1045.181. [DOI] [Google Scholar]
- Huang Y, Rong C, Zhang R, Liu S. Evaluating frontier orbital energy and HOMO/LUMO gap with descriptors from density functional reactivity theory. J Mol Model. 2017;23(1):1–12. doi: 10.1007/s00894-016-3175-x. [DOI] [PubMed] [Google Scholar]
- Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Antimicrobial Resistance. 2001;32:1608–1614. doi: 10.1086/320532. [DOI] [PubMed] [Google Scholar]
- Huovinen P, Sundstrom L, Swedberg G, Skold O. Trimethoprim and Sulfonamide Resistance. Antimicrob Agents Chemother. 1995;39:279–289. doi: 10.1128/aac.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huschek G, Hollmann D, Kurowski N, Kaupenjohann M, Vereecken H. Re-evaluation of the conformational structure of sulfadiazine species using NMR and ab initio DFT studies and its implication on sorption and degradation. Chemosphere. 2008;72(10):1448–1454. doi: 10.1016/j.chemosphere.2008.05.038. [DOI] [PubMed] [Google Scholar]
- Igwe CN, Okoro UC (2014) Synthesis, characterization, and evaluation for antibacterial and antifungal activities of N-heteroaryl substituted benzene sulphonamides. Organic Chem Int:1–5. 10.1155/2014/419518
- Isik K, Özdemir-Kocak F. Antimicrobial activity screening of some sulfonamide derivatives on some Nocardia species and isolates. Microbiol Res. 2009;164(1):49–58. doi: 10.1016/j.micres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Isik K, Özdemir-Kocak F. Antimicrobial activity screening of some sulfonamide derivatives on some Nocardia species and isolates. Microbiol Res. 2009;164(1):49–58. doi: 10.1016/j.micres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Islam MM, Moyon NS, Gashnga PM, Mitra S, Received Interaction of sulfadiazine with model water soluble proteins : a combined fluorescence spectroscopic and molecular modeling approach. J Fluoresc. 2013;24(2):579–588. doi: 10.1007/s10895-013-1330-7. [DOI] [PubMed] [Google Scholar]
- Islam MM, Sonu VK, Gashnga PM, Moyon NS, Mitra S. Caffeine and sulfadiazine interact differently with human serum albumin: a combined fluorescence and molecular docking study. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy. 2016;152:23–33. doi: 10.1016/j.saa.2015.07.051. [DOI] [PubMed] [Google Scholar]
- Ixit RBD, Atel TSP, Anparia SFV. DNA-binding interaction studies of microwave assisted synthesized sulfonamide substituted 8-hydroxyquinoline derivatives. Sci Pharm. 2011;79:293–308. doi: 10.3797/scipharm.1102-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Kim S, Han S, Seo J, Lee S, Park Y, Choi K, Kho YL, Kim PG, Park J, Choi K. Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment: are the current environmental concentrations safe? Ecotoxicology. 2012;21(7):2031–2050. doi: 10.1007/s10646-012-0956-6. [DOI] [PubMed] [Google Scholar]
- Karataş D, Tekin A, Çelik MS. Density functional theory computation of organic compound penetration into sepiolite tunnels. Clay Clay Miner. 2017;65(1):1–13. doi: 10.1346/CCMN.2016.064043. [DOI] [Google Scholar]
- Kato M, Jonassen HB, Fanning JC. Copper(II) complexes with subnormal magnetic moments. Chem Rev. 1964;64(2):99–128. doi: 10.1021/cr60228a003. [DOI] [Google Scholar]
- Khan SA, Maher S, Naheed N, Mahmood T, Ayub K, Farooq A, Khan SB, Shah Z (2018) Isolation, spectroscopic and density functional theory of two withanolide glycosides. J Mol Struct:1–26. 10.1016/j.molstruc.2018.09.078
- Khan DA, Knowles SR, Shear NH. Sulfonamide hypersensitivity: fact and fiction. Journal of Allergy and Clinical Immunology: In Practice. 2019;7(7):2116–2123. doi: 10.1016/j.jaip.2019.05.034. [DOI] [PubMed] [Google Scholar]
- Kohout J, Krätsmár-Šmogrovič J. Copper (II) Complexes with organic ligands (VII) magnetic properties of copper (II) acetate and copper (П) Sal icylate complexes of pyridine and quinoline N-oxides. Chem Pap. 1968;22:481–492. [Google Scholar]
- Kouroumkis P, Szabo S, Selye H. Effect of pharmacologic conditioning on sulfadiazine toxicity and concentrations in plasma. Pharmacology. 1974;329:321–329. doi: 10.1159/000136506. [DOI] [PubMed] [Google Scholar]
- Kracke, R. R. (1944). The Effects of Sulfonamide Drugs on the Blood. 191–199.
- Kraemer SA, Ramachandran A, Perron GG. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms. 2019;7:1–24. doi: 10.3390/microorganisms7060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk P. Time-dependent density functional theory calculations of the solvatochromism of some azo sulfonamide fluorochromes. J Mol Model. 2015;21(5):1–18. doi: 10.1007/s00894-015-2651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavanya R. Sulphonamides: a pharmaceutical review. International Journal of Pharmaceutical Science Invention. 2017;6(2):1–3. [Google Scholar]
- Laws M, Shaaban A, Rahman KM. Antibiotic resistance breakers: current approaches and future directions. FEMS Microbiol Rev. 2019;43(5):490–516. doi: 10.1093/femsre/fuz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AYC, Tsai YT. Occurrence of pharmaceuticals in Taiwan’s surface waters: impact of waste streams from hospitals and pharmaceutical production facilities. Sci Total Environ. 2009;407(12):3793–3802. doi: 10.1016/j.scitotenv.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao R, Shangguan D, Zhang H, Liu G. Novel sulfamethazine ligand used for one-step purification of immunoglobulin G from human plasma. J Chromatogr B. 2003;792:177–185. doi: 10.1016/s1570-0232(03)00263-0. [DOI] [PubMed] [Google Scholar]
- Liu K, Xu S, Zhang M, Kou Y, Zhou X, Luo K (2016) Estimation of the toxicity of sulfadiazine to Daphnia magna using negligible depletion hollow-fiber liquid-phase microextraction independent of ambient pH. Nat Publ Group:4–11. 10.1038/srep39798 [DOI] [PMC free article] [PubMed]
- Liu X, Huang F, Yu Y, Jiang Y, Zhao K, He Y. Chemosphere Determination and toxicity evaluation of the generated byproducts from sulfamethazine degradation during catalytic oxidation process. Chemosphere. 2019;226:103–109. doi: 10.1016/j.chemosphere.2019.03.125. [DOI] [PubMed] [Google Scholar]
- Long X, Zhang Z, Han S, Tang M, Zhou J, Zhang J, Xue Z, Li Y, Zhang R, Deng L, Dong A. Structural mediation on polycation nanoparticles by sulfadiazine to enhance DNA transfection efficiency and reduce toxicity. Appl Mater Interfaces. 2015;7:7542–7551. doi: 10.1021/am508847j. [DOI] [PubMed] [Google Scholar]
- Lu JIE, Rohani S. Synthesis and preliminary characterization of sulfamethazine-theophylline co-crystal. J Pharm Sci. 2010;99:4042–4047. doi: 10.1002/jps.22142. [DOI] [PubMed] [Google Scholar]
- Lv Y, Tan T, Svec F. Molecular imprinting of proteins in polymers attached to the surface of nanomaterials for selective recognition of biomacromolecules. Biotechnol Adv. 2013;31(8):1172–1186. doi: 10.1016/j.biotechadv.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Ma P, Zhou Z, Yang W, Tang B, Liu H, Xu W (2015) Preparation and application of sulfadiazine surface molecularly imprinted polymers with temperature-responsive properties. Journal Of Applied Polymer Science:1–12. 10.1002/app.41769
- Mahmood L. The metabolic processes of folic acid and vitamin B12 deficiency. J Health Res Rev. 2014;1:5–9. [Google Scholar]
- Majewsky M, Wagner D, Delay M, Bräse S, Yargeau V, Horn H. Antibacterial activity of sulfamethoxazole transformation products (TPs): general relevance for sulfonamide TPs modified at the para position. Chem Res Toxicol. 2014;27(10):1821–1828. doi: 10.1021/tx500267x. [DOI] [PubMed] [Google Scholar]
- Mansour AM (2014) DFT studies , spectral and biological activity evaluation of binary and ternary sulfamethazine Fe ( III ) complexes. J Coord Chem:37–41. 10.1080/00958972.2014.951345
- Mansour AM, Mohamed RR (2015) Sulfamethazine copper(II) complexes as antimicrobial thermal stabilizer and co- stabilizers for rigid PVC: spectroscopic, thermal, and DFT studies. RSC Adv:1–22
- Mansour AM, Radacki K. Experimental and DFT studies of sulfadiazine ‘piano-stool’ Ru(ii) and Rh(iii) complexes. RSC Adv. 2020;10:10673–10680. doi: 10.1039/d0ra01085e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathammal R, Jayamani N, Geetha N. Molecular structure, NMR, HOMO, LUMO, and vibrational analysis of O-anisic acid and anisic acid based on DFT calculations. Journal of Spectroscopy. 2013;1:1–19. doi: 10.1155/2013/171735. [DOI] [Google Scholar]
- Mathews SM, V, J. Thomas I, Panicker JT, Kuriakose LS. Sulfa drugs and the skin. World J Pharm Res. 2015;4(10):382–390. [Google Scholar]
- Matsumoto K. Binding of sulfonamides to erythrocyte proteins and possible drug-drug interaction. Chem Pharm Bull. 1989;37:2807–2810. doi: 10.1248/cpb.37.2807. [DOI] [PubMed] [Google Scholar]
- Mcardell CS, Suter MJ, Giger W. Trace determination of macrolide and sulfonamide antimicrobials, a human sulfonamide metabolite , and trimethoprim in wastewater using liquid chromatography coupled to electrospray tandem mass spectrometry. Anal Chem. 2004;76(16):4756–4764. doi: 10.1021/ac0496603. [DOI] [PubMed] [Google Scholar]
- McFarland MM, Zach SJ, Wang X, Potluri LP, Neville AJ, Vennerstrom JL, Davis PH. Review of experimental compounds demonstrating anti-toxoplasma activity. Antimicrob Agents Chemother. 2016;60(12):7017–7034. doi: 10.1128/AAC.01176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekala R, Mathammal R. Quantum computational and spectroscopic analysis of sulfamethazine. Int J Curr Res Rev. 2012;04(7):1–154. [Google Scholar]
- Naredla RR, Klumpp DA. Preparation of sulfonamides from N-silylamines. Tetrahedron Lett. 2013;54(45):5945–5947. doi: 10.1016/j.tetlet.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth J, Oesch G, Kuster SP. Bacteriostatic versus bactericidal antibiotics for patients with serious bacterial infections: systematic review and meta-analysis. J Antimicrob Chemother. 2015;70(2):382–395. doi: 10.1093/jac/dku379. [DOI] [PubMed] [Google Scholar]
- Ogruc-ildiz G, Akyuz S, Ozel AE. Experimental, ab initio and density functional theory studies on sulfadiazine. Journal of Molecular Structure. 2009;924–926:514–522. doi: 10.1016/j.molstruc.2008.12.067. [DOI] [Google Scholar]
- Pandya SB, Patel UH, Chaudhary KP, Socha BN, Patel NJ, Bhatt BS (2019) DNA interaction, cytotoxicity and molecular structure of cobalt complex of 4 - amino - N - ( 6 - chloropyridazin - 3 - yl ) benzene sulfonamide in the presence of secondary ligand pyridine. Applied Organometallic Chemistry, May:1–14. 10.1002/aoc.5235
- Pareek A, Rani P, Kishore D. A short review on: Sulphonamides. Int J Pharm Bio Sci. 2013;4(1):812–820. [Google Scholar]
- Peng FJ, Ying GG, Liu YS, Su HC, He LY. Joint antibacterial activity of soil-adsorbed antibiotics trimethoprim and sulfamethazine. Sci Total Environ. 2015;506–507:58–65. doi: 10.1016/j.scitotenv.2014.10.117. [DOI] [PubMed] [Google Scholar]
- Poirier LA, Doerge DR, Gaylor DW, Miller MA, Lorentzen RJ, Casciano DA, Kadlubar FF, Schwetz BA. An FDA Review of Sulfamethazine Toxicity. Regul Toxicol Pharmacol. 1999;30:217–222. doi: 10.1006/rtph.1999.1348. [DOI] [PubMed] [Google Scholar]
- Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathogens and Global Health. 2015;109(7):309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir MA, Ahmed M, Iqbal M. Synthesis, characterization, and antibacterial activities of novel sulfonamides derived through condensation of amino group containing drugs, amino acids, and their analogs Muhammad. Biomed Res Int. 2015;34(6):1099–1106. doi: 10.1155/2015/938486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radha Mothilal SKK, Thamaraichelvan AE. Synthesis, characterization and biological studies of sulfadiazine drug based transition metal complexes. J Chem Pharm Res. 2016;8(8):202–211. doi: 10.9734/irjpac/2016/29451. [DOI] [Google Scholar]
- Rajendiran N, Thulasidhasan J. Binding of sulfamerazine and sulfamethazine to bovine serum albumin and nitrogen purine base adenine : a comparative study. International Letters of Chemistry, Physics and Astronomy. 2015;59:170–187. doi: 10.18052/www.scipress.com/ILCPA.59.170. [DOI] [Google Scholar]
- Rama A, Lucatello L, Benetti C, Galina G, Bajraktari D. Assessment of antibacterial drug residues in milk for consumption in Kosovo. J Food Drug Anal. 2017;25(3):525–532. doi: 10.1016/j.jfda.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool N, Kanwal A, Rasheed T, Ain Q, Mahmood T, Ayub K, Zubair M, Khan KM, Arshad MN, Asiri AM, Zia-ul-haq M, Jaafar HZE. One pot selective arylation of 2-bromo-5-chloro thiophene; molecular structure investigation via density functional theory (DFT), X-ray analysis, and their biological activities. Int J Mol Sci. 2016;17:1–16. doi: 10.3390/ijms17070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi M, Marathe NP, Gillings MR, Flach C, Kristiansson E, Larsson DGJ. Discovery of the fourth mobile sulfonamide resistance gene. Microbiome. 2017;5:1–12. doi: 10.1186/s40168-017-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy NS, Rao AS, Chari MA, Kumar VR, Jyothy V, Himabindu V. Synthesis and antibacterial activity of sulfonamide derivatives at C-8 alkyl chain of anacardic acid mixture isolated from a natural product cashew nut shell liquid (CNSL) J Chem Sci. 2012;124(3):723–730. doi: 10.1007/s12039-012-0253-1. [DOI] [Google Scholar]
- Robertson LP, Moodie LWK, Holland DC, Jandér KC, Göransson U. Sulfadiazine masquerading as a natural product from scilla madeirensis (Scilloideae) J Nat Prod. 2020;83(4):1305–1308. doi: 10.1021/acs.jnatprod.0c00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root K, Barylyu K, Schwab A, Thelemanna J, Illarionovb B, Geista J, Gräwertb T, Bacherc A, Fischerb M, François Diedericha RZ. Aryl bis-sulfonamides bind to the active site of a homotrimeric isoprenoid biosynthesis enzyme IspF and extract the essential divalent metal cation cofactor Katharina. R Soc Chem. 2013;00:1–11. doi: 10.1039/C8SC00814K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz HS (1972) Silver sulfadiazine: antimicrobial agents and chemotherapy. 2(5):373–383 [DOI] [PMC free article] [PubMed]
- Ross, L. J., & Plainfield, N. (1968). Preparation of sulfamethazine and acetyl sulfamethazine. United States Patent Office, 3–6.
- Sajid M, Hamad A. Sulfadiazine binds and unfolds bovine serum albumin: an in vitro study. Mol Biol Rep. 2013;40:6081–6090. doi: 10.1007/s11033-013-2719-8. [DOI] [PubMed] [Google Scholar]
- Seneca H (2015) Long-Acting Sulfonamides in Urinary Tract Infections. JAMA:975–980 [PubMed]
- Seydel JK. Sulfonamides, structure-activity relationship, and mode of action. J Pharm Sci. 1968;57:1455–1478. doi: 10.1002/jps.2600570902. [DOI] [PubMed] [Google Scholar]
- Shah TJ, Moshirfar M, Hoopes PC. “Doctor, I have a Sulfa Allergy”: clarifying the myths of cross-reactivity. Ophthalmol Therapy. 2018;7(2):211–215. doi: 10.1007/s40123-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmukler BE, Brugnara C, Alper SL. Structure and genetic polymorphism of the mouse KCC1 gene. Biochim Biophys Acta. 2000;1492:353–361. doi: 10.1016/s0167-4781(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Shun-ichi Yamada T, Fujita T, Arita J. Synthesis of sulfadiazine. Osaka Res Lab. 1950;350:1949–1951. [Google Scholar]
- Sonu, Parveen BR, Pal SP, H A short review on sulphonamides with antimicrobial activity. Int J Pharm Chem. 2017;07(05):70–73. [Google Scholar]
- Sonu VK, Rajkumar I, Bhattacharjee K, Joshi SR (2018) Interaction of caffeine and sulfadiazine with Lysozyme adsorbed at colloidal metal nanoparticle interface: influence on drug transport ability and antibacterial activity. Journal of Biomolecular Structure and Dynamics:1–42. 10.1080/07391102.2018.1426497 [DOI] [PubMed]
- Sulfamethazine and its sodium salt . IARC Monographs on the evaluation of carcinogenic risks to humans. 2001. pp. 341–359. [PMC free article] [PubMed] [Google Scholar]
- Sultan EA (2015) Pathophysiologic mechanisms of immune-mediated drug hypersensitivity reactions to sulfonamides. Electronic Thesis and Dissertation Repository:1–125
- Supuran CT. Special issue: Sulfonamides. Molecules. 2017;22(10):1–5. doi: 10.3390/molecules22101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran CT, Casini A, Scozzafava A. Protease inhibitors of the sulfonamide type: anticancer, antiinflammatory, and antiviral agents. Med Res Rev. 2003;23(5):535–558. doi: 10.1002/med.10047. [DOI] [PubMed] [Google Scholar]
- Tacic A, Nikolic V, Nikolic L, Savic I. Antimicrobial sulfonamide drugs. Advanced Technologies. 2017;6(1):58–71. doi: 10.5937/savteh1701058t. [DOI] [Google Scholar]
- Tahir IM, Iqbal T, Saleem S, Mehboob H. Effect of acetaminophen on sulfamethazine acetylation in male volunteers. Int J Immunopathol Pharmacol. 2016;29:17–22. doi: 10.1177/0394632015593238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailor SM, Patel UH. Synthesis, spectroscopic characterization, antimicrobial activity and crystal structure of silver and copper complexes of sulfamethazine. J Coord Chem. 2015;68(13):2192–2207. doi: 10.1080/00958972.2015.1055258. [DOI] [Google Scholar]
- Taneja N, Sharma M. Antimicrobial resistance in the environment: the Indian scenario. Indian J Med Res. 2019;149:119–128. doi: 10.4103/ijmr.IJMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Yang J, Zhou S, Xiao A, Li W, Ying G. Chemical oxidation of sulfadiazine by the Fenton process : Kinetics, pathways, toxicity evaluation chemical oxidation of sulfadiazine by the Fenton process: kinetics , pathways , toxicity evaluation. Journal of Environmental Science and Health , Part B :Pesticides, Food Contaminants, and Agricultural Wastes. 2014;49:37–41. doi: 10.1080/03601234.2014.951572. [DOI] [PubMed] [Google Scholar]
- Thiele-Bruhn S, Beck IC. Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere. 2005;59(4):457–465. doi: 10.1016/j.chemosphere.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Tilles SA. Practical issues in the management of hypersensitivity reactions: sulfonamides. South Med J. 2001;94(1–10):817–824. doi: 10.1097/00007611-200108000-00013. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Miyazaki M, Mashima K, Takagi S, Hara S, Kamimura H, Jimi S. The effects of silver sulfadiazine on methicillin-resistant staphylococcus aureus biofilms. Microorganisms. 2020;8(10):1–12. doi: 10.3390/microorganisms8101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlemann T, Berden G, Oomens J. Preferred protonation site of a series of sulfa drugs in the gas phase revealed by IR spectroscopy. European Physical Journal D. 2021;75(1):1–13. doi: 10.1140/epjd/s10053-020-00027-x. [DOI] [Google Scholar]
- Ullah Z, Rauf A, Yaseen M, Hassan W, Tariq M, Ayub K, Tahir AA, Ullah H (2015) Density functional theory and phytochemical study of 8-hydroxyisodiospyrin. J Mol Struct:1–32. 10.1016/j.molstruc.2015.04.027
- Vagdevi GSHM. Synthesis and in vitro antibacterial activity of novel substituted N-1, 3-benzoxazol-2yl benzene sulfonamides. Int J Sci Res (IJSR) 2018;7(12):715–717. [Google Scholar]
- Van Mourik T, Bühl M, Gaigeot MP. Density functional theory across chemistry, physics and biology. Philos Trans R Soc A Math Phys Eng Sci. 2014;372:1–5. doi: 10.1098/rsta.2012.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola, C. L. (2015). The antibiotic resistance crisis part 1: causes and threats. P&T, 40(4), 277–283. [PMC free article] [PubMed]
- Verma DK (2018) Density functional theory (DFT) as a powerful tool for designing corrosion inhibitors in aqueous phase. Advanced Engineering Testing:87–105. 10.5772/intechopen.78333
- Wang N, Yang X, Jiao S, Zhang J, Ye B, Gao S. Sulfonamide-resistant bacteria and their resistance genes in soils fertilized with manures from Jiangsu Province, Southeastern China Na. PLoS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0112626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Ge H, Liu C, Zhang S, Tian G. Mechanistic and conformational studies on the interaction of sulfamethazine with human immunoglobulin G by molecular modeling and multi-spectroscopic approach in vitro. Luminescence. 2015;30:798–804. doi: 10.1002/bio.2822. [DOI] [PubMed] [Google Scholar]
- Wang S, Du K, Yuan R, Chen H, Wang F, Zhou B. Effects of sulfonamide antibiotics on digestion performance and microbial community during swine manure anaerobic digestion. Environ Eng Res. 2021;26(1):1–12. doi: 10.4491/eer.2019.471. [DOI] [Google Scholar]
- Warrington R, Silviu-Dan F, Wong T. Drug allergy. Allergy, Asthma Clin Immunol. 2018;14:130–139. doi: 10.1186/s13223-018-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SJ, Hartman TJ, Stolzenberg-solomon R, Pietinen P, Barrett MJ, Taylor PR, Virtamo J, Albanes D. Null association between prostate cancer and serum folate, Vitamin B 6 , Vitamin B 12 , and Homocysteine. Cancer Epidemiology, Biomarkers & Prevention. 2003;12:1271–1272. [PubMed] [Google Scholar]
- White RJ, Cooper R (2003) Silver sulphadiazine: a review of the evidence. Clinical REVIEW This:51–61
- Wiedemann B, Heisig A, Heisig P. Uncomplicated urinary tract infections and antibiotic resistance-epidemiological and mechanistic aspects. Antibiotics. 2014;3(3):341–352. doi: 10.3390/antibiotics3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KJ, Janney FR. Sulfadiazine review of its use in treatment of children. American Journal of Diseases of Children. 2015;734(June 14):702–712. [Google Scholar]
- Won JS, Kaewsuk J, Jo JH, Lim D, Seo GT. A density functional theory study on the ozone oxidation of sulfonamide antibiotics. J Adv Oxid Technol. 2015;18(1):31–38. [Google Scholar]
- Wood WB, Austrain R (1941) Studies on the antibacterial action of the sulfonamide drugs. 7:383–394 [DOI] [PMC free article] [PubMed]
- Wood EM, Yasutake WT, Johnson HE, Yasutake WT, Acute HEJ (1957) Acute sulfamethazine toxicity in young salmon TOXICITY. The Progressive Fish-Culturist:64–67. 10.1577/1548-8659(1957)19
- Xu Z, Lu X, Li Y, Wei S. Theoretical analysis on heteroleptic Cu ( I )—based complexes for dye-sensitized Solar cells: effect of. Molecules. 2020;25:1–15. doi: 10.3390/molecules25163681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef F, Mansour O, Herbali J. Sulfonamides: historical discovery development (Structure-aactivity relationship notes) Iiij. 2018;1(1):1–15. [Google Scholar]
- Zawodniak A, Lochmatter P, Beeler A, Pichler WJ. Cross-reactivity in drug hypersensitivity reactions to sulfasalazine and sulfamethoxazole. Int Arch Allergy Immunol. 2010;153(2):152–156. doi: 10.1159/000312632. [DOI] [PubMed] [Google Scholar]
- Zessel K, Mohring S, Hamscher G, Kietzmann M, Stahl J. Biocompatibility and antibacterial activity of photolytic products of sulfonamides. Chemosphere. 2014;100:167–174. doi: 10.1016/j.chemosphere.2013.11.038. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lin Y, Lin C (2014) Study on the synthesis of sulfonamide derivatives and their interaction with bovine serum albumin. Luminescence:1–11. 10.1002/bio.2725 [DOI] [PubMed]