Abstract
The gut microbiome and the intestinal immune system are driving contributors to inflammatory bowel diseases (IBD). Both have an important signalling factor in common: short-chain fatty acids (SCFAs). SCFAs (acetate, propionate and butyrate) are produced by bacterial fermentation in the gut and exert several effects on host metabolism and immune system. This review provides an overview of the current knowledge of these effects, with specific focus on energy metabolism, intestinal barrier, immune system, and disease activity in IBD. To conclude, more research is needed on the cross-feeding mechanisms in the gut microbiome, as well as on the therapeutic potential of SCFAs on different disease models. Also randomized controlled trials and prospective cohort studies should investigate the clinical impact of SCFA administration.
Keywords: Short chain fatty acids, Inflammatory bowel disease, Acetate, propionate, Butyrate, Gut
1. Introduction
The human gut microbiota is a complex and dynamic microbial community of bacteria, fungi/yeasts, and viruses [1,2]. It is integral to the maintenance of health and the regulation of the host immune system [2]. Alterations in microbial composition have been linked to multiple pathologies, such as diabetes mellitus, obesity, colorectal cancer, and inflammatory bowel diseases (IBD) [2,3]. Especially bacterial species that feed on non-digestible dietary fibers play an important role in human health as they produce metabolites, such as SCFA. Next to being important cross-feeding products, these metabolites also beneficially affect the intestinal mucosa [4,5]. Due to their role in metabolic and immunologic homeostasis, and gut barrier integrity, they are of interest for therapeutic development, for instance in IBD which include Crohn's disease (CD) and ulcerative colitis (UC). These diseases are characterized by a complex interplay between the environment, the gut microbiome, and the host immune system in genetically susceptible individuals. However, the exact pathogenesis is still unknown. Consequently, therapeutics are mainly based on dampening the immune system [5] in a more or less targeted way. However, results are not satisfying and only 30% of patients at most achieve and maintain clinical and endoscopic remission [6,7]. Considering the pivotal role of the microbiota in IBD, new therapeutic approaches manipulating or replacing the gut microbiome have been proposed to restore a normal interaction between the immune system and the gut microbiome [8]. However, therapies such as prebiotics, probiotics, antibiotics, and fecal microbial transplantation (FMT) have so far shown inconsistent results.
The increasing knowledge on the importance of the gut microbiome in health and disease has led to the quest for new or next-generation probiotics, seeking a better selection, and formulation of administrated strains [9]. For the reasons mentioned above, SCFAs and SCFA-producing organisms might be the missing piece of the puzzle. We here review the current knowledge of the effects of SCFAs on human metabolism, on intestinal barrier, and on inflammation in IBD patients, to assess the utility of such next-generation probiotics.
2. Bacterial fermentation and production of SCFA
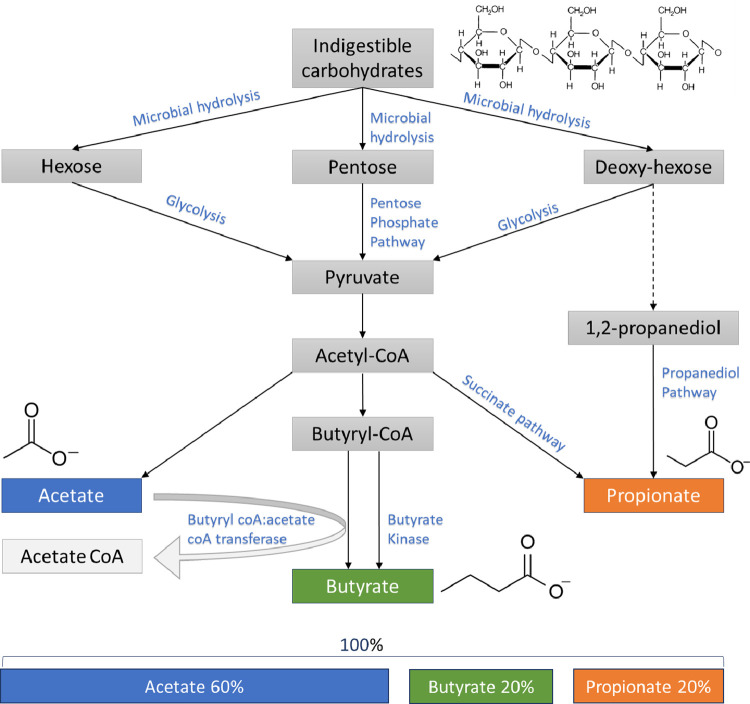
Many members of the intestinal microbiota are able to obtain carbon and energy from the fermentation of complex carbohydrates, which are indigestible by the human host [10]. This process of fermentation leads to the production of SCFAs in the colon [10]. The 3 major SCFAs produced by gut bacteria are acetate, propionate, and butyrate, which are aliphatic carboxylic acids containing 1 carbon in the carboxylic function and respectively 1, 2 and 3 carbons in the aliphatic tail [5]. Recent advances in metagenomics enabled the description of bacteria responsible for this production [4]. After microbial hydrolysis (Fig. 1), pyruvate, the main precursor for SCFAs, is produced through the glycolytic pathway for (deoxy-) hexoses and the pentose phosphate pathway for pentoses [10]. The pathways for acetate production are commonly spread among bacterial classes and attains the highest concentration in the intestinal lumen [11], in contrast to propionate and butyrate pathways which are more conserved and substrate specific [4]. To produce propionate, two pathways are possible, namely, the succinate pathway used by Bacteroidetes and Negativicutes (Firmicutes phylum), and the propanediol pathway used by Lachnospiraceae [11]. Butyrate production is mediated by the key enzymes, butyrate kinase, or butyryl CoA:acetate CoA transferase [5].The majority of the butyrate producers, amongst Faecalibacterium prausnitzii, Eubacterium rectale and Eubacterium hallii use the latter pathway whereas only a few butyrogenic taxa such as Coprococcus comes and Coprococcus eutactus are known to use the butyrate kinase route [4,12].
Fig. 1.
Overview of the pathways of bacterial fermentation resulting in the production of SCFA hydrolysis, including molar ratios of SCFAs in the colon on a total of 100%.
After fermentation, millimolar SCFA concentrations are available in the lumen from where they are absorbed by the colonic epithelium through passive and active transport [10]. Depending on the available substrates, SCFA concentrations [13] reach relative molar ratios for acetate-propionate-butyrate of approximately 60-20-20 respectively and for all human colonic regions (Fig. 1). Absolute concentrations are higher in the proximal colon due to higher availability of carbohydrates and SCFA uptake by the epithelium. After absorption, the proportion of butyrate decreases to 8% in the portal blood and the propionate proportion to 12%. Eventually, this drop was thought to show that the metabolism of propionate and butyrate is mainly restricted to the intestinal mucosa [5,13]. Contrarily, acetate concentrations remain measurable after uptake in the peripheral blood [13], pointing in the direction of systemic functions for acetate [5]. However, this should be nuanced as a recent randomized, cross-over study in healthy subjects exposed that during fermentation of carbohydrates labelled propionate and butyrate can double, as acetate may only increase with 10% [14]. Moreover, acetate does not exclusively originate from the colon, it is also produced endogenously from fatty acid oxidation [15].
Next to the butyrate producing commensals in the gut, a few gut pathogens also possess butyrogenic pathways [16]. However, they have divergently evolved to produce butyrate using distinct pathways. All gut commensals ferment pyruvate for butyrate production, whereas the pathogenic bacteria, for example Fusobacterium, utilizes different pathways like those for Glutamate (4-aminobutyrate) and Lysine, which are associated with a release of harmful byproducts like ammonia [16].
3. Impact of the gut environment on bacterial growth and SCFA production
Several factors influence the production of SCFA in the gastrointestinal tract [11]. First, carbohydrates that lead to high SCFA production, lower the pH in the colon, which sequentially affects the microbial composition and because of this, the SCFA production [12]. Moreover, most SCFA are absorbed in the proximal colon in exchange for bicarbonate, which neutralizes the luminal pH. As a result, the pH in the cecum is lower (5˒5) than the rectum (6˒5) [12]. For example, in vitro studies exploiting single-stage fermentor systems showed restricted growth of Bacteroides species fed on soluble polysaccharide, in mildly acidic pH [17]. This phenomenon can be explained by the lower capacity of Bacteroides compared to Firmicutes species to tolerate the presence of SCFA at pH 5˒5 than at pH 6˒5 resulting in a microbial composition shift [17]. This shift limits propionate and stimulates butyrate production [17,18]. Another example are bacteria using the butyryl CoA – acetate CoA transferase pathway that display higher acetate consumption and butyrate production at mildly acidic pH in comparison to near neutral pH [18]. Moreover, the lower pH prevents overgrowth by pH-sensitive pathogenic bacteria [12].
Besides pH, the presence of growth factors is needed, more specifically, iron has proven to be essential for SCFA production [11]. In iron-deficient rats, significantly lower concentrations of faecal butyrate and propionate were retrieved [19] and the bacterial composition was strongly modified, involving a higher abundance of Lactobacilli and Enterobacteriaceae and a significant decrease of Roseburia species and Eubacterium rectale, which are major butyrate producers [19]. In the same study, repletion of iron by the administration of FeSO4 led to increased caecal butyrate concentrations and partially restored microbial populations [19]. Similarly, the effects of iron on the production of SCFA have been examined in an in vitro model for the human gut [20]. Herein, very strong iron deficiency leads to a decreased production of butyrate and lower butyryl CoA- acetate CoA transferase gene expression. Conversely, under high iron conditions, Ruminococcus intestinalis displayed higher butyrate and higher gene expression [20]. Thus, iron depletion and repletion affect microbial composition and metabolic activity in the rodent gastrointestinal tract [19,20].
Third, intestinal gasses have an impact on SCFA production. Oxygen concentrations differ over different areas of the gastrointestinal tract and this is mainly caused by a different microbial composition with other oxygen sensitivity and metabolic capacity [11] and vice versa, the oxygen level affects the composition [21]. For example, Faecalibacterium prausnitzii is an obligate anaerobe which has a growth optimum at low, but not zero, oxygen levels [22]. Thus, oxygen levels influence the fermentation process and production of SCFA. In addition, bacteria consuming hydrogen affect the hydrogen partial pressure that by consequence influences the overall fermentation balance, and thus the SCFA production [11,23].
4. Effects of SCFA on the human metabolism
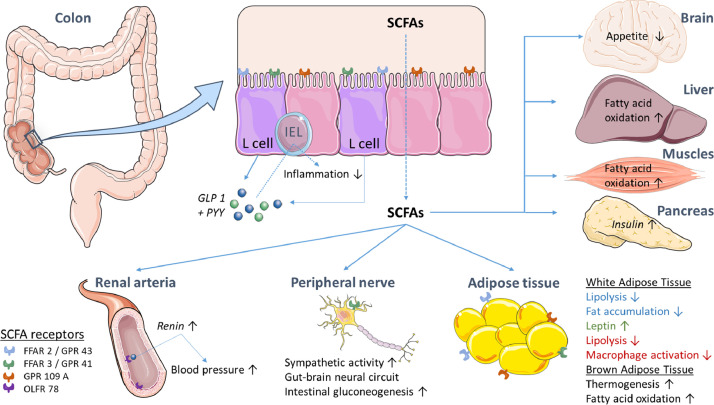
SCFA exert different beneficial effects on the human energy metabolism, including glucose, lipid, and cholesterol metabolism in several tissue types [4,12]. SCFA are transported through the colonocytes, which exploit butyrate as their main energy source after apical uptake [24]. The fraction of SCFAs that is not consumed by the colonic mucosa is transported through the basolateral membrane towards the portal blood stream [12]. The transport mechanisms for SCFAs from the blood to different tissue types is still largely unknown [12]. SCFA have shown to be present throughout the human body as several SCFA receptors have been identified, however, these are not involved in transportation (Table 1 and Fig. 2).
Table 1.
Overview of the SCFA-receptors, their expression, activation, production and function. (*)= Indirect effect, A= Acetate, AI= Anti-inflammatory, B= Butyrate, P= Propionate.
| Receptor | Expression | Activation | Production | Function |
|---|---|---|---|---|
| FFAR 2/ GPR 43 | IEL-cells | A > P > B | PYY, GLP 1 | Glucose and lipid metabolism Appetite regulation* |
| Adipose tissue | / | Regulation lipid homeostasis, Insulin regulation | ||
| Immune cells | AI signals (Treg & IL10↑) | Anti-inflammatory properties | ||
| FFAR 3/ GPR 41 | IEL-cells | B > P> A | PYY, GLP 1 | Glucose metabolism,Appetite regulation* |
| Sympatic ganglia | P | / | HR↑, E expenditure↑ | |
| GPR 109 A | Colonic epithelial cells | (β-OH-) B | AI-signals | Colonic inflammation ↓, carcinogenesis ↓ |
| Adipose tissue | / | Regulate lipid homeostasis | ||
| Immune cells (M, DC) | AI-signals (Treg & IL10↑) | Anti-inflammatory properties | ||
| OLFR 78 | Renal blood vessels | Microbial A and P | Renine* | Blood pressure regulation |
Fig. 2.
Overview of the direct and indirect effects of SCFAs on different tissues and human metabolism. FFAR= Free Fatty Acid Receptor, GLP 1=Glucagon-like receptor, GPR= G-coupled protein receptor, IEL=Intraepithelial lymphocyte, OLFR= Olfactory receptor, PYY=Peptide tyrosine tyrosine.
The SCFA receptors (Table 1) include free fatty acid receptors (FFAR) which are G-protein coupled receptors (GPRs) [24,25]. FFAR 2 or GPR 43 has been recognized on intestinal endocrine L-cells and the receptor is activated mainly by acetate and propionate [24,26]. For example, intestinal IgA production is promoted and mediated by FFAR2 upon acetate activation [27]. In contrast, FFAR 3 or GPR 41 is preferably activated by propionate and butyrate over acetate [24,25]. Activation of both receptors leads to PYY and GLP 1 secretion by the L-cells, which affect several tissues including the cardiovascular system, the pancreas and the brain [24].
More recently, GPR 109A and Olfactory receptor 78 (OLF 78) have also been identified as SCFA receptors. These receptors exert different effects depending on their location [24]. GPR 109A is expressed by colonic epithelial cell and upon activation by (β-hydroxy-) butyrate, it mediates anti-inflammatory properties like IL10 production [24,28,29]. Contrarily, OLFR 78 is mainly expressed by renal blood vessels, and is involved in blood pressure regulation [30].
Together, SCFAs are able to affect the metabolism and energy homeostasis [24]. However, many of the biological roles of SCFA have been investigated in vitro and in in vivo studies whereas little is known about their impact in humans, mainly due to a lack of quantitative data on SCFA kinetics, and clinical trials [12]. More extensive reading on the specific effects of SCFA on the energy homeostasis and metabolism can be found in the paper of Canfora et al [31].
5. Effects of SCFA on epithelial barrier and immune system
The innate immune system consists of components which are important in the first defense against infections, including the intestinal barrier. These components prevent the entrance of micro-organisms and their multiplication in the body. Furthermore, these mechanisms are regulated by the adaptive immune system, which requires more time and is cell-specific, unlike the innate response [32].
5.1. Innate immune system
5.1.1. Intestinal barrier integrity
The intestinal barrier is a complex, multi-component system in which the epithelium and innate immune cells contribute to its integrity and hence prevent unwanted translocation of components from the lumen to the body [33]. When this barrier is disturbed, bacteria can infiltrate and cause a dysregulated immune response, which is thought to be an important primary event in the pathogenesis of IBD [34].
The mechanisms that disturb barrier integrity include the downregulation of epithelial cadherin in tight junctions, a thinner mucus layer, abnormal goblet cell functioning including mucin 2 and resistin-like molecule β (RELMβ); and dysfunctional Paneth cell associated mechanisms, including secretion of antimicrobial products, nucleotide binding oligomerization domain protein 2 (NOD2), and autophagy-related 16-like I (ATG16LI) gene associated functions [35].
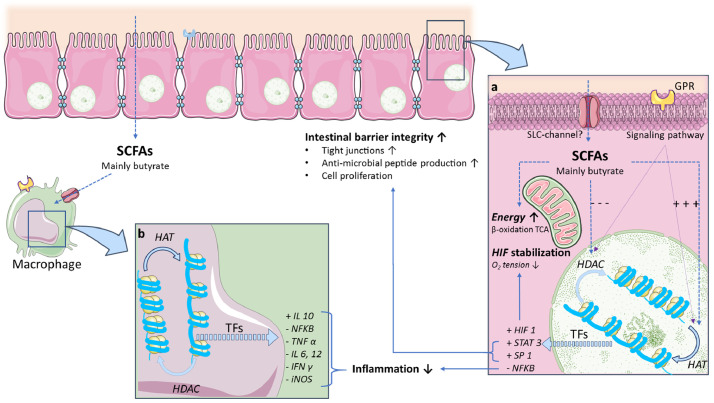
Butyrate and other SCFAs provide abundant energy to the intestinal epithelial cells by β-oxidation in the mitochondrial tricarboxylic acid cycle (TCA cycle) [5,34] (Fig. 3 A). Second, butyrate also acts as a hypoxia inducible factor 1 (HIF 1) stabilizer, a transcription factor that coordinates barrier protection [5,36]. Butyrate encouraged barrier protection, unless HIF-1β is absent [36]. Third, butyrate promotes barrier integrity through the activation of genes coding for tight-junction components and protein reassembly through transcription factors (Fig. 3A), such as STAT3 and SP1 [5]. This activation results in the maintenance and/or increased transepithelial electrical resistance (TEER) in human, rodent, and porcine experiments [37], [38], [39]; even after exposure to inflammatory conditions [40]. Similar effects were achieved through the supplementation of CD microbiota with a butyrate-producing candidate probiotic, namely Butyrococcus pullicaecorum. These in vitro observations provided evidence for the beneficial effects of butyrate on the intestinal barrier [41]. This is relevant for IBD as butyrate-producing bacteria are often depleted and intestinal barrier is proposed to be an important therapeutic target [5,42].
Fig. 3.
Mechanisms of SCFA, mainly butyrate, to protect the intestinal barrier and control inflammation by inhibition of HDAC and activation of HAT in colonocytes (a) and macrophages (b). This inhibition leads to translation of the DNA and the production transcription factors resulting in HIF stabilization, increased intestinal barrier integrity and downregulation of inflammatory mediators. GPR= G-protein coupled receptor, HAT= histone acetyl transferase, HDAC= histone deacetylase, HIF= hypoxia inducible factor, SLC= solute carrier, TCA= tricarboxylic acid cycle, TF= transcription factor.
Although positive effects on the intestinal barrier have been noted in rodent, porcine, and human experiments, contradictory data has been achieved in some in vitro studies [72]. Vancamelbeke et al. demonstrated that butyrate (8mM, for 48h) – when applied to a human primary colonic monolayer – induced beneficial effects on TEER [42]. In the same experiments, butyrate did not improve epithelial barrier integrity in the presence of inflammatory stimulators and was even shown to be detrimental there [42]. Similar observations have been noted regarding the protection of colonic stem cells against microbial metabolites [43]. Thus, butyrate may have dual effects and even negatively affect the gut barrier, especially in the presence of inflammation. Therefore, additional studies are needed to provide a more nuanced explanation for the effects of butyrate on the intestinal barrier [42,43].
5.1.2. Effects of SCFAs on neutrophils, monocytes and macrophages
SCFAs also affect neutrophils, monocytes and macrophages (Fig. 3 B) by downregulating histone deacetylase (HDAC) [39,44] and nuclear factor-ƘB (NFƘB) [24]. Consequently, these gut metabolites are able to manipulate gene expression. In neutrophils, this mechanism is thought to inhibit TNF production in the presence of lipopolysaccharides (LPS) [45]. Furthermore, high SCFA concentrations inducing IL8, IL6 and IL1β in these cells, while lower concentrations do not [28]. Moreover, SCFAs affect chemotaxis of neutrophils, induced by inflammatory mediators and chemokines, and their viability [29].
Many studies have determined the specific effects of SCFAs on monocytes and macrophages. A study by Cox et al showed the anti-inflammatory capacity of SCFA by regulating cytokine and prostaglandin E2 (PGE2) production [46]. Decreased IL10 was observed after LPS-stimulation, as well as a dose-dependent inhibition of cytokine CCL2. Similarly, in PBMC's a drop in LPS-induced TNFα and IFNγ have been noticed. SCFAs do appear to reduce the responsiveness of lamina propria macrophages to commensal bacteria via nitric oxide, IL6, and IL12 [47]. However, another study identified pro-inflammatory effects, as augmented concentrations of IL1β, IL6, and IL8 were observed [48]. Yet, these different observations seem to be dependent on the studied tissue, the applied stimulations, and environmental conditions [28]. Nevertheless, it is clear that SCFAs affect monocytes and macrophages in their immune function [28].
5.1.3. Effects on dendritic cells
SCFAs may affect the differentiation and functions of dendritic cells (DC). In In vitro studies butyrate could inhibit the maturation of DCs when incubated with different inflammatory inducers [28]. The delay in human DCs maturation was explained by inhibition of dendrite formation and expression of surface markers88. Moreover, butyrate altered the production of cytokines, such as IL10 and IL12 [49]. These pre-treated DCs with butyrate showed a lower capacity to stimulate T cells [49]. Also downregulation of DC development by butyrate has been reported, although the functional maturation was not affected after LPS stimulation [50]. Most likely butyrate and propionate can inhibit the histone deacetylase (HDAC) and thereby suppress the expression of transcription factors [50], which acetate cannot [51]. These observations point in the direction of unique characteristics for each SCFA.
5.2. Adaptive immune system
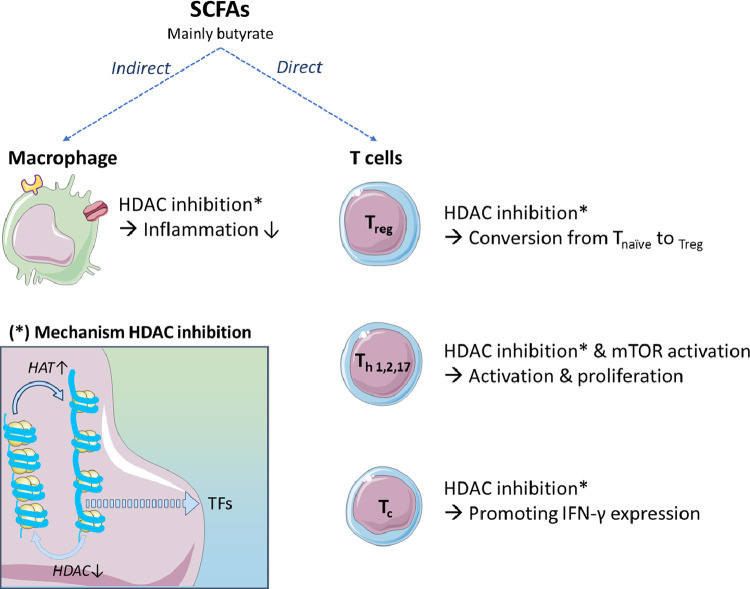
SCFAs are able to modulate the adaptive immune system by influencing the macrophages and dendritic cells as well as exerting direct effects on the adaptive immune system. First of all, the activation of GPR109A-receptor by butyrate in macrophages and DCs (Fig. 3B and Fig. 4) appears to be crucial for maintaining the equilibrium between pro-and anti-inflammatory T cells [52]. GPR109A-deficient KO mice exhibit a reduced IL10 and an increased IL17 production by T cells which increases the susceptibility to colitis [52].
Fig. 4.
Schematic overview of the (in)direct effects of SCFAs, mainly butyrate, on the adaptive immune system. The HDAC-inhibition mechanism is shown in the square (*) and promotes translation of transcription factors. HAT= histone acetyl transferase, HDAC= histone deacetylase, mTOR= mammalian target of rapamycin, TF= transcription factor.
Second, these microbial metabolites can potentiate the conversion of naïve T cells to regulatory T cells. This might be explained by taking together the effects on DCs and the results of in vitro studies that showed an increase of two enzymes, indoleamine 2˒3-dioxygenase 1 (indoleamine) and aldehyde dehydrogenase 1A2, which are known to diminish immune activation through tryptophan depletion and generation of retinoid acid [53]. In this mechanism the SLC5a8 transporter might be involved, as well as the inhibition of HDAC [50].
5.2.1. Regulatory T cells
Increased numbers of regulatory T cells are thought to be caused by the inhibition of HDAC. For example, butyrate has been demonstrated to facilitate extrathymic peripheral polarization towards regulatory T cells in vitro and in vivo [51]. Clostridia spp. producing butyrate can induce colonic regulatory T cells in mice. The causal mechanism might be the enhancement of H3 acetylation in the promotor and the conserved non-coding sequence regions of the Foxp3 locus by butyrate, which increases the expression of this transcription factor [54]. The activation of FFAR2 might also be involved in this shift towards regulatory T cells. Contrarily, acetate does not directly show HDAC inhibition, therefore, the conversion towards butyrate by the microbiota might be an interesting target [50].
5.2.2. Helper T cells
Next to the polarization of regulatory T cells, SCFAs are also affecting polarization and activation of Th1, Th2, and Th17 by HDAC inhibition. For example, studying allergic airway inflammation, SCFAs have been shown to cause an impaired FFAR3 mediated Th2 polarization [55]. In this regard, a recent study demonstrated the protection of a high fiber diet and acetate administration against the development of allergic airways disease [56]. Acetate, propionate and butyrate also promote the generation of Th1 and Th17 in vitro [57]. Additionally, in vivo mice studies showed less inflammation after supplementing acetate in the drinking water in combination with anti-CD3 administration. This observation might be the consequence of simultaneous production of the anti-inflammatory cytokine IL10 [28,57]. These effects were again mediated by HDAC inhibition, nevertheless, mTOR (mammalian target of rapamycin) activation was also proposed to be involved [57]. Moreover, the same HDAC mechanism is exploited to induce Fas-mediated apoptosis of T cells by butyrate. In vitro studies showed that butyrate inhibits the proliferation of T lymphocytes at lower concentrations whereas higher concentrations induce apoptosis in activated T cells [58].
5.2.3. Cytotoxic T cells
The effects of SCFAs, especially butyrate and propionate, on cytotoxic T cells (CTL) have been investigated [59]. An in vitro Study by Luu et al. (2018) showed independence of SCFA-receptors FFAR2 and -3 in the butyrate-mediated regulation of CTLs [59]. However, similarly to other T cells, HDAC inhibition by butyrate and propionate was identified to modulate the gene expression of CTLs and thereby promoting their IFN-γ expression. Acetate was suggested to enhance this IFN-γ production as well, conversely by operating as a metabolic substrate for CTLs [59]. Moreover, increased acetate concentrations are required for optimal memory CD8+ T cell function in vitro and in vivo during bacteremia in mice [60].
6. Relevance of SCFA-producing bacteria and SCFAs in Inflammatory Bowel Diseases: an update of recent work
IBD is characterized by an altered microbial composition, often mentioned as dysbiosis [5]. Generally, microbial dysbiosis in CD and UC patients is associated with fewer SCFA-producing bacteria. Many of the depleted bacteria belong to the Ruminococcaceae and Lachnospiraceae families of the Firmicutes phylum. Both families represent most of the butyrate-producing bacteria in the gut. The most repetitive finding is the reduced abundance of F. prausnitzii in patients with active IBD. In UC, alterations in Roseburia species have been observed. In CD, a higher abundance of Ruminococcus gnavus, and a lower abundance of F. prausnitzii, Bifidobacterium adolescentis, Dialister invisus and other SCFA-producing microbiota has been reported [5,61]. The presence of Eubacterium and Roseburia species, as well as SCFA biosynthesis and secondary bile acids has been associated with remission in fecal microbial transplantation studies in IBD patients [62]. Whereas, absence of remission has been linked to Fusobacterium, Suterella, and Escherichia spp., as well as increased heme [62]. Moreover, acetate-producing bifidobacteria are known to exhibit various probiotic effects against enteropathogenic infections [63]. Furthermore, co-culturing studies with bifidobacteria and Faecalibacterium spp. demonstrated proven cross-feeding activities between both bacterial groups. Additionally, acetate is a crucial source for butyrate producing bacteria. This production has already proven its importance in the immune system and highlights the relevance of studies on acetate [24,44]. Therefore, cross-feeding is an important metabolic interaction mechanism and includes features such as the utilization of acetate by butyrate-producing bacteria [64].
Moreover, crossfeeding might explain the indirect effects of acetate on the human body, especially the immune system [64]. For example, although acetate did not show a direct inhibition of the HDAC mechanism [56], administration of acetate was shown to have protective effects, mediated by HDAC inhibition [55]. This points in the direction of its utilization by microbiota to produce butyrate. Yet, increasing acetate might not be enough, and it might be necessary to increase the abundance of the butyrate-producing bacteria, as well as fiber in the diet [5]. Therefore, more research on this topic is necessary.
Next to crossfeeding, different strategies might be pursued to obtain higher SCFA concentrations in the colon [65]. First, the administration of a prodrug, for example tributyrin, may increase serum butyrate level. However, up today, there are no reports of clinical trials with this compound in IBD [65]. Second, highly fermentable dietary fibers such as prebiotics can cause a significant rise in plasma SCFA levels. Moreover, administration on long term basis increases fecal levels of total SCFA and butyrate [65]. Although prebiotics might be good inducers of SCFA, they are often not well tolerated in IBD patients because of bloating and abdominal cramps [65]. Third, direct postbiotic delivery of SCFAs in the colon might be obtained by enema-based administration or orally slow-release formulations [65]. Fourth, exploiting endogenous metabolic processes that produce SCFA to attain health benefits is another interesting possibility [65]. For example, low alcohol consumption creates a temporary increase in plasma acetate levels and is associated with a lower risk of heart disease and diabetes [65]. Different methods might be integrated, for example, combining dietary and postbiotic administration by esterification of SCFAs into resistant starch, protects them from release and absorption in the small intestine [65].
Furthermore, SCFAs measured in feces of IBD patients are lower compared to healthy controls. A decrease in acetate and propionate, yet not in butyrate concentrations was observed in UC patients [61], while another study showed decreased propionate and butyrate [66]. The reduction in SCFA in IBD has furthermore been associated with disease activity, and patients in remission have higher levels of butyrate than patients with severe active disease [67]. Recently, butyrate has been shown to promote the growth of SCFA-producing bacteria in IBD patients [68]. However, further investigation is needed to determine its clinical implication.
Supplementation of butyrate-producing bacteria, especially Butyrococcus pullicaecorum, improved epithelial barrier integrity in CD-based simulations [41]. Boesmans et al. positively assessed the safety and tolerance of this species in healthy individuals [69]. Nevertheless, addition of butyrate on biopsies of inflamed and non-inflamed UC patients showed that its potency to downregulate gene expression in inflammatory pathways was greater in non-inflamed controls [70]. Moreover, TNFα has been identified to reduce the responsiveness of the intestinal epithelium to butyrate, which might undermine its potential efficacy in IBD patients with active inflammation [71] and may rather suggest a prophylactic administration to prevent disease flares.
In this respect, in vivo mice experiments showed contradicting results, for example, oral administration of butyrate aggravates dextran sodium sulphate (DSS)-induced colitis, while daily intraperitoneal butyrate injection mildly improved disease activity [72]. Another study by Tye et al. (2018) demonstrated an aggravation of DSS colitis in mice by limiting butyrate-producing organisms due to nucleotide-binding oligomerization leucine rich repeat and pyrin domain containing (NLRP1), which is an inflammasome sensor [73]. Loss of this inflammasome significantly ameliorated the induced colitis [73].
Recently a multi-omics study of the gut microbial ecosystem in IBD showed that SCFAs were generally reduced in dysbiotic patients [74]. The reduction of especially butyrate is consistent with the observed depletion of butyrate producers such as F. prausnitzii and R. hominis [74]. Most clinical studies are randomized trials, and only very limited numbers of cohort studies are available on this topic [74]. There is especially a lack of large prospective cohort studies [74].
Considering the differences in UC and CD presentation [3], there might indeed be a different response between these two clinical phenotypes upon SCFA administration. Although speculative, protective effect of SCFA administration may be more observed in UC or CD colitis compared to CD ileitis, or perhaps in general more in patients with a milder disease manifestation. However, to our knowledge, there are no studies available on this topic.
Up till now, most studies used butyrate and its potential to decrease symptoms, and inflammation. However, crossfeeding in IBD might be an interesting target as well. For example, acetate supplementation in colitis induced mice have been showed to play a crucial role in the response of the gut to insults and tissue repair [75]. Moreover, administration of butyrate may be more toxic than acetate when directly added to the intestinal cells [59]. Together, more research is needed to determine the nuanced effects of acetate, acetate-producing organisms and propionate when targeting inflammatory bowel disease.
7. Conclusion and research gaps
Microbial SCFAs exert several beneficial effects on the human metabolism by intervening in glucose homeostasis, lipid metabolism, and appetite regulation. Moreover, SCFAs affect intestinal barrier integrity and hence the host's immunity, mainly by inhibiting the HDAC-mechanism, and are therefore of increasing interest in therapy development. So far, most research has been performed on the effects of butyrate, pointing in the direction of a prebiotic and/or a probiotic treatment to support management in IBD. Restoring dysbiosis in CD and UC by raising colonization of butyrate-producing organisms seems promising, although acetate might be an alternative target.
To date, in vitro studies have been mainly executed in epithelial monocultures. The development of coculture models combining the microbiome and the immune system is mandatory to make progress. Additionally, most in vivo studies have been based on chemically induced colitis models in mice. Consequently, adding different models, like immune mediated models, instead of chemically induced ones would broaden the knowledge on this topic. Subsequently, more research is needed on the cross-feeding mechanisms in the gut microbiome, as well as on the therapeutic potential of SCFAs on different disease models, including determination of optimal dose and composition. Also randomized controlled trials and prospective cohort studies should investigate the clinical impact of SCFA administration.
8. Outstanding questions
It is unclear how SCFA administration might affect microbiome composition, and immune system in IBD patients. To bridge this knowledge gap, it is important to decipher the crossfeeding mechanisms, as well as the therapeutic potential of SCFA. Do acetate and propionate have the potential to be therapeutic targets, and what would be the optimal dose and indication? How does crossfeeding work, and how can it be used to support IBD management? Are there any differences in potential effects in CD vs UC? How relevant are disease activity and extent of the lesions? Given that a reduction of Faecalibacterium prausnitzii is associated with postoperative Crohn's recurrence, could SCFAs be indicated in the setting of Crohn's disease postoperative prevention of recurrence? A better fundamental understanding of these aspects will provide evidence for the support by pre-, pro-, or synbiotics in the management of IBD.
9. Search strategy and selection criteria
Data for this review were identified by searches of PubMed and references from relevant articles using the search terms “short chain fatty acids”, “SCFA”, “butyrate”, “acetate”, “propionate”, “inflammatory bowel disease”, “IBD”, “gut microbiome”, and any combinations of these terms. Only articles published in English between 2010 and 2020 were included, earlier articles were only added if highly relevant.
Contributors
SD has written the manuscript and SD, KM, KV, JR and SV had substantial contributions to all of the following: (1) drafting the article or revising it critically for important intellectual content and (2) final approval of the final version of the submitted manuscript.
Declaration of Competing Interest
KM reports employment as a Pfizer Medical advisor in inflammation and immunology since 5/01/2021. JR reports grants from J&J, Beneo, Colruyt, Prodigest, Aphea.Bio, Cargill, other from Yakult, Nutricia, GSK Vaccines, MRM health, Aphea.Bio, Janssen Pharmaceuticals, Roche, Takeda, Tsumura, ABInbev, DSM, Nestle, Pfizer, BMS, Ferring, outside the submitted work; KV reports grants from Nestlé, personal fees from Yakult, personal fees from Biocodex, outside the submitted work; SV reports grants from AbbVie, J&J, Pfizer, and Takeda, other from AbbVie, Arena Pharmaceuticals, Avaxia, Boehringer Ingelheim, Celgene, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, Hospira, Janssen, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Takeda, Theravance, and Tillots Pharma AG, outside the submitted work; The remaining authors disclose no conflicts.
Acknowledgements
This work was supported by the VIB Grand Challenges Program and European Research Council (ERC) [grant number 694679]; K.M. is a postdoctoral fellow and S.V. is senior clinical investigator of the Fund for Scientific Research-Flanders, Belgium (FWO-Vlaanderen). The funders had no role in review design, interpretation and writing of this work.
The figures have been created using Servier Medical Art from https:// smart.servier.com.
References
- 1.Stange E.F., Schroeder B.O. Microbiota and mucosal defense in IBD: an update. Expert Rev Gastroenterol Hepatol. 2019;13:963–976. doi: 10.1080/17474124.2019.1671822. [DOI] [PubMed] [Google Scholar]
- 2.Vrancken G., Gregory A.C., Huys G.R.B., Faust K., Raes J. Synthetic ecology of the human gut microbiota. Nat Rev Microbiol. 2019 doi: 10.1038/s41579-019-0264-8. [DOI] [PubMed] [Google Scholar]
- 3.Eisenstein M. A slow-motion epidemic. Nature. 2016;540 doi: 10.1038/540S98a. S98-S99. [DOI] [PubMed] [Google Scholar]
- 4.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venegas D.P. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagnini C., Pizarro T.T., Cominelli F. Novel pharmacological therapy in inflammatory bowel diseases: beyond anti-tumor necrosis factor. Front Pharmacol. 2019;10:1–11. doi: 10.3389/fphar.2019.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiner P. Mechanism-based treatment strategies for IBD: cytokines, cell adhesion molecules, JAK inhibitors, gut flora, and more. Inflamm Intestinal Dis. 2019;4:79–96. doi: 10.1159/000500721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pigneur B., Sokol H. Fecal microbiota transplantation in inflammatory bowel disease: the quest for the holy grail. Mucosal Immunol. 2016;9:1360–1365. doi: 10.1038/mi.2016.67. [DOI] [PubMed] [Google Scholar]
- 9.El Hage R., Hernandez-Sanabria E., Van de Wiele T. Emerging trends in ‘smart probiotics’: Functional consideration for the development of novel health and industrial applications. Front Microbiol. 2017;8:1–11. doi: 10.3389/fmicb.2017.01889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugenholtz F., Mullaney J.A., Kleerebezem M., Smidt H., Rosendale D.I. Modulation of the microbial fermentation in the gut by fermentable carbohydrates. Bioactive Carbohydr Dietary Fibre. 2013;2:133–142. [Google Scholar]
- 11.Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 12.Den Besten G. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings J.H., Pomare E.W., Branch H.W.J., Naylor C.P.E., MacFarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boets E. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Layden B.T., Angueira A.R., Brodsky M., Durai V., Lowe W.L. Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013;161:131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Anand S., Kaur H., Mande S.S. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front Microbiol. 2016;7:1–12. doi: 10.3389/fmicb.2016.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker A.W., Duncan S.H., Carol McWilliam Leitch E., Child M.W., Flint H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71:3692–3700. doi: 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kettle H., Louis P., Holtrop G., Duncan S.H., Flint H.J. Modelling the emergent dynamics and major metabolites of the human colonic microbiota. Environ Microbiol. 2015;17:1615–1630. doi: 10.1111/1462-2920.12599. [DOI] [PubMed] [Google Scholar]
- 19.Dostal A. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J Nutr. 2012;142:271–277. doi: 10.3945/jn.111.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dostal A. Iron modulates butyrate production by a child gut microbiota in vitro. mBio. 2015;6:1–12. doi: 10.1128/mBio.01453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L., Kelly C.J., Colgan S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol – Cell Physiol. 2015;309 doi: 10.1152/ajpcell.00191.2015. C350-C360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan M.T. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J. 2012;6:1578–1585. doi: 10.1038/ismej.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf P.G., Biswas A., Morales S.E., Greening C., Gaskins H.R. H2 metabolism is widespread and diverse among human colonic microbes. Gut Microbes. 2016;7:235–245. doi: 10.1080/19490976.2016.1182288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasubuchi M., Hasegawa S., Hiramatsu T., Ichimura A., Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers E.S., Morrison D.J., Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74:328–336. doi: 10.1017/S0029665114001657. [DOI] [PubMed] [Google Scholar]
- 26.Mohammad S. Role of free fatty acid receptor 2 (FFAR2) in the regulation of metabolic homeostasis. Curr Drug Targets. 2015;16:771–775. doi: 10.2174/1389450116666150408103557. [DOI] [PubMed] [Google Scholar]
- 27.Wu W. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrêa-Oliveira R., Fachi J.L., Vieira A., Sato F.T., Vinolo M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:1–8. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohira H., Tsutsui W., Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotlo K. The olfactory G protein-coupled receptor (Olfr-78/OR51E2) modulates the intestinal response to colitis. Am J Physiol-Cell Physiol. 2020;318:C502–C513. doi: 10.1152/ajpcell.00454.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 32.Peterson L.W., Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 33.van Hemert S., Skonieczna-Żydecka K., Loniewski I., Szredzki P., Marlicz W. Microscopic colitis—microbiome, barrier function and associated diseases. Ann Transl Med. 2018;6 doi: 10.21037/atm.2017.03.83. 39-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong J.-P. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin Med J. 2019;132:1951–1958. doi: 10.1097/CM9.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos G.P., Papadakis K.A. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly C.J. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao W. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCβ2. Int J Mol Sci. 2016;17:1–12. doi: 10.3390/ijms17101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valenzano M.C. Remodeling of tight junctions and enhancement of barrier integrity of the CACO-2 intestinal epithelial cell layer by micronutrients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng L. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J Immunol. 2017;199:2976–2984. doi: 10.4049/jimmunol.1700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan H., Ajuwon K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One. 2017;12:1–20. doi: 10.1371/journal.pone.0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geirnaert A. Butyrate-producing bacteria supplemented in vitro to Crohn's disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vancamelbeke M. Butyrate does not protect against inflammation-induced loss of epithelial barrier function and cytokine production in primary cell monolayers from patients with ulcerative colitis. J Crohn’s Colitis. 2019;13:1351–1361. doi: 10.1093/ecco-jcc/jjz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiko G. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;165:1708–1720. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratajczak W. Immunomodulatory potential of gut microbiome-derived shortchain fatty acids (SCFAs) Acta Biochim Pol. 2019;66:1–12. doi: 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- 45.Vinolo M.A.R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22:849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Cox M.A. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E2 and cytokines. World J Gastroenterol. 2009;15:5549–5557. doi: 10.3748/wjg.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012;277:66–73. doi: 10.1016/j.cellimm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Mirmonsef P. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am J Reprod Immunol. 2012;67:391–400. doi: 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nastasi C. Butyrate and propionate inhibit antigen-specific CD8+ T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-15099-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh N. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arpaia N. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh N. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveira L.D.M., Teixeira F.M.E., Sato M.N. Impact of retinoic acid on immune cells and inflammatory diseases. Mediators Inflamm. 2018 doi: 10.1155/2018/3067126. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao T. The epigenetic modification during the induction of Foxp3 with sodium butyrate. Immunopharmacol Immunotoxicol. 2018;40:309–318. doi: 10.1080/08923973.2018.1480631. [DOI] [PubMed] [Google Scholar]
- 55.Trompette A. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 56.Thorburn A.N. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6 doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 57.Park J. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bailón E. Butyrate in vitro immune-modulatory effects might be mediated through a proliferation-related induction of apoptosis. Immunobiology. 2010;215:863–873. doi: 10.1016/j.imbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Luu M. Regulation of the effector function of CD8+ T cells by gut microbiota-derived metabolite butyrate. Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-018-32860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balmer M.L. Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity. 2016;44:1312–1324. doi: 10.1016/j.immuni.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Machiels K. A decrease of the butyrate-producing species roseburia hominis and faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 62.Paramsothy S. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019;156 doi: 10.1053/j.gastro.2018.12.001. 1440-1454.e2. [DOI] [PubMed] [Google Scholar]
- 63.Fukuda S. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–549. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 64.Rios-Covian D., Gueimonde M., Duncan S.H., Flint H.J., De Los Reyes-Gavilan C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol Lett. 2015;362:1–7. doi: 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 65.Gill P.A., van Zelm M.C., Muir J.G., Gibson P.R. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48:15–34. doi: 10.1111/apt.14689. [DOI] [PubMed] [Google Scholar]
- 66.Huda-Faujan N. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J. 2010;4:53–58. doi: 10.2174/1874091X01004010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumari R., Ahuja V., Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J Gastroenterol. 2013;19:3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Facchin S. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol Motil. 2020:13–25. doi: 10.1111/nmo.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boesmans L. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of butyricicoccus pullicaecorum to healthy volunteers. mSystems. 2018;3:1–11. doi: 10.1128/mSystems.00094-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magnusson M.K., Isaksson S., Öhman L. The anti-inflammatory immune regulation induced by butyrate is impaired in inflamed intestinal mucosa from patients with ulcerative colitis. Inflammation. 2020;43:507–517. doi: 10.1007/s10753-019-01133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrer-Picón E. Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm Bowel Dis XX. 2019:1–13. doi: 10.1093/ibd/izz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berndt B.E. Butyrate increases IL-23 production by stimulated dendritic cells. Am J Physiol – Gastrointestinal Liver Physiol. 2012;303:1384–1392. doi: 10.1152/ajpgi.00540.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tye H. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lloyd-Price J. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laffin M. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-48749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]