Abstract
Objective
Binge‐eating disorder (BED) disrupts dopamine neuron function, in part by altering dopamine transporter (DAT) activity. This study characterized the effects of high‐fat bingeing on presynaptic dopamine terminals and tested the hypothesis that acute low‐dose amphetamine would restore DAT function.
Methods
C57BL/6 mice were given limited access (LimA) to a high‐fat diet (2 h/d, 3 d/wk) or standard chow (control). After 6 weeks, ex vivo fast‐scan cyclic voltammetry was used to characterize dopamine‐terminal adaptations in the nucleus accumbens. Prior to undergoing fast‐scan cyclic voltammetry, some mice from each group were given amphetamine (0.5 mg/kg intraperitoneally).
Results
Escalation of high fat intake, termed bingeing, occurred in the LimA group and coincided with increased phasic dopamine release, reduced dopamine uptake rates, and increased dopamine receptor 2 (D2) autoreceptor function. Acute amphetamine selectively reversed dopamine uptake changes in the LimA group and restored the potency of amphetamine to inhibit uptake.
Conclusions
High‐fat bingeing enhanced dopaminergic signaling in the nucleus accumbens by promoting phasic dopamine release and reducing clearance. This study’s data show that amphetamine was efficacious in restoring impaired DAT function caused by high‐fat bingeing but did not reduce dopamine release to normal. These presynaptic changes should be considered if amphetamine‐like dopamine releasers are used as treatments for BED.
Study Importance.
What is already known?
-
►
Binge‐eating disorder (BED) is linked to alterations in dopamine system function.
-
►
Dopamine release in the nucleus accumbens (NAc) contributes to appetitive and consummatory aspects of food intake.
-
►
Some amphetamine‐like dopamine agonists are used to treat BED off label.
What does this study add?
-
►
Bingeing on high‐fat food reduced the dopamine uptake rate in the NAc and augmented the capacity for phasic dopamine release.
-
►
Bingeing on high‐fat food enhanced dopamine receptor 2 (D2) autoreceptor function in the NAc.
-
►
Acute amphetamine administration (intraperitoneal) reversed dopamine‐terminal changes in the NAc by restoring the dopamine uptake rate in mice that binged on high‐fat food.
How might these results change the direction of research or the focus of clinical practice?
-
►
Our data provide mechanisms as to how bingeing on high‐fat food changes dopamine‐terminal function in the NAc and as to how low‐dose amphetamine can restore dopamine neurotransmission in mice that binge. These results show how dopamine agonists are efficacious with limited clinical use but highlight neurochemical changes that may contribute to abuse potential with longer‐term use.
Introduction
Binge‐eating disorder (BED), which was acknowledged as a distinct eating disorder in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders, was previously categorized as a maladaptive eating behavior and grouped with bulimia nervosa. BED is defined as eating a larger portion of food than usual in a discrete period, accompanied by lack of control over intake. Additional criteria outline psychological constructs like embarrassment, disgust, and guilt centered around episodic binges. BED can occur in the absence of obesity (clinically defined as BMI ≥ 30 kg/m2), but patients with BED have a higher prevalence of obesity and develop metabolic disorders earlier in life (1). Evaluating the emotional perceptions of binge eating is unique to clinical studies in humans; however, rodent models of BED have been developed to examine the appetitive and consummatory aspects, as well as the neurochemical adaptations that contribute to, or arise from, binge‐like intake of palatable food (2). Elucidating changes in neurochemistry may also provide insight into susceptibility to common comorbidities like depression, anxiety, and substance‐use disorders.
Clinical evidence of BED altering dopamine neurochemistry is linked to the dopamine transporter (DAT) and dopamine receptor 2 (D2) protein levels and/or function (3). Patients with BED are reported to have an increased incidence of polymorphisms of the DAT gene (solute carrier family 6 member 3 [SLC6A3]) that reduce DAT function but increase sensitivity to the anorexic effects of methylphenidate (4) and enhance the dopaminergic response to visual food cues in the presence of DAT uptake inhibitors (5). Similarly, polymorphisms in the Taq1A allele, which has been linked with increased D2 availability, are common in individuals with BED (6). Increased D2 availability with BED is distinct from reduced D2 binding in patients with obesity who carry the 1A allele (6), indicating unique genotypes for each clinical phenotype. Collectively, clinical evidence of reduced DAT function and altered D2 binding in BED shows that dysregulated dopamine neurotransmission occurs in clinical BED, with DAT and D2 likely influencing dysregulated food intake.
Although clinical studies implicate DAT and D2 in BED, there are limited reports on how BED causes specific neuroadaptations to dopamine terminals, where DAT and D2 facilitate dopamine neurotransmission. Instead, most reports showing adaptations to the dopamine system use obesogenic models with continuous access to palatable foods that engender hyperphagia, not episodic binges. Hyperphagic models of obesity align with clinical reports in patients with obesity and show impaired DAT‐mediated dopamine uptake (7, 8), along with reduced DAT protein levels (9) and reduced D2 function that is associated with compulsive eating (10) and addiction‐like reward deficiency (11). Evidence that reduced DAT and D2 function in rodent models of obesity influences reward circuitry is supported by reports of behavioral cross‐sensitization between amphetamine or cocaine with palatable foods (12, 13). Engagement of the mesolimbic reward system is a common feature of substance abuse; however, whether dysregulated food intake should be labeled as a type of “food addiction” is up for debate.
Animal models of BED engage the mesolimbic reward system in a manner similar to that of obesogenic food intake. After bingeing on high‐fat food, mice exhibit elevated c‐Fos expression (a marker of neuronal activity) in the nucleus accumbens (NAc), ventral tegmental area, and lateral hypothalamus (14). These brain regions coordinate dopaminergic responses to food (15), specifically in the NAc core, where the reinforcing properties of abused drugs and palatable food are encoded (16). Only a few preclinical studies have directly examined DAT and D2 with binge eating. One study by Halpern and colleagues (17) reported that a D2 antagonist, raclopride, blocked the ability of deep brain stimulation to reduce high‐fat binges in mice. Another by the Corwin group (18) showed that D2 in the prefrontal cortex may limit binge consumption. Additionally, binge‐like consumption of high‐fat food in mice increased D2 autoreceptor sensitivity and enhanced behavioral sensitization to amphetamine (19). Interestingly, repeated exposure to amphetamine in mice that binge on high‐fat food attenuated amphetamines’ effects on dopamine release and uptake, indicating a resilience to these adaptations in the NAc. Considering reports that amphetamine dose‐dependently decreased high‐fat bingeing in mice (20) and that a mouse model of high‐fat bingeing blocked NAc terminal adaptations to repeated amphetamine administration along with paradoxically reduced DAT function but enhanced sensitivity to DAT agonists in patients with BED, the purpose of this study was to further characterize DAT function in the NAc core of mice after bingeing on high‐fat food by (1) measuring how high‐fat bingeing impacts phasic dopamine release, which resembles intrinsic dopamine neuron activity in response to salient cues or rewarding stimuli (21, 22), and (2) characterizing the effect of acute amphetamine administration on dopamine terminals in the NAc in mice that binge on high‐fat food.
Methods
Animals and diet
Six‐week‐old male C57BL/6 mice (N = 28) (Jackson Laboratories, Bar Harbor, Maine) were housed in groups of two to three in shoe box cages and were maintained on a reverse light–dark cycle. Mice were separated into a control group given standard rodent chow (3.46 kcal/g; 26% protein, 14% fat, and 60% carbohydrate) (Prolab RMH 3000, catalog number 5P00; LabDiet, St. Louis, Missouri) (Figure 1A) or into a limited‐access (LimA) group given 2‐hour access to a high‐fat diet (5.21 kcal/g; 20% protein, 60% fat, and 20% carbohydrate) (Diet‐Induced Obesity [DIO], catalog number D12492; Research Diets, New Brunswick, New Jersey) (online Supporting Information) 3 d/wk beginning 3 hours into the dark cycle (Figure 1B). Standard chow was not available during the 2‐hour high‐fat access. Food intake for each cage was measured daily for both groups and after 30‐minute and 2‐hour high‐fat access periods in the LimA group. Cage intake was divided by number of mice per cage to calculate daily kilocalories. Animal protocols were approved by the Institutional Animal Care and Use Committee of the Wake Forest School of Medicine, in accordance with the NIH and Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Figure 1.
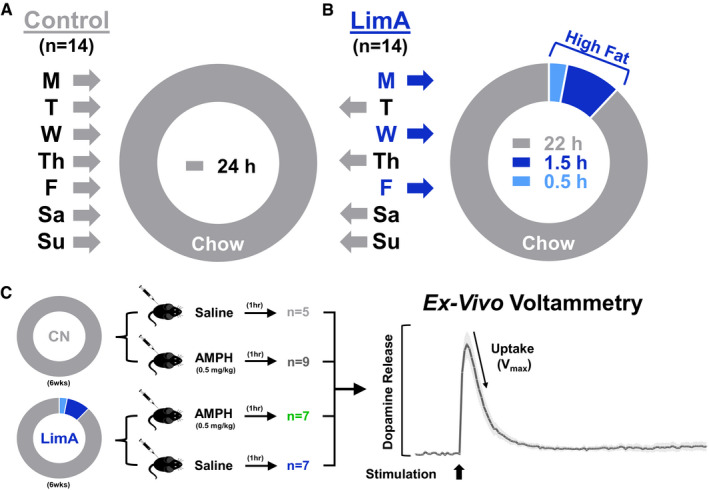
Experimental design. Mice were divided into two dietary groups: (A) the control (CN) group received standard rodent chow exclusively (n = 14), and (B) the limited‐access (LimA) group (n = 14) received 24‐hour access to standard chow on Tuesday (T), Thursday (Th), Saturday (Sa), and Sunday (Su) and limited access to a high‐fat diet for 2 hours on Monday (M), Wednesday (W), and Friday (F), with standard chow the remaining 22 hours on high‐fat days. (C) After 6 weeks on each feeding paradigm, mice from each group received 0.5 mg/kg of intraperitoneal amphetamine (AMPH) or an equal volume of saline 1 hour before proceeding to ex vivo voltammetry to measure dopamine release and uptake in the nucleus accumbens. Vmax, maximal rate of dopamine uptake.
Fast‐scan cyclic voltammetry
Fast‐scan cyclic voltammetry was used to measure dopamine in the NAc core. One hour prior to decapitation, an intraperitoneal (i.p.) bolus of amphetamine (0.5 mg/kg) or saline vehicle was given in a total volume of 0.1 mL (Figure 1B). This amphetamine dose has been shown to reverse dopamine‐terminal changes caused by cocaine self‐administration (23, 24). All voltammetry experiments began 3 to 4 hours into the dark cycle on a day just before mice would have received limited access to the high‐fat food, and they were performed as previously described (8). Briefly, mice were anesthetized using isoflurane and decapitated, and then their brains were swiftly removed. One hemisphere of the brain was coronally sliced (300‐µm slices), and NAc tissue was excised from the other hemisphere for Western blotting. Brain slices equilibrated for 60 minutes at 32.5ºC in oxygenated (95% O2 to 5% CO2) artificial cerebral spinal fluid (126mM NaCl, 25mM NaHCO3, 11mM d‐glucose, 2.5mM KCl, 2.4mM CaCl2, 1.2mM MgCl2, 1.2mM NaH2PO4, and 0.4mM l‐ascorbic acid; pH adjusted to 7.4) at a flow rate of 1 m/min. We used a triangular wave form scanning from −0.4 to +1.2 to −0.4 V/s versus Ag/AgCl at 400 V/s every 100 milliseconds, using carbon fiber microelectrodes to detect the peak dopamine release evoked with 4‐millisecond pulse stimulations (monophasic, 350 μA) from a bipolar electrode (catalog number 8IMS3033SPCE; Plastics One, Roanoke, Virginia) every 3 minutes. This technique measures synaptic dopamine every 100 milliseconds by detecting a change in the current when dopamine is oxidized at +0.6 V on the voltage ramp and is then reduced when the voltage sweeps back to −0.4 V. The current reflects synaptic dopamine concentrations and DAT‐mediated dopamine removal from the synapse. We report dopamine release as the dopamine concentration when the current reaches its peak. Phasic dopamine release was measured by applying stimulation trains of five pulses at 5, 10, 20, 40, or 100 Hz. Data were acquired and modeled using Demon Voltammetry Software (Wake Forest Innovations, Winston‐Salem, North Carolina), based on Michaelis‐Menten kinetics using 160 nM as the affinity of dopamine for the DAT (Km) (25). Dose–response curves for quinpirole (catalog number 1061; Tocris, Bristol, UK) or d‐amphetamine (catalog number A5880; Sigma‐Aldrich, St. Louis, Missouri) were applied to ex vivo slices in a cumulative half‐log manner. Electrodes were calibrated after each experiment with 3μM dopamine (catalog number 3548; Tocris). The total numbers of brain slices and animals are reported in each figure.
Western blot protein quantification
NAc tissue was flash frozen in 2‐methylbutane (catalog number M32631; Sigma‐Aldrich) and stored at −80ºC. Membrane and cytosolic fractions were isolated using differential centrifugation in a sucrose fractionation buffer (250mM sucrose, 20mM HEPES, 10mM KCl, 1.5mM MgCl2, 1mM ethylenediaminetetraacetic acid (EDTA), and 1mM EGTA, with Halt Protease Inhibitor Cocktail [catalog number 78430; Thermo Fisher Scientific, Waltham, Massachusetts]). Tissue was homogenized and centrifuged at 720g for 5 minutes, and then the supernatant was removed and centrifuged at 10,000g for 10 minutes. The resulting supernatant was removed and ultracentrifuged at 100,000g for 1 hour. After ultracentrifugation, the supernatant retained was used as the cytosolic fraction. The pellet was lysed (Pierce IP Lysis Buffer, catalog number 87787; Thermo Fisher Scientific) for an enriched membrane fraction. Blots used 15 µg of protein in NuPAGE 4% to 12% Bis‐Tris protein gels (catalog number NP0322; Thermo Fisher Scientific) and were then transferred to polyvinylidene fluoride membranes (catalog number 88518; Thermo Fisher Scientific). Primary antibodies for DAT (catalog number AB2231; Millipore, Burlington, Massachusetts) and β‐actin (catalog number A2228; Sigma‐Aldrich) were diluted 1:8,000 and 1:20,000, respectively, in Pierce blocking buffer (catalog number 37570; Thermo Fisher Scientific). Secondary anti‐rabbit and anti‐mouse antibodies (catalog numbers G‐21234 and A16072; Thermo Fisher Scientific) were diluted 1:5000 for DAT and 1:8,000 for β‐actin. Imaging was performed on a ChemiDoc XRS using chemiluminescence, and densitometry was analyzed using Quantity One software (Bio‐Rad Laboratories, Hercules, California).
Statistics
GraphPad Prism version 8 (GraphPad Software, San Diego, California) was used for statistical analyses. Two‐way repeated‐measures ANOVA compared differences within and between treatment groups for body weight and voltammetry dose–response curves. Two‐way ANOVA also tested the effect of diet (control or LimA) and drug (saline or amphetamine) as sources of variation for all voltammetry experiments. Tukey or Sidak post hoc analysis determined significant differences between experimental groups when appropriate. Data normality and outlier analysis were assessed using a D’Agostino‐Pearson omnibus K2 test followed by the Grubbs test (α = 0.05). Group data are presented as the group mean ± SEM.
Results
LimA to high‐fat food engendered binge‐like consumption of high‐fat food
Weight gain was similar between groups (Figure 2A), consistent with comparable average daily energy intakes (Figure 2B). However, on days with high‐fat access, the LimA group consumed 61% of daily kilocalories in the 2‐hour high‐fat access widow (Figure 2C), including 48% within the first 30 minutes and only 13% in the following 1.5 hours. This indicated a binge‐like feeding pattern of the high‐fat food. Moreover, the percentage of kilocalories consumed during the first 30 minutes and throughout the 2‐hour access to high‐fat food was significantly greater than the percentage of kilocalories consumed during the entire 22‐hour access to standard chow (39%) (P = 0.035 and P < 0.0001, respectively) (Figure 2B). The numbers of total kilocalories consumed were similar between the LimA and control groups in weeks 1 and 6 (Figure 2D); however, in the LimA group, kilocalories from chow were significantly reduced (P = 0.025) from week 1 (63%) to week 6 (38.5%) (Figure 2E), indicating a shift in preference toward the high‐fat food and a voluntary restriction of the standard chow. We also observed escalated binge‐like feeding on high‐fat food, measured by an increase in the percentage of total daily kilocalories consumed during these feeding bins over time: 2 hours (37%‐61%, weeks 1‐6; P = 0.013) (Figure 2F) and 30 minutes (27%‐48%, weeks 1‐6; P = 0.037) (Figure 2G).
Figure 2.
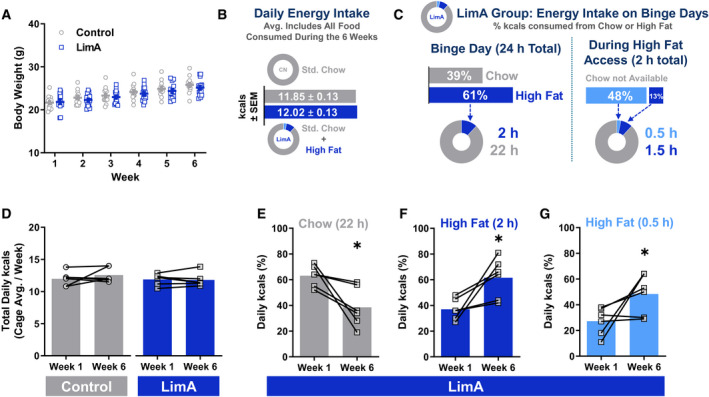
Body weight and food intake. (A) Body weight gain over the 6‐week feeding period. (B) Daily energy intake in kilocalories averaged per mouse, including all food over 6 weeks. (C) Percentage breakdown of kilocalories consumed from chow or high‐fat food in the limited‐access (LimA) group on days with access to high‐fat food. (D) The overall kilocalorie intake was stable from week 1 to week 6, but (E) mice in the LimA group consumed significantly less standard chow in week 6 than in week 1 (P < 0.05), coinciding with a significantly higher percentage of kilocalories from high‐fat food (F) after 2 hours (P < 0.05) and (G) after 30 minutes (P < 0.05) using paired Student t tests. All data reflect the mean ± SEM (*P < 0.05).
High‐fat bingeing increased phasic dopamine release and reduced presynaptic dopamine uptake rate
Next, we sought to determine whether high‐fat bingeing altered dopamine release and uptake in the NAc. Dopamine release evoked by a single pulse of electrical stimulation is considered to model “tonic,” low‐frequency release events, and it was not statistically different between the control (2.8 ± 0.5 µM) and LimA (1.9 ± 0.3 µM) groups or between the control group receiving saline and the control group receiving 0.5 mg/kg of i.p. amphetamine (CN‐AMPH) 1 hour before decapitation (2.6 ± 0.3 µM). However, a nearly significant increase in the dopamine release occurred in the LimA group receiving 0.5 mg/kg of i.p. amphetamine (LimA‐AMPH) compared with the LimA mice receiving i.p. saline (3.3 ± 0.5 µM and 1.9 ± 0.3 µM, respectively; P = 0.057) (Figure 3A). Dopamine release after a “phasic” burst of five stimulation pulses at 5, 10, 20, 40, or 100 Hz was greater in the LimA group than in the control group (Figure 3B), with main effects of diet (F (1,10) = 5.751; P = 0.037) and stimulation frequency (F (4,40) = 48.47; P < 0.001). Amphetamine (i.p.) did not alter phasic dopamine release in the CN‐AMPH or LimA‐AMPH mice (Figure 3C‐3D).
Figure 3.
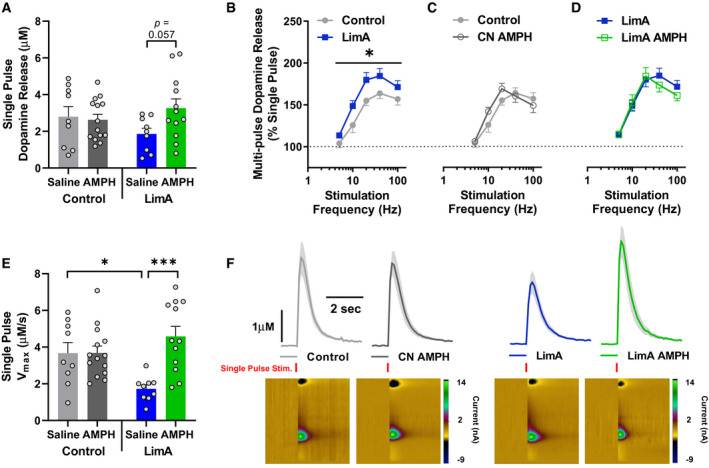
Difference in dopamine release and uptake in the nucleus accumbens after intraperitoneal (i.p.) saline or amphetamine (AMPH) administered in vivo. (A) Dopamine release evoked by a single pulse in mice receiving i.p. saline or 0.5 mg/kg of AMPH 1 hour prior to brain removal. (B‐D) Dopamine release evoked by phasic five‐pulse stimulation over 5‐, 10‐, 20‐, 40‐, and 100‐Hz frequencies. (E) Maximal rate of dopamine uptake (Vmax). (F) Visual comparison of averaged line traces showing dopamine release (peak height) and Vmax (downward slope) representing the mean ± SEM current in the shaded area with a representative pseudocolor plot for each group. The control group included n = 9 slices from n = 5 mice, the limited‐access (LimA) group included n = 9 slices from n = 7 mice, the control group receiving 0.5 mg/kg of i.p. AMPH (CN‐AMPH) included n = 14 slices from n = 9 mice, and the LimA group receiving 0.5 mg/kg of i.p. AMPH (LimA‐AMPH) included n = 12 slices from n = 7 mice. All data reflect the mean ± SEM (*P < 0.05; ***P < 0.001).
Regarding the maximal rate of dopamine uptake (Vmax), we observed a main effect of i.p. amphetamine (F(1,40) = 9.520; P = 0.0037) and an amphetamine‐by‐diet interaction (F (1,40) = 9.327; P = 0.004). The Vmax in the LimA group (1.7 ± 0.2 µM/s) was significantly attenuated compared with that in the control group (3.7 ± 0.6 µM/s) (P = 0.019). Amphetamine (i.p.) did not affect the Vmax differently in the control group receiving saline and in CN‐AMPH (3.7 ± 0.4 µM/s) but significantly increased the Vmax in the LimA‐AMPH group (4.6 ± 0.5 µM/s) compared with the LimA group receiving i.p. saline (P < 0.001) (Figure 3E). Visual representations of single‐pulse dopamine release and uptake are illustrated, with aggregated line traces showing group differences in dopamine release (peak height) and uptake (angle of the descending slope) (Figure 3F).
DAT function was impaired after high‐fat bingeing but was restored with i.p. amphetamine administration
The reduced Vmax in the LimA group indicates that bingeing on high‐fat food impaired DAT‐mediated dopamine uptake. To further characterize DAT function, we measured the potency of amphetamine as an uptake inhibitor using voltammetry and compared DAT protein levels in membrane and cytosolic fractions with Western blotting. Amphetamine potency was determined by bathing ex vivo brain slices with amphetamine in a dose–response curve and identifying changes in the affinity (apparent Km) of the DAT for dopamine. Increased potency is measured by greater uptake inhibition at a lower concentration of amphetamine (26). A two‐way ANOVA identified a main effect of amphetamine dose on uptake inhibition within groups (F (4,100) = 101.2; P < 0.001) and a treatment group‐by‐dose interaction (F (12,100) = 2.059; P = 0.027) (Figure 4A‐4D). Tukey post hoc analysis revealed significantly reduced amphetamine potency in the LimA group compared with the control groups (P < 0.05) (Figure 4A) and LimA‐AMPH (P < 0.01) (Figure 4C) at 10µM. Interestingly, i.p. amphetamine restored the potency of amphetamine as an uptake inhibitor after high‐fat bingeing while not changing the potency in the control group. Aggregated line traces show uptake inhibition in response to 10µM amphetamine (Figure 4E). Finally, amphetamine caused a dose‐dependent reduction in dopamine release in all groups (Figure 4F), consistent with amphetamine causing depletion of exocytotic vesicles and DAT‐mediated reverse transport of dopamine over time.
Figure 4.
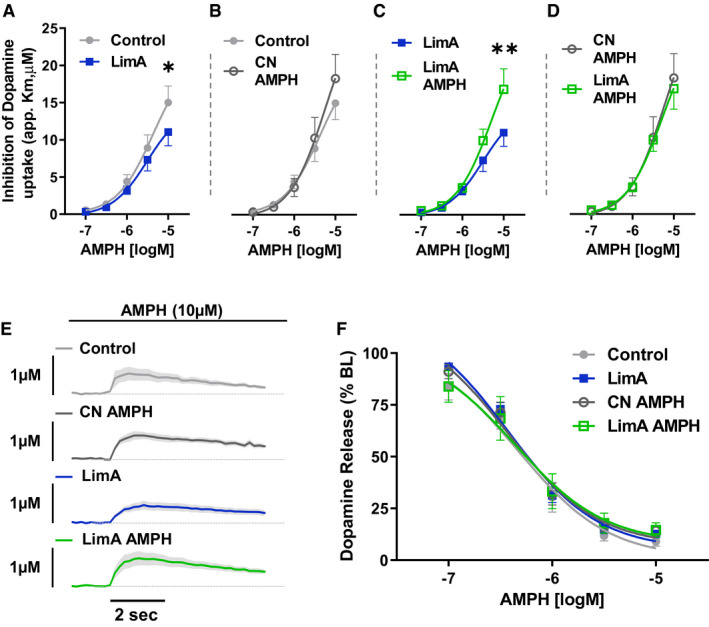
Amphetamine (AMPH) potency at the dopamine transporter (DAT). (A‐D) The potency of AMPH as an uptake inhibitor at the DAT, measured as apparent Km (app. Km) for dopamine in the presence of AMPH, applied cumulatively to the slice bath in half‐log doses. (E) Averaged dopamine traces showed impaired clearance in the presence of 10µM AMPH. (F) Finally, AMPH dose‐dependently depleted stimulated dopamine release similarly in all treatment groups. Two‐way repeated‐measures ANOVA was used to determine the effects of the drug and diet in the experimental group. The control group included n = 7 slices from n = 5 mice, the limited‐access (LimA) group included n = 6 slices from n = 6 mice, the control group receiving 0.5 mg/kg of intraperitoneal (i.p.) AMPH (CN‐AMPH) included n = 8 slices from n = 8 mice, and the LimA group receiving 0.5 mg/kg of i.p. AMPH (LimA‐AMPH) included n = 7 slices from n = 6 mice. All data reflect the mean ± SEM (*P < 0.05; **P < 0.01).
To examine whether our reported effects on amphetamine potency corresponded with DAT protein levels, we conducted Western blot analysis on enriched membrane and cytosolic fractions of NAc tissue. A one‐way ANOVA of DAT band density, relative to β‐actin, identified a significant effect of treatment group (F (3,20) = 8.672; P < 0.001) on the 68‐kDa molecular weight band in the plasma membrane fraction (considered the immature DAT isoform), with Tukey post hoc analysis indicating significantly lower DAT levels in the LimA (P < 0.001), CN‐AMPH (P < 0.05), and LimA‐AMPH (P < 0.05) groups compared with the control group receiving saline (Figure 5A,5C). There were no treatment group differences in membrane levels of the mature DAT isoform (78 kDa) or the 150‐kDa molecular weight band corresponding with DAT dimers. No treatment effects were observed on any DAT isoform in cytosolic fractions from the NAc.
Figure 5.
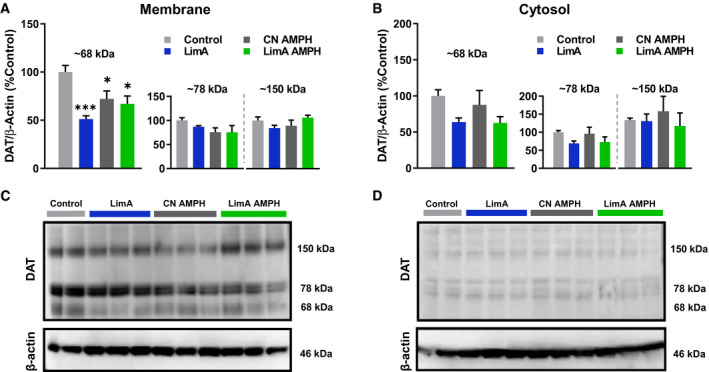
Dopamine transporter (DAT) protein levels. Western blot quantification of DAT protein (normalized to β‐actin) from enriched membrane and cytosolic fractions of nucleus accumbens brain tissue. Normalized DAT protein in each group was expressed as a percentage of that of the control group. (A) Histological representation of DAT band density at ~150, 78, and 68 kDa in membrane fractions and (B) cytosolic fractions showed a reduced density of only the 68‐kDa DAT band in all groups compared with the control group. Representative blots showing DAT band density at 150, 78, and 68 kDa, as well as the 46‐kDa β‐actin band containing (C) membrane and (D) cytosolic samples. All data reflect the mean ± SEM (*P < 0.05; ***P < 0.001).
High‐fat bingeing increased presynaptic sensitivity to the dopamine D2 receptor agonist quinpirole
To examine presynaptic control of dopamine release by dopamine D2 autoreceptors, the dopamine D2 agonist quinpirole was used to measure autoreceptor sensitivity. Two‐way repeated‐measures ANOVA revealed main effects of diet (F (1,6) = 8.047; P < 0.05) and quinpirole dose (F (4,24) = 153.6; P < 0.0001), with Sidak post hoc analysis identifying a significant difference between the LimA and control groups at 10 nM quinpirole (P < 0.05) (Figure 6A). There was also a main effect of diet (F (1,14) = 52.10; P < 0.0001) and a diet‐by‐amphetamine (i.p.) interaction (F (1,14) = 62.17; P < 0.0001) on the quinpirole half‐maximal inhibitory concentration (IC50), with a significantly lower IC50 in the LimA group (6.8 nM) than in the control group (16.3 nM) (P < 0.0001) (Figure 6D) indicating enhanced sensitivity to quinpirole‐induced inhibition of dopamine release. In controls, i.p. amphetamine reduced the IC50 compared with i.p. saline (P < 0.0001).
Figure 6.
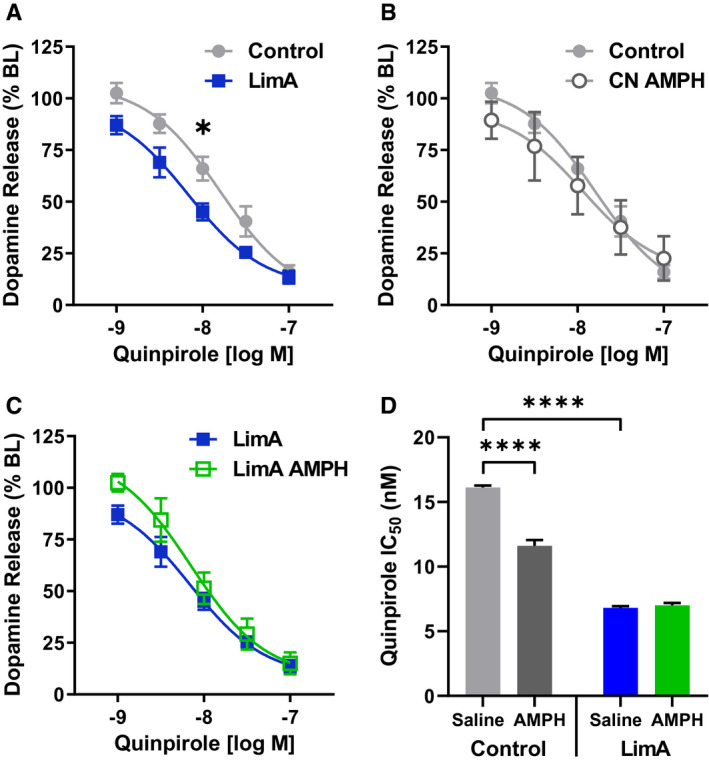
Presynaptic dopamine receptor 2/dopamine receptor 3 (D2/D3) autoreceptor function. All groups showed a dose‐dependent reduction in evoked dopamine release with increasing concentrations of the D2/D3 agonist quinpirole. (A) Two‐way repeated‐measures ANOVA revealed a significant increase in D2/D3 sensitivity in the limited‐access (LimA) group compared with the control group, whereas post hoc analysis identified a significant difference between groups at 10 nM (−8.0 log M) (P < 0.05). The quinpirole dose responses were similar between (B) the control group and the control group receiving 0.5 mg/kg of intraperitoneal (i.p.) amphetamine (CN‐AMPH) and (C) the LimA group and the LimA group receiving 0.5 mg/kg of i.p. amphetamine (LimA‐AMPH). Overall, increased sensitivity is indicated by a leftward shift in the regression‐curve fit and (D) a reduction in the half‐maximal inhibitory concentration (IC50) for quinpirole in the LimA group compared with the control group, and amphetamine reduced the IC50 for quinpirole in the control group. The control group included n = 4 slices from n = 4 mice, the LimA group included n = 4 slices from n = 4 mice, the CN‐AMPH group included n = 5 slices from n = 4 mice, and LimA‐AMPH included n = 5 slices from n = 4 mice. All data reflect the mean ± SEM (*P < 0.05; ****P < 0.0001).
Discussion
Here, we report that intermittent limited access to high‐fat food engendered binge‐like feeding that escalated over time and corresponded with presynaptic dopamine‐terminal adaptations in the NAc core. We showed that high‐fat bingeing slowed the rate of dopamine uptake, enhanced phasic dopamine release, and increased the sensitivity of D2 autoreceptors. Increased phasic release, coupled with reduced uptake, suggests augmented synaptic dopamine signaling after high‐fat bingeing, possibly leading to an adaptive increase in D2 autoreceptor activity to counter increased synaptic dopamine. It is possible that high‐fat bingeing primes phasic dopamine‐release capacity, which could heighten dopamine‐dependent processes like the salience of drugs, palatable high‐fat foods, or cues predicting their availability. We also showed that i.p. amphetamine administration selectively increased dopamine uptake and restored the reduced amphetamine potency in mice that binged on high‐fat food but had no effect in control mice. This is consistent with previous reports of amphetamine reversing dopamine‐terminal adaptations caused by cocaine self‐administration, presumably through restoration of normal DAT function (23, 24).
Dopamine neurons fire with a tonic pacemaker‐like frequency (<5 Hz), but salient stimuli initiate phasic burst firing at 15 to 20 Hz (22). Burst firing facilitates learning and memory, arousal, assignment of emotional valence, and contextual awareness that collectively contribute to the reward salience of abused substances (21). Phasic release elevates synaptic dopamine, which increases binding to postsynaptic dopamine receptor 1 (D1) and D2 and contributes to behavioral outcomes downstream of the NAc (22). We observed that high‐fat bingeing had no effect on tonic dopamine release (single pulse) but significantly elevated dopamine release in response to phasic five‐pulse 20‐Hz stimulations. Microdialysis experiments show a gradual elevation in dopamine with food intake (27). The dopamine rise is likely an accumulation of phasic dopamine release in response to food, showing a gradual rise in dopamine from baseline after meal onset. The magnitude of phasic dopamine release in response to food is related to visual cues and the amount of palatable food received (28) but is also triggered by unpredicted food delivery (29). Interestingly, depleting dopamine in the NAc reduced operant responding for food but enhanced ad libitum food intake (30). Together, these data suggest that phasic dopamine release drives appetitive aspects of food intake, whereas reduced tonic levels may encourage hyperphagia. This is consistent with the anhedonia theory of food intake, which posits that food consumption may compensate for reduced dopamine levels (11), and with the “signal‐to‐noise” theory, which postulates that a greater change in synaptic dopamine is achieved with phasic burst firing to stimuli (i.e., palatable food) and that a lower baseline would increase the dopamine signal relative to the background noise (21). It is possible that high‐fat bingeing primes the NAc for phasic dopamine signaling, which may increase dopaminergic responses to palatable food or food cues. Intact insulin signaling may contribute to this dopaminergic response, given that insulin enhances flavor preferences and synaptic dopamine release (31). We have previously reported normal insulin levels in this limited access paradigm (19). Primed phasic signaling would be consistent with previous findings that show that intermittent, but not continuous, access to high‐fat food enhanced the progressive ratio of responding to the food (32). Additionally, conditioning to the limited access schedule of high‐fat delivery may also contribute to the synaptic adaptations we observed, as dopamine release is known to shift from the reward to cues signaling its future availability with behavioral training (33). Together, increased phasic capacity and dietary entrainment may promote escalated high‐fat bingeing, as we observed in the LimA group. Although we cannot measure psychological criteria like embarrassment, disgust, or a sense of lacking control, the LimA group matched the Diagnostic and Statistical Manual of Mental Disorders criteria for food consumption: that is, eating significantly more food in a discrete period of time than normal and eating more rapidly than normal. Moreover, escalation of high‐fat bingeing resembles binge intake of cocaine or alcohol in similar restricted‐access paradigms (34, 35), indicating a similar consummatory phenotype between palatable food binges and drug self‐administration. Importantly, mice that binged did not develop obesity in our study. Clinically, there is a higher prevalence of obesity with BED, and a possible limitation of our study is the short length of exposure that did not engender obesogenic weight gain. However, our model does show neurochemical similarities to behavioral comorbidities like substance‐use disorders.
We also observed reduced dopamine uptake after bingeing on high‐fat food, which is consistent with previous reports (19). Reduced uptake may prolong dopaminergic signaling, particularly when coupled with enhanced phasic release. Reduced uptake did not correspond with changes in the mature DAT protein isoform (78 kDa) at the plasma membrane, which contain multiple N‐glycosylation sites associated with a Vmax that is enhanced in comparison with that of the immature less N‐glycosylated isoform (36). That there were no changes in the mature isoform suggests that high‐fat bingeing impairs DAT function without affecting membrane protein levels. Clinical reports of reduced DAT function in patients with BED are linked with polymorphisms in the DAT gene (SLC6A3), resulting in a short allele variant containing seven or nine tandem repeats (37). This short DAT variant is linked to reduced DAT binding availability (38), whereas a longer variant with 10 repeats enhanced binding of DAT ligands by ~50%. With reduced binding availability in patients with BED expressing short DAT variants, it is interesting that patients with BED are more sensitive to the appetite‐suppressive effects of DAT uptake inhibitors (4). However, if basal dopamine is reduced by high‐fat bingeing, as reported in mice (19), the elevation in synaptic dopamine by DAT uptake inhibition may be sufficient to activate postsynaptic D2 receptors and reduce the threshold of burst firing needed to produce enough dopamine to occupy lower‐affinity D1 receptors, both of which suppress food intake (39, 40). It is interesting that patients with BED are reported to have increased D2 availability but that clinical obesity is associated with reduced D2 binding (6). With respect to BED, this is counterintuitive, considering that D2 activation generally reduces food intake. This highlights the complexity of the brain’s neurochemical changes in BED and suggests that D2 projections via an “indirect pathway” and other neurotransmitters in downstream circuits are worthy of future investigation. Consistent with elevated D2 in BED, we observed an increase in presynaptic D2 autoreceptor function, which was similar to enhanced D2 sensitivity in alcohol withdrawal (41). In this study, however, it is likely that greater phasic‐release capacity at dopamine terminals caused an adaptive increase in D2 autoreceptor sensitivity to limit dopamine activity.
Amphetamine derivatives like methylphenidate, phentermine, and lisdexamfetamine are dopamine agonists and DAT ligands used for weight management and attention‐deficit disorder but they are sometimes used off label to treat BED. Long‐term use of these drugs is discouraged, however, because of high abuse potential. We found that acute amphetamine restored DAT function, which likely contributes to the short‐term efficacy of these DAT ligands for BED treatment. It is also important to note that the amphetamine dose we found to improve DAT function in our study was not found to be anorectic in mice and it may cause a protracted increase in food intake (20). It remains to be seen whether slightly higher doses of amphetamine that are anorectic also restore DAT function. Amphetamine has a transient effect on DAT protein levels, causing membrane recruitment in the short term (42) but internalization over time (43). Amphetamine also disrupts DAT oligomeric complexes (44) into more efficient monomer and dimer isoforms. These mechanisms contribute to amphetamine’s ability to reverse dopamine‐terminal changes caused by cocaine, by restoring DAT‐mediated uptake after a single low‐dose treatment (23, 24). Repeated amphetamine exposure produces behavioral sensitization and increases DAT‐mediated uptake (19). Interestingly, intermittent access to high‐fat food attenuates this effect and further enhances behavioral sensitization to amphetamine (19). It appears that acute amphetamine administration normalizes NAc terminal changes caused by high‐fat bingeing, but repeated amphetamine exposure is unable to sustain these corrective effects in mice that binge. This resistance to lasting beneficial NAc‐terminal changes may contribute to the abuse liability of prolonged amphetamine‐derived treatments for weight management or BED. This raises an important question: does enhancing amphetamine potency in a BED phenotype known to have twice the prevalence of stimulant and polydrug use (45) and higher impulsivity support a transition to stimulant drug use? Considering this question, it may be necessary to reexamine whether dopamine agonists that alter DAT function are appropriate for a population that may be more susceptible to the neurological effects of these pharmacotherapies. If so, it begs the question of what treatment frequency or duration can be used safely. Future studies should examine whether high‐fat bingeing increases the reinforcing efficacy of amphetamine in behavioral studies to further outline treatment parameters and possible abuse potential.
Funding agencies
This work was supported by grants R15DK119897 (SCF) and R01DA048490, U01AA014091, P50AA026117, and P50DA006634 (SRJ).
Disclosure
The authors declared no conflict of interest.
Author contributions
Conceptualization and study design: SCF and SRJ; data collection and analysis: SCF; manuscript preparation: SCF and SRJ.
Supporting information
Supporting Information
References
- 1. McCuen‐Wurst C, Ruggieri M, Allison KC. Disordered eating and obesity: associations between binge‐eating disorder, night‐eating syndrome, and weight‐related comorbidities. Ann N Y Acad Sci 2017;1411:96‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corwin RL, Babbs RK. Rodent models of binge eating: are they models of addiction? ILAR J 2012;53:23‐34. [DOI] [PubMed] [Google Scholar]
- 3. Naef L, Pitman KA, Borgland SL. Mesolimbic dopamine and its neuromodulators in obesity and binge eating. CNS Spectr 2015;20:574‐583. [DOI] [PubMed] [Google Scholar]
- 4. Davis C, Levitan RD, Kaplan AS, et al. Dopamine transporter gene (DAT1) associated with appetite suppression to methylphenidate in a case‐control study of binge eating disorder. Neuropsychopharmacology 2007;32:2199‐2206. [DOI] [PubMed] [Google Scholar]
- 5. Wang G‐J, Geliebter A, Volkow ND, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 2011;19:1601‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis CA, Levitan RD, Reid C, et al. Dopamine for “wanting ” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17:1220‐1225. [DOI] [PubMed] [Google Scholar]
- 7. Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One 2013;8:e58251. doi: 10.1371/journal.pone.0058251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fordahl SC, Jones SR. High‐fat‐diet‐induced deficits in dopamine terminal function are reversed by restoring insulin signaling. ACS Chem Neurosci 2017;8:290‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Narayanaswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet‐induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes (Lond) 2013;37:1095‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction‐like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 2010;13:635‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blum K, Braverman ER, Holder JM, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 2000;32(suppl 1):1‐112. [DOI] [PubMed] [Google Scholar]
- 12. Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross‐sensitization to a low dose of amphetamine. Neuroscience 2003;122:17‐20. [DOI] [PubMed] [Google Scholar]
- 13. Puhl MD, Cason AM, Wojnicki FHE, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behav Neurosci 2011;125:930‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valdivia S, Patrone A, Reynaldo M, Perello M. Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model. PLoS One 2014;9:e87478. doi: 10.1371/journal.pone.0087478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrario CR, Labouèbe G, Liu S, et al. Homeostasis meets motivation in the battle to control food intake. J Neurosci 2016;36:11469‐11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev 2002;1:281‐296. [DOI] [PubMed] [Google Scholar]
- 17. Halpern CH, Tekriwal A, Santollo J, et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci 2013;33:7122‐7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corwin RLW, Wojnicki FHE, Zimmer DJ, et al. Binge‐type eating disrupts dopaminergic and GABAergic signaling in the prefrontal cortex and ventral tegmental area. Obesity (Silver Spring) 2016;24:2118‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fordahl SC, Locke JL, Jones SR. High fat diet augments amphetamine sensitization in mice: role of feeding pattern, obesity, and dopamine terminal changes. Neuropharmacology 2016;109:170‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. West KS, Lawson V, Swanson AM, Dunigan AI, Roseberry AG. Amphetamine dose‐dependently decreases and increases binge intake of fat and sucrose independent of sex. Obesity (Silver Spring) 2019;27:1874‐1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wanat MJ, Willuhn I, Clark JJ, Phillips PEM. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev 2009;2:195‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 2016;6:123‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferris MJ, Calipari ES, Rose JH, et al. A single amphetamine infusion reverses deficits in dopamine nerve‐terminal function caused by a history of cocaine self‐administration. Neuropsychopharmacology 2015;40:1826‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siciliano CA, Mauterer MI, Fordahl SC, Jones SR. Modulation of striatal dopamine dynamics by cocaine self‐administration and amphetamine treatment in female rats. Eur J Neurosci 2019;50:2740‐2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine‐induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 2011;202:158‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calipari ES, Ferris MJ, Siciliano CA, Jones SR. Differential influence of dopamine transport rate on the potencies of cocaine, amphetamine, and methylphenidate. ACS Chem Neurosci 2015;6:155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a microdialysis study. Pharmacol Biochem Behav 1996;55:297‐302. [DOI] [PubMed] [Google Scholar]
- 28. Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science 2005;307:1642‐1645. [DOI] [PubMed] [Google Scholar]
- 29. Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward‐predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci 2011;34:1997‐2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav 1994;49:85‐91. [DOI] [PubMed] [Google Scholar]
- 31. Stouffer MA, Woods CA, Patel JC, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 2015;6:8543. doi: 10.1038/ncomms9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wojnicki FHE, Babbs RK, Corwin RLW. Reinforcing efficacy of fat, as assessed by progressive ratio responding, depends upon availability not amount consumed. Physiol Behav 2010;100:316‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 2007;10:1020‐1028. [DOI] [PubMed] [Google Scholar]
- 34. Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self‐administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology 2009;34:796‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S. Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol 2013;18:496‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li LB, Chen N, Ramamoorthy S, et al. The role of N‐glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem 2004;279:21012‐21020. [DOI] [PubMed] [Google Scholar]
- 37. Shinohara M, Mizushima H, Hirano M, et al. Eating disorders with binge‐eating behaviour are associated with the s allele of the 3’‐UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci 2004;29:134‐137. [PMC free article] [PubMed] [Google Scholar]
- 38. Heinz A, Goldman D, Jones DW, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000;22:133‐139. [DOI] [PubMed] [Google Scholar]
- 39. Terry P, Gilbert DB, Cooper SJ. Dopamine receptor subtype agonists and feeding behavior. Obes Res 1995;3(suppl 4):515S‐523S. [DOI] [PubMed] [Google Scholar]
- 40. O’Connor EC, Kremer Y, Lefort S, et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 2015;88:553‐564. [DOI] [PubMed] [Google Scholar]
- 41. Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend 2015;150:24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live‐cell imaging using total internal reflection fluorescence microscopy. J Neurosci 2009;29:3328‐3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahlig KM, Lute BJ, Wei Y, et al. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Pharmacol 2006;70:542‐548. [DOI] [PubMed] [Google Scholar]
- 44. Siciliano CA, Saha K, Calipari ES, et al. Amphetamine reverses escalated cocaine intake via restoration of dopamine transporter conformation. J Neurosci 2018;38:484‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Root TL, Pisetsky EM, Thornton L, Lichtenstein P, Pedersen NL, Bulik CM. Patterns of co‐morbidity of eating disorders and substance use in Swedish females. Psychol Med 2010;40:105‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


