Abstract
Several antiviral peptides (AVPs) from aquatic organisms have been effective in interfering with the actions of infectious viruses, such as Human Immunodeficiency Virus-1 and Herpes Simplex Virus-1 and 2. AVPs are able to block viral attachment or entry into host cells, inhibit internal fusion or replication events by suppressing viral gene transcription, and prevent viral infections by modulating host immunity. Therefore, as promising therapeutics, the potential of aquatic AVPs for use against the COVID-19 pandemic caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is considered. At present no therapeutic drugs are yet available. A total of 32 AVPs derived from fish and shellfish species are discussed in this review paper with notes on their properties and mechanisms of action in the inhibition of viral diseases both in humans and animals, emphasizing on SARS-CoV-2. The molecular structure of novel SARS-CoV-2 with its entry mechanisms, clinical signs and symptoms are also discussed. In spite of only a few study of these AVPs against SARS-CoV-2, aquatic AVPs properties and infection pathways (entry, replication and particle release) into coronaviruses are linked in this paper to postulate an analysis of their potential but unconfirmed actions to impair SARS-CoV-2 infection in humans.
Keywords: Antiviral peptides, Aquatic organism, COVID-19, SARS-CoV-2, Virus
1. Introduction
The global morbidity and mortality caused by viral infections are increasing incrementally, drawing attention to the importance of developing effective therapeutics against viruses (Barlow et al., 2014). Effective treatments are costly and require early and specific viral identification, requiring extensive processing time for vaccine development and testing of antiviral therapeutics (Falco et al., 2009; Findlay et al., 2013). The emergence of the new 2019 novel coronaviruses (nCoV) pandemic strain underscores the necessity of broader-spectrum antiviral drugs (Zhang Wen et al., 2020) and research efforts are underway to identify novel antiviral drugs or therapeutics (Gurwitz, 2020; Kuebutornye et al., 2020; Wang, Chen, H et al., 2020; Wang, Cheng, W et al., 2020; Wang, M et al., 2020; Wang, Y et al., 2020). Alternatively, antimicrobial peptides (AMPs) could have utility because of their wide range of activities against infectious viruses in both humans and animals.
AMP molecules composed of 20–50 amino acid residues, known as antiviral peptides (AVPs) (Boas et al., 2019), have emerged as new agents to combat viral infections (Boas et al., 2019; Qureshi et al., 2014; Skalickova et al., 2015). According to those reports, mechanisms of action of antiviral drugs are mainly of two types, i.e. virus targeting and host targeting that can act upon various transcription and replication related enzymes to destroy a viral pathogen or inactivate their infectiveness (Boas et al., 2019; Lou et al., 2014). Mechanisms of action AVPs against viruses include binding with specific viral subunits, blockage of viral entry into host cells, interaction with viral envelopes and modulation of host immune pathways. Small defensin peptides can inhibit infection by human immunodeficiency virus (HIV)-1 (Chang et al., 2005), influenza A virus (IAV) (Hartshorn et al., 2006), herpes simplex virus (HSV) (Hazrati et al., 2006), human adenovirus (HAdV) (Bastian and Schäfer, 2001) and human papillomavirus (Buck et al., 2006).
A number of potentially valuable AVPs have been characterized from fish, shellfish, marine sponge and other aquatic organisms. Recently, fish- and shellfish-derived AVPs have been found to have preventative actions against several fish infectious viruses such as viral haemorrhagic septicaemia virus (VHSV), Singapore grouper iridovirus (SGIV), nervous necrosis virus (NNV), white spot syndrome virus (WSSV) and others (Guo et al., 2012; Jin et al., 2010). Moreover, microspinosamide and Pa-MAP from sponge (Sidonops microspinosa) and winter flounder (Pleuronectes americanus) exhibited activities against HIV-1 (Rashid et al., 2001) and HSV-1 and 2 (Migliolo et al., 2012). AVPs like HR2P and mutant mucropin-M1 has gained recognition for their activities against Middle East respiratory syndrome (MERS)-CoV (Hilchie et al., 2013) and severe acute respiratory syndrome (SARS)-CoV (Li et al., 2011). In addition, two peptides (K12 and K29) derived from SARS-CoV non-structural protein nsp10 reportedly inhibit replication of SARS-CoV (Ke et al., 2012). Similarly, mouse β-defensin-4 demonstrated potent and broad-spectrum antiviral effects against multiple respiratory infectious viruses in vitro and in vivo (Zhao et al., 2016).
In late December 2019, a cluster of cases of pneumonia of uncertain aetiology was reported to China National Health Commission, subsequently leading to the discovery of a new coronavirus in 07 January 2020 from patients in Wuhan (Chen Wang et al., 2020). The World Health Organization (WHO) consequently named this infection as novel coronaviruses disease 2019 (COVID-19) on 11 February 2020 and declared it a pandemic on 11 March 2020 (Ul Qamar et al., 2020), after its spread to at least 219 countries and territories (WHO, 2020). Presently, the world is heavily impacted by and struggling to deal with SARS-CoV-2, which led to ⁓111 million confirmed cases and 2.5 million deaths as of 20th February 2021 (WHO, 2020). Different kind of activities like genome sequencing (Zhang and Holmes, 2020), trialling existing drugs and medicines e.g. remdisivir (Beigel et al., 2020), hydroxycholoroquine and azithromycin (Gautret et al., 2020), including drug suggestions by bioinformatics tools namely drug repurposing and molecular docking approach (Hasan et al., 2020; Parvez et al., 2020) were evaluated as potential treatments of COVID-19 pathology. At present no reports are available on the use of AVPs from aquatic organisms or any other sources against SARS-CoV-2 infection. However, as there are some AVPs that inhibit viruses similar to SARS-CoV-2, like SARS-CoV (Ke et al., 2012), MERS-CoV (Hilchie et al., 2013) and some other respiratory viruses (Zhao et al., 2016), which are also very common in the aquatic organisms like in fish, shellfish and even in aquatic plants. The present study discusses properties, history and actions of AVPs from aquatic organisms that are used against infectious viruses including CoVs in human and animals. In addition, the potential of aquatic AVPs for the inactivation and destruction of CoV-2 infection pathway in human is explored according to their mechanisms of action and history of interactions with similar types of viruses. This discussion on aquatic AVPs and their possible use against SARS-CoV-2 might illuminate the prospects of developing fisheries-based therapeutics for the treatment of COVID-19.
2. History of antiviral peptides
Nisin, a 34-residue peptide produced by the lactic acid bacterium Lactococcus lactis, was the first AMP reported in 1928 (Desriac et al., 2013). It contains unsaturated stereo-inverted amino acids and thioether bridges in the structure, as explained by Gross and Morell (1971). Another AMP, germicidin, isolated from soil bacterium Bacillus brevis, is active against gram-positive microorganisms in vivo (Dubos, 1939) and is among the first commercially produced antibiotics (Van Epps, 2006). Although, the discovery of AMPs from eukaryotes dated back to 1896 (Jago and Jago, 1926), AMP isolated from wheat (Triticum astivum) endosperm in 1942 and was found to be active against Pseudomonas solanacearum and Xanthomonas campestris (De Caleya et al., 1972). Another of the initially-discovered AMPs is melittin, a 26-non-modified residue peptide derived from the venom of European honeybee (Apis mellifera) (Fischer and Neumann, 1961). However, isolation and characterization of AMP gained momentum after the discovery of penicillin and streptomycin in 1943. To date, 123 peptides from human, 222 from mammals, 1057 from amphibians (Wang et al., 2016), and 122 from fish and shellfish have been characterized (Masso-Silva and Diamond, 2014).
Pardaxin is the first AMP characterized in fish, moses sole (Pardachirus marmoratus) found in the Red Sea (Primor and Tu, 1980). In 1997, a 25-residues amino acid peptide pleurocidin was identified from the secreted mucous of winter flounder (Cole et al., 1997) and another 21-residue amino acid containing AMP misgurin was characterized from mudfish (Misgurnus anguillicaudatus) with strong antimicrobial activity (Park et al., 1997). Some additional AMPs have been isolated from various fish, shellfish, sponge and other marine organisms, displaying antiviral properties either in vitro, in vivo or both. For instance, hepcidins from turbot (Scophthalmus maximus; Pereiro et al., 2012), medaka (Oryzias melastigmus; Cai et al., 2012); EcDefensin (Guo et al., 2012), β-defensin (Jin et al., 2010) from orange-spotted grouper (Epinephelus coioides), Scygonadin from green mud crab (Scylla paramamosain, Peng, Liu, Chen, Hao, & Wang, 2012), and microspinosamide from marine sponge (Rashid et al., 2001).
Despite the introduction of specific therapy and drugs for treating viral infections in 1946, AMPs are a promising alternative in the design of therapeutics to control viral diseases (Field and De Clercq, 2004). In the 1980s, only a few AVPs were reported from different sources. Of those, Didemnins are among the earliest AVPs identified, from the Caribbean tunicate Trididemnum; this compound is capable of inhibiting activities of DNA and RNA viruses in vitro and in leukemic cells (Rinehart et al., 1981). Ganz et al. (1985) identified a defensin from human neutrophils named HNP, which was effective inactivating HSV-1 directly (Ganz et al., 1985). Later, defensins (HNP-1, HNP-2 and HNP-3) were extensively tested and found to inactivate HSV-2, cytomegalovirus (CMV), vesicular stomatitis virus (VSV) and IAV (Daher et al., 1986). In the next decade, several AVPs were isolated from various sources, viz. tachyplesin (Morimoto et al., 1991) and polyphemusin (Nakashima et al., 1993) from horseshoe crab (Tachypleus tridentatus), PAP from pokeweed (Phytolacca americana; Chen et al., 1991) and magainin from the aquatic frog (Xenopus laevis, (Aboudy et al., 2009). The majority of the AVPs from fish and shellfish have been isolated in the 21st century. Among them, Pa-MAP was isolated from winter flounder and found interactive with viral envelope of HSV (Migliolo et al., 2012). In addition, Chia et al. (2010) described Epinecidin-1, tilapia hepcidin (TH) 1–5 from the euryhaline tilapia (Oreochromis mossambicus) and cyclic shrimp anti-lipopolysaccharide factor (cSALF) from tiger shrimp (Penaeus monodon), which induced clumping of fish NNV particle or virion. AVPs isolated from aquatic organisms are listed in Table 1 .
Table 1.
Properties and activities of AVPs from aquatic organisms effective against viruses.
| Peptide | Source | Target virus | Mechanisms of action | Reference |
|---|---|---|---|---|
| Tilapia hepcidin (TH) 1–5 | Tilapia (Oreochromis mossambicus) |
IPNV | Positive modulation of interleukin, annexin, and other anti-viral genes expressions | (Rajanbabu and Chen, 2011) |
| TH 1–5 | Tilapia | Grouper NNV | Agglutination of virions into clumps to inhibit their entry into host cells | (Chia et al., 2010) |
| TH 1–5 | Tilapia | JEV | Modulation of immune responsive gene expressions | (Huang et al., 2011) |
| Hepcidin 1–5 | Tilapia | NNV | Disruption or digestion of viral capsid proteins | (Wang et al., 2010) |
| Hepcidin-1 and hepcidin-2 | Turbot (Scophthalmus maximus) |
Megalocytivirus RBIV-C1 | Disruption of viral envelope or capsid | (Zhang, J et al., 2014; Zhang, M et al., 2014) |
| Hepcidin-1 and hepcidin-2 | Turbot | VHSV | Increase peptide in host body tissues to suppress viral infection | (Pereiro et al., 2012) |
| EC-hepcidin1 and EC-hepcidin2 | Orange-spotted grouper (Epinephelus coioides) | SGIV | Inhibition of viral replication | (Zhou et al., 2011) |
| Om-hep1 and Pro-omhep1 | Medaka (Oryzias melastigmus) | WSSV | Inhibition of viral replication | (Cai et al., 2012) |
| SA-hepcidin2 | Spotted scat (Scatophagus argus) | SCRV and MsReV | Not characterized | (Gui et al., 2016) |
| HAMP2-1/4 and HAMP2-3 | Large yellow croaker (Larimichthys crocea) | SGIV | Suppression of viral ORF072 and ORF086 gene expression; Inhibition of viral replication | (Mu et al., 2018) |
| omBD-1 | Rainbow trout (Oncorhynchus mykiss) | VHSV | Modulation of immunity by upregulating MX1 gene and IFN-related antiviral response | (Falco et al., 2008) |
| zfBD2 | Zebrafish (Danio rerio) |
SVCV | Modulation of immunity by upregulating IFN stimulated MX gene | (García-Valtanen et al., 2014) |
| EcDefensin | Orange-spotted grouper | SGIV and NNV | Modulation of immunity and suppression of viral genes expressions; Reduction of structural proteins (MCP and CP) accumulation; Inhibition of viral replication | (Guo et al., 2012) |
| Grouper β-defensin | Orange-spotted grouper | RGV | Not characterized | (Jin et al., 2010) |
| TroBD | Golden pompano (Trachinotus ovatus) | NNV | Not characterized | (Zhou et al., 2019) |
| Epinecidin-1 | Orange-spotted grouper | NNV | Reduction of virion spreading through aggregation | (Wang et al., 2010) |
| Piscidin 1 N, 1H, 2 and 3 | Hybrid striped bass (Morone saxatilis × Morone chrysops) | CCV and FV3 | Reduction of viral infectivity | (Chinchar et al., 2004) |
| TO24 | Red drum (Sciaenops ocellatus) | ISKNV | Not characterized | (He et al., 2018) |
| Pa-map | Winter flounder (Pleuronectes americanus) |
HSV | Disruption of viral envelope | (Migliolo et al., 2012) |
| MDPle | Common dab (Limanda limanda) | VHSV | Disruption of viral membrane | (Falco et al., 2009) |
| NKLP27 (CsNKL1 derived) | Tongue sole (Cynoglossus semilaevis) | Megalocytivirus RBIV-C1 | Inhibition of viral infection by upregulating expression of genes (TLR9 and Myd88) | (Zhang, M et al., 2014) |
| NK-lysin (NKl) | European bass, (Dicentrarchus labrax) | Nodavirus | Not characterized | (Valero et al., 2020) |
| Cyclic shrimp anti-lipopolysaccharide factor (cSALF) | Tiger Shrimp (Penaeus monodon) |
NNV | Modulation of gene (MyD88, Toll like receptor 4, and MX) expressions | (Chia et al., 2010) |
| PEN5 | Tiger Shrimp | WSSV | Increase the peptide load in WSSV infected shrimp to inhibit infection | (Woramongkolchai et al., 2011) |
| ALFpm3 (P. monodon anti-lipopolysaccharide factor 3) |
Tiger Shrimp | WSSV | Binds with the virus envelope protein WSSV189 | (Somboonwiwat et al., 2005) |
| rLvHcL48 | Pacific white shrimp (Litopenaeus vannamei) | WSSV | Suppression of viral wsv069 and wsv421 gene expression | (Zhan et al., 2019) |
| Mj-sty | Kuruma shrimp (Marsupenaeus japonicas) | WSSV | Modulation of post-infection immune response | (Liu et al., 2015) |
| LBD (AFLs domain) | Chinese shrimp (Fenneropenaeus chinensis) | WSSV | Inhibition of viral replication | (Li et al., 2015) |
| Scygonadin | Green mud crab (Scylla paramamosain) | WSSV | Inhibition of virus replication through reducing IE1 gene expression | (Peng et al., 2012) |
| SpALF1 and Sp-ALF2 | Green mud crab | WSSV | Inhibition of virus replication through reducing IE1 gene expression | (Liu et al., 2012) |
| rCqALF | Red claw crayfish, Cherax quadricarinatus |
WSSV | Disruption of virus envelop; Inhibition of virus replication through reducing IE1 gene expression | (Lin et al., 2016) |
| Microspinosamide | Marine sponge (Sidonops microspinosa) | HIV | Inhibition of cytopathic effect of HIV-1 infection | (Rashid et al., 2001) |
CCV: Channel catfish virus; FV3: Frog virus 3; GCRV: Grass carp reovirus; HIV: Human immunodeficiency virus; HSV: Herpes simplex virus; IPNV: Infectious pancreatic necrosis virus; ISKNV: Spleen and kidney necrosis virus; JEV: Japanese encephalitis virus; MsReV: Micropterus salmoides (largemouth bass) reovirus; NNV: Nervous necrosis virus; VHSV: Viral haemorrhagic septicaemia virus; RGV: Rana grylio virus; SGIV: Singapore grouper iridovirus; SCRV: Siniperca chuatsi rhabdovirus; SVCV: Spring viraemia of carp virus; WSSV: White spot syndrome virus.
Recently, AVPs have drawn attention for their contribution in combating SARS-CoV-2. Two peptides S2P25 and S2P26, which inhibited SARS-CoV-1 previously, were found through molecular dynamics simulation to have some promise of blocking the cellular entry of SARS-CoV-2 (Chowdhury et al., 2020). Other studies have predicted the efficacy of antiviral peptides against SARS-CoV-2 through computational approaches. For instance, Dermaseptin-s9 peptide showed the best affinity to the active site of SARS-CoV-2 macromolecule in protein-peptide docking simulation (Fakih, 2020). Similarly, Ling et al. (2020) designed antiviral peptides through in silico modelling seeking compounds capable of preventing SARS-CoV-2 membrane fusion, which could be used in the prevention and treatment of COVID-19 pandemic.
3. Properties of AVPs from aquatic organisms
AMPs are small, gene encoded, amphipathic peptides with molecular weight < 13 kDa, and majority of them are positively charged with a few known to be anionic in nature (Hancock and Sahl, 2006; Zasloff, 2002). Cationic AMPs typically possess 20–50 amino acid residues and are enriched with basic amino acids like lysine and arginine (Hancock, 2001). AMPs have demonstrable broad-spectrum antimicrobial abilities against bacteria, fungi, parasites, viruses and even tumor cells (Cuesta et al., 2008; Mihajlovic and Lazaridis, 2010). Since AMPs are evolutionarily preserved components of innate immunity, their presence in organisms ranging from prokaryotes to higher animals including aquatic organisms are well documented (Bowdish et al., 2005; Lai and Gallo, 2009). AMPs are a primary defensive weapon of fishes innate immune systems (Smith et al., 2010). Based on the structure, 122 AMPs are reported from fish into five families namely hepcidins, β-defensins, histone-derived peptides, cathelicidins and fish-specific piscidins (Wang et al., 2016), of which the first three were antiviral against certain viral infections in aquatic organisms and humans (Table 1).
Hepcidins are versatile cysteine rich cationic peptides first characterized in human liver and therefore known as liver expressed-antimicrobial peptide or LEAP (Park et al., 2001). Moreover, it is found in tissues of different vertebrates including fish, amphibians and reptiles, and acts as a regulator of iron absorption in the intestines (Nemeth, 2004; Park et al., 2001). However, a diverse group of hepcidins were identified in teleosts and the first one was reported from hybrid striped sea bass, Morona chrysops × M. saxatilis (Shike et al., 2002). Since then, hepcidins with antiviral properties have been identified in more than thirty different fish species. For instance, tilapia derived TH 1–5 showed antiviral activity against infectious pancreatic necrosis virus (IPNV) (Rajanbabu and Chen, 2011), NNV (Wang et al., 2010), grouper NNV (Chia et al., 2010) and Japanese encephalitis virus (JEV) (Huang et al., 2011). In addition, antiviral effects of TH 1–5 and epinecidin-1 against NNV of Japanese rice fish (Oryzias latipes) were noted in vivo (Table 1). Further verification through reverse transcriptase polymerase chain reaction (RT-PCR) confirmed down regulation of NNV and interferon gene expressions (Wang et al., 2010) similarly of TroBD isolated from Golden pompano (Trachinotus ovatus) (Zhou et al., 2019). Besides, TH 1–5 and cSALF peptide limited the viral entry into cells in vitro by agglutinating NNV virions into clump (Chia et al., 2010). Although different functions of most hepcidin peptides area as yet inconclusive, some other peptides, viz. hepcidin-1, 2 characterized from turbot showed inhibitory effects against megalocytivirus RBIV-C1 and VHSV (Pereiro et al., 2012; Zhang, J et al., 2014). Moreover, EC-hepcidin 1, 2 and HAMP2-1/4, 2–3 also inhibited SGIV infection (Mu et al., 2018; Zhou et al., 2011). Similarly, SA-hepcidin 2 isolated from spotted scat (Scatophagus argus) was inhibitory against rhabdovirus and reovirus (Gui et al., 2016). Study with synthesized peptides SmHep1P and SmHep2P of hepcidin 1, 2, showed significant reduction in megalocytivirus RBIV-C1 infections, being SmHep2P more effective than SmHep1P (Zhang, J et al., 2014). Further, NKLP27 from tongue sole (Cynoglossus semilaevis) inhibited megalocytivirus RBIV-C1 by upregulating expressions of TLR9 and Myd88 genes (Zhang, M et al., 2014).
Recently, two variants of hepcidine Om-hep1 and 2 have been isolated from medaka (O. melastigmas), and those transformed into synthetic mature Om-hep1 and recombinant pro-Omhep1 leading to a dramatic inhibitory activity against WSSV in the hematopoietic (Hpt) cells of crayfish (Cherax quadricarinatus; Cai et al., 2012). Besides, scygonadin, a novel crab antimicrobial peptide biologically produced in Pichia pastoris reportedly interferes with WSSV replication in vitro in Hpt cells of crayfish (Peng et al., 2012).
Defensins are cysteine-rich, 3–6 kDa cationic antimicrobial peptides acting as a first line defence against both enveloped and non-enveloped viruses predominantly found in plants, vertebrates and invertebrates (Bals et al., 1998; Bulet et al., 2004; Weinberg et al., 2006; Zhu, 2008). Defensins consist of α-helical and β-pleated sheet structures stabilized by disulphide bridges (Selsted et al., 1985), and broadly categorized into α, β and θ defensins subfamilies based on their intra-molecular disulphide bonds. Reported of the antiviral activity of defensins in mammalian hosts have detailed inhibitory effects against HIV-1, IAV, HSV-1, 2, HAdV, human papillomavirus, parainfluenza virus 3 (PIV-3), respiratory syncytial virus (RSV), vaccinia and chandipura viruses (Bastian and Schäfer, 2001; Buck et al., 2006; Chang et al., 2005; Chattopadhyay et al., 2006; Grubor et al., 2004; Hartshorn et al., 2006; Hazrati et al., 2006; Howell et al., 2007; Meyerholz et al., 2007; Zhang et al., 2002).
EcDefensin, a defensin peptide from the liver cells of orange-spotted grouper exhibited strong antiviral activity against enveloped DNA virus and non-enveloped RNA virus like SGIV and NNV (Guo et al., 2012). Similarly, β-defensin from grouper pituitary cDNA showed higher expression on both pituitary and testis. The antiviral effect revealed by β-defensin transfected EPC cells exhibited pivotal role of the peptide in regulation of both innate immunity and endocrine activity (Jin et al., 2010). Furthermore, β-defensin has demonstrated antiviral activity against frog-specific Rana grylio virus (RGV). Falco et al. (2008)confirmed antiviral activity of rainbow trout (Oncorhynchus mykiss) derived β -defensin through fish cells transfected with omBD-1 peptide against one the devastating rhabdorvirus VHSV. Interestingly, defensin was considered as an AMP in the early stages of research (Kagan et al., 1990), but subsequent studies revealed other vital roles of defensins such as cell signalling, immature dendritic cell recruitment to the place of infection and immunomodulation (Biragyn et al., 2002). García-Valtanen et al. (2014) suggested zebrafish (Danio rerio) derived β-defensin 2 (zfBD2) as potent adjuvant of viral DNA vaccine possessing both antiviral and immunomodulatory properties against VHSV in vivo and in vitro (Table 1).
Peptide piscidins 1 N, 1H, 2 and 3 are a family of amphipatic cationic α-helical AVPs recognized from the mast cells of hybrid striped bass (Silphaduang and Noga, 2001); these have exhibited antiviral activity against both enveloped channel catfish virus and non-enveloped frog virus 3 (Chinchar et al., 2004). Other AVPs, anti-lipopolysaccharide factors (ALF) have been isolated and characterized from crustaceans (Table 1), with potential inhibitory activity against WSSV in crustaceans (Lin et al., 2016; Liu et al., 2012). These small protein molecules bind and neutralize lipopolysaccharides found in hemocytes of horseshoe crab (Morita et al., 1985). Several studies have reported inhibition of WSSV replication through reduction of early gene transcription by ALFs, isolated and characterized from Chinese shrimp (Fenneropenaeus chinensis), red claw crayfish (Li et al., 2015; Lin et al., 2016) and by Mj-sty peptide from Kuruma shrimp (Marsupenaeus japonicas; Hong-tao Liu et al., 2015). Furthermore, ALFpm3 (Somboonwiwat et al., 2005) and PEN5 (Woramongkolchai et al., 2011) from tiger shrimp were found to inhibit WSSV by disrupting the virus envelope protein WSSV189 and increasing the peptide load after infection. Similarly, expression of wsv069 and wsv421 genes of WSSV have been suppressed by rLvHcL48 peptide isolated from Pacific white shrimp (Litopenaeus vannamei; Zhan et al., 2019).
The Pa-MAP peptide from winter flounder inhibited HSV-1 at 80% and HSV-2 at 90%, although 90 μM maximum non-toxic concentrations (MNTC) raised the level of inhibition to 94 and 97% for HSV-1 and HSV-2, respectively, with ability to disrupt the viral envelop or capsid (Migliolo et al., 2012; see Table 1). In addition, Pa-MAP characterized from marine sponge was inhibitory against HIV-1 infection through a cell proliferation (XTT) in vitro with approximately 0.2 μg/mL of EC50 (Rashid et al., 2001).
However, FASTA sequence of three common AVPs such as ‘Defensin’, ‘Hepcidin’ and ‘Piscidin’ were retrieved from NCBI (https://www.ncbi.nlm.nih.gov/) and RCSB PDB databases (https://www.rcsb.org/) and considered for multiple sequence alignment (MSA) by using Clustal Omega software (https://www.ebi.ac.uk/Tools/msa/clustalo/). These attempts were employed for brief documentation and analysis of the conserved regions or identical sites of AVPs from aquatic organisms with reported purified or commercial AVPs from human or synthetic origins. For example, in case of defensins, four alpha-defensins from human source were retrieved, and allowed to MSA with five other AVPs from aquatic organisms including Atlantic cod (Gadus morhua), Atlantic salmon (Salmo salar), puffer fish (Tetraodon nigroviridis), crocodile icefish (Pseudochaenichthys georgianus), and killifish (Fundulus heteroclitus). MSA revealed nine conserved positions among the sequences including different identical positions. Again, five hepcidin sequences of aquatic source (striped_bass, Morone saxatilis; Rio pearlfish, Nematolebias whitei; European sea bass, Dicentrarchus labrax; tuberculated flathead, Sorsogona tuberculata) were aligned with two hepcidins of human origin. Here, around nineteen positions were conserved including the availability of a specific site ‘CCNCC’ (Cysteine-Asperginie-Cysteine-Cysteine) among all hepcidin sequences. In addition, MSA of two synthetic constructs of piscidin and five homologous piscidin sequences from striped bass also exhibited the conserved pattern and identical sites. Though the analysis had been done within a minimal number of sequences, results indicated that the AVPs from aquatic sources shared conserved regions with human or commercial synthetic constructs of AVPs. The detailed alignment results are illustrated in Fig. 1 .
Fig. 1.
Multiple sequence alignment of AVPs (Defensin, Hepcidin and Piscidin) from aquatic organisms with reported purified or commercial AVPs from human or synthetic origins. FASTA sequences were retrieved from NCBI and RCSB PDB databases, and MSA done by Clustal Omega. Highlighted portion indicated the conserved regions. Colour letters of sequences showed identical regions.
4. AVPs mechanisms of action against viruses
AVPs, isolated from various animals, plants, insects, fish and humans reportedly display broad antiviral activities against viruses through different mechanisms of action, such as blocking virus fusion or entry into the host cells, preventing the spread of virus through suppression of its gene expression, and inhibition of translation through an immune modulatory mechanism (Jenssen et al., 2006; Mulder et al., 2013) (Fig. 2 ).
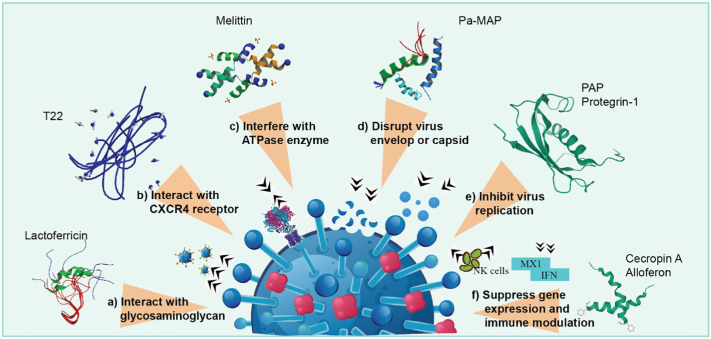
Fig. 2.
Mechanisms of action of AVPs against virus – a) block the virus before entry through interaction with glycosaminoglycans, b) prevent virus entry into the host cell by interacting with CXCR4 receptor, c) inhibit cell fusion by interference with ATPase, d) host protection by disrupting virus envelope or capsid, e) avert seeding of virus within cell by inhibiting their replication or protein synthesis, and f) suppression of virus gene expression and modulation of host immunity by stimulating NK cells.
AVP blocks viral entry through interaction with heparan sulfate, which is the most important glycosaminoglycan molecule related to viral attachment with host cells (Fig. 2a). It has been reported that lactoferricin is able to inhibit HSV infection by binding to heparan sulfate (Andersen et al., 2003; Jenssen et al., 2004). Similarly, human α-defensin, LL-37 and magainin also bind with glycosaminoglycans to prevent viral attachment with host cells (Jenssen et al., 2006). AVPs utilize another mechanism that interacts with specific cellular receptors to inhibit viral entry into the hosts (Fig. 2b). Polyphemusin analogue T22 has been shown to have potent antiviral activity against HIV-1, 2 in vitro, where the peptide binds to a chemokine receptor CXCR4, a co-receptor for the entry of HIV-1 into T cells (Nakashima et al., 1992; Tamamura et al., 1998). Besides, θ-defensin interacts with HSV-2 glycoprotein B and HIV-1 glycoprotein 120 with high affinity, thus blocking the entry of HSV-2 and HIV-1 into host cells (Münk et al., 2003; Yasin et al., 2004).
A proposed mechanism of action has suggested that melittin obtained from honey bee inhibited HSV-1 by preventing cell fusion through interference with the activity of ATPase enzyme (Fig. 2c), thereby disrupting the membrane fusion process (Matanic and Castilla, 2004). AVPs protect the host cell from different viral infection through interaction with the virus envelop or disrupting virus envelop/capsid (Fig. 2d). To exert anti-HIV activity, dermaseptin directly interacts with the viral particle, eventually disrupting the membrane of the virus (Lorin et al., 2005). Moreover, peptide Pa-MAP isolated from the winter flounder has been found to inhibit HSV-1, 2 infection by interacting with viral envelop (Migliolo et al., 2012; Teixeira et al., 2013).
Inhibition of viral replication or protein synthesis is another mechanism triggered by various peptides to prevent virus spreading within the host cell (Fig. 2e). The PAP from American pokeweed, for example, prevented tobacco mosaic virus (TMV) infection by inactivating ribosomes, thereby blocking viral protein synthesis (Taylor et al., 1994). A similar mechanism was also exhibited by protegrin-1 to exert antiviral activity against HIV-1 (Steinstraesser et al., 2005). Suppression of viral gene expression and modulation of immunity are two important inhibitory mechanisms of AVPs (Fig. 2f), as in the cases of melittin and cecropin A, both found to inhibit HIV-1 infection by suppression of viral gene expression (Brack-Werner et al., 1998). The peptide omBD1 isolated from rainbow trout inhibited VHSV infection by upregulating the expression of mx1 gene, suggesting an immune modulatory mechanism of peptides (Falco et al., 2007).
5. Aquatic AVPs against human and animal viruses
A number of studies reported the efficacy of AVPs against human and animal viruses, but their application as therapeutics is limited in animals. Peptide TH 1–5 was found effective against IPNV (Rajanbabu and Chen, 2011) and NNV (Wang et al., 2010). Likewise, cecropin B and its synthetic analogue CF17 were active against a range of fish viruses including infectious hematopoietic necrosis virus (IHNV), VHSV, snakehead rhabdovirus (SHRV) and IPNV (Chiou et al., 2002).
Moreover, several peptides are found active against human viruses, viz. influenza, rabies, HIV, HSV, hepatitis C virus (HCV) and West Nile virus (WNV) (Thakur et al., 2012). Peptides isolated from invertebrates such as melittin, cecropin and alloferon were inhibitory against different infectious viruses. Melittin, an antitumor peptide has been reported to inhibit the activity of HIV-1, HSV-1 and Junin virus through a membrane fusion process (Matanic and Castilla, 2004). Alloferon-1, 2 are two potential biopharmaceutical compounds having the ability to improve host innate immune response as they shown activity against influenza virus (Chernysh et al., 2002). Another AVP T22, which is synthesized by a solid-phase method based on 9-fluorenyl-methyloxycarbonyl (Fmoc) (Akaji et al., 1989), has shown strong activity against HIV-1, 2 strains in vitro through virus-cell fusion, or uncoating process after virus adsorption (Nakashima et al., 1992).
Mulder et al. (2013) reviewed a number of peptides from vertebrates that are active against diseases causing viruses in humans like defensins HNP-1, which is a type of α-defensin peptide capable of inactivating HSV-1, 2, CMV, VSV and IAV/WSN (Daher et al., 1986). Retrocyclin is a θ-defensin 18-residue peptide capable of blocking the entry of HIV-1 into host cell (Münk et al., 2003). In addition, lactoferricin (LfcinB) peptide derived from multifunctional glycoprotein lactoferrin has shown inhibitory action against HIV-1, CMV (Swart et al., 1998) and HSV-1, 2 (Andersen et al., 2003). Another peptide isolated from bovine neutrophils, i.e. indolicidin has shown antiviral mechanism while interacting with HIV-1 (Robinson et al., 1998). There are other examples of peptides that are active against human viruses, such as PAP (Kaur et al., 2011) and dermaseptin (Belaid et al., 2002) against HIV and HSV, and Hp1090 against HCV (Yan et al., 2011).
Peptides originated from marine organisms have exhibited antiviral activity against both animal and human viruses. As for instance, microspinosamide from sponge has been found to be active against HIV-1 infection (Rashid et al., 2001). Pa-Map isolated from winter flounder interacts with viral envelopes and has inhibited HSV-1 and HSV-2 (Migliolo et al., 2012). Moreover, nine peptides from different sources were studied and among them tachyplesin-1, clavanin A, penaeidin-3 and ALF have shown antiviral activity against HSV-1, adenovirus-5 and rotavirus SA11 (Carriel-Gomes et al., 2007).
Recent updates report that many peptides are active against infectious viruses causing illness in humans and animals. For example, P1 peptide derived from a phage displayed peptide library found to inhibit JEV in vitro and in vivo (Wei et al., 2020). Besides this, Brice and Diamond (2020) suggested innate antiviral defense by three human host peptides, namely α- and β-defensins and the sole human cathelicidin LL-37 against viral infections and their potential to develop antiviral therapeutic agents. Peptides 229E-HR1P and 229E-HR2P designed from HR1 and HR2 regions of human coronavirus (HCoV)-229E have demonstrated inhibitory activity against HCoV-229E spike protein (Xia et al., 2018). Similarly, a defensin-like peptide P9R has exhibited pH dependent activity against respiratory viruses like SARS-CoV and MERS-CoV (Zhao et al., 2020a).
6. Insights of AVPs against human coronaviruses and SARS-CoV-2
Potentially effective vaccine and antiviral therapeutics options are being studied with different collaborative efforts toward the development of anti-SARS-CoV-2 drug compounds. Though some likely therapeutants appear to have prospects for treatment of COVID-19, AVPs or AMPs have not been given a similar level of attention to date (Caly et al., 2020; Ledford, 2020; Ohashi et al., 2020; Vanden Eynde, 2020; Wang, Chen, H et al., 2020; Wang, Cheng, W et al., 2020; Wang, M et al., 2020; Wang, Y et al., 2020). Few recent reports have strong evidence that AVPs could provide alternative options of therapeutic agents against the SARS-CoV-2. For example, a recent study suggested that a natural lectin-like defensins-5 (HD5) peptide (ATCYCRTGRC ATRESLSGVCEISGRLYRLCCR) can successfully block the ACE2 receptors on the host, in which it is already established that ACE2 receptor plays vital role in the entry of SARS-CoV-2 into the human cellular system (Mahendran et al., 2020; Cheng Wang et al., 2020). Another analysis of the likely effectiveness of AVPs against coronaviruses was reported by Xia et al. (2019). From their experimental data, they suggested that ‘EK1’ (SLDQINVTFLDLEYEMKKLEEAIKKLEESYIDLKEL) exhibited significant level of cross-reactivity against all MERS-CoV and SARS-CoV (Xia et al., 2019). EK1 was found to be effective in the inhibition of viral fusion entry as well as the challenges of HCoVOC43, alpha-coronavirus and MERS-CoV virus also could be protected by its intranasal uses (Mahendran et al., 2020; Xia et al., 2020, Xia et al., 2019). In addition, the viral envelope protein of SARS-CoV, MERS-CoV, and influenza H5N1 viruses were be disrupted by the strong electrostatic affinity with a AVP named ‘mucroporin-M1’ (LFRLIKSLIKRLVSAFK), which was designed as peptide analogue from the parent peptide mucroporin (LFGLIPSLIGGLVSAFK) extracted the venom of the scorpion Lychas mucronatus (Dai et al., 2008; Li et al., 2011). Viral uncoating for the successful release of viral RNA is a critical step during the disease progression by this group of pathogens, and hence endosomal acidification is crucial for pH-dependent activation of viral fusion proteins. This endosomal acidification step was found to be misled by the mouse b-defensins-4 derived P9 (NGAICWGPCPTAFRQ IGNCGHFKVRCCKIR) in MERS-CoV (Zhao et al., 2016). Moreover, another cyclic peptide RTD-1 (GFCRCLCRRGVCRCICTR) was also reported against the SARS-CoV infection which could act as an immunomodulatory effector molecule via a cytokine response combating against SARS-CoV progression (Wohlford-Lenane et al., 2009). Responding against the pandemic and infectious disease outbreaks is urgently needed for defending against COVID-19. The above mentioned reports suggested that AVPs could be an alternative option of effective drug response against the coronaviruses. Additionally, aquatic organisms have already been described as promising sources of AVPs. Hence, aquatic AVPs might be among preferred therapeutic candidates of COVID-19 for evaluation through proper scientific experimental and clinical validation.
6.1. Possible action of aquatic AVPs against SARS-CoV-2
6.1.1. Structure of SARS-CoV-2, entry mechanisms and disease symptoms
The whole genome sequence of novel SARS CoV-2 was 1st published on 10 January 2020 which has facilitated the identification of SARS CoV-2-infected patients using RT-PCR (Zhu et al., 2020). The very first literature (published on 22 January 2020) described SARS CoV-2 belonging to β-coronavirus group carrying ancestry of bat coronavirus HKU9-1, which is similar to other SARS-CoVs as its spike protein strongly interacts with human angiotensin-converting enzyme (ACE)-2 receptor (Xu et al., 2020). The viral genome of SARS-CoV-2 is ~29.8 kilobase with a GC content of ~38%, and virus diameter and surface spike length varies from 60 to 140 nm and 8–12 nm, respectively (Chan et al., 2020; Zhou et al., 2020). Genetic analysis demonstrated that SARS-CoV-2 matches 88%, 79% and 50% identity with two bat SARSr-CoV, normal SARS-CoV, and MERS-CoV, respectively, indicating distinct/novel characteristics of SARS-CoV-2 (Lu et al., 2020). Pangolins and bat identified CoVs showed maximum similarity ~85.5–92.4% and 96.2%, respectively, with CoV-2 (Lam et al., 2020; Zhou et al., 2020), although the intermediate host of CoV-2 is still unknown (Benvenuto et al., 2020).
Under electron microscope, this single stranded RNA virus showed spherical shape with spike-like projections on surface similar to crowns, and named after the Latin meaning of corona (Barcena et al., 2009). Its molecular arrangement consists of 5′ and 3′ untranslated region (UTR), nonstructural proteins encoded replicase complex, spike protein (S), envelope protein (E), membrane protein (M), nucleocapsid protein (N) and some unidentified nonstructural open reading frames (ORF) (Ul Qamar et al., 2020; Zhu et al., 2020). Viral envelope (human cell membrane derived lipid bilayer) anchored S, E and M proteins. Entry of CoVs into the host cell is mediated mainly by S glycoprotein, which possesses two functional subunits described as S1 and S2 domains (S1/S2 sites) to bind with host cells and trigger the fusion of viral and cellular membranes (Tortorici and Veesler, 2019). CoV-2 and other CoVs have affinity for binding with human ACE2 and host proteases cleaved S, whereas S2 domain placed upstream of fusion peptide (S2′ site) (Millet and Whittaker, 2015). Interestingly, CoV-2 has a furin-like protease cleavage site (RRAR↓SV) between S1 and S2 boundary (AYT↓M) which is processed during biogenesis, but the comparable sites are absent in other CoVs (Coutard et al., 2020; Walls et al., 2020). Among three (S, M and E) different proteins in viral envelope of CoVs, M protein is comparatively abundant and responsible to provide virus shape, whereas combination of M and E protein orchestrates virus internal assembly and helps to mature the outer envelope (Siu et al., 2008). Transfer of virus particles into the host cell is performed by E protein and N protein which binds/interacts with viral RNA during particle assembly to form nucleocapside (Ashour et al., 2020; Masters, 2006). In β-CoVs cis acting RNA extends 3′ to 5′ into ORF and association between 5′ and 3′ UTR act as a potential molecular switch for the synthesis of subgenomic RNA (Yang and Leibowitz, 2015).
Although fatality rates of SARS, MERS and SARS-CoV-2 are 10, 36, and 2.3%, respectively (Wu and McGoogan, 2020), CoV-2 S protein showed 10 to 20-fold higher affinity to bind with human ACE2 compared to others, making CoV-2 a super-spreader during 2020 pandemic (Wrapp et al., 2020). Like other CoVs, CoV-2 entry into host cells depends on recognition and binding of S protein with ACE2 receptor. After cleaving of S protein by host proteases, S1 further divided into N- and C-terminal domains having ACE2 receptor binding entity, and S2 causing the fusion of viral and host cell membranes (Lu et al., 2013; Zhang Wei, et al., 2020). Protease enzymes activity of S1/S2 cleaving completely dependent on amino acid profile of virus, and similar to SARS-CoV, S protein of CoV-2 is cleaved by cathepsin L and TMPRSS2 at S2′ site during cellular entry (Ashour et al., 2020). Fusion of host cell membrane and viral envelope triggers the entry of viral genome into the cell cytoplasm for multiplication. Following receptor binding SARS-CoV S1/S2 site (ATY↓M) is cleaved by cathepsin L during entry in the host cell endosome (Bosch et al., 2008). Human organs like alveolar epithelial cells in lung or enterocytes in small intestine are enriched with higher amount of ACE2, the potential site of SARS-CoV-2 attack (Zou et al., 2020). The development of cathepsin L inhibitor or neutralizing antibodies may be useful to control SARS-CoV-2 infection in humans.
Pneumonia has characteristics in common with the SARS-CoV-2 infection, now known as COVID-19 disease. Similar to other influenza viruses, signs and symptoms of COVID-19 include high temperature, continuous cough, expectoration, headache, pharyngalgia, dyspnoea and diarrhoea. Recently, the UK government included “loss of smell and taste” to the list of symptoms induced by COVID-19. Zheng (2020) reported that 90, 68 and 96% infected patients showed fever, cough and fatigue, respectively. Besides these, bilateral and unilateral pneumonia as well as multiple mottling and ground glass opacity were identified in lungs as revealed from chest X-ray and CT scanning (Chen et al., 2020). The U.S. Centers for Disease Control and Prevention reported that COVID-19 cause respiratory illness and in particular, that elderly, immune compromised and people with other health related problems are more vulnerable to this disease (Gralinski and Menachery, 2020). Since COVID-19 has spread across the world, mutation over time can make it more virulent and for this reason, proper surveillance and research are necessary to monitor and understand the change of its genetic materials in different environments. Incidentally, Asian males showed higher expression levels of ACE-2 receptor that are more susceptible to COVID-19 (Zhao et al., 2020b) compared to non-Asian counterparts (European and North American countries) demonstrating severe symptoms and sickness (Rothe et al., 2020).
6.1.2. Inhibitory mechanism of aquatic AVPs against SARS-CoV-2
Peptide based antimicrobial therapy has become a promising field in pharmaceutical research and a large number of peptides are being currently tested for medicinal use (Fosgerau and Hoffmann, 2015). AVP derived from aquatic organisms could initiate a new avenue in searching candidate drugs against SARS-CoV-2. Features of APV that are preferable as antiviral agents include – (i) interfering with protein-protein interactions, (ii) blocking the substrate binding site of key viral proteins, (iii) having high half-life in cellular environment, and (iv) minimum marketable time (Kaspar and Reichert, 2013). The absence of effective remedy against SARS-CoV-2 has drawn attention to unique therapeutics candidates with the possibility of combating adverse health complications during the COVID-19 pandemic. Most of the antiviral peptides including AVPs from aquatic organisms are found to inhibit or destabilize key proteins of virus (Reddy et al., 2004). The life cycle of SARS-CoV-2 and other coronaviruses have following steps – (i) recognition of viral spike protein by host receptor, (ii) viral entry into the host cell, (iii) release of viral RNA for replication, (iv) translation of viral replicase polyproteins using the translation machinery of host cell, (v) synthesis of viral structural and nonstructural proteins, and (vi) assembly and release of viral particles from the host cell through exocytosis (Bar-On et al., 2020; Wan et al., 2020).
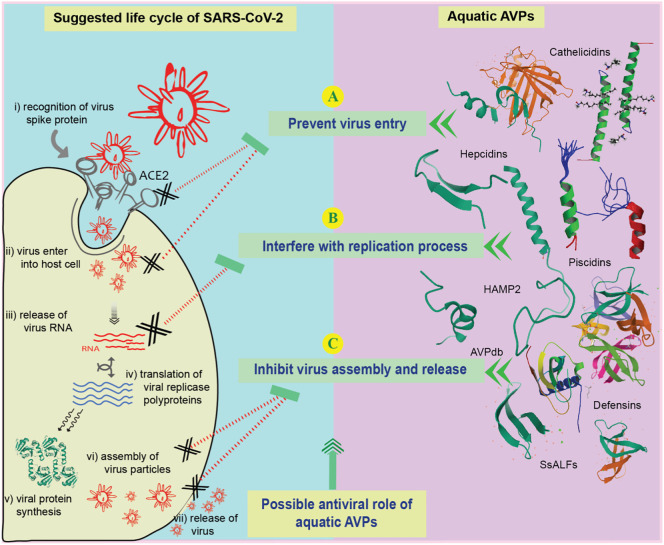
Candidate drugs with the most therapeutic potential focus on the above-mentioned steps, without which normal process of viral disease progression in the host environment may be disturbed or interrupted. The possible mechanisms behind the action of aquatic APVs against coronaviruses include – (1) inhibition of the viral entry through interfering with viral protein and host receptor-binding protein, (2) blocking of key proteins involved in the replication process, and (3) inhibition of the assembly and release of viral particles. These three mechanisms against SARS-CoV-2 including the known actions of aquatic AVPs against other member of coronaviruses are illustrated in Fig. 3 .
Fig. 3.
The life cycle of SARS-CoV-2 in host cells (i–vii), and possible antiviral role of aquatic AVPs against different stages of SARS-CoV-2 (A–C).
6.1.2.1. Inhibition of viral entry
The entry of SARS-CoV-2 into human cell is initiated by S protein. S1 subunit of S protein usually interacts with ACE2 of human cell and S2 subunit strengthens the fusion between human cell membranes and viral envelope protein (Coutard et al., 2020; Holmes, 2003). Since S protein plays vital roles in the membrane fusion and viral entry into the host cellular environment, it could be a potential therapeutic target for SARS-CoV-2. Aquatic AVPs are reported to interfere with this entry step of different viral pathogens (Fig. 3A), including CoVs. β-defensin-like peptides isolated from aquatic sources (Zou et al., 2007) are biologically similar to human and other organism derived defensins (Cuesta et al., 2011). Besides, β-defensin was able to combat the emerging respiratory viruses such as H1N1, H7N7, H3N2, H7N9, H5N1, SARS-CoV and MERS-CoV. It was found that P9, one of the 11 investigated β-defensins, could bind to the viral glycoproteins leading to blockade of membrane fusion for respiratory viruses (Zhao et al., 2016). Moreover, it could block the entry of HSV by binding its envelope glycoprotein (Hazrati et al., 2006). RSV β-defensin could destabilize the envelope protein responsible for inhibiting viral entry into the intracellular host environment (Kota et al., 2008). Immunomodulatory effects were reported for IAV that promote the uptake of IAV by neutrophils before viral entry (Tecle et al., 2007). Piscidins are another group of widely reported aquatic AVPs (such as Piscidin-1) isolated from the mast cells of the hybrid striped bass and other fish species (Silphaduang et al., 2006; Silphaduang and Noga, 2001). Potent antiviral activity by obstructing viral entry (such as HIV-1) into the host cellular environment has been reported (Wang, 2012; Wang et al., 2010). Moreover, polyphemusins, isolated from horseshoe crab, and their modified derivative (T22) have also been investigated as viral fusion inhibitor (Hikichi et al., 2016). Another aquatic antiviral peptide Cathelicidins is capable of inhibiting viral entry, hence that AVP may be an effective therapeutic against SARS-CoV-2. Cathelicidins peptide LL-37 also showed potent antiviral efficacy against two important respiratory viruses, RSV and IAV (Mansbach et al., 2012; Tripathi et al., 2013). LL-37 initiates peptide-mediated membrane disruption with influenza virus leading to the inhibition of viral fusion and viral propagation within the host cell (Tripathi et al., 2013). Moreover, LL-37 mediated membrane disruption was reported with vaccinia virus (Dean et al., 2010). Cathelicidins peptides were successfully evaluated as potential antiviral therapeutics against HIV, HSV and Adenovirus (Levinson et al., 2012; Matanic and Castilla, 2004; Smith et al., 2010) and thus, cathelicidins also appear to be among prospective therapeutics against SARS-CoV-2.
6.1.2.2. Blocking proteins in replication process
The expression of several structural and nonstructural proteins coded within the viral genome usually precede the replication process, using host cell machineries. SARS-CoV-2 releases genomic materials into the host cytoplasm for initiating replication (Channappanavar et al., 2016; Chu et al., 2020). Replicase polyproteins are synthesized from viral RNA which lead to the formation of RNA-dependent RNA polymerase (RdRp) and helicase with other non-structural proteins. These RdRp and helicase and other nonstructural proteins (NSP 3, 4, 6) participate in the translation of important proteins and full-length positive-strand genomic RNA for further processing of new viral particles (Gordon et al., 2020; Li et al., 1999). Aquatic AVPs hold some potential as options for targeting the replication process of SARS-CoV-2 (Fig. 3B). CoVs are mostly dependent on iron-containing enzymes to complete their replication processes (Adedeji and Lazarus, 2016; Jia et al., 2019). Hence, depriving iron supply could be a better therapeutic option for the treatment of COVID-19. Incidentally, human ferroportin protein expressed in the duodenal enterocytes and macrophages can govern iron release, and human hepcidins underregulate this ferroportin leading to iron deficiency and compromised viral replication (Ganz, 2007; Liu et al., 2016). Hepcidins (cysteine rich peptides isolated from hybrid striped bass and other fish species; Shike et al. (2002), could also play a role in interference with normal iron supplies within the host cell. Although extensive research on iron regulation in COVID-19 patients and hepcidins supplements is underway, adjuvant based therapeutic option for battling against SARS-CoV-2 could be a promising alternative (Liu et al., 2020). Further, cathelicidins AVPs were found to inhibit RT enzyme in HIV (Bergman et al., 2007). Several other aquatic AVPs such as Scygonadin, SpALF1, SpALF2, rCqALF and HAMP2 also have demonstrated their capacity to inhibit the replication cycle of different viral pathogens (Cheung et al., 2014; Lin et al., 2016; Neves et al., 2017), raising another option for retarding the replication of SARS-CoV-2.
6.1.2.3. Prevent assembly and release of virus particles
There are limited investigations into the coronavirus assembly and release process, although some studies suggest that viral nucleocapsid proteins are involved in the genomic RNA assembly and viral particles are released through exocytosis process (Kota et al., 2008; Stertz et al., 2007). This step or information could be useful for targeting candidate AVPs after extensive research. The AVP database suggested that only a few peptides have potential inhibition efficacy during the release of SARS CoVs particles (Fig. 3C). The database also includes several peptides with potential antiviral activity showing various mechanisms of action against different CoVs including MERS and SARS (Qureshi et al., 2014). A modified peptide sequence ‘dec-RVKR-cmk’ was reported to be efficient for blocking coronavirus release (Bergeron et al., 2005). But there are no aquatic AVPs known to be effective in this step, which requires further research.
Aquatic AVPs have already been inhibitory against different coronaviruses including other deadly viruses. Therefore, AVPs derived from aquatic sources have the potential ability to combat SARS-CoV-2, although validation is necessary to determine the effectiveness of AVPs as a therapeutic option for COVID-19 patients. Overall, peptide based therapeutics, especially aquatic AVPs could be promising as they have established capability to inhibit the life cycle of a variety of respiratory viruses.
7. Concluding remarks
AVPs produced by aquatic organisms (fish, shellfish, aquatic plants and other organisms) have potential as novel therapeutics or drugs against both aquatic and terrestrial infectious viruses. Despite having antiviral activity against numerous viruses, no studies have been reported on the application of aquatic AVPs as drugs against viral infections. The mechanisms of action of some AVPs derived from aquatic sources are yet to be characterized (Gui et al., 2016; He et al., 2018; Jin et al., 2010; Valero et al., 2020; Zhou et al., 2019). Some aquatic AVPs showing in vitro activity against viruses might lack similar actions in vivo. Also, AVPs induced immune pathways in humans or animals are still under investigation (Chia et al., 2010; Rashid et al., 2001). However, aquatic organisms derived AVPs could inhibit SARS-CoV-2 in a number of mechanisms, even though there is no previous study in relevant of this topic. The potential of aquatic AVPs according to the history of other AVPs and the mechanism of their inhibition of viruses are discussed here which could broaden the research scopes of the development of anti-SARS-CoV-2 therapeutics and hence could defeat COVID-19.
Funding
This work was supported by Korea Basic Science Institute (National Research Facilities and Equipment Center) grant (2020R1A6C101A201) funded by the Ministry of Education, Republic of Korea.
Author statement
Authors confirm that this research work is original and has not been published elsewhere nor is it currently under consideration for publication elsewhere. The final version of the manuscript being submitted has been seen and approved by all authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aboudy Y., Mendelson E., Shalit I., Bessalle R., Fridkin M. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int. J. Pept. Protein Res. 2009;43:573–582. doi: 10.1111/j.1399-3011.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Adedeji A.O., Lazarus H. Biochemical characterization of middle east respiratory syndrome coronavirus helicase. mSphere. 2016;1 doi: 10.1128/mSphere.00235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaji K., Fujii N., Tokunaga F., Miyata T., Iwanaga S., Yajima H. Studies on peptides. CLXVIII.: syntheses of three peptides isolated from horseshoe crab hemocytes, tachyplesin I, tachyplesin II, and polyphemusin I. Chem. Pharm. Bull. 1989;37:2661–2664. [Google Scholar]
- Andersen J.H., Jenssen H., Gutteberg T.J. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antivir. Res. 2003;58:209–215. doi: 10.1016/S0166-3542(02)00214-0. [DOI] [PubMed] [Google Scholar]
- Ashour H.M., Elkhatib W.F., Rahman M.M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R., Goldman M.J., Wilson J.M. Mouse β-Defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect. Immun. 1998;66:1225–1232. doi: 10.1128/IAI.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena M., Oostergetel G.T., Bartelink W., Faas F.G.A., Verkleij A., Rottier P.J.M., Koster A.J., Bosch B.J. Cryo-electron tomography of mouse hepatitis virus: insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. 2009;106:582–587. doi: 10.1073/pnas.0805270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P.G., Findlay E.G., Currie S.M., Davidson D.J. Antiviral potential of cathelicidins. Future Microbiol. 2014;9:55–73. doi: 10.2217/fmb.13.135. [DOI] [PubMed] [Google Scholar]
- Bar-On Y.M., Flamholz A., Phillips R., Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020;9 doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian A., Schäfer H. Human α-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 2001;101:157–161. doi: 10.1016/S0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of covid-19 — final report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaid A., Aouni M., Khelifa R., Trabelsi A., Jemmali M., Hani K. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 2002;66:229–234. doi: 10.1002/jmv.2134. [DOI] [PubMed] [Google Scholar]
- Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J. Med. Virol. 2020;92:455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron E., Vincent M.J., Wickham L., Hamelin J., Basak A., Nichol S.T., Chrétien M., Seidah N.G. Implication of proprotein convertases in the processing and spread of severe acute respiratory syndrome coronavirus. Biochem. Biophys. Res. Commun. 2005;326:554–563. doi: 10.1016/j.bbrc.2004.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman P., Walter-Jallow L., Broliden K., Agerberth B., Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007;5:410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- Biragyn A., Ruffini P.A., Leifer C.A., Klyushnenkova E., Shakhov A., Chertov O., Shirakawa A.K., Farber J.M., Segal D.M., Oppenheim J.J., Kwak L.W. Toll-like receptor 4-dependent activation of dendritic cells by -defensin 2. Science (80-. ). 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Boas L.C.P.V., Campos M.L., Berlanda R.L.A., de Carvalho Neves N., Franco O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019;76:3525–3542. doi: 10.1007/s00018-019-03138-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., Bartelink W., Rottier P.J.M. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82:8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish D.M.E., Davidson D.J., Lau Y.E., Lee K., Scott M.G., Hancock R.E.W. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R., Erfle V., von Pechmann N., Neumann M., Winder D., Kleinschmidt A., Salmons B., Ludvigsen A., Wachinger M., Holle R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- Brice D.C., Diamond G. Antiviral activities of human host defense peptides. Curr. Med. Chem. 2020;27:1420–1443. doi: 10.2174/0929867326666190805151654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C.B., Day P.M., Thompson C.D., Lubkowski J., Lu W., Lowy D.R., Schiller J.T. Human -defensins block papillomavirus infection. Proc. Natl. Acad. Sci. 2006;103:1516–1521. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulet P., Stocklin R., Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol. Rev. 2004;198:169–184. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- Cai L., Cai J.J., Liu H.P., Fan D.Q., Peng H., Wang K.J. Recombinant medaka (Oryzias melastigmus) pro-hepcidin: multifunctional characterization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012;161:140–147. doi: 10.1016/j.cbpb.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriel-Gomes M.C., Kratz J.M., Barracco M.A., Bachére E., Barardi C.R.M., Simões C.M.O. In vitro antiviral activity of antimicrobial peptides against herpes simplex virus 1, adenovirus, and rotavirus. Mem. Inst. Oswaldo Cruz. 2007;102:469–472. doi: 10.1590/S0074-02762007005000028. [DOI] [PubMed] [Google Scholar]
- Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.L., Vargas J., DelPortillo A., Klotman M.E. Dual role of α-defensin-1 in anti–HIV-1 innate immunity. J. Clin. Invest. 2005;115:765–773. doi: 10.1172/JCI21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal Pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S., Sinha N.K., Banerjee S., Roy D., Chattopadhyay D., Roy S. Small cationic protein from a marine turtle has β-defensin-like fold and antibacterial and antiviral activity. Proteins Struct. Funct. Bioinforma. 2006;64:524–531. doi: 10.1002/prot.20963. [DOI] [PubMed] [Google Scholar]
- Chen Z.C., White R.F., Antoniw J.F., Lin Q. Effect of pokeweed antiviral protein (PAP) on the infection of plant viruses. Plant Pathol. 1991;40:612–620. doi: 10.1111/j.1365-3059.1991.tb02426.x. [DOI] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernysh S., Kim S.I., Bekker G., Pleskach V.A., Filatova N.A., Anikin V.B., Platonov V.G., Bulet P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. 2002;99:12628–12632. doi: 10.1073/pnas.192301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung R.C.F., Wong J.H., Pan W.L., Chan Y.S., Yin C.M., Dan X.L., Wang H.X., Fang E.F., Lam S.K., Ngai P.H.K., Xia L.X., Liu F., Ye X.Y., Zhang G.Q., Liu Q.H., Sha O., Lin P., Ki C., Bekhit A.A., Bekhit A.E.-D., Wan D.C.C., Ye X.J., Xia J., Ng T.B. Antifungal and antiviral products of marine organisms. Appl. Microbiol. Biotechnol. 2014;98:3475–3494. doi: 10.1007/s00253-014-5575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia T.-J., Wu Y.-C., Chen J.-Y., Chi S.-C. Antimicrobial peptides (AMP) with antiviral activity against fish nodavirus. Fish Shellfish Immunol. 2010;28:434–439. doi: 10.1016/j.fsi.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Chinchar V., Bryan L., Silphadaung U., Noga E., Wade D., Rollins-Smith L. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology. 2004;323:268–275. doi: 10.1016/j.virol.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Chiou P.P., Lin C.M., Perez L., Chen T.T. Effect of cecropin B and a synthetic analogue on propagation of fish viruses in vitro. Mar. Biotechnol. 2002;4(3):294–302. doi: 10.1007/s10126-002-0021-1. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.M., Talukder S.A., Khan A.M., Afrin N., Ali M.A., Islam R., Parves R., Al Mamun A., Sufian M.A., Hossain M.N., Hossain M.A., Halim M.A. Antiviral peptides as promising therapeutics against SARS-CoV-2. J. Phys. Chem. B. 2020;124:9785–9792. doi: 10.1021/acs.jpcb.0c05621. [DOI] [PubMed] [Google Scholar]
- Chu H., Chan J.F.-W., Wang Y., Yuen T.T.-T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.-P., Zhou J., Yuan S., Kok K.-H., To K.K.-W., Chan I.H.-Y., Zhang A.J., Sit K.-Y., Au W.-K., Yuen K.-Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020;71:1400–1409. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A.M., Weis P., Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta A., Meseguer J., Esteban M.Á. The antimicrobial peptide hepcidin exerts an important role in the innate immunity against bacteria in the bony fish gilthead seabream. Mol. Immunol. 2008;45:2333–2342. doi: 10.1016/j.molimm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Cuesta A., Meseguer J., Esteban M.Á. Molecular and functional characterization of the gilthead seabream β-defensin demonstrate its chemotactic and antimicrobial activity. Mol. Immunol. 2011;48:1432–1438. doi: 10.1016/j.molimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Daher K.A., Selsted M.E., Lehrer R.I. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C., Ma Y., Zhao Z., Zhao R., Wang Q., Wu Y., Cao Z., Li W. Mucroporin, the first cationic host defense peptide from the venom of lychas mucronatus. Antimicrob. Agents Chemother. 2008;52:3967–3972. doi: 10.1128/AAC.00542-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caleya R.F., Gonzalez-Pascual B., Garcia-Olmedo F., Carbonero P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl. Microbiol. 1972;23:998–1000. doi: 10.1128/am.23.5.998-1000.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R.E., O’Brien L.M., Thwaite J.E., Fox M.A., Atkins H., Ulaeto D.O. A carpet-based mechanism for direct antimicrobial peptide activity against vaccinia virus membranes. Peptides. 2010;31:1966–1972. doi: 10.1016/j.peptides.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Desriac F., Jégou C., Brillet B., Le Chevalier P., Fleury Y. In: Utilization of Fish Waste. Gálvez R.P., Bergé J.-P., editors. CRC Press; Boca Raton, Florida: 2013. Antimicrobial peptides from fish; pp. 106–141. [Google Scholar]
- Dubos R.J. Studies on a bactericidal agent extracted from a soil bacillus. J. Exp. Med. 1939;70:11–17. doi: 10.1084/jem.70.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih T.M. Dermaseptin-based antiviral peptides to prevent COVID-19 through in silico molecular docking studies against SARS-CoV-2 spike protein. Pharm. Sci. Res. 2020;7:65–70. doi: 10.7454/psr.v7i4.1079. [DOI] [Google Scholar]
- Falco A., Mas V., Tafalla C., Perez L., Coll J.M., Estepa A. Dual antiviral activity of human alpha-defensin-1 against viral haemorrhagic septicaemia rhabdovirus (VHSV): inactivation of virus particles and induction of a type I interferon-related response. Antivir. Res. 2007;76:111–123. doi: 10.1016/j.antiviral.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Falco A., Chico V., Marroquí L., Perez L., Coll J.M., Estepa A. Expression and antiviral activity of a β-defensin-like peptide identified in the rainbow trout (Oncorhynchus mykiss) EST sequences. Mol. Immunol. 2008;45:757–765. doi: 10.1016/j.molimm.2007.06.358. [DOI] [PubMed] [Google Scholar]
- Falco A., Ortega-Villaizan M., Chico V., Brocal I., Perez L., Coll J.M., Estepa A. Antimicrobial peptides as model molecules for the development of novel antiviral agents in aquaculture. Mini-Rev. Med. Chem. 2009;9:1159–1164. doi: 10.2174/138955709789055171. [DOI] [PubMed] [Google Scholar]
- Field H.J., De Clercq E. Antiviral drugs-a short history of their discovery and development. Microbiol. Today. 2004;31:58–61. [Google Scholar]
- Findlay E.G., Currie S.M., Davidson D.J. Cationic host defence peptides: potential as antiviral therapeutics. BioDrugs. 2013;27:479–493. doi: 10.1007/s40259-013-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F.G., Neumann W.P. The venom of the honeybee. III. On the chemical knowledge of the principle active constituent (melittin) Biochem. Z. 1961;335:51–61. [PubMed] [Google Scholar]
- Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov. Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Ganz T. Molecular control of iron transport. J. Am. Soc. Nephrol. 2007;18:394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M.E., Szklarek D., Harwig S.S., Daher K., Bainton D.F., Lehrer R.I. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Valtanen P., Martinez-Lopez A., Ortega-Villaizan M., Perez L., Coll J.M., Estepa A. In addition to its antiviral and immunomodulatory properties, the zebrafish β-defensin 2 (zfBD2) is a potent viral DNA vaccine molecular adjuvant. Antivir. Res. 2014;101:136–147. doi: 10.1016/j.antiviral.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12:135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Morell J.L. Structure of nisin. J. Am. Chem. Soc. 1971;93:4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- Grubor B., Gallup J.M., Meyerholz D.K., Crouch E.C., Evans R.B., Brogden K.A., Lehmkuhl H.D., Ackermann M.R. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin. Diagn. Lab. Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L., Zhang P., Zhang Q., Zhang J. Two hepcidins from spotted scat (Scatophagus argus) possess antibacterial and antiviral functions in vitro. Fish Shellfish Immunol. 2016;50:191–199. doi: 10.1016/j.fsi.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Guo M., Wei J., Huang X., Huang Y., Qin Q. Antiviral effects of β-defensin derived from orange-spotted grouper (Epinephelus coioides) Fish Shellfish Immunol. 2012;32:828–838. doi: 10.1016/j.fsi.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R.E.W. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001;1:156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- Hancock R.E.W., Sahl H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Hartshorn K.L., White M.R., Tecle T., Holmskov U., Crouch E.C. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 2006;176:6962–6972. doi: 10.4049/jimmunol.176.11.6962. [DOI] [PubMed] [Google Scholar]
- Hasan M., Parvez M.S.A., Azim K.F., Imran A.S., Raihan T., Gulshan A., Muhit S., Akhand R.N., Uddin M.B., Ahmed S.S.U. Main protease inhibitors and drug surface hotspot for the treatment of COVID-19: drug repurposing and molecular docking approach. ChemRxiv (Preprint) 2020;12118857 doi: 10.1016/j.biopha.2021.111742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati E., Galen B., Lu W., Wang W., Ouyang Y., Keller M.J., Lehrer R.I., Herold B.C. Human α- and β-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 2006;177:8658–8666. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- He S., Wang J., Du X., Yue B., Wang G., Zhou S., Xie B., Zhang M. A teleost TFPI-2 peptide that possesses a broad antibacterial spectrum and immune-stimulatory properties. Fish Shellfish Immunol. 2018;82:469–475. doi: 10.1016/j.fsi.2018.08.051. [DOI] [PubMed] [Google Scholar]
- Hikichi Y., Yokoyama M., Takemura T., Fujino M., Kumakura S., Maeda Y., Yamamoto N., Sato H., Matano T., Murakami T. Increased HIV-1 sensitivity to neutralizing antibodies by mutations in the Env V3-coding region for resistance to CXCR4 antagonists. J. Gen. Virol. 2016;97:2427–2440. doi: 10.1099/jgv.0.000536. [DOI] [PubMed] [Google Scholar]
- Hilchie A.L., Wuerth K., Hancock R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013;9:761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- Holmes K.V. SARS-associated coronavirus. N. Engl. J. Med. 2003;348:1948–1951. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- Howell M.D., Streib J.E., Leung D.Y.M. Antiviral activity of human β-defensin 3 against vaccinia virus. J. Allergy Clin. Immunol. 2007;119:1022–1025. doi: 10.1016/j.jaci.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-N., Rajanbabu V., Pan C.-Y., Chan Y.-L., Hui C.-F., Chen J.-Y., Wu C.-J. Modulation of the immune-related gene responses to protect mice against Japanese encephalitis virus using the antimicrobial peptide, tilapia hepcidin 1-5. Biomaterials. 2011;32:6804–6814. doi: 10.1016/j.biomaterials.2011.05.053. [DOI] [PubMed] [Google Scholar]
- Jago W., Jago W. Toxic action of wheat flour to brewer’s yeast. Ind. Ferment. 1926;128–167 [Google Scholar]
- Jenssen H., Andersen J.H., Uhlin-Hansen L., Gutteberg T.J., Rekdal Ø. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antivir. Res. 2004;61:101–109. doi: 10.1016/j.antiviral.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Yan L., Ren Z., Wu L., Wang J., Guo J., Zheng L., Ming Z., Zhang L., Lou Z., Rao Z. Delicate structural coordination of the Severe Acute Respiratory Syndrome coronavirus Nsp13 upon ATP hydrolysis. Nucleic Acids Res. 2019;47:6538–6550. doi: 10.1093/nar/gkz409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.-Y., Zhou L., Wang Y., Li Z., Zhao J.-G., Zhang Q.-Y., Gui J.-F. Antibacterial and antiviral roles of a fish β-defensin expressed both in pituitary and testis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B.L., Selsted M.E., Ganz T., Lehrer R.I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc. Natl. Acad. Sci. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar A.A., Reichert J.M. Future directions for peptide therapeutics development. Drug Discov. Today. 2013;18:807–817. doi: 10.1016/j.drudis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Kaur I., Gupta R.C., Puri M. Ribosome inactivating proteins from plants inhibiting viruses. Virol. Sin. 2011;26:357–365. doi: 10.1007/s12250-011-3223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke M., Chen Y., Wu A., Sun Y., Su C., Wu H., Jin X., Tao J., Wang Y., Ma X., Pan J.-A., Guo D. Short peptides derived from the interaction domain of SARS coronavirus nonstructural protein nsp10 can suppress the 2′-O-methyltransferase activity of nsp10/nsp16 complex. Virus Res. 2012;167:322–328. doi: 10.1016/j.virusres.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota S., Sabbah A., Chang T.H., Harnack R., Xiang Y., Meng X., Bose S. Role of human β-defensin-2 during tumor necrosis factor-α/NF-κB-mediated innate antiviral response against human respiratory syncytial virus. J. Biol. Chem. 2008;283:22417–22429. doi: 10.1074/jbc.M710415200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebutornye F.K.A., Wang Z., Lu Y., Abarike E.D., Sakyi M.E., Li Y., Xie C.X., Hlordzi V. Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020;97:83–95. doi: 10.1016/j.fsi.2019.12.046. [DOI] [PubMed] [Google Scholar]
- Lai Y., Gallo R.L. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T.-Y., Jia N., Zhang Y.-W., Shum M.H.-H., Jiang J.-F., Zhu H.-C., Tong Y.-G., Shi Y.-X., Ni X.-B., Liao Y.-S., Li W.-J., Jiang B.-G., Wei W., Yuan T.-T., Zheng K., Cui X.-M., Li J., Pei G.-Q., Qiang X., Cheung W.Y.-M., Li L.-F., Sun F.-F., Qin S., Huang J.-C., Leung G.M., Holmes E.C., Hu Y.-L., Guan Y., Cao W.-C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- Levinson P., Choi R.Y., Cole A.L., Hirbod T., Rhedin S., Payne B., Guthrie B.L., Bosire R., Cole A.M., Farquhar C., Broliden K. HIV-neutralizing activity of cationic polypeptides in cervicovaginal secretions of women in HIV-serodiscordant relationships. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.-P., Huang P., Park S., Lai M.M.C. Polypyrimidine tract-binding protein binds to the leader RNA of mouse hepatitis virus and serves as a regulator of viral transcription. J. Virol. 1999;73:772–777. doi: 10.1128/JVI.73.1.772-777.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhao Z., Zhou D., Chen Y., Hong W., Cao L., Yang J., Zhang Y., Shi W., Cao Z., Wu Y., Yan H., Li W. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides. 2011;32:1518–1525. doi: 10.1016/j.peptides.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Guo S., Li F., Xiang J. Functional diversity of anti-lipopolysaccharide factor isoforms in shrimp and their characters related to antiviral activity. Mar. Drugs. 2015;13:2602–2616. doi: 10.3390/md13052602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Gao Y., Wang H., Zhang Q., Zeng C., Liu H. Identification of an anti-lipopolysacchride factor possessing both antiviral and antibacterial activity from the red claw crayfish Cherax quadricarinatus. Fish Shellfish Immunol. 2016;57:213–221. doi: 10.1016/j.fsi.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Ling R., Dai Y., Huang B., Huang W., Yu J., Lu X., Jiang Y. In silico design of antiviral peptides targeting the spike protein of SARS-CoV-2. Peptides. 2020;130:170328. doi: 10.1016/j.peptides.2020.170328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Chen R., Zhang Q., Wang Q., Li C., Peng H., Cai L., Zheng C., Wang K. Characterization of two isoforms of antiliopolysacchride factors (Sp-ALFs) from the mud crab Scylla paramamosain. Fish Shellfish Immunol. 2012;33:1–10. doi: 10.1016/j.fsi.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang J., Mao Y., Liu M., Niu S., Qiao Y., Su Y., Wang C., Zheng Z. Identification and expression analysis of a novel stylicin antimicrobial peptide from Kuruma shrimp (Marsupenaeus japonicus) Fish Shellfish Immunol. 2015;47:817–823. doi: 10.1016/j.fsi.2015.09.044. [DOI] [PubMed] [Google Scholar]
- Liu J., Sun B., Yin H., Liu S. Hepcidin: a promising therapeutic target for iron disorders: a systematic review. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zhang S., Nekhai S., Liu S. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr. Clin. Microbiol. Reports. 2020;7:13–19. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin C., Saidi H., Belaid A., Zairi A., Baleux F., Hocini H., Bélec L., Hani K., Tangy F. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology. 2005;334:264–275. doi: 10.1016/j.virol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lou Z., Sun Y., Rao Z. Current progress in antiviral strategies. Trends Pharmacol. Sci. 2014;35:86–102. doi: 10.1016/j.tips.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., Zhang B., Shi Y., Yan J., Gao G.F. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendran A.S.K., Lim Y.S., Fang C.-M., Loh H.-S., Le C.F. The potential of antiviral peptides as COVID-19 therapeutics. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.575444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach J.M., Piedra P.A., Borregaard N., Martineau A.R., Neuman M.I., Espinola J.A., Camargo C.A. Serum cathelicidin level is associated with viral etiology and severity of bronchiolitis. J. Allergy Clin. Immunol. 2012;130 doi: 10.1016/j.jaci.2012.07.044. 1007-1008.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masso-Silva J., Diamond G. Antimicrobial peptides from fish. Pharmaceuticals. 2014;7:265–310. doi: 10.3390/ph7030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matanic V.C.A., Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents. 2004;23:382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Meyerholz D.K., Gallup J.M., Lazic T., De Macedo M.M.A., Lehmkuhl H.D., Ackermann M.R. Pretreatment with recombinant human vascular endothelial growth factor reduces virus replication and inflammation in a perinatal lamb model of respiratory syncytial virus infection. Viral Immunol. 2007;20:188–196. doi: 10.1089/vim.2006.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliolo L., Silva O.N., Silva P.A., Costa M.P., Costa C.R., Nolasco D.O., Barbosa J.A.R.G., Silva M.R.R., Bemquerer M.P., Lima L.M.P., Romanos M.T.V., Freitas S.M., Magalhães B.S., Franco O.L. Structural and functional characterization of a multifunctional alanine-rich peptide analogue from Pleuronectes americanus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihajlovic M., Lazaridis T. Antimicrobial peptides in toroidal and cylindrical pores. Biochim. Biophys. Acta Biomembr. 2010;1798:1485–1493. doi: 10.1016/j.bbamem.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M., Mori H., Otake T., Ueba N., Kunita N., Niwa M., Murakami T., Iwanaga S. Inhibitory effect of tachyplesin I on the proliferation of human immunodeficiency virus in vitro. Chemotherapy. 1991;37:206–211. doi: 10.1159/000238855. [DOI] [PubMed] [Google Scholar]
- Morita T., Ohtsubo S., Nakamura T., Tanaka S., Iwanaga S., Ohashi K., Niwa M. Isolation and biological activities of limulus anticoagulant (AntiLPS factor) which interacts with lipopolysaccharide (LPS) J. Biochem. 1985;97:1611–1620. doi: 10.1093/oxfordjournals.jbchem.a135218. [DOI] [PubMed] [Google Scholar]
- Mu Y., Huo J., Guan Y., Fan D., Xiao X., Wei J., Li Q., Mu P., Ao J., Chen X. An improved genome assembly for Larimichthys crocea reveals hepcidin gene expansion with diversified regulation and function. Commun. Biol. 2018;1:195. doi: 10.1038/s42003-018-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder K.C.L., Lima L.A., Miranda V.J., Dias S.C., Franco O.L. Current scenario of peptide-based drugs: the key roles of cationic antitumor and antiviral peptides. Front. Microbiol. 2013;4:1–23. doi: 10.3389/fmicb.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münk C., Wei G., Yang O.O., Waring A.J., Wang W., Hong T., Lehrer R.I., Landau N.R., Cole A.M. The θ-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retrovir. 2003;19:875–881. doi: 10.1089/088922203322493049. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Masuda M., Murakami T., Koyanagi Y., Matsumoto A., Fujii N., Yamamoto N. Anti-human immunodeficiency virus activity of a novel synthetic peptide, T22 ([Tyr-5,12, Lys-7]polyphemusin II): a possible inhibitor of virus-cell fusion. Antimicrob. Agents Chemother. 1992;36:1249–1255. doi: 10.1128/AAC.36.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H., Yamamoto N., Masuda M., Fujii N. Defensins inhibit HIV replication in vitro. Aids. 1993;7:1129. doi: 10.1097/00002030-199308000-00019. [DOI] [PubMed] [Google Scholar]
- Nemeth E. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (80-. ). 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Neves J.V., Ramos M.F., Moreira A.C., Silva T., Gomes M.S., Rodrigues P.N.S. Hamp1 but not Hamp2 regulates ferroportin in fish with two functionally distinct hepcidin types. Sci. Rep. 2017;7:14793. doi: 10.1038/s41598-017-14933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., Shirai T., Kanaya S., Ito Y., Kim K.S. Multidrug treatment with nelfinavir and cepharanthine against COVID-19. bioRxiv. 2020 doi: 10.1101/2020.04.14.039925. [DOI] [Google Scholar]
- Park C.B., Lee J.H., Park I.Y., Kim M.S., Kim S.C. A novel antimicrobial peptide from the loach, Misgurnus anguillicaudatus. FEBS Lett. 1997;411:173–178. doi: 10.1016/S0014-5793(97)00684-4. [DOI] [PubMed] [Google Scholar]
- Park C.H., Valore E.V., Waring A.J., Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- Parvez M.S.A., Karim M.A., Hasan M., Jaman J., Karim Z., Tahsin T.…Hosen M.J. Prediction of potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2 using comprehensive drug repurposing and molecular docking approach. International journal of biological macromolecules. 2020;163:1787–1797. doi: 10.1016/j.ijbiomac.2020.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H., Liu H.-P., Chen B., Hao H., Wang K.-J. Optimized production of scygonadin in Pichia pastoris and analysis of its antimicrobial and antiviral activities. Protein Expr. Purif. 2012;82:37–44. doi: 10.1016/j.pep.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Pereiro P., Figueras A., Novoa B. A novel hepcidin-like in turbot (Scophthalmus maximus L.) highly expressed after pathogen challenge but not after iron overload. Fish Shellfish Immunol. 2012;32:879–889. doi: 10.1016/j.fsi.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Primor N., Tu A.T. Conformation of pardaxin, the toxin of the flatfish Pardachirus marmoratus. Biochim. Biophys. Acta - Protein Struct. 1980;626:299–306. doi: 10.1016/0005-2795(80)90124-5. [DOI] [PubMed] [Google Scholar]
- Qureshi A., Thakur N., Tandon H., Kumar M. AVPdb: a database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 2014;42:D1147–D1153. doi: 10.1093/nar/gkt1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanbabu V., Chen J.Y. Antiviral function of tilapia hepcidin 1-5 and its modulation of immune-related gene expressions against infectious pancreatic necrosis virus (IPNV) in Chinook salmon embryo (CHSE)-214 cells. Fish Shellfish Immunol. 2011;30:39–44. doi: 10.1016/j.fsi.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Rashid M.A., Gustafson K.R., Cartner L.K., Shigematsu N., Pannell L.K., Boyd M.R. Microspinosamide, a New HIV-inhibitory cyclic depsipeptide from the marine sponge Sidonops microspinosa. J. Nat. Prod. 2001;64:117–121. doi: 10.1021/np0002379. [DOI] [PubMed] [Google Scholar]
- Reddy K.V.R., Yedery R.D., Aranha C. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents. 2004;24:536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Rinehart K., Gloer J., Hughes R., Renis H., McGovren J., Swynenberg E., Stringfellow D., Kuentzel S., Li L. Didemnins: antiviral and antitumor depsipeptides from a caribbean tunicate. Science (80-. ). 1981;212:933–935. doi: 10.1126/science.7233187. [DOI] [PubMed] [Google Scholar]
- Robinson W.E., McDougall B., Tran D., Selsted M.E. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 1998;63:94–100. doi: 10.1002/jlb.63.1.94. [DOI] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., Seilmaier M., Drosten C., Vollmar P., Zwirglmaier K., Zange S., Wölfel R., Hoelscher M. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M.E., Harwig S.S., Ganz T., Schilling J.W., Lehrer R.I. Primary structures of three human neutrophil defensins. J. Clin. Invest. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shike H., Lauth X., Westerman M.E., Ostland V.E., Carlberg J.M., Van Olst J.C., Shimizu C., Bulet P., Burns J.C. Bass hepcidin is a novel antimicrobial peptide induced by bacterial challenge. Eur. J. Biochem. 2002;269:2232–2237. doi: 10.1046/j.1432-1033.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- Silphaduang U., Noga E.J. Peptide antibiotics in mast cells of fish. Nature. 2001;414:268–269. doi: 10.1038/35104690. [DOI] [PubMed] [Google Scholar]
- Silphaduang U., Colorni A., Noga E.J. Evidence for widespread distribution of piscidin antimicrobial peptides in teleost fish. Dis. Aquat. Org. 2006;72:241–252. doi: 10.3354/dao072241. [DOI] [PubMed] [Google Scholar]
- Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S.M., Bruzzone R., Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalickova S., Heger Z., Krejcova L., Pekarik V., Bastl K., Janda J., Kostolansky F., Vareckova E., Zitka O., Adam V., Kizek R. Perspective of use of antiviral peptides against influenza virus. Viruses. 2015;7:5428–5442. doi: 10.3390/v7102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V.J., Desbois A.P., Dyrynda E.A. Conventional and unconventional antimicrobials from fish, marine invertebrates and micro-algae. Mar. Drugs. 2010;8:1213–1262. doi: 10.3390/md8041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somboonwiwat K., Marcos M., Tassanakajon A., Klinbunga S., Aumelas A., Romestand B., Gueguen Y., Boze H., Moulin G., Bachere E. Recombinant expression and anti-microbial activity of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2005;29:841–851. doi: 10.1016/j.dci.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Steinstraesser L., Tippler B., Mertens J., Lamme E., Homann H.-H., Lehnhardt M., Wildner O., Steinau H.-U., Überla K. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology. 2005;2:2. doi: 10.1186/1742-4690-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A., Weber F., Kochs G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart P.J., Kuipers E.M., Smit C., Van Der Strate B.W., Harmsen M.C., Meijer D.K. In: Advances in Lactoferrin Research. Advances in Experimental Medicine and Biology. Spik G., Legrand D., Mazurier J., Pierce A., Perraudin J.P., editors. Springer; Boston, MA: 1998. Lactoferrin; pp. 205–213. [PubMed] [Google Scholar]
- Tamamura H., Xu Y., Hattori T., Zhang X., Arakaki R., Kanbara K., Omagari A., Otaka A., Ibuka T., Yamamoto N., Nakashima H., Fujii N. A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem. Biophys. Res. Commun. 1998;253:877–882. doi: 10.1006/bbrc.1998.9871. [DOI] [PubMed] [Google Scholar]
- Taylor S., Massiah A., Lomonossoff G., Roberts L.M., Lord J.M., Hartley M. Correlation between the activities of five ribosome-inactivating proteins in depurination of tobacco ribosomes and inhibition of tobacco mosaic virus infection. Plant J. 1994;5:827–835. doi: 10.1046/j.1365-313X.1994.5060827.x. [DOI] [PubMed] [Google Scholar]
- Tecle T., White M.R., Gantz D., Crouch E.C., Hartshorn K.L. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J. Immunol. 2007;178:8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- Teixeira L.D., Silva O.N., Migliolo L., Fensterseifer I.C.M., Franco O.L. In vivo antimicrobial evaluation of an alanine-rich peptide derived from Pleuronectes americanus. Peptides. 2013;42:144–148. doi: 10.1016/j.peptides.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Thakur N., Qureshi A., Kumar M. AVPpred: collection and prediction of highly effective antiviral peptides. Nucleic Acids Res. 2012;40:W199–W204. doi: 10.1093/nar/gks450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S., Tecle T., Verma A., Crouch E., White M., Hartshorn K.L. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013;94:40–49. doi: 10.1099/vir.0.045013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020;10:313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero Y., Arizcun M., Cortés J., Ramírez-Cepeda F., Guzmán F., Mercado L., Esteban M.Á., Chaves-Pozo E., Cuesta A. NK-lysin, dicentracin and hepcidin antimicrobial peptides in European sea bass. Ontogenetic development and modulation in juveniles by nodavirus. Dev. Comp. Immunol. 2020;103:103516. doi: 10.1016/j.dci.2019.103516. [DOI] [PubMed] [Google Scholar]
- Van Epps H.L. René Dubos: unearthing antibiotics. J. Exp. Med. 2006;203:259. doi: 10.1084/jem.2032fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Eynde J.J. COVID-19: a brief overview of the discovery clinical trial. Pharmaceuticals. 2020;13:65. doi: 10.3390/ph13040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.058. 281-292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. Natural antimicrobial peptides as promising anti-HIV candidates. Curr. Top. Pept. Protein Res. 2012;13:93. [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-D., Kung C.-W., Chen J.-Y. Antiviral activity by fish antimicrobial peptides of epinecidin-1 and hepcidin 1–5 against nervous necrosis virus in medaka. Peptides. 2010;31:1026–1033. doi: 10.1016/j.peptides.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Wang G., Li X., Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Chen, Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Cheng, Wang S., Li D., Zhao X., Han S., Wang T., Zhao G., Chen Y., Chen F., Zhao J. Lectin-like intestinal defensin inhibits 2019-nCoV spike binding to ACE2. bioRxiv. 2020 doi: 10.1101/2020.03.29.013490. [DOI] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yeming, Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Yi, Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Hameed M., Wang X., Zhang J., Guo S., Anwar M.N., Pang L., Liu K., Li B., Shao D., Qiu Y., Zhong D., Zhou B., Ma Z. Antiviral activity of phage display-selected peptides against Japanese encephalitis virus infection in vitro and in vivo. Antivir. Res. 2020;174:104673. doi: 10.1016/j.antiviral.2019.104673. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Quiñones-Mateu M.E., Lederman M.M. Role of Human β-defensins in HIV Infection. Adv. Dent. Res. 2006;19:42–48. doi: 10.1177/154407370601900109. [DOI] [PubMed] [Google Scholar]
- WHO WHO Coronavirus Disease (COVID-19) Dashboard [WWW Document] 2020. https://covid19.who.int/ (accessed 10.30.20)
- Wohlford-Lenane C.L., Meyerholz D.K., Perlman S., Zhou H., Tran D., Selsted M.E., McCray P.B. Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J. Virol. 2009;83:11385–11390. doi: 10.1128/JVI.01363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woramongkolchai N., Supungul P., Tassanakajon A. The possible role of penaeidin5 from the black tiger shrimp, Penaeus monodon, in protection against viral infection. Dev. Comp. Immunol. 2011;35:530–536. doi: 10.1016/j.dci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (80-. ). 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xia S., Xu W., Wang Q., Wang C., Hua C., Li W., Lu L., Jiang S. Peptide-based membrane fusion inhibitors targeting HCoV-229E spike protein HR1 and HR2 domains. Int. J. Mol. Sci. 2018;19:487. doi: 10.3390/ijms19020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.-T.K., Wang Q., Du L., Tan W., Wilson I.A., Jiang S., Yang B., Lu L. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhao Z., He Y., Wu L., Cai D., Hong W., Wu Y., Cao Z., Zheng C., Li W. A new natural α-helical peptide from the venom of the scorpion Heterometrus petersii kills HCV. Peptides. 2011;32:11–19. doi: 10.1016/j.peptides.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Yang D., Leibowitz J.L. The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 2015;206:120–133. doi: 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin B., Wang W., Pang M., Cheshenko N., Hong T., Waring A.J., Herold B.C., Wagar E.A., Lehrer R.I. θ defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 2004;78:5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhan S., Aweya J.J., Wang F., Yao D., Zhong M., Chen J., Li S., Zhang Y. Litopenaeus vannamei attenuates white spot syndrome virus replication by specific antiviral peptides generated from hemocyanin. Dev. Comp. Immunol. 2019;91:50–61. doi: 10.1016/j.dci.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181:223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yu W., He T., Yu J., Caffrey R.E., Dalmasso E.A., Fu S., Pham T., Mei J., Ho J.J., Zhang W., Lopez P., Ho D.D. Contribution of human -defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science (80-. ). 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yu L., Li M., Sun L. Turbot (Scophthalmus maximus) hepcidin-1 and hepcidin-2 possess antimicrobial activity and promote resistance against bacterial and viral infection. Fish Shellfish Immunol. 2014;38:127–134. doi: 10.1016/j.fsi.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Zhang M., Li M., Sun L. NKLP27: a teleost NK-lysin peptide that modulates immune Response, induces degradation of bacterial DNA, and inhibits bacterial and viral infection. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Wei, Du R.H., Li B., Zheng X.S., Yang X. Lou, Hu B., Wang Y.Y., Xiao G.F., Yan B., Shi Z.L., Zhou P. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Wen, Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhou J., Zhang K., Chu H., Liu D., Poon V.K.-M., Chan C.C.-S., Leung H.-C., Fai N., Lin Y.-P., Zhang A.J.-X., Jin D.-Y., Yuen K.-Y., Zheng B.-J. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci. Rep. 2016;6:22008. doi: 10.1038/srep22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Toe K.K.W., Sze K.-H., Yung T.T.-M., Bian M., Lam H., Li C., Chu H., Yuen K.-Y. A broad-spectrum virus-and host-targeting antiviral peptide against SARS-CoV-2 and other respiratory viruses. Res. Sq. 2020 doi: 10.21203/rs.3.rs-18687/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-Cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.-G., Wei J.-G., Xu D., Cui H.-C., Yan Y., Ou-Yang Z.-L., Huang X.-H., Huang Y.-H., Qin Q.-W. Molecular cloning and characterization of two novel hepcidins from orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2011;30:559–568. doi: 10.1016/j.fsi.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Lei Y., Cao Z., Chen X., Sun Y., Xu Y., Guo W., Wang S., Liu C. A β-defensin gene of Trachinotus ovatus might be involved in the antimicrobial and antiviral immune response. Dev. Comp. Immunol. 2019;92:105–115. doi: 10.1016/j.dci.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. Discovery of six families of fungal defensin-like peptides provides insights into origin and evolution of the CSαβ defensins. Mol. Immunol. 2008;45:828–838. doi: 10.1016/j.molimm.2007.06.354. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Mercier C., Koussounadis A., Secombes C. Discovery of multiple beta-defensin like homologues in teleost fish. Mol. Immunol. 2007;44:638–647. doi: 10.1016/j.molimm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;1–8 doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]