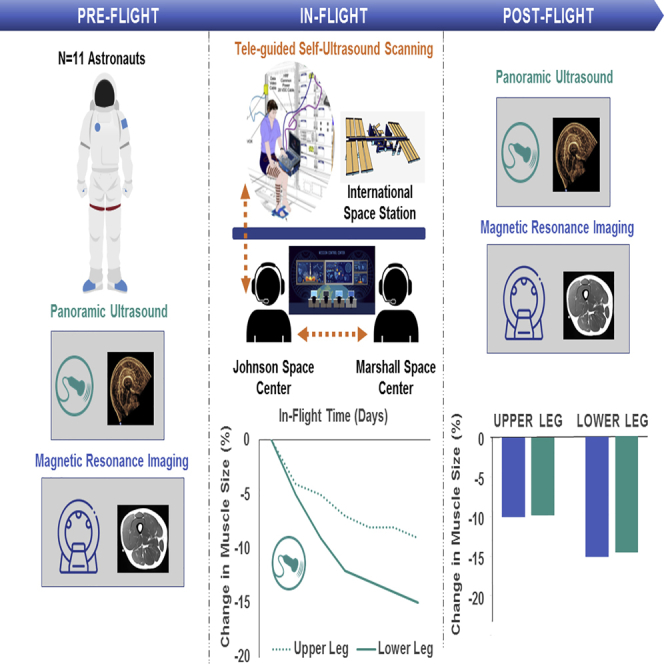
Summary
Loss of muscle mass is a major concern for long duration spaceflight. However, due to the need for specialized equipment, muscle size has only been assessed before and after spaceflight where ~20% loss is observed. Here, we demonstrate the utility of teleguided self-ultrasound scanning (Tele-SUS) to accurately monitor leg muscle size in astronauts during spaceflight. Over an average of 168 ± 57 days of spaceflight, 74 Tele-SUS sessions were performed. There were no significant differences between panoramic ultrasound images obtained by astronauts seven days prior to landing and expert sonographer after flight or between change in muscle size assessed by ultrasound and magnetic resonance imaging. These findings extend the current capabilities of ultrasound imaging to allow self-monitoring of muscle size with remote guidance.
Subject areas: Medicine, Medical Imaging, Biological Sciences
Graphical abstract
Highlights
-
•
We examined teleguided self-ultrasound to monitor leg muscle size on the ISS
-
•
Muscle thickness ultrasound does not detect change in muscle size during spaceflight
-
•
Panoramic ultrasound accurately monitors change in muscle size compared to MRI
-
•
Teleguided self-ultrasound reveals upper and lower leg muscle loss during spaceflight
Medicine; Medical Imaging; Biological Sciences
Introduction
Remote monitoring has been used for over six decades to characterize spaceflight-induced multisystem toxicity (Scott et al., 2019). However, due to the need for specialized equipment such as computerized axial tomography (CT) or magnetic resonance imaging (MRI) (Trappe et al., 2009), muscle size has only been assessed before and after spaceflight where large decreases in lower leg (~10–20%) and upper leg (~7–15%) muscle size have been reported (LeBlanc et al., 1995, 2000a; Trappe et al., 2009). Given that loss of strength often exceeds loss of muscle size (Kress and Hall, 2014), there is a significant need for a tool that can monitor the time course and magnitude of atrophic remodeling for future human exploration missions to the Moon, Mars, and beyond (Fitts et al., 2010).
The delivery of a new ultrasound machine to the International Space Station (ISS) in 2010 provided the first opportunity to remotely monitor muscle size during spaceflight. Here, we evaluated a teleguided self-ultrasound scanning (Tele-SUS) framework that extends the current capabilities of ultrasound imaging to remotely monitor lower extremity muscle size.
Results
Teleguided self-ultrasound scanning of muscle thickness during spaceflight does not detect change in muscle size
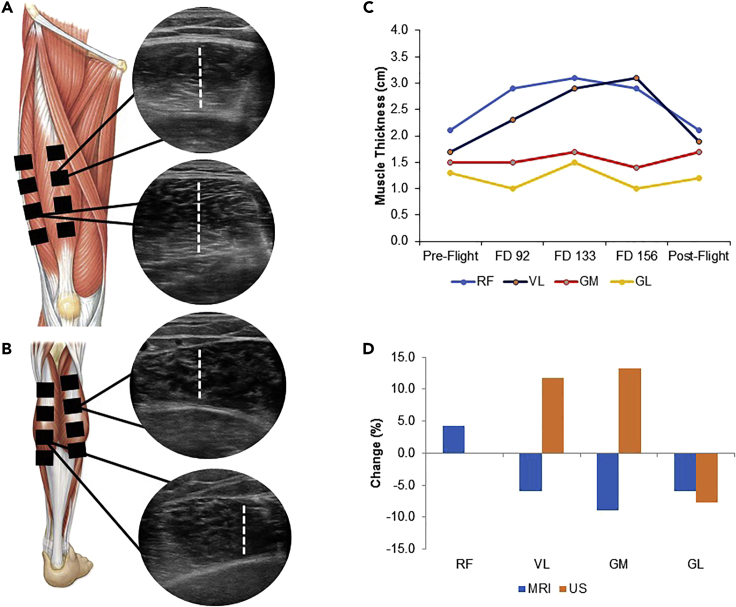
Ultrasound assessment of muscle size is typically performed by evaluating thickness of the rectus femoris (RF) at a single point along the thigh (Puthucheary et al., 2013). However, single point muscle thickness is associated with poor reliability (Mourtzakis et al., 2017), and other lower extremity muscles are more susceptible to atrophic remodeling than the RF (Ploutz-Snyder et al., 2018; Scott et al., 2020). To augment reliability and evaluate other functionally important muscles, a six-member team of sonographers, ultrasound experts, and remote guidance specialists developed a standardized Tele-SUS protocol (transparent methods in supplemental information) that evaluated muscle thickness at four points along four muscles: the RF, vastus lateralis (VL), gastrocnemius lateralis (GM), and gastrocnemius lateralis (GL) (Figures 1A and 1B; Figures S1A–S1D). We piloted muscle thickness Tele-SUS in one astronaut. First, ultrasound images were acquired by an expert sonographer approximately 40 days before spaceflight (pre-flight) and compared against MRI cross-sectional area (CSA) images. During spaceflight, the Tele-SUS protocol was performed by two ground-based teams and the astronaut located on the ISS on flight days 90, 133, and 156 using one-way video from the ISS to the ground and two-way voice communication. After 182 days of spaceflight, ultrasound and MRI images were acquired 1 day after flight. We found high variability in muscle thickness throughout spaceflight with both increases and decreases in all muscles (Figure 1C). In assessment of pre-flight to post-flight change, there was discordance between MRI (RF: +4.3%; VL: −6.0%; GM: −9.0%; GL: −6.0%) and ultrasound (RF: 0%; VL: +11.8%; GM: +13.3%; GL: 7.7%) (Figure 1D). We concluded that ultrasound assessment of muscle thickness was not a valid tool to detect change in muscle size during spaceflight.
Figure 1.
Teleguided self-ultrasound scanning of muscle thickness in spaceflight
(A) Schematic of upper leg muscles and ultrasound image of RF and VL muscle thickness.
(B) Schematic of lower leg muscles and ultrasound image of GM and GL muscle thickness.
(C) Time course of change in muscle thickness before, during, and after spaceflight.
(D) Pre-flight to post-flight percentage change in muscle size from MRI and ultrasound images.
See also Figures S1A–S1D.
Abbreviations are as follows: FD, flight day; MRI, magnetic resonance imaging; US, ultrasound; RF, rectus femoris; VM, vastus medialis; VL, vastus lateralis; GM, gastrocnemius medialis; GL, gastrocnemius lateralis.
Panoramic ultrasound assessment of muscle cross-sectional area is reliable and valid
We therefore conducted a series of studies evaluating panoramic ultrasound because it is a technique that, similar to MRI, permits automatic construction of CSA. Panoramic ultrasound requires consistent motion in a stable plane for optimal image construction. To ensure correct translocation of the probe during image acquisition, we first developed a prototype ultrasound guide using a flexible kitchen cutting board (Figures S2A and S2B). In 10 healthy participants (5 males, 5 females; age: 34.8 ± 9.4 years; body mass: 69.5 ± 11.2 kg), we then acquired panoramic ultrasound and matched MRI images at multiple points along the RF, vastus medialis (VM), VL, GM, and GL (Figures 2A and 2B); two trained raters manually traced all images (ultrasound: MATLAB, MathWorks, USA; MRI: ImageJ, National Institutes of Health, Bethesda, MD, USA, version 1.42). The coefficient of variation (CV) and intraclass coefficient for panoramic ultrasound between two raters ranged from 2.39% to 4.05% and 0.96 to 0.99, respectively (Table S1), demonstrating high reliability, and based on Bland-Altman plots, panoramic ultrasound was valid compared to MRI (Figures S3A–S3D). We subsequently developed three different-sized upper and lower leg ultrasound guides (Figures S4A and S4B) and, as previously reported (Scott et al., 2012), evaluated the reliability and validity of panoramic ultrasound against MRI in 9 healthy participants (8 male, 1 female; age: 34.5 ± 8.2 years; body mass: 74.7 ± 10.5 kg) before, during, and after 14 days of bed rest, a spaceflight analog. The absolute difference in CSA between MRI and panoramic ultrasound was small, ranging from 0.3 ± 1.0cm2 (RF) to 3.3 ± 2.1cm2 (VL) (Scott et al., 2012). We then assessed utility of panoramic ultrasound for longitudinal monitoring of muscle size in comparison with MRI among 27 participants (data previously reported: 26 male, 1 female; age: 34.6 ± 7.8 years; body mass: 77.5 ± 10.0 kg) randomized to 70 days of bed rest with or without exercise (Scott et al., 2017). In analysis of 698 panoramic ultrasound and 698 MRI images, we previously reported that the concordance between panoramic ultrasound and MRI was excellent in the quadriceps (Lin's concordance correlation coefficient: 0.78; p < 0.0001), and compared to MRI, panoramic ultrasound demonstrated high accuracy in detecting quadriceps atrophy and hypertrophy (sensitivity: 73.7%; specificity: 74.2%) and gastrocnemius atrophy (sensitivity: 83.1%) (Scott et al., 2017).
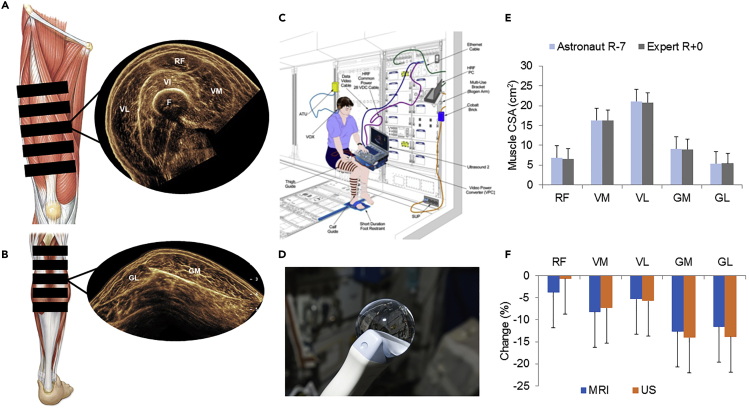
Figure 2.
Teleguided self-ultrasound scanning of panoramic imaging in spaceflight
(A) Schematic of upper leg muscles and panoramic ultrasound CSA image. Images were acquired in one motion in order to capture all quadriceps muscles (VM, RF, VL). The femur is also visible.
(B) Schematic of lower leg muscles and panoramic ultrasound image of GM and GL.
(C) Panoramic ultrasound image acquisition set up on the International Space Station.
(D) Water droplet on ultrasound probe in microgravity.
(E) Muscle CSA obtained using Tele-SUS on the ISS seven days prior to landing and expert sonographers on landing day.
(F) Pre-flight to post-flight percentage change in muscle CSA from MRI and panoramic ultrasound images. Data are represented as mean ± standard deviation.
See also Figures S4A, S4B, and S5, Tables S3 and S4.
Abbreviations are as follows: RF, rectus femoris; VM, vastus medialis; VL, vastus lateralis; VI, vastus intermedius; F, femur; GM, gastrocnemius medialis; GL, gastrocnemius lateralis, CSA, cross-sectional area; R, recovery; MRI, magnetic resonance imaging; Tele-SUS, teleguided self-ultrasound scanning.
Teleguided self-ultrasound with panoramic scanning during spaceflight accurately monitors change in muscle size
We therefore modified the original Tele-SUS protocol for acquisition of panoramic ultrasound during spaceflight (transparent methods). Panoramic ultrasound and MRI images were obtained in 11 astronauts (10 males, 1 female; age: 47.5 ± 5.9 years; body mass: 78.6 ± 10.6 kg) approximately 40 days before flight. The in-flight Tele-SUS protocol was performed as previously described with three major modifications. First, panoramic ultrasound with customized ultrasound guides was used (Figure 1C). Second, we hypothesized that due to molecular adhesion in microgravity, water reclaimed from the water recovery system could be used as an alternative acoustic medium to ultrasound gel. Water placed directly within the guide slices was an excellent acoustic medium and was used for all in-flight scans (Figure 2D). Finally, we only captured images during Ku-band availability and checked images against pre-flight image references. We repeated acquisition until image quality was sufficiently comparable to image references or acquisition window ended. Ultrasound images were acquired by an expert sonographer 1 day after flight, and MRI images were obtained within 4 days of ultrasound. Over an average of 168 ± 57 days of spaceflight, 74 Tele-SUS sessions were performed. Ninety-one percent (449 of 492 performed) of the images were rated high quality. The number of attempts to obtain high quality images ranged from 2.7 ± 1.5 (GL) to 4.3 ± 2.1 (VM). Acquisition time ranged from 33 min to 82 min (Table S3); there was a 19 ± 12 min improvement in acquisition time from first (72 ± 12 min) to second (54 ± 8 min) and subsequent (53 ± 11 min) sessions. In a subset of 256 in-flight images, CV between two raters ranged from 3.7% to 10.6% (Table S4), similar to our previous findings from images acquired from expert sonographers (Scott et al., 2012). On average, there was a decline during spaceflight in all muscles except the RF (Figure S5). There were no significant differences between panoramic ultrasound images obtained by astronauts seven days prior to landing and expert sonographer after flight (Figure 2E) or between ultrasound and MRI CSA change (Figure 2F).
Discussion
We developed an integrated framework that extends the current capabilities of ultrasound imaging to monitor muscle size using Tele-SUS. In addition to accurately assessing and monitoring leg muscle size, we demonstrated that astronauts completing long-duration spaceflight missions can successfully acquire high-quality self-scanned ultrasound images with remote guidance.
Spaceflight-induced muscle loss is a significant concern for astronauts and was observed following even short-duration (~14 day) Mercury, Gemini, and Apollo missions (Dietlein, 1974). More recently, LeBlanc et al. reported a 6% decrease in leg muscle volume following an 8-day mission (LeBlanc et al., 2000b), and decreases in the soleus (~10–20%), gastrocnemius (~10–15%), and knee extensor and flexor (~7–15%) muscle size have been reported following long-duration (6 months) missions, even with exercise countermeasures (English et al., 2020; Trappe et al., 2009). Tele-SUS accurately monitored change in leg muscle size in astronauts during ISS missions, suggesting that ultrasound could be used to monitor muscle size on future human exploration missions. Additional studies are needed to determine whether monitoring muscle loss and initiating targeted countermeasures can offset atrophy compared with usual care and to evaluate whether fully remote self-ultrasound scanning (i.e., non-teleguided) can be used for muscle monitoring given the ~20min time delay between astronauts and ground personnel on missions to Mars.
Muscle mass loss is also significant clinical concern for millions of patients with chronic diseases such as cancer, heart failure, and chronic obstructive pulmonary disease, as well as adults older than 70 years (Anker et al., 1997; Caron et al., 2009; Evans et al., 2008; Kim and Choi, 2013; Morley et al., 2014; Prado et al., 2009). Moreover, muscle atrophy can develop in younger individuals through immobilization and reduced physical activity (Trappe et al., 2001). Low muscle size and decreased muscle size over time are associated with an increased risk of mortality, treatment failure, hospital re-admission, and cancer therapy-induced toxicity (Evans et al., 2008; Greening et al., 2015; Kuroki et al., 2015; Prado et al., 2009; Stene et al., 2015). Evaluation of muscle size with current imaging techniques (e.g., CT, MRI) is time consuming, expensive, may involve exposure to low dose radiation, and requires large, specialized equipment (Prado and Heymsfield, 2014). To this end, ultrasound is one of the most widely used diagnostic imaging modalities throughout the world, and in many remote and operational settings, sonography constitutes the only imaging modality. Panoramic ultrasound therefore has distinct advantages in safety, cost, and portability compared to standard tools. Teleguided ultrasound consists of a geographically separated expert providing real-time direction for ultrasound image acquisition by an inexperienced sonographer. Recent studies have found that with minimal training, ultrasound-naive examiners can obtain high-quality ultrasound images when teleguided by a trained sonographer (Biegler et al., 2013; Chalumeau-Lemoine et al., 2009; Levine et al., 2015). However, the majority of studies to date consist of a teleguided operator scanning another individual (Marsh-Feiley et al., 2018). Whether Tele-SUS coupled with hand-held ultrasound probes (Francis et al., 2020) could provide a lower cost and portable approach to monitoring change in leg muscle size in patients is not known; however, at least one clinical trial is assessing the feasibility and utility of teleguided at-home ultrasound monitoring (NCT04131920).
We developed an integrated framework that allows non-expert operators to perform high-quality panoramic imaging using Tele-SUS. The evolution of Tele-SUS will complement the burgeoning field of telemedicine not only as a tool to monitor muscle mass but also as a potential method for monitoring other systems.
Limitations of the study
Our study limitations require consideration. First, although we quantified inter-rater reliability, intra-rater acquisition and analysis variability was not assessed. Small but significant inconsistencies during image acquisition could contribute to variability in muscle size. Second, due to limitations in positioning for self-scanning and astronaut time constraints, we quantified the anterior thigh and posterior calf muscles because of their sentinel role in ambulation. However, prior studies have reported substantial atrophy in the anterior calf, as well as the high rate of atrophy in the hamstrings (Miokovic et al., 2012; Scott et al., 2020). Ultrasound could likely be used to quantify the magnitude and trajectory of atrophy in many other superficial muscles. Finally, our results are limited by a relatively small number of astronauts; external validation of Tele-SUS in a larger cohort is warranted.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Jessica Scott (scottj1@mskcc.org).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The data sets that support the findings of this study are available from NASA's Life Sciences Data Archive (LSDA) (https://lsda.jsc.nasa.gov/) upon reasonable request. The LSDA is the repository for all human and animal research data, including that associated with this study. The LSDA team provides the appropriate processes, tools, and secure infrastructure for archival of experimental data and dissemination while complying with applicable rules, regulations, policies, and procedures governing the management and archival of sensitive data and information. The LSDA team enables data and information dissemination to the public or to authorized personnel either by providing public access to information or via an approved request process for information and data from the LSDA in accordance with NASA Institutional Review Board direction. MATLAB ultrasound analysis code is available on request. Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Jessica Scott (scottj1@mskcc.org).
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
This study was supported by the Human Research Program of the National Aeronautics and Space Administration (NASA) and National Space Biomedical Research Institute (NSBRI). J.M.S. is supported by a research grant from the National Cancer Institute and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Author contributions
Conceptualization, J.M.S., M.D., D.S.M., R.P.-S., and L.P.-S; methodology, J.M.S., M.D., D.S.M., E.H., L.S., and D.R.P.; investigation, J.M.S., M.D., D.S.M., E.H., L.S., D.R.P., and D.C.; software, N.A.; data curation, J.M.S., M.D., D.S.M., and D.C.; formal analysis, R.P.-S.; writing – original draft, J.M.S., M.D., and L.P.-S.; writing – review & editing, all authors; funding acquisition, L.P.-S.; project administration, J.M.S., M.D., and L.P.-S.
Declaration of interests
L.W.J. has stock ownership in Pacylex, Inc.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102344.
Supplemental information
References
- Anker S.D., Ponikowski P., Varney S., Chua T.P., Clark A.L., Webb-Peploe K.M., Harrington D., Kox W.J., Poole-Wilson P.A., Coats A.J. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- Biegler N., McBeth P.B., Tiruta C., Hamilton D.R., Xiao Z., Crawford I., Tevez-Molina M., Miletic N., Ball C.G., Pian L. The feasibility of nurse practitioner-performed, telementored lung telesonography with remote physician guidance - 'a remote virtual mentor. Crit. Ultrasound J. 2013;5:5. doi: 10.1186/2036-7902-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M.A., Debigare R., Dekhuijzen P.N., Maltais F. Comparative assessment of the quadriceps and the diaphragm in patients with COPD. J. Appl. Physiol. 2009;107:952–961. doi: 10.1152/japplphysiol.00194.2009. [DOI] [PubMed] [Google Scholar]
- Chalumeau-Lemoine L., Baudel J.L., Das V., Arrive L., Noblinski B., Guidet B., Offenstadt G., Maury E. Results of short-term training of naive physicians in focused general ultrasonography in an intensive-care unit. Intensive Care Med. 2009;35:1767–1771. doi: 10.1007/s00134-009-1531-3. [DOI] [PubMed] [Google Scholar]
- Dietlein L.F. NASA Johnson Space Center; 1974. The Proceedings of the Skylab Life Sciences Symposium. [Google Scholar]
- English K.L., Downs M., Goetchius E., Buxton R., Ryder J.W., Ploutz-Snyder R., Guilliams M., Scott J.M., Ploutz-Snyder L.L. High intensity training during spaceflight: results from the NASA Sprint Study. NPJ Microgravity. 2020;6:21. doi: 10.1038/s41526-020-00111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W.J., Morley J.E., Argiles J., Bales C., Baracos V., Guttridge D., Jatoi A., Kalantar-Zadeh K., Lochs H., Mantovani G. Cachexia: a new definition. Clin. Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Fitts R.H., Trappe S.W., Costill D.L., Gallagher P.M., Creer A.C., Colloton P.A., Peters J.R., Romatowski J.G., Bain J.L., Riley D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010;588:3567–3592. doi: 10.1113/jphysiol.2010.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J.R., Fairhurst H., Whalley G., Kaethner A., Ralph A., Yan J., Cush J., Wade V., Monteiro A., Remenyi B. The RECARDINA Study protocol: diagnostic utility of ultra-abbreviated echocardiographic protocol for handheld machines used by non-experts to detect rheumatic heart disease. BMJ Open. 2020;10:e037609. doi: 10.1136/bmjopen-2020-037609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening N.J., Harvey-Dunstan T.C., Chaplin E.J., Vincent E.E., Morgan M.D., Singh S.J., Steiner M.C. Bedside assessment of quadriceps muscle by ultrasound after admission for acute exacerbations of chronic respiratory disease. Am. J. Respir. Crit. Care Med. 2015;192:810–816. doi: 10.1164/rccm.201503-0535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.N., Choi K.M. Sarcopenia: definition, epidemiology, and pathophysiology. J. Bone Metab. 2013;20:1–10. doi: 10.11005/jbm.2013.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress J.P., Hall J.B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014;371:287–288. doi: 10.1056/NEJMc1406274. [DOI] [PubMed] [Google Scholar]
- Kuroki L.M., Mangano M., Allsworth J.E., Menias C.O., Massad L.S., Powell M.A., Mutch D.G., Thaker P.H. Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann. Surg. Oncol. 2015;22:972–979. doi: 10.1245/s10434-014-4040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc A., Lin C., Shackelford L., Sinitsyn V., Evans H., Belichenko O., Schenkman B., Kozlovskaya I., Oganov V., Bakulin A. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 2000;89:2158–2164. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- LeBlanc A., Rowe R., Schneider V., Evans H., Hedrick T. Regional muscle loss after short duration spaceflight. Aviat. Space Environ. Med. 1995;66:1151–1154. [PubMed] [Google Scholar]
- LeBlanc A., Schneider V., Shackelford L., West S., Oganov V., Bakulin A., Voronin L. Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet. Neuronal. Interact. 2000;1:157–160. [PubMed] [Google Scholar]
- Levine A.R., McCurdy M.T., Zubrow M.T., Papali A., Mallemat H.A., Verceles A.C. Tele-intensivists can instruct non-physicians to acquire high-quality ultrasound images. J. Crit. Care. 2015;30:871–875. doi: 10.1016/j.jcrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Marsh-Feiley G., Eadie L., Wilson P. Telesonography in emergency medicine: a systematic review. PLoS One. 2018;13:e0194840. doi: 10.1371/journal.pone.0194840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miokovic T., Armbrecht G., Felsenberg D., Belavy D.L. Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J. Appl. Physiol. 2012;113:1545–1559. doi: 10.1152/japplphysiol.00611.2012. [DOI] [PubMed] [Google Scholar]
- Morley J.E., Anker S.D., von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle. 2014;5:253–259. doi: 10.1007/s13539-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourtzakis M., Parry S., Connolly B., Puthucheary Z. Skeletal muscle ultrasound in critical care: a tool in need of translation. Ann. Am. Thorac. Soc. 2017;14:1495–1503. doi: 10.1513/AnnalsATS.201612-967PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploutz-Snyder L.L., Downs M., Goetchius E., Crowell B., English K.L., Ploutz-Snyder R., Ryder J.W., Dillon E.L., Sheffield-Moore M., Scott J.M. Exercise training mitigates multisystem deconditioning during bed rest. Med. Sci. Sports Exerc. 2018;50:1920–1928. doi: 10.1249/MSS.0000000000001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado C.M., Baracos V.E., McCargar L.J., Reiman T., Mourtzakis M., Tonkin K., Mackey J.R., Koski S., Pituskin E., Sawyer M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- Prado C.M., Heymsfield S.B. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J. Parenter. Enteral Nutr. 2014;38:940–953. doi: 10.1177/0148607114550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthucheary Z.A., Rawal J., McPhail M., Connolly B., Ratnayake G., Chan P., Hopkinson N.S., Phadke R., Dew T., Sidhu P.S. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- Scott J.M., Dolan L.B., Norton L., Charles J.B., Jones L.W. Multisystem toxicity in cancer: lessons from NASA's countermeasures Program. Cell. 2019;179:1003–1009. doi: 10.1016/j.cell.2019.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.M., Downs M., Buxton R., Goetchius E., Crowell B., Ploutz-Snyder R., Hackney K.J., Ryder J., English K., Ploutz-Snyder L.L. Disuse-induced muscle loss and rehabilitation: the national Aeronautics and space administration bed rest study. Crit. Care Explor. 2020;2:e0269. doi: 10.1097/CCE.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.M., Martin D.S., Ploutz-Snyder R., Caine T., Matz T., Arzeno N.M., Buxton R., Ploutz-Snyder L. Reliability and validity of panoramic ultrasound for muscle quantification. Ultrasound Med. Biol. 2012;38:1656–1661. doi: 10.1016/j.ultrasmedbio.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Scott J.M., Martin D.S., Ploutz-Snyder R., Matz T., Caine T., Downs M., Hackney K., Buxton R., Ryder J.W., Ploutz-Snyder L. Panoramic ultrasound: a novel and valid tool for monitoring change in muscle mass. J. Cachexia Sarcopenia Muscle. 2017;8:475–481. doi: 10.1002/jcsm.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene G.B., Helbostad J.L., Amundsen T., Sorhaug S., Hjelde H., Kaasa S., Gronberg B.H. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015;54:340–348. doi: 10.3109/0284186X.2014.953259. [DOI] [PubMed] [Google Scholar]
- Trappe S., Costill D., Gallagher P., Creer A., Peters J.R., Evans H., Riley D.A., Fitts R.H. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 2009;106:1159–1168. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- Trappe S.W., Trappe T.A., Lee G.A., Widrick J.J., Costill D.L., Fitts R.H. Comparison of a space shuttle flight (STS-78) and bed rest on human muscle function. J. Appl. Physiol. 2001;91:57–64. doi: 10.1152/jappl.2001.91.1.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets that support the findings of this study are available from NASA's Life Sciences Data Archive (LSDA) (https://lsda.jsc.nasa.gov/) upon reasonable request. The LSDA is the repository for all human and animal research data, including that associated with this study. The LSDA team provides the appropriate processes, tools, and secure infrastructure for archival of experimental data and dissemination while complying with applicable rules, regulations, policies, and procedures governing the management and archival of sensitive data and information. The LSDA team enables data and information dissemination to the public or to authorized personnel either by providing public access to information or via an approved request process for information and data from the LSDA in accordance with NASA Institutional Review Board direction. MATLAB ultrasound analysis code is available on request. Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Jessica Scott (scottj1@mskcc.org).