Abstract
Theta oscillations in the hippocampal local field potential (LFP) appear during translational movement and arousal, modulate the activity of principal cells, and are associated with spatial cognition and episodic memory function. All known anxiolytics slightly but consistently reduce hippocampal theta frequency. However, whether this electrophysiological effect is mechanistically related to the decreased behavioral expression of anxiety is currently unclear. Here, we propose that a reduction in theta frequency affects synaptic plasticity and mnemonic function and that this can explain the reduction in anxiety behavior. We test this hypothesis in a biophysical model of contextual fear conditioning. First, we confirm that our model reproduces previous empirical results regarding the dependence of synaptic plasticity on presynaptic firing rate. Next, we investigate how theta frequency during contextual conditioning impacts learning. These simulations demonstrate that learned associations between threat and context are attenuated when learning takes place under reduced theta frequency. Additionally, our simulations demonstrate that learned associations result in increased theta activity in the amygdala, consistent with empirical data. In summary, we propose a mechanism that can account for the behavioral effect of anxiolytics by impairing the integration of threat attributes of an environment into the cognitive map due to reduced synaptic potentiation.
Keywords: anxiety, anxiolytic, contextual fear, fear recall, theta rhythm
1. INTRODUCTION
Neural oscillations in the theta band are among the most prominent feature of the hippocampal local field potential (LFP; Buzsáki, 2002; O'Keefe & Nadel, 1978). Theta oscillatory activity is implicated in mnemonic function (Düzel, Penny, & Burgess, 2010), spatial cognition (Buzsáki & Moser, 2013), and behavior control under survival threat (Tovote, Fadok, & Lüthi, 2015). A large body of research has investigated antecedents of theta power or amplitude on the one hand and the mechanisms by which theta oscillations modulate the dynamics of individual neurons on the other. For example, hippocampal theta power increases in environments containing potential threats (Adhikari, Topiwala, & Gordon, 2010; Khemka, Barnes, Dolan, & Bach, 2017) and in response to threat‐predicting cues (Likhtik, Stujenske, A Topiwala, Harris, & Gordon, 2014; Seidenbecher, Laxmi, Stork, & Pape, 2003). The theta phase of individual spikes affects the magnitude and direction of ensuing synaptic plasticity (Hölscher, Anwyl, & Rowan, 1997; Hyman, Wyble, Goyal, Rossi, & Hasselmo, 2003), and hippocampal place cells exhibit a theta phase code for location (O'Keefe & Recce, 1993) suggestive of a role in spatial coding (Eichenbaum, Dudchenko, Wood, Shapiro, & Tanila, 1999).
The frequency of theta oscillations is ~4–12 Hz in rodent species (Colgin, 2013) and is known to increase with running speed during locomotion (Sławińska & Kasicki, 1998; Wells et al., 2013). Notably, theta frequency is slightly (0.5–2 Hz; McNaughton, Richardson, & Gore, 1986) but consistently reduced by anxiolytic drugs—compounds that alleviate rodent anxiety‐like behavior (for a review, see Nemeroff, 2003a), human anxiety‐like behavior (e.g., Bach, Korn, Vunder, & Bantel, 2018; Biedermann et al., 2017; Korn et al., 2016) and clinical anxiety in humans (McNaughton & Coop, 1991). In anesthetized rodents, the slowing of theta frequency is so robust that it has been proposed as a screening test for clinically effective anxiolytics (McNaughton, Kocsis, & Hajos, 2007; Yeung, Treit, & Dickson, 2012). Importantly, this frequency decrease is independent of the relationship between theta frequency and running speed (Monaghan, Chapman, & Hasselmo, 2017; Wells et al., 2013). Theta slowing has been observed for all known anxiolytic agents (McNaughton & Coop, 1991). This includes barbiturates and benzodiazepines (McNaughton et al., 1986), sometimes categorized together as “classical anxiolytics” (Riva, Gaudio, & Dakanalis, 2015), as well as many “novel” anxiolytic agents of more recent discovery such as buspirone (Coop & McNaughton, 1991), the selective serotonin reuptake inhibitor fluoxetine (Munn & McNaughton, 2008), the tricyclic antidepressant imipramine (Zhu & McNaughton, 1995), and the GABA agonist pregabalin (Siok, Taylor, & Hajós, 2009). Theta slowing is also seen in drugs that have anxiolytic properties but are not primarily used to that end, such as somatostatin (Engin, Stellbrink, Treit, & Dickson, 2008).
Strikingly, despite their common effects on theta frequency and behavior, the molecular targets of these various anxiolytics are clearly distinct. Specifically, barbiturates and benzodiazepines interact with the neurotransmission of γ‐aminobutyric acid (GABA) and have been shown to affect only specific GABA‐A receptor subunits (Macdonald & Olsen, 1994); and pregabalin increases GABA levels (Nemeroff, 2003b). In contrast, buspirone, selective serotonin reuptake inhibitors, and tricyclic antidepressants target the serotonergic (5‐hydroxytryptamine; 5‐HT) system, albeit via different pharmacological mechanisms (Hiemke & Hartter, 2000; Mahmood & Sahajwalla, 1999). This suggests that the behavioral expression of anxiolysis is more closely related to a decrease in theta frequency than to the proximal molecular mechanism of action. Nonetheless, these observations are merely correlational, and there is no data to confirm or refute a suggestion that anxiolytic theta slowing is mechanistically related to a reduction of anxiety‐like behavior.
In this paper, we sought to provide a possible mechanistic link between changes in theta frequency and anxiety‐like behavior using a biophysical proof‐of‐concept model. From the many assays of anxiety‐like behavior, we chose to simulate contextual fear conditioning (Grillon & Ernst, 2020; Likhtik et al., 2014; Maren & Hobin, 2007; Phillips & LeDoux, 1992), which is well amenable to computational modeling due to its high level of experimental control. Contextually conditioned freezing is consistently reduced by anxiolytic drugs (Ehrlich et al., 2009; Luyten, Vansteenwegen, Van Kuyck, Gabriëls, & Nuttin, 2011; Sanger & Joly, 1985). Our model system is composed of a population of putative amygdalar neurons that respond selectively to noxious stimuli and receive feed‐forward excitation from a population of theta‐modulated hippocampal place cells (Jung, Wiener, & McNaughton, 1994; O'Keefe & Nadel, 1978; Ranck, 1973). Building on a standard model of synaptic plasticity, we show that a small reduction in theta frequency substantially reduces the potentiation of these feedforward connections during simulated contextual fear conditioning, in line with recent empirical work that demonstrated a correlation between theta frequency and spatial learning (Young, Ruan, & McNaughton, 2020). This leads to reduced expression of contextual fear (i.e., reduced firing rates in simulated amygdalar neurons) during subsequent exposure to the conditioned context. To validate our model, we finally show that it can also account for the occurrence of amygdalar theta oscillations upon presentation of a conditioned stimulus, as has been observed experimentally (Lesting et al., 2011; Likhtik et al., 2014; Seidenbecher et al., 2003). In summary, we demonstrate a network‐level mechanism that is capable of accounting for the common impact of anxiolytic drugs on anxiety‐like behavior via their impact on theta frequency, despite their heterogeneous molecular targets.
2. METHODS
2.1. Dendritic spine model
To investigate the effect of theta frequency on synaptic plasticity and contextual fear conditioning, we simulate postsynaptic dendritic spines on a population of N “fear cells” that receive synaptic input from a population of M hippocampal place cells. These fear cells are active whenever the simulated agent perceives a noxious stimulus, analogous to neurons in the lateral nucleus of the amygdala (LA; Paré & Collins, 2000; Romanski, Clugnet, Bordi, & LeDoux, 1993), while place cells produce theta modulated spike trains whenever the animal is located at a particular location within the environment (O'Keefe & Nadel, 1978; Thompson & Best, 1989). In these simulations, we assume that during salient or novel experiences, acetylcholine (ACh) is released into the hippocampus to promote learning by enhancing synaptic plasticity but reducing the strength of intrahippocampal connections that might generate recall. Conversely, during familiar experiences, levels of ACh in the hippocampus are low, promoting recall by enhancing intrahippocampal connections but reducing synaptic plasticity that might disrupt existing associations. This relationship is supported by empirical data (Douchamps, Jeewajee, Blundell, Burgess, & Lever, 2013; Hasselmo, 2006). For example, intracerebral administration of cholinergic antagonists into either the hippocampus or basolateral amygdala (BLA) suppresses the acquisition of conditioned fear (Wilson & Fadel, 2017), as does the optogenetic inhibition of cholinergic activity in the BLA during training (Jiang et al., 2016). Conversely, optogenetic enhancement of cholinergic neurons in the medial septum—the main ACh input to the hippocampus—enhances contextual fear conditioning (Hersman et al., 2017).
The membrane potential at each dendritic spine is a linear sum of two components: excitatory postsynaptic potentials (EPSPs) generated by input from hippocampal place cells, and backpropagating action potentials (BPAPs) from the soma. Each of these inputs generates depolarization away from the resting membrane potential Vr:
| (1) |
EPSPs generated by an input spike at time ti are modeled as the sum of two exponential functions with time constants and , respectively, modulated by the presence of acetylcholine ([ACh]; Hasselmo, 2006), and a normalization parameter s chosen to produce peak depolarization of 8 mV:
| (2) |
The choice of the parameter s is in line with empirical reports (Rosenkranz, 2012), although we note that is makes little qualitative difference to the results.
BPAPs generated by an output spike at time t0 are modeled as the sum of two exponential functions with time constants and that correspond to a fast spike and slower after‐depolarising potential, respectively (following Shouval, Bear, & Cooper, 2002). The relative amplitude of the fast and slow BPAP components is dictated by the parameters and :
| (3) |
The value of the parameters of the dendritic spine model used in the simulations are summarized in Table 1.
TABLE 1.
Model parameters used to simulate the contextual conditioning protocols (Shouval et al., 2002)
| Parameter | Description | Value | |
|---|---|---|---|
| τ | Time constant of the neuronal membrane | 20 ms | |
| vrest | Neuronal resting potential | −65 mV | |
| v* | Neuronal firing threshold | −55 mV | |
| vreset | Neuronal reset potential | −75 mV | |
| λ | Synaptic strength decay constant | 0.1 | |
|
|
Slow EPSP time constant | 50 ms | |
|
|
Fast EPSP time constant | 5 ms | |
| α1 | Parameter used in the definition of Ω (Equation ((5)) | 0.35 | |
| α2 | Parameter used in the definition of Ω (Equation ((5)) | 0.55 | |
| β1 | Parameter used in the definition of Ω (Equation ((5)) | 80 | |
| β2 | Parameter used in the definition of Ω (Equation ((5)) | 80 | |
| P1 | Parameter used in the definition of η (Equation (7)) | 0.1 s | |
| P2 | Parameter used in the definition of η (Equation (7)) | P1 /10−4 | |
| P3 | Parameter used in the definition of η (Equation (7)) | 3 | |
| P4 | Parameter used in the definition of η (Equation (7)) | 1 s | |
| τCa | Calcium time constant | 50 ms | |
| P0 | Probability of NMDAr opening after action potential | 0.5 | |
| GNMDA | NMDAr conductance | −1/500 [μM/(ms · mV)] | |
| If | Fast NMDAr current component intensity | 0.5 | |
| Is | Slow NMDAr current component intensity | 0.5 | |
| τf | Fast NMDAr current component time constant | 50 ms | |
| τs | Slow NMDAr current component time constant (NMDAr) | 200 ms | |
| Vr | Reversal potential for calcium | 130 mV | |
| Aθ | Amplitude of the intracellular theta oscillation | 3 mV | |
|
|
Fast BPAP component intensity | 0.75 | |
|
|
Slow BPAP component intensity | 0.25 | |
|
|
Fast BPAP component time constant | 3 ms | |
|
|
Slow BPAP component time constant | 25 ms | |
| K | Number of simulated input spikes | 900 | |
| rsafe | Fear cells' firing rate in the safe compartment | 0.85 Hz | |
| rthreat | Fear cells' firing rate in the threatening compartment | 1.85 Hz |
2.2. Synaptic plasticity model
Consistent with previous empirical (Bear, Cooper, & Ebner, 1987; Lisman, 1989) and theoretical studies, we assume that activity‐dependent changes in synaptic strength W at each dendritic spine are governed by intracellular Calcium concentration [Ca2+], in accordance with the calcium control hypothesis (Shouval et al., 2002). The values of the parameters of the synaptic plasticity model described below are listed in Table 1.
Calcium concentration in the dendritic spine increases in proportion to the current influx through NMDA receptors, INMDA, which does not contribute to the membrane potential, and subsequently decays with a constant τCa:
| (4) |
NMDA currents, in turn, are governed by the channel opening probability P0, the maximum channel conductance GNMDA, the time constants of rising and decay τf and τs, respectively, and a voltage‐dependent term characterizing the blockade of NMDA channels by magnesium H(Vs), where [Mg2+] represents extracellular magnesium concentration and the calcium reversal potential:
| (5) |
| (6) |
Finally, the magnitude and direction of changes in synaptic strength are governed by a non‐linear function of calcium concentration Ω([Ca2+]), which is modulated by a learning rate η([Ca2+]) and the presence of ACh (Hasselmo, 2006):
| (7) |
| (8) |
| (9) |
| (10) |
2.3. Stimulation protocols
First, to confirm that the plasticity model above can replicate changes in synaptic weight observed in vitro (e.g., O'Connor, Wittenberg, & Wang, 2005), we subjected dendritic spines to “tetanic stimulation” with trains of 100 spikes delivered at varying frequency fθ in the absence of any output spiking activity, and with λ = 1 and s = 1.45 (Shouval et al., 2002).
Next, we sought to examine the impact of changes in theta frequency on learning during a simulated contextual fear conditioning paradigm. We divided the M hippocampal place cells into two subpopulations that were each active in one of two contexts: a threatening compartment T+ and a safe compartment T− (see Figure 1). While active, place cells fired rhythmic, inhomogeneous Poisson spike trains according to the rate function rPC, with theta frequency fθ varying across simulations and a gain factor K set to produce an average of one spike per oscillatory cycle:
| (11) |
FIGURE 1.
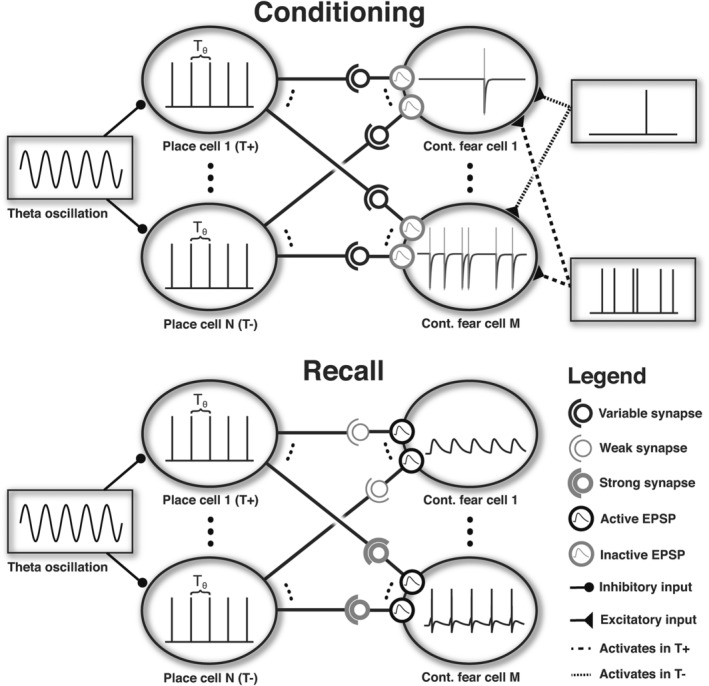
Schematic representation of the network configuration during conditioning and recall. Top—During conditioning, high levels of ACh inhibit EPSPs but support plasticity. Each fear cell receives location‐dependent inputs from the hippocampus, which activate NMDA receptors, and produce Poisson spike trains that reflect the absence or presence of noxious stimuli in the safe and threatening compartments, respectively. Bottom—During recall, low levels of ACh enhance EPSPs while inhibiting further synaptic plasticity. Noxious stimuli are no longer delivered, and the activity of fear cells is thus determined by place cell inputs and the synaptic weights induced by prior conditioning. As a result, place cells active in T+ elicit stronger activity in fear cells. In both panels, Tθ indicate the period of the theta rhythm
While in the safe compartment T−, fear cells fired homogenous Poisson spike trains with a rate of rsafe; while in the threatening compartment T+, fear cells fired homogenous Poisson spike trains with an increased rate of rthreat. During conditioning, the simulated animal spent a time interval corresponding to 100 theta periods in each compartment (Likhtik et al., 2014; Maren & Hobin, 2007; Phillips & LeDoux, 1992), and the level of acetylcholine was set to [ACh] = 1 to promote synaptic plasticity but eliminate the recall of previously encoded associations by reducing EPSP amplitude (Equations (2) and (7)).
After conditioning, we tested the simulated agent's ability to recall contextual fear. The level of acetylcholine was set to [ACh] = 0 to eliminate further synaptic plasticity but promote the recall of previously encoded associations by enhancing EPSP amplitude. Hippocampal theta frequency was set to fθ = 5 Hz and the simulated agent spent another interval of 25 theta periods in each compartment. In this case, we were interested in the output firing rate of fear cells generated by input from the corresponding place cell population, assuming that elevated fear cell firing rate elevates levels of freezing behavior, a typical behavioral measure of fear. We assumed that the somatic membrane potential of fear cells Vsoma was equal to the average membrane potential of all dendritic spines, and that output spikes were fired whenever the somatic membrane potential exceeded a threshold Vthr, after which the membrane potential of all spines was set to the reset membrane potential Vreset. We quantified the probability of freezing in each compartment (T+ or T−) as the fraction of 100 simulations in which the average firing rate of the fear cell population exceeded the threshold rfreeze =1.5 Hz.
2.4. Postsynaptic spike train analysis
Finally, we sought to quantify the theta modulation of output fear cell spike trains before and after the simulated fear conditioning protocol described above. To do so, we first computed the temporal auto‐correlation of spikes fired by each fear cell in 10 ms bins for lags of up to 1 s. We subsequently computed the fast Fourier transform of the mean‐normalized temporal auto‐correlation for frequencies up to 50 Hz and smoothed the resulting power spectra with a Gaussian kernel of 2 Hz width.
3. RESULTS
3.1. Synaptic plasticity model
Despite their different molecular mechanisms of action, anxiolytic drugs generate both a reduction in anxiety behavior, including contextual fear conditioning (Ehrlich et al., 2009; Luyten et al., 2011; Sanger & Joly, 1985), as well as a small but consistent decrease in hippocampal theta frequency. Here, we sought to examine whether the latter phenomenon could potentially explain the altered behavioral phenotype. To this end, we built a neural model of theta modulated hippocampal place cells projecting to amygdalar “fear cells” through synapses that followed a standard calcium‐dependent plasticity rule (Shouval et al., 2002). First, to establish that this plasticity rule could account for the empirically observed dependence of synaptic modifications on the frequency of trains of afferent stimuli (Dudek & Bear, 1992, 1993; Mulkey & Malenka, 1992), we simulated a presynaptic “tetanic stimulation” protocol at different stimulation frequencies (see Methods). Consistent with previous empirical (O'Connor et al., 2005) and theoretical (Shouval et al., 2002) data, synaptic strength increased as a function of presynaptic stimulation frequency (Figure 2). Interestingly, the greatest change in synaptic strength was observed approximately between 4 and 9 Hz, that is, within a frequency band that roughly overlaps with rodent hippocampal theta frequency (Colgin, 2016).
FIGURE 2.
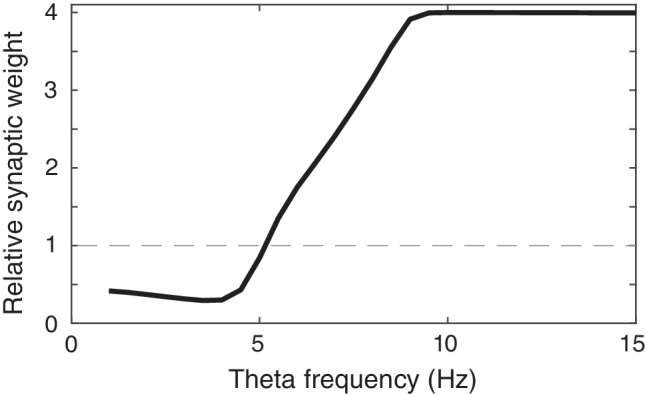
Dependence of synaptic strength on theta frequency during simulation of a presynaptic rate‐induced plasticity protocol
3.2. Contextual fear conditioning
Next, to quantify whether this dependency was sufficient to explain the behavioral effect of anxiolytics, we simulated a standard contextual fear conditioning protocol (Likhtik et al., 2014; Figure 1, Top). During conditioning, our simulated agent explored an arena divided into two parts: a safe compartment and a threatening compartment (Figure 3a). Different subpopulations of theta‐modulated hippocampal place cells were active in each compartment, while fear cells fired Poisson spike trains at a higher rate in the threatening compartment than in the safe compartment (Paré & Collins, 2000; Romanski et al., 1993). During acquisition, high levels of ACh supported synaptic plasticity and inhibited the recall of existing associations (Hasselmo, 2006). Hippocampal theta frequency was either set to a baseline value of 6 Hz, or a reduced value of 5.5 Hz to reflect the small but significant reduction in frequency associated with the administration of anxiolytics. While conditioning increased the strength of synaptic inputs from place cells active in both compartments and at both theta frequencies, potentiation was stronger after conditioning at 6 Hz (consistent with the tetanic stimulation results illustrated in Figure 2) and when post‐synaptic firing rates were greater in the threatening compartment (Figure 3b).
FIGURE 3.
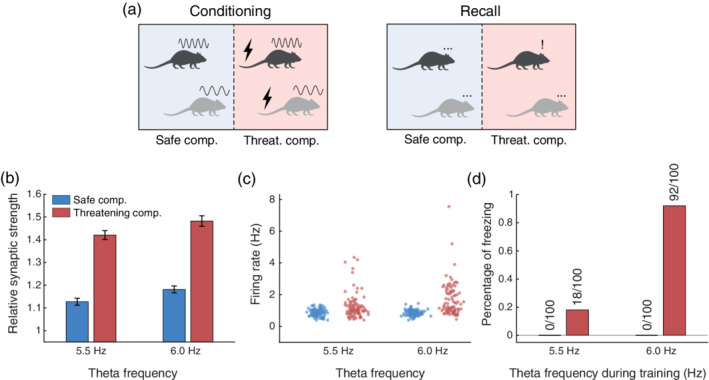
(a) Schematic of the simulated experimental protocol. Conditioning: High and low frequency sinusoids above the rodent's head represent conditioning at either high or low theta frequency, respectively, whereas the bolt symbol indicates the presence of noxious stimuli. Recall: The symbols above the rodent's head illustrate the behavior predicted by the model, with the three dots indicating no behavioral response and the exclamation mark a freezing response to the contextual cue. (b) Relative average synaptic strength obtained after simulating contextual conditioning during epochs of theta activity at 5.5 or 6 Hz in the threatening and safe compartment; error bars represent SEM. (c) Firing rate distribution of fear cells during recall at both theta frequencies in both compartments. (d) Percentage probability of freezing over 100 simulations
3.3. Contextual fear recall
Following conditioning, we assessed the behavioral expression of learned anxiety—here modeled as contextual fear—during a subsequent recall phase in which noxious stimuli were no longer present (Figure 1, Bottom). In this case, the simulated agent was again exposed to each compartment of the conditioning arena, with the level of ACh reduced to promote recall and inhibit further synaptic plasticity. In this case, activity in the fear cell population was generated by input from theta modulated hippocampal place cells, and output firing rates reflected the relative strength of synaptic inputs generated during the acquisition phase. Hence, the firing rate of fear cells was greater in the threatening compartment, and greater following conditioning with a higher theta frequency (i.e., in the absence of anxiolytics, Figure 3c).
To relate this result to behavior, we sought to quantify the probability of freezing in each condition, which we assumed to occur whenever the average firing rate of the fear cell population exceeded a threshold. Under this assumption, the simulated agent exhibited no freezing in the safe compartment, regardless of theta frequency during conditioning. Conversely, in the threatening compartment, freezing occurred in 92% of simulations after conditioning with a theta frequency of 6 Hz, but only in 18% of the simulations after conditioning at 5.5 Hz (Figure 3d). We emphasize that any choice of freezing threshold and theta frequencies (within the 4–9 Hz range), would produce qualitatively similar results: a small reduction in theta frequency (as small as 0.5 Hz) is sufficient to significantly reduce synaptic potentiation, and therefore impair the acquisition of conditioned contextual fear when the freezing threshold is set accordingly. Below this range, no synaptic potentiation occurs; above this range, synaptic potentiation is saturated. In either case, changes in frequency have no effect. In summary, our model predicts a substantial difference between the behavioral expression of anxiety following contextual fear conditioning with hippocampal theta frequencies that varied by only 0.5 Hz. This suggests that a decrease in theta frequency within the range generated by the administration of anxiolytics can account for a significant reduction in anxiety in a model of contextual fear conditioning.
3.4. Model validation: Postsynaptic theta
Previous empirical studies have revealed theta‐band oscillatory activity in the rodent lateral amygdala (LA) during the presentation of conditioned threat cues, and these oscillations are in phase coherence with ongoing hippocampal theta oscillations (Likhtik et al., 2014; Seidenbecher et al., 2003). To validate our model, we sought to demonstrate that the simulated fear cells (which are analogous to LA neurons; Romanski et al., 1993) also exhibit this property, as their output spike trains are primarily dictated by increased synaptic input from theta modulated hippocampal place cells after the acquisition of contextual fear. We computed power spectra for fear cell spike trains in each condition (see Methods) and found a peak in the theta frequency band in the threatening compartment following conditioning at 6.0 Hz. At 5.5 Hz, we found a similar peak but with amplitude lower by an order of magnitude (Figure 4). Hence, our model can account for the appearance of theta‐band oscillations in LA following contextual fear conditioning, consistent with empirical observations (Likhtik et al., 2014; Seidenbecher et al., 2003).
FIGURE 4.
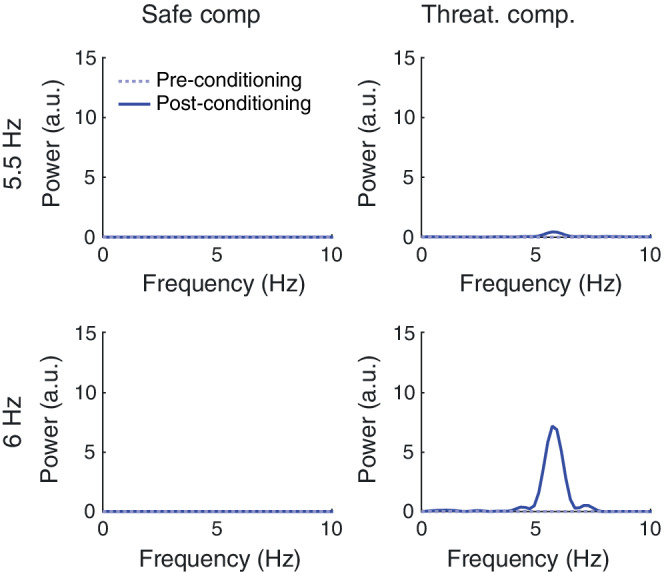
Power spectra of fear cell output spike trains in the safe (left) or threatening compartment (right). The solid cyan line and the blue dashed line indicate the spectrum before and after conditioning, respectively, with either low (5.5 Hz; top) or high (6.0 Hz; bottom) hippocampal theta frequency [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In spite of their different molecular mechanisms of action, all known anxiolytic drugs reduce the frequency of hippocampal theta oscillations. This reduction is usually small—between 0.5 and 2 Hz (McNaughton et al., 1986)—but its specificity to anxiolytics led researchers to regard it as a possible test for anxiolytic agents (McNaughton et al., 2007; Yeung et al., 2012). In this study, we investigated whether this theta frequency reduction could mechanistically explain the behavioral effect of these drugs on anxiety behavior. We based our analysis on contextual fear conditioning, a paradigm in which conditioned freezing is reduced by anxiolytic drugs (Ehrlich et al., 2009; Luyten et al., 2011; Sanger & Joly, 1985). Our simulations demonstrate that even small frequency reductions can have a significant effect on synaptic plasticity, sufficient to disrupt the contextual association between environment and threat.
Put differently, our model predicts that increasing theta frequency during contextual threat learning would increase the strength of these associations, thereby increasing the behavioral expression of contextual fear. Two factors contribute to this effect. First, the relationship between synaptic potentiation and the frequency of presynaptic activity is steep in a frequency band that approximately corresponds to rodent theta (4–9 Hz; Figure 2 in the main text; see also Figure 3b in Shouval et al., 2002). Therefore, small increases in hippocampal theta frequency can have a substantial effect on the synaptic potentiation of (presynaptic) hippocampal place cell inputs to (postsynaptic) neurons in the amygdala (“fear cells”). Second, freezing behavior (the measure of conditioned fear) is assumed to be elicited when average firing rates in the amygdala exceed some threshold, which is less likely if synaptic inputs from hippocampal place cells encoding context are weaker. Hence, small reductions in theta frequency can substantially reduce the strength of inputs to the amygdala, decreasing firing rates in the amygdala and thus the probability of freezing (or vice versa). Although the empirical relationship between theta power and memory is long established (Düzel et al., 2010), and experiments have shown memory impairments following the abolition of theta (McNaughton, Ruan, & Woodnorth, 2006; Winson, 1978), this is, to the best of our knowledge, the first model demonstrating a possible relation between theta frequency and memory, and thus providing a possible mechanistic role for the frequency perturbations caused by anxiolytics.
Importantly, our model additionally accounts for the empirical observation that theta oscillations coherent with hippocampal activity appear in the rodent LA after fear conditioning (Likhtik et al., 2014; Seidenbecher et al., 2003). The model posits that potentiated hippocampal inputs at theta frequency elicit firing in the LA, which collectively yields oscillations of the LFP at the same frequency. A similar phenomenon has been reported in the medial prefrontal cortex (mPFC) of rodents at the decision point of a maze after successful learning of task rules (Benchenane et al., 2010). This suggests that post‐learning theta synchronization might reflect functional pairing between the hippocampus and other brain regions.
We examined contextual fear conditioning because it can be more easily controlled in empirical studies and the underlying neural circuit is well‐described, whereas innate anxiety is likely to originate from hard‐wired adaptive tendencies with a partly unknown neural basis. However, we note that anxiolytics also affect behavior in a range of tests not involving conditioning (Choleris, Thomas, Kavaliers, & Prato, 2001; Pellow, Chopin, File, & Briley, 1985). For instance, the open field test is a common approach/avoidance anxiety test for rodents, consisting of an open arena in which the rodent can roam freely. The natural rodents' propensity toward exploring the arena conflicts with the preferential avoidance of exposed central regions, causing them to spend longer time along the walls in the periphery (Choleris et al., 2001). However, as in contextual conditioning, both the firing rate of LA neurons and avoidance behavior build up during exposure to the environment over minutes, suggesting that even so‐called innate anxiety behavior may arise from learning processes (Wang et al., 2011). Since the animal is usually placed in innate anxiety tests only once during their lifetime, it appears possible that they learn a cognitive map of the threatening environment features during the test. This learning might similarly be impaired by slowing theta frequency. Nonetheless, our model is not intended to address behavioral or neural responses associated with inherently aversive stimuli, which proceed in the absence of learning, although theta frequency coupling between medial temporal lobe regions during such experience may emerge as well (Zheng et al., 2017).
Our model predicts that the behavioral effect of anxiolytics originates from impairing the creation of neural associations between context and potential threat. This further predicts that anxiolytics do not impair already formed associations. In support of this, previous studies showed that benzodiazepines block fear conditioning if administered just before the conditioning epoch, but they are ineffective when administered just before recall (Sanger & Joly, 1985). This effect is not limited to learned anxiety. Multiple studies have shown that once animals experience the elevated plus‐maze without drug, anxiolytics are rendered ineffective in later exposure to the test—a phenomenon called “one trial tolerance” (File, 1993; File, Mabbutt, & Hitchcott, 1990).
Whether our model could also account for the clinical effect of anxiolytics—a reduction in subjective feelings of anxiety—remains unclear, as the neurobiological basis of these feelings is incompletely understood (Bach & Dayan, 2017; LeDoux, 2014). Importantly, we note that the anxiolytic effect in clinical contexts is immediate and does not vanish on repeated exposure to an anxiety‐generating context (Escarabajal, Torres, & Flaherty, 2003). It may therefore be that the drug effect on anxiety behavior, and subjective feelings of anxiety, are mediated by distinct mechanisms.
Importantly, however, the particular theta frequency values used in these simulations do not affect the qualitative nature of our results—that reductions in theta frequency will reduce synaptic potentiation and therefore impair learning. This is the case for any pair of (baseline and anxiolytic reduced) theta frequencies in the ~4–9 Hz range, below which no learning takes place, and above which increases in synaptic weight begin to saturate (as illustrated by Figure 2). Our model also suggests that larger theta reduction will cause a proportionally larger reduction in learning, although the downstream effect on the behavioral expression of freezing depends on the firing threshold that elicits freezing, which induces a non‐linearity.
A testable prediction of our model is that reduction of theta frequency alone, that is, without administration of anxiolytics, has an impact on learning and memory, and on anxiety behavior. To empirically demonstrate this, one could exploit the variability between epochs of theta activity and investigate correlations between theta frequency during conditioning and memory, as indexed by behavioral expression of anxiety during later recall. Other approaches require experimental manipulation of theta frequency. Previous studies have shown that this is possible by reducing the temperature of the brain (Whishaw & Vanderwolf, 1971). However, the fine frequency tuning necessary for probing the proposed effect might be difficult, and the secondary effects of temperature reduction cannot be ruled out easily. More recently, experimentally‐controlled theta oscillations have been induced with electrical patterned microstimulation (Lesting et al., 2011) and optogenetic approaches (Korotkova & Ponomarenko, 2017), providing viable methods for testing the proposed relation. This would also mitigate a concern that anxiolytic drugs have a wider array of effects beyond decreasing theta frequency, possibly encompassing an impact on nociception, which were not modeled here.
In summary, we presented a biophysical model suggesting that the behavioral effect of anxiolytics could be mediated by the effect that anxiolytics exert on theta frequency. The model accounts for a range of experimental findings and makes novel predictions about the effect of theta frequency on contextual fear conditioning, which may be tested in future experimental investigations.
ACKNOWLEDGMENT
DRB receives funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant agreement No. ERC‐2018 CoG‐816564 ActionContraThreat) and from the National Institute for Health Research (NIHR) UCLH Biomedical Research Centre. The Wellcome Centre for Human Neuroimaging is supported by core funding from the Wellcome Trust [203147/Z/16/Z].
Castegnetti G, Bush D, Bach DR. Model of theta frequency perturbations and contextual fear memory. Hippocampus. 2021;31:448–457. 10.1002/hipo.23307
Giuseppe Castegnetti and Daniel Bush contributed equally to this work.
Funding information H2020 European Research Council, Grant/Award Number: ERC‐2018 CoG‐816564; Wellcome Trust, Grant/Award Number: 203147/Z/16/Z
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study. The codes used in this study can be downloaded from github.com/gxcastegnetti/plathe.
REFERENCES
- Adhikari, A. , Topiwala, M. A. , & Gordon, J. A. (2010). Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron, 65, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, D. R. , & Dayan, P. (2017). Algorithms for survival: A comparative perspective on emotions. Nature Reviews. Neuroscience, 18, 311–319. [DOI] [PubMed] [Google Scholar]
- Bach, D. R. , Korn, C. W. , Vunder, J. , & Bantel, A. (2018). Effect of valproate and pregabalin on human anxiety‐like behaviour in a randomised controlled trial. Translational Psychiatry, 8, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear, M. F. , Cooper, L. N. , & Ebner, F. F. (1987). A physiological basis for a theory of synapse modification. Science (80‐), 237, 42–48. [DOI] [PubMed] [Google Scholar]
- Benchenane, K. , Peyrache, A. , Khamassi, M. , Tierney, P. L. , Gioanni, Y. , Battaglia, F. P. , & Wiener, S. I. (2010). Coherent theta oscillations and reorganization of spike timing in the hippocampal‐ prefrontal network upon learning. Neuron, 66, 921–936. [DOI] [PubMed] [Google Scholar]
- Biedermann, S. V. , Biedermann, D. G. , Wenzlaff, F. , Kurjak, T. , Nouri, S. , Auer, M. K. , … Fuss, J. (2017). An elevated plus‐maze in mixed reality for studying human anxiety‐related behavior. BMC Biology, 15, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki, G. (2002). Theta oscillations in the hippocampus. Neuron, 33, 325–340. [DOI] [PubMed] [Google Scholar]
- Buzsáki, G. , & Moser, E. I. (2013). Memory, navigation and theta rhythm in the hippocampal‐entorhinal system. Nature Neuroscience, 16, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris, E. , Thomas, A. W. , Kavaliers, M. , & Prato, F. S. (2001). A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neuroscience and Biobehavioral Reviews, 25, 235–260. [DOI] [PubMed] [Google Scholar]
- Colgin, L. L. (2013). Mechanisms and functions of theta rhythms. Annual Review of Neuroscience, 36, 295–312. [DOI] [PubMed] [Google Scholar]
- Colgin, L. L. (2016). Rhythms of the hippocampal network. Nature Reviews. Neuroscience, 17, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coop, C. F. , & McNaughton, N. (1991). Buspirone affects hippocampal rhythmical slow activity through serotonin1A rather than dopamine D2 receptors. Neuroscience, 40, 169–174. [DOI] [PubMed] [Google Scholar]
- Douchamps, V. , Jeewajee, A. , Blundell, P. , Burgess, N. , & Lever, C. (2013). Evidence for encoding versus retrieval scheduling in the hippocampus by theta phase and acetylcholine. The Journal of Neuroscience, 33, 8689–8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek, S. M. , & Bear, M. F. (1992). Homosynaptic long‐term depression in area CA1 of hippocampus and effects of N‐methyl‐D‐aspartate receptor blockade. Proceedings of the National Academy of Sciences, 89, 4363–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek, S. M. , & Bear, M. F. (1993). Bidirectional long‐term modification of synaptic effectiveness in the adult and immature hippocampus. The Journal of Neuroscience, 13, 2910–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düzel, E. , Penny, W. D. , & Burgess, N. (2010). Brain oscillations and memory. Current Opinion in Neurobiology, 20, 245–257. [DOI] [PubMed] [Google Scholar]
- Ehrlich, I. , Humeau, Y. , Grenier, F. , Ciocchi, S. , Herry, C. , & Lüthi, A. (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron, 62, 757–771. [DOI] [PubMed] [Google Scholar]
- Eichenbaum, H. , Dudchenko, P. , Wood, E. , Shapiro, M. , & Tanila, H. (1999). The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron, 23, 209–226. [DOI] [PubMed] [Google Scholar]
- Engin, E. , Stellbrink, J. , Treit, D. , & Dickson, C. T. (2008). Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: Behavioral and neurophysiological evidence. Neuroscience, 157, 666–676. [DOI] [PubMed] [Google Scholar]
- Escarabajal, M. D. , Torres, C. , & Flaherty, C. F. (2003). The phenomenon of one‐trial tolerance to the anxiolytic effect of chlordiazepoxide in the elevated plus‐maze test is abolished by previous administration of chlordiazepoxide or buspirone. Life Sciences, 73, 1063–1074. [DOI] [PubMed] [Google Scholar]
- File, S. E. (1993). The interplay of learning and anxiety in the elevated plus‐maze. Behavioural Brain Research, 58, 199–202. [DOI] [PubMed] [Google Scholar]
- File, S. E. , Mabbutt, P. S. , & Hitchcott, P. K. (1990). Characterisation of the phenomenon of “one‐trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus‐maze. Psychopharmacology, 102, 98–101. [DOI] [PubMed] [Google Scholar]
- Grillon, C. , & Ernst, M. (2020). A way forward for anxiolytic drug development: Testing candidate anxiolytics with anxiety‐potentiated startle in healthy humans. Neuroscience and Biobehavioral Reviews, 119, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo, M. E. (2006). The role of acetylcholine in learning and memory. Current Opinion in Neurobiology, 16, 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersman, S. , Cushman, J. , Lemelson, N. , Wassum, K. , Lotfipour, S. , & Fanselow, M. S. (2017). Optogenetic excitation of cholinergic inputs to hippocampus primes future contextual fear associations. Scientific Reports, 7, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemke, C. , & Hartter, S. (2000). Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacology and Therapeutics, 85, 11–28. [DOI] [PubMed] [Google Scholar]
- Hölscher, C. , Anwyl, R. , & Rowan, M. J. (1997). Stimulation on the positive phase of hippocampal theta rhythm induces long‐term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo. The Journal of Neuroscience, 17, 6470–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, J. M. , Wyble, B. P. , Goyal, V. , Rossi, C. A. , & Hasselmo, M. E. (2003). Stimulation in hippocampal region CA1 in behaving rats yields long‐term potentiation when delivered to the peak of theta and long‐term depression when delivered to the trough. The Journal of Neuroscience, 23, 11725–11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, L. , Kundu, S. , Lederman, J. D. D. , López‐Hernández, G. Y. Y. , Ballinger, E. C. C. , Wang, S. , … Role, L. W. W. (2016). Cholinergic signaling controls conditioned fear behaviors and enhances plasticity of cortical‐amygdala circuits. Neuron, 90, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, M. W. , Wiener, S. I. , & McNaughton, B. L. (1994). Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. The Journal of Neuroscience, 14, 7347–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemka, S. , Barnes, G. , Dolan, R. J. , & Bach, D. R. (2017). Dissecting the function of hippocampal oscillations in a human anxiety model. The Journal of Neuroscience, 37, 6869–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn, C. W. , Vunder, J. , Mirò, J. , Fuentemilla, L. , Hurlemann, R. , & Bach, D. R. (2016). Amygdala lesions reduce anxiety‐like behavior in a human benzodiazepine‐sensitive approach‐avoidance conflict test. Biological Psychiatry, 82(7), 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova, T. , & Ponomarenko, A. (2017). Optogenetic manipulations of neuronal network oscillations: combination of optogenetics and electrophysiological recordings in behaving mice. In In Vivo Neuropharmacology and Neurophysiology (pp. 67–88). New York, NY: Humana Press. [Google Scholar]
- LeDoux, J. E. (2014). Coming to terms with fear. Proceedings of the National Academy of Sciences of the United States of America, 111, 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting, J. , Narayanan, R. T. , Kluge, C. , Sangha, S. , Seidenbecher, T. , & Pape, H. C. (2011). Patterns of coupled theta activity in amygdala‐hippocampal‐prefrontal cortical circuits during fear extinction. PLoS One, 6, e21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik, E. , Stujenske, J. M. , A Topiwala, M. , Harris, A. Z. , & Gordon, J. A. (2014). Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neuroscience, 17, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman, J. (1989). A mechanism for the Hebb and the anti‐Hebb processes underlying learning and memory. Proceedings of the National Academy of Sciences of the United States of America, 86, 9574–9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten, L. , Vansteenwegen, D. , Van Kuyck, K. , Gabriëls, L. , & Nuttin, B. (2011). Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cognitive, Affective, & Behavioral Neuroscience, 11, 228–244. [DOI] [PubMed] [Google Scholar]
- Macdonald, R. L. , & Olsen, R. W. (1994). GABA A receptor channels. Annual Review of Neuroscience, 17, 569–602. [DOI] [PubMed] [Google Scholar]
- Mahmood, I. , & Sahajwalla, C. (1999). Clinical pharmacokinetics and pharmacodynamics of buspirone, an anxiolytic drug. Clinical Pharmacokinetics, 36, 277–287. [DOI] [PubMed] [Google Scholar]
- Maren, S. , & Hobin, J. A. (2007). Hippocampal regulation of context‐dependent neuronal activity in the lateral amygdala. Learning & Memory, 14, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton, N. , & Coop, C. F. (1991). Neurochemically dissimilar anxiolytic drugs have common effects on hippocampal rhythmic slow activity. Neuropharmacology, 30, 855–863. [DOI] [PubMed] [Google Scholar]
- McNaughton, N. , Kocsis, B. , & Hajos, M. (2007). Elicited hippocampal theta rhythm: A screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behavioural Pharmacology, 18, 329–346. [DOI] [PubMed] [Google Scholar]
- McNaughton, N. , Richardson, J. , & Gore, C. (1986). Reticular elicitation of hippocampal slow waves: Common effects of some anxiolytic drugs. Neuroscience, 19, 899–903. [DOI] [PubMed] [Google Scholar]
- McNaughton, N. , Ruan, M. , & Woodnorth, M.‐A. (2006). Restoring theta‐like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus, 16, 1102–1110. [DOI] [PubMed] [Google Scholar]
- Monaghan, C. K. , Chapman, W. G. I. , & Hasselmo, M. E. (2017). Systemic administration of two different anxiolytic drugs decreases local field potential theta frequency in the medial entorhinal cortex without affecting grid cell firing fields. Neuroscience, 364, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey, R. M. , & Malenka, R. C. (1992). Mechanisms underlying induction of homosynaptic long‐term depression in area CA1 of the hippocampus. Neuron, 9, 967–975. [DOI] [PubMed] [Google Scholar]
- Munn, R. G. K. , & McNaughton, N. (2008). Effects of fluoxetine on hippocampal rhythmic slow activity and behavioural inhibition. Behavioural Pharmacology, 19, 257–264. [DOI] [PubMed] [Google Scholar]
- Nemeroff, C. B. (2003a). Anxiolytics: Past, present, and future agents. The Journal of Clinical Psychiatry, 64, 3–6. [PubMed] [Google Scholar]
- Nemeroff, C. B. (2003b). The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacology Bulletin, 37, 133–146. [PubMed] [Google Scholar]
- O'Connor, D. H. , Wittenberg, G. M. , & Wang, S. S. H. (2005). Dissection of bidirectional synaptic plasticity into saturable unidirectional processes. Journal of Neurophysiology, 94, 1565–1573. [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. , & Nadel, L. (1978). The hippocampus as a cognitive map. Oxford: Clarendon Press. [Google Scholar]
- O'Keefe, J. , & Recce, M. L. (1993). Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus, 3, 317–330. [DOI] [PubMed] [Google Scholar]
- Pare, D. , & Collins, D. R. (2000). Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. Journal of Neuroscience, 20(7), 2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow, S. , Chopin, P. , File, S. E. , & Briley, M. (1985). Validation of open: Closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. Journal of Neuroscience Methods, 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Phillips, R. G. , & LeDoux, J. E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106, 274–285. [DOI] [PubMed] [Google Scholar]
- Ranck, J. B. (1973). Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. Experimental Neurology, 41, 532–555. [DOI] [PubMed] [Google Scholar]
- Riva, G. , Gaudio, S. , and Dakanalis, A. (2015). The Neuropsychology of (Oxford).
- Romanski, L. M. , Clugnet, M. C. , Bordi, F. , & LeDoux, J. E. (1993). Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behavioral Neuroscience, 107, 444–450. [DOI] [PubMed] [Google Scholar]
- Rosenkranz, J. A. (2012). In vivo voltage‐dependent influences on summation of synaptic potentials in neurons of the lateral nucleus of the amygdala. Neuroscience, 226, 101–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger, D. J. , & Joly, D. (1985). Anxiolytic drugs and the acquisition of conditioned fear in mice. Psychopharmacology, 85, 284–288. [DOI] [PubMed] [Google Scholar]
- Seidenbecher, T. , Laxmi, T. R. , Stork, O. , & Pape, H. C. (2003). Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science (80‐), 301, 846–850. [DOI] [PubMed] [Google Scholar]
- Shouval, H. Z. , Bear, M. F. , & Cooper, L. N. (2002). A unified model of NMDA receptor dependent bidirectional synaptic plasticity. Proceedings of the National Academy of Sciences of the United States of America, 99, 10831–10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok, C. J. , Taylor, C. P. , & Hajós, M. (2009). Anxiolytic profile of pregabalin on elicited hippocampal theta oscillation. Neuropharmacology, 56, 379–385. [DOI] [PubMed] [Google Scholar]
- Sławińska, U. , & Kasicki, S. (1998). The frequency of rat's hippocampal theta rhythm is related to the speed of locomotion. Brain Research, 796, 327–331. [DOI] [PubMed] [Google Scholar]
- Thompson, L. T. , & Best, P. J. (1989). Place cells and silent cells in the hippocampus rats of. The Journal of Neuroscience, 9, 2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote, P. , Fadok, J. P. , & Lüthi, A. (2015). Neuronal circuits for fear and anxiety. Nature Reviews. Neuroscience, 16, 317–331. [DOI] [PubMed] [Google Scholar]
- Wang, D. V. , Wang, F. , Liu, J. , Zhang, L. , Wang, Z. , & Lin, L. (2011). Neurons in the amygdala with response‐selectivity for anxiety in two ethologically based tests. PLoS One, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, C. E. , Amos, D. P. , Jeewajee, A. , Douchamps, V. , Rodgers, J. , O'Keefe, J. , … Lever, C. (2013). Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. The Journal of Neuroscience, 33, 8650–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw, I. Q. , & Vanderwolf, C. H. (1971). Hippocampal EEG and behavior: Effects of variation in body temperature and relation of EEG to vibrissae movement, swimming and shivering. Physiology & Behavior, 6, 391–397. [DOI] [PubMed] [Google Scholar]
- Wilson, M. A. , & Fadel, J. R. (2017). Cholinergic regulation of fear learning and extinction. Journal of Neuroscience Research, 95, 836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson, J. (1978). Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science (80‐), 201, 160–163. [DOI] [PubMed] [Google Scholar]
- Yeung, M. , Treit, D. , & Dickson, C. T. (2012). A critical test of the hippocampal theta model of anxiolytic drug action. Neuropharmacology, 62, 155–160. [DOI] [PubMed] [Google Scholar]
- Young, C. K. , Ruan, M. , & McNaughton, N. (2020). Speed modulation of hippocampal theta frequency and amplitude predicts water maze learning. Hippocampus, 31, 201–212. [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Anderson, K. L. , Leal, S. L. , Shestyuk, A. , Gulsen, G. , Mnatsakanyan, L. , … Lin, J. J. (2017). Amygdala‐hippocampal dynamics during salient information processing. Nature Communications, 8(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. o. , & McNaughton, N. (1995). Effects of long‐term administration of phenelzine on reticular‐elicited hippocampal rhythmical slow activity. Neuroscience Research, 21, 311–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study. The codes used in this study can be downloaded from github.com/gxcastegnetti/plathe.


