To the Editor:
The existing vaccination strategies for prevention of adult Streptococcus pneumoniae lung infections are only partially effective (1) and novel preventive approaches are required. Recent data have shown that adults develop immunity to S. pneumoniae through repeated episodes of asymptomatic nasopharyngeal colonization (2–6). This naturally acquired immunity includes protective responses to both protein and capsular antigens (2–6) and is boosted by recolonization events (4, 7). These data suggest that deliberate nasopharyngeal administration of live S. pneumoniae could prevent serious S. pneumoniae infections by strengthening preexisting cross-serotype protective immunity that inhibits nasopharyngeal colonization with virulent strains, increases antigen-specific systemic immunity, and perhaps strengthens alveolar macrophage–mediated innate immunity (2, 3, 6, 7). This strategy would require S. pneumoniae strains able to stimulate protective immunity but unable to cause disease in a population with an underlying increased susceptibility to S. pneumoniae. Here we describe the development and preclinical characterization of two live attenuated S. pneumoniae strains with these characteristics that are suitable for future use in human trials. Animal procedures were approved by the local ethical review process and conducted in accordance with UK national guidelines under project license PPL70/6510.
Fourteen mutant strains of the BHN418 6B S. pneumoniae strain containing deletions of known or potential virulence determinants (selected using data from published virulence screen and transcriptomic studies) were screened in mouse infection models for their virulence phenotypes and ability to induce protective immunity after nasopharyngeal colonization. Eight mutations reduced virulence without affecting colonization (data not shown). Of these, deletion of fhs or proABC (both with poorly understood roles during S. pneumoniae infections) caused particularly strong impairments of virulence. These genes were selected along with piaA (which encodes an iron transporter required for systemic virulence) as targets to make double mutant strains for investigation as candidate strains for prevention of S. pneumoniae infections. Double mutations were made to increase the degree of virulence attenuation and minimize the risk of revertant strains developing when used in human studies. Target genes were replaced from start to stop codon with the kanamycin or spectinomycin antibiotic resistance cassettes using established transformation techniques (8) to create the ∆fhs/piaA (BHN418 fhs::aad9; piaA::aphIII; spcR kanR) and ∆proABC/piaA (BHN418 proABC::aad9; piaA::aphIII; spcR kanR) strains. Whole-genome sequencing (MicrobesNG, Birmingham University) identified 11 and 2 nonsynonymous SNPs in the ∆fhs/piaA and ∆proABC/piaA strains, respectively, but no other major unexpected mutations compared with the parental 6B strain (data not shown).
The ∆fhs/piaA and ∆proABC/piaA strains were strongly reduced in systemic virulence in a mouse model of pneumonia yet in a colonization model maintained nasopharyngeal colony-forming unit densities similar to the wild-type strain (Table 1). After two episodes of colonization with the double mutant strains, mice developed significant serum IgG responses to the homologous BHN418 6B strain and two heterologous S. pneumoniae strains (TIGR4 and D39) (Figures 1A and 1B). Serum IgG responses from mice colonized with wild-type, ∆proABC/piaA, or ∆fhs/piaA strains were increased against the unencapsulated compared with encapsulated 6B strain (Figure 1A), and when assessed using flow cytometry, recognized whole wild-type 6B, TIGR4, and D39 S. pneumoniae bacteria (data not shown). Together, these data suggest that colonization with the virulence-attenuated strains induced significant antibody responses mainly against noncapsular antigens. Compatible with these data, no significant anticapsular responses were detected in serum from colonized mice using a Meso Scale Discovery multimeric bead assay (6) (data not shown). Instead, immunoblots confirmed that serum IgG from colonized mice recognized multiple protein bands in lysates of S. pneumoniae BHN418 6B, TIGR4, and D39 strains (data not shown).
Table 1.
In Vivo Phenotype Analysis of ∆proABC/piaA and ∆fhs/piaA Mutant Strains in Mouse Infection Models
| Infection Conditions and Model | Target Organ and (if Applicable) Group | 6B Wild Type |
∆proABC/piaA |
∆fhs/piaA |
PBS Control Mice |
||||
|---|---|---|---|---|---|---|---|---|---|
| Log10 cfu/ml | n | Log10 cfu/ml | n | Log10 cfu/ml | n | Log10 cfu/ml | n | ||
| Virulence models | |||||||||
| Pneumonia | Lungs | 5.26 (0.55) | 5 | 3.78 (1.50)* | 5 | 4.54 (0.35) | 5 | N/A | — |
| Blood | 3.30 (0.77) | 5 | 0 (0)† | 5 | 0 (0)† | 5 | N/A | — | |
| Colonization | Nasal wash | 4.13 (0.41) | 10 | 3.99 (0.05) | 5 | 3.55 (0.46) | 5 | N/A | — |
| Colonization-then-challenge models | |||||||||
| 6B pneumonia‡ | Lungs | 3.70 (1.86) | 15 | 2.51 (2.03) | 16 | 2.40 (3.24)† | 11 | 3.66 (2.68) | 16 |
| Blood | 0 (0)§ | 15 | 0 (0)§ | 16 | 0 (0)§ | 11 | 2.09 (3.89) | 16 | |
| BALF | 2.00 (2.18)† | 15 | 2.00 (2.38) | 16 | 0 (1.09)* | 11 | 2.83 (1.20) | 16 | |
| 6B pneumonia in μMT mice | Lungs, wild-type control mice | 4.54 (0.86) | 6 | 4.10 (0.50) | 6 | 3.11 (1.46) | 6 | N/A | — |
| Lungs, μMT mice | 4.08 (0.32) | 3 | 4.18 (0.88) | 6 | 4.28 (0.39) | 6 | N/A | — | |
| Blood, wild-type control mice | 0 (0) | 6 | 0 (0) | 6 | 0 (0) | 6 | N/A | — | |
| Blood, μMT mice | 3.54 (0.77)† | 3 | 0 (2.48) | 6 | 3.23 (0.43)* | 6 | N/A | — | |
| 6B pneumonia in CD4+ cell–depleted mice | Lungs, untreated control mice | 3.27 (2.91) | 6 | 3.59 (2.89) | 6 | 3.24 (2.73) | 6 | N/A | — |
| Lungs, CD4+ cell–depleted mice | 3.54 (2.19) | 5 | 1.33 (2.88) | 6 | 3.47 (1.40) | 6 | N/A | — | |
| Blood, untreated control mice | 0 (0) | 6 | 0 (0) | 6 | 0 (0) | 6 | N/A | — | |
| Blood, CD4+ cell–depleted mice | 0 (0) | 5 | 0 (0) | 6 | 0 (0) | 6 | N/A | — | |
| 6B colonization‡ | Nasal wash | 2.71 (1.46)† | 10 | 1.54 (1.81)§ | 10 | 2.54 (0.94)* | 10 | 3.88 (0.16) | 10 |
| TIGR4 colonization | Nasal wash, untreated control mice | 1.00 (1.78)* | 5 | 1.98 (2.99)† | 6 | 1.75 (1.66)† | 6 | 3.84 (0.47) | 6 |
| Nasal wash, CD4+ cell–depleted mice | 2.52 (0.92) | 6 | 2.90 (1.03) | 6 | 3.38 (0.40) | 6 | 3.36 (0.73) | 6 | |
Definition of abbreviations: BALF = BAL fluid; N/A = not applicable; PBS = phosphate-buffered saline; S. pneumoniae = Streptococcus pneumoniae.
For the pneumonia model, colony-forming units were obtained 28 hours after intranasal inoculation with 1 × 107 S. pneumoniae CFU in 50 μl PBS under deep isoflurane anesthesia, and for the colonization model, 7 days after colonization with 1 × 107 cfu in 10 μl PBS under light isoflurane anesthesia (4, 6, 8). For colonization-then-challenge experiments, mice underwent two episodes of colonization (Days 0 and 14) with 1 × 107 cfu in 10 μl PBS under light isoflurane anesthesia of wild-type BHN418 6B, ∆proABC/piaA, or ∆fhs/piaA S. pneumoniae strains or sham colonization with PBS before challenge between Days 30 and 42. For these, the data presented are pooled from two experiments except the colonization-with-∆proABC/piaA-and-then-challenge experiment, which includes pooled data from three experiments. The μMT−/− mice were in the C57B/6J background (kind gift from Claudia Mauri, University College London); all other experiments used CD1 mice aged 4–8 weeks. CD4+ cells were depleted by intraperitoneal injection of 250 μg anti-CD4 mAb (GK 1.5; BioxCell) 48 and 24 hours before S. pneumoniae challenge (6); flow cytometry confirmed >99% depletion of splenic CD4+ cells (data not shown). Data are presented as the median of log10 cfu/ml (interquartile range) recovered from target organs after infection with wild-type, ∆proABC/piaA, ∆fhs/piaA, or PBS control mice. P values were obtained using Kruskal-Wallis tests with Dunn’s post hoc test comparing groups with the wild-type 6B strain (for virulence models) or the PBS sham-colonized group (for colonization-then-challenge data). The Mann-Whitney test was used for experiments with μMT−/− mice and CD4+ cell–depleted mice, comparing wild-type/untreated mouse data with μMT−/−/CD4+ cell–depleted mouse data, respectively, for each target organ and S. pneumoniae strain combination.
P < 0.01.
P < 0.05.
Combined data from two repeated experiments.
P < 0.001.
Figure 1.
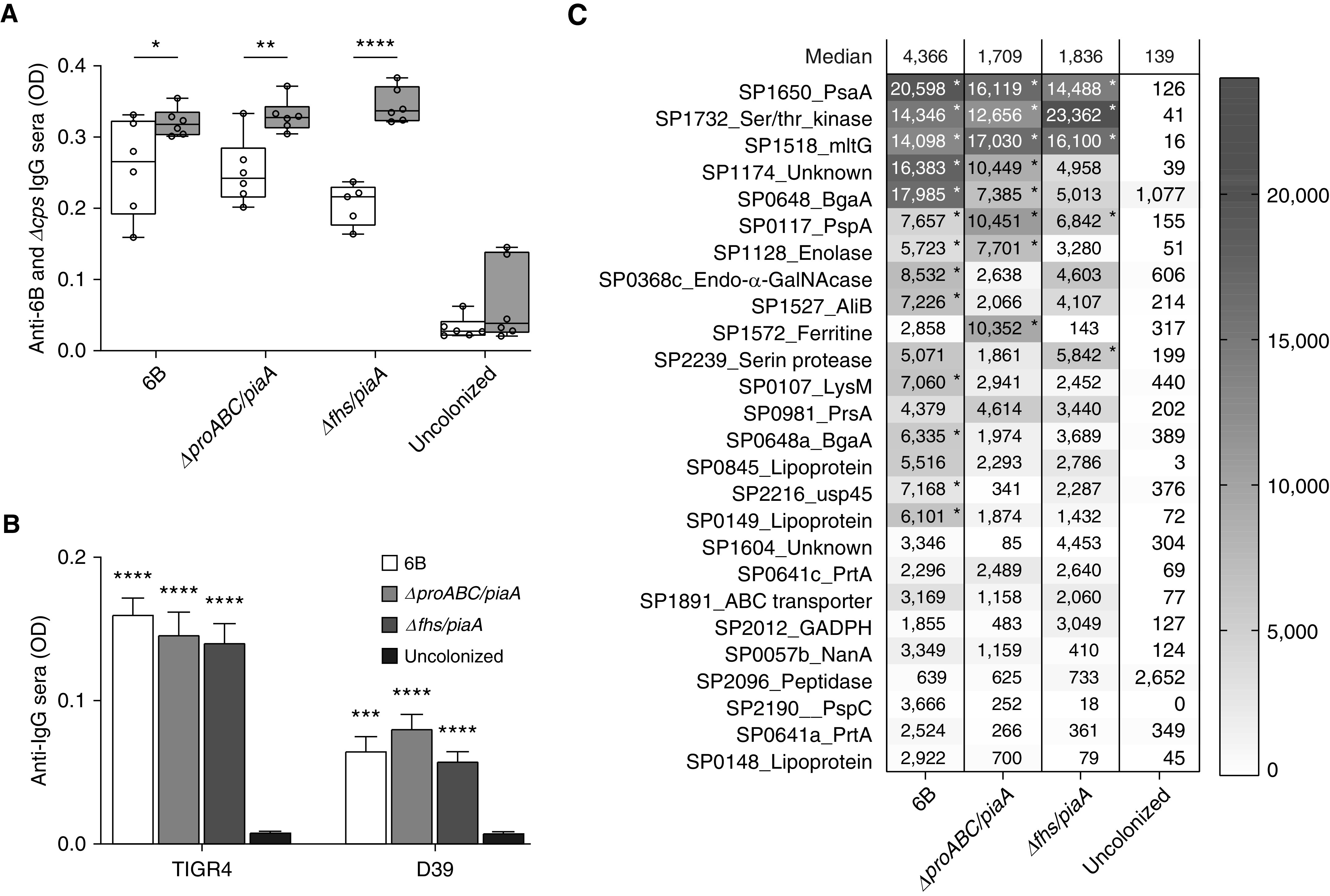
Serological responses in serum from mice colonized with wild-type and the ∆proABC/piaA or ∆fhs/piaA Streptococcus pneumoniae strains. (A and B) Whole-cell IgG ELISAs were performed as previously described (4, 6) using mouse sera recovered 28 days after colonization with the wild-type BHN418 6B or double mutant strains compared with uncolonized control mice. (A) Whole-cell ELISAs to the homologous wild-type BHN418 6B (white) or unencapsulated ∆cps BHN418 6B mutant (gray) strains (serum concentration 1 in 50). (B) Whole-cell ELISAs to the heterologous TIGR4 or D39 strains (serum concentration 1 in 50). Data in A are presented as box-and-whisker plots (whiskers represent the full range of the data) and in B as means (with error bars representing SDs); asterisks represent statistical significance between wild-type and unencapsulated strains in A or uncolonized control mice in B (Kruskal-Wallis test with Dunn’s correction for multiple comparisons; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001). (C) Data on IgG binding to an S. pneumoniae protein antigen array probed with sera from mice colonized twice with the 6B strain, ∆proABC/piaA, or ∆fhs/piaA strains. The protein array contains 289 S. pneumoniae proteins selected for their known antigenicity in humans and high degree of conservation among S. pneumoniae strains (5). The array was constructed using cell-free in vitro transcription/translation expression and printing onto nitrocellulose-coated glass AVID slides (Grace Bio-Labs, Inc.), then probed with 1:25 mouse serum, and images were acquired and analyzed using an ArrayCAM Imaging System from Grace Bio-Labs (5). (C) Heat map of mean IgG binding levels to the top 26 proteins recognized by IgG in colonized mouse sera (n = 6 mice). Results with an asterisk are statistically significantly different from the sham-colonized group (Kruskal-Wallis test to identify significant differences between groups, P < 0.05 uncorrected for multiple comparisons). OD = optical density.
The S. pneumoniae protein antigens recognized by serum IgG from colonized mice were identified by probing a protein array containing the majority of conserved S. pneumoniae proteins recognized by naturally acquired IgG found in human sera (5). Significant IgG responses were detected to 26 proteins (Figure 1C), with considerable overlap in the antigens recognized between mice colonized with the mutant and wild-type strains. These included well-recognized immunodominant S. pneumoniae antigens (e.g., PsaA, PspA, and SktP) as well as conserved proteins with few data on their utility as protective antigens (e.g., MltG, Bga, and PhtE). Importantly, subsequent pneumonia challenge in mice previously colonized with the double mutant strains was not associated with enhanced pulmonary or systemic cytokine responses (measured by Meso Scale Discovery), or major changes in recruited inflammatory cell subsets (assessed by flow cytometry of lung preparations) compared with sham (with phosphate-buffered saline) or S. pneumoniae wild-type 6B-colonized control mice (data not shown).
When challenged using the S. pneumoniae BHN418 6B strain pneumonia model, mice previously colonized with the wild-type BHN418 6B, ∆proABC/piaA, or ∆fhs/piaA strains were totally protected against bacteremia (Table 1). In addition, mice colonized with the wild-type or ∆fhs/piaA strains had reduced lung colony-forming units (Table 1). Repeat colonization and 6B pneumonia rechallenge experiments in B cell–deficient μMT mice or mice depleted of CD4+ cells before challenge demonstrated an important role for antibody rather than CD4+ cells for colonization-induced protection against septicemia (Table 1). Previous colonization with the ∆proABC/piaA or ∆fhs/piaA strains also protected against recolonization of the nasopharynx with the wild-type homologous BHN418 6B strain or the heterologous TIGR4 strain, reducing nasal wash colony-forming units by >1.5 log10 7 days after recolonization challenge (Table 1). If replicated in human studies, this reduction in nasopharyngeal colony-forming units is likely to impair successful nasopharyngeal colonization by S. pneumoniae and thereby reduce the incidence of subsequent invasive infections. Compatible with published data showing that protection against recolonization is mediated by CD4+ effector cells targeting protein antigens (6, 9, 10), CD4+ cell depletion abrogated the protective effect of prior colonization against recolonization (Table 1).
To summarize, we propose that administering to the nasopharynx mutant attenuated S. pneumoniae strains could be a novel strategy to overcome some of the limitations of the existing vaccines. Here, we have described the design and preclinical evaluation of the ∆fhs/piaA or ∆proABC/piaA BHN418 6B mutant strains and demonstrated they are good candidate strains for testing this strategy in humans. These mutant strains can now be investigated for their protective efficacy against S. pneumoniae colonization in a trial using an established controlled human S. pneumoniae infection challenge model (3, 7).
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Prof. Birgitta Henriques Normark (Karolinska Institute) for providing the 6B strain BHN418.
Footnotes
E.R.-S. and G.E. are supported by Medical Research Council grants R/N02687X/1 and MR/R001871/1, respectively. This work was undertaken at University College London Hospitals/University College London, who received a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centre’s funding scheme. R.S.H. is supported through the NIHR Global Health Research Unit on Mucosal Pathogens using UK aid from the UK Government, and D.G. receives support from the NIHR Great Ormond Street Institute of Child Health Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author Contributions: E.R.-S. contributed to conceiving, designing, conducting, and analyzing experiments; designing the study; and writing the manuscript. E.R.-S., G.E., P.F., R.R.d.A., and R.N. contributed to conducting and analyzing experiments. R.S.H., S.B.G., D.M.F., and J.S.B. contributed to conceiving and designing the study. E.R.-S., P.F., D.G., and R.S.H. contributed to designing and analyzing experiments. J.S.B. contributed to designing and analyzing experiments and writing the manuscript. All authors have read and approved the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202011-4161LE on December 17, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.José RJ, Brown JS. Adult pneumococcal vaccination: advances, impact, and unmet needs. Curr Opin Pulm Med. 2017;23:225–230. doi: 10.1097/MCP.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Sevillano E, Ercoli G, Brown JS. Mechanisms of naturally acquired immunity to Streptococcus pneumoniae. Front Immunol. 2019;10:358. doi: 10.3389/fimmu.2019.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira DM, Neill DR, Bangert M, Gritzfeld JF, Green N, Wright AK, et al. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med. 2013;187:855–864. doi: 10.1164/rccm.201212-2277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson R, Cohen JM, Reglinski M, Jose RJ, Chan WY, Marshall H, et al. Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS Pathog. 2017;13:e1006137. doi: 10.1371/journal.ppat.1006137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croucher NJ, Campo JJ, Le TQ, Liang X, Bentley SD, Hanage WP, et al. Diverse evolutionary patterns of pneumococcal antigens identified by pangenome-wide immunological screening. Proc Natl Acad Sci USA. 2017;114:E357–E366. doi: 10.1073/pnas.1613937114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, Brown JS. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015;8:627–639. doi: 10.1038/mi.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitsi E, Carniel B, Reine J, Rylance J, Zaidi S, Soares-Schanoski A, et al. Nasal pneumococcal density is associated with microaspiration and heightened human alveolar macrophage responsiveness to bacterial pathogens. Am J Respir Crit Care Med. 2020;201:335–347. doi: 10.1164/rccm.201903-0607OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandavilli S, Homer KA, Yuste J, Basavanna S, Mitchell T, Brown JS. Maturation of Streptococcus pneumoniae lipoproteins by a type II signal peptidase is required for ABC transporter function and full virulence. Mol Microbiol. 2008;67:541–557. doi: 10.1111/j.1365-2958.2007.06065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


