Abstract
Objective
This work attempted to isolate, identify, and correlate the composition of essential oils (EOs) and pharmacological properties of two imported Amomum subulatum fruit samples. These samples were collected from Indian and KSA local supermarkets to ensure consistency in their therapeutic effects.
Methods
EOs were extracted from Indian and KSA A. subulatum fruit samples using a hydro-distillation method and identified by gas chromatography-mass spectrometry (GC–MS). Antimicrobial activity against gram-negative bacteria (Pseudomonas aeruginosa, Escherichia coli, and Acinetobacter baumannii) was determined using minimum inhibitory (MIC) and minimum bactericidal concentration methods. Antioxidant and anti-inflammatory activities were determined using a 2,2-diphenyl-1-picrylhydrazyl-induced free radical assay, and a bovine albumin inhibitory assay, respectively. These analyses were performed to evaluate the pharmacological activities of the substances.
Results
GC–MS retention times of both samples demonstrated 56 bioactive ingredients with different percentages. The principal bioactive compounds in the Indian and Saudi Arabian EO samples were 1,8-cineole (44.24% and 46.22%, respectively), α-terpineol (7.47% and 7.04%, respectively), terpinen-4-ol (5.01% and 4.83%, respectively), geraniol D (4.05% and 3.54%, respectively), and β-pinene (3.38% and 3.98%, respectively). Superior antimicrobial activity against the selected strains was observed for both samples, with an MIC range of 0.5%–1%. Antioxidant assays demonstrated moderate activity in both samples. Moreover, the Indian and Saudi Arabian samples exhibited IC50 values of 53.12% and 55.26 μg/mL, respectively, in albumin denaturation inhibition assays. This indicated an outstanding anti-inflammatory potential comparable to ibuprofen.
Conclusions
The composition of EOs from both samples exhibited similar qualitative but different quantitative variability. No major variations in the pharmacological properties of EOs were observed. More studies are essential for further validation of our study findings.
Keywords: Amomum subulatum, Essential oils, Gas-chromatography, Marketed samples, Pharmacological activity
الملخص
أهداف البحث
في هذا العمل حاولنا فصل وتحديد تركيبة الزيت العطري والخصائص الدوائية لنوعين من ثمار حب الهيل الأسود المستوردة التي تم شراؤها من المتاجر المحلية الهندية والسعودية للتأكد من علاقة التركيب الكيميائي بالتأثيرات العلاجية.
طرق البحث
لقد تم استخلاص الزيت العطري من عينات ثمار حب الهيل الأسود الهندية والسعودية باستخدام طريقة التقطير المائي، ثم تم تحديد تركيب الزيت العطري بواسطة جهاز مطياف الكتلة المرتبط بالكروماتوغرافيا الغازية. بعدها تم تقييم فاعلية تلك الزيوت كمضاد جرثومي ضد البكتيريا سالبة الجرام (الزائفة الزنجارية والإشريكية القولونية وأسينوباكتر بوماني) باستخدام طريقتي تحديد قيمة الحد الأدنى من التركيز المثبط وتحديد التركيز الأدنى القاتل للجراثيم. هذا بالإضافة إلى أنه قد تم تحديد الفاعلية المضادة للأكسدة باستخدام طريقة مكافحة الجذور الحرة التي يسببها مركب (٢,٢-ثنائي الفينيل ١- بيكريل الهيدرازيل) مع الفاعلية المضادة للالتهابات بواسطة فحص مثبطات الألبومين البقري وذلك لتقييم التأثيرات الدوائية لتلك الزيوت.
النتائج
لقد أظهرت نتائج تحليل الزيوت العطرية المستخلصة بواسطة جهاز مطياف الكتلة المرتبط بالكروماتوغرافيا الغازية لكلتا العينتين وجود ٥٦ مكونا ذا فاعلية حيوية بنسب مختلفة حيث كانت المركبات الرئيسة في عينات الزيوت المستخلصة الهندية منها والسعودية هي: ١.٨- سينول (بتركيز ٤٤.٢٤٪ و٤٦.٢٢٪ على التوالي) ومركب الفا-تيربينول (بتركيز ٧.٤٧٪ و٧.٠٤٪ على التوالي) ومركب تيربينين-٤-ول (بتركيز ٥.٠١٪ و٤.٨٣٪ على التوالي) ومركب جرانيول د (بتركيز ٤.٠٥٪ و٣.٥٤٪ على التوالي) وبيتا-بينين (بتركيز ٣.٣٨٪ و٣.٩٨٪ على التوالي). كما لوحظ نشاط فائق مضاد للجراثيم ضد السلالات المختارة في كلتا العينتين بحد أدنى من التركيز المثبط الذي تراوح بين ٠.٥-١٪. كما كان التأثير المضاد للأكسدة ذو نشاط معتدل في كلتا العيّنتين. علاوةً على ذلك لقد أظهرت عينات السوق الهندي والسعودي قيمتي التأثير المضاد للالتهابات المعرفة بتركيز المادة الموافقة للتثبيط النصفي بنسبة ٥٣.١٢٪ و٥٥.٢٦ ميكروغرام/مل على التوالي في اختبار تثبيط تمسخ الألبومين. مما يشير إلى إمكانات مضادة للالتهابات مماثلة أو شبيهة لتأثير دواء الإيبوبروفين.
الاستنتاجات
أن مكونات تركيبة الزيتين من كلتا العيّنتين متشابهتين نوعيا ولكن مع وجود بعض الاختلافات الكمية لكل مركب تم التعرف عليه. كما أنه لم يلحظ أي اختلافات كبيرة في الخصائص الدوائية لهما مما يتطلب إجراء المزيد من الدراسات لمزيد من التأكيد.
الكلمات المفتاحية: حب الهيل الأسود, العينات المسوَقة, الزيوت الأساسية, الكروماتوغرافيا الغازية, النشاط الدوائي
Introduction
Greater cardamom (Amomum subulatum, F: Zingiberaceae) is native to India, Nepal, and Bhutan. The fruit of this plant is mainly used as a flavoring agent in the cuisines of those countries and is considered as an aphrodisiac in the Middle East. The seeds of this plant are used as diuretics, astringents, and appetisers.1 A. subulatum is mainly grown as a cash crop in Eastern Nepal, India (Sikkim, West Bengal, Uttarakhand, Assam, Nagaland, Himachal Pradesh, and Arunachal Pradesh), and Southern Bhutan. Fruit or seeds of this plant are generally used for the treatment of cough, nausea, vomiting, congestive jaundice, gonorrhoea, headache, ischemic heart disease, pulmonary tuberculosis, and skin cancer, as well as in producing antioxidant and anti-inflammatory compounds.2
The fruit of A. subulatum contains 2%–3% essential oils (EOs). The main component of the EOs is the oxygenated monoterpene ‘eucalyptol’ or 1,8-ceniole (65%–80%), the concentration of which varies across cultivars and geographical conditions of cultivation.1 Variable chemical composition has been reported by different investigators. Kaskoos et al. reported 1,8-cineole, β-myrcene, α-terpineol and terpinen-4-ol as the major constituents of EO in the Indian (Sikkim) varieties,3 whereas Satyal et al. reported 1,8-cineole, alpha- and beta-pinene, and alpha-terpineol as the major consituents in the Nepalese varieties.4 In another study, Joshi et al. reported 1,8-cineole, α-terpineol, limonene, nerolidol,4-terpineol, δ-terpineol, δ-3-carene, β-myrcene and germacrene in the EO of Himachal Pradesh (Indian) cultivars.5 Shrestha reported a completely different composition of EOs of Nepalese cultivars, consisting of mainly α-terpineol, terpine-4-ol, pinocarvone, nerolidol, and pinocarveol.6 Hence, the composition of EOs in the known cultivars has been reported to be variable. Since then, A. subulatum has become one of the most widely investigated plants. Active compounds, including both simple and oxygenated monoterpenes and sesquiterpenes, have been reported in the EO of A. subulatum fruits.7,8
The EOs of this plant exhibit antifungal and antibacterial activities. Variable potency of antimicrobial activity has been reported for similar microbial strains.9,10 Additionally, anticancerous, nematocidal, and insecticidal activities have been reported by several researchers.3,4,8 In addition to the EOs, different solvent-soluble extracts of the fruit have been investigated for food preservative, anti-scabies, anticancer, immune suppression, and various other properties.11,12
Thus, the composition and pharmacological properties of the EOs of A. subulatum are still the subjects of extensive research interests. In the current study, we investigated and compared the EO composition of A. subulatum fruit available in local Indian and Saudi Arabian markets to assess the uniformity in the composition and pharmacological properties of the EOs by assaying in vitro antimicrobial activity against unique gram-negative microbial strains, by assessing antioxidant, and anti-inflammatory activities that have not been reported to date.
Materials and Methods
Sample collection and authentication
Fruit of A. subulatum was purchased from India (New Friend's Colony, East Delhi) and KSA (Al Kharj, Riyadh Region). Fruit samples were deposited and authenticated in an herbarium (2020/3/SOP/AS/027), dated 02-03-2020 at the School of Pharmacy, Sharda University, Greater Noida (UP). Fruits were pulverized using a grinder. Approximately 150 g of each ground sample was stored in a wide-mouth airtight amber coloured glass container for further study.
Hydrodistillation and percentage yield
A Clevenger-type apparatus (10 mL volume capacity, lighter than water, and fitted with a condenser), chiller (Buchi B-741, Switzerland), 2000 mL round bottom flask (RBF), and heating mantle (2000 mL) were used for the extraction of EOs. The powder (50 g) was transferred into the RBF, 1 L distilled water was added, the apparatus was fixed, the chiller was switched on, and the temperature was adjusted to 70 °C. The process was continued for 3 h, the volume of extracted EO was recorded, and the percentage yield was calculated. The experiment was repeated 3 times, and the percentage ± standard deviation was determined. The extracted EOs of each sample were then dried using anhydrous Na2CO3 and stored in amber coloured borosilicate glass vials at 4 °C for further analysis of sample composition and antimicrobial activity.
Gas chromatography–mass spectrometry analysis
The identification of metabolites in the EOs of the Indian and Saudi Arabian samples was performed using gas chromatography (GC) (HP 5890, Hewlett–Packard, Agilent Technologies, Palo Alto, CA, USA) coupled to a mass spectrometer (MS) (HP 5972A) equipped with a flame ionisation detector. An HP-5 MS (30 m × 250 μm × 0.25 μm film thickness) capillary column was used. The temperatures of the injector and detector were maintained at 270 °C and 300 °C, respectively. The oven temperature was initially set at 40 °C for 1 min, and then increased at a rate of 10 °C/min to 110 °C, maintained for 1 min, again increased at the rate of 10 °C/min to 300 °C, and then held for 5 min. Three microliters (3 μL) of the diluted sample (10% in acetone) was injected at a split ratio of 1:100. The flow rate for the carrier gas (helium) was adjusted to 3.0 mL per min. The process was repeated 3 times for each EO. The scan mass ranged from 50 to 550 m/z. Normal scanning was used for EO spectra.
Linear retention indices (RIs) of metabolites were calculated using a homologous series of n-alkanes (C8–C30) under similar conditions of temperature-programmed GC. Metabolites were identified by comparing linear RIs with those reported in the literature13 and mass spectra with those of NIST 05 and Wiley 275 inherent mass spectral library.
Analysis of different classes of terpenes
From the GC–MS spectra for each sample, the structure of each composition was identified.5,14 The composition of each sample was divided into different groups of terpenes such as monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, diterpene hydrocarbons, and non-terpenes.
Estimation of antibacterial activities of EOs
Micro-organisms
The in vitro antimicrobial activity of EOs was assessed in the 3 selected gram-negative bacterial strains, namely Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 35218), and Acinetobacter baumannii (ATCC BAA747). The strains were sub-cultured on Mueller–Hinton medium (MHA) at 37 °C prior to antibacterial assays.
Disc diffusion method
The antimicrobial activity of A. subulatum EOs was compared using a disc diffusion method.15 MHA plates were inoculated with 0.1 mL of appropriately diluted (2.5 × 10−6 CFU/mL) freshly grown cultures. Sterile discs (6-mm in diameter) impregnated with 10 μL of EOs were mounted. Solvent and MH broth were used as controls. Plates were incubated for 18–24 h at 37 °C to assess growth inhibition around the disc. All assays were performed in triplicate, and inhibition zone diameters were measured in mm following the CLSI guidelines.16
Minimum inhibitory/bactericidal concentrations
Serial EO sample dilutions of 0.125%, 0.25%, 0.5%, 1%, 2%, 4%, and 8% in analytical grade ethanol were used to determine MIC values by the broth macrodilution method.17 The lowest EO concentration at which there was no visible growth following incubation was considered to be the MIC. The minimum bactericidal concentration (MBC) was determined by subculturing 100 μL from each negative test tube on agar following a previous method.15 The experiment was repeated 3 times and the lowest concentration with no visible growth after 24 h incubation at 35 °C was considered to be the MBC. Means ± standard deviation values were calculated using Excel 2013 (Microsoft).
Free radical scavenging (2,2-diphenyl-1-picrylhydrazyl) assay
The in vitro antioxidant properties of EOs were analyzed by determining the free radical scavenging (FRS) ability induced by 2,2-diphenyl-1-picrylhydrazyl (DPPH) using a previous method with certain modifications.8 The DPPH-cation (10 mL; 0.1 mM) was prepared in methanol. Different dilutions of EOs and ascorbic acid (62.5.1000 μg/mL) were prepared in the same solvent separately. Approximately 2 mL each of different dilution of the mixture and DPPH solutions were vortexed and kept for 30 min at 37°C. After incubation, the optical absorbance (Ab) was recorded against a blank (a mixture of 2 mL DPPH and 2 mL methanol) using a UV-VIS spectrophotometer at 517 nm. The test was performed in triplicate. The percentage FRS ability of EOs was compared using the following equation:
| % Inhibition of DPPH-cation = [(1 − Absample /Abcontrol) × 100] |
Inhibition of albumin (BSA) denaturation assay
The in vitro anti-inflammatory activity of EOs was assessed using a bovine soluble albumin (BSA) denaturation method, with certain modifications in the process followed by Gunathilake et al..18 To perform the assay, seven dilutions (ranging from 6.25 to 100 μg/mL) of EOs and standard (Ibuprofen) were prepared in phosphate-buffered saline (PBS; pH = 6.8). Aliquots of 100 μL sample or standard, 1000 μL of 1% BSA, and 1400 μL of PBS were mixed thoroughly, and the reaction mixture was incubated at 37 °C for 15 min, heated at 72 °C for 5 min, and then cooled. The optical absorbance was measured at 660 nm against a blank containing a mixture of 1000 μL and 1500 μL of BSA (1%) and PBS, respectively, using a UV-VIS spectrophotometer. The test was performed in triplicate, and the percentage of protein denaturation (inhibition) by EOs was compared using the following equation:
| % inhibition BSA = [(1 − Absample /Abcontrol) × 100] |
Statistical analysis
All experiments were repeated three times. Regression-analysis was used to estimate the IC50 values for antioxidant and anti-inflammatory activities. All analyses were performed using Microsoft (MS 2010) Excel software.
Results
Percentage yield of EO
Hydrodistillation using Clevenger apparatus of fruit samples of A. subulatum from local Saudi Arabian and Indian markets yielded 1.9. ± 0.24% and 1.7. ± 0.11% of EO, respectively, and the colour of both samples was light yellow.
Composition of EOs
Table 1 presents the composition of EOs obtained from Saudi Arabian and Indian samples of A. subulatum fruit. The oils of A. subulatum samples were characterized by a high percentage of volatile components (97.01%–98.68%). The percentage of eucalyptol (1,8-Cineole), was identified as the main compound in the EOs of both Saudi Arabian (46.22%) and Indian (44.24%) samples. The EOs of Saudi Arabian and Indian samples also contained α-terpineol (7.04% and 7.47%, respectively), terpinen-4-ol (4.83% and 5.01%, respectively), β-pinen (3.98% and 3.38%, respectively), trans-p-mentha-1(7),8-dien-2-ol (3.54% and 2.34%, respectively), α-selinene (2.91% and 3.14%, respectively), β-myrcene (2.53% and 2.26%, respectively), and linalool (2.08% and 2.14%, respectively) in considerable amounts. Other compounds constituting less than 2%, namely α-pinene, γ-terpinene, α-terpinolene, cis-carveol, limonene, and β-selinene, were also identified in both samples. Compounds such as perillaldehyde, trans-geranic acid methyl-ester, methyl cinnamate, isoledene, elemene, and tetrasiloxane decamethyl were identified only in Saudi Arabian samples, whereas bicyclo[4.4.0]decane, α-springene, and arsenous acid were identified only in Indian samples.
Table 1.
Composition of volatile oil hydro-distilled from the Indian and Saudi Arabian A. subulatum.
| Composition (A) |
Percentage Area (B) |
RI Lit. (C) | RI Exp. (D) | ||
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | Indian | Saudi Arab | |||
| 1. | β-Thujene | 0.28 | 0.3 | 925 | 928 |
| 2. | α-Pinene | 1.41 | 1.87 | 932 | 938 |
| 3. | β-Pinene | 3.38 | 3.98 | 974 | 976 |
| 4. | β-Myrcene | 2.53 | 2.26 | 988 | 992 |
| 5. | 3-Carene | 0.26 | 0.26 | 1005 | 1011 |
| 6. | d-Limonene | 1.13 | 1.29 | 1024 | 1028 |
| 7. | beta.-Ocimene | 0.17 | 0.18 | 1048 | 1055 |
| 8. | γ-Terpinene | 1.64 | 1.79 | 1054 | 1058 |
| 9. | α- Terpinolen | 1.62 | 1.71 | 1086 | 1088 |
| 10. | p-Mentha-1,3,8-triene | 0.34 | 0.30 | 1118 | 1121 |
| % Monoterpene hydrocarbons | 12.76% | 13.94% | |||
| Oxygenated monoterpene | |||||
| 11. | 1,8 Ceniole | 44.24 | 46.22 | 1026 | 1032 |
| 12. | cis-Sabinene hydrate | 0.34 | 0.33 | 1065 | 1070 |
| 13. | Linalool | 2.14 | 2.08 | 1095 | 1093 |
| 14. | trans-Sabinene hydrate | 0.59 | 0.55 | 1098 | 1099 |
| 15. | cis-p-Menth-2-en-1-ol | 0.31 | 0.29 | 1124 | 1118 |
| 16. | p-Mentha-1(7),3-diene | 0.46 | 0.41 | 1160 | 1161 |
| 17. | p-Mentha-1,5-dien-8-ol | 1.16 | 1.06 | 1170 | 1166 |
| 18. | Terpinen-4-ol | 5.01 | 4.83 | 1180 | 1077 |
| 19. | p-Menth-1-en-9-al | 0.23 | 0.23 | 1191 | 1188 |
| 20. | α-Terpineol | 7.47 | 7.04 | 1194 | 1193 |
| 21. | cis-Carveol | 0.86 | 0.70 | 1206 | 1202 |
| 22. | 2-exo-Hydroxy-8-cineole | 0.31 | 0.23 | 1222 | 1228 |
| 23. | trans-Carveol | 0.18 | 0.16 | 1226 | 1231 |
| 24. | cis Citral | 0.49 | 0.43 | 1227 | 1235 |
| 25. | d-Carvone | 0.81 | 0.29 | 1241 | 1245 |
| 26. | Cis-Geraniol | 4.05 | 3.54 | 1256 | 1254 |
| 27. | trans Citral | 0.63 | – | 1260 | 1256 |
| 28. | L-Perillaldehyde | – | 0.11 | 1271 | 1273 |
| 29. | trans-Bornyl acetate | 0.33 | 0.29 | 1290 | 1285 |
| 30. | trans-Pinocarveyl acetate | t | 0.12 | 1309 | 1302 |
| 31. | trans-Geranic acid methyl ester | – | 1.26 | 1322 | 1323 |
| 32. | trans-p-mentha-1(7),8-diene-2-ol | 2.46 | 2.13 | 1332 | 1333 |
| 33. | α-Terpinyl propionate | 0.63 | 0.60 | 1428 | 1420 |
| % Oxygenated monoterpene | 72.79% | 72.9% | |||
| Sesquiterpenes hydrocarbons | |||||
| 34. | β-Elemene | 0.45 | 0.40 | 1389 | 1392 |
| 35. | 10s,11s-Himachala-3(12),4-diene | 0.15 | 0.11 | 1399 | 1402 |
| 36. | Aromadendrene | 0.77 | 0.38 | 4140 | 1437 |
| 37. | Isoledene | – | 0.24 | 1457 | 1460 |
| 38. | α-Elemene | – | 0.11 | 1477 | 1475 |
| 39. | Germacrene D | 0.28 | 0.24 | 1476 | 1481 |
| 40. | γ-Muurolene | 0.96 | 0.57 | 1488 | 1485 |
| 41. | α-selinene | 3.14 | 2.91 | 1489 | 1486 |
| 42. | β-selinene | 1.15 | 1.00 | 1496 | 1495 |
| 43. | Bisabolene | 0.18 | t | 1499 | 1505 |
| 44. | (−)-.α-Panasinsen | 0.19 | 0.16 | 1527 | 1530 |
| % Sesquiterpenes hydrocarbons | 7.27% | 6.18% | |||
| Oxygenated sesquiterpenes | |||||
| 45. | Nerolidol | 2.35 | 2.40 | 1560 | 1565 |
| 46. | Farnesol | t | 0.11 | 1740 | 1727 |
| % Oxygenated sesquiterpenes | 2.43% | 2.51% | |||
| Non-terpenoid | |||||
| 47. | Tetrasiloxane, decamethyl- | – | 1.17 | 1033 | 1035 |
| 48. | Octanal | 0.27 | 0.27 | 1068 | 1073 |
| 49. | 1,3,7-Nonatriene, 4,8-dimethyl- | 0.39 | 0.39 | 1105 | 1113 |
| 50. | Cyclododecene, (Z)- | 0.11 | t | 1193 | 1190 |
| 51. | Bicyclo[4.4.0]decane | 0.12 | – | 1241 | 1244 |
| 52. | Methyl cinnamate | – | 0.74 | 1375 | 1381 |
| 53. | 4,8,12-trimethyltrideca-1,3,7,11-tetraene | 0.24 | 0.16 | 1581 | 1592 |
| 54. | Estrone methyl ether | 0.13 | 0.11 | 2045 | 2060 |
| 55. | 9-Octadecenoic acid (Z)-, methyl ester | 0.31 | 0.26 | 2096 | 2172 |
| 56. | Arsenous acid, tris(tert-butyldimethylsilyl) ester | 0.19 | – | – | 2249 |
| % Non-terpenoid | 1.76% | 3.15% | |||
T = trace (less than 0.09%), (−) = not detected, (A): Composition of total essential oils (computed from the total GC peak area), (B): Percentage area, (C): RI exp. – KI Kovats' retention index relative to C8–C3 and 2n-alkanes. (D): RI Lit-KI Kovats' retention index.13
Antibacterial activity
In the present study, the assessed EOs exhibited superior antimicrobial potency against all selected microbes; however, the level of microbial growth inhibition was found to be dependent on the concentration of EOs and the microbial strain involved (Table 2).
Table 2.
Antimicrobial activity of essential oils extracted from Indian and Saudi Arabian A. subulatum.
| Gram-negative bacteria | Al-Mehran (KSA) |
AS (Delhi) |
||||
|---|---|---|---|---|---|---|
| ZI(MM) | MIC (% V/V) | MBC (% V/V) | ZI(MM) | MIC (% V/V) | MBC (% V/V) | |
| P. aeruginosa | 15.00 ± 0.00 | 0.5 | 1 | 15.00 ± 0.81 | 0.5 | 1 |
| E. coli | 16.00 ± 0.00 | 0.5 | 1 | 14.66 ± 0.94 | 0.5 | 2 |
| A. baumanii | 12.33 ± 0.94 | 1 | 4 | 12.66 ± 0.47 | 1 | 4 |
Zone of inhibition (ZI, mean ± SD of triplicates).
Antioxidant activities
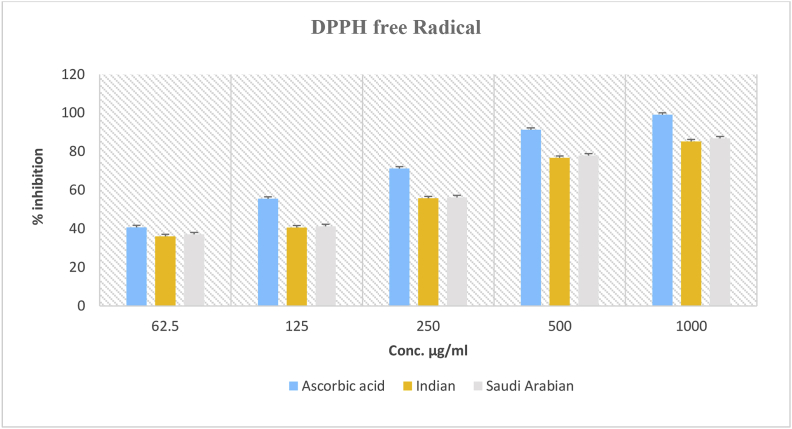
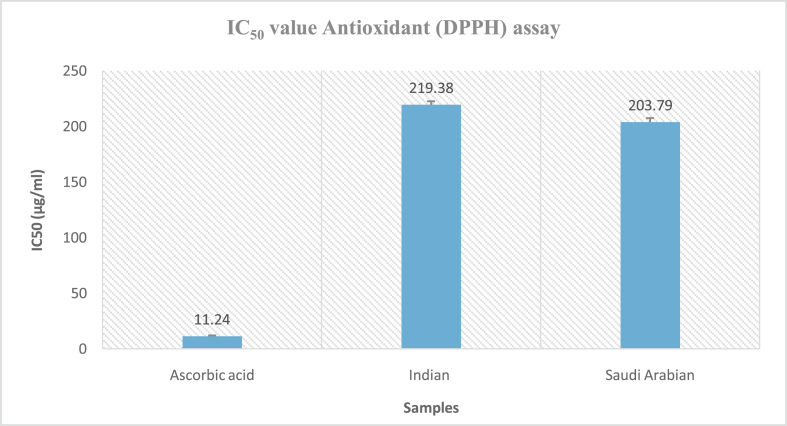
The FRS activity against DPPH-induced free radicals in Indian and Saudi Arabian samples at 1000 μg/mL was approximately 85.27% and 86.86%, respectively (Figure 1). The EOs of both samples exhibited similar antioxidant contents. The IC50 values of EOs for the Indian samples (219.38 μg/mL) were marginally higher than the Saudi Arabian samples (203.79 μg/mL) (Figure 2).
Figure 1.
Antioxidant activity of essential oils from A. subulatum (Indian and Saudi Arabian samples), and ascorbic acid using DPPH FRS assay.
Figure 2.
IC50 value of DPPH FRS assays of essential oils from A. subulatum (Indian and Saudi Arabian samples), and ascorbic acid.
Anti-inflammatory activity
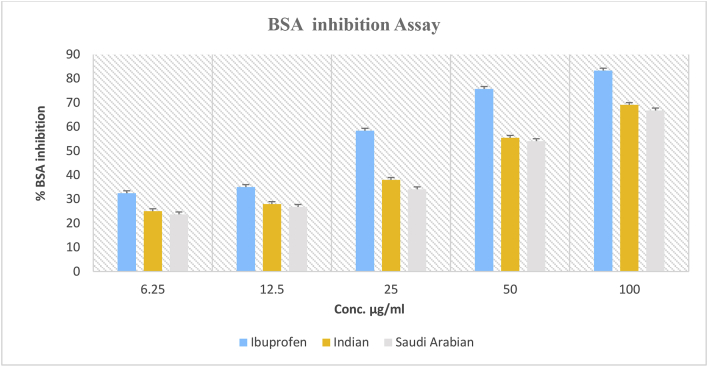
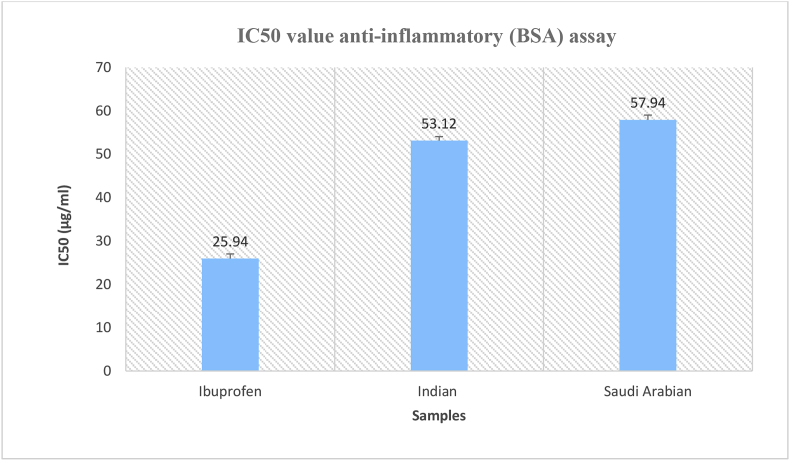
The percent inhibition of protein denaturation by Ibuprofen was approximately 83.33% at 100 μg/mL. The EOs of Indian and Saudi Arabian A. subulatum samples exhibited inhibition of 69.09% and 66.81%, respectively (Figure 3). The IC50 value for Ibuprofen was 25.94%, whereas the EOs of Indian and Saudi Arabian A. subulatum samples exhibited IC50 values of 53.12 μg/mL and 57.94 μg/mL, respectively (Figure 4).
Figure 3.
BSA inhibitory assays for essential oils from A. subulatum (Indian and Saudi Arabian samples), and Ibuprofen.
Figure 4.
IC50 value of BSA inhibitory assays for essential oils from A. subulatum (Indian and Saudi Arabian samples), and Ibuprofen.
Discussion
The EO yields of both A. subulatum samples were similar to those reported in Sikkim and Himachal Pradesh (India) cultivars and in local varieties,5 but lower than that of Nepalese varieties.2,4
The results of the current study demonstrated a high proportion of oxygenated monoterpenes, such as 1,8-cineole, α-terpineol, terpinen-4-ol, trans-p-mentha-1(7),8-dien-2-ol, and linalool in the extracted EOs. The obtained compositions are dissimilar to those reported in the literature. Kaskoos et al. reported high amounts of monoterpenes, including eucalyptol (77.4%) and β-myrcene (5.0%), and low amounts of α-terpineol (4.9%), terpinen-4-ol (2.3%), and caryophyllene (2.3%).3 Bhandari et al. also reported a completely different composition of EOs in a sample obtained from Uttarakhand (India) and demonstrated high amounts of eucalyptol (73.27%), along with α-terpineol, limonene, α-terpinyl acetate, α-pinene, and other compounds.19 Noumi et al. analysed EOs of A. subulatum fruits obtained from Jeddah, KSA, and demonstrated the presence of 1,8-cineole (41.7 ± 1.6%), similar to our results; however, other reported components exhibited only qualitative similarity with our results while exhibiting quantitative variations.10 Different types of terpenes, such as monoterpenes, sesquiterpenes, and diterpenes, resulting from the combination of 2, 3, and 4 units of isoprene (C5 units), respectively, exhibit a wide range of pharmacological activities and represent a large class of natural products.14 Several studies have reported a similar range of different classes of terpenoids.5,10,20 In several studies, quantitative variation in the composition of A. subulatum EO has been attributed to changes in the environmental, geographical, and genetic variability of the cultivated area.
EOs from Indian and Saudi Arabian samples exhibited higher antibacterial activity against gram-negative bacteria, with inhibition zones ranging from 15 to 12.33 mm. The present data demonstrated that A. subulatum EOs exhibit antimicrobial inhibitory activity against all selected gram-negative bacteria in the range of 0.5%v/v–1%v/v for MIC and 1%–4% for MBC. No major differences were observed between the samples. Among bacteria, P. aeruginosa and E. coli exhibited equal susceptibility to EOs obtained from both samples, whereas A. baumannii exhibited less susceptibility. Bachir and Benali demonstrated slightly higher sensitivity of gram-negative bacteria to EOs compared with that of gram-positive bacteria.21
Generally, gram-positive bacteria are more sensitive to antibiotics and EOs than gram-negative bacteria. The lower sensitivity of gram-negative bacteria could be attributed to their additional cell walls, which act as barriers to restricting the entry of hydrophobic compounds. Contrary to the general myth, Mith et al., after studying the effects of 15 commercial EOs against 8 bacterial strains, reported that a majority of EOs exhibit activity against gram-positive bacteria; however, Origanum majorana EO was more active against gram-negative bacteria than gram-positive bacteria.15 The antibacterial activity of A. subulatum is due to the presence of active antibacterial components in the EO.
Antimicrobial components identified in the oil of both samples included 1,8-cineole, α-pinene, β-pinen, geranic acid methyl-ester, β-myrcene, nerolidol, γ-terpinene, and α-terpinolen. These compounds may contribute to the activity against gram-negative bacteria. Eucalyptol (1,8-cineole) is the major compound identified in A. subulatum. Hendry et al. studied the antimicrobial activity of 1,8-cineole against gram-negative bacteria, including E. coli and P. aeruginosa, and reported higher activity of 1,8-cineole against gram-negative bacteria. Overall, their results indicated that EOs containing 1,8-cineole are more active than 1,8-cineole alone.22 Li et al. conducted a study regarding EOs of C. longepaniculatum leaf containing 1,8-cineole (48.55%) and reported excellent activity against gram-negative bacteria, which may be due to the hydrophobicity of 1,8-cineole.23
Other constituents, such as α-terpineol, terpinen-4-ol, linalool, limonene, p-menthane chemotype, α-pinene, α-selinene and β-selinene, nerolidol, terpinene, and terpinolene also contribute to antibacterial activity against gram-negative bacteria.24, 25, 26, 27, 28 Several other studies have also demonstrated the antimicrobial activity of A. subulatum EO against gram-negative bacteria.4,6,9,10,29 Dose-dependent increases in antioxidant effects of both samples with increasing concentration was also observed (p < 0.001). Ascorbic acid (standard) exhibited greater antioxidant activity than EOs obtained from both samples. The antioxidant activity of A. subulatum is mainly due to the presence of a high content of 1,8-cineole,30 α-terpineol,31 terpinen-4-ol,32 β-pinene and α-pinene,33 linalool,34 and β-myrcene.30 Furthermore, our results are aligned with reports by several other investigators. Such studies also directly support the antioxidant potential of A. subulatum EO.8,35 Protein denaturation % inhibition is normally the degree of protein stabilization measured against the control. The anti-inflammatory drug Ibuprofen and EOs showed a reduction in protein denaturation, and confirmed the anti-inflammatory activity of A. subulatum fruit Eos which may be helpful in the management of inflammatory conditions.36 A previous study demonstrated concentration-based bovine albumin denaturation inhibition by EOs, which is consistent with our study.37 The higher anti-inflammatory activity of Indian A. subulatum samples compared with KSA samples may be due to the presence of higher percentages of 1,8-cineole, α-terpineol, β-pinene, α-pinene, linalool, among several other components.32, 33, 34, 35, 36, 37, 38
Conclusions
EOs from the fruit of A. subulatum obtained from KSA and India exhibited qualitatively similar, but quantitatively different, compositions. No significant differences in pharmacological properties were observed while correlating the activities of both samples. Overall, higher antibacterial activity against selected gram-negative bacteria, moderate antioxidant activity comparable to that of standard ascorbic acid, and excellent anti-inflammatory activity similar to Ibuprofen were observed in both samples. Thus, EO of A. subulatum may be a suitable candidate as a novel alternative antibacterial and anti-inflammatory agent. Further studies involving marketed samples are required to confirm the useful pharmacological properties.
Recommendations
Further pharmacological evaluations should be carried out to determine the extent of other medicinal properties of A. subulatum EOs from different geographical origins.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study does not contain any experimental research with humans or animals performed by any of the authors. Ethical approval was exempted.
Authors' contributions
AA collected samples, conducted the research, interpreted the data, and wrote the entire manuscript. VS conceived and designed the study, provided logistic support, and revised a draft of the article, as well as interpreted the data of the article. Both authors have critically reviewed and approved the final draft, and are responsible for the content and similarity index of the manuscript.
Acknowledgments
The authors are grateful to Dr. Raees, College of Pharmacy, King Saud University, Riyadh, for advice regarding antioxidant and antimicrobial activity, and Dr. Afroz Bakht, College of Science, Prince Sattam Bin Abdulaziz University, Al-Kharj, for advice regarding GC–MS analysis.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Shrestha K.P., Shrestha J. Value chain analysis of large cardamom in Ilam District of Nepal. Azarian J Agric. 2018;5(6):179–189. http://azarianjournals.ir/wp-content/uploads/aja18110401.pdf [Google Scholar]
- 2.Agnihotri S., Wakode S. Antimicrobial activity of essential oil and various extracts of fruits of greater cardamom. Indian J Pharmaceut Sci. 2010;72(5):657–659. doi: 10.4103/0250-474X.78542. https://pubmed.ncbi.nlm.nih.gov/21695005/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaskoos R.A., Mir S.R., Kapoor R., Ali M. Essential oil composition of the fruits of Amomum subulatum Roxb. J Essent Oil-Bear Plants. 2008;11:184–187. doi: 10.1080/0972060X.2008.10643617. [DOI] [Google Scholar]
- 4.Satyal P., Dosoky N.S., Kincer B.L., Setzer W.N. Chemical compositions and biological activities of Amomum subulatum essential oils from Nepal. Nat Prod Commun. 2012;7:1233–1236. doi: 10.1177/1934578X1200700935. [DOI] [PubMed] [Google Scholar]
- 5.Joshi R., Sharma P., Sharma V., Prasad R., Sud R.K., Gulati A. Analysis of the essential oil of large cardamom (Amomum subulatum Roxb.) growing in different agro-climatic zones of Himachal Pradesh, India. J Sci Food Agric. 2013;93:1303–1309. doi: 10.1002/jsfa.5886. [DOI] [PubMed] [Google Scholar]
- 6.Shrestha R.L. GC-MS analysis, antibacterial and antioxidant study of Amomum subulatum Roxb. J Med Plants Stud. 2017;5(6):126–129. http://www.plantsjournal.com/archives/2017/vol5issue6/PartB/5-6-21-999.pdf [Google Scholar]
- 7.Rout P.K., Sahoo D., Jena K.S., Rao Y.R. Analysis of the oil of large cardamom (Amomum subulatum Roxb.,) growing in Sikkim. J Essent Oil Res. 2003;15:265–266. doi: 10.1080/10412905.2003.9712138. [DOI] [Google Scholar]
- 8.Alam A., Majumdar R.S. Antioxidant activity of essential oil of three cultivars of Amomum subulatum and standardization of high performance thin layer chromatography (HPTLC) method for the estimation of 1,8-cineole. Afr J Biotechnol. 2018;17:1129–1137. doi: 10.5897/AJB2018.16508. [DOI] [Google Scholar]
- 9.Alam A., Majumdar R.S., Alam P. Comparative study of metabolites and antimicrobial activities of essential oils extracted from three Amomum subulatum cultivars. Asian J Pharmaceut Clin Res. 2019;12:219–223. doi: 10.22159/ajpcr.2019.v12i6.33215. [DOI] [Google Scholar]
- 10.Noumi E., Snoussi M., Alreshidi M.M., Rekha P.D., Saptami K., Caputo L. Chemical and biological evaluation of essential oils from cardamom species. Molecules. 2018;23:2818. doi: 10.3390/molecules23112818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor I.P.S., Singh B., Sing G. Essential oil and oleoresins of cardamom (Amomum subulatum Roxb.) as natural food preservatives for sweet orange (Citrus sinensis) juice. J Food Process Eng. 2011;34:1101–1113. doi: 10.1111/j.1745-4530.2009.00525.x. [DOI] [Google Scholar]
- 12.Sharma B., Vasudeva N., Sharma S. Essential oil composition and anti-scabies potential of Amomum subulatum Roxb. Leaves. Anti Infect Agent. 2019;17:1. doi: 10.2174/2211352517666190919143631. [DOI] [Google Scholar]
- 13.Adams R.P. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. Identification of essential oil components by gas chromatography/mass spectrometry. [Google Scholar]
- 14.Mahdavi B., Yaacob W.A., Din L.B., Aisha M.A.S. Essential oil composition of three air-dried parts of etlingera brevilabrum. J Essent Oil-Bear Plants. 2013;16:17–22. doi: 10.1080/0972060X.2013.764200. [DOI] [Google Scholar]
- 15.Mith H., Duré R., Delcenserie V., Zhiri A., Daube G., Clinquart A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci Nutr. 2014;2:403–416. doi: 10.1002/fsn3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI . Performance standards for antimicrobial susceptibility testing. 28th ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2018. CLSI supplement M100. [Google Scholar]
- 17.Sakkas H., Economou V., Gousia P., Bozidis P., Sakkas V.A., Petsios S. Antibacterial efficacy of commercially available essential oils tested against drug-resistant gram-positive pathogens. Appl Sci. 2018;8:2201. doi: 10.3390/app8112201. [DOI] [Google Scholar]
- 18.Gunathilake K.D.P.P., Ranaweera K.K.D.S., Rupasinghe H.P.V. In Vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines. 2018;6(4):107. doi: 10.3390/biomedicines6040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhandari A.K., Bisht V.K., Negi J.S., Baunthiyal M. 1, 8-Cineole: a predominant component in the essential oil of large cardamom (Amomum subulatum Roxb.) J Med Plants Res. 2013;7(26):1957–1960. doi: 10.5897/JMPR2013.5131. [DOI] [Google Scholar]
- 20.Gurudutt K.N., Naik J.P., Srinivas P., Ravindranath B. Volatile constituents of large cardamom (Amomum subulatum Roxb.) Flavour Fragrance J. 1996;11:7–9. doi: 10.1002/(sici)1099-1026(199601)11:1<7::aid-ffj542>3.0.co;2. [DOI] [Google Scholar]
- 21.Bachir R.G., Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac J Trop Biomed. 2012;2(9):739–742. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendry E.R., Worthington T., Conway B.R., Lambert P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J Antimicrob Chemother. 2009;64(6):1219–1225. doi: 10.1093/jac/dkp362. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Li Z.W., Yin Z.Q., Wei Q., Jia R.Y., Zhou L.J. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int J Clin Exp Med. 2014;7:1721–1727. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4132134/ [PMC free article] [PubMed] [Google Scholar]
- 24.Carson C.F., Riley T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol. 1995;78:264–269. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 25.Zengin H., Baysal A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19(11):17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghaffari T., Kafil H.S., Asnaashari S., Farajnia S., Delazar A., Baek S.C. Chemical composition and antimicrobial activity of essential oils from the aerial parts of pinus eldarica grown in Northwestern Iran. Molecules. 2019;24(17):3203. doi: 10.3390/molecules24173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebouças de Araújo Í.D., Coriolano de Aquino N., Véras de Aguiar Guerra A.C., Ferreira de Almeida Júnior R., Mendonça Araújo R., Fernandes de Araújo Júnior R. Chemical composition and evaluation of the antibacterial and cytotoxic activities of the essential oil from the leaves of Myracrodruon urundeuva. BMC Compl Alternative Med. 2017;17(1):419. doi: 10.1186/s12906-017-1918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padalia R.C., Verma R.S., Chauhan A., Goswami P., Singh V.R., Verma S.K. p-Menthenols chemotype of Cymbopogon distans from India: composition, antibacterial and antifungal activity of the essential oil against pathogens. J Essent Oil Res. 2018;30(1):40–46. doi: 10.1080/10412905.2017.1375035. [DOI] [Google Scholar]
- 29.Naveed R., Hussain I., Tawab A., Tariq M., Hameed S., Mahmood M.S. Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria. BMC Compl Alternative Med. 2013;13:265. doi: 10.1186/1472-6882-13-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciftci O., Ozdemir I., Tanyildizi S., Yildiz S., Oguzturk H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol Ind Health. 2011;27(5):447–453. doi: 10.1177/0748233710388452. [DOI] [PubMed] [Google Scholar]
- 31.Bicas J.L., Neri-Numa I.A., Ruiz A.L.T.G., Carvalho J.E.D., Pastore G.M. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Toxicol. 2011;49(7):1610–1615. doi: 10.1016/j.fct.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Shapira S., Pleban S., Kazanov D., Tirosh P., Arber N. Terpinen-4-ol: a novel and promising therapeutic agent for human gastrointestinal cancers. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0156540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salehi B., Upadhyay S., Erdogan O.I., Kumar J.A., Jayaweera S.L.D., Dias D.A. Therapeutic potential of α- and β-pinene: a miracle gift of nature. Biomolecules. 2019;9(11):738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seol G.H., Kang P., Lee H.S., Seol G.H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016;16:17. doi: 10.1186/s12883-016-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ndoye Foe F.M., Tchinang T.F.K., Nyegue A.M., Abdou J.P., Yaya A.J.B., Tchinda A.T. Chemical composition, in vitro antioxidant and anti-Inflammatory properties of essential oils of four dietary and medicinal plants from Cameroon. BMC Compl Alternative Med. 2016;16:117. doi: 10.1186/s12906-016-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latif A., Ashiq K., Ashiq S., Ali E., Anwer I.S., Qamar S. Phytochemical analysis and in vitro investigation of anti- inflammatory and xanthine oxidase inhibition potential of root extracts of Bryophyllum pinnatum. J Anim Plant Sci. 2020;30:219–228. doi: 10.36899/JAPS.2020.1.0025. [DOI] [Google Scholar]
- 37.Juergens U.R., Dethlefsen U., Steinkamp G., Gillissen A., Repges R., Vetter H. Anti-inflammatory activity of 1,8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003;97(3):250–256. doi: 10.1053/rmed.2003.1432. https://doi:10.1053/rmed.2003.1432 [DOI] [PubMed] [Google Scholar]
- 38.Kim M.G., Kim S.M., Min J.H., Kwon O.K., Park M.H., Park J.W. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int Immunopharm. 2019;74:105706. doi: 10.1016/j.intimp.2019.105706. https://doi:10.1016/j.intimp.2019.105706 [DOI] [PubMed] [Google Scholar]