Abstract
Tris(pentafluorophenyl)borane has been found to catalyze the two‐fold C(sp3)−H silylation of various trialkylamine derivatives with dihydrosilanes, furnishing the corresponding 4‐silapiperidines in decent yields. The multi‐step reaction cascade involves amine‐to‐enamine dehydrogenation at two alkyl residues and two electrophilic silylation reactions of those enamines, one inter‐ and one intramolecular.
Keywords: amines, boron, C−H activation, Si−H activation, silicon
Acyclic tertiary amines with alkyl substitution undergo two consecutive C(sp3)−H silylation reactions with dihydrosilanes to form 4‐silapiperidines. Bond formation occurs β to the nitrogen atom at two of the alkyl residues. The reaction is catalyzed by the strong boron Lewis acid B(C6F5)3 and involves enamine intermediates generated by dehydrogenation.
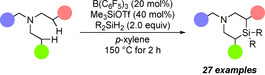
Selective functionalization of C(sp3)−H bonds is an important goal in synthetic chemistry. [1] One way to achieve this is by transition‐metal‐catalyzed C(sp3)−H silylation,[ 2 , 3 ] and recently selected boron Lewis acids also emerged as catalysts for this purpose. [4] For example, B(C6F5)3 has been shown to abstract hydride from α‐C(sp3)−H bonds of amines to result in the formation of iminium ions and the borohydride; [5] that iminium ion is C−H acidic and can be deprotonated by another molecule of the amine, affording the corresponding enamine along with the ammonium borohydride[ 6 , 7 ] (Scheme 1, gray box). The net reaction is a dehydrogenation that enables subsequent bond formation with electrophiles in the β‐position to the nitrogen atom, thereby representing a formal activation of the β‐C(sp3)−H bond. This process has already been employed for silylation, [8] alkylation, [9] deuteration, [10] and olefination [11] of the β‐carbon atom of various (a)cyclic tertiary amines (Scheme 1, top). Of note, Park and Chang merged the C(sp3)−H silylation with a B(C6F5)3‐catalyzed intramolecular Friedel–Crafts‐type silylation [12] for the synthesis of bridged silicon‐containing nitrogen heterocycles starting from N‐arylated piperidines. [8a] However, the undirected silylation of acyclic tertiary amines [3c] as well as their challenging two‐fold C(sp3)−H silylation are unprecedented. We disclose here a β,β′‐selective C(sp3)−H silylation of acyclic tertiary amines and dihydrosilanes catalyzed by B(C6F5)3 to directly arrive at sila analogues of piperidines (Scheme 1, bottom left). These are valuable building blocks in medicinal chemistry, [13] for example, for the dopamine receptor antagonist sila‐haloperidol (Scheme 1, bottom right). [14] Different from our approach, established syntheses typically start from divinyl‐substituted silanes employing a sequence of hydrobromination or hydroboration–oxidation–sulfonylation followed by dialkylation of a primary amine. [15]
Scheme 1.
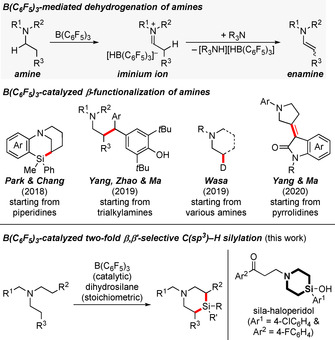
B(C6F5)3‐catalyzed β‐C(sp3)−H functionalization of tertiary amines. R groups=various aryl and alkyl groups as well as H; Ar=aryl group.
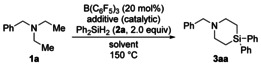
We began our investigation with optimizing the two‐fold C(sp3)−H silylation of benzyldiethylamine (1 a→3 aa; Table 1). Treatment of 1 a and Ph2SiH2 (2 a, 2.0 equiv) with 20 mol % of B(C6F5)3 in p‐xylene at 150 °C afforded 3 aa after 15 h in 56 % yield (Table 1, entry 1). Previous reports had indicated that the use of a metal oxide [8a] or a silyl triflate [5c] as an additive could improve the reactivity. [16] However, substoichiometric amounts of CaO or SrO decreased the yield (Table 1, entries 2 and 3). The addition of 40 mol % of a silyl triflate improved the reactivity (Table 1, entries 4–6), and a 75 % yield of 3 aa was obtained with Me3SiOTf as the additive. That yield was somewhat lower when using less and more Me3SiOTf, respectively (Table 1, entries 7 and 8). The reaction was completed within 2 h while a further shortened reaction time to 1 h resulted in a lower yield (Table 1, entries 9 and 10). Other arene solvents were tested but none provided a better outcome (Table 1, entries 11–13). A control experiment verified that Me3SiOTf is unable to mediate the reaction in the absence of B(C6F5)3 (Table 1, entry 14). Less B(C6F5)3 or Ph2SiH2 (2 a) as well as lowering the temperature to 120 °C led to a decreased reactivity (Table 1, entries 15–17). The volume of the reaction vessel was also examined, and the results indicate that vessels smaller than 10 mL are detrimental (Table 1, entries 18 and 19). We ascribe this to catalyst inhibition by dihydrogen at high pressure. [7] A good yield was restored on a 5.0 mmol scale when performing the two‐fold C(sp3)−H silylation in an open system with a continuous flow of nitrogen gas (Table 1, entry 20).
Table 1.
Selected examples of the optimization of B(C6F5)3‐catalyzed two‐fold C(sp3)−H silylation.[a]
|
Entry |
Additive (mol %) |
Solvent |
t [h] |
Yield [%][b] |
|---|---|---|---|---|
|
1 |
– |
p‐xylene |
15 |
56 |
|
2 |
CaO (50) |
p‐xylene |
15 |
48 |
|
3 |
SrO (50) |
p‐xylene |
15 |
50 |
|
4 |
Me3SiOTf (40) |
p‐xylene |
15 |
75 |
|
5 |
tBuMe2SiOTf (40) |
p‐xylene |
15 |
66 |
|
6 |
iPr3SiOTf (40) |
p‐xylene |
15 |
62 |
|
7 |
Me3SiOTf (20) |
p‐xylene |
15 |
67 |
|
8 |
Me3SiOTf (80) |
p‐xylene |
15 |
60 |
|
9 |
Me3SiOTf (40) |
p‐xylene |
2 |
75 (73) |
|
10 |
Me3SiOTf (40) |
p‐xylene |
1 |
42 |
|
11 |
Me3SiOTf (40) |
toluene |
2 |
74 |
|
12 |
Me3SiOTf (40) |
benzene |
2 |
62 |
|
13 |
Me3SiOTf (40) |
C6H5Cl |
2 |
55 |
|
14[c] |
Me3SiOTf (40) |
p‐xylene |
2 |
0 |
|
15[d] |
Me3SiOTf (40) |
p‐xylene |
2 |
49 |
|
16[e] |
Me3SiOTf (40) |
p‐xylene |
2 |
68 |
|
17[f] |
Me3SiOTf (40) |
p‐xylene |
15 |
61 |
|
18[g] |
Me3SiOTf (40) |
p‐xylene |
2 |
60 |
|
19[h] |
Me3SiOTf (40) |
p‐xylene |
2 |
34 |
|
20[i,j] |
Me3SiOTf (40) |
p‐xylene |
12 |
(65) |
[a] All reactions were performed on a 0.050 mmol scale in a 10 mL sealed tube. [b] Yields determined by 1H NMR spectroscopy using mesitylene as an internal standard; isolated yields in parentheses. [c] Without B(C6F5)3. [d] 10 mol % B(C6F5)3 used. [e] 1.5 equiv Ph2SiH2 (2 a) used. [f] Run at 120 °C. [g] 5.0 mL sealed tube used. [h] 1.0 mL sealed tube used. [i] Open system with a continuous flow of nitrogen gas. [j] 5.0 mmol scale.
We continued exploring the scope under the optimized reaction setup (Scheme 2; cf. Table 1, entry 9). It must be noted that reductive C(sp3)−N bond cleavage [17] is competing in any of the reactions summarized in Scheme 2, and secondary amines are the major byproducts (not quantified because of their volatility). N‐Benzylated diethylamine derivatives bearing various electron‐donating or ‐withdrawing substituents on the aryl moiety reacted with Ph2SiH2 (2 a) to furnish the corresponding 4‐silapiperidines in moderate to good yields (1 b–l→3 ba–la; gray box). All halo groups (1 g–j) and a trifluoromethyl group (1 k) were compatible. Tertiary amine 1 l containing a methyl ether underwent demethylation/silylation, and the free phenol was isolated in 50 % yield after purification by flash chromatography on silica gel (1 l→3 la). A lower yield was obtained for a naphth‐2‐ylmethyl instead of the benzyl group (1 m→3 ma). The bis(4‐silapiperidine) 3 na was formed in 47 % yield by four‐fold C(sp3)−H silylation of 1 n. Replacing the benzyl group by an alkyl group was feasible (1 o‐q→3 oa‐qa). Notably, the two‐fold C(sp3)−H silylation of substrate 1 o bearing two ethyl groups and one cyclohexyl group proceeded chemoselectively at the ethyl groups to form 3 oa. Substituted 4‐silapiperidine derivatives were obtained from tertiary amines with groups other than ethyl (1 r–t→3 ra–ta). As expected, 1 t gave 3 ta with essentially no diastereoselectivity (cis/trans=58:42). Attempted but failed cyclizations included tertiary benzylamines as precursors having two isopropyl, cyclohexyl, isobutyl, or phenethyl groups as well as 1‐benzylazepane (see the Supporting Information for details).
Scheme 2.
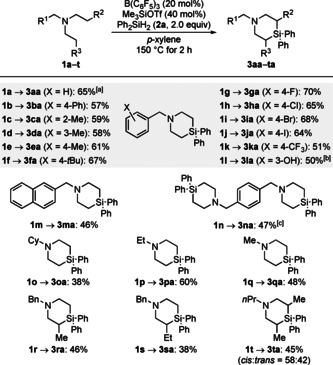
Scope I: Variation of the tertiary amine. Reaction conditions (0.10 mmol scale): B(C6F5)3 (20 mol %), Me3SiOTf (40 mol %), Ph2SiH2 (2 a, 2.0 equiv), and p‐xylene (0.80 mL) at 150 °C for 2 h. Yields are isolated yields. [a] See Table 1, entry 20. [b] Starting from N‐ethyl‐N‐(3‐methoxybenzyl)ethanamine (1 l). [c] 40 mol % of B(C6F5)3, 80 mol % of Me3SiOTf, and 4.0 equiv of Ph2SiH2 (2 a) used. Bn=benzyl, Cy=cyclohexyl.
We also tried the silylation of the tertiary aniline derivative 1 u which did not react under Park's and Chang's catalytic system (Scheme 3). [8a] Bicyclic 4 ua and tricyclic 5 ua formed in yields of 50 % and 8 %, respectively. The proportion of 5 ua increased at longer reactions times, for example, 44 % yield of 4 ua and 25 % yield of 5 ua after 3 h. As for the aforementioned method, [8a] intramolecular Friedel–Crafts C(sp2)−H silylation [12] is favored over intramolecular C(sp3)−H silylation.
Scheme 3.
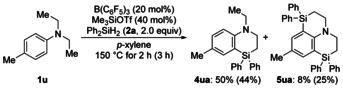
Consecutive C(sp3)−H/C(sp2)−H silylation of an aniline derivative.
We next assessed the dihydrosilane scope in the reaction of model substrate 1 a (Table 2). Diarylsilanes 2 b–e exhibited good reactivity, furnishing the corresponding products in the same yield range as compared to 2 a (1 a→3 ab–ae; Table 2, entries 1–4). No reaction was seen with sterically hindered dimesitylsilane (2 f; Table 2, entry 5). Modest yield was obtained with MePhSiH2 (2 g in 1 a→3 ag; Table 2, entry 6) but the synthesis of a spirocyclic derivative with 1‐silaindane (2 h) was low yielding (Table 2, entry 7). [18] The dialkylsilane Et2SiH2 (2 i) afforded desired 3 ai in moderate yield (Table 2, entry 8), but again, there was no reaction with bulky tBu2SiH2 (2 j; Table 2, entry 9). The reaction of the primary hydrosilane PhSiH3 yielded only trace amounts of the 4‐silapiperidine (not shown).
Table 2.
Scope II: Variation of the hydrosilane.[a]
|
Entry |
Hydrosilane |
R |
R′ |
Yield [%][b] |
|---|---|---|---|---|
|
1 |
2 b |
4‐MeC6H4 |
4‐MeC6H4 |
65 (3 ab) |
|
2 |
2 c |
4‐tBuC6H4 |
4‐tBuC6H4 |
65 (3 ac) |
|
3 |
2 d |
4‐FC6H4 |
4‐FC6H4 |
67 (3 ad) |
|
4 |
2 e |
Ph |
Naphth‐1‐yl |
68 (3 ae) |
|
5 |
2 f |
Mes |
Mes |
no reaction (3 af) |
|
6 |
2 g |
Ph |
Me |
40 (3 ag) |
|
7 |
2 h |
1‐silaindan‐1,1‐diyl |
traces (3 ah) |
|
|
8 |
2 i |
Et |
Et |
42 (3 ai) |
|
9 |
2 j |
tBu |
tBu |
no reaction (3 aj) |
[a] Reaction conditions (0.10 mmol scale): B(C6F5)3 (20 mol %), Me3SiOTf (40 mol %), hydrosilane 2 (2.0 equiv), and p‐xylene (0.80 mL) at 150 °C for 2 h. [b] Isolated yield. Mes=mesityl.
The benzyl group in 4‐silapiperidines such as 3 aa serves as a linchpin for further manipulations (Scheme 4). Debenzylation was achieved by treatment with 1‐chloroethyl chloroformate followed by the reaction of the resulting carbamate with MeOH (3 aa→6). The benzyl group can also be converted into a benzoyl group by oxidation with KMnO4 in the presence of BnNEt3Cl (3 aa→7).
Scheme 4.
Elaboration of an N‐benzylated 4‐silapiperidine. [a] 1) 1‐chloroethyl chloroformate (1.2 equiv), CH2Cl2, 0 °C to Δ, 1 h; RT, 20 h; 2) MeOH, Δ, 1 h; [b] KMnO4 (3.0 equiv), BnNEt3Cl (3.0 equiv), CH2Cl2, Δ, 3 h.
To gain insight into the reaction mechanism of this two‐fold C(sp3)−H silylation, deuterium‐labeling experiments and stoichiometric experiments were performed (Scheme 5). The reaction of 1 a with Ph2SiD2 (2 a‐d 2) under standard conditions gave 3 aa‐d 3 in the expected yield with 41 % deuterium incorporation in the benzylic position as well as at the α carbon atoms (Scheme 5, top). This result confirms the known reversible hydride abstraction from C(sp3)−H bonds α to an amine nitrogen atom. [6] Importantly, 6 % deuterium incorporation was also detected for the β carbon atoms, which is evidence for hydrogenation of the enamine intermediate. In the case of diethyl‐substituted 1 a, silylation is faster than this backward reaction. Conversely, di‐n‐butyl‐substituted 1 v shows a different outcome (Scheme 5, top). None of the hypothetical 4‐silapiperidine 3 va‐d 3 was found (not shown) but instead 1 v‐d 3 with the usual deuteration in the α‐positions. However, the deuteration grade in the β‐positions was 24 %, demonstrating that enamine hydrogenation is now a competitive if not the only reaction pathway for more hindered alkyl chains. To inspect the influence of the Me3SiOTf additive, we mixed 1 a and B(C6F5)3 in an equimolar ratio (Scheme 5, bottom). This known reaction [6] led to the formation of the three boron species 8 a–10 a in 52 %, 30 %, and 17 % yield, respectively, and this product distribution was not affected by the addition of 1.0 equiv of Me3SiOTf.
Scheme 5.
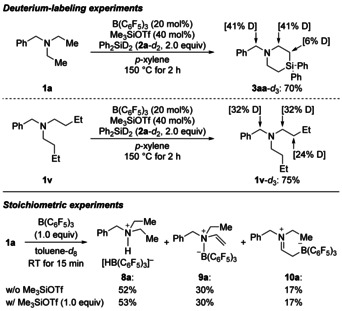
Deuterium‐labeling and stoichiometric experiments. Individual deuteration grades were estimated by 1H NMR spectroscopy. The overall deuteration grades of 2.87 D for 3 aa‐d 3 and 2.98 D for 1 v‐d 3 were determined by mass spectrometry.
On the basis of the above experimental results and the literature precedent[ 6 , 8 ] as well as DFT calculations by Park and Dang, [8b] a plausible reaction mechanism is proposed (Scheme 6). B(C6F5)3 promotes hydride abstraction from the tertiary amine 1 a to generate the iminium borohydrides 11 a and 11 a′ in equilibrium. Their subsequent deprotonation by unreacted 1 a yields enamine 12 a and FLP‐type dihydrogen adduct 8 a; these can regenerate the free amine 1 a and the catalyst B(C6F5)3 along with release of dihydrogen.[ 7 , 19 ] The thus‐formed enamine 12 a then engages in the rate‐determining B(C6F5)3‐catalyzed intermolecular hydrosilylation [8b] through the Piers mechanism [20] with 12 a as a carbon nucleophile (B(C6F5)3→13 a→15 aa). Alternatively, B(C6F5)3‐activated hydrosilane 13 a can also react with the amine nitrogen nucleophile 1 a to equilibrate with silylammonium borohydride 14 aa, the resting species of the overall process.[ 8a , 8b ] Initially formed 15 aa stands in equilibrium with regioisomeric 15 aa′ and 15 aa′′, and 15 aa′′ can undergo another deprotonation affording enamine 16 aa. That enamine again enters the catalytic cycle of the B(C6F5)3‐promoted, now intramolecular hydrosilylation to eventually arrive at the title compound 3 aa.
Scheme 6.
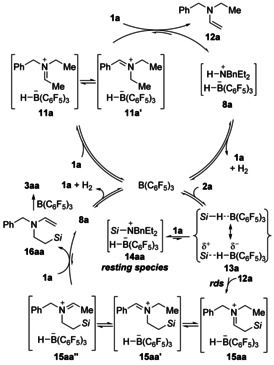
Plausible mechanism for the formation of 3 aa from 1 a and 2 a (Si=HPh2Si). rds=rate‐determining step.
In summary, we have developed a B(C6F5)3‐catalyzed two‐fold β,β′‐selective (formal) C(sp3)−H silylation of acyclic tertiary amines with dihydrosilanes to construct 4‐silapiperidines and its derivatives. The reaction involves two amine‐to‐enamine dehydrogenation reactions each followed by an inter‐ and an intramolecular electrophilic enamine silylation, respectively.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
H.F. gratefully acknowledges the Alexander von Humboldt Foundation for a postdoctoral fellowship (2018–2020), and K.X. thanks the China Scholarship Council for a predoctoral fellowship (2019–2023). M.O. is indebted to the Einstein Foundation Berlin for an endowed professorship. We also thank Dr. Maria Schlangen (TU Berlin) for expert advice with the MS measurements. Open access funding enabled and organized by Projekt DEAL.
H. Fang, K. Xie, S. Kemper, M. Oestreich, Angew. Chem. Int. Ed. 2021, 60, 8542.
Dedicated to Professor Siegfried Blechert on the occasion of his 75th birthday
Contributor Information
Dr. Huaquan Fang, http://www.organometallics.tu‐berlin.de.
Prof. Dr. Martin Oestreich, Email: martin.oestreich@tu-berlin.de.
References
- 1. He C., Whitehurst W. G., Gaunt M. J., Chem 2019, 5, 1031–1058, and references therein. [Google Scholar]
- 2.For authoritative reviews, see:
- 2a. Richter S. C., Oestreich M., Trends Chem. 2020, 2, 13–27; [Google Scholar]
- 2b. Hartwig J. F., Romero E. A., Tetrahedron 2019, 75, 4059–4070; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2c. Fukumoto Y., Chatani N. in Organosilicon Chemistry: Novel Approaches and Reactions (Eds.: Hiyama T., Oestreich M.), Wiley-VCH, Weinheim, 2019, pp. 171–211. [Google Scholar]
- 3.For examples of transition-metal-catalyzed C(sp3)−H silylation α or β to an amine nitrogen atom, see:
- 3a. Mita T., Michigami K., Sato Y., Chem. Asian J. 2013, 8, 2970–2973 (α, directed, and intermolecular); [DOI] [PubMed] [Google Scholar]
- 3b. Fang H., Hou W., Liu G., Huang Z., J. Am. Chem. Soc. 2017, 139, 11601–11609 (α and intramolecular); [DOI] [PubMed] [Google Scholar]
- 3c. Su B., Lee T., Hartwig J. F., J. Am. Chem. Soc. 2018, 140, 18032–18038 (β and intramolecular). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For a review, see:
- 4a. Park S., Chin. J. Chem. 2019, 37, 1057–1071; for a review dedicated to transition-metal-free C−H silylation, see: [Google Scholar]
- 4b. Schuman D. P., Liu W.-B., Nesnas N., Stoltz B. M. in Organosilicon Chemistry: Novel Approaches and Reactions (Eds.: Hiyama T., Oestreich M.), Wiley-VCH, Weinheim, 2019, pp. 213–240. [Google Scholar]
- 5.For examples of B(C6F5)3-catalyzed α-functionalization of tertiary amines, see:
- 5a. Shang M., Chan J. Z., Cao M., Chang Y., Wang Q., Cook B., Torker S., Wasa M., J. Am. Chem. Soc. 2018, 140, 10593–10601; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Maier A. F. G., Tussing S., Zhu H., Wicker G., Tzvetkova P., Flörke U., Daniliuc C. G., Grimme S., Paradies J., Chem. Eur. J. 2018, 24, 16287–16291; [DOI] [PubMed] [Google Scholar]
- 5c. Tian J.-J., Zeng N.-N., Liu N., Tu X.-S., Wang X.-C., ACS Catal. 2019, 9, 295–300; [Google Scholar]
- 5d. Chan J. Z., Chang Y., Wasa M., Org. Lett. 2019, 21, 984–988; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5e. Chan J. Z., Yesilcimen A., Cao M., Zhang Y., Zhang B., Wasa M., J. Am. Chem. Soc. 2020, 142, 16493–16505; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5f. Basak S., Alvarez-Montoya A., Winfrey L., Melen R. L., Morrill L. C., Pulis A. P., ACS Catal. 2020, 10, 4835–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For a review, see:
- 6a. Focante F., Mercandelli P., Sironi A., Resconi L., Coord. Chem. Rev. 2006, 250, 170–188; for a key publication, see: [Google Scholar]
- 6b. Millot N., Santini C. C., Fenet B., Basset J. M., Eur. J. Inorg. Chem. 2002, 3328–3335. [Google Scholar]
- 7.These ammonium borohydrides are dihydrogen adducts of amine/borane FLPs (FLP=frustrated Lewis pair). While release of dihydrogen is slow at room temperature, reversible dihydrogen activation occurs at elevated temperatures. Sumerin V., Schulz F., Nieger M., Leskelä M., Repo T., Rieger B., Angew. Chem. Int. Ed. 2008, 47, 6001–6003; [Google Scholar]; Angew. Chem. 2008, 120, 6090–6092. [Google Scholar]
- 8.
- 8a. Zhang J., Park S., Chang S., J. Am. Chem. Soc. 2018, 140, 13209–13213; see also: [DOI] [PubMed] [Google Scholar]
- 8b. Zhou M., Park S., Dang L., Org. Chem. Front. 2020, 7, 944–952; [Google Scholar]
- 8c. Zhang J., Chang S., J. Am. Chem. Soc. 2020, 142, 12585–12590. [DOI] [PubMed] [Google Scholar]
- 9. Li R., Chen Y., Jiang K., Wang F., Lu C., Nie J., Chen Z., Yang G., Chen Y.-C., Zhao Y., Ma C., Chem. Commun. 2019, 55, 1217–1220. [DOI] [PubMed] [Google Scholar]
- 10. Chang Y., Yesilcimen A., Cao M., Zhang Y., Zhang B., Chan J. Z., Wasa M., J. Am. Chem. Soc. 2019, 141, 14570–14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y., Wan H.-L., Huang Y., Liu S., Wang F., Lu C., Nie J., Chen Z., Yang G., Ma C., Org. Lett. 2020, 22, 7797–7803. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Ma Y., Wang B., Zhang L., Hou Z., J. Am. Chem. Soc. 2016, 138, 3663–3666; [DOI] [PubMed] [Google Scholar]
- 12b. Yin Q., Klare H. F. T., Oestreich M., Angew. Chem. Int. Ed. 2016, 55, 3204–3207; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 3256–3260; for a review, see: [Google Scholar]
- 12c. Bähr S., Oestreich M., Angew. Chem. Int. Ed. 2017, 56, 52–59; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 52–59. [Google Scholar]
- 13.For a review, see:
- 13a. Franz A. K., Wilson S. O., J. Med. Chem. 2013, 56, 388–405; for recent work on silicon-containing nitrogen heterocycles, see:23061607 [Google Scholar]
- 13b. Barraza S. J., Denmark S. E., J. Am. Chem. Soc. 2018, 140, 6668–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The 4-silapiperidine core was prepared in several steps with a ring-forming dialkylation of a primary amine as the key step (90 °C in an autoclave for 16 h): Tacke R., Heinrich T., Bertermann R., Burschka C., Hamacher A., Kassack M. U., Organometallics 2004, 23, 4468–4477. [Google Scholar]
- 15.
- 15a. Gerlach M., Jutzi P., Stasch J.-P., Przuntek H., Z. Naturforsch. B 1982, 37, 657–662; [Google Scholar]
- 15b. Kim B. M., Cho J. H., Tetrahedron Lett. 1999, 40, 5333–5336; for hydroamination with lithium amides, see: [Google Scholar]
- 15c.Ref. [15b]; for formal hydroamination by aminomercuration–reduction, see:
- 15d. Barluenga J., Jiménez C., Nájera C., Yus M., Synthesis 1982, 414–417. [Google Scholar]
- 16.The roles of these additives are not entirely clear. The metal oxides are believed to facilitate the deprotonation step[8a] (iminium ion→enamine; Scheme 1, gray box) while silyl triflates are thought to enhance hydride release from the borohydride through the intermediate formation of a pentacoordinate silicon hydride as a hydride shuttle.[5c]
- 17.
- 17a. Fang H., Oestreich M., Angew. Chem. Int. Ed. 2020, 59, 11394–11398; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 11491–11495; see also: [Google Scholar]
- 17b.Ref. [8c].
- 18.Such spirocyclic systems are also relevant in medicinal chemistry: Tacke R., Handmann V. I., Bertermann R., Burschka C., Penka M., Seyfried C., Organometallics 2003, 22, 916–924. [Google Scholar]
- 19.
- 19a. Maier A. F. G., Tussing S., Schneider T., Flörke U., Qu Z.-W., Grimme S., Paradies J., Angew. Chem. Int. Ed. 2016, 55, 12219–12223; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 12407–12411; [Google Scholar]
- 19b. Kojima M., Kanai M., Angew. Chem. Int. Ed. 2016, 55, 12224–12227; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 12412–12415. [Google Scholar]
- 20.
- 20a. Parks D. J., Blackwell J. M., Piers W. E., J. Org. Chem. 2000, 65, 3090–3098; [DOI] [PubMed] [Google Scholar]
- 20b. Rendler S., Oestreich M., Angew. Chem. Int. Ed. 2008, 47, 5997–6000; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 6086–6089; [Google Scholar]
- 20c. Sakata K., Fujimoto H., J. Org. Chem. 2013, 78, 12505–12512; [DOI] [PubMed] [Google Scholar]
- 20d. Houghton A. Y., Hurmalainen J., Mansikkamki A., Piers W. E., Tuononen H. M., Nat. Chem. 2014, 6, 983–988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


