Abstract
Dysregulated protein synthesis is frequently involved in oncogenesis and cancer progression. Translation initiation is thought to be the rate-limiting step in protein synthesis, and the mRNA 5’ cap-binding protein eukaryotic translation initiation factor 4E (eIF4E) is a pivotal factor that initiates translation. The activities of eIF4E are regulated at multiple levels, one of which is through its phosphorylation at Serine 209 by the mitogen-activated protein kinase-interacting kinases (MNKs, including MNK1 and MNK2). Benefiting from novel mouse genetic tools and pharmacological MNK inhibitors, our understanding of a role for eIF4E phosphorylation in tumor biology and cancer therapy has greatly evolved in recent years. Importantly, recent studies have found that the level of eIF4E phosphorylation is frequently upregulated in a wide variety of human cancer types, and phosphorylation of eIF4E drives a number of important processes in cancer biology, including cell transformation, proliferation, apoptosis, metastasis and angiogenesis. The MNK-eIF4E axis is being assessed as a therapeutic target either alone or in combination with other therapies in different cancer models. As novel MNK inhibitors are being developed, experimental studies bring new hope to cure human cancers that are not responsive to traditional therapies. Herein we review recent progress on our understanding of a mechanistic role for phosphorylation of eIF4E in cancer biology and therapy.
Keywords: cancer, translational control, eIF4E, phosphorylation, MNK
1. The cap-binding protein eIF4E and translational control
mRNA translation (protein synthesis) is the most energy-consuming step in gene expression and is highly regulated [1]. Translational control provides a fast adaptive response to environmental cues and is essential for maintaining protein homeostasis in cells [2]. mRNA translation in eukaryotes can includes three steps: initiation, elongation and termination[2, 3]. Protein synthesis is controlled primarily at the step of mRNA translation initiation [2]. Most eukaryotic mRNAs are translated by a cap-dependent mechanism. All the nuclear transcribed mRNAs are capped at their 5’ end by an N7-methylated guanosine linked to the first nucleotide of the mRNA [4].
Translation initiation in eukaryotes begins with the binding of the eukaryotic translation initiation factor 4F (eIF4F) complex to the m7GTP cap structure of the mRNA [5]. The eIF4F complex consists of eIF4E, eIF4G and eIF4A. Eukaryotic translation initiation factor 4E (eIF4E) is a highly conserved protein that recognizes and binds to the cap. The concave surface of eIF4E is combined with the cap, and the convex surface interacts with eIF4G, the scaffold protein[5]. eIF4A is the RNA helicase that unwinds the secondary structure of 5’UTR (untranslated region) and promotes ribosome binding and scanning of 5’UTR. eIF4F complex assembly is the rate limiting step for translation initiation and it depends largely on the availability of eIF4E. The poly(A)-binding protein (PABP) also associates with the eIF4F complex via eIF4G, and binds the poly-A tail of most eukaryotic mRNA molecules, thus forming a circular mRNA[6, 7]. It is thought that the circularization mediated by PABP / eIF4F enhances translation [7, 8]. The eIF4F complex interacts with eIF3 to recruit the 43S pre-initiation complex to the cap.
The eIF2-GTP complex binds to the methionyl transfer RNA and forms a ternary complex with the 40S ribosomal subunit [9]. Other initiation factors such as eIF5, eIF1 and eIF1A bind to the ternary complex and form the 43S pre-initiation complex. The pre-initiation complex scans the mRNA across the 5’ UTR in a 5’ to 3’ direction until the methionyl tRNA finds the start codon, usually (but not always) AUG. After attachment to the mRNA, the 43S complex scans to the initiation codon, whereupon it forms a 48S initiation complex. After identifying the start codon, the eIF2-GTP complex undergoes hydrolysis, triggering the release of itself as well as other eIFs from the 48S complex [10]. Dissociation of these factors allows for the binding of the 60S ribosomal subunit and the formation of the 80S ribosomal complex, which concludes the translation initiation process.
As it is of low abundance, eIF4E serves as a key checkpoint in controlling the rate of mRNA translation [11]. eIF4E abundance and activity are regulated by several mechanisms [12]. First of all, the level of eIF4E is regulated at the level of transcription and mRNA stability. Some study suggests that eIF4E is a myc target gene as it has an E-box in its promoter. Secondly, the eIF4E-binding proteins (4E-BPs) compete with eIF4G to bind to eIF4E. Once bound to 4E-BPs, eIF4E cannot bind to eIF4G and initiate translation. This can be relieved after 4E-BP phosphorylation by the mechanistic target of rapamycin (mTOR) signaling pathway [13, 14]. Thirdly, eIF4E activity can be regulated by its phosphorylation at Serine 209 [15] (see below).
Protein synthesis is frequently dysregulated in human diseases such as cancer [16]. Recent studies have found that eIF4E is often overexpressed and hyperphosphorylated in human cancers, which is correlated with disease progression and prognosis [17]. Experimental studies have found that phosphorylation of eIF4E plays a fundamental role in tumor biology. Here, we review recent progress on a role for eIF4E phosphorylation in tumor biology and its potential as a therapeutic target for cancer therapy.
2. Phosphorylation of eIF4E by MNKs
2.1. The MAPK-MNK signaling pathway
The activity of eIF4E is regulated through its phosphorylation by two MAP kinase-interacting kinases (MNKs, i.e. MNK1 and MNK2) at a single residue Ser209 [18, 19]. MNK1 and MNK2 belong to the serine/threonine protein kinase family and are activated by extracellular signal-regulated kinases (ERKs) or p38 MAPK under the stimulation of extracellular factors, such as mitogens, osmotic stress, heat shock and proinflammatory cytokines[20]. Extracellular signal–regulated kinases (ERKs, including ERK 1 and ERK2) are activated downstream of oncoproteins such as receptor tyrosine kinases, Ras and Raf, and in turn drive MNK1 activation and eIF4E phosphorylation. The p38 MAP kinases α and β are activated by stress signals such as arsenite and anisomycin and various cytokines [15].
In human cells, four MNK isoforms have been identified: MNK1a, MNK1b, MNK2a and MNK2b. The four isoforms are similar in their N-termini and each contain a nuclear localization signal and an eIF4G binding site[20]. MNKs are associated with the C-terminus of eIF4G, allowing the kinase to be close enough to phosphorylate eIF4E[21]. However, their C-terminal regions are different. MNK1a and MNK2a contain MAPK binding sites, which are lacking in the MNK1b and MNK2b. The MAPK-binding domain of MNK1a interacts with p38 MAPK and ERK1/2, whereas the MAPK-binding domain of MNK2a only binds to ERK1/2[20]. MNK1 has low basal activity in resting cells and is activated by ERK and p38 MAPK in response to mitogens and stress responses. In contrast, MNK2 has high basal activity, but is mainly regulated by ERKs and responds weakly to p38 MAPK. Previous evidence suggests that MAPK activates MNK1 to induce eIF4E phosphorylation, while MNK2 is primarily involved in constitutive phosphorylation of eIF4E under basal conditions[22]. (Figure 1).
Figure 1. Schematic illustration of the cellular signaling pathways that lead to eIF4E phosphorylation.
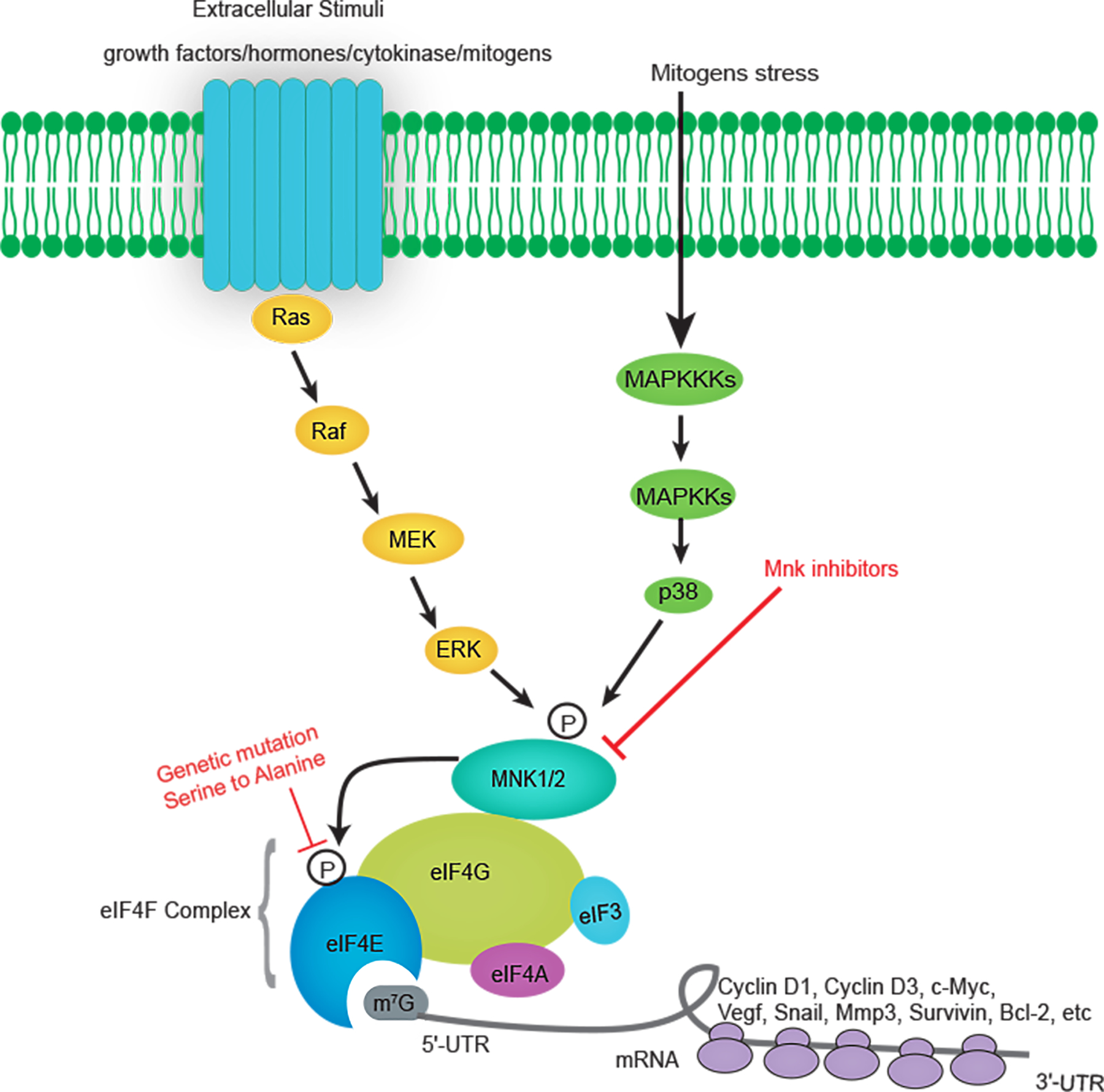
Extracellular stimuli and mitogens stress activate components of the MAPK pathway including the ERK and p38 MAP kinases. In turn, MNK1 and MNK2 are activated by ERK and p38 MAP kinases, bind to eIF4G and phosphorylate eIF4E at Ser209. Phosphorylation of eIF4E promotes mRNA translation of a variety of mRNAs involved in tumor biology, including Cyclin D1, Cyclin D3, C-myc, VEGF, SNAIL, MMP3, Survivin, Bcl-2, etc. As hyperphosphorylation of eIF4E is critical for its oncogenic activities, it can be repressed by genetic mutation of Ser209 or using MNK inhibitors.
2.2. The MNK and mTORC1 pathways converge on eIF4E to regulate mRNA translation
The mechanism/mammalian target of rapamycin (mTOR) is a highly evolutionarily conserved serine/threonine kinase that is a member of the phosphatidylinositol 3-kinase-related kinase (PIKK) family, which acts on downstream of phosphatidylinositol 3-kinase (PI3K) / the AKT pathway [23]. mTOR forms two polyprotein complexes: mTORC (mTOR complex)1 and mTORC2. Both complexes contain mTOR kinase, but the associated regulatory proteins are different. mTORC1 is defined by a regulatory protein associated with mTOR (RAPTOR) [24, 25] and has the key downstream substrates including 4E-BPs (including 4E-BP1, 2 and 3). 4E-BPs are translational repressors that bind to eIF4E and block its binding to eIF4G. The activities of 4E-BPs are regulated via phosphorylation by mTORC1[13,14]. After phosphorylation by mTORC1, 4E-BPs dissociate from eIF4E, thus allowing the formation of eIF4F complex and cap-dependent translation initiation. eIF4E is therefore a key converging point whereby the MNK and mTORC1 pathways control mRNA translation. Importantly, emerging evidence suggests significant crosstalk between MNK and mTORC1 pathways. For example, Brown et al. found that [26] MNK deletion can reduce basal mTORC1 signaling and MNK activation contributes to rapamycin resistance in cancer cells by sustaining mTORC1 activity following rapamycin treatment. Consistently, MNK inhibition alone only mildly suppresses the lymphoma cell growth, but the combined MNK and mTORC1 inhibition totally abrogates the growth, suggesting combined inhibition of mTORC1 and MNK exhibits synergistic effects in cancer therapy [27].
2.3. Biological functions of eIF4E phosphorylation
MNKs regulate mRNA translation through eIF4E phosphorylation. It, however, remains poorly understood how the phosphorylation of eIF4E affects its function. Biophysical studies show that phosphorylation of eIF4E markedly reduces its affinity for capped RNA, primarily due to an increased rate of dissociation [28, 29]. However, studies using the eIF4ES209A cells show that eIF4E phosphorylation positively regulates the translation of a variety of mRNAs that are involved in tumorigenesis, suggesting that eIF4E phosphorylation may have differential effects on mRNA translation [30]. For example, phosphorylation of eIF4E enhances translation of specific mRNAs involved in cell proliferation and survival, such as Cyclin D1 and D3, c-Myc, Snail, Mmp3, Hdm2 (Mdm2 in mouse), Survivin and Bcl-2 (B-cell lymphoma 2) [30–32]. Moreover, phosphorylation of nuclear eIF4E plays an important role in exporting a set of mRNAs from the nucleus to the cytoplasm (including Cyclin D1, Hdm2, and Odc) [33, 34]. However, phosphorylation of eIF4E is not required for cell survival, proliferation and development, as eIF4ES209A and MNK1/2 double knockout mice are viable and fertile [22,30].
In the nervous system, phosphorylation of eIF4E also plays an important role in synaptic plasticity [17], mood modulation, nociceptive plasticity and circadian rhythms. The genetic and pharmacological inhibition of mouse eIF4E phosphorylation leads to anxiety and depression-like behaviors[35, 36]. Recent studies also revealed that MNK-eIF4E signaling is a critical signaling pathway for the generation of nociceptive plasticity leading to acute pain responses to inflammation and the development of hyperalgesic priming and chronic pain[37, 38]. Moreover, eIF4E phosphorylation promotes the mRNA translation of the clock genes Period 1 and 2, thus facilitating circadian clock resetting and contributing to precise timekeeping[39].
3. eIF4E phosphorylation in tumor biology
The regulation of cell proliferation and survival by eIF4E phosphorylation has only been observed in cancer but not in normal tissues, and aberrant levels of eIF4E phosphorylation are found in a variety of human cancers, such as nasopharyngeal carcinoma, astrocytoma and melanoma. Increasing evidence supports that eIF4E phosphorylation is involved in a number of key processes in tumor biology including cell proliferation, transformation, apoptosis, tumor metastasis and angiogenesis (Figure 2).
Figure 2. eIF4E phosphorylation regulates an oncogenic network.
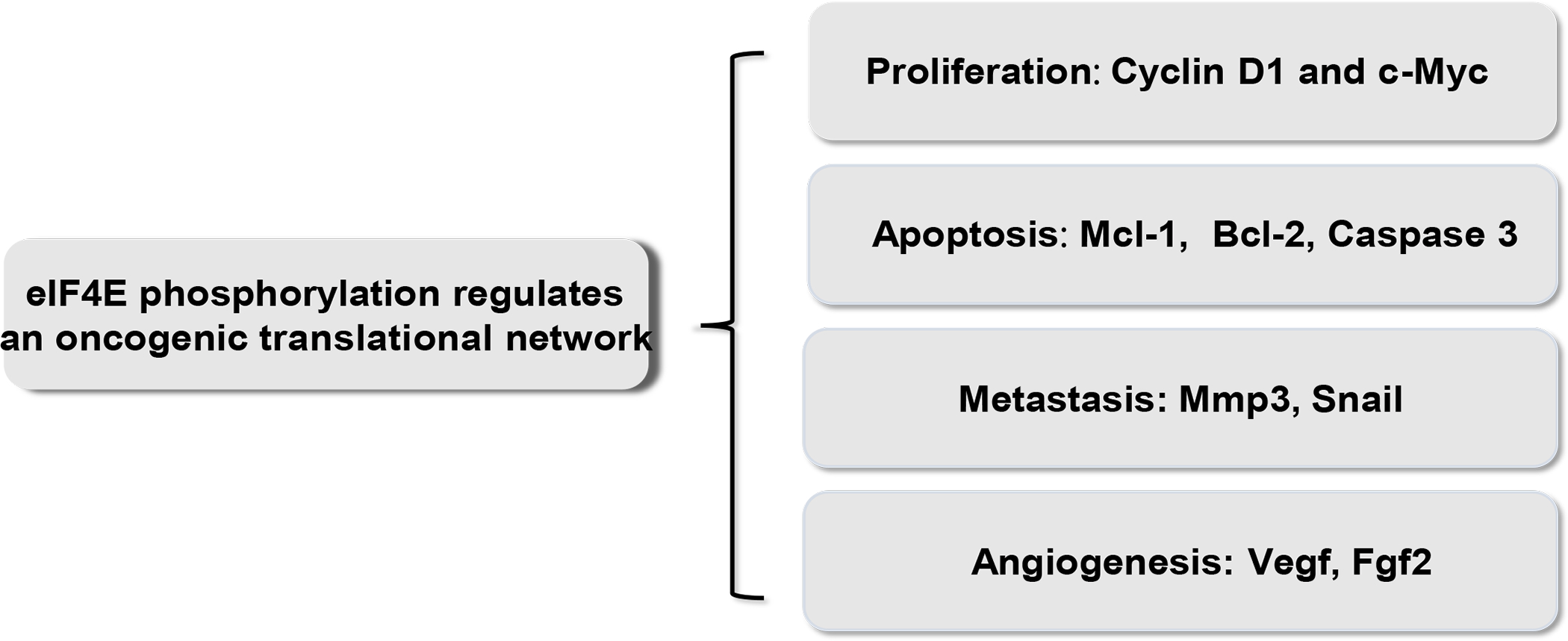
eIF4E phosphorylation regulates a translational network of mRNAs encoding protumorigenic factors, which are involved in multiple oncogenic processes including cell proliferation, apoptosis, metastasis, and angiogenesis.
3.1. eIF4E phosphorylation and cell proliferation
eIF4E overexpression can lead to increased levels of Cyclin D1 and c-Myc proteins, which are key mediators of cell proliferation[40]. Cyclin D1 is required for cells to enter the S phase, and its increased expression is related to cell proliferation[41]. c-Myc is ubiquitously expressed during embryogenesis and in post-developmental tissues with high proliferative capacity [42]. MNK inhibition reduces the synthesis of Cyclin D1 and represses the proliferation of breast cancer cells[43]. Conversely, MNK1 overexpression is associated with high levels of c-Myc expression in acute myeloid leukemia (AML), and inhibition of MNK1 activity by the inhibitor CGP57380 or kinase dead mutants of MNK1 significantly impairs proliferation of hematopoietic cells [44]. Similarly, the level of eIF4E phosphorylation is positively correlated with β-catenin signaling (including β-Catenin, Cyclin D1, c-Myc). CGP57380 downregulates β-catenin and suppresses the proliferation of nasopharyngeal carcinoma (NPC) cells [45]. However, it is worth mentioning that CGP57380 is a highly non-specific inhibitor and the results obtained with it can only suggest the involvement of eIF4E phosphorylation in a given process, but only more specific inhibitors and/or gene editing can confirm a more definitive role for eIF4E phosphorylation.
3.2. eIF4E phosphorylation and cell transformation
Cell transformation is the process whereby normal cells acquire the properties of cancer cells, which may occur as a primary process in normal tissue, or when malignant degeneration occurs in a previously existing benign tumor. eIF4E overexpression and its phosphorylation may contribute to cancer cell transformation. As a proto-oncogene, eIF4E overexpression leads to immortalization of NIH 3T3 murine fibroblasts and transformation of human epithelial cells by selectively and disproportionately increasing the expression of proteins related to growth, angiogenesis, and survival factors [46]. Moderate reduction of eIF4E expression can reverse many phenotypes associated with RAS-induced malignant transformation [47]. Elevated eIF4E phosphorylation was first found in src-transformed cell lines[48]. Importantly, eIF4E double mutation (ST209/210AA) substantially eliminates its ability to transform cells as compared with wild-type eIF4E [33].
Furic et al. have also shown that eIF4ES209A MEFs are resistant to RAS and C-MYC induced transformation, demonstrating that its phosphorylation of eIF4E is indispensable for its transforming activity [30]. Furthermore, transformation is also inhibited in MNK1/2 double knockout MEF that overexpress eIF4E, demonstrating sufficiency of MNK1/2 for eIF4E phosphorylation. By using adoptive transfer methods, Wendel et al. have showed ectopic expression of eIF4ES209A mutant failed to cause tumor transformation in the Myc driven B-cell leukemia/lymphoma model (Eμ-Myc). Accordingly, high levels of phosphorylated eIF4E effectively upregulate the expression of MCL-1 in human lymphomas[49]. In another study by Ueda et al., MNK1/2 double knockouts can also inhibit RAS-mediated oncogenesis[50]. Taken together, these results indicate that phosphorylation of eIF4E by MNKs can promote cell transformation.
3.3. eIF4E phosphorylation and cell apoptosis
A hallmark of cancer is the ability of malignant cells to evade apoptosis, which is a rapid and irreversible process to eliminate dysfunctional cells. Mcl-1 is a Bcl-2 family protein that controls apoptosis, and it is involved at an early stage in a cascade of events that lead to the release of cytochrome c from mitochondria, thus promoting cell survival. Mcl-1 is a mRNA substrate of eIF4E and its expression can be reduced by inhibiting eIF4E phosphorylation [49]. MEFs overexpressing eIF4E or MNK1 contain more Mcl-1 mRNA in the polyribosome fractions compared with controls, while the total mRNA levels remain unchanged [49]. eIF4E overexpression can lead to increased Cyclin D1 levels, which have anti-apoptotic effects [51]. Caspase 3, a key regulator of cell apoptosis, promotes repopulation of surviving tumor cells via activation of the caspase 3/PKCδ/p38/MNK1 signaling pathway in irradiated pancreatic tumor cells. Accordingly, pharmacologic inhibition of MNK1 with CGP57380 and Ribavirin can significantly repress tumor cell repopulation and tumor cell survival [52]. Thus, eIF4E phosphorylation decreases tumor cell apoptosis and promotes survival.
3.4. eIF4E phosphorylation and tumor metastasis
Metastasis is the spread of cancer cells to new areas of the body by way of the lymph system or bloodstream and it is one of the leading causes of failure in cancer treatment and death of patients. In many advanced tumors, epithelial-to-mesenchymal transition (EMT) renders tumor cells highly malignant and is required for metastasis. Phosphorylation of eIF4E is correlated with the increase of mesenchymal markers such as N-cadherin, fibronectin and vimentin that are mediators of cell invasiveness, thus promoting EMT, tumor invasion, and metastasis [31]. Transforming growth factor-beta (TGF-β) induces eIF4E phosphorylation, which in turn stimulates translation of matrix metalloproteinase 3 (Mmp3) and Snail mRNAs and induces EMT [31]. eIF4ES209A mice are resistant to lung metastasis in a mammary tumor model and cells isolated from these mice showed impaired invasion in vitro [32]. Consistently, MNK1 signaling promotes translation of SMAD2 mRNA as well as TGF-β–induced cell motility and vimentin (a marker for EMT) expression in glioblastoma cells [53]. Conversely, MNK inhibitors can inhibit EMT, cell invasiveness and metastasis in the nasopharyngeal cancer[45].
3.5. eIF4E phosphorylation and angiogenesis
Tumor angiogenesis is the process of generating new blood vessels, which is required to provide sustained supply of oxygen and nutrients to tumor cells, as well as to excrete metabolic wastes from the tumor tissue. Overexpression of eIF4E increases the secretion of vascular endothelial growth factor (VEGF) without affecting its mRNA levels[54]. Similarly, fibroblast growth factor 2 (FGF-2) mRNA is also loaded onto polysomes in cells that overexpress eIF4E, resulting in increased FGF-2 secretion[55]. VEGF and FGF-2 are key regulators of tumor progression by promoting angiogenesis.
However, few studies focused directly on a role for eIF4E phosphorylation in angiogenesis. Angiogenesis is known to play an important role in the progression of hepatocellular carcinoma (HCC) and resistance to chemotherapy. Blockage of eIF4E phosphorylation by the MNK inhibitor cercosporamide impairs hepatocellular carcinoma (HCC) angiogenesis by inhibiting the formation, migration, proliferation and survival of capillary networks of HCC endothelial cells [56]. Vascular recovery or angiogenesis after radiation therapy plays an important role in tumor recurrence. eIF4E phosphorylation may mediate caspase 3 regulated VEGF-α expression. Importantly, by activating the NF-κB/COX-2/PGE2 axis and p-eIF4E/VEGF-A signaling, caspase 3 helps to establish a pro-angiogenic microenvironment in post-irradiation dying glioma cells. Accordingly, caspase 3 inhibition disrupts the proangiogenic effect of glioma cells and reduces tumorigenicity [57]. Thus, decreasing eIF4E phosphorylation can increase the sensitivity of tumor cells to chemotherapy or radiotherapy.
4. Targeting eIF4E phosphorylation in experimental cancer therapy
As phosphorylation of eIF4E plays an important role in many fundamental processes of tumor biology, repressing eIF4E phosphorylation can be used as a potential strategy to treat cancer patients. One strategy is to pharmacologically inhibit MNKs, since they are the only kinases that can phosphorylate eIF4E. The candidate compounds are CGP57380, cercosporamide and 5-(2-(phenylamino) pyrimidin-4-yl) thiazole-2(3H)-one derivatives. CGP57380 is a potent MNK1 inhibitor with an IC50 of 2.2 μM in vitro [58]. However, it can also inhibit many other kinases [59]. For example, CGP57380 inhibits phosphorylation of multiple substrates of the MAPK/MNK/RSK and PI3K/mTOR/S6K1 pathways in vitro, which can inhibit the assembly of polysomes and target multiple components known to regulate cap-dependent translation [60]. It also targets CK1 with similar potency as MNK1 and inhibits protein kinases including Aurora B, DYRK, SGK, BRSK2, and LCK [59]. Cercosporamide, an antifungal agent, is also a potent MNK inhibitor. The IC50 of cercosporamide is 116 nM for MNK1 and 11 nM for MNK2 in vitro [61]. Notably, oral administration of cercosporamide to mice bearing tumor xenografts can inhibit eIF4E phosphorylation in xenograft tumors and in mouse liver tissue as quickly as 30 min after administration[61]. The 5-(2-(Phenylamino) pyrimidin-4-yl) thiazole-2(3H)-one derivatives were synthesized by screening a library of kinase inhibitors [62]. Mechanistic studies have confirmed that these inhibitors can reduce eIF4E phosphorylation and induce apoptosis in tumor cell lines by reducing the expression of anti-apoptotic proteins Mcl-1 [62].
As MNK1 and MNK2 are the two kinases that phosphorylate eIF4E, their genetic mutants can also be used to study functions of MNKs as well as eIF4E phosphorylation [22]. The Mnk1, Mnk2 and Mnk1/2 double knockout mice are powerful genetic tools to study MNK functions in vivo [22]. Moreover, as eIF4E can be phosphorylated only on Serine 209, eIF4ESer209A mice were created [30]. These mouse genetic tools can be used to study functions of MNKs and eIF4E phosphorylation in vivo. In addition, the advent of CRISPR/Cas9 gene editing technology has the potential to be used to investigate the direct role of MNK1/2 in multiple tumors without relying on mostly non-specific inhibitors. Increasing studies have demonstrated the efficacy of pharmacologically and/or genetically targeting MNK kinase and eIF4E phosphorylation in experimental cancer therapy, which are summarized in Table 1.
Table 1.
Targeting MNK and/or eIF4E phosphorylation in different types of cancer
| Cancer Types | Intervention Methods | Effects | Mechanisms | Reference |
|---|---|---|---|---|
| Breast cancer | Serine to Alanine mutation at eIF4E Serine 209 | eIF4ES209A mice are resistant to lung metastases in a mammary tumor model (MMTV-PyMT) | Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3 | [31] |
| MNK inhibitor CGP57380 | CGP57380 inhibits proliferation of cell lines SKBr3, BT474, ZR75.1, T47D, and MDA-MB-231 | CGP57380 may inhibit the synthesis of cyclin D1 by inhibiting MNK kinase activity | [43] | |
| MNK inhibitor CGP57380 | CGP57380 reduces colony formation of AU565 cells | MNKs is activated in the HER2/Ras/Raf/ERK pathway and it correlates with HER2 overexpression | [63] | |
| MNK inhibitor CGP57380 | CGP57380 inhibits proliferation of MDA-MB 468 and MDA-MB 231 cells | CGP57380 associates with an increase of E-cadherin and β-catenin protein levels by downregulating p-eIF4E levels | [64] | |
| Retinoic acid metabolism blocking agents (RAMBAs) VNLG-147, −152 and −153, etc | RAMBAs inhibited growth, colonization, invasion, and migration and induce apoptosis of MDA-MB-231 and MDA-MB-468 cells | RAMBA retinamides degrade MNK rather than inhibiting its kinase activity | [65] | |
| MNK inhibitor CGP57380 | CGP57380 restored tamoxifen sensitivity | eIF4E phosphorylation is increased in tamoxifen-resistant breast cancers cell lines | [66] | |
| Chemotherapeutic drugs doxorubicin, cyclophosphamide, dasatinib and MNK inhibitor CGP57380 | CGP57380 enhances response of LCC1 and LCC9 cells to chemotherapy | MNK inhibition abolishes chemotherapeutic drugs-mediated β-catenin activation in breast cancer cells | [67] | |
| MNK inhibitor CGP57380 | CGP57380 decreased SUM149 cells dissemination within window chamber model with GFP-tagged SUM149 | MNK signaling promotes XIAP expression and NFκB activity | [68] | |
| MNK inhibitors CGP57380 and cercosporamide | Synergistic combination of MNK1/2 and PI3K inhibitors slow the rate of cell migration in MDA-MB-231 cells | The combination of MNK1/2 and mTORC1/2 inhibition induces G1 cell cycle arrest | [69] | |
| Novel ferrocene analogues | Compound 5 reduces cell proliferation and the IC50 in MDA-MB-231 cells (triple negative breast cancer cells) | Novel ferrocene analogues-compound 5 inhibits MNK1/2 kinase activity | [70] | |
| MNK1/2 protein degraders, racemic VNLG-152 | VNLG-152 exhibits remarkable antitumor and antimetastatic activities against the MDA-MB-231 cell line and patient-derived TNBC xenograft models | MNK-eIF4E signaling pathway regulates downstream factors involved in cell cycle regulation, apoptosis, pro-inflammatory cytokines/chemokines secretion, epithelial-mesenchymal transition (EMT) and metastasis | [71] | |
| MNK-7g containing thieno [2,3-d] pyrimidine scaffold | MNK-7g blocks the migration of MDA-MB-231 cells | MNK-7g targeting MNK1/2 does not affect other signaling pathways tested and have no adverse effects on cell viability | [72] | |
| Ovarian cancer | MNK inhibitor CGP57380 | CGP57380 significantly suppresses OVCAR-5 cell proliferation | MNK1 regulates the mRNA translation of proliferation-related proteins through phosphorylating eIF4E in ovarian cancer cell | [73] |
| MEK inhibitor U0126 | U0126 combined with chemotherapeutic agents significantly enhances growth inhibition and apoptosis induction of SK-OV-3 cells | Chemotherapy agents activate ERK/MNK/eIF4E in a MEK-dependent manner | [74] | |
| Cervical cancer | MNK inhibitor CGP57380 or MNK siRNAs | CGP57380 is effective in inhibiting proliferation and migration, and inducing apoptosis in cervical cancer cells CaLo, SiHa and C-33A | CGP57380 or MNK siRNAs can effectively reduce the phosphorylation of eIF4E and β-catenin, thereby reducing β-catenin activity and Wnt target gene transcription levels in cervical cancer cells | [75] |
| Prostate cancer | Serine to Alanine mutation at eIF4E Serine 209 | eIF4ES209A mice are resistant to tumorigenesis in a prostate cancer model. | eIF4E phosphorylation increases the translation efficiency of a subset of mRNAs encoding pro-tumorigenic factors | [30] |
| Novel retinamides (NRs) | NRs induce cell cycle arrest, apoptosis, and inhibit proliferation and migration of PC-3, C4-2B and 22Rv1 cells | NRs target both AR signaling and eIF4E translation via enhancing AR and MNK degradation through ubiquitin-proteasome pathway | [76] | |
| VNHM-1–81, VNHM-1–66 VNHM-1–73 | Retinamides inhibit cell proliferation and migration and induce apoptosis in MDA-MB-231 human breast and CWR22Rv1 prostate cancer sells | These novel C-4 azolyl retinamides (NRs) induce MNK1/2 degradation via the ubiquitin-proteasome pathway with resultant depletion of p-eIF4E | [77] | |
| Galeterone and VNPT55 | VNPT55 profoundly inhibits migration and invasion of PC-3, DU145 and CWR22Rv1 cells | Galeterone and VNPT55 downregulate protein expression of several EMT markers (Snail, Slug, N-Cadherin, Vimentin and MMP-2/−9) via antagonizing the MNK-eIF4E axis | [78] | |
| MNK inhibitor CGP57380 | CGP57380 sensitizes CRPC cells to RAD001 and bicalutamide | CGP57380 reduces eIF4E phosphorylation and sensitizes survivin levels to RAD001 | [79] | |
| a novel retinamide VNLG-152 | VNLG-152 suppresses growth and metastasis of aggressive CWR22Rv1 tumors xenograft | The retinamide VNLG-152 decreases cyclin D1 and Bcl-2, suppresses EMT in CWR22Rv1 tumors | [80] | |
| Leukemia | MNK inhibitor CGP57380 | CGP57380 enhances myeloid differentiation of HL60 and 32D cells | MNK1 expression is associated with high levels of c-Myc expression | [44] |
| MNK inhibitor CGP57380 or MNK siRNAs | CGP57380 or MNK siRNAs enhances the suppressive effects of low cytarabine concentrations on CFU-L of U937 cells | MNK kinase is negatively regulated in the generation of chemotherapy-induced antileukemic responses | [81] | |
| MNK inhibitor CGP57380 | CGP57380 significantly reduces serial replating efficiency of blast crisis (BC) progenitors and prevents BC granulocyte macrophage progenitors from serially transplanting immunodeficient mice | CGP57380 prevents β-catenin activation | [82] | |
| MNK inhibitor cercosporam-ide | Cercosporamide suppresses effects on primitive leukemic progenitors (CFU-L) of U937 cells and suppresses growth of MV4-11 AML xenograft tumors | Cercosporamide suppresses phosphorylation of eIF4E and exhibits antileukemic effects | [83] | |
| Selective MNK2 inhibitor (MNKI-85) and a dual-specific MNK1 and MNK2 inhibitor (MNKI-19) | MNKI-85 and MNKI-19 are effective in inhibiting the growth of FLT3- internal tandem duplication (ITDs) expressed AML cells | MNKI-19 and MNK-85 reduce the level of phosphorylated eIF4E, induce G1 phase cell cycle arrest and apoptosis | [84] | |
| Ribavirin | Ribavirin inhibits cell proliferation and induces apoptosis of K562 cells | Ribavirin reduces the expression of Mcl-1 at protein synthesis level but not mRNA transcriptional level by decreasing eIF-4E phosphorylation | [85] | |
| MNK inhibitor MNKI-8e and short hairpin RNA (shRNA) mediated knockdown | MNKI-8e and MNK shRNAs enhances the ability of cytarabine to induce apoptosis of MV4-11 AML cells | MNKI-8e and MNK shRNAs downregulates the expression of anti-apoptotic Mcl-1 protein | [86] | |
| Merestinib | Merestinib suppresses cell growth of AML patient-derived cells and tumor growth in a MM6 cell xenograft model | Merestinib blocks Mnk kinase activity and eIF4E phosphorylation in acute myeloid leukemia cells | [87] | |
| Niclosamide and dasatinib | The combination of niclosamide and dasatinib significantly inhibit proliferation and induces apoptosis in a panel of CML cell lines | Niclosamide can inhibit phosphorylation of Erk, MNK1 and eIF4E in CML cells | [88] | |
| MNK inhibitor CGP57380 and everolimus | CGP57380 produces a synergistic growth inhibitory effect with everolimus in T-cell acute lymphoblastic leukemia (T-ALL) cells | CGP57380 overcomes everolimus-mediated eIF4E phosphorylation and sensitizes T-ALL cells to everolimus | [89] | |
| N-phenyl-4-(1H-pyrrol-3-yl) pyrimidin-2-amine derivatives | Most of these compounds demonstrate potent anti-proliferative activity against MV4-11 AML cells | These compounds reduce eIF4E phosphorylation and induced apoptosis by down-regulating Mcl-1 and by cleaving PARP | [90] | |
| Lymphoma | Serine to Alanine mutation at eIF4E Serine 209; an constitutively activated or kinase-dead form of MNK1 | MNK1 activation promotes tumorigenesis and MNK1 repression inhibits tumor cell proliferation | eIF4E phosphorylation suppresses apoptosis by up-regulating the anti-apoptotic protein Mcl-1 | [49] |
| MNK inhibitor CGP57380 | CGP57380 causes inhibition of cell proliferation and cell death in HKBML cell line | MNK inhibitor reduces cyclin D1 expression | [91] | |
| MNK inhibitor 4-Amino-5-(4-fluoroanilino)-pyrazolo[3,4-d] pyrimidine | MNK Inhibitor suppresses growth of cutaneous T-Cell lymphoma (CTCL) cells and displays a minimal pro-apoptotic effect | MNK inhibitor induces cell apoptosis | [27] | |
| Pancreatic cancer | MNK inhibitor CGP57380 and ribavirin | CGP57380 and ribavirin significantly weaken Sox2-mediated repopulation of SW1990 and BxPc-3 cells | CGP57380 enhances tumor radiosensitivity by inhibiting eIF4E phosphorylation | [52] |
| MNK inhibitor MNK-I and gemcitabine | MNK-I significantly increased cell death in MiaPaCa2 cells exposed to gemcitabine | MNK-I strongly reduces gemcitabine-induced eIF4E phosphorylation | [92] | |
| MNK inhibitor CGP57380 | CGP57380 can reverse EMT, decrease migration,and limit growth of CD18-CR cells | CGP57380 increases E-cadherin, decreases vimentin and reduces migration of PDAC cells, decreases the protein expression of ZEB1 without reducing ZEB1 mRNA levels | [93] | |
| Gal/analogs (VNPT55, VNPP414 and VNPP433-3β) | Gal/analogs profoundly inhibit cell viability of gemcitabine-naive/resistance PDAC cell lines | Gal/analogs downregulate MNK1/2, peIF4E, NF-κB (p-p65), N-cadherin, MMP-1/−2/−9, Slug, Snail, CXCR4, β-Catenin, Nanog, BMI-1, Oct-4 and induced caspase 3-mediated cell-death | [94] | |
| Lung cancer | MNK inhibitor CGP57380 and mTOR inhibitor RAD001 | CGP57380 and RAD001 augment the antitumor efficacy through inhibiting proliferation and inducing apoptosis in A549 and H157 cells | CGP57380 suppresses eIF4E phosphorylation and sensitizes NSCLC cells to RAD001 | [95] |
| Rifabutin | Rifabutin is effectively against H3255, H1650 and H460 cells and H3255 xenograft mouse model through inhibiting proliferation and inducing apoptosis | Rifabutin suppresses eIF4E phosphorylation and decreases β-catenin activity | [96] | |
| Hepatocellular carcinoma | MNK inhibitor cercosporamide | Cercosporamide selectively suppresses angiogenesis, growth and survival of HepG2, HuH6, SNU-182 and Hep3 B cells | Cercosporamide blocks eIF4E phosphorylation and selectively exhibits anti-HCC activities | [56] |
| Glioma | MNK1/2 double knockdown and MNK inhibitor CGP57380 | MNK1 knockdown of U87MG cells shows reduced focus formation and enhanced apoptosis | eIF4E phosphorylation enhances the translation of pro-survival genes | [50] |
| MNK inhibitor CGP57380 and RAD001 | CGP57380 inhibits BS125 and LN319 cell growth and sensitizes GBM cells to rapamycin. | SMAD2-dependent TGF-β signaling pathway and vimentin expression are suppressed by CGP57380 | [53] | |
| MNK inhibitor CGP57380 and RAD001 | CGP57380 and RAD001 profoundly inhibit proliferation in U373, LN229, and U87MG cells and reduce tumor growth in U87MG-luc glioma cells xenograft mouse model | CGP57380 suppresses eIF4E phosphorylation and increases 4EBP1 binding to eIF4E combined with RAD001 | [97] | |
| MNK inhibitor merestinib | Merestinib inhibited growth of 83Mes, MD30, and GBM43 cells and improved overall survival in the U87 cell xenograft mouse model | Merestinib blocks phosphorylation of eIF4E in established GBM cell lines and patient-derived glioma stem cells (GSCs) | [98] | |
| MNK inhibitor CGP57380 and temozolomide (TMZ) | CGP57380 sensitive U373, LN229 cells to chemotherapeutic drugs TMZ | CGP57380 reduces eIF4E phosphorylation and induces association of inactive MNK1 with eIF4G1 | [99] | |
| MNK inhibitor CGP57380 or MNK1/2 siRNAs and arsenic trioxide (ATO) | CGP57380 sensitizes 83Mes cells to arsenic trioxide in neurosphere and apoptosis assays | CGP57380 suppresses MNK activation and eIF4E phosphorylation, which are activated by ATO | [100] | |
| Medulloblastoma | MNK inhibitor CGP57380 | CGP57380 and PI3Kα significantly reduce tumor formation and promote survival in subcutaneous and intracranial mouse D283 cell xenograft models | CGP57380 enhances the antineoplastic effects of PI3Kα inhibition by inhibiting MNK | [101] |
| Neurofibromin 1-mutant (NF1-mutant) cancers | Cabozantinib and MEK inhibitor PD-0325901 (PD901) | Cabozantinib cooperates with PD901 induces tumor regression in C57BL/6-Trp53tm1Tyj Nf1tm1Tyj (NPcis) mice | Cabozantinib suppresses eIF4ES209 phosphorylation in malignant peripheral nerve sheath tumors (MPNSTs) at even lower concentrations than CGP57380 | [102] |
| Multiple myeloma (MM) | MNK inhibitor CGP57380; Serine to alanine mutation at eIF4E serine 209 | CGP57380 prevents IL-6-induced stimulation of growth of ANBL-6 and 8226 MM cell lines; CGP57380 or curtailing eIF-4E phosphorylation with the phosphomutant will prevent MM growth in 8226 cell xenograft mice | CGP57380 or curtailing eIF-4E phosphorylation inhibit IL-6-induced MM cell expansion and gene expression involved in metabolic and proteotoxic responses | [103] |
| Nasopharyngeal Carcinoma | MNK inhibitor CGP57380 | CGP57380 decreases proliferation, cell cycle progression, migration, invasion, and metastasis in NP69, CNE1, HNE1, HNE2, 5–8F, and 6–10B cells and CNE1 cells xenograft mice | GP57380 downregulates β-catenin in the nucleus | [45] |
| Thyroid cancer | MNK inhibitors CGP57380, cercosporamide and cisplatin | MNK inhibitors inhibit proliferation and induces apoptosis of ATC cells and enhances the effects of cisplatin in in vitro and in vivo | MNK inhibitors enhance the efficacy of cisplatin by inhibiting cisplatin-induced eIF4E phosphorylation. | [104] |
| MNK inhibitor CGP57380 and MNK1/2 siRNA, BET inhibitors (BETis) | CGP57380 and MNK1/2 siRNA potentiate the effects of BETis at suppressing proliferation in K1, RO82-w-1, and FTC-133 cell lines and suppressing tumor growth in TBP-3868 thyroid cancer cells implanted mice | CGP57380 and MNK1/2 siRNA enhance the efficacy of BETis in suppressing proliferation of cancer cells in vitro and in a syngeneic mouse model by inhibiting MNKs | [105] |
4.1. Breast cancer
eIF4E phosphorylation is overexpressed in many types of breast cancers. Chrestensen et al. have shown that phosphorylation of eIF4E was increased in breast cancer cell lines with HER2 overexpression, and inhibition of MNKs by CGP57380 reduced proliferation of these cell lines [63]. Robichaud et al. have reported that eIF4ESer209A mice are resistant to lung metastasis of breast cancer, and cells isolated from these mice exhibit impaired invasiveness [31]. The same group has also found that eIF4E phosphorylation can promote neutrophil survival and accumulation, thus facilitating metastasis to the lung in a mouse model of breast cancer [32]. These studies point to a key role for the MNKs in the events underlying metastasis and indicate that a small molecule targeting MNKs can be used for treating breast cancer metastasis. Interestingly, as MNK signaling is located downstream of EGFR/HER2, it can promote XIAP expression and NFKB activity when activated in the inflammatory breast cancer [68]. Taken together, MNKs inhibition can be a promising way for breast cancer treatment.
4.2. Prostate cancer
PTEN is a tumor suppressor that is frequently mutated in the prostate cancer (PCa). In PCa cells with intact PTEN expression, the PI3K/AKT/mTOR pathway is effectively suppressed, while the MNK-dependent phosphorylation of eIF4E is elevated. Elevated levels of phosphorylated eIF4E are associated with prostate cancer progression in human patients [30]. Accordingly, the eIF4E Ser209A mice are resistant to tumorigenesis in a prostate cancer model[30]. D’Abronzo et al. [79] have found that eIF4E phosphorylation in localized PCa samples strongly correlates with the expression of Ki67, a marker of cell proliferation. Inhibition of MNK and eIF4E phosphorylation are more effective than rapamycin in suppressing proliferation of PTEN-expressing cells. Conversely, in PTEN mutated PCa cells, the PI3K/AKT/mTOR pathway is constitutively active and the level of eIF4E phosphorylation is low. When PTEN is absent in the prostate, it leads to early onset of prostatic intraepithelial neoplasia (PIN) and invasive cancer [106]. Inhibition of mTOR by rapamycin can induce eIF4E phosphorylation. Concomitant treatment with MNK inhibitors, therefore, demonstrate additive effects on inhibition of protein synthesis and cell cycle progression [107]. Together, these results support a role for eIF4E phosphorylation in the progression of prostate cancer.
4.3. Leukemia
An increasing body of evidence supports that dysregulation of the MNK-eIF4E signaling pathway is involved in hematologic malignancies. MNK1 activity is induced by several AML fusion genes and plays an important role in myeloid differentiation [44]. Suppressing the MNK-eIF4E axis by CGP57380 can inhibit the function of blast crisis leukemia stem cells (BC LSCs) by affecting the production of β-catenin without affecting the self-renewal ability of hematopoietic stem cells [44, 82]. Additionally, The MNK inhibitor cercosporamide has been shown to suppress MV4-11 AML xenograft tumor growth [83]. The MNK inhibitors therefore deserve further development and clinical evaluation in the treatment of leukemia.
4.4. Lymphoma
Several important discoveries about the role of eIF4E in tumor biology came from studies of lymphoma models. The transcription factor c-Myc is correlated with B-cell lymphomagenesis, and transgenic mice overexpressing Myc under the control of immunoglobulin heavy chain enhancer transcription factor (Eμ-Myc) develop B-cell lymphoma on average 3–4 months. By intercrossing βT-Eif4e and Eμ-Myc transgenic mouse lines, Ruggero et al. show that the onset of lymphoma was significantly accelerated and was evident at less than 1 month of age [108]. The anti-apoptotic protein Mcl-1 is a translational target of phosphorylated eIF4E and contributes to lymphomagenesis [49]. Accordingly, in the Eμ-Myc model, phosphorylated eIF4E promotes tumorigenesis by inhibiting apoptosis. Marzec et al. [27] employed a combination of the mTORC1 inhibitor rapamycin and an MNK inhibitor 4-Amino-5-(4-fluoroanilino)-pyrazolo[3,4-d]pyrimidine to treat T-cell lymphoma (CTCL) cells. The combined therapy results in markedly increased suppression of proliferation and more cell apoptosis compared with the treatment with rapamycin alone.
4.5. Pancreatic cancer
Pancreatic cancer is a highly malignant neoplasm. Studies have shown human pancreatic ductal adenocarcinoma (PDAC) tumors are associated with the aberrant activities of the MNK-eIF4E axis [93]. Pharmacological and genetic inhibition of MNKs can decrease growth and EMT of PDAC cells. Furthermore, it has been reported that the cytostatic effect of chemotherapeutic drugs can be synergistically enhanced by pharmacological or genetic inhibition of eIF4E phosphorylation in PDAC cells [92]. Repressing MNK kinases significantly inhibit Sox2-mediated tumor cell repopulation after radiotherapy [52]. Taken together, the MNK/eIF4E pathway represents a promising target to treat pancreatic cancer.
4.6. Brain cancer
Glioblastoma multiforme (GBM) is the most common and deadly brain tumor originating from glial cells. Individuals with grade IV astrocytoma have a median survival time of 17 weeks without treatments. Clinical evidence has demonstrated that the level of eIF4E phosphorylation is significantly elevated in astrocytoma compared with surrounding normal brain tissue [109–111]. Ueda et al. [50] have shown that knockdown of MNK1 in the human glioma cell line U87MG resulted in a significant reduction in tumor formation when injected into athymic nude mice. Grzmil et al. [53] have demonstrated that pharmacologically inhibiting MNK activity by CGP57380 or MNK1 knockdown can reduce GBM cell proliferation and colony formation. Microarray analysis of total RNA and polysomal RNA in MNK1-depleted GBM cells identified mRNAs involved in regulating the SMAD2-dependent TGF-β pathway. Furthermore, pharmacologic MNK inhibition targets mesenchymal glioma stem cells and prolongs survival in a mouse model of glioblastoma [98]. These studies indicate that the MNK/eIF4E axis represents a promising target in GBM treatment.
5. Clinical studies on eIF4E phosphorylation and MNKs in human cancer
High levels of eIF4E phosphorylation are correlated with poor clinical prognosis in human cancers. Clinical studies have found that eIF4E was significantly over-phosphorylated in a wide variety of human cancer tissues compared with the adjacent normal tissues [112]. Elevated levels of p-MNK1, p-eIF4E and p-p70S6K proteins are associated with tumor recurrence and poor prognosis in astrocytoma [110]. Overexpression of p-eIF4E and co-expression of p-MNK1, p-eIF4E and p-p70S6K proteins are inversely proportional to the overall survival of astrocytoma. Multivariate cox regression analysis further confirmed that overexpression of p-eIF4E and co-expression of p-MNK1, p-eIF4E and p-p70S6K proteins, regardless of age and WHO grade, were associated with poor prognosis of astrocytoma. Similarity, increased expression of eIF4E and phospho-eIF4E were found in melanoma[113]. By analyzing 149 specimens of melanocytic lesions from 114 patients, they found that phospho-eIF4E overexpression was highly associated with malignancy, metastatic potential and reduced survival.
The expression levels of p-MNK1 and p-eIF4E proteins in nasopharyngeal carcinoma (NPC) are significantly higher than those of non-cancerous nasopharyngeal epithelial proteins[114]. The RAS/MAPK pathway was also found hyperactivated in patients with undifferentiated pleomorphic sarcoma, therefore it can predict a higher risk of disease recurrence and impair overall survival[115]. MNK2 and p-eIF4E overexpression are observed in patients with non-small cell lung cancer and are correlated with proliferation, migration, invasion, and lower survival rates in patients with NSCLC [116]. Overall, it is suggested that overexpression of p-eIF4E can serve as an independent biomarker for unfavorable prognosis and is a potential therapeutic target in patients with different types of cancer.
One of the most important causes of death in cancer treatment is chemoresistance caused by continuous chemotherapy. eIF4E phosphorylation has been observed to be a common feature of advanced breast cancer patients and cell lines (including ERα positive lines and HER2, ERα and PR negative lines) and affects response to chemotherapy [67]. By comparing patient samples and all tested breast cancer cell lines, Li et al. found that all samples showed the lowest p-eIF4E levels before chemotherapy. Meanwhile, MNK kinase inhibitors CGP57380 and cercosporamide sensitize breast cancer cells to respond to chemotherapy in vitro and delay MCF7 and MDA-MB-231 tumor growth in vivo [67]. This indicates that eIF4E phosphorylation in breast cancer cells plays a role in cancer response to chemotherapy and increased eIF4E phosphorylation has significant implications in development of resistance to chemotherapeutic agents.
MNK single-nucleotide polymorphisms (SNPs) have been suggested as a predictive marker for response to chemotherapy in colorectal cancer patients. In a study by Berger et al. [117], MNK SNPs were analyzed in 567 patients with KRAS wild-type metastatic colorectal cancer (mCRC) in randomized phase III trials. AA genotype carriers of the MNK1 rs8602 single-nucleotide polymorphism had a shorter progression-free survival than those harboring any C. Additionally, AA carriers also had a decreased overall response rate than C carriers treated with chemotherapy. These results suggest that MNK1 rs8602 polymorphism may serve as a predictive marker in KRAS wild type mCRC patients and MNK1 may be a promising drug target for these patients.
6. Conclusions
In conclusion, phosphorylation of the cap-binding protein eIF4E is involved in a number of key biological processes in tumorigenesis and progression, including cell proliferation, transformation, apoptosis, tumor metastasis and angiogenesis. Clinical evidence supports that hyperphosphorylation of eIF4E is associated with poor prognosis of patients with several cancer types. MNK inhibitors and eIF4E Ser209 mutation can significantly suppress tumor proliferation and metastasis in vivo and in vitro in a variety of cancer models. MNK inhibition can significant enhance the efficacies of traditional chemotherapy and radiotherapy. As significant interplays exist between MNK and mTORC1 pathways, combined inhibition of these signaling pathways can be developed as novel therapeutic strategies. The development of novel, potent, and specific MNK inhibitors is important and deserves further investigation. Taken together, clinical use of small-molecule inhibitors targeting eIF4E phosphorylation to treat cancer is likely imminent.
Acknowledgements
We thank Dr. Robert Cormier for his critical reading of the manuscript. This study was supported by a National Institutes of Health Grant NS118026, a Faculty Start-Up Grant from the University of Minnesota Medical School, a Grant from University of Minnesota Foundation and a Grant from Whiteside Institute for Clinical Research to R.C., a Chinese National Science and Technology Major Project Grant (No. 2018ZX09711003) to W.Z.
Abbreviations
- eIF4E
eukaryotic translation initiation factor 4E
- MNK
mitogen activated protein kinase interacting protein kinase
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- PABP
poly(A) binding protein
- EMT
epithelial–mesenchymal transition
- PTEN
phosphatase and tensin homolog
- RSK
ribosomal s6 kinase
- MMP3
Matrix Metallopeptidase 3
- Mdm2
mouse double minute 2 homolog
- HDM2
human homolog of double minute 2
- PIN
prostatic intraepithelial neoplasia
- Bcl-2
B-cell lymphoma 2
- ODC
ornithine decarboxylase
- ASD
autism spectrum disorder
- AML
adult acute myeloid leukemia
- CML
chronic myelogenous leukemia
- TGFβ
transforming growth factor-beta
- SMAD2
mothers against decapentaplegic homolog 2
- VEGF
vascular endothelial growth factor
- FGF-2
fibroblast growth factor 2
- Sox2
SRY (sex determining region Y)-box2
- XIAP
X-linked inhibitor of apoptosis protein
- HCC
hepatocellular carcinoma
- GBM
glioblastoma multiforme
- PCa
prostate cancer
- NPC
nasopharyngeal carcinoma
- UPS
undifferentiated pleomorphic sarcoma
Footnotes
Disclosure statement
The content of this review article represents the collective contributions of the listed authors. None of the authors have commercial interests of any kind to declare that are of relevance to the contents of the article.
References
- 1.Lane N and Martin W, The energetics of genome complexity. Nature, 2010. 467(7318): p. 929–34. [DOI] [PubMed] [Google Scholar]
- 2.Sonenberg N and Hinnebusch AG, Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell, 2009. 136(4): p. 731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqui N and Sonenberg N, Signalling to eIF4E in cancer. Biochem Soc Trans, 2015. 43(5): p. 763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shatkin AJ, mRNA cap binding proteins: essential factors for initiating translation. Cell, 1985. 40(2): p. 223–4. [DOI] [PubMed] [Google Scholar]
- 5.Marcotrigiano J, et al. , Cocrystal structure of the messenger RNA 5’ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell, 1997. 89(6): p. 951–61. [DOI] [PubMed] [Google Scholar]
- 6.Groppo R and Richter JD, Translational control from head to tail. Curr Opin Cell Biol, 2009. 21(3): p. 444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svitkin YV and Sonenberg N, [Translational control by the poly(A) binding protein: a check for mRNA integrity]. Mol Biol (Mosk), 2006. 40(4): p. 684–93. [PubMed] [Google Scholar]
- 8.Kahvejian A, et al. , Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev, 2005. 19(1): p. 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinnebusch AG, The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem, 2014. 83: p. 779–812. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman RJ, Control of gene expression at the level of translation initiation. Curr Opin Biotechnol, 1994. 5(5): p. 550–7. [DOI] [PubMed] [Google Scholar]
- 11.Sonenberg N, eIF4E, the mRNA cap-binding protein: from basic discovery to translational research. Biochem Cell Biol, 2008. 86(2): p. 178–83. [DOI] [PubMed] [Google Scholar]
- 12.Raught B and Gingras AC, eIF4E Activity Is Regulated at Multiple Levels. Int J Biochem Cell Biol, 1999. 31(1): p. 43–57. [DOI] [PubMed] [Google Scholar]
- 13.Pause A, et al. , Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5’-cap function. Nature, 1994. 371(6500): p. 762–7. [DOI] [PubMed] [Google Scholar]
- 14.Gingras AC, Raught B, and Sonenberg N, eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem, 1999. 68: p. 913–63. [DOI] [PubMed] [Google Scholar]
- 15.Proud CG, Mnks, eIF4E phosphorylation and cancer. Biochim Biophys Acta, 2015. 1849(7): p. 766–73. [DOI] [PubMed] [Google Scholar]
- 16.Roux PP and Topisirovic I, Signaling Pathways Involved in the Regulation of mRNA Translation. Mol Cell Biol, 2018. 38(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bramham CR, Jensen KB, and Proud CG, Tuning Specific Translation in Cancer Metastasis and Synaptic Memory: Control at the MNK-eIF4E Axis. Trends Biochem Sci, 2016. 41(10): p. 847–858. [DOI] [PubMed] [Google Scholar]
- 18.Waskiewicz AJ, et al. , Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol, 1999. 19(3): p. 1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukunaga R and Hunter T, MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J, 1997. 16(8): p. 1921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waskiewicz AJ, et al. , Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J, 1997. 16(8): p. 1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyronnet S, et al. , Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J, 1999. 18(1): p. 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda T, et al. , Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol, 2004. 24(15): p. 6539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxton RA and Sabatini DM, mTOR Signaling in Growth, Metabolism, and Disease. Cell, 2017. 169(2): p. 361–371. [DOI] [PubMed] [Google Scholar]
- 24.Hara K, et al. , Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell, 2002. 110(2): p. 177–89. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, et al. , mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell, 2002. 110(2): p. 163–75. [DOI] [PubMed] [Google Scholar]
- 26.Brown MC and Gromeier M, MNK Controls mTORC1:Substrate Association through Regulation of TELO2 Binding with mTORC1. Cell Rep, 2017. 18(6): p. 1444–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzec M, et al. , Simultaneous inhibition of mTOR-containing complex 1 (mTORC1) and MNK induces apoptosis of cutaneous T-cell lymphoma (CTCL) cells. PLoS One, 2011. 6(9): p. e24849. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Scheper GC, et al. , Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem, 2002. 277(5): p. 3303–9. [DOI] [PubMed] [Google Scholar]
- 29.Zuberek J, et al. , Phosphorylation of eIF4E attenuates its interaction with mRNA 5’ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA, 2003. 9(1): p. 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furic L, et al. , eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A, 2010. 107(32): p. 14134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robichaud N, et al. , Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3. Oncogene, 2015. 34(16): p. 2032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robichaud N, et al. , Translational control in the tumor microenvironment promotes lung metastasis: Phosphorylation of eIF4E in neutrophils. Proc Natl Acad Sci U S A, 2018. 115(10): p. E2202–E2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topisirovic I, Ruiz-Gutierrez M, and Borden KL, Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res, 2004. 64(23): p. 8639–42. [DOI] [PubMed] [Google Scholar]
- 34.Phillips A and Blaydes JP, MNK1 and EIF4E are downstream effectors of MEKs in the regulation of the nuclear export of HDM2 mRNA. Oncogene, 2008. 27(11): p. 1645–9. [DOI] [PubMed] [Google Scholar]
- 35.Aguilar-Valles A, et al. , Translational control of depression-like behavior via phosphorylation of eukaryotic translation initiation factor 4E. Nat Commun, 2018. 9(1): p. 2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amorim IS, et al. , Loss of eIF4E Phosphorylation Engenders Depression-like Behaviors via Selective mRNA Translation. J Neurosci, 2018. 38(8): p. 2118–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moy JK, et al. , The MNK-eIF4E Signaling Axis Contributes to Injury-Induced Nociceptive Plasticity and the Development of Chronic Pain. J Neurosci, 2017. 37(31): p. 7481–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moy JK, et al. , eIF4E phosphorylation regulates ongoing pain, independently of inflammation, and hyperalgesic priming in the mouse CFA model. Neurobiol Pain, 2018. 4: p. 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao R, et al. , Light-regulated translational control of circadian behavior by eIF4E phosphorylation. Nat Neurosci, 2015. 18(6): p. 855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmer SG, DeBenedetti A, and Graff JR, Translational control of malignancy: the mRNA cap-binding protein, eIF-4E, as a central regulator of tumor formation, growth, invasion and metastasis. Anticancer Res, 2000. 20(3A): p. 1343–51. [PubMed] [Google Scholar]
- 41.Baldin V, et al. , Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev, 1993. 7(5): p. 812–21. [DOI] [PubMed] [Google Scholar]
- 42.Morrish F and Hockenbery D, Myc’s mastery of mitochondrial mischief. Cell Cycle, 2003. 2(1): p. 11–3. [DOI] [PubMed] [Google Scholar]
- 43.Wheater MJ, Johnson PW, and Blaydes JP, The role of MNK proteins and eIF4E phosphorylation in breast cancer cell proliferation and survival. Cancer Biol Ther, 2010. 10(7): p. 728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Worch J, et al. , The serine-threonine kinase MNK1 is post-translationally stabilized by PML-RARalpha and regulates differentiation of hematopoietic cells. Oncogene, 2004. 23(57): p. 9162–72. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, et al. , Suppression Of beta-catenin Nuclear Translocation By CGP57380 Decelerates Poor Progression And Potentiates Radiation-Induced Apoptosis in Nasopharyngeal Carcinoma. Theranostics, 2017. 7(7): p. 2134–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazaris-Karatzas A, Montine KS, and Sonenberg N, Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5’ cap. Nature, 1990. 345(6275): p. 544–7. [DOI] [PubMed] [Google Scholar]
- 47.Rinker-Schaeffer CW, et al. , Decreasing the level of translation initiation factor 4E with antisense RNA causes reversal of ras-mediated transformation and tumorigenesis of cloned rat embryo fibroblasts. Int J Cancer, 1993. 55(5): p. 841–7. [DOI] [PubMed] [Google Scholar]
- 48.Frederickson RM, Montine KS, and Sonenberg N, Phosphorylation of eukaryotic translation initiation factor 4E is increased in Src-transformed cell lines. Mol Cell Biol, 1991. 11(5): p. 2896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wendel HG, et al. , Dissecting eIF4E action in tumorigenesis. Genes Dev, 2007. 21(24): p. 3232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda T, et al. , Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci U S A, 2010. 107(32): p. 13984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polunovsky VA, et al. , Translational control of the antiapoptotic function of Ras. J Biol Chem, 2000. 275(32): p. 24776–80. [DOI] [PubMed] [Google Scholar]
- 52.Yu Y, et al. , eIF4E-phosphorylation-mediated Sox2 upregulation promotes pancreatic tumor cell repopulation after irradiation. Cancer Lett, 2016. 375(1): p. 31–38. [DOI] [PubMed] [Google Scholar]
- 53.Grzmil M, et al. , MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-beta signaling pathway in human glioblastoma. Cancer Res, 2011. 71(6): p. 2392–402. [DOI] [PubMed] [Google Scholar]
- 54.Kevil CG, et al. , Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: implications for tumor angiogenesis. Int J Cancer, 1996. 65(6): p. 785–90. [DOI] [PubMed] [Google Scholar]
- 55.Kevil C, et al. , Translational enhancement of FGF-2 by eIF-4 factors, and alternate utilization of CUG and AUG codons for translation initiation. Oncogene, 1995. 11(11): p. 2339–48. [PubMed] [Google Scholar]
- 56.Liu Y, et al. , Inhibition of eukaryotic initiation factor 4E phosphorylation by cercosporamide selectively suppresses angiogenesis, growth and survival of human hepatocellular carcinoma. Biomed Pharmacother, 2016. 84: p. 237–243. [DOI] [PubMed] [Google Scholar]
- 57.Feng X, et al. , Dying glioma cells establish a proangiogenic microenvironment through a caspase 3 dependent mechanism. Cancer Lett, 2017. 385: p. 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knauf U, Tschopp C, and Gram H, Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol, 2001. 21(16): p. 5500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bain J, et al. , The selectivity of protein kinase inhibitors: a further update. Biochem J, 2007. 408(3): p. 297–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M, et al. , Inhibition of polysome assembly enhances imatinib activity against chronic myelogenous leukemia and overcomes imatinib resistance. Mol Cell Biol, 2008. 28(20): p. 6496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konicek BW, et al. , Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res, 2011. 71(5): p. 1849–57. [DOI] [PubMed] [Google Scholar]
- 62.Diab S, et al. , Discovery of 5-(2-(phenylamino)pyrimidin-4-yl)thiazol-2(3H)-one derivatives as potent Mnk2 inhibitors: synthesis, SAR analysis and biological evaluation. ChemMedChem, 2014. 9(5): p. 962–72. [DOI] [PubMed] [Google Scholar]
- 63.Chrestensen CA, et al. , MNK1 and MNK2 regulation in HER2-overexpressing breast cancer lines. J Biol Chem, 2007. 282(7): p. 4243–52. [DOI] [PubMed] [Google Scholar]
- 64.Pons B, et al. , The effect of p-4E-BP1 and p-eIF4E on cell proliferation in a breast cancer model. Int J Oncol, 2011. 39(5): p. 1337–45. [DOI] [PubMed] [Google Scholar]
- 65.Ramalingam S, et al. , First MNKs degrading agents block phosphorylation of eIF4E, induce apoptosis, inhibit cell growth, migration and invasion in triple negative and Her2-overexpressing breast cancer cell lines. Oncotarget, 2014. 5(2): p. 530–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geter PA, et al. , Hyperactive mTOR and MNK1 phosphorylation of eIF4E confer tamoxifen resistance and estrogen independence through selective mRNA translation reprogramming. Genes Dev, 2017. 31(22): p. 2235–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z, et al. , Inhibiting the MNK-eIF4E-beta-catenin axis increases the responsiveness of aggressive breast cancer cells to chemotherapy. Oncotarget, 2017. 8(2): p. 2906–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans MK, et al. , XIAP Regulation by MNK Links MAPK and NFkappaB Signaling to Determine an Aggressive Breast Cancer Phenotype. Cancer Res, 2018. 78(7): p. 1726–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lineham E, et al. , Synergistic effects of inhibiting the MNK-eIF4E and PI3K/AKT/ mTOR pathways on cell migration in MDA-MB-231 cells. Oncotarget, 2018. 9(18): p. 14148–14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sansook S, et al. , Probing the Anticancer Action of Novel Ferrocene Analogues of MNK Inhibitors. Molecules, 2018. 23(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramalingam S, et al. , The Novel Mnk1/2 Degrader and Apoptosis Inducer VNLG-152 Potently Inhibits TNBC Tumor Growth and Metastasis. Cancers (Basel), 2019. 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin X, et al. , Design, synthesis and activity of Mnk1 and Mnk2 selective inhibitors containing thieno[2,3-d]pyrimidine scaffold. Eur J Med Chem, 2019. 162: p. 735–751. [DOI] [PubMed] [Google Scholar]
- 73.Hou S, et al. , Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol, 2017. 19(9): p. 1107–1116. [DOI] [PubMed] [Google Scholar]
- 74.Liu S, Zha J, and Lei M, Inhibiting ERK/Mnk/eIF4E broadly sensitizes ovarian cancer response to chemotherapy. Clin Transl Oncol, 2018. 20(3): p. 374–381. [DOI] [PubMed] [Google Scholar]
- 75.Zhang W, et al. , Inhibiting MNK Selectively Targets Cervical Cancer via Suppressing eIF4E-Mediated beta-Catenin Activation. Am J Med Sci, 2019. 358(3): p. 227–234. [DOI] [PubMed] [Google Scholar]
- 76.Ramamurthy VP, et al. , Simultaneous targeting of androgen receptor (AR) and MAPK-interacting kinases (MNKs) by novel retinamides inhibits growth of human prostate cancer cell lines. Oncotarget, 2015. 6(5): p. 3195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mbatia HW, et al. , Novel C-4 heteroaryl 13-cis-retinamide Mnk/AR degrading agents inhibit cell proliferation and migration and induce apoptosis in human breast and prostate cancer cells and suppress growth of MDA-MB-231 human breast and CWR22Rv1 human prostate tumor xenografts in mice. J Med Chem, 2015. 58(4): p. 1900–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwegyir-Afful AK, et al. , Galeterone and VNPT55 disrupt Mnk-eIF4E to inhibit prostate cancer cell migration and invasion. FEBS J, 2016. 283(21): p. 3898–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Abronzo LS, et al. , The androgen receptor is a negative regulator of eIF4E phosphorylation at S209: implications for the use of mTOR inhibitors in advanced prostate cancer. Oncogene, 2017. 36(46): p. 6359–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramamurthy VP, et al. , The retinamide VNLG-152 inhibits f-AR/AR-V7 and MNK-eIF4E signaling pathways to suppress EMT and castration-resistant prostate cancer xenograft growth. FEBS J, 2018. 285(6): p. 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Altman JK, et al. , Negative regulatory effects of Mnk kinases in the generation of chemotherapy-induced antileukemic responses. Mol Pharmacol, 2010. 78(4): p. 778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim S, et al. , Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci U S A, 2013. 110(25): p. E2298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altman JK, et al. , Inhibition of Mnk kinase activity by cercosporamide and suppressive effects on acute myeloid leukemia precursors. Blood, 2013. 121(18): p. 3675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teo T, et al. , Pharmacologic Inhibition of MNKs in Acute Myeloid Leukemia. Mol Pharmacol, 2015. 88(2): p. 380–9. [DOI] [PubMed] [Google Scholar]
- 85.Shi F, et al. , Ribavirin Inhibits the Activity of mTOR/eIF4E, ERK/Mnk1/eIF4E Signaling Pathway and Synergizes with Tyrosine Kinase Inhibitor Imatinib to Impair Bcr-Abl Mediated Proliferation and Apoptosis in Ph+ Leukemia. PLoS One, 2015. 10(8): p. e0136746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li P, et al. , Inhibition of Mnk enhances apoptotic activity of cytarabine in acute myeloid leukemia cells. Oncotarget, 2016. 7(35): p. 56811–56825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kosciuczuk EM, et al. , Merestinib blocks Mnk kinase activity in acute myeloid leukemia progenitors and exhibits antileukemic effects in vitro and in vivo. Blood, 2016. 128(3): p. 410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Z, et al. , Anthelmintic drug niclosamide enhances the sensitivity of chronic myeloid leukemia cells to dasatinib through inhibiting Erk/Mnk1/eIF4E pathway. Biochem Biophys Res Commun, 2016. 478(2): p. 893–9. [DOI] [PubMed] [Google Scholar]
- 89.Huang XB, et al. , MNK1 inhibitor CGP57380 overcomes mTOR inhibitor-induced activation of eIF4E: the mechanism of synergic killing of human T-ALL cells. Acta Pharmacol Sin, 2018. 39(12): p. 1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abdelaziz AM, et al. , Discovery of N-Phenyl-4-(1H-pyrrol-3-yl)pyrimidin-2-amine Derivatives as Potent Mnk2 Inhibitors: Design, Synthesis, SAR Analysis, and Evaluation of in vitro Anti-leukaemic Activity. Med Chem, 2019. 15(6): p. 602–623. [DOI] [PubMed] [Google Scholar]
- 91.Muta D, et al. , Inhibition of eIF4E phosphorylation reduces cell growth and proliferation in primary central nervous system lymphoma cells. J Neurooncol, 2011. 101(1): p. 33–9. [DOI] [PubMed] [Google Scholar]
- 92.Adesso L, et al. , Gemcitabine triggers a pro-survival response in pancreatic cancer cells through activation of the MNK2/eIF4E pathway. Oncogene, 2013. 32(23): p. 2848–57. [DOI] [PubMed] [Google Scholar]
- 93.Kumar K, et al. , Differential Regulation of ZEB1 and EMT by MAPK-Interacting Protein Kinases (MNK) and eIF4E in Pancreatic Cancer. Mol Cancer Res, 2016. 14(2): p. 216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kwegyir-Afful AK, et al. , Galeterone and its analogs inhibit Mnk-eIF4E axis, synergize with gemcitabine, impede pancreatic cancer cell migration, invasion and proliferation and inhibit tumor growth in mice. Oncotarget, 2017. 8(32): p. 52381–52402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wen Q, et al. , CGP57380 enhances efficacy of RAD001 in non-small cell lung cancer through abrogating mTOR inhibition-induced phosphorylation of eIF4E and activating mitochondrial apoptotic pathway. Oncotarget, 2016. 7(19): p. 27787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J, et al. , Antibiotic drug rifabutin is effective against lung cancer cells by targeting the eIF4E-beta-catenin axis. Biochem Biophys Res Commun, 2016. 472(2): p. 299–305. [DOI] [PubMed] [Google Scholar]
- 97.Grzmil M, et al. , MNK1 pathway activity maintains protein synthesis in rapalog-treated gliomas. J Clin Invest, 2014. 124(2): p. 742–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bell JB, et al. , MNK Inhibition Disrupts Mesenchymal Glioma Stem Cells and Prolongs Survival in a Mouse Model of Glioblastoma. Mol Cancer Res, 2016. 14(10): p. 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grzmil M, et al. , Inhibition of MNK pathways enhances cancer cell response to chemotherapy with temozolomide and targeted radionuclide therapy. Cell Signal, 2016. 28(9): p. 1412–21. [DOI] [PubMed] [Google Scholar]
- 100.Bell JB, et al. , Differential Response of Glioma Stem Cells to Arsenic Trioxide Therapy Is Regulated by MNK1 and mRNA Translation. Mol Cancer Res, 2018. 16(1): p. 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eckerdt F, et al. , Potent Antineoplastic Effects of Combined PI3Kalpha-MNK Inhibition in Medulloblastoma. Mol Cancer Res, 2019. 17(6): p. 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lock R, et al. , Cotargeting MNK and MEK kinases induces the regression of NF1-mutant cancers. J Clin Invest, 2016. 126(6): p. 2181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi Y, et al. , MNK1-induced eIF-4E phosphorylation in myeloma cells: a pathway mediating IL-6-induced expansion and expression of genes involved in metabolic and proteotoxic responses. PLoS One, 2014. 9(4): p. e94011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Hu K, et al. , Inhibition of Mnk-eIF4E pathway sensitizes the efficacy to chemotherapy in anaplastic thyroid cancer. Future Oncol, 2017. 13(6): p. 489–498. [DOI] [PubMed] [Google Scholar]
- 105.Pham TND, et al. , Induction of MNK Kinase-dependent eIF4E Phosphorylation by Inhibitors Targeting BET Proteins Limits Efficacy of BET Inhibitors. Mol Cancer Ther, 2019. 18(2): p. 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trotman LC, et al. , Pten dose dictates cancer progression in the prostate. PLoS Biol, 2003. 1(3): p. E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bianchini A, et al. , Phosphorylation of eIF4E by MNKs supports protein synthesis, cell cycle progression and proliferation in prostate cancer cells. Carcinogenesis, 2008. 29(12): p. 2279–88. [DOI] [PubMed] [Google Scholar]
- 108.Ruggero D, et al. , The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med, 2004. 10(5): p. 484–6. [DOI] [PubMed] [Google Scholar]
- 109.Martinez-Saez E, et al. , peIF4E as an independent prognostic factor and a potential therapeutic target in diffuse infiltrating astrocytomas. Cancer Med, 2016. 5(9): p. 2501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan W, et al. , Elevated levels of p-Mnk1, p-eIF4E and p-p70S6K proteins are associated with tumor recurrence and poor prognosis in astrocytomas. J Neurooncol, 2017. 131(3): p. 485–493. [DOI] [PubMed] [Google Scholar]
- 111.Bredel M, et al. , High-resolution genome-wide mapping of genetic alterations in human glial brain tumors. Cancer Res, 2005. 65(10): p. 4088–96. [DOI] [PubMed] [Google Scholar]
- 112.Fan S, et al. , Phosphorylated eukaryotic translation initiation factor 4 (eIF4E) is elevated in human cancer tissues. Cancer Biol Ther, 2009. 8(15): p. 1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carter JH, et al. , Phosphorylation of eIF4E serine 209 is associated with tumour progression and reduced survival in malignant melanoma. Br J Cancer, 2016. 114(4): p. 444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zheng J, et al. , Phosphorylated Mnk1 and eIF4E are associated with lymph node metastasis and poor prognosis of nasopharyngeal carcinoma. PLoS One, 2014. 9(2): p. e89220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Serrano C, et al. , RAS/MAPK pathway hyperactivation determines poor prognosis in undifferentiated pleomorphic sarcomas. Cancer, 2016. 122(1): p. 99–107. [DOI] [PubMed] [Google Scholar]
- 116.Guo Z, et al. , MAP kinase-interacting serine/threonine kinase 2 promotes proliferation, metastasis, and predicts poor prognosis in non-small cell lung cancer. Sci Rep, 2017. 7(1): p. 10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berger MD, et al. , Impact of Genetic Variations in the MAPK Signaling Pathway on Outcome in Metastatic Colorectal Cancer Patients Treated With First-Line FOLFIRI and Bevacizumab: Data From FIRE-3 and TRIBE Trials. Ann Oncol, 2017. 28(11): p. 2780–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]


