Summary
The effects of climate change on tropical forests will depend on how diverse tropical tree species respond to drought. Current distributions of evergreen and deciduous tree species across local and regional moisture gradients reflect their ability to tolerate drought stress, and might be explained by functional traits.
We measured leaf water potential at turgor loss (i.e. ‘wilting point’; πtlp), wood density (WD) and leaf mass per area (LMA) on 50 of the most abundant tree species in central Panama. We then tested their ability to explain distributions of evergreen and deciduous species within a 50 ha plot on Barro Colorado Island and across a 70 km rainfall gradient spanning the Isthmus of Panama.
Among evergreen trees, species with lower πtlp were associated with drier habitats, with πtlp explaining 28% and 32% of habitat association on local and regional scales, respectively, greatly exceeding the predictive power of WD and LMA. In contrast, πtlp did not predict habitat associations among deciduous species.
Across spatial scales, πtlp is a useful indicator of habitat preference for tropical tree species that retain their leaves during periods of water stress, and holds the potential to predict vegetation responses to climate change.
Keywords: deciduous, drought tolerance, evergreen, forest response, moisture, osmotic potential, species traits, water potential at turgor loss point
Introduction
Global climate change is altering precipitation patterns, resulting in drying trends and increasing frequency and severity of drought in many parts of the world (IPCC, 2015; Trenberth et al., 2011; Dai et al., 2018). Forest productivity is reduced under drier climates (e.g. Poorter et al., 2017; Taylor et al., 2017; Meakem et al., 2018) and in drought years (Hofhansl et al., 2015; Meakem et al., 2018). Drought can also cause elevated tree mortality (e.g. Zuleta et al., 2017; Klein & Hartmann, 2018), particularly among the large trees that contribute substantive fractions of total ecosystem biomass and productivity (Phillips et al., 2010; Bennet et al., 2015; Lutz et al., 2018; Meakem et al., 2018). These effects are likely to result in positive feedbacks to climate change through reduced ecosystem carbon sequestration and a shift towards lower biomass forests (Weltzin et al., 2003; McDowell et al., 2020). Modeling these dynamics is critical to reducing the large uncertainty surrounding the role of forests – particularly tropical forests – in shaping Earth’s future climate (Friedlingstein et al., 2006; Cavaleri et al., 2015). Within this broader context, an important challenge is characterizing how the distribution of phylogenetically and functionally diverse tropical tree species depends on moisture availability, against the background of the copious interactions of biotic and abiotic factors integrated over the deep history of the species and ecosystems (Condit et al., 1995; Wright, 2002; Engelbrecht et al., 2007; Brodribb et al., 2020).
To effectively predict distributions of traits or species across tropical moisture gradients, it is necessary to identify plant traits that are both strongly associated with moisture availability and can be readily measured across numerous species. Hydraulic traits – for example, hydraulic conductivity of sapwood and leaves and xylem tension at 50% or 88% loss of maximum hydraulic conductivity (P50, P88) – are mechanistic traits strongly linked to a physiological function, which have been found to be correlated with drought tolerance and species distributions across moisture gradients (e.g. Nardini & Luglio, 2014; Liu et al., 2019; Laughlin et al., 2020). However, measuring these traits is very time‐consuming, presenting a substantial barrier to their use in species‐rich tropical forests. More rapidly measurable traits, including wood density (WD) and leaf mass per area (LMA), have been collected for a broader array of species and shown to predict growth, survival and light requirements of species in diverse communities (e.g. Reich, 1995; Poorter & Bongers, 2006; Shipley et al., 2006). However, WD and LMA have limited power to predict species drought tolerance (McGregor et al., 2020) or distributions across moderate moisture gradients (Umaña et al., 2020). WD is often taken as a proxy for vulnerability to xylem embolism because it correlates with P50 within and among temperate species (Rosner, 2017; Savi et al., 2019). Studies from the tropics show that WD does not predict P50 in tropical species (Trueba et al., 2017; de Guzman et al., 2020), but relates to other hydraulic properties such as sapwood water release curves (de Guzman et al., 2020).
One trait that can be measured efficiently and is strongly correlated with drought tolerance and spatial distributions with respect to water availability is turgor loss point (πtlp) (Tyree et al., 1999; Bartlett et al., 2012a; Farrel et al., 2017). Species whose leaves lose turgor (i.e. wilt) at more negative water potentials are generally more drought‐tolerant (e.g. Baltzer et al., 2008; Bartlett et al., 2016a; Kunert, 2020; McGregor et al., 2020). The link between πtlp and drought tolerance has been observed across species differing in local distributions with respect to topographic habitats of varying water availability (Bartlett et al., 2016b; McFadden et al., 2019), across wet‐vs‐dry climate communities (Baltzer et al., 2008; Blackman et al., 2019; Medeiros et al., 2019), and across biomes (Bartlett et al., 2012b, 2016b; Zhu et al., 2018). A tendency for higher πtlp in wetter habitats has also been observed across a climatic gradient in northeast Spain (Rosas et al., 2019). Within the tropics, πtlp varies with respect to moisture on local scales (Bartlett et al., 2016b; McFadden et al., 2019) and is lower in species tolerant of seasonally or perennial dry habitats (Baltzer et al., 2008; Medeiros et al., 2019).
The association of traits with climatic distribution is complicated by the fact that trees can follow a wide spectrum of strategies for coping with seasonal water shortages or drought. The endpoints of this spectrum are adaptations to either avoid or tolerate dehydration under water stress (Grubb, 1998; Tyree et al., 2003). To avoid dehydration, trees need to establish access to water, reduce water loss or concentrate their growth in the period in which water is most available. Typical adaptations are deep rooting, early stomatal closure, low cuticular conductance, water storage in plant organs, osmotic adjustments and/or leaf shedding (Westoby et al., 2002). Such adaptations occur with increasing frequency as moisture availability decreases; for instance, the fraction of deciduous species and individuals increases with dry season intensity, particularly in the larger size classes (Condit et al., 2000a; Meakem et al., 2018; Alberton et al., 2019). In contrast, to tolerate dehydration, trees need physiological adaptations allowing them to maintain water transport, gas exchange and cell survival under water stress (i.e. at a low water potential). This can be achieved by increasing the ability of cells to remain functional at low water availability and water potential. The sensitivity to embolism represents an important constraint on tree functioning and survival during drought. Because the leaf is a bottleneck in the water transport pathway within the soil–plant–atmosphere continuum (Sack et al., 2005), leaf hydraulic and drought tolerance traits are expected to be important indicators of species’ ability to tolerate desiccation. In general, however, it remains poorly understood how drought strategy (i.e. avoidance vs tolerance) mediates the distribution of species traits across moisture gradients.
Leaf phenology, which is linked to the drought strategies described above, would logically interact with traits including πtlp, WD and LMA to shape species’ abilities to cope with drought. Tropical tree species exhibit adiversity of leaf phenological strategies ranging from drought‐deciduous (complete or partial deciduousness during the driest time of the year) to brevi‐deciduous and evergreen strategies (Reich, 1995; Powers & Tiffin, 2010; Park et al., 2019). Drought‐deciduous tree species lose their leaves at the driest time of the year as a dehydration avoidance strategy, and therefore there is little reason to expect that variation in πtlp, WD and LMA will be related to species distribution along a regional moisture gradient. Brevi‐deciduous species are completely deciduous for brief periods of c. 1‐3 wk, but this deciduous period is not aligned with the driest time of the year. Thus, for brevi‐deciduous and evergreen species, which both maintain leaves at the driest time of the year, it is logical to expect that these species would require greater desiccation tolerance adaptations in drier environments, whereas drought‐deciduous species would not. This remains to be tested.
Here, we test the abilities of a mechanistic leaf drought tolerance trait, πtlp, and the more widely measured plant functional traits of LMA and WD, to predict species distribution with respect to seasonal moisture variability in natural forest communities spanning local and regional moisture gradients in Panama. We hypothesized that across both local (within a 50 ha plot) and regional scales, evergreen and brevi‐deciduous species with more negative πtlp, higher WD and higher LMA would be associated with drier environments, but that there would be at most a weak relationship for deciduous species; and πtlp would be a much better predictor of species distributions across moisture gradients than LMA and WD.
Materials and Methods
Study sites and tree censuses
The study region features a rainfall gradient across the Isthmus of Panama from the wetter Caribbean side (3100–3800 mm annual precipitation) to the drier Pacific side (1800 mm; Engelbrecht et al., 2007). Barro Colorado Island (BCI), our focal study site, is located in the center of the gradient, and receives on average 2630 mm of rainfall per year. The proportion of deciduous species among canopy tree species increases from 14% on the wetter end to 41% on the drier end, with BCI intermediate at 28% (Condit et al., 2000a). The region has a tropical moist climate with a distinct dry season, averaging 131 d in length on BCI, between January and April (Paton, 2013). The vegetation is species‐rich tropical moist lowland forest, with 312 tree and shrub species in the 50 ha plot on BCI (Katabuchi et al., 2017), and 867 species in total in 72 plots in the region (including the BCI 50 ha plot; Condit et al., 2013).
A 50 ha long‐term monitoring plot was established in 1980 on the BCI plateau (Hubbell & Foster, 1983) and is now part of the Forest Global Earth Observatory network of large‐scale forest dynamics plots (ForestGEO; Anderson‐Teixeira et al., 2015). Regular tree censuses have recorded the diameter and location of all trees larger than 1 cm in diameter at 5 yr intervals, following the ForestGEO census protocol (Condit, 1998). This analysis uses data from the 2015 census, which were retrieved from the ForestGEO data portal (http://ctfs.si.edu/datarequest/), where it is available upon request.
Elevation varies by 38 m within the plot, leading to distinct small‐scale habitat heterogeneity and associated variation in water availability (Fig. 1a; Condit et al., 2000b; Harms et al., 2001; Kupers et al., 2019). Habitat types within the plot may be categorized into old secondary forest (c. 2 ha; Condit, 1998), high plateau, low plateau, slope, streamside and swamp areas (see Fig. 3a). For the purposes of analyses, we divided the plot into 1250 20×20 m quadrats, most of which could be assigned to one of those habitat categories (except 66 quadrates that were classified as mixed habitat). Quadrats with a slope less than 7° are designated plateau, and are further distinguished as high or low plateau based on elevation above or below 152 m (Harms et al., 2001). The habitat categories high and low plateau are well‐drained upland soils (Dietrich et al., 1996; Condit, 1998). Quadrats with a slope of more than 7° are classified as slope habitat; they are characterized by higher dry season soil moisture than the plateau areas (Kupers et al., 2019). Highest water availability is found along the stream, which contains water well into the dry season. The c. 1.5 ha swamp area is waterlogged during much of the wet season.
Fig. 1.
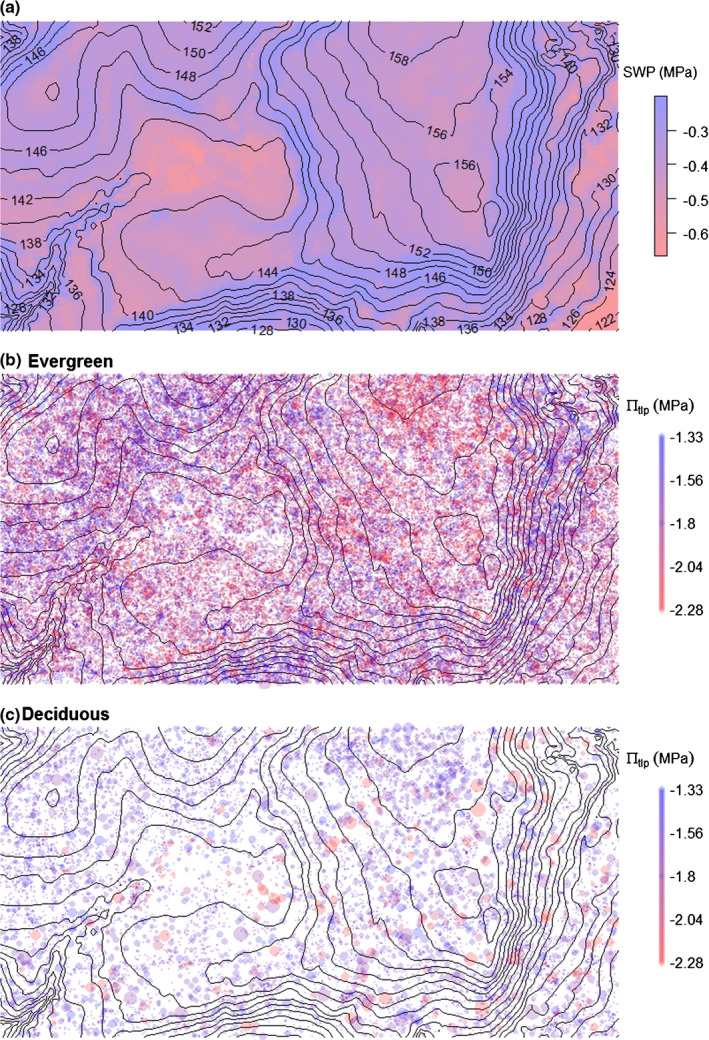
Map of the 50 ha ForestGEO plot on Barro Colorado Island showing the distribution of (a) dry season soil water potential of a regular dry year (from Kupers et al., 2019); (b) evergreen trees of varying πtlp – point size is scaled to tree diameter and color indicates πtlp; and (c) deciduous trees of varying πtlp (note: Dalbergia retusa does not occur in the plot). Trees for which πtlp data are not available are not plotted.
Fig. 3.
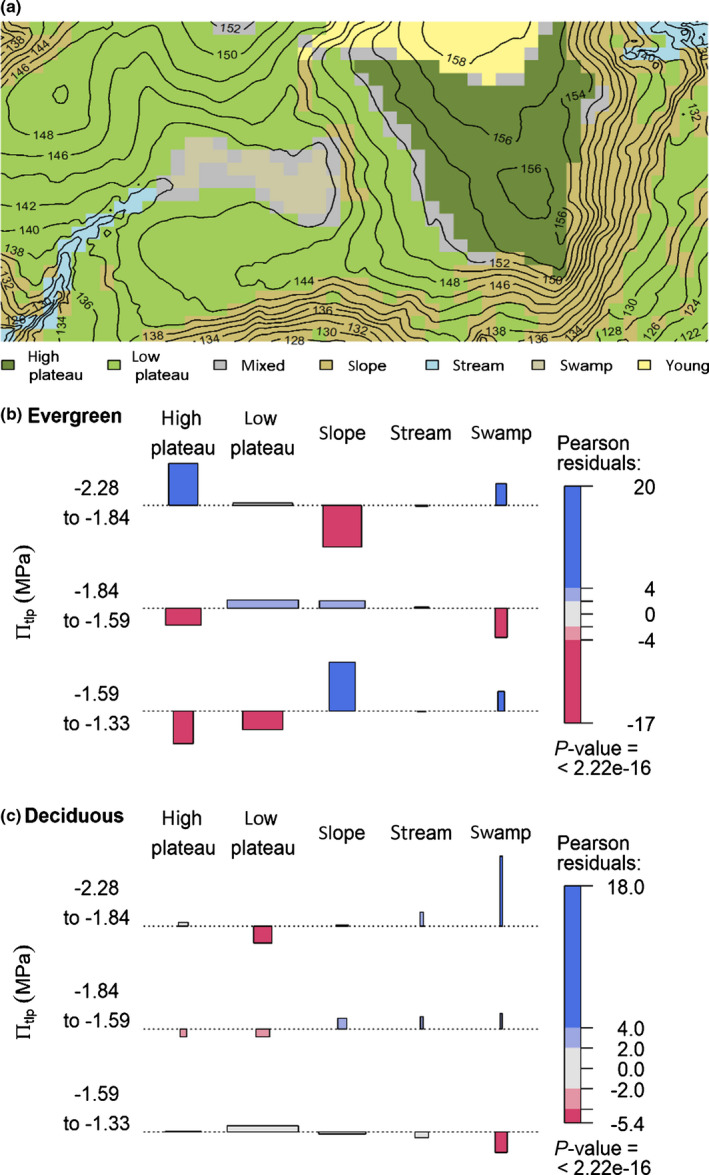
Habitat associations of evergreen and deciduous tree species to πtlp classes within the Barro Colorado Island 50 ha ForestGEO plot. (a) Map of the plot showing the habitat classifications of Condit (1998). (b,c) Association plots representing the residuals of a contingency table assuming independence of πtlp class and habitat for (b) evergreen and (c) deciduous species. The height of each bar is proportional to its signed contribution to the Pearson’s χ2 and bar width is proportional to the square root of the expected counts corresponding to the cell, so the area of each box is proportional to the difference between observed and expected frequencies. Horizontal dotted lines indicate no difference between observed and expected frequencies. The absolute size of the residuals is indicated by the coloration of the boxes: blue for positive, red for negative, very colorful for large residuals (> 4), less colorful for medium residuals (< 4 and > 2), light gray for small residuals (< 2). Under the assumption of independence and normality of residuals, 2 and 4 roughly correspond to a significant test with P < 0.05 and P < 0.0001 respectively. The P‐value given at the bottom of the legend corresponds to the overall chi‐square test’s P‐value.
Regional analyses were based on the BCI 50 ha plot in combination with tree inventories of 71 smaller plots (0.5–6 ha) in Central Panama (Condit et al., 2013). These plots span the rainfall gradient from an estimated 1756 mm of rainfall per year at Cerro La Torre (8.91182N, −79.5868W) to an estimated 4148 mm of rainfall per year at Esperanza (9.37642N, −79.3598W). There is also considerable variation in dry season moisture availability along this gradient, with a dry season water deficit of −579 mm in the southern region and only −237 mm in the northern region (Condit et al., 2013). Small‐plot inventories followed the same protocol as at the 50 ha long‐term tree census at BCI. For all plots, full census information (diameter and identity of every individual in multiple censuses) and climate data are available through the Smithsonian ForestGEO data portal (http://ctfs.si.edu/Public/Datasets/PanamaTreePlots/PanamaPlotInfo.php).
Species were assigned to deciduousness classes based on a combination of systematic surveys (Condit et al., 2000b) and expert knowledge, as detailed in Meakem et al. (2018). For analysis, we separated species into two groups, which we refer to as ‘evergreen’ and ‘deciduous’, while recognizing that each encompasses substantial variation in leaf phenology. We grouped brevi‐deciduous species with evergreen species (see Supporting Information Table S1) because they are foliated and transpiring during the peak of the dry season (Kunert et al., 2010). Our deciduous class thus is specifically a drought‐deciduous class, including semi‐deciduous and facultatively deciduous species, as well as considerable variation in the duration of deciduousness. In total, we had 34 evergreen species (of which seven were brevi‐deciduous), and 16 deciduous species.
Leaf sampling
We sampled leaves from 50 tree species, targeting dominant canopy species (Table S1), between April and July 2018. Specifically, we sampled 40 species represented by at least 10 individuals in the BCI 50 ha plot, prioritizing based on their biomass within the plot (estimated using allometries of Chave et al., 2014). Together, these comprised 60% of the total plot biomass. We also sampled several additional species that were very abundant or had drastic changes in their abundance since the first inventory in 1982 in the BCI 50 ha plot (e.g. Hybanthus prunifolius, Piper cordulatum; see Katabuchi et al., 2017), or that were ecologically important in the larger region (e.g. Dalbergia retusa). We sampled three adult individuals per species on BCI (in the 25 ha plot, which is located 100 m from the 50 ha plot, as no destructive sampling is allowed in the 50 ha plot) or in Gamboa in the case of one species that is rare on BCI (Dalbergia retusa). We collected one fully sun‐exposed branch per tree. For canopy species we chose mature trees reaching the upper canopy; for understory species we chose sun‐exposed individuals in canopy gaps. Canopy branches were sampled with a crossbow by shooting a bolt with an attached fishing line over a branch, and then using a rope chainsaw. Directly after cutting the branches from the trees, we placed the branches in humid and opaque plastic bags. The samples were transported to the laboratory as quickly as possible (<2 h between sampling and laboratory processing) and recut underwater at least two nodes distal to the original cut. The recut samples were placed in buckets with water, covered with plastic bags again and rehydrated overnight (c. 12 h or longer). We tested that rehydration time was sufficient by measuring the leaf water potential of rehydrated branches with a Scholander pressure chamber (for a subset of 25 species).
We measured WD (g cm−3) of a 2 to 4 cm long segment of each cut branch. We specifically sampled the lowest (most proximal) part of each branch, to ensure that all samples were fully lignified. The segment was placed in water overnight to allow full hydration. The next day, the bark was removed and the xylem was split in the center to remove the pith. Both parts of the xylem were submerged in a container with water put on a scale to measure the displaced volume of the water. The xylem was then dried in an oven at 60°C for at least 48 h and weighed. We used three leaves of different sizes (large, medium and small) from each collected branch to estimate LMA (g m−2). All leaves were scanned and then dried in the oven at 60°C for at least 48 h and weighed. Leaf area was derived from scanned images using an R script by G. Arellano.
Osmometric determination of the turgor loss point
We determined πtlp based on measurements of the sap osmotic potential of leaves (Bartlett et al., 2012a). The osmotic potential was measured with a vapor pressure osmometer (VAPRO 5520; Wescor, Logan, UT, USA). On the day following sample collection, we removed two mature, fully expanded leaves per branch from the rehydrated branches. Two sample disks were cut from the leaf centrally between the midrib and margin with a 4 mm diameter cork borer. Disks were tightly wrapped in aluminum foil and submerged in liquid nitrogen for at least 2 min (Bartlett et al., 2012a). We used the standard 10 μl chamber well of the osmometer. Immediately before putting the disks into the chamber of the osmometer, the disks were punctured with a dissection needle about 10–15 times to improve evaporation through the cuticle and to reduce equilibration time (Kikuta & Richter, 1992). The osmometer was set in the autorepeat mode and measurements were recorded until the equilibrium was indicated by an increase between measurements of < 0.01 MPa (about five osmometer readings). πtlp was calculated from the osmotic potential given by the osmometer (πosm) using the biophysically based calibration equation established by Bartlett et al., (2012a):
| (Eqn 1) |
This biophysically based calibration equation has been verified for a variety of tropical tree species by Maréchaux et al. (2016).
Statistical analysis
We calculated the mean and standard deviation of each trait for each species, and used the mean traits in interspecific analyses. To evaluate how traits were related across species, we calculated Pearson correlations for each pair of traits. For evergreen and deciduous species groups separately, we analyzed how species mean WD, LMA and πtlp related to both dry season soil water potential (Fig. 1a; Kuper et al., 2019) and to habitat types (see Fig. 3a; Harms et al., 2001) on the BCI 50 ha plot. For the former analysis, we used a map of mean dry season soil water potential in a regular dry year (Kupers et al., 2019; Fig. 1a), assigning each quadrat in the plot into categories of low, intermediate and high soil water potential, each spanning exactly one‐third of the total observed range. For the analysis of habitat categories, we adopted the habitat map of Harms et al. (2001), excluding the young forest (c. 2 ha of the entire plot; Condit, 1998) and mixed habitat (i.e. transition between any two other categories). We calculated the proportion of trees in a given soil water potential grouping or habitat as
| (Eqn 2) |
where N habitat is the number of trees in a given soil water potential grouping or habitat and N 50ha is the total number of trees across all habitats analyzed. We performed a chi‐square test to evaluate whether the observed proportion in habitat differed significantly from a null expectation representing independence of the trait of interest from habitat type, using the ‘assoc’ function from the R package vcd (Meyer et al., 2006). In this analysis, we grouped species based on their trait values into three classes of equal breadth. To test for sensitivity to the groupings, we repeated the analysis with two and five classes. To account for spatial nonindependence of different trees, we also applied the more conservative torus translation test to the habitat type analysis (Harms et al., 2001). Furthermore, we used a linear regression (function ‘lm’ from the R package stats) to test for correlations between traits (WD, LMA and πtlp) and species‐specific proportion in habitat.
To test for moisture association on the regional scale, we adopted a moisture association index (MAI) from Condit et al. (2013), which uses a hierarchical Gaussian logistic regression to model tree distributions across the 72 sample sites included in our regional analysis. The model includes estimated dry season moisture availability and seven soil factors. We define MAI as the species‐specific first‐order response parameter to standardized dry season moisture in this model, where a negative value means a negative species response to more moisture availability and a positive value a positive response to higher moisture availability. We used linear regression (function ‘lm’ from the R package stats) to quantify the association between the different traits (WD, LMA and πtlp) and MAI, analyzing evergreen and deciduous species separately. Our use of traits measured at one site for the regional analysis implicitly assumes that these trait values are indicative of species strategies more generally, notwithstanding probable regional intraspecific trait variation (Osazuwa‐Peters et al., 2017).
All statistical analyses were conducted using the R software (R Core Team 2019). Data are archived in Zenodo (https://doi.org/10.5281/zenodo.4431676).
Results
Inter‐ and intraspecific variation in traits
Mean species turgor loss point (πtlp) ranged from −1.34 to −2.05 MPa in evergreen species and from −1.25 to −2.30 MPa in deciduous species (Table S1). Intraspecific variation of πtlp was low, with the coefficient of variation (CV) below 5% in 33 of 50 species, and CV greater than 10% in only three species. Mean species LMA ranged from 39.0 to 153.4 g m−2 in evergreen species and from 31.7 to 110.6 g m−2 in deciduous species. Intraspecific variation of LMA was high, with CVs > 10% for 25 species. Mean species WD ranged from 0.228 to 0.894 g cm−3 in evergreen species and from 0.224 to 0.622 g cm−3 in deciduous species. Twelve species had < 5% intraspecific variation in WD, while 22 had variation > 10%. Relationships among these traits were weak, with Pearson correlations of 0.23 (P = 0.1088) for πtlp and LMA, –0.037 (P = 0.7998) for πtlp and WD, and 0.14 (P = 0.3432) for LMA and WD (Fig. S1).
Local scale
Species mean πtlp was associated with soil moisture characteristics and habitat distributions within the BCI 50 ha plot in evergreen species but not in deciduous species (Figs 1, 2, 3, 4). These patterns, which are visibly apparent in our maps of trees by πtlp (Fig. 1), were statistically confirmed by associations of evergreen species with soil water potential in a regular dry season year and habitat types (Figs 2, 3). Evergreen species with lower πtlp (−2.28 to −1.84 MPa) were positively associated with lower mean dry season soil water potential and negatively associated with higher soil moisture potential (Fig. 2a). Evergreen species with higher πtlp (−1.59 to −1.33 MPa) showed the opposite pattern. Deciduous species with lower πtlp (−2.28 to −1.84 MPa) were overrepresented in areas with lowest dry season soil water potential and underrepresented in habitats with intermediate soil water potential, but there were otherwise no significant associations of πtlp‐based species groupings to soil moisture potential classes (Fig. 2b). These trends were consistent when species were divided into different numbers of quartiles (division in median shown in Fig. S2).
Fig. 2.
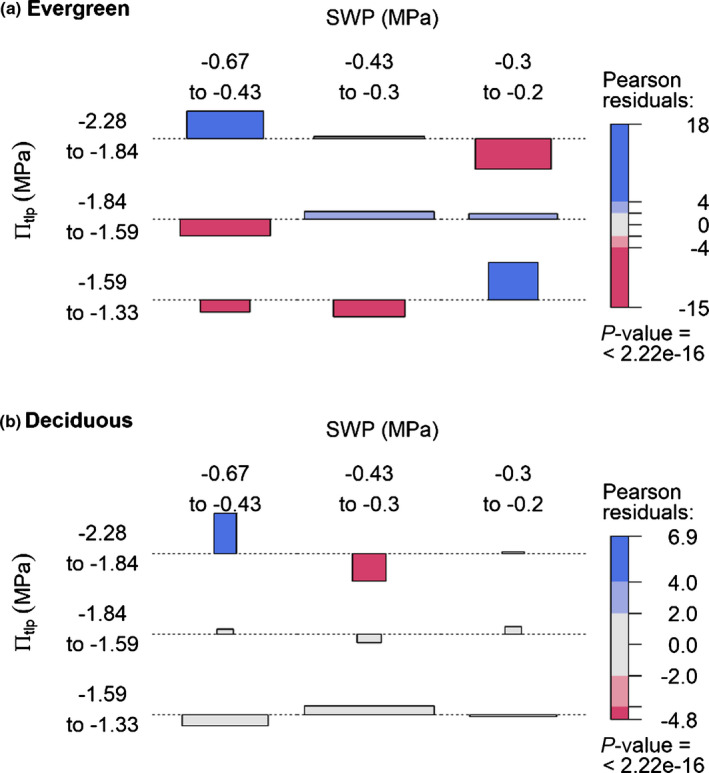
Soil moisture associations of evergreen and deciduous tree species to πtlp classes within the Barro Colorado Island 50 ha ForestGEO plot. Association plots representing the residuals of a contingency table assuming independence of πtlp class and mean dry season soil water potential (SWP) for (a) evergreen and (b) deciduous species. The height of each bar height is proportional to its signed contribution to the Pearson’s χ2 and width is proportional to the square root of the expected counts corresponding to the cell, so the area of each box is proportional to the difference between observed and expected frequencies. Horizontal dotted lines indicate no difference between observed and expected frequencies. The absolute size of the residuals is indicated by the coloration of the boxes: blue for positive, red for negative, very colorful for large residuals (> 4), less colorful for medium residuals (< 4 and > 2), light gray for small residuals (< 2). Under the assumption of independence and normality of residuals, 2 and 4 roughly correspond to a significant test with P < 0.05 and P < 0.0001 respectively. The P‐value given at the bottom of the legend corresponds to the overall chi‐square test’s P‐value.
Fig. 4.
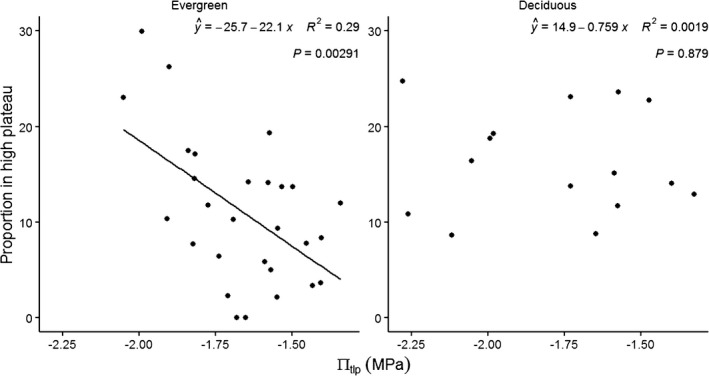
Relationships between the proportion of trees of each species growing in high plateau and πtlp. The linear regression line is shown when the relationship is significant (P < 0.05).
Analyzed in relation to habitat types, evergreen species with lower πtlp were overrepresented in the drier high plateau and underrepresented in the wetter slope, whereas those with higher πtlp showed the opposite pattern (Figs 1b, 3b). In contrast, deciduous species showed no clear relationship between πtlp and affinity with these habitats (Figs 1c, 3c). There were a number of significant associations based on πtlp in relation to the swamp habitat, which covers a very small area. Although this area is waterlogged during much of the wet season, it has the most negative surface soil water potential of any habitat in the dry season (Fig. 1a). Evergreen species with more negative πtlp (≤ −1.82 MPa) were overrepresented in the swamp, consistent with their overrepresentation in other dry habitats (Fig. 3b). However, evergreen species with less negative πtlp (≥ −1.59 MPa) were also overrepresented in the swamp, as were deciduous species with more negative πtlp.
On a species level, there was a significant negative relationship between the proportion of individuals in high plateau and species mean πtlp for evergreen species (Fig. 4 left panel; R 2 = 0.28, P = 0.004), contrasting with no relationship for deciduous species (Fig. 4 right panel; R 2 < 0.001, P = 0.914). The torus translation test, which is conservative in identification of species–habitat associations, identified significant associations to the high plateau or slope for only nine species (Fig. S3), yielding insufficient statistical power for a formal test. However, species with significant positive associations to the high plateau or negative associations to the slope (n = 2) had more negative πtlp (≤ −1.82 MPa) than those with negative associations to the high plateau or positive associations to the slope (n = 7; πtlp ≥ −1.74 MPa).
WD was not a significant predictor of the proportion in high plateau for either evergreen (R 2 = 0.008, P = 0.647) or deciduous species (R 2 = 0.001, P = 0.897). However, there was a significant positive association of evergreen species with low WD (< 0.483 g cm−3) to the slope, swamp and stream areas and a negative association of species with intermediate WD (= 0.483 g cm−3 WD ≤ 0.621 g cm−3) to the high plateau and the low plateau areas (Figs S4–S6). Deciduous species showed no relationship of WD to habitat association. LMA of evergreen species was negatively correlated with the species‐specific proportion in high plateau (R 2 = 0.20, P = 0.0181) – the opposite pattern of what would be explained based on physiology – whereas there was no significant relationship for deciduous species (R 2 < 0.001, P = 0.993; Figs S7–S9). Evergreen species with high LMA were negatively associated with high and low plateau areas, but low LMA was significantly associated with the plateau areas. A positive association was present for species with high LMA and slope, stream and swamp areas; however, low LMA species were negatively associated with those areas.
Regional scale
On the regional scale, πtlp was positively correlated with MAI (R 2 = 0.32, P = 0.003) among evergreen species (Fig. 5), indicating that low‐πtlp species are associated with drier habitats. In contrast, πtlp and MAI were not significantly correlated for deciduous species (Fig. 5; R 2 = 0.009, P = 0.715). There was no significant correlation of MAI with WD or LMA (see Figs S10, S11) for either evergreen (WD: R 2 = 0.005, P = 0.746; LMA: R 2 = 0.003, P = 0.779) or deciduous tree species (WD: R 2 = 0.065, P = 0.324; LMA: R 2 = 0.039, P = 0.447).
Fig. 5.
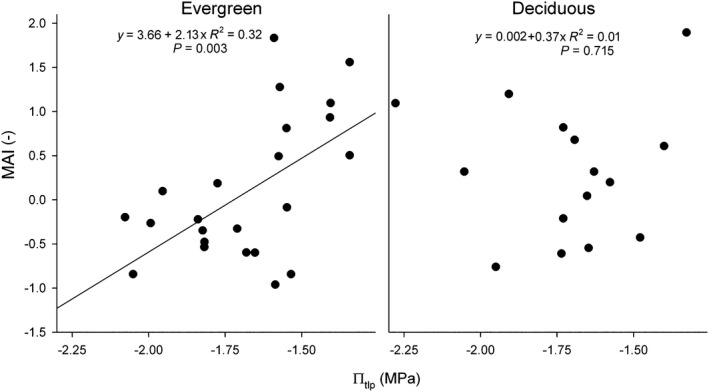
Regional‐scale correlation between species’ moisture association index (MAI) and πtlp for evergreen and deciduous species. MAI describes a species’ distribution across the Isthmus of Panama with respect to dry season moisture (positive values indicate a positive association with moisture), as modeled by Condit et al. (2013).
Discussion
Our results demonstrate that on both local and regional scales, low turgor loss point (πtlp) is associated with the ability of evergreen species to survive and persist in habitats with greater seasonal moisture stress. Evergreen (or brevi‐deciduous) species showed clear local‐scale habitat preference and regional distribution across a moisture gradient depending on their πtlp, indicating that leaf desiccation tolerance significantly affects a species’ ability to establish and survive in periodically water‐limited environments. In contrast, πtlp does not explain the soil moisture and habitat association of deciduous species, suggesting that a species’ ability to shed leaves under drought conditions neutralizes the benefits of low πtlp. The success of πtlp in predicting local‐ and regional‐scale species distributions contrasts with the failure of WD and LMA, which showed no consistent, physiologically congruent relationships with species distributions on local or regional scales (Figs S4–S11). We therefore conclude from this extensive data collection that πtlp is a useful trait for predicting species’ drought tolerance across diverse tropical species.
Turgor loss point drives habitat association and species distribution
We show that πtlp is a physiological parameter influencing ecological drought tolerance of diverse tropical tree species, including on local (Figs 1, 2, 3, 4) and regional (Fig. 5) scales. πtlp can be understood as a leaf parameter that scales with whole plant–water relationships, defining a species’ requirements of certain minimum soil water availability (Bartlett et al., 2012b). In other words, πtlp is describing the permanent wilting point (Bartlett et al., 2012b), which refers to the soil water potential or plant‐available soil water content when trees start wilting irreversibly (Veihmeyer & Hendrickson, 1928). The fact that πtlp is directly related to wilting explains the association of later wilting species to drier habitats, where they can survive in niches with naturally occurring water limitations and outcompete less drought‐adapted species. Therefore, species adapted to drier forest habitats can be expected to either have the ability to shed their leaves or maintain turgor under more negative water potentials than tree species adapted to moist habitats (Poorter & Markesteijn, 2008). Accordingly, tree species in seasonally dry forests are generally able to maintain leaf turgor at lower water potential (more negative πtlp) than those in less seasonal moist forests. This indicates that dry‐season intensity is one of the main drivers of tree distributions across environmental gradients. Consequently, dry‐season performance, especially hydraulic resistance against dry season water stress among evergreen tree species, is a crucial factor for population dynamics and species distribution patterns in a community context (Engelbrecht et al., 2000, 2007). On a regional scale, πtlp explained 35% of the variation in evergreen species association to moisture across a gradient of dry season moisture (Condit et al., 2013). This is consistent with the finding that more negative πtlp was positively associated with more seasonal climates across the Thai‐Malay peninsula (Baltzer et al., 2008), but in this case expands to a larger number of species.
Avoidance or persistence
Unlike evergreen tree species, deciduous tree species exhibited no clear relationship between their habitat association and π tlp. π tlp of deciduous species explained neither habitat association and soil moisture requirements in the 50 ha plot on BCI nor species distributions across the regional moisture gradient. We explain the lack of correlation between π tlp and species moisture associations by the simple fact that deciduous trees are avoiding the water‐limited time of the year by shedding leaves and being dormant. Deciduousness is a well‐known strategy of canopy trees to avoid drought stress in tropical forests (Poorter & Markesteijn, 2008). Thus, deciduous trees are relatively insensitive to water limitation during the dry season, as their water demands are minimal while deciduous (Kunert et al., 2010). This means that deciduous species characterized with a less negative πtlp can survive potentially in very dry habitats like the high plateau areas. However, those species were more abundant in the slope areas (Condit et al., 2013), probably due to higher soil fertility in slopes compared to the high plateau. In general, seasonal drought favors the development of deciduous trees when sufficient soil nutrients are available, whereas infertile soils favor the long‐lived evergreen leaves to preserve limited nutrients (Givnish, 2002). In a meta‐analysis, Bartlett et al. (2012b) showed that πtlp is a good indicator of a species’ drought tolerance if other factors do not become equally or more important than soil moisture availability. Nutrient limitation would be such a case, where nutrient use efficiency is more important for the trees than water availability. Tree species distributions in Panama depend not only on dry‐season moisture but also on phosphorus availability (Condit et al., 2013). For example, the deciduous species Cavanillesia platanifolia (πtlp = −1.71 MPa) is restricted to slope areas with sufficient phosphorus availability. Thus, deciduousness and the dry season avoidance strategy comes at the cost that the seasonal production of new leaves requires a minimum of soil fertility.
Diverging π tlp on temporary waterlogged swamps
A very interesting aspect of our results was that species in the most and least negative πtlp classes were positively associated with the swamp area, whereas those with intermediate πtlp were negatively associated (Fig. 3). Two adaptations might explain this phenomenon. First, the physiological response of trees to waterlogging is similar to drought response in that waterlogging also causes stomatal closure (Kreuzwieser & Rennenberg, 2014). Anaerobic root conditions during waterlogging cause a reduction in root conductance, especially under conditions with a high evaporative demand, and wilting can occur (Bradford & Hsiao, 1982). Physiological responses to waterlogging also include a drastic increase in the concentration of soluble carbohydrates in leaves (Ferner et al., 2012), which would directly affect their osmotic potential and thus turgor loss point. Indeed, comparing two congeneric Mora species in Guyana, one occupying a well‐drained habitat and the other a seasonally waterlogged habitat, the species associated with seasonally waterlogged soils had a more negative πtlp than the species growing in a well‐drained habitat and regularly drought‐stressed habitat (ter Steege, 1994). The second explanation might be that trees growing on seasonally waterlogged sites have shallow rooting systems (Fan et al., 2017). In seasonal climates, this can potentially cause a shift in seasonal stress situations from seasonally waterlogged to seasonal soil water limitation. Whereas the trees try to avoid anoxic conditions during the wet season by developing shallow rooting systems, their shallow roots cannot reach the moisture as the swamp dries out during the dry seasons. Indeed, the topsoil layer in the swamp area on BCI dries out relatively fast and reaches even more negative soil water potentials than the plateau areas during the dry season (Fig. 1a; Kupers et al., 2019).
Conclusions
We deliver evidence that in tropical forests a tree species’ osmotic adaptation to a certain niche with a given moisture availability is expressed in the species‐specific leaf water potential at turgor loss point (πtlp). This applies for nutrient‐preserving evergreen tree species but not deciduous species, which avoid dehydration via leaf loss but face nutrient limitations in constructing new leaves. Further research will be required to understand how seasonal moisture limitation and nutrient availability interactively constrain πtlp and leaf phenology and shape species distributions. In the meantime, we suggest that πtlp can be parameterized for the application in forest ecosystem models to predict future changes of species composition and distribution patterns under climate change.
Author contributions
NK, KAT, LS and SJD designed the research. NK and JZ collected the trait data in Panama. RP, RCC and SPH led collection of tree census data. LS, HCML, SJW and SMM contributed methods and expertise. Data analyses were performed by VH and NK. NK, KAT and LS interpreted the results. NK and KAT wrote the first draft of the manuscript, and all authors contributed to revisions.
Supporting information
Fig. S1 Correlation plot indicating the relationships between πtlp, LMA and WD.
Fig. S2 Habitat associations of evergreen and deciduous tree species by πtlp within the Barro Colorado Island 50 ha ForestGEO plot.
Fig. S3 Linear relationship between the proportion of trees of each species growing in high plateau and πtlp, highlighting significant Torus associations (Harms et al., 2004).
Fig. S4 Map of the 50 ha ForestGEO plot on Barro Colorado Island showing the distribution of WD within the evergreen trees, and WD within the deciduous trees.
Fig. S5 Habitat associations of evergreen and deciduous tree species by WD within the Barro Colorado Island 50 ha ForestGEO plot.
Fig. S6 Linear relationship between the proportion of trees growing in high plateau and WD, highlighting significant Torus associations (Harms et al., 2004).
Fig. S7 Map of the 50 ha ForestGEO plot on Barro Colorado Island showing the distribution of LMA within the evergreen trees, and within the deciduous trees.
Fig. S8 Habitat associations of evergreen and deciduous tree species by LMA within the Barro Colorado Island 50 ha ForestGEO plot.
Fig. S9 Linear relationship between the proportion of trees growing in high plateau and LMA, highlighting significant Torus associations (Harms et al., 2004).
Fig. S10 Moisture association index (MAI of Condit et al., 2013) as a function of wood density (WD).
Fig. S11 Moisture association index (MAI of Condit et al., 2013) as a function of leaf mass per area (LMA).
Table S1 Summary of observed mean turgor loss point (πtlp), leaf mass area (LMA), wood density (WD) and leaf phenology. Standard deviation is given for all mean values.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank Gabrielle Arellano for providing the R‐script for the leaf area analysis. We thank the staff of BCI for help with fieldwork logistics. We are grateful to Andres Hernandez and Janice Harwood for helping to identify specimens. This study was funded primarily by Smithsonian’s Forest Global Earth Observatory (ForestGEO, and was supported in part by the Next Generation Ecosystem Experiments‐Tropics, funded by the US Department of Energy, Office of Science, Office of Biological and Environmental Research.
References
- Alberton B, da Silva TR, Sanna Freire Silva T, Rocha HR, Moura M, Morellato LPC. 2019. Leafing patterns and drivers across seasonally dry tropical communities. Remote Sensing 11: 2267. [Google Scholar]
- Anderson‐Teixeira KJ, Davies SJ, Bennett AC, Gonzalez‐Akre EB, Muller‐Landau HC, Wright SJ, Abu Salim K, Almeyda Zambrano AM, Alonso A, Baltzer JL et al. 2015. CTFS‐ForestGEO: a worldwide network monitoring forests in an era of global change. Global Change Biology 21: 528–549. [DOI] [PubMed] [Google Scholar]
- Baltzer JL, Davies SJ, Bunyavejchewin S, Noor NSM. 2008. The role of desiccation tolerance in determining tree species distributions along the Malay‐Thai Peninsula. Functional Ecology 22: 221–231. [Google Scholar]
- Bartlett MK, Klein T, Jansen S, Choat B, Sack L. 2016a. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proceedings of the National Academy of Sciences, USA 113: 13098–13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett MK, Scoffoni C, Ardy R, Zhang Y, Sun S, Cao K, Sack L. 2012a. Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods in Ecology and Evolution 3: 880–888. [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L. 2012b. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta‐analysis. Ecology Letters 15: 393–405. [DOI] [PubMed] [Google Scholar]
- Bartlett MK, Zhang Y, Yang J, Kreidler N, Sun SW, Lin L, Hu YH, Cao KF, Sack L. 2016b. Drought tolerance as a driver of tropical forest assembly: resolving spatial signatures for multiple processes. Ecology 97: 503–514. [DOI] [PubMed] [Google Scholar]
- Bennett A, McDowell N, Allen C. 2015. Larger trees suffer most during drought in forests worldwide. Nature Plants 1: 15139. [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Creek D, Maier C, Aspinwall MJ, Drake JE, Pfautsch S, O’Grady A, Delzon S, Medlyn BE, Tissue DT et al. 2019. Drought response strategies and hydraulic traits contribute to mechanistic understanding of plant dry‐down to hydraulic failure. Tree Physiology 39: 910–924. [DOI] [PubMed] [Google Scholar]
- Bradford KJ, Hsiao TC. 1982. Stomatal behavior and water relations of waterlogged tomato plants. Plant Physiology 70: 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Powers J, Cochard H, Choat B. 2020. Hanging by a thread? Forests and drought. Science 368: 261–266. [DOI] [PubMed] [Google Scholar]
- Cavaleri MA, Reed SC, Smith WK, Wood TE. 2015. Urgent need for warming experiments in tropical forests. Global Change Biology 21: 2111–2121. [DOI] [PubMed] [Google Scholar]
- Chave J, Réjou‐Méchain M, Búrquez A, Chidumayo E, Colgan MS, Delitti WB, Duque A, Eid T, Fearnside PM, Goodman RC et al. 2014. Improved allometric models to estimate the aboveground biomass of tropical trees. Global Change Biology 20: 3177–3190. [DOI] [PubMed] [Google Scholar]
- Condit R. 1998. Tropical forest census plots: Methods and results from Barro Colorado Island, Panama and a comparison with other plots. Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Condit R, Ashton PS, Baker P, Bunyavejchewin S, Gunatilleke S, Gunatilleke N, Hubbell SP, Foster RB, Itoh A, LaFrankie JV et al. 2000b. Spatial patterns in the distribution of tropical tree species. Science 288: 1414–1418. [DOI] [PubMed] [Google Scholar]
- Condit R, Engelbrecht BMJ, Pino D, Pérez R, Turner BL. 2013. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proceedings of the National Academy of Sciences, USA 110: 5064–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R, Hubbell SP, Foster RB. 1995. Mortality rates of 205 Neotropical tree and shrub species and the impact of a severe drought. Ecological Monographs 65: 419–439. [Google Scholar]
- Condit R, Watts K, Bohlman SA, Pérez R, Foster RB, Hubbell SP. 2000a. Quantifying the deciduousness of tropical forest canopies under varying climates. Journal of Vegetation Science 11: 649–658. [Google Scholar]
- Dai A, Zhao T, Chen J. 2018. Climate change and drought: a precipitation and evaporation perspective. Current Climate Change Reports 4: 301–312. [Google Scholar]
- De Guzman ME, Acosta‐Rangel A, Winter K, Meinzer FC, Bonal D, Santiago LS. 2020. Hydraulic traits of Neotropical canopy liana and tree species across a broad range of wood density: implications for predicting drought mortality with models. Tree Physiology 41: 24–34. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Windsor M, Dunne T. 1996. Geology, climate and hydrology of Barro Colorado Island. In: Leigh JE, Rand A, Windsor DM, eds. The ecology of a tropical forest. Seasonal rhythms and annual changes, 2nd edn. Washington, DC, USA: Smithsonian Institution Press, 21–46. [Google Scholar]
- Engelbrecht BMJ, Comita LS, Condit R, Kursar TA, Tyree MT, Turner BL, Hubbell SP. 2007. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447: 80. [DOI] [PubMed] [Google Scholar]
- Engelbrecht BMJ, Velez V, Tyree MT. 2000. Hydraulic conductance of two co‐occuring neotropical understory shrubs with different habitat preferences. Annal of Forest Science 57: 201–208. [Google Scholar]
- Fan Y, Miguez‐Macho G, Jobbágy EG, Jackson RB, Otero‐Casal C. 2017. Hydrologic regulation of plant rooting depth. Proceedings of the National Academy of Sciences, USA 114: 10572–10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell C, Szota C, Arndt SK. 2017. Does the turgor loss point characterize drought response in dryland plants? Plant, Cell & Environment 40: 1500–1511. [DOI] [PubMed] [Google Scholar]
- Ferner E, Rennenberg H, Kreuzwieser J. 2012. Effect of flooding on C metabolism of flood‐tolerant (Quercus robur) and non‐tolerant (Fagus sylvatica) tree species. Tree Physiology 32: 135–145. [DOI] [PubMed] [Google Scholar]
- Friedlingstein P, Cox P, Betts R, Bopp L, von Bloh W, Brovkin V, Cadule P, Doney S, Eby M, Bala FIB et al. 2006. Climate‐carbon cycle feedback analysis: results from the C4MIP model intercomparison. Journal of Climate 19: 3337–3353. [Google Scholar]
- Givnish T. 2002. Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fennica 36: 703–743. [Google Scholar]
- Grubb PJ. 1998. A reassessment of the strategies of plants which cope with shortages of resources. Perspectives in Plant Ecology, Evolution and Systematics 1: 3–31. [Google Scholar]
- Harms KE, Condit R, Hubbell SP, Foster RB. 2001. Habitat associations of trees and shrubs in a 50 ha neotropical forest plot. Journal of Ecology 89: 947–959. [Google Scholar]
- Hofhansl F, Schnecker J, Singer G, Wanek W. 2015. New insights into mechanisms driving carbon allocation in tropical forests. New Phytologist 205: 137–146. [DOI] [PubMed] [Google Scholar]
- Hubbell SP, Foster RB. 1983. Diversity of canopy trees in a neotropical forest and implications for conservation. In: Whitmore T, Chadwick A, Sutton A, eds. Tropical rain forest: ecology and management. Oxford, UK: The British Ecological Society, 25–41. [Google Scholar]
- IPCC . 2015. Climate change 2014: synthesis report. Geneva, Switzerland: Intergovernmental Panel on Climate Change. [Google Scholar]
- Katabuchi M, Wright SJ, Swenson NG, Feeley KJ, Condit R, Hubbell SP, Davies SJ. 2017. Contrasting outcomes of species and community level analyses of the temporal consistency of functional composition. Ecology 98: 2273–2280. [DOI] [PubMed] [Google Scholar]
- Kikuta SB, Richter H. 1992. A simplified pressure‐volume method for the estimation of osmotic adjustment with the pressure chamber. Bodenkultur 43: 307–318. [Google Scholar]
- Klein T, Hartmann H. 2018. Climate change drives tree mortality. Science 362: 758. [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Rennenberg H. 2014. Molecular and physiological responses of trees to waterlogging stress. Plant, Cell & Environment 37: 2245–2259. [DOI] [PubMed] [Google Scholar]
- Kunert N. 2020. Preliminary indications for diverging heat and drought sensitivities in Norway spruce and Scots pine in Central Europe. iForest ‐ Biogeosciences and Forestry 13: 89–91. [Google Scholar]
- Kunert N, Schwendenmann L, Hölscher D. 2010. Seasonal dynamics of tree sap flux and water use in nine species in Panamanian forest plantations. Agricultural and Forest Meteorology 150: 411–419. [Google Scholar]
- Kupers SJ, Wirth C, Engelbrecht BMJ, Rüger N. 2019. Dry season soil water potential maps of a 50 hectare tropical forest plot on Barro Colorado Island, Panama. Scientific Data 6: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin DC, Delzon S, Clearwater MJ, Bellingham PJ, McGlone MS, Richardson SJ. 2020. Climatic limits of temperate rainforest tree species are explained by xylem embolism resistance among angiosperms but not among conifers. New Phytologist 226: 727–740. [DOI] [PubMed] [Google Scholar]
- Liu H, Gleason SM, Hao G, Hua L, He P, Goldstein G, Ye Q. 2019. Hydraulic traits are coordinated with maximum plant height at the global scale. Science Advances 5: eaav1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz JA, Furniss TJ, Johnson DJ, Davies SJ, Allen D, Alonso A, Anderson‐Teixeira KJ, Andrade A, Baltzer J, Becker KML et al. 2018. Global importance of large‐diameter trees. Global Ecoogy and Biogeography 27: 849–864. [Google Scholar]
- Maréchaux I, Bartlett MK, Gaucher P, Sack L, Chave J. 2016. Causes of variation in leaf‐level drought tolerance within an Amazonian forest. Journal of Plant Hydraulics 3: e004. [Google Scholar]
- McDowell NG, Allen CG, Anderson‐Teixeira K, Aukema BH, Bond‐Lamberty B, Chini L, Clark JS, Dietze M, Grossiord C, Hanbury‐Brown A et al. 2020. Pervasive shifts in forest dynamics in a changing world. Science 368: aaz9463. [DOI] [PubMed] [Google Scholar]
- McFadden IR, Bartlett MK, Wiegand T, Turner BL, Sack L, Valencia R, Kraft NJB. 2019. Disentangling the functional trait correlates of spatial aggregation in tropical forest trees. Ecology 100: e02591. [DOI] [PubMed] [Google Scholar]
- McGregor IR, Helcoski R, Kunert N, Tepley AJ, Gonzalez‐Akre EB, Herrmann V, Zailaa J, Stovall AE, Bourg NA, McShea WJ et al. 2020. Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytologist. doi: 10.1111/nph.16996. [DOI] [PubMed] [Google Scholar]
- Meakem V, Tepley AJ, Gonzalez‐Akre EB, Herrmann V, Muller‐Landau HC, Wright SJ, Hubbell SP, Condit R, Anderson‐Teixeira KJ. 2018. Role of tree size in moist tropical forest carbon cycling and water deficit responses. New Phytologist. 219: 947–958. [DOI] [PubMed] [Google Scholar]
- Medeiros CD, Scoffoni C, John GP, Bartlett MK, Inman‐Narahari F, Ostertag R, Cordell S, Giardina C, Sack L. 2019. An extensive suite of functional traits distinguishes Hawaiian wet and dry forests and enables prediction of species vital rates. Functional Ecology 33: 712–734. [Google Scholar]
- Meyer D, Zeileis A, Hornik K. 2006. The Strucplot framework: visualizing multi‐way contingency tables with vcd. Journal of Statistical Software 17: 48. [Google Scholar]
- Nardini A, Luglio J. 2014. Leaf hydraulic capacity and drought vulnerability: possible trade‐offs and correlations with climate across three major biomes. Functional Ecology 28: 810–818. [Google Scholar]
- Osazuwa‐Peters OL, Wright SJ, Zanne AE. 2017. Linking wood traits to vital rates in tropical rainforest trees: Insights from comparing sapling and adult wood. American Journal of Botany 104: 1464–1473. [DOI] [PubMed] [Google Scholar]
- Park JY, Muller‐Landau HC, Lichstein JW, Rifai SW, Dandois JP, Bohlman SA. 2019. Quantifying leaf phenology of individual trees and species in a tropical forest using unmanned aerial vehicle (UAV) images. Remote Sensing 11: 1534. [Google Scholar]
- Paton S. 2013. Meteorological and hydrological summary for Barro Colorado Island. Panama City, Panama: Smithsonian Tropical Research Institute, 201. [Google Scholar]
- Phillips OL, van der Heijden G, Lewis SL, López‐González G, Aragão LEOC, Lloyd J, Malhi Y, Monteagudo A, Almeida S, Dávila EA et al. 2010. Drought‐mortality relationships for tropical forests. New Phytologist 187: 631–646. [DOI] [PubMed] [Google Scholar]
- Poorter L, Bongers F. 2006. Leaf traits are predictors of plant performance across 53 rain forest species. Ecology 87: 1733–1743. [DOI] [PubMed] [Google Scholar]
- Poorter L, Markesteijn L. 2008. Seedling traits determine drought tolerance of tropical tree species. Biotropica 40: 321–331. [Google Scholar]
- Poorter L, van der Sande MT, Arets EJMM. 2017. Biodiversity and climate determine the functioning of Neotropical forests. Global Ecology and Biogeography 26: 1423–1434. [Google Scholar]
- Powers JS, Tiffin P. 2010. Plant functional type classifications in tropical dry forests in Costa Rica: leaf habit versus taxonomic approaches. Functional Ecology 24: 927–936. [Google Scholar]
- R Core Team . 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL https://www.R‐project.org/ [accessed 14 December 2020]. [Google Scholar]
- Reich PB. 1995. Phenology of tropical forests: patterns, causes, and consequences. Canadian Journal of Botany 73: 164–174. [Google Scholar]
- Rosas T, Mencuccini M, Barba J, Cochard H, Saura‐Mas S, Martínez‐Vilalta J. 2019. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytologist 223: 632–646. [DOI] [PubMed] [Google Scholar]
- Rosner S. 2017. Wood density as a proxy for vulnerability to cavitation: size matters. Journal of Plant Hydraulics 4: e001. [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. 2005. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist 167: 403–413. [DOI] [PubMed] [Google Scholar]
- Savi T, Tintner J, Da Sois L, Grabner M, Petit G, Rosner S. 2019. The potential of Mid‐Infrared spectroscopy for prediction of wood density and vulnerability to embolism in woody angiosperms. Tree Physiology 39: 503–510. [DOI] [PubMed] [Google Scholar]
- Shipley B, Vile D, Granier E. 2006. From plant traits to plant communities: a statistical mechanistic approach to biodiversity. Science 314: 812–814. [DOI] [PubMed] [Google Scholar]
- ter Steege H. 1994. Flooding and drought tolerance in seeds and seedlings of two Mora species segregated along a soil hydrological gradient in the tropical rainforest of Guyana. Oecologia 100: 356–367. [DOI] [PubMed] [Google Scholar]
- Taylor PG, Cleveland CC, Wieder WR, Sullivan BW, Doughty CE, Dobrowski SZ, Townsend AR. 2017. Temperature and rainfall interact to control carbon cycling in tropical forests. Ecology Letters 20: 779–788. [DOI] [PubMed] [Google Scholar]
- Trenberth KE. 2011. Changes in precipitation with climate change. Climate Research 47: 123–138. [Google Scholar]
- Trueba S, Pouteau R, Lens F, Feild TS, Isnard S, Olson ME, Delzon S. 2017. Vulnerability to xylem embolism as a major correlate of the environmental distribution of rain forest species on a tropical island. Plant, Cell & Environment 40: 277–289. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Engelbrecht BMJ, Vargas G, Kursar TA. 2003. Desiccation tolerance of five tropical seedlings in Panama. Relationship to a field assessment of drought performance. Plant Physiology 132: 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sobrado MA, Stratton LJ, Becker P. 1999. Diversity of hydraulic conductance in leaves of temperate and tropical species: possible causes and consequences. Journal of Tropical Forest Science 11: 47–60. [Google Scholar]
- Umaña MN, Condit R, Pérez R, Turner BL, Wright SJ, Comita LS. 2020. Shifts in taxonomic and functional composition of trees along rainfall and phosphorus gradients in central Panama. Journal of Ecology 109: 51‐61 [Google Scholar]
- Veihmeyer FJ, Hendrickson AH. 1928. Soil moisture at permanent wilting of plants. Plant Physiology 3: 355–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzin JF, Loik ME, Schwinning S, Williams DG, Fay PA, Haddad BM, Harte J, Huxman TE, Knapp AK, Lin G et al. 2003. Assessing the response of terrestrial ecosystems to potential changes in precipitation. BioScience 53: 941–952. [Google Scholar]
- Westoby M, Falster DS, Moles AD, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. [Google Scholar]
- Wright JS. 2002. Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130: 1–14. [DOI] [PubMed] [Google Scholar]
- Zhu SD, Chen YJ, Ye Q, He PC, Liu H, Li RH, Fu PL, Jiang GF, Cao KF. 2018. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiology 38: 658–663. [DOI] [PubMed] [Google Scholar]
- Zuleta D, Duque A, Cardenas D, Muller‐Landau HC, Davies SJ. 2017. Drought‐induced mortality patterns and rapid biomass recovery in a terra firme forest in the Colombian Amazon. Ecology 98: 2538–2546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Correlation plot indicating the relationships between πtlp, LMA and WD.
Fig. S2 Habitat associations of evergreen and deciduous tree species by πtlp within the Barro Colorado Island 50 ha ForestGEO plot.
Fig. S3 Linear relationship between the proportion of trees of each species growing in high plateau and πtlp, highlighting significant Torus associations (Harms et al., 2004).
Fig. S4 Map of the 50 ha ForestGEO plot on Barro Colorado Island showing the distribution of WD within the evergreen trees, and WD within the deciduous trees.
Fig. S5 Habitat associations of evergreen and deciduous tree species by WD within the Barro Colorado Island 50 ha ForestGEO plot.
Fig. S6 Linear relationship between the proportion of trees growing in high plateau and WD, highlighting significant Torus associations (Harms et al., 2004).
Fig. S7 Map of the 50 ha ForestGEO plot on Barro Colorado Island showing the distribution of LMA within the evergreen trees, and within the deciduous trees.
Fig. S8 Habitat associations of evergreen and deciduous tree species by LMA within the Barro Colorado Island 50 ha ForestGEO plot.
Fig. S9 Linear relationship between the proportion of trees growing in high plateau and LMA, highlighting significant Torus associations (Harms et al., 2004).
Fig. S10 Moisture association index (MAI of Condit et al., 2013) as a function of wood density (WD).
Fig. S11 Moisture association index (MAI of Condit et al., 2013) as a function of leaf mass per area (LMA).
Table S1 Summary of observed mean turgor loss point (πtlp), leaf mass area (LMA), wood density (WD) and leaf phenology. Standard deviation is given for all mean values.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


